Abstract
We surveyed 323 members of the Pediatric Infectious Diseases Society about their clinical practices for skin abscess management based on the 2011 Infectious Diseases Society of America guidelines and contemporary evidence. Despite this guideline and recent randomized trials, variability exists among pediatric infectious diseases clinicians in current skin and soft tissue infection management practices.
Keywords: antibiotics, decolonization, skin and soft tissue infection, SSTI management, Staphylococcus aureus
Since the community-associated methicillin-resistant Staphylococcus aureus (MRSA) USA300 clone emerged in the 1990s, the incidence of skin and soft tissue infections (SSTIs) has risen dramatically [1–4]. Recurrent SSTI poses a significant burden, occurring in >50% of individuals [5]. In 2008, coincident with the circulation of these novel strains, Creech et al surveyed infectious diseases physicians regarding treatment and prevention of SSTIs in pediatric patients, identifying wide variability in practice [6]. In 2011, the Infectious Diseases Society of America (IDSA) published MRSA treatment and prevention clinical guidelines, based largely upon expert opinion [7]. Between 2011 and 2017, several large, randomized controlled trials (RCTs) assessed the role of systemic antibiotics in the treatment of acute SSTIs [8, 9] and the effectiveness of decolonization measures in preventing recurrent SSTIs [5, 10, 11]. Thus, we aimed to evaluate current SSTI management practices by pediatric infectious diseases clinicians in the context of current clinical practice guidelines and contemporary evidence.
METHODS
Three infectious diseases physicians (S. A. F., R. C. O., J. G. N.) designed a survey comprised of 5 increasingly complex SSTI clinical scenarios. For each vignette, the survey queried clinical practice for SSTI management: use and duration of systemic antibiotics for acute SSTIs and recommendation of additional preventive efforts including hygiene measures, topical antibiotic ointment application, antiseptic body washes, systemic antibiotics for decolonization, and household contact measures. The electronic survey was disseminated by the Pediatric Infectious Diseases Society (PIDS) to all members via email in July 2018 (Supplementary Methods). Answers remained anonymous. Statistical analyses were performed via Pearson χ 2, Fisher exact, and independent samples t tests (SPSS version 25, IBM SPSS, Chicago, Illinois).
The vignettes consisted of 5 clinical presentations (see the Supplementary Methods for complete vignettes):
“Primary SSTI”: Previously healthy 3-year-old girl with a primary skin abscess. She attends daycare.
“Recurrent SSTI”: Same 3-year-old girl, now experiencing a recurrent skin abscess.
“Athlete-household SSTI”: Previously healthy 15-year-old boy wrestler with a primary skin abscess and family members with history of SSTIs.
“Multiple-recurrent SSTI”: 8-year-old girl with a skin abscess and a history of 5 prior MRSA SSTIs.
“Refractory SSTI”: Same 8-year-old girl; all household members performed decolonization regimen after the most recent SSTI, yet patient experienced another skin abscess.
RESULTS
Of 1053 PIDS members, 323 respondents (31%) indicated that they evaluate patients with S aureus infections and completed the survey. Respondents were attending-level physicians (80%) or fellows (16%) specializing in pediatric infectious diseases (Supplementary Table 1). Median years of clinical practice was 9 (interquartile range, 4–19). Ninety-one percent practiced in an academic medical center; 39% reported having institutional SSTI management guidance.
SSTI Management
The majority of respondents sent abscess fluid for culture and antibiotic susceptibility testing (Figure 1A). Most (72%) recommended that the primary SSTI patient return to daycare immediately with a bandage over the healing incision. Many (65%) recommended that the athlete-household SSTI patient wait for the incision to heal completely before returning to wrestling, while 32% recommended returning to practice immediately with a bandage over the healing incision.
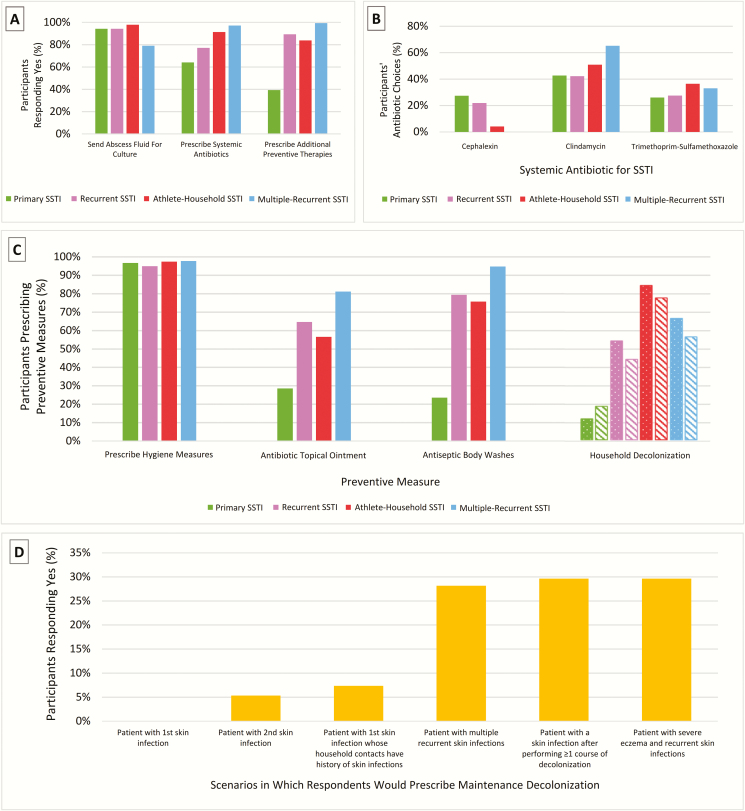
Figure 1.
A, Acute skin and soft tissue infection (SSTI) management respondents indicating “yes” by scenario (primary SSTI, recurrent SSTI, athlete-household SSTI, multiple-recurrent SSTI) to the questions “Would you send the abscess drainage fluid to the clinical microbiology laboratory for culture and antibiotic susceptibility testing?”; “Would you prescribe a systemic antibiotic (intravenous, intramuscular, oral) for this patient?”; and “Would you prescribe any additional management measures to prevent recurrent abscesses (eg, hygiene measures, topical antimicrobials) for this patient?” B, Systemic antibiotic selection for acute SSTI. The 3 most frequently prescribed antibiotics across all scenarios were clindamycin (51%), trimethoprim-sulfamethoxazole (31%), and cephalexin (12%). Amoxicillin, amoxicillin-clavulanate, doxycycline, and linezolid accounted for <5% of responses. C, Index patient and household contact preventive measures. For respondents stating that they would recommend additional measures to prevent recurrent SSTI, specific preventive measures are demonstrated by scenario. Solid bars indicate that the response is applied to the index patient. Patterned bars indicate household contacts: Dots indicate topical antibiotic, and stripes indicate antiseptic body washes. D, Scenario(s) for which respondents would prescribe maintenance decolonization. Respondents were more likely to prescribe maintenance decolonization for patients presenting with multiple, recurrent skin infections, patients presenting with a skin infection after previously performing at least one course of decolonization measures, and patients with severe eczema and recurrent skin infections.
Respondents were less likely to prescribe systemic antibiotics for acute SSTI in less complex scenarios (Figure 1A). The most commonly prescribed systemic antibiotics were clindamycin, trimethoprim-sulfamethoxazole, and cephalexin (Figure 1B). While there was no significant difference in systemic antibiotic prescribing between attendings/fellows and institutions with/without standard SSTI guidance, there was a significant difference in mean years of clinical practice between respondents who would (11.9 years) and would not (22.3 years) prescribe systemic antibiotics for the multiple-recurrent SSTI scenario (P = .05). When informed that the culture from the recurrent SSTI patient’s abscess grew S aureus resistant to the prescribed antibiotic, and the abscess was healing, 48% of respondents stopped antibiotic therapy, 46% changed the antibiotic, and 5% continued current therapy.
Preventive Measures
While only 39% of respondents would prescribe additional preventive measures (eg, enhanced hygiene, decolonization with topical antimicrobials and antiseptics) for the primary SSTI patient, 84%–98% would do so for increasingly complex scenarios (Figure 1C). There was no significant difference in recommendations for preventive measures between attendings/fellows, mean years of clinical practice, or institutions with/without standard SSTI guidance. Free-response prevention recommendations included avoiding tight/restrictive clothing, hand hygiene, alcohol-based nasal decolonization agents, antiseptic gargles, expediting toilet training and/or changing diapers frequently, using hot water and/or bleach to wash clothes/linens, and taking pets to the veterinarian.
Hygiene Measures
Of respondents prescribing preventive measures, nearly all (95%–98%) prescribed hygiene measures to index patients (Figure 1C). The most common measures were “do not share personal hygiene items,” “change underwear daily,” “keep fingernails short,” and “wash bed linens weekly” (Supplementary Table 2).
Topical Antibiotic Ointment
As the scenarios became more complex, respondents were increasingly likely to prescribe topical antibiotics to the index patient, most commonly mupirocin to the anterior nares, twice daily, for 5 days (Table 1).
Table 1.
Treatment and Prevention Measures Recommended by Scenario
| Measure | Primary SSTI | Recurrent SSTI | Athlete-Household SSTI | Multiple-Recurrent SSTI | Refractory SSTIa |
|---|---|---|---|---|---|
| Systemic antibiotic therapy | 196/306 (64) | 222/289 (77) | 252/276 (91) | 259/267 (97) | … |
| Antibiotic prescribed for acute infectionb | |||||
| Amoxicillin | 0/196 (0) | 0/222 (0) | 0/252 (0) | 1/259 (0.5) | … |
| Amoxicillin-clavulanate | 4/196 (2) | 2/222 (1) | 1/252 (0.5) | 0/259 (0) | … |
| Cephalexin | 61/196 (31) | 58/222 (26) | 12/252 (5) | 0/259 (0) | … |
| Clindamycin | 95/196 (48) | 112/222 (50) | 148/252 (59) | 182/259 (70) | … |
| Doxycycline | 2/196 (1) | 2/222 (1) | 18/252 (7) | 3/259 (1) | … |
| Linezolid | 3/196 (2) | 4/222 (2) | 3/252 (1) | 1/259 (0.5) | … |
| TMP-SMX | 58/196 (30) | 73/222 (33) | 106/252 (42) | 92/259 (35) | … |
| Duration of antibiotics for acute infection | |||||
| 3 days | 10/196 (5) | 5/222 (2) | 4/252 (2) | 2/259 (1) | … |
| 5 days | 67/196 (34) | 72/222 (32) | 70/252 (28) | 57/259 (22) | … |
| 7 days | 99/196 (51) | 108/222 (49) | 122/252 (48) | 126/259 (49) | … |
| 10 days | 19/196 (10) | 36/222 (16) | 50/252 (20) | 59/259 (23) | |
| 14 days | 1/196 (1) | 1/222 (1) | 5/252 (2) | 13/259 (5) | … |
| Recommend additional preventive measures | 120/306 (39) | 257/288 (89) | 231/276 (84) | 265/267 (99) | … |
| Hygiene measures | 115/120 (96) | 244/257 (95) | 224/231 (97) | 259/265 (98) | … |
| Topical antibiotic ointment | 34/119 (29) | 166/257 (65) | 130/231 (56) | 215/259 (83) | 111/155 (72) |
| Siteb | |||||
| Nose | 13/34 (38) | 146/166 (88) | 121/130 (93) | 208/215 (97) | 108/111 (97) |
| Axilla | 3/34 (9) | 8/166 (5) | 11/130 (8) | 20/215 (9) | 10/111 (9) |
| Fingernails | 3/34 (9) | 9/166 (4) | 7/130 (5) | 13/215 (6) | 10/111 (9) |
| Umbilicus | 1/34 (2) | 1/166 (1) | 1/130 (1) | 7/215 (3) | 7/111 (6) |
| Perineum/rectum | 8/34 (24) | 27/166 (16) | 13/130 (10) | 39/215 (18) | 20/111 (18) |
| Site of infection | 20/34 (59) | 15/166 (9) | 6/130 (5) | 4/215 (2) | 0/111 (0) |
| Frequency of application | |||||
| 1x/day | 5/34 (15) | 11/166 (7) | 11/130 (9) | 18/215 (8) | 12/111 (11) |
| 2x/day | 18/34 (53) | 132/166 (80) | 107/130 (82) | 177/215 (82) | 88/111 (79) |
| 3x/day | 9/34 (27) | 19/166 (11) | 10/130 (8) | 19/215 (9) | 10/111 (9) |
| Application duration | |||||
| 3 days | 3/34 (9) | 9/166 (5) | 2/130 (2) | 5/215 (2) | 6/111 (5) |
| 5 days | 13/34 (38) | 73/166 (44) | 63/130 (49) | 97/215 (45) | 53/111 (48) |
| 7 days | 13/34 (38) | 58/166 (35) | 47/130 (36) | 74/215 (34) | 32/111 (29) |
| 10 days | 1/34 (3) | 15/166 (9) | 8/130 (6) | 26/215 (12) | 8/111 (7) |
| Antiseptic body wash | 28/119 (24) | 204/257 (79) | 174/230 (76) | 251/259 (97) | 155/155 (100) |
| CHG | 10/28 (36) | 87/204 (43) | 110/174 (63) | 134/251 (53) | 71/155 (46) |
| Bleach | 16/28 (57) | 116/204 (57) | 58/174 (33) | 113/251 (45) | 81/155 (52) |
| Capful of bleach into bath water | 0/16 (0) | 15/116 (13) | 7/58 (12) | 14/113 (12) | 9/81 (11) |
| 1/4 cup to full bathtub | 5/16 (31) | 32/116 (28) | 23/58 (40) | 38/113 (34) | 31/81 (38) |
| 1/4 cup to half-full bathtub | 4/16 (25) | 41/116 (35) | 16/58 (28) | 40/113 (35) | 24/81 (30) |
| 1/4 cup to quarter-full bathtub | 7/16 (44) | 19/116 (16) | 8/58 (14) | 16/113 (14) | 11/81 (14) |
| Frequency of body washes | |||||
| ≥1x/day | 12/28 (43) | 93/204 (46) | 84/174 (48) | 127/251 (51) | 16/155 (10) |
| Every other day | 10/28 (36) | 93/204 (46) | 67/174 (39) | 104/251 (41) | 22/155 (14) |
| 1x/week | 6/28 (21) | 13/204 (6) | 0/174 (0) | 0/251 (0) | 41/155 (27) |
| 2x/week | … | … | … | … | 67/155 (43) |
| Duration of body washesc | |||||
| 3 days | 1/28 (4) | 12/203 (6) | 2/174 (1) | 7/251 (3) | … |
| 5 days | 3/28 (11) | 35/203 (17) | 31/174 (18) | 44/251 (17) | … |
| 7 days | 11/28 (39) | 75/203 (37) | 61/174 (35) | 90/251 (36) | … |
| 10 days | 2/28 (7) | 21/203 (10) | 27/174 (16) | 41/251 (16) | … |
| 1–3 months | 8/28 (29) | 33/203 (16) | 21/174 (12) | 28/251 (11) | 75/155 (48) |
| 6 months | … | … | … | … | 58/155 (37) |
| 12 months | … | … | … | … | 15/155 (10) |
| Systemic antibiotics for decolonization | 2/119 (2) | 3/257 (1) | 4/229 (2) | 27/259 (10) | 15/155 (10) |
Data are presented as no./No. (%). Denominators vary based on REDCap survey branch logic and missing responses.
Abbreviations: CHG, chlorhexidine gluconate; SSTI, skin and soft tissue infection; TMP-SMX, trimethoprim-sulfamethoxazole.
aScenario included only questions regarding decolonization; results reported for ongoing (maintenance) decolonization regimen.
bRespondents could select >1 option.
cFor duration of antiseptic body washes for the male wrestler, 9 of 174 (5%) wrote in the response “during wrestling season.”
Antiseptic Body Washes
Fewer respondents prescribed antiseptic body washes for the primary SSTI patient (24%) than for more complex scenarios (79%–100%). Chlorhexidine washes and dilute bleach water baths were both frequently prescribed once daily for 7 days. The most common bleach dilution was 1/4 cup per half-full or full bathtub (Table 1).
Maintenance Decolonization
For the refractory SSTI patient, 60% of respondents would recommend maintenance decolonization, most commonly dilute bleach water baths (1/4 cup bleach per full bathtub), twice weekly for 1–3 months and mupirocin ointment to the anterior nares, twice daily, for 5 days, monthly for 6 months (Table 1). Few respondents (10%) recommending maintenance decolonization also prescribed oral antibiotics.
In their clinical practice overall, 50% of respondents reported prescribing maintenance decolonization measures for their patients. Maintenance decolonization was most commonly prescribed for patients with recurrent SSTI, including those who had already performed ≥1 course of decolonization, and those with severe eczema (Figure 1D).
Primary Versus Recurrent SSTI
Respondents were more likely to prescribe systemic antibiotics for the acute infection (odds ratio [OR], 1.9; 95% confidence interval [CI], 1.4–2.7), topical antibiotic ointment (OR, 3.8; 95% CI, 2.9–4.8), and antiseptic body washes (OR, 8.6; 95% CI, 6.5–11.3) for patients with recurrent infections compared to those with primary infections (Figure 1A).
Family History Versus No Family History of SSTI
Respondents were more likely to prescribe systemic antibiotics to a patient whose family members report prior SSTI (91%) than to patients without family history (64%) (OR, 5.9; 95% CI, 3.7–9.5). Compared to the patient with primary SSTI without family history, respondents would more often prescribe topical antibiotics (OR, 6.7; 95% CI, 4.4–10.3), antiseptic body washes (OR, 15.1; 95% CI, 9.5–24.0), and household decolonization measures (OR, 47.3; 95% CI, 21.6–103.7) to the patient with primary SSTI plus family history (Figure 1C).
DISCUSSION
To assess adherence to guidelines and contemporary evidence, we aimed to understand current SSTI management practices for treatment of acute skin infections and prevention of recurrent infections by pediatric infectious diseases clinicians. The 2011 IDSA guideline states that incision and drainage is the primary treatment for skin abscesses, and adjunctive antibiotic therapy may not be necessary for simple skin abscesses. However, recent seminal RCTs demonstrate improved outcomes with administration of systemic antibiotics in conjunction with incision and drainage for acute skin abscesses, regardless of size [8, 9, 12]. In the present survey, only 60% of respondents would recommend systemic antibiotics for a primary skin abscess. Thus, while the majority would be in compliance with IDSA guidelines, this finding suggests that contemporary evidence has not been fully implemented into clinical practice. Based on our findings and these new RCTs, an updated and/or pediatric-specific guideline for S aureus SSTI treatment and prevention is warranted.
More than half of patients with SSTI will experience recurrent infections [5]. Thus, the IDSA guideline recommends preventive measures, including education regarding appropriate wound care, personal hygiene, and avoiding sharing personal hygiene items [7]. Enhanced hygiene was the most frequently recommended preventive measure cited by our respondents. For patients with recurrent SSTI despite implementing hygiene measures, and in situations with multiple household members experiencing SSTI, IDSA recommends that clinicians consider decolonization with topical antimicrobials and antiseptic baths. Our respondents were significantly more likely to recommend decolonization to the index patient in scenarios in which there was recurrent SSTI or family history of SSTI.
MRSA transmission and reacquisition frequently occurs among household members. An RCT demonstrated that a household decolonization approach (compared to decolonization of the index patient alone) reduced SSTI incidence among index patients and household contacts [5]. Among our respondents, decolonization for household contacts was most commonly recommended when there was a family history of SSTI and less often for scenarios without affected household contacts. Considering the aforementioned trial, Creech et al recommend a 5-day decolonization protocol consisting of antiseptic body washes and intranasal mupirocin for index patients experiencing recurrent infections and their household contacts [13]. Additionally, as environmental surfaces serve as reservoirs of transmission, targeted household environmental cleaning may be an important component of infection prevention [14].
This study has limitations. Our survey targeted pediatric infectious diseases clinicians, who often evaluate more complex cases via referrals, and may take a more conservative approach. However, these specialists are often approached by primary care pediatricians and emergency medicine clinicians to provide general recommendations regarding the treatment and prevention of common infectious diseases, such as SSTI. The response rate was 31%, a rate consistent with similar surveys published by other disciplines [15, 16]. Last, recall and/or response bias is possible among respondents.
In conclusion, this study reveals that SSTI management practices supported by recent RCTs are not consistently recommended among pediatric infectious diseases clinicians. This information can provide guidance for improved knowledge translation through dissemination and implementation of evidence-based practices.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Patrick Reich, MD, MSCI, Andrew Janowski, MD, and David Rosen, MD, PhD for their involvement in the survey piloting and revision process, and the Pediatric Infectious Diseases Society for survey dissemination.
Disclaimer. These funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Agency for Healthcare Research and Quality (AHRQ).
Financial support. This work was supported by the AHRQ (grant numbers R01-HS021736, R01-HS024269 to S. A. F.) and the NIH/National Center for Advancing Translational Sciences (grant number UL1-TR002345 to S. A. F.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–91. [DOI] [PubMed] [Google Scholar]
- 2. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. ; EMERGEncy ID Net Study Group Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355:666–74. [DOI] [PubMed] [Google Scholar]
- 3. Pallin DJ, Egan DJ, Pelletier AJ, et al. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–8. [DOI] [PubMed] [Google Scholar]
- 4. Lee BY, Singh A, David MZ, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 2013; 19:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fritz SA, Hogan PG, Hayek G, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis 2012; 54:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Creech CB, Beekmann SE, Chen Y, Polgreen PM. Variability among pediatric infectious diseases specialists in the treatment and prevention of methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J 2008; 27:270–2. [DOI] [PubMed] [Google Scholar]
- 7. Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 8. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med 2016; 374:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daum RS, Miller LG, Immergluck L, et al. ; DMID 07-0051 Team A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med 2017; 376:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fritz SA, Camins BC, Eisenstein KA, et al. Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: a randomized trial. Infect Control Hosp Epidemiol 2011; 32:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaplan SL, Forbes A, Hammerman WA, et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis 2014; 58:679–82. [DOI] [PubMed] [Google Scholar]
- 12. Talan DA, Moran GJ, Krishnadasan A, et al. Subgroup analysis of antibiotic treatment for skin abscesses. Ann Emerg Med 2018; 71:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Creech CB, Al-Zubeidi DN, Fritz SA. Prevention of recurrent staphylococcal skin infections. Infect Dis Clin North Am 2015; 29:429–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hogan PG, Mork RL, Boyle MG, et al. Interplay of personal, pet, and environmental colonization in households affected by community-associated methicillin-resistant Staphylococcus aureus. J Infect 2019; 78:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metz V, Köchl B, Fischer G. Should pregnant women with substance use disorders be managed differently? Neuropsychiatry (London) 2012; 2:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol 2015; 15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.