Abstract
Objective: Our objective was to test the antidepressant effect of transcranial photobiomodulation (t-PBM) with near-infrared (NIR) light in subjects suffering from major depressive disorder (MDD).
Background: t-PBM with NIR light is a new treatment for MDD. NIR light is absorbed by mitochondria; it boosts cerebral metabolism, promotes neuroplasticity, and modulates endogenous opioids, while decreasing inflammation and oxidative stress.
Materials and methods: We conducted a double-blind, sham-controlled study on the safety and efficacy [change in Hamilton Depression Rating Scale (HAM-D17) total score at end-point] of adjunct t-PBM NIR [823 nm; continuous wave (CW); 28.7 × 2 cm2; 36.2 mW/cm2; up to 65.2 J/cm2; 20–30 min/session], delivered to dorsolateral prefrontal cortex, bilaterally and simultaneously, twice a week, for 8 weeks, in subjects with MDD. Baseline observation carried forward (BOCF), last observation carried forward (LOCF), and completers analyses were performed.
Results: The effect size for the antidepressant effect of t-PBM, based on change in HAM-D17 total score at end-point, was 0.90, 0.75, and 1.5 (Cohen's d), respectively for BOCF (n = 21), LOCF (n = 19), and completers (n = 13). Further, t-PBM was fairly well tolerated, with no serious adverse events.
Conclusions: t-PBM with NIR light demonstrated antidepressant properties with a medium to large effect size in patients with MDD. Replication is warranted, especially in consideration of the small sample size.
Keywords: : depression, low-level laser therapy, randomized controlled trial
Introduction
Twenty-one million Americans (about 10%) suffered from a depressive episode over the past year.1 Major depressive disorder (MDD) is the third leading cause of global disability2; the global cost of mental illness is expected to more than double by 2030, with depressive disorders at the forefront.3 Sadly, 43% of primary care patients who experience a 6-month anxiety or depressive disorder diagnosis do not receive treatment, with most preferring self-management.4 Although registered medications are proven cost-effective antidepressants,5 many people in mild to moderate psychological distress prefer self-help over professional help or prescription medications.6 Limiting factors—which might contribute to undertreatment—with current first-line intervention for MDD are as follows: (1) the burdensome side effects associated with pharmacological treatments7,8 and (2) the need for frequent sessions and specialized professionals when opting for evidence-based psychotherapies.9 Device-based interventions for MDD, such as electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation, are approved as third- or fourth-line treatments for resistant depression.10 Device-based antidepressant treatment, which is safe, inexpensive, and easy to administer at home, could represent a valuable first-line option for depressed patients who prefer complementary and alternative medicine.
Transcranial photobiomodulation (t-PBM) with near-infrared radiation (NIR) has emerged as a potential antidepressant treatment in both animal models11–15 and human studies.16–19 t-PBM consists of delivering NIR—or red light—to the scalp of the patient, which penetrates the skull and modulates function of the adjacent cortical areas of the brain. PBM with red light and/or NIR appears to increase brain metabolism (by activating the cytochrome C oxidase in the mitochondria), to increase neuroplasticity, and to modulate endogenous opioids, while decreasing inflammation and oxidative stress.20–26 t-PBM penetrates deeply into the cerebral cortex,27–29 modulates cortical excitability,30,31 and improves cerebral perfusion32–34 and oxygenation.35 Studies have suggested that it can significantly improve cognition in healthy subjects,36–38 and in subjects with traumatic brain injury (TBI).39–41 The safety of t-PBM has been studied in a sample of acute 1410 stroke patients, with no significant differences in rates of adverse events between t-PBM and sham exposure.42–44 Uncontrolled studies suggest an antidepressant effect of t-PBM in subjects suffering from MDD.16–19
Aims of the study
We report here on the first, randomized, double-blind, sham-controlled, pilot trial of the antidepressant effect of t-PBM in MDD patients.
Materials and Methods
This single-site study—Evaluation of LEDs Therapeutic Effect in Depression (ELATED-2)—was approved by the Massachusetts General Hospital (MGH) institutional review board (IRB). Recruitment began in February 2014, and the study was completed in August 2015. The main sources of recruitment were weekly Craigslist advertisements and people calling in to the general research line of the MGH Depression Clinical and Research Program.
Inclusion and exclusion criteria
Adult subjects (18–65 years of age) meeting the (Structured Clinical Interview for the DSM-IV—Diagnostic Statistical Manual, Fourth Edition) criteria for MDD, with at least a moderate degree of depression severity [Hamilton Depression Rating Scale (HAM-D17) total score ranging 14–24], were included in the study after providing written informed consent. The MGH IRB required a maximum permitted HAM-D17 score of 24 to prevent inclusion of patients at greater risk of suicide. During this episode, subjects could have failed no more than one FDA-approved antidepressant medication (for at least 6 weeks) and no more than one course of structured psychotherapy for depression (for at least 8 weeks). Other exclusionary conditions included active substance use disorders (prior 6 months), lifetime psychotic episodes, bipolar disorder, active suicidal ideation, and homicidal ideation, in addition to unstable medical illness and recent stroke (prior 3 months). Women of child-bearing potential were required to use a birth control method if sexually active; pregnancy and lactation were exclusionary. To allow maximum light penetration and minimize potential risks of local tissue damage from the use of NIR, the following conditions were also exclusionary: (1) having a forehead skin condition likely to impede light penetration, such as tattoo or birth mark; (2) taking a light-activated medication (prior 14 days); and (3) having a head implant; the latter criterion to prevent possible dislodgement associated with vasodilation, as in the case of endovascular stents.
Study design and treatment
Eligible subjects were randomized to an 8-week study with twice weekly double-blind t-PBM NIR versus sham treatment. At each treatment session, NIR or sham was administered to the forehead bilaterally, simultaneously [Omnilux New U, light emitting diode, manufactured by Photomedex, Inc., Montgomeryville, PA—see Supplement on Technology section in Supplementary Data (see Supplementary Data at www.liebertpub.com/pho) and Figs. 1 and 2 for placements and dosimetry]. The choice of an LED device, as opposed to a laser device, was supported by a prior study on t-PBM for MDD.16 The device used for this study emitted NIR at a wavelength of 823 nm, corresponding to the peak absorption spectrum for our biological target: cytochrome C oxidase.45 In cadaver heads, the same device delivered 2% of the light at a penetration depth of 1 cm from the skin surface on frontal areas.27 A 2% penetration rate allows an NIR energy density equivalent to the fluence inducing neurological benefit in animal models [fluence: 0.85–1.27 J/cm2, not accounting for blood-related attenuation of light on the prefrontal cortex (i.e., optical energy per unit area, expressed in J/cm2)].22 As we were targeting the dorsolateral prefrontal cortex (dlPFC), we simultaneously directed the NIR to the F3 (left) and F4 (right) sites on the forehead—derived from the electroencephalography (EEG) placement map.16 Since previous studies suggested that 1 and 6 sessions of t-PBM were insufficient to determine a sustained antidepressant response,16,18 we extended the course of t-PBM to 8 weeks with a total of 16 sessions (out of 18 planned visits to the clinic); twice a week sessions had been acceptable and well tolerated in our proof-of-concept study.18 The study clinician had the option to adjust the duration of light exposure after completion of week 3 and 5 (after 6 and 10 sessions, respectively) from 20 min (∼40 J/cm2) to 25 (∼50 J/cm2) and 30 min (∼60 J/cm2), respectively. Instructions were to increase exposure per protocol, as tolerated, to maximize the antidepressant effect. Each device had a treatment window of 28.7 cm2, so the maximum cumulative dose over the entire treatment course was 43.7 kJ. The exposure time was designed to allow a fluence of 60 J/cm2, despite relatively low power density (irradiance) of 33.2 mW/cm2—based on settings reported by the manufacturer. Similar and greater NIR fluences have been associated with antidepressant response and improved cognition in prior reports.16,18,36 All, but three subjects remained on stable antidepressant treatment during the trial; their data were censored after change in concomitant psychoactive therapies (their last available assessment before change in therapies was used as end-point).
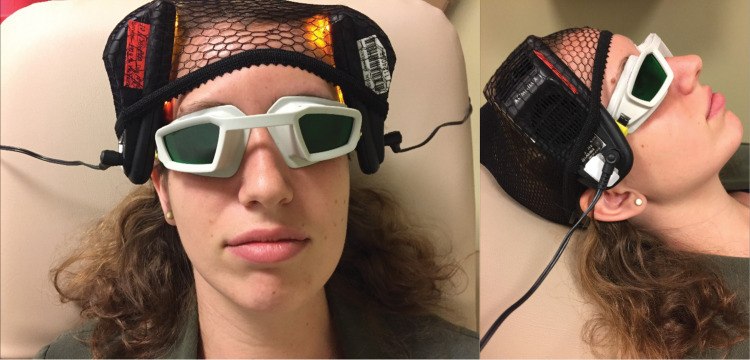
FIG. 1.
The pictures show the handheld portions of the Omnilux New U devices, which are pressed against the forehead of the subject, bilaterally and simultaneously on the F3 and F4 sites (reference to EEG placement sites).
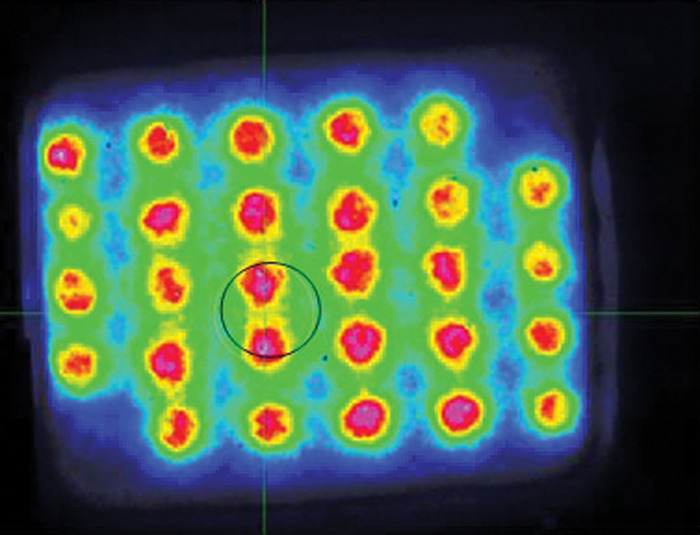
FIG. 2.
The picture shows the intensity gradient for the NIR produced by the 28 LEDs of the Omnilux New U device (with a color scale depicting the highest intensity in red). A spot of maximum average intensity across 1 cm2 aperture is indicated by the black circle. Active device #1124053. NIR, near infrared.
Randomization and blinding
Two t-PBM device types were available for each modality (NIR and sham). The apparent behavior of the devices was identical for both modalities: noise upon working, feeling of warmth, and visual signs were indistinguishable. However, only NIR mode t-PBM device produced the therapeutic NIR energy. NIR light is invisible and undetectable to patients and physicians. The study research assistant used permuted block randomization with varying block sizes to randomize subjects in 1:1 manner to each pair of instruments as “A” and “B.” Only the research assistant was able to identify each pair of instruments as “A” and “B.” The investigators and the subjects remained blind to the subject assignment, since the label on each device was covered before treatment administration. Photomedex, Inc. provided the blinding codes of NIR and sham for each labeled pair of devices, which were kept in a sealed envelope at the study site.
Clinical outcome measures
The primary outcome measure was the total score of the HAM-D17 for depressive symptoms,46 in accordance to our initial report before study enrollment (Clinicaltrials.gov). The HAM-D17 scale—with 17 items—aims to quantify the degree of depression in patients who already have a diagnosis of MDD. Questions focus on neurovegetative and other depressive symptoms experienced over the past 7 days. Answers to questions are rated on a scale of 0–4 or 0–2, with higher scores indicating more severe pathology. Scores on the HAM-D17 typically fall into the following ranges: (1) not depressed = 0–7; (2) mildly depressed = 8–13; (3) moderately depressed = 14–18; (4) severely depressed = 19–22; and (5) very severely depressed = 23 and over. The Clinical Global Impression−Improvement (CGI-I) subscale was adopted as a secondary measure for the overall clinical benefit.47 The total score of the Quick Inventory of Depressive Symptomatology (QIDS) was used as a self-rated outcome measure of depression.48 Tolerability was assessed with an Adverse Event Form, which allowed the recording of an adverse event's description, start and end dates, intensity and seriousness, relation to the treatment, as well as any action taken and final outcome. A specific semistructured scale, the Transcranial Light Therapy Self-Report Questionnaire (T-SR-Q) was completed by participants at week 4 and 8. The scale explored any discomfort related to the treatment sessions, to detect potential reasons of unblinding. Blinding questionnaires also tracked the subject and clinician beliefs of assignment to active treatment at week 4 and at week 8.
Study sample and data cleaning
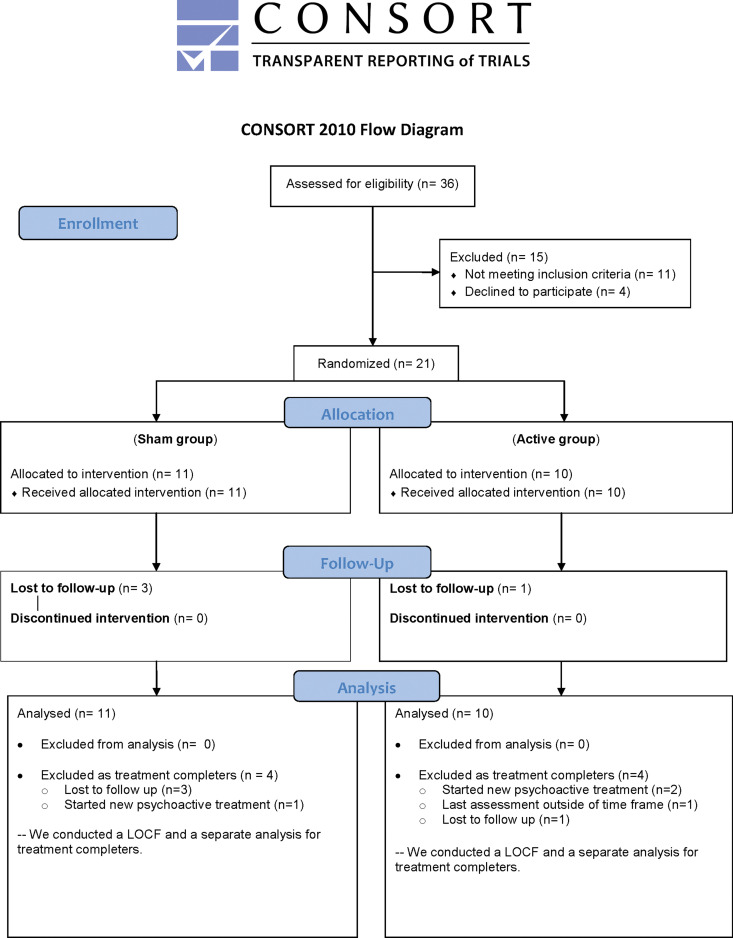
Twenty-one subjects were randomized, received at least one t-PBM session, and were included in the analyses (Table 1A, B). For self-rated scales (secondary measures), the sample is limited to 20 subjects, since 1 subject (#4) randomized to NIR consistently skipped several answers across scales and for the duration of the study. This subject carried a diagnosis of attention-deficit disorder and reported significant difficulties concentrating while off stimulants. The completers—who were followed for the entire 8-week study period and who received a clinical assessment immediately after—were 13 subjects. As briefly mentioned, three subjects (#6, #10, #19) were censored as part of the a-priori data cleaning process due to their starting a new psychoactive treatment during the course of the study. One more subject (#2), who completed the 8 weeks of t-PBM sessions, performed follow-up assessment outside the permitted timeframe and the end-point was set a priori as the penultimate assessment. Four more subjects were lost to follow-up: two of them after 2 and 3 weeks of t-PBM sessions (#5, #14), while the remaining two subjects (#20, #21) failed to return to the clinic for their 1-week visit and therefore had no post-treatment assessments (see Fig. 3 for the subjects' flow chart).
Table 1.
Diagnosis, Treatment, Depression Outcome, and Adverse Events in Subjects Treated with Transcranial Photobiomodulation NIR Mode (A) and Sham Mode (B)
| Subjects on t-PBM NIR mode | Age | Gender | Current diagnosis | Concomitant antidepressant treatment (duration) | Treatment sites on forehead | Number of t-PBM sessions (NIR) | Maximum duration of t-PBM session (min) | Baseline HAM-D17 | Last visit HAM-D17 (weeks of t-PBM) | Adverse events (day of onset) |
|---|---|---|---|---|---|---|---|---|---|---|
| A. NIR mode t-PBM group | ||||||||||
| #1 | 49 | F | MDD, PD; SAD; PTSD | Sertraline 200 mg/day (≥6 weeks) | F3, F4 proximity | 16 | 30 | 19 | 10 (8 weeks) | None |
| #2 | 58 | F | MDD | Psychotherapy (>12 weeks) | F3, F4 proximity | 14 | 30 | 19 | 18 (7 weeks) | Day 47: gastrointestinal sickness |
| #3 | 64 | M | MDD, PTSD | None | F3, F4 | 14 | 30 | 21 | 3 (8 weeks) | Day 0: restless sleep Day 2: headaches Day 7: taste illusion Day 10: abdominal bloating Day 50: gout |
| #4 | 24 | F | MDD, PTSD, OCD | None | Fp1, Fp2 | 10 | 30 | 28 | 16 (8 weeks) | Day 50: self-harm |
| #5 | 27 | M | MDD, Anxiety NOS | None | F3, F4 | 4 | 20 | 16 | 6 (2 weeks) | Day 2: insomnia |
| #6 | 39 | M | MDD | None | F3, F4 | 10 | 25 | 18 | 18 (5 weeks) | None |
| #7 | 41 | M | MDD | Psychotherapy (>2 years) | F3, F4 | 15 | 30 | 21 | 3 (8 weeks) | Day 0: insomnia Day 14: irritable mood |
| #8 | 44 | F | MDD | Venlafaxine 75 mg/day (6 weeks) | F3, F4 proximity | 16 | 30 | 21 | 0 (8 weeks) | Day 0: visual illusions (vivid colors) |
| #9 | 50 | F | MDD | None | F3, F4 proximity | 15 | 25 | 21 | 5 (8 weeks) | Day 0: visual illusion (vivid colors) Day 35: irritable mood Day 47: decreased memory; word finding difficulties Day 56: irritable mood |
| #10 | 50 | F | MDD, GAD, PTSD | Nortriptylinea 50 mg/day (>2 years) Citalopram 40 mg/day (24 weeks) |
F3, F4 | 1 | 20 | 22 | 19 (3 weeks) | None |
| B. Sham mode t-PBM group | ||||||||||
| #11 | 52 | M | MDD, PTSD, GAD | Psychotherapy (≥8 weeks) | F3, F4 proximity | 14 | 30 | 19 | 19 (8 weeks) | None |
| #12 | 50 | F | MDD, Anxiety NOS | None | Fp1, Fp2 | 16 | 30 | 19 | 22 (8 weeks) | None |
| #13 | 64 | F | MDD | None | F3, F4 | 14 | 30 | 26 | 17 (8 weeks | None |
| #14 | 48 | M | MDD, GAD, SAD | None | F3, F4 | 6 | 20 | 20 | 19 (3 weeks) | None |
| #15 | 29 | M | MDD, GAD, SAD | None | F3, F4 | 15 | 30 | 22 | 10 (8 weeks) | Day 15: left torso pain Day 28: insomnia |
| #16 | 64 | F | MDD, GAD | Venlafaxine 75 mg/day Psychotherapy (both 2 years) |
Fp1, Fp2 | 16 | 30 | 19 | 5 (8 weeks) | None |
| #17 | 24 | F | MDD | None | F3, F4 | 16 | 30 | 17 | 20 (8 weeks) | None |
| #18 | 60 | M | MDD | Fluoxetine 20 mg/day (1 year) |
F3, F4 proximity | 16 | 30 | 16 | 2 (8 weeks) | Day 14: headaches Day 13: belching |
| #19 | 53 | F | MDD | None | F3, F4 | 8 | 25 | 14 | 10 (4 weeks) | None |
| #20 | 62 | F | MDD | None | F3, F4 proximity | 2 | 20 | 22 | — (1 week) | — |
| #21 | 52 | F | MDD, PTSD | Wellbutrin 300 mg/day Quetiapine 75 mg/day (both ≥6 weeks) Psychotherapy (≥8 weeks) |
F3, F4 | 2 | 20 | 21 | — (1 week) | — |
Grayed rows: for study subjects who did not complete the 8-week study or missed the assessment right after.
Day 0: less than 24 h from first t-PBM session.
“—” missing assessment due to early dropout.
Subject #10 was already on nortriptyline maintenance when she experienced her index episode and failed to respond to citalopram during her index episode.
F3, F4, EEG mapping sites corresponding to the dorsolateral prefrontal cortex; Fp1, Fp2, EEG mapping sites corresponding to the frontal poles.
EEG, electroencephalography; GAD, generalized anxiety disorder; HAM-D17, Hamilton Depression Rating Scale; MDD, major depressive disorder; NIR, near infrared radiation; OCD, obsessive compulsive disorder; PD, panic disorder; PTSD, post-traumatic stress disorder; SAD, social anxiety disorder; t-PBM, transcranial photobiomodulation.
FIG. 3.
CONSORT clinical trial flow diagram.
Analyses
We tested our study hypothesis that t-PBM NIR mode will decrease HAM-D17 scores in study subjects significantly more than the sham. The dependent variable was our primary outcome of depression severity (as measured by the HAM-D17 total score); the independent variable was our comparison, the NIR and sham groups. We used three approaches: (1) intent-to-treat approach with baseline observation carried forward (BOCF; n = 21),49 (2) intent-to-treat approach with last observation carried forward (LOCF; n = 19),50 and (3) completers sample (n = 13). The LOCF approach allows the inclusion in analyses of subjects who received treatments and had at least one assessment after baseline; their last available assessment is considered the end-point and used for the analyses. The BOCF approach differs from the LOCF as it also allows the inclusion in the analyses of subjects who received treatments, but did not complete any assessments afterwards; for these isolated cases, the baseline assessment is also the end-point (all other subjects' data are computed as in the LOCF). The completers' approach restricts the analyses to subjects who finished the 8-week trial and therefore, for all completers, the assessment after completion is used as end-point for the analyses. A Mann–Whitney U test was chosen—after noting skewed distribution of outcome data—comparing the change in the total severity score from baseline to end-point. To calculate the effect size of t-PBM, we adopted the Cohen's d formula for the change of HAM-D17 total score from baseline to end-point. We examined post hoc the self-rated QIDS total score for depression (BOCF, LOCF, and completers approach). We also compared the overall rates of antidepressant response and remission at end-point for the two groups. Rates of antidepressant response and remission were calculated according to the HAM-D17 total score (≥50% decrease and score ≤7, respectively) and the CGI-I scale (response equal to score 1 or 2). All response and remission rates were compared by Pearson's chi-square test. For any type of adverse event, we reported its frequency and described its characteristics, relation to the treatment, any action taken, and final outcome. Baseline characteristics for the two groups were compared by Mann–Whitney U test and Pearson's chi-square test, respectively, for continuous and nominal variables. To assess blinding, Pearson's chi-square test was used to determine if the distribution of subjects across treatment groups differed from the relative guess of clinicians and subjects; a percentage was reported to indicate the proportion of subjects whose assignment was correctly guessed. For all analyses, significance was set at p ≤ 0.05.
Results
Baseline characteristics
There were no significant differences among the two groups at baseline in terms of demographic and clinical characteristics as well as concurrent antidepressant treatment (Table 2), except for a history of more MDD episodes in the t-PBM NIR group (mean 4.3 ± 1.7 vs. 2.6 ± 1.8; z = 1.988, p = 0.047).
Table 2.
Baseline Demographic and Clinical Characteristics of Subjects Receiving Transcranial Photobiomodulation in NIR and Sham Mode
| NIR (n = 10) | Sham (n = 11) | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years, mean (SD) | 45.0 (12.8) | 50.7 (13.3) | ns |
| Gender, female, n (%) | 6 (60) | 6 (56) | ns |
| Race, n (%) | ns | ||
| White | 9 (90) | 10 (91) | |
| Black or African American | 0 | 1 (9) | |
| Asian | 1 (10) | 0 | |
| Ethnicity, n (%) | |||
| Non-Hispanic | 10 (100) | 11 (100) | |
| Clinical characteristics, mean (SD) | |||
| HAM-D17 total score | 20.6 (3.2) | 20.2 (4.3) | ns |
| CGI-severity | 4.4 (0.5) | 4.4 (0.5) | ns |
| Months of current MDD episode | 14.5 (15.4) | 19.9 (22.0) | ns |
| Age of MDD onset | 15.5 (6.7) | 31.6 (19.6) | ns |
| Number of MDD episodes | 4.3 (1.7) | 2.6 (1.8) | 0.047 |
| Number of hospitalizations | 1.0 (1.3) | 1.3 (2.4) | ns |
| Current antidepressant treatment, n (%) | |||
| None | 5 (50) | 7 (64) | |
| Psychotherapy alone | 2 (20) | 1 (9) | |
| Antidepressant medication(s)—no psychotherapy | 3 (30) | 1 (9) | |
| Antidepressant medication(s) and psychotherapy | 0 | 2 (18) | |
ns, nonsignificant (p > 0.05).
CGI, Clinical Global Impression; HAM-D17, Hamilton Depression Rating Scale; MDD, major depressive disorder; NIR, near infrared radiation; SD, standard deviation.
Prior and baseline antidepressant treatment
Roughly half of the sample in the NIR mode (40%; n = 4) and in the sham mode (64%; n = 7) groups had not received an antidepressant medication or psychotherapy during this MDD episode. Three subjects per group had tried psychotherapy during this episode. Three NIR and two sham subjects had tried one antidepressant medication during this episode. Two and one subjects in the NIR and sham group, respectively, had undergone two medication trials. During the study, all subjects continued their baseline antidepressant treatment, if any (Table 1A, B), except subject #8 who discontinued her psychotherapy at baseline. During the study, the maximum exposure time (min) per session of t-PBM was nearly identical in the two groups (mean 26.5 ± 4.12 vs. 26.8 ± 4.62; z = −0.316, p = 0.752).
Antidepressant effect according to clinician-rated measurement
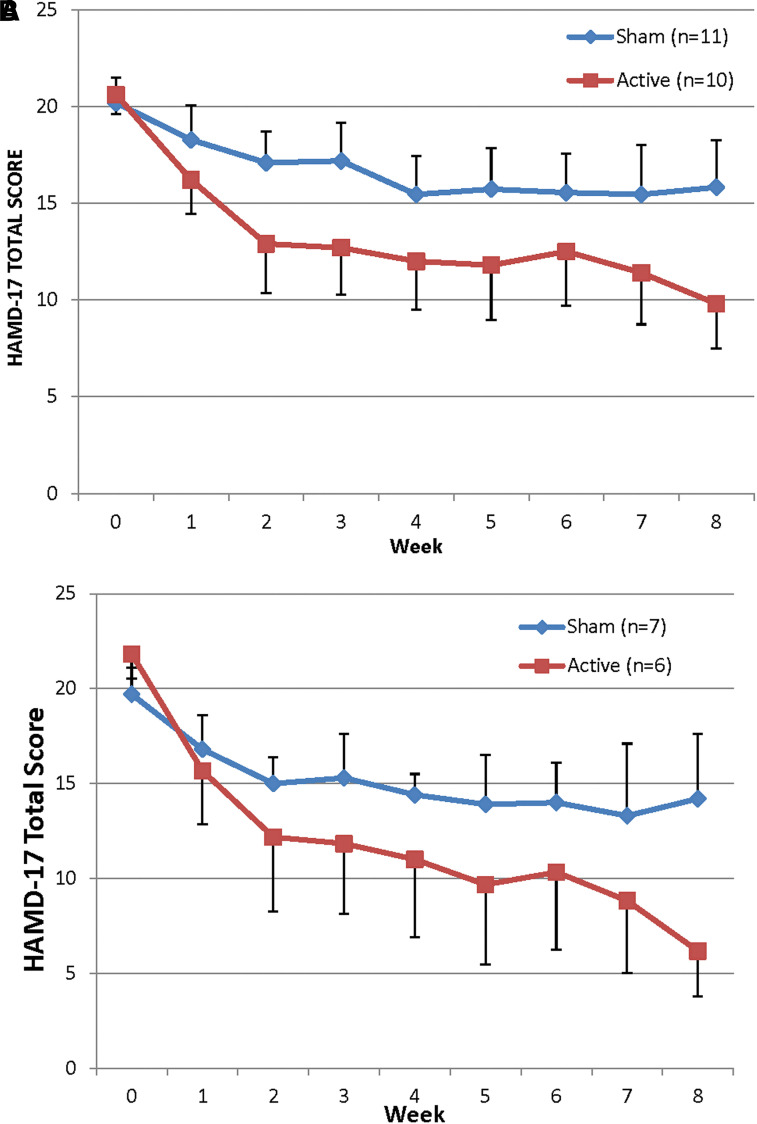
The mean change in HAM-D17 total score in subjects receiving t-PBM in NIR mode was significantly greater than in subjects receiving sham mode with the BOCF approach [NIR (n = 10) −10.8 ± 7.55 vs. sham (n = 11) −4.4 ± 6.65; z = 1.982, p = 0.047] and with the completers approach [NIR (n = 6) −15.7 ± 4.41 vs. sham (n = 7) −6.1 ± 7.86; z = 2.158, p = 0.031]. However, the threshold for significance was not reached with the LOCF approach [NIR (n = 10) −10.8 ± 7.55 vs. sham (n = 9) −5.33 ± 7.04; z = 1.556, p = 0.119]. Figure 4 illustrates the mean HAM-D17 total scores over the course of the study for the two t-PBM groups (BOCF and completers). The effect size for the antidepressant effect of t-PBM, based on change in HAM-D17 total score at end-point, was 0.90, 0.75, and 1.5 (Cohen's d), respectively, for BOCF, LOCF, and completers.
FIG. 4.
Graph of mean HAM-D17 total score in subjects treated with NIR light (red line) and in subjects treated with sham (blue line); (A) all study subjects included (n = 21); (B) only completers included (n = 13). (A) All subjects (intent to treat—end-point carried forward; mean ± SE). (B) Completers only (imputed values for three missing data points from interim visits; mean ± SE). Error bars on one side of each line. HAM-D17, Hamilton Depression Rating Scale; SE, standard error.
Antidepressant effect according to self-rated measurement
In the post hoc analyses, the antidepressant effect of t-PBM NIR mode, measured by self-rated QIDS total scores, approached significance only in completers (BOCF: n = 20, −5.3 ± 5.81 vs. −3.0 ± 3.00; z = 0.877, p = 0.380; LOCF: n = 18, −5.3 ± 5.81 vs. −3.7 ± 2.91; z = 0.576, p = 0.565; and completers: n = 12, −9.8 ± 4.09 vs. −4.3 ± 3.04; z = 1.874, p = 0.061).
Antidepressant response and remission rates
At end-point, response and remission per the HAM-D17 occurred in 5 out of 10 (50%) subjects in the NIR mode. In the sham mode, response and remission occurred in 3 and 2 subjects out of 11, respectively (27% and 18%) (response: n = 21; χ2 = 1.15; df = 1; p = 0.284 and remission: n = 21; χ2 = 2.39; df = 1; p = 0.122). Response in the NIR mode was attained after 2 weeks of t-PBM (n = 3) and after 3 and 4 weeks (n = 1 for each time point); in the sham mode, it occurred after 3, 4, and 5 weeks of t-PBM (n = 1 for each time point). At end-point, 60% of NIR versus 18% of sham subjects were at least “much improved” according to the CGI (n = 21; χ2 = 3.88; df = 1; p = 0.049).
Blinding of subjects and clinicians
w% rate of correct guesses at week 4 (n = 15; χ2 = 1.03; df = 1; p = 0.310) and 54% at week 8 (n = 11; χ2 = 0.24; df = 1; p = 0.621). However, clinicians' guesses were significantly different among the two groups at both week 4 (n = 14; χ2 = 4.66; df = 1; p = 0.031) and week 8 (n = 10; χ2 = 4.28; df = 1; p = 0.038), with a 79% and 80% rate of correct guesses, respectively.
Adverse events
The t-PBM sessions were well tolerated. None of the adverse events was serious and all, but one had resolved at study end. Only one subject (#9) in the NIR mode group required dose adjustment due to irritable mood, which first became apparent at day 35 (after eight t-PBM sessions and after reaching 25 min of irradiation/session). While some irritable mood persisted with the lower t-PBM dose (20 min), the subject was able to complete the study. After study completion, this subject was contacted by phone by study staff (P.C.), reported complete resolution of irritability. In terms of the relationship of adverse events to the study intervention (t-PBM), only two subjects receiving the sham mode developed an adverse event at least “possibly related” to the intervention according to the investigators: (#15) experienced insomnia at day 28 and (#18) headaches at day 14. Instead, in the NIR mode, five subjects developed one or more adverse events at least “possibly related” to the intervention: three subjects (#3, #5, #7) experienced insomnia (at day-0, 2, 14); three subjects (#8, #9, #3) experienced illusions such as “seeing vivid colors” or “tasting from an ashtray” (at day-0, 0, 7); two subjects (#7, #9) experienced irritable mood (at day-14, 35) and subject #3 experienced headaches at day 2 and abdominal bloating at day 10. Overall, the NIR mode group experienced more adverse events and more early-onset events (first 7 days). Noticeably, in the same group, the onset of several adverse events occurred within 24 h from the first t-PBM session (day-0) and with some adverse events—such as headaches and vivid illusions—occurring within 1 h from the t-PBM session. Illusionary phenomena were sporadic and typically present only after the first sessions and were short lasting (30 min). All, at least “possibly related,” adverse events were either mild or moderate, except in two cases (#3 with severe restless sleep, headaches, and taste illusions and #8 with severe visual illusions). One subject (#4), who only partially responded to t-PBM NIR mode superficially cut her wrists and took five pills of acetominophen with the intent to self-harm (day-50). The subject denied suicidal intent and this event—considered “unrelated” by the study investigator—was neither life-threatening nor required medical attention; the subject reported being upset after an argument with her boyfriend and wanting his attention.
Discussion
Our study demonstrated preliminary evidence of an at least medium effect size for the antidepressant efficacy of t-PBM NIR (Cohen's d ≥ 0.75 for all analyses). t-PBM was fairly well tolerated with none of the adverse events causing study discontinuation and only one case requiring dose adjustment. Attrition rates were the average for clinical trials. The one episode of self-harm during the study was considered unrelated to t-PBM by the study investigator and was neither life-threatening nor required medical attention. While it is unclear if t-PBM might worsen suicidal ideation in some cases, a recent report suggests the opposite.51
Our results are consistent with open-label reports that also demonstrated an antidepressant effect for t-PBM in MDD patients16–19 and with a sham-controlled study on enhancement of attention bias modification for depression with t-PBM.52 The neuromodulatory effect of t-PBM has also been documented in double-blind, randomized, sham-controlled studies in healthy subjects, demonstrating cognitive enhancement of t-PBM.36–38 Our detection of a medium to large effect size of t-PBM (0.75–1.5) in MDD is also noteworthy, however, common for small studies. Interestingly, early-onset adverse events occurred exclusively in the NIR mode group (5/9 subjects) and included unusual and unanticipated perceptual disturbances, such as visual and taste illusions (3/9 subjects). It is possible that raters were biased toward positive outcomes by the report of early adverse events in the t-PBM NIR-group, as raters' correct prediction of t-PBM assignment was 80%. The post hoc analyses of the self-report measure of the antidepressant effect—while not reaching statistical significance in a smaller sample size—showed similar trends in terms of effect size and p-value in completers (p = 0.06), despite the prediction of t-PBM assignment by subjects did not exceed chance (50%).
From a cellular and molecular perspective, the beneficial effect of t-PBM (NIR) on brain metabolism is the primary putative mechanism for its antidepressant effect. In experimental and animal models, PBM (NIR and red light) is absorbed by cytochrome C oxidase and, by stimulating the mitochondrial respiratory chain, leads to increased ATP production.23–25 In a study on healthy subjects, t-PBM (NIR) improved cerebral oxygenation, supposedly through an increase in cerebral blood flow coupled with increased oxygen demands.35 Similarly, a study in MDD subjects found that t-PBM led to a nearly significant increase in regional cerebral blood flow.16 Both studies relied on functional NIR spectroscopy to assess oxygenation and blood flow. In a case report of chronic TBI, the improvement in brain perfusion subsequent to t-PBM was imaged with single-photon emission computerized tomography (SPECT).32 Multiple studies have reported regional and global brain hypometabolism in MDD;53–57 moreover, metabolic abnormalities seem to improve after antidepressant treatment or after recovery.58–60
From a neurophysiological perspective, decreased activity in the prefrontal areas has been implicated in the dysfunction of emotion regulation in depressed patients.61 The emotion regulation circuitry is dependent on top-down regulation, mediated by the prefrontal cortex, in particular by the dlPFC.61 In our study, t-PBM (NIR mode) was simultaneously directed to the F3 F4 EEG reference points of the scalp or in close proximity—in all, but one subject—suggesting that modulation of the dlPFC and the emotion-regulation circuitry might be the mechanism for the antidepressant effect.
Our study has six main limitations: (1) the small sample size precludes generalizability and warrants replication in larger cohorts of MDD patients; the low placebo effect might partly explain the medium to large effect size and the significant findings, despite the small sample; (2) the emergence of early-onset adverse events might have affected the quality of blinding for the clinicians, however, not for study subjects; (3) the two-arm design, with only one NIR regimen, precludes any inference on the ideal parameters of stimulation with regard to clinical efficacy and tolerability; (4) the use of BOCF and of LOCF does not model for potential improvement or worsening of depression after dropout; yet it provides information on overall outcome—accounting for known early dropout in sham-treated subjects62—while the completers analysis is exempt from modeling biases; (5) the lack of long-term follow-up precludes the evaluation of sustainability of the therapeutic effect and long-term tolerability; and (6) the study was not designed to investigate mechanisms; specific studies are needed to correlate clinical effects with the effect of NIR on brain circuitry and metabolism.
Conclusions and Summary
t-PBM with NIR could be a novel intervention for patients suffering from MDD, who have demonstrated intolerance or refractoriness to antidepressants or prefer nonpharmacological approaches. t-PBM is mechanistically different from other existing device-based treatments for MDD, which typically employ electromagnetic modulation of cortical neurons. Numerous NIR LED devices are FDA approved for sale over the counter; they are considered safe for personal use and—provided FDA approval for this specific indication—may represent an inexpensive option for the home treatment of MDD, under appropriate medical supervision. If t-PBM were to be confirmed as an effective and safe treatment for MDD, it would be well suited for adoption among the expanding range of therapeutic options for MDD.
Supplementary Material
Acknowledgments
The Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award) and the Brain and Behavior Research Foundation (NARSAD Young Investigator Award; Grant No. 19159) supported this investigation. Two pairs of study devices (active and sham) were provided by Photomedex, Inc. (Orangeburg, NY), and underwent independent dosimetry testing (real and sham), provided by Litecure, Inc. (Newark, DE). None of the funding sources had any involvement in the study design; in the collection, analysis, or interpretation of study data; in the writing of the report; or in the decision to submit the article for publication.
Author Disclosure Statement
Dr. Cassano's salary was supported by the Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award), by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and by the Photothera, Inc. unrestricted grant. Drug donation was from TEVA. Travel reimbursement was from Pharmacia-Upjohn. Dr. Cassano has received consultation fees from Janssen Research and Development. Dr. Cassano has filed a provisional patent related to the use of near-infrared light in psychiatry. PhotoMedex, Inc. supplied four devices for this clinical study. Since completion of the study and of the article, Dr. Cassano is/has (1) received unrestricted funding from Litecure, Inc. to conduct an additional study on transcranial photobiomodulation for the treatment of major depressive disorder; (2) received unrestricted funding from Cerebral Sciences to conduct a study on transcranial photobiomodulation for the treatment of generalized anxiety disorder; (3) co-founded, member of the board of directors and consultant of Niraxx Light Therapeutics, Inc., a company focused on the development of new modalities of treatment based on near-infrared light.
Dr. Mischoulon has received research support from the FisherWallace, Nordic Naturals, Methylation Sciences, Inc. (MSI), and PharmoRx Therapeutics. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.”
Dr. Katnani: Research funding from NINDS 5R01NS086422.
Mr. De Taboada receives salary from LiteCure LLC (Newark, DE) as V.P. of research and Development.
Disclosures Regarding Patents: Mr. De Taboada is a named inventor in multiple patents for the treatment of neurological disorders and injuries, including MDD.
Dr. M. Hamblin: Vielight, Inc., Scientific Advisory Board; Global Photon, Scientific Advisory Board; Lexington, Inc, Scientific Advisory Board; LaserCap Scientific Advisory Board; and USHIO, Inc, Consultant.
Research Support: Dr. J. Alpert: Abbott Laboratories, Alkermes, Lichtwer Pharma GmbH, Lorex Pharmaceuticals; Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Cyberonics, Eli Lilly & Company, Forest Pharmaceuticals, Inc., GlaxoSmithkline, J&J Pharmaceuticals, National Institutes of Health, NARSAD, Novartis, Organon, Inc., PamLab, LLC, Pfizer, Inc., Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth-Ayerst Laboratories.
Advisory Board/Consultant: Eli Lilly & Company, Luye Pharmaceuticals, PamLab LLC, and Pharmavite LLC.
Speaking/Publishing: MGH Academy, Reed Medical Education, Primedia, Nevada Psychiatric Association, American Society of Clinical Psychopharmacology, American Psychiatric Association, Belvoir Media Group, Eli Lilly & Company, Xian-Janssen, Organon, and Psicofarma.
Research Support: Dr. M. Fava: Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; BioResearch; BrainCells, Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck, Inc.; MedAvante; Methylation Sciences, Inc.; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer, Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Takeda Pharmaceuticals; Tal Medical; VistaGen); and Wyeth-Ayerst Laboratories.
Advisory Board/Consultant: Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma, Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells, Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co., Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai, Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm, Inc.; Lorex Pharmaceuticals; Lundbeck, Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; PamLab, LLC.; Pfizer, Inc.; PharmaStar; Pharmavite LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor, Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceutical; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; and VistaGen.
Speaking/Publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer, Inc.; PharmaStar; United BioSource, Corp.; and Wyeth-Ayerst Laboratories.
Stock/Other Financial Options: Equity Holdings: Compellis; PsyBrain, Inc.
Royalty/Patent, Other Income: Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven.
Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte. Ltd.
Dr. D. Iosifescu: In the past 5 years, Dr. Iosifescu was a consultant for Avanir, Axsome, CNS Response, INSYS Therapeutics, Lundbeck, Otsuka, Servier, and Sunovion; he has received research support (through the Icahn School of Medicine at Mount Sinai) from Alkermes, Astra Zeneca, Brainsway, Euthymics, Neosync, Roche, and Shire, and he has received speaker honoraria from the Massachusetts General Hospital Psychiatry Academy, Medscape, and Global Medical Education.
All other authors have no conflict of interests to declare.
References
- 1. Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 2012;21:169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond Engl 2016;388:1545–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloom DE, Cafiero E, Jané-Llopis E, et al. The Global Economic Burden of Noncommunicable Diseases. Program on the Global Demography of Aging. 2012. Available at: https://ideas.repec.org/p/gdm/wpaper/8712.html (Last accessed July11, 2018).
- 4. van Beljouw I, Verhaak P, Prins M, Cuijpers P, Penninx B, Bensing J. Reasons and determinants for not receiving treatment for common mental disorders. Psychiatr Serv 2010;61:250–257 [DOI] [PubMed] [Google Scholar]
- 5. Barrett B, Byford S, Knapp M. Evidence of cost-effective treatments for depression: a systematic review. J Affect Disord 2005;84:1–13 [DOI] [PubMed] [Google Scholar]
- 6. Jorm AF, Griffiths KM, Christensen H, Parslow RA, Rogers B. Actions taken to cope with depression at different levels of severity: a community survey. Psychol Med 2004;34:293–299 [DOI] [PubMed] [Google Scholar]
- 7. Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry 2004;16:15–25 [DOI] [PubMed] [Google Scholar]
- 8. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry 2016;61:540–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parikh SV, Quilty LC, Ravitz P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 2. Psychological treatments. Can J Psychiatry 2016;61:524–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milev RV, Giacobbe P, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. Neurostimulation treatments. Can J Psychiatry 2016;61:561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohammed HS. Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers Med Sci 2016;31:1651–1656 [DOI] [PubMed] [Google Scholar]
- 12. Salehpour F, Rasta SH. The potential of transcranial photobiomodulation therapy for treatment of major depressive disorder. Rev Neurosci 2017;28:441–453 [DOI] [PubMed] [Google Scholar]
- 13. Salehpour F, Rasta SH, Mohaddes G, Sadigh-Eteghad S, Salarirad S. Therapeutic effects of 10-Hz pulsed wave lasers in rat depression model: a comparison between near-infrared and red wavelengths. Lasers Surg Med 2016;48:695–705 [DOI] [PubMed] [Google Scholar]
- 14. Xu Z, Guo X, Yang Y, et al. Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol 2017;54:4551–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu X, Alberico SL, Moges H, De Taboada L, Tedford CE, Anders JJ. Pulsed light irradiation improves behavioral outcome in a rat model of chronic mild stress. Lasers Surg Med 2012;44:227–232 [DOI] [PubMed] [Google Scholar]
- 16. Schiffer F, Johnston AL, Ravichandran C, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016;3:031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassano P, Cusin C, Mischoulon D, et al. Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatry J 2015;2015:352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caldieraro MA, Sani G, Bui E, Cassano P. Long-term near-infrared photobiomodulation for anxious depression complicated by Takotsubo cardiomyopathy. J Clin Psychopharmacol 2018;38:268–270 [DOI] [PubMed] [Google Scholar]
- 20. Mirzaii-Dizgah I, Ojaghi R, Sadeghipour HR, Sadeghipour-Roodsari HR, Karimian SM, Sohanaki H. Attenuation of morphine withdrawal signs by low level laser therapy in rats. Behav Brain Res 2009;196:268–270 [DOI] [PubMed] [Google Scholar]
- 21. Hagiwara S, Iwasaka H, Hasegawa A, Noguchi T. Pre-irradiation of blood by gallium aluminum arsenide (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Anesth Analg 2008;107:1058–1063 [DOI] [PubMed] [Google Scholar]
- 22. Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012;40:516–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 1997;66:866–871 [DOI] [PubMed] [Google Scholar]
- 24. Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg 2007;25:180–182 [DOI] [PubMed] [Google Scholar]
- 25. Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 2002;323:207–210 [DOI] [PubMed] [Google Scholar]
- 26. Zalewska-Kaszubska J, Obzejta D. Use of low-energy laser as adjunct treatment of alcohol addiction. Lasers Med Sci 2004;19:100–104 [DOI] [PubMed] [Google Scholar]
- 27. Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One 2012;7:e47460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med 2015;47:312–322 [DOI] [PubMed] [Google Scholar]
- 29. Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat 2015;11:2191–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Konstantinović LM, Jelić MB, Jeremić A, Stevanović VB, Milanović SD, Filipović SR. Transcranial application of near-infrared low-level laser can modulate cortical excitability. Lasers Surg Med 2013;45:648–653 [DOI] [PubMed] [Google Scholar]
- 31. Chaieb L, Antal A, Masurat F, Paulus W. Neuroplastic effects of transcranial near-infrared stimulation (tNIRS) on the motor cortex. Front Behav Neurosci 2015;9:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henderson TA, Morries LD. SPECT perfusion imaging demonstrates improvement of traumatic brain injury with transcranial near-infrared laser phototherapy. Adv Mind Body Med 2015;29:27–33 [PubMed] [Google Scholar]
- 33. Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg 2012;30:231–233 [DOI] [PubMed] [Google Scholar]
- 34. Salgado ASI, Zângaro RA, Parreira RB, Kerppers II. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med Sci 2015;30:339–346 [DOI] [PubMed] [Google Scholar]
- 35. Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med 2016;48:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013;230:13–23 [DOI] [PubMed] [Google Scholar]
- 37. Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2017;11:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hwang J, Castelli DM, Gonzalez-Lima F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci 2016;31:1151–1160 [DOI] [PubMed] [Google Scholar]
- 39. Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma 2014;31:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg 2011;29:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat 2015;11:2159–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hacke W, Schellinger PD, Albers GW, et al. Transcranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke J Cereb Circ 2014;45:3187–3193 [DOI] [PubMed] [Google Scholar]
- 43. Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke J Cereb Circ 2007;38:1843–1849 [DOI] [PubMed] [Google Scholar]
- 44. Zivin JA, Albers GW, Bornstein N, et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009;40:1359–1364 [DOI] [PubMed] [Google Scholar]
- 45. Jöbsis-VanderVliet FF, Piantadosi CA, Sylvia AL, Lucas SK, Keizer HH. Near-infrared monitoring of cerebral oxygen sufficiency. I. Spectra of cytochrome c oxidase. Neurol Res 1988;10:7–17 [DOI] [PubMed] [Google Scholar]
- 46. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Targum SD, Busner J, Young AH. Targeted scoring criteria reduce variance in global impressions. Hum Psychopharmacol 2008;23:629–633 [DOI] [PubMed] [Google Scholar]
- 48. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573–583 [DOI] [PubMed] [Google Scholar]
- 49. Liu-Seifert H, Zhang S, D'Souza D, Skljarevski V. A closer look at the baseline-observation-carried-forward (BOCF). Patient Prefer Adherence 2010;4:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Woolley SB, Cardoni AA, Goethe JW. Last-observation-carried-forward imputation method in clinical efficacy trials: review of 352 antidepressant studies. Pharmacotherapy 2009;29:1408–1416 [DOI] [PubMed] [Google Scholar]
- 51. Henderson T, Morries L. Multi-watt near-infrared phototherapy for the treatment of comorbid depression: an open-label single-arm study. Front Psychiatry 2017;8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Disner SG, Beevers CG, Gonzalez-Lima F. Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimulat 2016;9:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry 1997;154:116–118 [DOI] [PubMed] [Google Scholar]
- 54. Volz HP, Rzanny R, Riehemann S, et al. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci 1998;248:289–295 [DOI] [PubMed] [Google Scholar]
- 55. Renshaw PF, Parow AM, Hirashima F, et al. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry 2001;158:2048–2055 [DOI] [PubMed] [Google Scholar]
- 56. Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry 2008;63:1127–1134 [DOI] [PubMed] [Google Scholar]
- 57. Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand 2000;101:11–20 [DOI] [PubMed] [Google Scholar]
- 58. Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000;48:830–843 [DOI] [PubMed] [Google Scholar]
- 59. Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol 2002;12:527–544 [DOI] [PubMed] [Google Scholar]
- 60. Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry 2007;164:778–788 [DOI] [PubMed] [Google Scholar]
- 61. Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry 2015;20:311–319 [DOI] [PubMed] [Google Scholar]
- 62. Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed Laser Surg 2017;35:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.