Abstract
Despite significant treatment advances, diabetes outcomes remain suboptimal and health care costs continue to rise. There are limited data on the feasibility and financial implications of integrating a diabetes-specific care team in the primary care setting (ie, where the majority of diabetes is treated). This pragmatic quality improvement project investigated whether a cardiometabolic care team intervention (CMC-TI) could achieve greater improvements in clinical, behavioral, and cost outcomes compared to usual diabetes care in a large primary care group in Southern California. Over 12 months, n = 236 CMC-TI and n = 239 usual care patients with type 1 or 2 diabetes were identified using the electronic medical record. In the CMC-TI group, a registered nurse (RN)/certified diabetes educator care manager, medical assistant health coach, and RN depression care manager utilized electronic medical record-based risk stratification reports, standardized decision-support tools, live and remote tailored treatments, and coaching to manage care. Results indicated that the CMC-TI group achieved greater improvements in glycemic and lipid control, diabetes self-management behaviors, and emotional distress over 1 year compared with the usual care group (all P < .05). The CMC-TI group also had a significant 12.6% reduction in total health care costs compared to a 51.7% increase in the usual care group during the same period and inclusive of CMC-TI program costs. Patients and providers reported high satisfaction with CMC-TI. These findings highlight that team-based care management interventions that utilize nurses, medical assistant health coaches, and behavioral specialists to support diabetes patients can help primary care practices achieve value-based targets of improved health, cost, and patient experience.
Keywords: diabetes, cardiometabolic, care management, clinical outcomes, financial outcomes
Introduction
Diabetes mellitus is the prototypical chronic illness because of prevalence and long-term complications, but also because of the proven benefit of several therapeutic interventions for risk reduction. It also is one of the most frequently diagnosed chronic diseases in the world; global diabetes prevalence has nearly doubled since 1980, rising from 4.7% to 8.5% in 2014.1 As of 2017, 9.4% of US adults had diabetes2 and the total estimated cost of diagnosed diabetes was $327 billion, representing a staggering 26% increase from 2012.3 The current health care model too often fails to assure that patients receive effective interventions.4 As a result, diabetes care is often poorly coordinated, and many individuals show inadequate clinical control, which in turn diminishes quantity and quality of life.
It is well established that optimizing cardiometabolic control, as demonstrated by improving glycosylated hemoglobin (HbA1c), blood pressure (BP), and lipids, can lead to significant reductions in morbidity and mortality.5–7 However, this can be particularly difficult to achieve in primary care (ie, where the majority of diabetes care is delivered). The 15-minute visit does not allow a physician enough time to provide effective and evidence-based acute, chronic, and preventive care while also building and maintaining rapport with a patient.8 More than 40% of primary care physicians report not having adequate time to spend with their patients, and research has found that 50% of patients leave an office visit without understanding providers' recommendations.8,9
Growing demand for adult primary care services (eg, because of aging baby boomers, the diabetes and obesity epidemics) coupled with a shortage of medical students opting into primary care as a career has contributed to a primary care demand-capacity.10 In turn, very large patient panel sizes limit primary care physicians' ability to respond effectively to the significant and growing chronic disease needs of their patients. Several individual-level factors (eg, age, multimorbidity, complex medical regimens, psychosocial concerns) also pose additional challenges to diabetes management in general.11–13 Despite significant advances in treatment over the past decade, outcomes remain suboptimal in diabetes, suggesting a need to redesign approaches to care delivery.14
Team-based care, or training non-licensed clinic personnel to “share the care” and serve as panel managers and health coaches, has been posited as a foundational element that can reduce the primary care demand-capacity imbalance and improve chronic disease care processes and outcomes.10 Indeed, many US settings are shifting responsibilities to medical assistants (MAs) – one of the fastest growing and widely available allied health professions – and implementing higher MA/physician ratios. Recent reviews have summarized the effectiveness of health coaching for chronic disease outcomes overall,15 and for diabetes specifically,16 including among underserved groups.17,18 Qualitative studies have highlighted methods for enhancing the success of MA health coaches,19 and also have provided evidence of primary care providers' (PCPs') satisfaction with (and acceptance of) delegating health coaching responsibilities to non-clinician staff.20
As another example, Project Dulce is an American Diabetes Association (ADA)-recognized, team-based care management program designed to improve health and access to care of at-risk individuals with type 2 diabetes. In conjunction with the PCP, Project Dulce's nurse-led multidisciplinary team provides clinical management, while trained peer educators deliver diabetes self-management education and support. Studies evaluating Project Dulce have demonstrated positive effects on clinical, behavioral, and cost outcomes, including decreased emergency room and hospital utilization.21–27
Rather than universally offering the same team-based care to all individuals, evidence suggests that tailoring interventions according to clinical risk represents a cost-effective way to meet the needs of the ever-growing numbers of individuals with chronic disease.28–30 Further, the improving functionality of electronic medical record (EMR) systems offers an increasingly efficient way to identify and stratify at-risk people in a health care setting.28 However, limited research has examined the clinical and financial benefits of embedding an integrated care team to improve cardiometabolic control among diabetes patients in an EMR-equipped primary care environment. The few studies that have incorporated simple risk stratification models in primary care settings have reported varying results.28,31–33
The Chronic Care Model (CCM) is a well-established approach for improving care.22,25,34–36 The present study capitalized on the CCM framework, EMR-based risk stratification, and tailored intervention to improve care and outcomes. The Scripps Whittier Diabetes Institute, in collaboration with Scripps Coastal Medical Group, conducted a pragmatic study in Southern California to evaluate the effects of a CCM-based, cardiometabolic care team intervention (CMC-TI) for patients with diabetes and cardiovascular risk factors in primary care. This evaluation examined the impact of CMC-TI on clinical, financial, behavioral, and psychosocial outcomes, and patient and provider satisfaction – outcomes of particular relevance to both patients and health care systems where value-based reimbursement methods also may be tied to these outcomes.
Methods
Procedures
A pragmatic, quasi-experimental, case control design was used to evaluate clinical and cost outcomes among patients with type 1 or 2 diabetes at 2 large primary care clinics (n = 1 intervention, n = 1 usual care) between March 2012 and September 2014. At the intervention clinic, the 12-month CMC-TI was delivered to all qualifying patients; surveys were administered to evaluate relevant patient-reported outcomes over 12 months, and a process evaluation gauged CMC-TI feasibility and satisfaction. Institutional review board approval and clinicaltrials.gov registration were not required as CMC-TI was a clinic-wide quality improvement program.
Environment
Scripps Health is a large health system in San Diego, CA comprised of 4 hospitals on 5 campuses, with nearly 70,000 admissions each year. Two large partnering medical groups deliver primary and specialty care services to more than 700,000 patients/year who are covered predominantly by private/commercial insurance and/or Medicare. The intervention and usual care clinics were proximally-located, part of the same Scripps medical group, and similar in demographics and size (20 and 15 providers, 1175 and 892 diabetes patients, respectively).
Participants
Between March 2012 and August 2013, n = 236 CMC-TI and n = 239 usual care clinic patients with type 1 or type 2 diabetes were identified using the EMR. Because of the pragmatic nature of the evaluation, and in order to enhance generalizability of the findings, no exclusion criteria were imposed. All N = 475 patients were stratified as “high,” “moderate,” or “low” risk according to HbA1c, BP, and low-density lipoprotein cholesterol (LDL-C) for descriptive purposes (Table 1). At the CMC-TI clinic these strata also were used to filter and prioritize higher risk patients on a clinical data dashboard for more immediate and frequent outreach.
Table 1.
Baseline Characteristics
| CMC-TI N (%) | Usual care N (%) | |
|---|---|---|
| N | 236 | 239 |
| Age in years (mean ± SD) | 61.94 ± 11.44 | 60.52 ± 10.45 |
| Male | 121 (51.3) | 127 (53.1) |
| Type 2 diabetes | 229 (97.0) | 236 (98.7) |
| BMI (kg/m2) (mean ± SD) | 32.63 ± 6.83 | 33.03 ± 6.85 |
| Clinical outcomesa (mean ± SD) | ||
| HbA1c (%) | 7.3 ± 1.6 | 7.2 ± 1.6 |
| LDL-C (mg/dL) | 91.67 ± 32.41 | 97.67 ± 35.61 |
| SBP (mmHg) | 126.22 ± 16.87 | 131.39 ± 17.65 |
| Clinical risk levelb | ||
| High | 44 (18.6) | 40 (16.7) |
| Moderate | 112 (47.5) | 116 (48.5) |
| Low | 80 (33.9) | 83 (34.7) |
| Positive PHQ-9 | 60 (10.6) | —c |
All values reported are N(%) unless otherwise noted. All between-group P values >.05.
Experimental Conditions: In the CMC-TI group, an RN/CDE care manager, medical assistant health coach, and RN depression care manager utilized electronic medical record-based risk stratification reports, standardized decision-support tools, live and remote tailored treatments, and coaching to manage care. Usual care is usual diabetes care in the clinic.
Primary outcome analyses controlled for differences in baseline clinical values.
High risk: HbA1c ≥9%, OR HbA1c ≥8% and LDL ≥100 mg/dl and BP ≥140/90 mmHg. Moderate risk: HbA1c ≥8% and/or LDL ≥100 mg/dl and/or BP ≥140/90 mmHg. Low risk: HbA1c <8% and LDL <100 mg/dl and BP <140/90 mmHg.
Measure was collected as part of CMC-TI, and therefore data were not available for usual care participants.
BMI, body mass index; CDE, certified diabetes educator; CMC-TI, cardiometabolic care team intervention; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; PHQ-9, Patient Health Questionnaire-9; RN, registered nurse; SBP, systolic blood pressure; SD, standard deviation.
Treatment groups
CMC-TI
The CMC-TI team included a registered nurse/certified diabetes educator (RN/CDE) care manager, MA health coach, and RN depression care manager. Decision-support tools guided therapy for glucose, BP, LDL-C, and depression. Competencies were established for team members and a decision support toolbox was adapted from Staged Diabetes Management, 3rd edition from the International Diabetes Center.
Eligible patients at the intervention clinic were scheduled for an initial CMC-TI appointment with the RN/CDE care manager who conducted a health history and physical exam. When medication changes were indicated, the RN/CDE care manager sent an EMR “task” to the PCP and, if approved, proceeded with changes. Patients who scored positive on validated depressive symptomatology screeners37,38 were referred to the RN depression care manager who conducted a comprehensive intake followed by group-based cognitive-behavioral therapy, as needed.
Following the initial visit, patients were supported by CMC-TI for 12 months. Level of follow-up care was determined by the patient's ability to achieve individualized targets for several key metabolic parameters; fasting self-monitored blood glucose (SMBG), HbA1c, LDL-C value, and home and clinic BP values. Individualized patient targets were established in collaboration with the PCP and were guided by the ADA Standards of Care. The RN/CDE care manager used decision-support tools and protocols for therapeutic interventions when initial values were out of range and targets were not met. Fasting SMBG values, home BP monitoring, and a repeat lipid profile were collected by the CMC-TI team over the first 4 weeks of intervention. Those with metabolic parameter(s) not meeting their individualized targets after 4 weeks were asked to return to see the RN/CDE care manager. Repeat visits were scheduled every 3–4 weeks to review the patient's information and adjust therapy.
Patients who noted improvements in their values and met their individualized targets reverted to quarterly visits and were subsequently managed via remote telephone follow-up by the MA/health coach. The MA/health coach was trained and scripted to utilize between-visit calls to assess for any barriers or challenges identified by the patient, and used motivational interviewing and collaborative goal-setting techniques to encourage positive behavior change. CMC-TI was implemented as the standard diabetes care at the intervention clinic, and as with other medical services, patients were able to decline any CMC-TI components.
Usual care
Both clinics provided standard diabetes care over the program period; patients were encouraged to continue with routine PCP visits, and referrals to specialists and/or diabetes self-management education (DSME) were initiated per PCP discretion. Providers received monthly Diabetes Registry Reports listing all diabetes patients not at system targets for lab draw frequency and clinical control. An ADA-accredited curriculum was used to deliver DSME at both sites.
Measures
Primary outcomes
Consistent with ADA Standards of Care,7 Scripps recommends assessment of HbA1c every 3 months, lipids annually, and BP at every visit. All samples were analyzed centrally by Scripps Laboratory Services using standard protocols. Scripps staff measured BP according to protocol39 using Welch Allyn Mobile Monitors. Clinical and financial data for CMC-TI and usual care patients were extracted from electronic databases by Scripps analysts.
Direct variable costs were derived for ambulatory (office visits, labs, imaging, outpatient surgery) and inpatient services (hospitalizations, emergency department [ED]) utilized during the pre period (ie, the 1 year period prior to program start) and post period (ie, the 1 year period after program start). Direct variable costs include labor (eg, physician, procedure-specific staff services) and supplies used to deliver services that are directly attributable to the treatment and care provided to a specific patient during a hospitalization. Not included in this metric (nor in the present analyses) are the supporting overhead or indirect costs that include resources such as utilities, information technology support, and administrative resources that are required for hospital operations and not directly tied to a specific patient's care. Additionally, pharmacy costs and hospitalizations outside of the Scripps Health system could not be captured, and thus are not factored into the health care costs of CMC-TI or usual care patients.
Patient-reported outcomes and process evaluation (CMC-TI patients only)
The Summary of Diabetes Self-Care Activities (SDSCA),40 Diabetes Distress Scale (DDS),41,42 and Patient Assessment of Chronic Illness Care (PACIC)43,44 were administered at month 0/program start, month 6, and month 12/program end, and entered into Research Electronic Data Capture (REDCap).45 Focus groups and interviews provided qualitative data on program feasibility and satisfaction.
Statistical analyses
Data analysis was performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corporation, Armonk, NY) and HLM7 (Scientific Software International, Lincolnwood, IL) following an intent-to-treat approach. Data exhibiting significant deviations from normality were transformed for significance testing; however, untransformed data are presented for interpretation purposes. Mixed models controlling for age and sex examined whether CMC-TI and usual care groups evidenced differential rates of change over time on clinical and cost outcomes (ie, time-by-group interactions). Secondary analyses were limited to patients in the moderate- and high-risk groups at baseline and examined change in the percentage of patients “at target” on labs at each clinic across time. Finally, within-group change on the SDSCA, DDS, and PACIC was examined for CMC-TI patients only.
Results
Sample descriptives
Over 19 months n = 236 patients with type 1 or type 2 diabetes were enrolled by the CMC-TI team and managed for a 12-month time period. Most patients were obese (64.1% with body mass index [BMI] ≥30) and 50 years or older (85.9%); sex was split evenly (52.2% male). The comparison group (n = 239) was similarly matched on demographics and clinical risk (all P > .05; Table 1).
Primary outcomes
Clinical outcomes
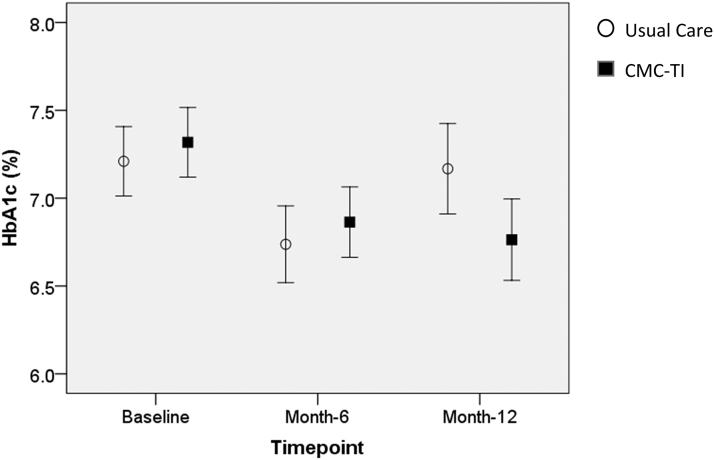
The CMC-TI group exhibited significantly greater improvements in HbA1c over 1 year compared with usual care (−0.5% vs. 0.0%; time-by-group interaction P = 0.011, Figure 1). No statistically significant group differences were observed for LDL-C or systolic BP in the overall sample.
FIG. 1.
HbA1c means and 95% CIs for the CMC-TI and usual care groups over 1 year. Time-by-group interaction effect (P = 0.011) indicated that the CMC-TI group exhibited significantly greater improvements in HbA1c over 1 year compared with usual care. Total N = 475. CI, confidence interval; CMC-TI, cartiometabolic care team intervention; HbA1c, glycosylated hemoglobin.
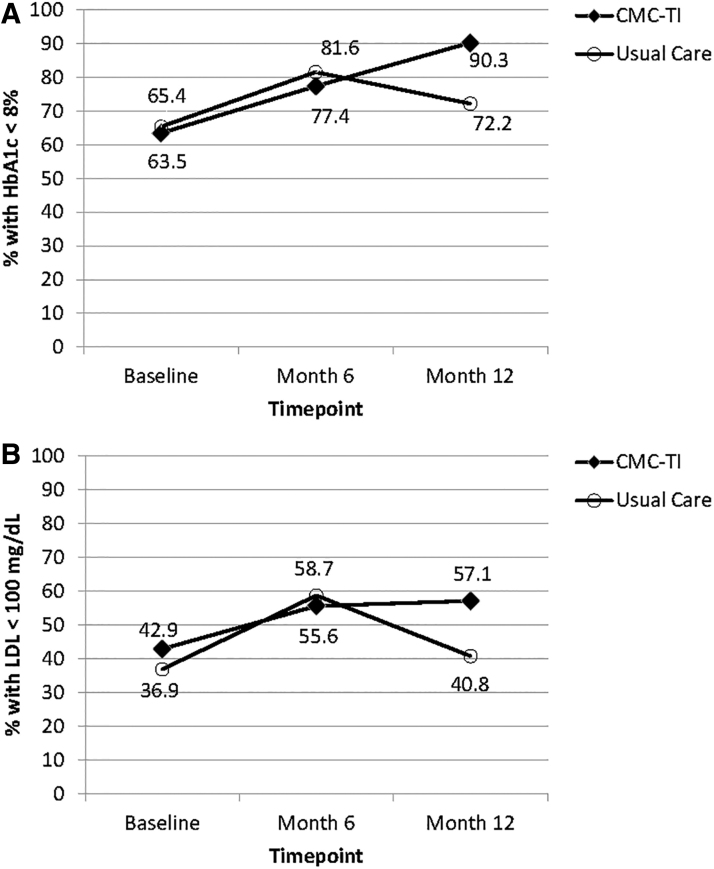
Secondary analyses limited to patients in the moderate- and high-risk groups at baseline (CMC-TI n = 156; usual care n = 156) indicated that, over 1 year, the percentage of patients with HbA1c <8% and LDL-C <100 mg/dL increased significantly in the CMC-TI group (P < 0.001 and P = 0.045, respectively) but not in usual care (P = 0.172 and P = 0.401; Figure 2). Between-group differences at month 12 were statistically significant for HbA1c <8% (P = 0.005) and LDL-C <100 mg/dL (P = .043).
FIG. 2.
Over 1 year, the percentage of moderate-/high-risk patients with (A) HbA1c <8% and (B) LDL <100 mg/dL increased significantly in the CMC-TI group (+26.8%, P = 0.001 and +14.2%, P = 0.045, respectively) but not in usual care (+8.7%, P = 0.172 and +3.9%, P = 0.401). Between-group differences at month 12 were statistically significant for HbA1c <8% (P = .005) and LDL-C <100 mg/dL (P = .043). Total N = 312. CMC-TI, cartiometabolic care team intervention; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein cholesterol.
Financial outcomes
Ambulatory and inpatient utilization data for CMC-TI and usual care patients during the pre and post periods are presented in Table 2.
Table 2.
Mean Inpatient Utilization, and Inpatient and Ambulatory Health Care Costs Per Patient Over the 1-Year Pre and 1-Year Post Periods
| Pre period |
Post period |
Pre vs. post |
|||||
|---|---|---|---|---|---|---|---|
| A |
B |
A vs. B |
C |
D |
C vs. D |
(C – A) vs. (D – B) |
|
| CMC-TI Mean (SD) |
Usual care Mean (SD) |
Pa | CMC-TI Mean (SD) |
Usual care Mean (SD) |
Pb | Pc | |
| Inpatient utilization | |||||||
| N (%) with ≥1 inpatient encounterd | 33 (12%) | 28 (14%) | 0.46 | 12 (5%) | 39 (16%) | <.001 | 0.001 |
| ED visits | 0.08 (0.37) | 0.10 (0.51) | 0.95 | 0.04 (0.26) | 0.17 (0.73) | 0.002 | 0.013 |
| Hospitalizations | 0.15 (0.56) | 0.11 (0.44) | 0.44 | 0.05 (0.33) | 0.19 (0.73) | 0.004 | 0 .004 |
| Inpatient encountersd | 0.23 (0.76) | 0.21 (0.75) | 0.46 | 0.09 (0.55) | 0.36 (1.17) | <.001 | <.001 |
| Direct variable costs | |||||||
| Inpatient | $1909 (11,174) | $928 (4379) | 0.42 | $932 (7845) | $1802 (8175) | <.001 | <.001 |
| Ambulatory | |||||||
| Office | $1292 (981) | $976 (1216) | <.001 | $1291 (1572) | $1142 (1504) | 0.43 | <.001 |
| Surgical | $109 (617) | $41 (314) | 0.26 | $114 (154) | $41 (155) | 0.158 | 0.62 |
| Imaging | $243 (374) | $176 (314) | 0.071 | $256 (427) | $234 (389) | 0.134 | 0.003 |
| Laboratory | $257 (755) | $179 (213) | <.001 | $218 (320) | $270 (228) | <.001 | <.001 |
| Subtotale | $1901 (1709) | $1372 (1537) | <.001 | $1878 (2029) | $1687 (1790) | 0.27 | <.001 |
| CMC-TI costs | – | – | – | $517 (0) | – | – | – |
| Total ambulatoryf | $1901 (1709) | $1372 (1537) | <.001 | $2397 (2029) | $1687 (1790) | <.001 | 0.65 |
| Total (inpatient + ambulatory) | $3810 (11,981) | $2300 (5522) | <.001 | $3329 (8338) | $3489 (8974) | 0.37 | 0.002 |
Pre period reflects the 1-year period prior to program start, whereas post period includes the 1-year period after program start. All values presented are Mean (SD) unless otherwise specified. Significance tests were performed with transformed data because of positive skew; however, data are presented in their original metric for interpretation purposes.
Experimental conditions: in the CMC-TI group, an RN/CDE care manager, medical assistant health coach, and RN depression care manager utilized electronic medical record-based risk stratification reports, standardized decision-support tools, live and remote tailored treatments, and coaching to manage care. Usual care is usual diabetes care in the clinic.
P values from independent sample t tests or chi-square (for ≥1 inpatient encounter) comparing CMC-TI and usual care group means during pre period only.
P values from independent sample t tests or chi-square (for ≥1 inpatient encounter) comparing CMC-TI and usual care group means during post period only.
P values for time-by-group interaction effects to determine whether the CMC-TI and usual care groups evidenced differential rates of change from pre period to post period.
Inpatient encounters include ED visits and hospitalizations.
Subtotal reflects total ambulatory health care utilization costs.
Total reflects total ambulatory health care utilization costs plus CMC-TI program implementation costs of $517 per CMC-TI patient added to the post period.
CDE, certified diabetes educator; CMC-TI, cardiometabolic care team intervention; ED, emergency department; RN, registered nurse; SD, standard deviation.
The CMC-TI group exhibited significantly greater reductions on all inpatient utilization and cost outcomes relative to usual care (all time-by-group interaction P < 0.05). Follow-up analyses limited to the post period indicated that the percentage of patients with ≥1 inpatient encounter of any kind, mean number of ED visits and inpatient hospitalizations, and mean inpatient health care costs per patient were significantly lower in CMC-TI versus usual care (all P < .01) (Table 2). Notably, no such differences were observed between groups during the pre period (all P > .05).
During the post period, there were no statistically significant differences in total ambulatory costs between groups. However, when CMC-TI program implementation costs ($517.33/CMC-TI patient) were added, CMC-TI patients did indeed exhibit higher total ambulatory costs during the post period; however, this between-group differential approximated that of the year prior (time-by-group interaction P = .65), indicating a shift of expenditures for CMC-TI patients from routine health care costs to program implementation (versus an overall cost increase for this group) after program start (Table 2).
Taken together, CMC-TI patients exhibited a significantly greater decrease in total health care costs [combined inpatient + ambulatory (CMC-TI program costs included)] over time compared with usual care (P = .002).
Patient-reported outcomes
CMC-TI participants achieved statistically significant improvements in healthful eating (4.20 to 4.96 days/week), exercise (3.01 to 4.52), blood glucose monitoring (3.82 to 5.46), and foot-checking (2.68 to 5.40) (all P < .05); no significant change was observed for self-reported medication adherence. Small, but statistically significant decreases were observed for diabetes distress over 1 year (1.75 to 1.46, P < .05); the percentage of individuals reporting “moderate” distress (DDS ≥2) decreased from 24.1% to 14.3% during this period.
Process evaluation
Mean in-person encounters were similar between individuals who started in the low- (M = 4), moderate-, and high-risk categories (M = 5). Average number of telephone follow-ups was higher in the moderate- and high-risk groups (M = 7) compared with low risk (M = 3). Overall, total encounters/patient were higher in the moderate- and high-risk groups (M = 12) compared to low risk (M = 7).
PACIC survey data indicated statistically significant improvements in CMC-TI patients' perceptions of CCM-based health care delivery components. Over 1 year, increases were observed in the overall PACIC scale (3.05 to 4.10) and all subscales: follow-up/care coordination (2.73 to 3.97 out of 5), support for patient activation (3.04 to 3.97) and self-management (goal-setting, 3.00 to 4.13; problem-solving, 3.14 to 4.21), and delivery system design/decision support (3.36 to 4.25) (all P < .05).
In 4 post-study focus groups (N = 21) participants reported high satisfaction with the program, and described CMC-TI visits and health coach calls as appropriate in content and duration/frequency. Those supported by the RN depression care manager expressed high satisfaction with the intervention received. Participants noted redundant lab orders and challenges reaching CMC-TI staff by telephone as areas for improvement. Interviews conducted with CMC-TI clinic providers revealed similar perspectives (ie, positive feedback from their patients, but duplicate labs). When asked to gauge CMC-TI impact on workload, responses ranged from no impact (0.0%) to a 25.0% decrease in workload. Providers rated communication with the CMC-TI team as fair-to-good, but noted some uncertainty in how CMC-TI differed from traditional DSME.
Discussion
Results demonstrate that a team-based chronic care management program implemented in a primary care clinic significantly improved clinical, financial, behavioral, and psychosocial outcomes – all highly desirable in the current health care climate. Glucose control was achieved within the first 6 months of CMC-TI and was maintained for an additional 6 months thereafter. Furthermore, in moderate- and high-risk patients the proportion with HbA1c <8% and LDL-C <100 mg/dl increased significantly over 1 year among CMC-TI but not usual care patients. Perhaps even more relevant were hospital and ED cost reductions of >50% over 1 year, and containment of total ambulatory and hospital costs from the pre- to the postintervention period, even after CMC-TI program costs were included in post period calculations. This is especially noteworthy when compared to the usual care clinic, which had increases in all costs across the same period.
Interestingly, the increase in ED and hospital utilization in the usual care group was relatively larger among the moderate-/high- versus low-risk usual care patients (+10% vs. +1%), while the decrease in utilization in the CMC-TI group was larger among the moderate-/high-risk versus low-risk CMC-TI patients (−15% vs. −6%). Thus, as predicted, the moderate- and high-risk groups carry potential to drive utilization/cost in the absence of intervention, yet represent the greatest opportunity for improvement in the presence of intervention. Also noteworthy, the pre- to post-period reduction in the ratio of hospitalizations-to-ED visits (without admission to hospital), was larger in the CMC-TI group than usual care. One could speculate that a reduction in this ratio is a proxy for a reduction in the severity of the presenting problem (thus more could be addressed in the ED, and fewer of them required admission to the hospital); however, further investigation would be needed to confirm this.
Consistent with the positive clinical and cost outcomes, CMC-TI patients also achieved significant improvements in diabetes self-management behaviors and diabetes distress, and reported significant improvements in CCM-based quality of care over 1 year. Overall satisfaction with this approach was clearly articulated by patients. Collectively, these outcomes are highly desirable for patients and health systems and are in line with the broader mandate proposed by the Institute for Healthcare Improvement to achieve the Quadruple Aim of “enhancing patient experience, improving population health, reducing costs, and improving the work life of health care providers.”46
Managing BP and lipids to recommended targets can significantly reduce complications, all-cause mortality, and costs but also can be challenging.7,47–49 CMC-TI leveraged the EMR to identify the highest risk patients, monitor their progress, and tailor therapeutic approaches using decision-support tools to guide medical and behavioral management with the consent of and communication with the PCP. Interestingly, both groups had initial improvements in metabolic parameters but only the CMC-TI group was able to maintain the improvements over 1 year.
The study team speculates that providers may have initiated medication changes in response to registry “not at target” reports received monthly, but were reluctant to further manage complex regimens over time (clinical inertia), whereas decision-support tools used by the care manager encouraged ongoing medication adjustments to ensure the targets were maintained. Furthermore, over time patients may encounter adherence barriers that overwhelm them, but CMC-TI health coaching may have enabled the patients to work through these challenges. Thus, the team proposes that a primary way in which CMC-TI achieved longer term improvements compared to usual care is that it provided the necessary clinical decision support (to facilitate dose adjustment) and health coaching (to promote medication adherence, routine primary care follow-up) needed to sustain clinical improvements from 6 to 12 months, and in turn, meaningfully impact cost. It also is possible that CMC-TI served as a multilevel intervention (ie, CMC-TI could have had some influence on physician behavior in addition to patient behavior).
Health costs are progressing relentlessly in the United States where “1 in 4 healthcare dollars are spent on the treatment of diabetes and its complications.”3 This study demonstrated that team-based interventions provided to all qualified patients regardless of payer, can significantly reduce costs in a short time frame, almost exclusively related to hospital and ED savings, yet translation and expansion of chronic care team integration in a primary care setting may pose one of the biggest hurdles to overcome in the current health system model. Although value-based and accountable care models continue to penetrate the market, most health care systems in California and across the nation are still operating in a mixed payor environment. This can lead to misaligned incentives between the entities providing care and an environment in which shared savings have not been easily attainable for CCMs that reduce hospital admission rates.
The present study conducted at Scripps Health, a large hospital health system in Southern California with a contractual partnership and an Accountable Care Organization relationship with Scripps Coastal Medical Group, may extend an opportunity for the translation of such a model. With decreasing reimbursement from Medicare and other payors, health systems must develop ongoing strategies for tailoring approaches to optimize care that avoids needless and costly interventions. The opportunity is ripe for incorporating collaborative team-based care.
It is through pragmatic, primary care-based quality improvement projects such as this that the feasibility and effectiveness of interventions in a real-world setting can be examined, without the influence of constraints of rigorous research methods that limit the ecological validity of well-controlled randomized controlled trials. However, there also are important drawbacks related to this methodology that warrant consideration.
First, CMC-TI did not include active outreach to primary care absent patients (ie, individuals who are not engaged in routine medical follow-up in primary care), and thus cannot be generalized to that population segment. However, participants in this pragmatic trial likely were more representative of the general primary care population than participants in highly controlled trials that require an individual to “opt in” and entail time-intensive research protocols. Second, although patient-level statistics on race and socioeconomic status were not available, the 2 study clinics serve a predominantly middle-class, non-Hispanic white patient base and cannot be generalized to care settings that serve lower income diverse populations.
Third, random assignment of clinics was not feasible because of practical limitations. Rather, one clinic was selected to be the intervention site based on availability of clinic space for the CMC-TI team and the other served as a usual care comparator for the clinical and cost evaluations. However, both sites used the same EMR and clinic processes, and had access to and the ability to refer to the same diabetes education program and endocrine specialists. Further, there were no significant differences in any available clinical or demographic variables at baseline between the 2 clinic samples. Nonetheless, it is still possible that other factors that were not captured here contributed to the outcomes observed, and thus replication of these findings in future research will be valuable.
The study only measured direct costs and did not include nonmedical costs (eg, lost productivity, transport). Although pharmacy costs could not be quantified and hospitalizations outside of the Scripps health system could not be captured, it is likely that these measurement issues impacted CMC-TI and usual care estimates equally. Finally, because of the pragmatic nature of this study, patient refusal of intervention components was not tracked and an intensive treatment fidelity evaluation was not conducted – thus limiting the ability to report on patient satisfaction with or differential effectiveness of individual CMC-TI components.
Although beyond the scope of the present study, future investigations should examine the effectiveness of CMC-TI-like programs beyond 1 year; the potential (multilevel) impact of a team-based intervention on physician behavior (eg, prescribing, dose-titrating); the types of hospitalizations and ED visits that chronic care management programs such as this one effectively reduce, and the potential differential impact on hospitalization versus ED costs; and the possible variance in intervention response and/or patient satisfaction between type 1 and 2 diabetes subgroups.
Conclusion
Despite these limitations, the results remain impressive. The clinical and financial differences noted within 2 very similar sites within the same health system, using comparable provider and support staff and comparable quality support processes, resulted in notable differences. In a health care environment where all costs are capitated, this would translate into significant cost savings and value to the system.
Acknowledgment
We would like to thank the staff at the Scripps Coastal Medical Group for their collaboration in conducting this program; Lindsay Wagner, RN/depression care manager, Cindy Garvey, RN/CDE, and Joyce Guillermo-Preciado, Magdalena Hernandez, Lauren McDonnell, and Alma Ayala, medical assistant/health coaches, for their dedication to patient care; and the Scripps finance analyst team for their support in providing the data needed for the final analysis.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
Funding Information
Financial support was provided through Scripps Health.
References
- 1. Roglic G. Global report on diabetes. Geneva: World Health Organization, 2016. [Google Scholar]
- 2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf Accessed June5, 2018
- 3. American Diabetes Association. Economic Costs of Diabetes in the U.S in 2017. http://care.diabetesjournals.org/content/41/5/917 DOI: 10.2337/dci18-0007 Accessed June5, 2018 [DOI]
- 4. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 5. Palmer AJ, Roze S, Valentine WJ, et al. . Impact of changes in HbA1c, lipids and blood pressure on long-term outcomes in type 2 diabetes patients: an analysis using the CORE diabetes model. Curr Med Res Opin 2004;20 suppl 1:S53–S58 [DOI] [PubMed] [Google Scholar]
- 6. Eddy DM, Pawlson LG, Schaaf D, et al. . The potential effects of HEDIS performance measures on the quality of care. Health Aff (Millwood) 2008;27:1429–1441 [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Summary of Revisions: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41(suppl 1):S4–S6 [DOI] [PubMed] [Google Scholar]
- 8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med 2007;5:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roter DL, Hall JA. Studies of doctor-patient interaction. Annu Rev Public Health 1989;10:163–180 [DOI] [PubMed] [Google Scholar]
- 10. Bodenheimer TS, Smith MD. Primary care: proposed solutions to the physician shortage without training more physicians. Health Aff (Millwood) 2013;32:1881–1886 [DOI] [PubMed] [Google Scholar]
- 11. Delahanty LM, Grant RW, Wittenberg E, et al. . Association of diabetes-related emotional distress with diabetes treatment in primary care patients with type 2 diabetes. Diabet Med 2007;24:48–54 [DOI] [PubMed] [Google Scholar]
- 12. Jones A, Olsen MZ, Perrild HJ, Willaing I. The psychological impact of living with diabetes: descriptive findings from the DAWN2 study in Denmark. Prim Care Diabetes 2016;10:83–86 [DOI] [PubMed] [Google Scholar]
- 13. Aghili R, Polonsky WH, Valojerdi AE, et al. . Type 2 diabetes: model of factors associated with glycemic control. Can J Diabetes 2016;40:424–430 [DOI] [PubMed] [Google Scholar]
- 14. Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013;36:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kivela K, Elo S, Kyngas H, Kaariainen M. The effects of health coaching on adult patients with chronic diseases: a systematic review. Patient Educ Couns 2014;97:147–157 [DOI] [PubMed] [Google Scholar]
- 16. Pirbaglou M, Katz J, Motamed M, Pludwinski S, Walker K, Ritvo P. Personal health coaching as a type 2 diabetes mellitus self-management strategy: a systematic review and meta-analysis of randomized controlled trials. Am J Health Promot 2018;32:1613–1626 [DOI] [PubMed] [Google Scholar]
- 17. Ruffin L Health coaching strategy to improve glycemic control in African- American adults with type 2 diabetes: an integrative review. J Natl Black Nurses Assoc 2017;28:54–59 [PubMed] [Google Scholar]
- 18. Dennis SM, Harris M, Lloyd J, Powell Davies G, Faruqi N, Zwar N. Do people with existing chronic conditions benefit from telephone coaching? A rapid review. Aust Health Rev 2013;37:381–388 [DOI] [PubMed] [Google Scholar]
- 19. Willard-Grace R, Najmabadi A, Araujo C, et al. . “I don't see myself as a medical assistant anymore”: learning to become a health coach, in our own voices. Inquiry Educ 2013;4:1–18. http://digitalcommons.nl.edu/ie/vol4/iss2/2 Accessed January3, 2014 [Google Scholar]
- 20. Margolius D, Wong J, Goldman ML, Rouse-Iniguez J, Bodenheimer T. Delegating responsibility from clinicians to nonprofessional personnel: the example of hypertension control. J Am Board Fam Med 2012;25:209–215 [DOI] [PubMed] [Google Scholar]
- 21. Gilmer TP, Philis-Tsimikas A, Walker C. Outcomes of project dulce: a culturally specific diabetes management program. Ann Pharmacother 2005;39:817–822 [DOI] [PubMed] [Google Scholar]
- 22. Gilmer TP, Roze S, Valentine WJ, et al. . Cost-effectiveness of diabetes case management for low-income populations. Health Serv Res 2007;42:1943–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilmer TP, Walker C, Johnson ED, Philis-Tsimikas A, Unutzer J. Improving treatment of depression among Latinos with diabetes using project dulce and IMPACT. Diabetes Care 2008;31:1324–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fortmann AL, Gallo LC, Philis-Tsimikas A. Glycemic control among Latinos with type 2 diabetes: the role of social-environmental support resources. Health Psychol 2011;30:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Philis-Tsimikas A, Walker C, Rivard L, et al. . Improvement in diabetes care of underinsured patients enrolled in project dulce: a community-based, culturally appropriate, nurse case management and peer education diabetes care model. Diabetes Care 2004;27:110–115 [DOI] [PubMed] [Google Scholar]
- 26. Philis-Tsimikas A, Gallo LC. Implementing community-based diabetes programs: the Scripps Whittier Diabetes Institute experience. Curr Diab Rep 2014;14:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Philis-Tsimikas A, Gilmer TP, Schultz J, Walker C, Fortmann AL, Gallo LC. Community-created programs: can they be the basis of innovative transformations in our health care practice? Implications from 15 years of testing, translating, and implementing community-based, culturally tailored diabetes management programs. Clin Diabetes 2012;30:156–163 [Google Scholar]
- 28. Rosenman MB, Holmes AM, Ackermann RT, et al. . The Indiana chronic disease management program. Milbank Q 2006;84:135–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feldman I, Hellstrom L, Johansson P. Heterogeneity in cost-effectiveness of lifestyle counseling for metabolic syndrome risk groups-primary care patients in Sweden. Cost Eff Resour Alloc 2013;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lauritzen T, Sandbaek A, Skriver MV, Borch-Johnsen K. HbA1c and cardiovascular risk score identify people who may benefit from preventive interventions: a 7 year follow-up of a high-risk screening programme for diabetes in primary care (ADDITION), Denmark. Diabetologia 2011;54:1318–1326 [DOI] [PubMed] [Google Scholar]
- 31. Evans CD, Eurich DT, Taylor JG, Blackburn DF. The collaborative cardiovascular risk reduction in primary care (CCARP) study. Pharmacotherapy 2010;30:766–775 [DOI] [PubMed] [Google Scholar]
- 32. Ajay VS, Tian M, Chen H, et al. . A cluster-randomized controlled trial to evaluate the effects of a simplified cardiovascular management program in Tibet, China and Haryana, India: study design and rationale. BMC Public Health 2014;14:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian M, Ajay VS, Dunzhu D, et al. . A cluster-randomized, controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard trial) in rural Tibet, China, and Haryana, India. Circulation 2015;132:815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78 [DOI] [PubMed] [Google Scholar]
- 35. Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: a systematic review. Prev Chronic Dis 2013;10:E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood) 2009;28:75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–1292 [DOI] [PubMed] [Google Scholar]
- 38. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. American Medical Group Foundation. Measure Up/Pressure Down Toolkit. http://www.measureuppressuredown.com/hcprof/toolkit.pdf Accessed April2, 2019
- 40. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 41. Polonsky WH, Fisher L, Earles J, et al. . Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 42. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the patient assessment of chronic illness care (PACIC). Med Care 2005;43:436–444 [DOI] [PubMed] [Google Scholar]
- 44. Glasgow RE, Whitesides H, Nelson CC, King DK. Use of the patient assessment of chronic illness care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care 2005;28:2655–2661 [DOI] [PubMed] [Google Scholar]
- 45. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med 2014;12:573–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. The Accord Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 49. Cholesterol Treatment Trialists' Collaborators, Kearney PM, Blackwell L, et al. . Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]