Abstract
Enhanced cognitive–behavioral therapy (CBT-E) is one of the primary evidence-based treatments for adults with eating disorders (EDs). However, up to 50% of individuals do not respond to CBT-E, likely because of the high heterogeneity present even within similar diagnoses. This high heterogeneity, especially in regard to presenting pathology, makes it difficult to develop a treatment based “on averages” and for clinicians to accurately pinpoint which symptoms should be targeted in treatment. As such, new models based at both the group, and individual level, are needed to more accurately refine targets for personalized evidence-based treatments that can lead to full remission. The current study (Expected N = 120 anorexia nervosa, atypical anorexia nervosa, and bulimia nervosa) will build both group and individual longitudinal models of ED behaviors, cognitions, affect, and physiology. We will collect data for 30 days utilizing a mobile application to assess behaviors, cognition, and affect and a sensor wristband that assesses physiology (heart rate, acceleration). We will also collect outcome data at 1- and 6-month follow-ups to assess ED outcomes and remission status. These data will allow for identification of “on average” and “individual” targets that maintain ED pathology and test if these targets predict outcomes, including ED remission.
Keywords: eating disorders, longitudinal modeling, network analysis, physiology, remission
1 |. INTRODUCTION
Eating disorders (EDs) are serious mental illnesses (Arcelus, Mitchell, Wales, & Nielsen, 2011; Zipfel, Löwe, Reas, Deter, & Herzog, 2000). Most individuals with EDs never achieve full remission, with approximately 60% of those with anorexia nervosa (AN) never remitting, even after 20 years of illness, or experiencing multiple relapses and descending into a chronic illness course (Fichter, Quadflieg, Crosby, & Koch, 2017; Keel & Brown, 2010). We urgently need personalized, effective, empirically valid treatments for adults with EDs that can promote full ED remission.
The primary model for understanding and conceptualizing EDs is based on cognitive–behavioral theory for EDs (CBT-E; Fairburn, 2008; Fairburn, Cooper, Shafran, & Wilson, 2008). Cognitive–behavioral theory posits that cognitions, behaviors, affect, and physiological symptoms interact to maintain and exacerbate psychopathology (Fairburn, Jones, Peveler, Hope, & O’Connor, 1993). CBT-E is centered on the notion that disrupting associations between illness pathways (e.g., cognitions and behaviors associated with eating, shape, and weight) drives symptom remission (Fairburn et al., 1993). Crucial to this premise is that disruption among symptoms is only possible if there is a thorough understanding of the intricate pathways between cognitive, behavioral, affective, and physiological symptomatology, such that precision interventions can be developed to target these unique pathways and symptoms that fall at the center of the illness (Hamburg & Collins, 2010; Levinson, Vanzhula, & Brosof, 2018). CBT-E one of the most utilized evidence-based treatment for adult EDs, however in its current “one-size-fits-all” format it does not address the high symptom heterogeneity, which is extremely common across EDs, making it difficult for patients to reach full remission (i.e., current form it is efficacious in only 50% of individuals; Bulik, Berkman, Brownley, Sedway, & Lohr, 2007). To personalize CBT-E (and other related treatments) to the individual, first, we need data that can identify individual targets (central symptoms) and if these targets predict remission.
The identification of precise illness pathways that intersect multiple units of analysis (i.e., cognitive, behavioral, affective, physiological) will lead to treatments aimed at specific individual targets. However, no data currently exist illuminating specific pathways among symptoms AND within individuals, primarily because methods to collect (i.e., sensor technology) and analyze (i.e., network analysis; NA) such complex data have only been recently developed. For example, to confidently estimate individual networks using NA we need intensive, real-time data with multiple (~100) time points per person (Zhang, Klein, Walsh, Lu, & Sazonov, 2014). Such data do not currently exist. We also need to model data integrating cognitive–affective–behavioral symptoms with physiology, as physiological symptoms, such as electrodermal activity (EDA: skin resistance and conductance variation) and acceleration also maintain EDs (Alberti et al., 2013; Dong, Scisco, Wilson, Muth, & Hoover, 2013; Farooq & Sazonov, 2016).
Network theory offers a data-driven way to use cognitive–behavioral symptom data to identify core symptoms and illness pathways that maintain an individual’s ED (Borsboom & Cramer, 2013; McNally, 2016). One application of network theory or NA identifies how core symptoms maintain and promote the spread of psychopathology within individuals (Epskamp, Deserno, & Bringmann, 2019; Epskamp, Waldorp, Mõttus, & Borsboom, 2018). NA is directly congruent with cognitive–behavioral theories proposition that (a) real-time cognitions, behaviors, affect, and physiological symptoms interact with each other to exacerbate and maintain illness and that (b) core symptoms are theorized to drive all other symptoms of the disorder. Targeting such symptoms at the core of each individual’s symptom network should lead to novel intervention targets for personalized treatment.
NA characterizes core symptoms (e.g., central features of the disorder such as overvaluation of shape or “trigger” symptoms) within networks of illness (using measures of centrality which identify core symptoms of pathology), as well as illness pathways (i.e., edges, defined by partial correlations accounting for the variance of all symptoms of the disorder) among symptoms of a disorder. Recent developments in NA provide a statistical approach to identify specific core “trigger” symptoms and symptoms pathways for each individual and how these symptoms differ from the average (Epskamp et al., 2018; Levinson, Vanzhula, & Brosof, 2018). From a cognitive–behavioral perspective, these core “trigger” symptoms are central to the maintenance of psychopathology. Therefore, intervening on core symptoms that are highly related to the largest number of other symptoms in the network should maximize the impact of the intervention on the other behaviors, thoughts, affect, and physiology related to the core symptom (Anderson & Maloney, 2001; Fisher et al., 2019).
Recent research using NA has identified overvaluation of weight and shape as a core symptom of ED pathology across individuals, (Christian et al., 2019; Levinson, Vanzhula, & Brosof, 2018), that core symptoms predict important ED treatment outcomes and prognosis (i.e., body mass index [BMI], depression; Levinson et al., 2018; Levinson et al., 2017; Olatunji, Levinson, & Calebs, 2018; Sala, Brosof, & Levinson, 2019), and that central symptoms are highly heritable (Olatunji, Christian, Strachan, & Levinson, 2020), supporting the idea that interventions targeted at core symptoms should maximize treatment efficacy and ultimately lead to remission. However, given the heterogeneous nature of EDs, it is theorized, and has been found, that core symptoms significantly vary across individuals (Levinson, Vanzhula, & Brosof, 2018). Once identified, core ED “trigger” symptoms can be directly targeted to disrupt the spread or “activation” of ED behaviors (Anderson & Maloney, 2001), which would help patients achieve remission.
The current study will answer three primary questions (a) Which individual core symptoms and illness pathways maintain EDs and predict the onset of ED behaviors? (b) How do core-maintaining symptoms differ across individuals? (c) How do physiological symptoms interact with cognitive, behavioral, and affective symptoms to predict outcomes (e.g., remission)? We hypothesize that, consistent with CBT-E theory, overvaluation of weight and shape will be identified as the most prevalent central symptoms. However, we also hypothesize that there will be high variability in central symptoms, such that certain core symptoms and illness pathways will vary across individuals (e.g., weight vs. loss of control fears), while other pathways will be invariant (e.g., pathways between binge eating and purging). We also hypothesize that symptoms identified as most central will predict ED outcomes at follow-ups. The current protocol extends prior research by including a large sample size, longer period of assessment, enhanced assessment of mobile-reported and physiological symptoms, and a follow-up assessment to capture remission outcomes. These aspects of the protocol allow us to, with greater power, model individual networks of pathology that encompass the entire spectrum of ED symptoms, and then test if central symptoms predict longer-term outcomes.
2 |. METHOD
2.1 |. Participants
We will recruit 120 adults (ages 18–65) with a diagnosis of AN, bulimia nervosa (BN), and other specified feeding and eating disorder: Atypical AN. We will recruit from these populations because (a) the primary empirically based treatment for adults with these disorders is based on CBT theory (i.e., CBT-E) and (d) these populations have the least evidence for effective ED treatments. Participants will be recruited from ED centers and social media. Participation is all remote and open to participants across the United States.
Participants will be diagnosed using formal structured clinical interviews (see below). Inclusion criteria include a current DSM-5 diagnosis of AN, BN, or Atypical AN. The weight threshold for an AN diagnosis will be less than 90% of expected body weight at the time of assessment, according to population norms and adjusted for age, sex, and height. Participants with a diagnosis of Atypical AN must meet all diagnostic criteria for AN with the exception of the weight criteria. Exclusion criteria include active suicidal intent, active psychosis, active mania, inability to read or write English, and/or medical instability. Participants are not required to be receiving treatment currently or in the past and we expect that most participants will not be receiving treatment (this will be assessed using the Treatment Interview, see below). This type of procedure ensures we can capture the natural progression of ED symptoms and how that progression leads to both short- and longer-term outcomes (remission), rather than how treatment impacts changes in symptoms.
2.2 |. Procedure
All participants will give informed consent and complete structured clinical interviews (Figure 1). The interviewer will provide instructions on using the mobile app and sensor wristband to included participants. Participants will complete self-report measures, including current and highest BMI, and use of psychotropic medications, stimulant/steroid use, and other medications. After baseline assessment, participants complete 30 days of mobile app surveys and sensor-technology monitoring (real-time data) to assess ED cognitions, behaviors, affect, and physiological responses. ED outcomes, including current ED symptoms, remission, treatment, and BMI will be assessed at 1- and 6-month follow-ups.
FIGURE 1.
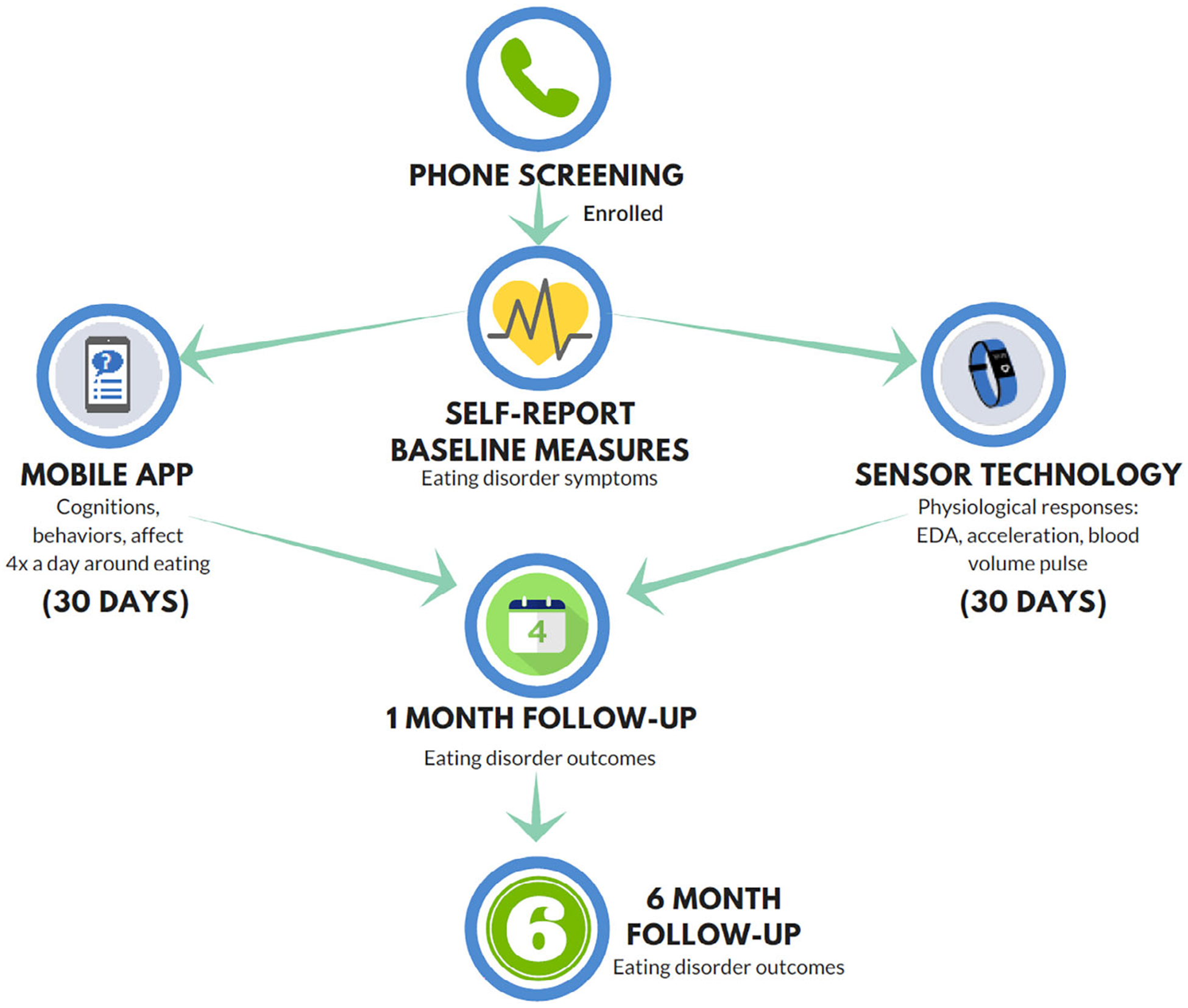
Procedure
2.2.1 |. Mobile application procedure
Mobile app surveys will assess cognitions, behaviors, and affect (see Table 1) delivered directly to the participant’s personal phone or tablet. Participants will complete four daily assessments over 30 days (120 assessment points) of approximately 3–5 min each. Participants are reimbursed based on the number of assessments they complete. Directions ask participants to focus on “what they are experiencing or feeling now.” Participants will be given directions on how to use the 0–100 scale, which is needed to ensure high variability for NA.
TABLE 1.
Mobile-application assessment items
| Content | Item |
|---|---|
| ED cognitions | |
| Fear of weight gain | I am terrified of gaining weight |
| Weight/shape dissatisfaction | I feel dissatisfied with my weight or shape |
| Fear of food | I am worried about eating |
| Drive for thinness | I am preoccupied with the desire to be thinner |
| Overvaluation of shape/weight | My worth as a person (how I think about and judge myself) is influenced by my weight and/or shape |
| Fear of losing control | I am afraid of losing control of my eating |
| Body dissatisfaction | I do not like how my body looks |
| Feel fat | I feel fat |
| Social appearance anxiety | I am worried people are judging the way I look negatively |
| Eating anxiety | I felt anxious when I ate |
| Hunger anxiety | When I felt hungry, it made me anxious |
| Repetitive thoughts about food | I am having thoughts about food that I cannot stop |
| Physical sensation of eating | I did not like the physical sensations I felt when eating |
| Urge to restrict | I have the urge to restrict |
| Difficulty drinking in public | I found it difficult to drink something in front of a group of people |
| Difficulty eating in public | I am concerned about other people seeing me eat |
| ED behaviors | |
| Restraint | I have been consciously trying to restrict my food intake to influence my shape or weight |
| Fasting | I have skipped meals or snacks in order to influence my shape or weight |
| Food rules | I have tried to follow definite rules regarding eating in order to influence shape or weight |
| Purging | I vomited in order to lose weight |
| Laxative/diuretic use | I used laxatives or diuretics in order to lose weight |
| Binge eating | I ate a very large amount of food in a short period of time and felt out of control |
| Chewing and spitting | I have chewed and spit |
| Body checking | I have squeezed, looked at, or touched parts of my body to determine their size or form |
| Excessive exercise | I felt compelled to exercise or that I must exercise for a certain amount of time and/or in a strenuous manner |
| Food avoidance | I avoided certain foods because they make me anxious |
| Small portions | I ate very small portions |
| Affect/comorbidities/maintaining mindsets | |
| Anxiety | I feel anxious |
| Stress | I feel stressed |
| Fear | I feel scared |
| Worthlessness | I feel worthless |
| Feeling ineffective | I feel ineffective |
| Guilt | I feel guilty |
| Shame | I feel ashamed |
| Depression | I feel so sad and unhappy I cannot stand it |
| Active suicidal ideation | I am thinking about hurting or killing myself |
| Passive suicidal ideation | I felt like I would be better off dead |
| Nonsuicidal self-injury | I have engaged in self-harm behaviors |
| Fatigue | I feel tired and fatigued |
| Agitation | I feel agitated |
| Difficulty relaxing | I am unable to relax right now |
| Obsessions | Right now I am upset about unpleasant thoughts that come into my mind against my will |
| Compulsion | I felt compelled to act in a certain way to get rid of thoughts I am having, even if it is senseless or excessive |
| Thought suppression | I frequently got uncomfortable thoughts and felt I must do something in order to get rid of them |
| Impulsivity | I had trouble controlling my impulses |
| Bad person | I am having the thought that I am a bad person |
| Emotional avoidance | I tried to avoid my emotions |
| Emotions overwhelming | I experienced my emotions as overwhelming and out of control |
| Post-event processing | I had thoughts or images about an event that occurred over and over again, that is resulting in my feelings getting worse and worse |
| Post-traumatic intrusive thoughts | I had repeated, disturbing memories, thoughts, or images about a stressful experience from the past |
| Heart racing | My heart raced for no good reason |
| Worries overwhelming | My worries overwhelmed me |
| Social interaction anxiety | When I was in a social situation, I felt uncomfortable |
| Social anxiety | I am afraid that others will not approve of me and reject me |
| Interoceptive awareness | I felt very sensitive to changes in my internal bodily sensations |
| Physical sensation discomfort | I had uncomfortable physical sensations in my body |
| Concern over mistakes | I am afraid of making mistakes |
| High standards | I am setting higher goals for myself than most people |
| Intolerance of uncertainty | I cannot stand not knowing what is going to happen in the future |
| Fear of attracting attention | I worried I might do something to attract the attention of other people |
| Avoidance | I avoided a difficult or scary situation |
Note: Directions for cognitions and affect will ask participants to focus on what they are thinking and feeling at the moment. Directions for behaviors will ask participants to report on behaviors since the last time they answered questions through the mobile app. All questions are on a 0–100 scale to ensure high enough variability to model individual networks. Participants are asked these questions four times a day for 30 days. Anchors for most questions range from 0 not at all, 50 somewhat, to 100 extremely.
2.2.2 |. Empatica procedure
The Empatica E4 or Embrace sensor-technology band will assess EDA, acceleration (movement), and blood volume pulse [heart rate and heart rate variability (HRV)]. Participants wear the band for 30 days corresponding to the days they complete the mobile app questions. When participants first receive the Empatica band, they will be asked to complete a 10-min period wearing the band where they sit quietly and move as little as possible while reading a passage of neutral text to obtain baseline measurements (Graham et al., 2019). During this time period they will be asked to sit in a familiar environment to eliminate potential activation from any novel stimuli in the environment.
2.2.3 |. Follow-up procedure
1- and 6-month follow-ups
At each follow-up, participants will complete the Structured Clinical Interview for DSM-5 (SCID-5), Eating Disorder Diagnostic Inventory (EDDI), and Treatment Interview semi-structured clinical interviews, as well as the Eating Disorder Examination Questionnaire (EDE-Q), Eating Pathology Symptoms Inventory (EPSI), and weight and height measures. We have chosen these time points based on research suggesting that most individuals with an ED are likely to relapse within the first 6 months following discharge from an intensive treatment center, with the highest likelihood in the first 3 months (Khalsa, Portnoff, McCurdy-McKinnon, & Feusner, 2017), and many of our participants will be recruited after discharge.
2.3 |. Measures
2.3.1 |. Diagnostic and treatment measures
SCID-5
The SCID-5 is a semi-structured interview used to arrive at DSM-5 diagnoses (First, Williams, Karg, & Spitzer, 2015). Participants will complete the ED modules. The SCID-5 has strong psychometric properties (First et al., 2015).
EDDI
The EDDI is a semi-structured interview based on the diagnostic criteria in the DSM-5 and derived from the SCID-5. The EDDI is used to examine the frequency and intensity of ED symptoms. It includes items that assess ED behaviors, cognitions, and physiological factors. The EDDI has excellent test–retest reliability (Heiss, Boswell, & Hormes, 2018).
MINI
The MINI is a semi-structured interview used to assess DSM-5 diagnoses. The anxiety and depression modules will be used to assess for comorbid anxiety and mood disorders. The suicidality, mania/hypomania, and psychosis modules will be used to assess for exclusion criteria. The MINI has strong psychometric properties (Sheehan et al., 1998).
Treatment interview
We will assess all current and past treatment experiences (partial hospitalization, residential, etc.), as well as dates of treatment.
2.3.2 |. Self-report measures
EDE-Q 6.0
The EDE-Q is a self-report questionnaire that assesses ED behaviors and thoughts (Fairburn & Beglin, 1994). The EDE-Q has strong psychometric properties (Berg, Peterson, Frazier, & Crow, 2012).
EPSI
The EPSI is a 45-item self-report measure used to assess ED symptoms. The EPSI has strong psychometric properties (Forbush et al., 2013).
Weight, height, weight suppression, and medical status
We will assess weight and height, and highest adult weight to assess weight suppression (i.e., difference between highest weight since reaching adult height and current body weight), to partially account for medical, nutritional status, as well as the impact of weight loss and/or gain.
2.3.3 |. Mobile application measures
All questions will be asked in the present tense to assess current cognitions, behaviors, and affect (Table 1).
2.3.4 |. Sensor data
We will assess EDA, acceleration (movement), and HRV in real-time to be included in group between and within-person models. We will analyze sensor data recorded in the 10-min period preceding the completion of each mobile assessment. Sensor data will be time-aligned, normalized, and resampled to match data across channels. Band-pass filters will isolate phasic (event-related) signals from basal (tonic) EDA. De-trending and discrete Fourier transformations will isolate signal-from-noise in accelerometer data. Feature extraction (mean; SD; root-mean-squared; min, max, interquartile range; peak frequency/amplitude; linear regression slope) will characterize the data within each 10-min window. Each 10-min window will be compared to data recorded during the 10-min baseline period (reading a neutral text) to determine the change from baseline in sensor data.
2.3.5 |. Outcome measures
Remission definition
Remission is defined as (a) no longer meeting diagnostic criteria for an ED, (b) no binge eating, purging, or fasting in the past 3 months, (c) BMI >18.5 kg/m2, and (d) scores on the EDE-Q global score within 1 SD of age-matched community norms. Partial Remission is defined as meeting the physical (BMI) and behavioral criteria but not cognitive criteria (EDE-Q) (Bardone-Cone et al., 2010). Since many participants will be recruited after discharge from intensive treatments, we expect that many will be classified as partial remission when they enter the study.
Treatment status
We will also consider progression from partial remission to active ED for any participant who enters into a more intensive treatment level during the course of the study (e.g., moving from outpatient to partial hospitalization).
2.3.6 |. Data analytic plan
All codes will be made open-source. The statistical analysis plan is modified from Levinson, Brosof, et al. (2018). We will conduct both within-person group level and individual person networks. These analyses allow us to examine symptoms both within one individual (n = 1), as well as averages within multiple individuals (n > 1). Missing data are estimated using the Kalman filter (Chen, Liu, Zhao, & Principe, 2017; de Haan-Rietdijk, Voelkle, Keijsers, & Hamaker, 2017). For any missing data not automatically estimated we will impute the data using the multiple imputation methods in the MICE package in R (van Buuren et al., 2015) as is recommended for NA (i.e., Levinson, Brosof, et al., 2018). MICE conducts multiple imputation using fully conditional specification (FCS) and Gibbs sampling. FCS creates separate models for the imputation of each individual variable by creating “plausible” values based on the other variables in the data set.
Model building and testing
We will include cognitive, behavioral, affective, and physiological data (HRV, EDA, and acceleration) in the group-level models. We will model both group and individual longitudinal models. We will also test for diagnostic differences (AN, Atypical AN, BN) in our group networks.
Individual models
Because we are collecting a large amount of mobile app data, we are unable to include all symptoms in each model. For our individual models, we will include the nine symptoms with a combination of the highest means and variances, meaning, they are the most active symptoms for those particular participants. For individual networks we will conduct intra-individual networks for each individual and then use count data to detect the most common central symptoms (based on prior research; Levinson, Vanzhula, & Brosof, 2018). We will model any participant who completed at least 30% of the data, which is 36 data points.
Group-level models
Each symptom was chosen based on theoretical and empirical reasoning and to reflect a comprehensive model of ED psychopathology. If stability is not adequate, we will use the Goldbricker function to reduce our symptom set (Jones, 2018; Levinson, Vanzhula, & Brosof, 2018) to minimize overlapping items, which is likely given the large number of items we plan to assess. Goldbricker identifies potential issues with item redundancy and the best_goldbricker function suggests which items should be removed. We will also include HRV, EDA, and acceleration in our group-level model. From our final models we will identify the top three central symptoms determined via out-strength (e.g., the impact the symptom has on the remaining symptoms relative to all other symptoms in the model). See Epskamp and Fried (2018) for additional details on computing stability, centrality indices, and partial correlations in our models.
Nomothetic and idiographic network estimation
We will use the multilevel vector autoregressive (VAR) model (mlVAR package, version 0.4 in R) to estimate the network structure of ED symptoms (cognitions, affect, behaviors, and physiology). VAR models capture intra-individual dynamics, and offer insights on the group-level (the average process of all participants), on the personal level (every participant obtains a personalized network), and differences across participants (see Epskamp, van Borkulo, et al., 2018 for a detailed description). These analyses are unique in that they statistically identify the most important symptoms and symptom variations both between and within individuals and across time. For the group level, mlVAR estimation yields three different network structures: a temporal network (prospective prediction or how do these symptoms predict later symptoms), a contemporaneous network (how processes are associated within the same measurement point while accounting for temporal effects), and between-subject networks (capturing, in general, whether participants high on a given node [central, “trigger” symptom] are also high on other nodes during the course of the study). Contemporaneous and temporal networks are also obtained per individual utilizing graphicalVAR (Wild et al., 2010), allowing for unique idiographic insights into individuals. At the individual level, the contemporaneous network is an undirected partial correlation network that demonstrates the relations between symptoms at the same time point, while the temporal network suggests which symptoms predict one another over time. Both contemporaneous and temporal idiographic networks provide important information on potential dynamics between symptoms for each individual, allowing for personalized, hypothesis-driven intervention (Epskamp, van Borkulo, et al., 2018; Wild et al., 2010).
Our hypotheses will be tested in the following steps:
Computation of both group longitudinal (between-person, contemporaneous, temporal) networks and individual longitudinal networks (contemporaneous and temporal; see below and see Epskamp et al. and Levinson et al. for examples; Epskamp, van Borkulo, et al., 2018; Levinson, Vanzhula, & Brosof, 2018).
Identification of symptoms with the highest centrality and unique partial correlations among symptoms in each network for each type of network.
Symptoms with the highest centrality (i.e., most central symptoms) will be entered as independent variables (IVs) into a regression model with the ED outcome (ED behaviors and remission) as the dependent variable (DV).
Most variable partial correlations (i.e., associations between symptoms that vary the most across participants) will be entered as IVs into a regression model with the ED outcome (ED behaviors and remission) as the DV.
Power analysis
Our ability to detect even weak effect sizes (0.20) is strong in our regression analyses (power >0.92). Power for mlVAR models is determined by the sample size, number of time points, and number of symptoms included in the model (Schultzberg & Muthén, 2018). With a sample size of 100 participants with 100 time points (including 120 participants with 120 time points and 27 symptoms), we would achieve more than adequate power. See also supplementary materials from Epskamp, Waldorp, et al. (2018) which reports simulation results for mlVAR, showing that mlVAR models are excellent in recovering the fixed effect structure with quite few data. We propose to collect 120 participants with 120 time points to account for expected compliance of ~74% (based on preliminary data) and missing data.
3 |. CONCLUSIONS
The current study aims to model both longitudinal group and individual ED pathology consisting of cognitive, behavioral, affective, and physiological symptoms using network analysis. We then plan to test if central symptoms and illness pathways relate to ED outcomes and remission. Identification of specific group and individual targets will set the stage for the development of precision treatments aimed at evidence-based intervention points. Specifically, if we are able to identify central symptoms that are predictive of remission, we can develop personalized treatments designed specifically to target these symptoms and we can use network algorithms to extend personalized treatments to additional populations.
ACKNOWLEDGMENTS
This study has been registered at Open Science Framework (osf.io/2tnvd) : Registration DOI: 10.17605/OSF.IO/7MU8C.
Funding information
National Institute of Mental Health, Grant/Award Number: 1R15MH121445-01
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
DATA AVAILABILITY STATEMENT
Data will be open source as per NIH policy.
REFERENCES
- Alberti M, Galvani C, El Ghoch M, Capelli C, Lanza M, Calugi S, & Dalle Grave R (2013). Assessment of physical activity in anorexia nervosa and treatment outcome. Medicine and Science in Sports and Exercise, 45(9), 1643–1648. 10.1249/MSS.0b013e31828e8f07 [DOI] [PubMed] [Google Scholar]
- Anderson DA, & Maloney KC (2001). The efficacy of cognitive–behavioral therapy on the core symptoms of bulimia nervosa. Clinical Psychology Review, 21(7), 971–988. 10.1016/S0272-7358(00)00076-3 [DOI] [PubMed] [Google Scholar]
- Arcelus J, Mitchell AJ, Wales J, & Nielsen S (2011). Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies. Archives of General Psychiatry, 68(7), 724–731. 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- Bardone-Cone AM, Harney MB, Maldonado CR, Lawson MA, Robinson DP, Smith R, & Tosh A (2010). Defining recovery from an eating disorder: Conceptualization, validation, and examination of psychosocial functioning and psychiatric comorbidity. Behaviour Research and Therapy, 48(3), 194–202. 10.1016/j.brat.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, & Crow SJ (2012). Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. International Journal of Eating Disorders, 45(3), 428–438. 10.1002/eat.20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, & Cramer AO (2013). Network analysis: An integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. 10.1146/annurev-clinpsy-050212-185608 [DOI] [PubMed] [Google Scholar]
- Bulik CM, Berkman ND, Brownley KA, Sedway JA, & Lohr KN (2007). Anorexia nervosa treatment: A systematic review of randomized controlled trials. International Journal of Eating Disorders, 40(4), 310–320. 10.1002/eat.20367 [DOI] [PubMed] [Google Scholar]
- Chen B, Liu X, Zhao H, & Principe JC (2017). Maximum correntropy Kalman filter. Automatica, 76, 70–77. 10.1016/j.automatica.2016.10.004 [DOI] [Google Scholar]
- Christian C, Perko VL, Vanzhula IA, Tregarthen JP, Forbush KT, & Levinson CA (2019). Eating disorder core symptoms and symptom pathways across developmental tages: A network analysis. Journal of Abnormal Psychology, 129, 177–190. 10.1037/abn0000477 [DOI] [PubMed] [Google Scholar]
- de Haan-Rietdijk S, Voelkle MC, Keijsers L, & Hamaker EL (2017). Discrete-vs. continuous-time modeling of unequally spaced experience sampling method data. Frontiers in Psychology, 8, 1849 10.3389/fpsyg.2017.01849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Scisco J, Wilson M, Muth E, & Hoover A (2013). Detecting periods of eating during free-living by tracking wrist motion. IEEE Journal of Biomedical and Health Informatics, 18(4), 1253–1260. 10.1109/JBHI.2013.2282471 [DOI] [PubMed] [Google Scholar]
- Epskamp S, Deserno MK, & Bringmann LF (2019). mlVAR: multi-level vector autoregression. R Package Version 0.4.4.
- Epskamp S, & Fried EI (2018). A tutorial on regularized partial correlation networks. Psychological Methods, 23(4), 617–634. 10.1037/met0000167 [DOI] [PubMed] [Google Scholar]
- Epskamp S, van Borkulo CD, van der Veen DC, Servaas MN, Isvoranu AM, Riese H, & Cramer AO (2018). Personalized network modeling in psychopathology: The importance of contemporaneous and temporal connections. Clinical Psychological Science, 6(3), 416–427. 10.1177/2167702617744325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Waldorp LJ, Mõttus R, & Borsboom D (2018). The Gaussian graphical model in cross-sectional and time-series data. Multivariate Behavioral Research, 53(4), 453–480. 10.1080/00273171.2018.1454823 [DOI] [PubMed] [Google Scholar]
- Fairburn CG (2008). Eating disorders: The transdiagnostic view and the cognitive behavioral theory In Fairburn CG (Ed.), Cognitive behavior therapy and eating disorders (pp. 7–22). England: Guilford Press. [Google Scholar]
- Fairburn CG, & Beglin SJ (1994). Assessment of eating disorders: Interview or self-report questionnaire?. International journal of eating disorders, 16(4), 363–370. [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Shafran R, & Wilson GT (2008). Eating disorders: A transdiagnostic protocol In Barlow DH (Ed.), Clinical handbook of psychological disorders: A step-by-step treatment manual (pp. 578–614). England: The Guilford Press. [Google Scholar]
- Fairburn CG, Jones R, Peveler RC, Hope RA, & O’Connor M (1993). Psychotherapy and bulimia nervosa: Longer-term effects of interpersonal psychotherapy, behavior therapy, and cognitive behavior therapy. Archives of General Psychiatry, 50(6), 419–428. 10.1001/archpsyc.1993.01820180009001 [DOI] [PubMed] [Google Scholar]
- Farooq M, & Sazonov E (2016). A novel wearable device for food intake and physical activity recognition. Sensors, 16(7), 1067 10.3390/s16071067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter MM, Quadflieg N, Crosby RD, & Koch S (2017). Long-term outcome of anorexia nervosa: Results from a large clinical longitudinal study. International Journal of Eating Disorders, 50(9), 1018–1030. 10.1002/eat.22736 [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV) (pp. 1–94). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Fisher AJ, Bosley HG, Fernandez KC, Reeves JW, Soyster PD, Diamond AE, & arkin J (2019). Open trial of a personalized modular treatment for mood and anxiety. Ehaviour Research and Therapy, 116, 69–79. 10.1016/j.brat.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Forbush KT, Wildes JE, Pollack LO, Dunbar D, Luo J, Patterson K, … Watson D (2013). Development and validation of the Eating Pathology Symptoms Inventory (EPSI). Psychological Assessment, 25(3), 859–878. 10.1037/a0032639 [DOI] [PubMed] [Google Scholar]
- Graham SA, Jeste DV, Lee EE, Wu TC, Tu X, Kim HC, & Depp CA (2019). Associations between heart rate variability measured with a wrist-worn sensor and older adults’ physical function: observational study. JMIR mHealth and uHealth, 7(10), e13757 10.2196/13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg MA, & Collins FS (2010). The path to personalized medicine. New England Journal of Medicine, 363(4), 301–304. 10.1056/NEJMp1006304 [DOI] [PubMed] [Google Scholar]
- Heiss S, Boswell JF, & Hormes JM (2018). Confirmatory factor analysis of the Eating Disorder Examination-Questionnaire: A comparison of five factor solutions across vegan and omnivore participants. International Journal of Eating Disorders, 51(5), 418–428. 10.1002/eat.22848 [DOI] [PubMed] [Google Scholar]
- Jones PJ (2018). networktools: Tools for identifying important nodes in networks. R Package Version 1.2.1.
- Keel PK, & Brown TA (2010). Update on course and outcome in eating disorders. International Journal of Eating Disorders, 43(3), 195–204. 10.1002/eat.20810 [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Portnoff LC, McCurdy-McKinnon D, & Feusner JD (2017). What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. Journal of Eating Disorders, 5(1), 20 10.1186/s40337-017-0145-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson CA, Brosof LC, Ma J, Fewell L, & Lenze EJ (2017). Fear of food prospectively predicts drive for thinness in an eating disorder sample recently discharged rom intensive treatment. Eating Behaviors, 27, 45–51. 10.1016/j.eatbeh.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson CA, Brosof LC, Vanzhula I, Christian C, Jones P, Rodebaugh TL, … Menatti A (2018). Social anxiety and eating disorder comorbidity and underlying vulnerabilities: Using network analysis to conceptualize comorbidity. International Journal of Eating Disorders, 51(7), 693–709. 10.1002/eat.22890 [DOI] [PubMed] [Google Scholar]
- Levinson CA, Vanzhula I, & Brosof LC (2018). Longitudinal and personalized networks of eating disorder cognitions and behaviors: Targets for precision intervention a proof of oncept study. International Journal of Eating Disorders, 51(11), 1233–1243. 10.1002/eat.22952 [DOI] [PubMed] [Google Scholar]
- McNally RJ (2016). Can network analysis transform psychopathology? Behaviour Research and Therapy, 86, 95–104. 10.1016/j.brat.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Christian C, Strachan E, & Levinson CA (2020). Central and peripheral symptoms in network analysis are differentially heritable a twin study of anxious misery. Journal of Affective Disorders. 10.1016/j.jad.2020.05.045 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Levinson C, & Calebs B (2018). A network analysis of eating disorder symptoms and characteristics in an inpatient sample. Psychiatry Research, 262, 270–281. 10.1016/j.psychres.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Sala M, Brosof LC, & Levinson CA (2019). Repetitive negative thinking predicts eating disorder behaviors: A pilot ecological momentary assessment study in a treatment seeking eating disorder sample. Behaviour Research and Therapy, 112, 12–17. 10.1016/j.brat.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Schultzberg M, & Muthén B (2018). Number of subjects and time points needed for multilevel time-series analysis: A simulation study of dynamic structural equation modeling. Structural Equation Modeling: A Multidisciplinary Journal, 25(4), 495–515. 10.1080/10705511.2017.1392862 [DOI] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33. [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, Robitzsch A, Vink G, Doove L, & Jolani S (2015). Package ‘mice’. Computer software version 2.22. https://mran.microsoft.com/snapshot/2015-06-19/web/packages/mice/mice.pdf. [Google Scholar]
- Wild B, Eichler M, Friederich HC, Hartmann M, Zipfel S, & Herzog W (2010). A graphical vector autoregressive modelling approach to the analysis of electronic diary data. BMC Medical Research Methodology, 10(1), 28 10.1186/1471-2288-10-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Klein DA, Walsh T, Lu J, & Sazonov ES (2014, June). Android TWEETY—A wireless activity monitoring and biofeedback system designed for people with anorexia nervosa In 2014 IEEE international symposium on medical measurements and applications (MeMeA) (pp. 1–5). Lisboa, Portugal: IEEE; 10.1109/MeMeA.2014.6860132 [DOI] [Google Scholar]
- Zipfel S, Löwe B, Reas DL, Deter HC, & Herzog W (2000). Long-term prognosis in anorexia nervosa: Lessons from a 21-year follow-up study. The Lancet, 355(9205), 721–722. 10.1016/S0140-6736(99)05363-5 [DOI] [PubMed] [Google Scholar]


