Abstract
The objective of this study was to investigate whether juvenile Iberian pigs with diet-induced nonalcoholic fatty liver disease (NAFLD), cholestasis, and gut dysbiosis would develop histological and metabolic markers of neurodegeneration in the frontal cortex (FC) and whether supplementing probiotics would influence the response to the diet. Twenty-eight juvenile Iberian pigs were fed for 10 wk either a control (CON) or high-fructose high-fat (HFF) diet with or without a commercial probiotic mixture. Compared with CON, HFF-fed pigs had a decreased number of neurons and an increase in reactive astrocytes in FC tissue. There was also a decrease in one-carbon metabolites choline and betaine and a marked accumulation of bile acids, cholesteryl esters, and polyol pathway intermediates in FC of HFF-fed pigs, which were associated with markers of neurodegeneration and accentuated with the severity of NAFLD. Betaine depletion in FC tissue was negatively correlated with choline-derived phospholipids in colon content, whereas primary conjugated bile acids in FC were associated with cholestasis. Plasma kynurenine-to-tryptophan quotient, as a marker of indoleamine 2,3-dioxygenase activity, and intestinal dysbiosis were also correlated with neuronal loss and astrogliosis. Recognition memory test and FC levels of amyloid-β and phosphorylated Tau did not differ between diets, whereas probiotics increased amyloid-β and memory loss in HFF-fed pigs. In conclusion, our results show evidence of neurodegeneration in FC of juvenile Iberian pigs and establish a novel pediatric model to investigate the role of gut-liver-brain axis in diet-induced NAFLD.
Keywords: bile acids, frontal cortex, metabolomics, microbiome, one-carbon metabolism
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver condition in Western populations, with ∼83 million individuals affected in the United States and a prevalence predicted to increase by 21% between 2015 and 2030 (16). NAFLD ranges from lipid accumulation in hepatocytes (i.e., steatosis) to nonalcoholic steatohepatitis (NASH), characterized by inflammation, fibrosis, hepatocellular degeneration, and, in advanced cases, cirrhosis and liver failure (14, 33, 59). In addition to liver injury, clinical studies suggest a link between NAFLD and neurodegeneration, with smaller brain volume (66) and impaired cognitive performance (62) observed in NAFLD patients. Further evidence is shown in rodent models of NASH, in which steatosis and hepatocellular injury paralleled the development of neuropathological hallmarks of Alzheimer’s disease (AD) (34). A liver-brain axis of neurodegeneration has been established through chronic inflammation (34), hepatic synthesis of ceramides (13), and decreased hepatic amyloid-β peptide (Aβ) clearance (17) as well as common risk factors such as insulin resistance and obesity (53). Moreover, changes in gut microbiota associated with cognitive decline in cirrhotic patients (4) and the improvement of hepatic encephalopathy after the administration of oral antibiotics (43, 54) also suggest a role for intestinal dysbiosis in the pathogenesis of liver-induced brain disease.
Magnetic resonance imaging studies show that the human brain continues to grow throughout childhood, with total brain volume increasing until puberty, making this a period of increased susceptibility to injury (30, 37). Nonetheless, despite the rapid increase of pediatric NAFLD (61, 67) and the potential long-term cognitive consequences associated with early brain damage, the effect of liver disease in neurodegeneration has not been investigated in children. Pigs represent an excellent translational model for evaluating the effects of insults on human brain development because of their perinatal brain growth, with no significant changes in the number of neurons between neonates and adult animals (10, 29). Additionally, brain features such as gray and white matter distribution, myelination, and electrical activity in neonate pigs are also similar to human infants (38). We have previously established a pig model of pediatric NAFLD in which juvenile Iberian pigs fed a high-fructose high-fat (HFF) diet for 10 wk developed steatosis, hepatocellular injury and inflammation, cholestasis, and dysregulation of enterohepatic bile acid (BA) signaling similar to that observed in child NAFLD populations (25). We also found colonic hyperplasia and compositional shifts in intestinal microbial populations, which by promoting trimethylamine synthesis and possibly disrupting gut barrier function may have caused damage to the liver (25).
Given that altered hepatic enzymes (49), plasma BAs (40, 50), and gut microbiome (8) have all been linked to neurodegenerative disorders in older patients and that neurons in frontal cortex (FC) are among the first to deteriorate in AD (28), the first goal of our study was to investigate whether juvenile Iberian pigs with diet-induced NAFLD would develop histological and metabolic markers of neurodegeneration in FC and whether these changes would be associated with cholestasis and intestinal dysbiosis. In addition, the second goal of the study was to assess whether a dietary probiotic intervention, by modulating gut microbiome, would be an effective therapeutic strategy to prevent brain injury in our pig model of pediatric NAFLD.
MATERIALS AND METHODS
Animals and experimental design.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of California State University (#1611) and conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. The characterization of the NAFLD phenotype in the Iberian pigs used in this study, including biochemistry, histology, metabolomics and microbiome in liver, blood and gut, has been described in a recent report (25). An outline of the study is presented in Supplemental Fig. S1A (see https://doi.org/10.6084/m9.figshare.12462566). Briefly, 28 Iberian pigs from the Iberian Pig Research Colony at California Polytechnic State University were weaned at 13 days of age and moved into a temperature-controlled room with a 12-h light-dark cycle. Pigs were housed in pairs in 1.5 × 1.5 m pens balanced for sex and weight and randomly assigned to receive one of four liquid diets (g·kg body wt−1·day−1): 1) control (CON-N; n = 8): 0 g of fructose, 11.2 g of fat, and 199.3 kcal of metabolizable energy (ME); 2) high-fructose high-fat (HFF-N; n = 6): 10 g of fructose, 20.6 g of fat, and 314.8 kcal of ME; 3) CON + 6.2 × 104 cfu/mL probiotics (CON-P, n = 8), and 4) HFF + 6.2 × 104 cfu/mL probiotics (HFF-P; n = 6). Animals were fed 45 mL/kg body wt at 6-h intervals to match the physiological volume of milk consumed by pigs during lactation. The probiotic mixture contained Pediococcus acidilactici and pentosaceus, Lactobacillus plantarum and Bacillus amyloliquefaciens (MultiBio 3PS; BiOWiSH Technologies, Cincinnati, OH) and was selected because of its hypocholesterolemic effects (6) and in vitro bile salt hydrolase activity (25). Detection and viability of probiotics in the diets and pig gastrointestinal tract are presented in Supplemental File S1 (see https://doi.org/10.6084/m9.figshare.12462512).
Blood was collected from the jugular vein on day 65 at 2 h postfeeding and from the left ventricle on day 70 after an overnight fast (8 h). All pigs were euthanized on day 70 of the study (83 days of age) at 8 h postfeeding using an intramuscular injection (4 mg/kg) of tiletamine and zolazepam (Telazol; Zoetis, Parsippany, NJ), followed by an intracardiac injection of pentobarbital sodium (0.4 mL/kg body wt; Schering-Plough, Union, NJ). Brains were removed immediately after euthanasia and weighed. Tissue from frontal cortex (FC) was washed for 5 s in ice-cold saline solution and frozen in liquid nitrogen or placed in plastic cassettes (Tissue-Tek Cryomold Standard; Sakura, Torrance, CA), covered with optimum cutting temperature compound (Tissue-Tek OCT; Sakura), and slowly frozen in liquid nitrogen-cooled 2-methylbutane (TCI, Portland, OR). Tissues were kept at −80°C until processing.
Pen activity and novel object recognition test.
To quantify pen physical activity, wired video cameras (Defender 4K; Defender, Cheektowaga, NY) with 4.0 MP (2,560 × 1,440) resolution at 30 frames/s were mounted from the ceiling at a 75° angle to the floor to have a clear view of the pens. Videos for activity annotation were recorded daily between days 60 and 70 of the study from 8:30 AM to 12:30 PM to encompass a 4-h period between feedings. Manual behavioral observations were carried out retrospectively using Behavioral Observation Research Interactive Software (BORIS; version 7.9) (19) by two experimenters blinded to the treatment based on the ethogram shown in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.12473471). The duration and number of pigs performing each behavior were annotated individually for each pen.
To assess recognition memory, animals were tested on the novel object recognition paradigm between weeks 7 and 9 of the study, as previously described (18). Tests were conducted with 7-day intervals, because 5-wk-old domestic pigs have been shown to remember objects for up to 6 days (21). Animals were tested in their home pens to avoid cross-contamination between probiotic and non-probiotic-treated groups. Objects were affixed to the pen gates with zip ties to prevent them from being removed while allowing slight movements when touched physically by the pigs. Sample and novel objects were of the same color, but the novel object differed in shape (Fig. 2B). Tests were conducted during the light cycle, starting 1 h after morning feeding. Pigs were given 10 min to explore the two sample objects (sample phase), and then, after a 1-h interval, an additional 10 min to explore one of the sample objects plus the novel object (test phase). The novel object was placed always on the right side of the gate and separated 1 m from the sample object. All objects were washed with water and soap to remove smell and feces between pens. During the testing, pigs were continuously recorded by ceiling video cameras with the same parameters as described for physical activity. Videos were analyzed using BORIS by two experimenters blinded to the treatment based on the ethogram presented in Supplemental Table S1. Data are presented as recognition index (RI; time spent investigating novel object/time investigating both objects).
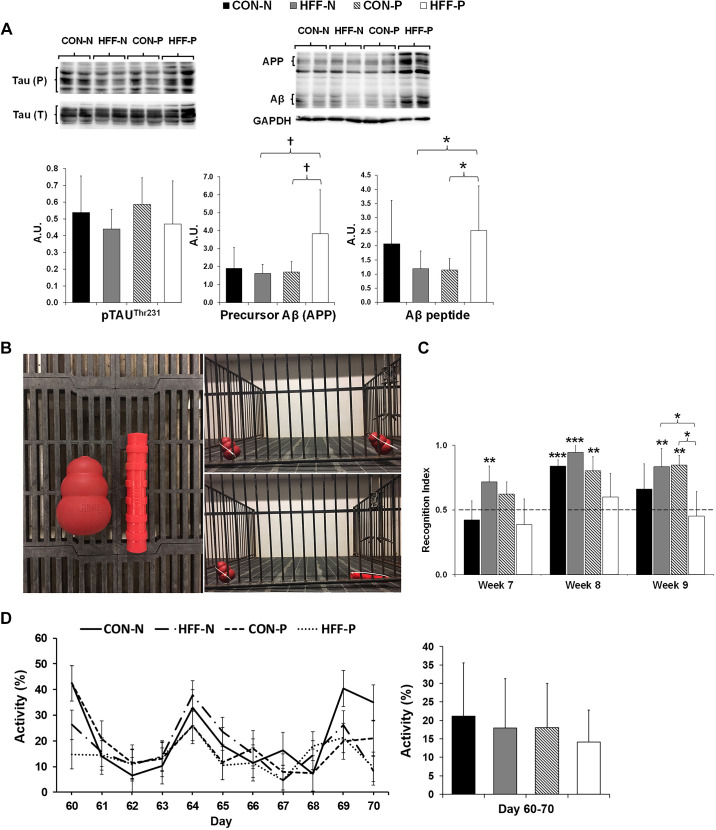
Fig. 2.
Markers of Alzheimer’s disease, cognitive function, and physical activity did not differ between control (CON) and high-fructose high-fat (HFF) diet-fed pigs. A: representative Western blots (top) and histograms (bottom) with the quantification of bands expressed as arbitrary units (AU), measuring the abundance and phosphorylation of Alzheimer’s disease hallmarks: microtubule-associated protein Tau, precursor of amyloid-β peptide (Aβ), and Aβ in frontal cortex of juvenile Iberian pigs. Histograms represent means ± SD from quantification of 6 protein bands from 3 independent Western blots, with each band representing 1 animal. GAPDH was used as a loading control. P values were adjusted for multiple testing with Tukey’s post hoc test. †P ≤ 0.1; *P ≤ 0.05. B, left: sample object used in the test is located on the left portion of the image and the novel object on the right portion of the image. Right: representative pictures showing the location of sample and novel objects during the test: learning phase (top) and memory phase (bottom). C: the recognition index (RI) was >0.5 for control (CON-N) in week 8, for high-fructose high-fat (HFF-N) in weeks 7, 8, and 9, and for CON + 6.2 × 104 cfu/mL probiotics (CON-P) in weeks 8 and 9 and increased for CON-P and HFF-N as compared with HFF + 6.2 × 104 cfu/mL probiotics (HFF-P) in week 9. Histograms represent the RI in CON-N (n = 4), CON-P (n = 4), HFF-N (n = 6), and HFF-P (n = 6). RI was calculated based on the formula (time spent investigating novel object/time investigating both objects). All values are means ± SD. D: pen activity, measured daily between days 60 and 70 of the study from 8:30 AM to 12:30 PM (left), did not show differences between treatments. Overall pen activity for each treatment during the 10-day interval (right). All values are means ± SD. Significant P values for 1-tailed t tests and P values adjusted for multiple testing with Tukey’s post hoc tests, are expressed as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. APP, amyloid-β precursor protein.
Cytokine and Western blot analysis in frontal cortex.
Analysis of proinflammatory cytokines tumor necrosis factor-α, interleukin 1α, and transforming growth factor-β in FC was performed by the Cytokine Core Laboratory at University of Maryland (Baltimore, MD), using pig-specific enzyme-linked immunosorbent assays. For Western blots, 100 mg of FC was homogenized in 800 µL of ice-cold lysis buffer containing 50 mM Tris·HCl (RPI, Mount Prospect, IL), 150 mM NaCl (Thermo Fisher Scientific, Rockford, IL), 1% Nonidet P 40 substitute (VWR Life Sciences, Solon, OH), 0.5% sodium dodecyl sulfate (SDS; 20% solution; RPI), 50 mM β-glycerophosphate (Millipore Sigma, Burlington, MA), 10 mM EDTA (Invitrogen, Rockford, IL), 10 mM NaF (VWR Life Sciences), 1 mM sodium orthovanadate (Alfa Aesar, Ward Hill, MA), 0.1 mM PMSF (Thermo Fisher Scientific), 1 µg of leupeptin (Millipore Sigma), and protease inhibitor mix (Pierce; Thermo Fisher Scientific). Homogenates were sonicated, incubated on a rotator for 2 h at 4°C, and centrifuged at 13,000 rpm for 15 min at 4°C. Collected supernatant was centrifuged again at 13,000 rpm for 15 min at 4°C. Protein concentration was measured using a protein assay kit (Pierce BCA; Thermo Fisher Scientific), following manufacturer’s protocol. Thirty five micrograms of protein samples were separated on SDS-PAGE (7% gel for proteins with molecular weight of 70–180 kDa, 10% for 44–62 kDa, and 15% gel for 14–25 kDa), transferred to polyvinylidene fluoride membrane (Thermo Fisher Scientific), and blocked with 5% bovine serum albumin (VWR Life Science) in Tris-buffered saline [2.42 g Tris Base (RPI), 8 g of NaCl (Thermo Fisher Scientific), and Tween 20 (0.1%; VWR Life Sciences)] for 1 h at room temperature (RT). Membranes were incubated overnight at 4°C with a primary antibody and for 1 h at RT with a secondary antibody, both diluted in 5% bovine serum albumin TBS-Tween 20. Antibody catalog numbers and dilutions are provided in Supplemental Table S2 (see https://doi.org/10.6084/m9.figshare.12462521). Membranes were developed using an enhanced chemiluminescence kit (Amersham ECL Prime; GE Healthcare, Little Chalfont, UK) and analyzed with a ChemiDoc-It Imaging System (Bio-Rad, Hercules, CA). Specific bands were quantified using Image Laboratory Software (version 6.0.1; Bio-Rad).
Immunofluorescence in frontal cortex.
Frontal cortex samples were embedded in optimum cutting temperature compound (Sakura), cut at a thickness of 12 µm with an OTF5000 cryostat microtome (Bright Instruments Ltd, Luton, UK), placed on positively charged microscope slides (Diamond White Glass; Globe Scientific, Mahwah, NJ) and kept at −20°C until immunostaining. Tissue sections were thawed for 15 min at RT, fixed in cold acetone (VMR Chemicals, Radnor, PA) for 7 min, washed three times for 10 min with phosphate buffered saline (PBS), and blocked in 2% bovine serum albumin (VWR Life Science) and 10% animal free blocker (Vector Laboratories, Burlingame, CA) in PBS for 1 h at RT. Tissue sections were then incubated overnight at 4°C in PBS with primary antibodies against glial fibrillary acidic protein (GFAP; Novus Biologicals, Littleton, CO) or neuronal nuclei (NeuN; MilliporeSigma) (Supplemental Table S2). Subsequently, sections were washed three times for 10 min and incubated for 2 h at RT in 0.5% bovine serum albumin and 10% animal free blocker in PBS with either Cyanine Cy3 AffiniPure F(ab′)2 Fragment Goat Anti-Rabbit IgG (H+L) (Jackson Laboratories Inc., West Grove, PA), or DyLight 594 Horse Anti-Mouse IgG (H+L) (Vector Laboratories). Following washing, coverslips were mounted with fluorescence protective medium (Vectashield Antifade Mounting Medium; Vector Laboratories), and images were taken with a FluoView 500 Confocal Laser Scanning Microscope (Olympus, Center Valley, PA) using a ×40 objective. Ten different areas per tissue section were randomly selected, and 10–12 consecutive pictures were taken from each area. Images were then converted into a z-projection using ImageJ software (60), and the average staining intensity was quantified and reported as a percentage of total area.
Analysis of metabolites.
Metabolomics assays for primary metabolomics, aminomics, lipidomics, and BAs were performed on FC samples using protein precipitation extraction with ultra-performance liquid chromatography tandem quadrupole mass spectrometry, as described in our previous study (25). Ceramides in FC tissue were isolated using a 96-well Ostro Pass-through Sample Preparation (Waters, Milford, MA) (26). Briefly, between 50 and 60 mg FC was added to the plate wells and spiked with a 5-μL anti-oxidant solution (0.2 mg/ml solution of BHT-EDTA in 1:1 methanol water) and 10 μL of a 1,000 nM ceramide C17 surrogate (Avanti Polar Lipids, Alabaster, AL). Next, isopropanol with 10 mM ammonium formate and 1% formic acid (150 μL) was added and aspirated three times to mix. The mixture was than eluted into glass inserts and dried by centrifugal vacuum evaporation with a Genovac EZ-2 (SP Scientific, Stone Ridge, NY) for 35 min on the medium boiling point setting. Residues were reconstituted with methanol containing the internal standard 1-cyclohexylureido, 3-dodecanoic acid (MilliporeSigma). The reconstituted solution was vortexed for 1 min and filtered through a 0.1-µm Durapore PVDF membrane (MilliporeSigma) by centrifugation at 9,500 rpm for 3 min at room temperature. Ceramides were separated using a Waters UPLC Acquity I-Class system (Waters) equipped with a 2.1 × 100 mm, 1.7 µm BEH C18 column interfaced with a 4000 QTRAP LC-MS/MS System (SCIEX, Concord, ON, Canada) operated in positive mode electrospray ionization and detected by multiple reaction monitoring. Metabolite intensities were normalized to that of internal standards (Cayman Chemical Company, Ann Arbor, MI; and Avanti Polar Lipids) added during the extraction and to sample weight to account for small variations in starting tissue. General metabolites were expressed as peak areas under the curve, whereas BAs and ceramides were expressed as absolute concentration (nM). In addition, the BA hydrophobicity index in FC was calculated using the BA molar fractions, as previously described (27).
Statistical analysis.
Protein expression, immunofluorescence, brain weight, and RI were analyzed by a two-way ANOVA using a mixed model in SAS 9.2 (Proc Mixed; SAS Institute Inc., Cary, NC) that included diet × probiotic as fixed effect and pen nested in diet × probiotic as random effect. Because only eight FC samples per blot were loaded (i.e., 2 CON-N, 2 CON-P, 2 HFF-N, and 2 HFF-P), the statistical model for protein expression also included blot as a random effect to account for interblot variability. All parameters were also analyzed according to the severity of liver disease using a one-way ANOVA that included the histological classification of liver injury as fixed effect (i.e., no steatosis, steatosis, and NASH), as previously described (25). One-tailed t tests were conducted to investigate whether RI was different from 0.50 by comparing it to a nonpreference score of 0.50 (18). Daily activity levels were analyzed by a three-way ANOVA that included 1) diet × probiotic × day as fixed effect, 2) pen nested in diet × probiotic as random effect, and 3) a repeated-measurement statement with day as repeated factor and pen nested in diet × probiotic as subject. The structure of the covariance was selected based on the smallest Akaike information criterion. Normality of the residuals and presence of outliers were assessed, and nonnormally distributed parameters were power transformed by a parameter, φ, whose optimal value was estimated using the maximum likelihood method (55). Data are presented as means ± SD. Multiple comparisons were corrected with Tukey’s post hoc test, and significant effects were considered at P ≤ 0.05. Correlations were determined using the PROC CORR in SAS, and Pearson correlation coefficient was considered significant at P ≤ 0.05.
Metabolomics data were imported into the Primer-E software (version 7; Primer-E Ltd., Plymouth, UK), log-transformed into a normal distribution approximation, and analyzed with a Euclidean distance matrix (25). Identification of metabolites differentially expressed between groups was performed by a two-way ANOVA with the same random and fixed effects described above. False discovery rate using the Benjamin-Hochberg procedure (5) was also calculated and provided, although the values were not used as part of the threshold to select significant metabolites in FC. Data are presented as fold change and significance levels by HFF compared with CON. Significant associations for metabolites altered by diet between colon content and FC were assessed by Spearman’s correlations in the R Statistical Language (version 3.6.0). Relative abundance data for each metabolomics platform were combined, and pigs were dropped if they had >30% of metabolite data missing in either the FC or colon data set. A total of six samples met these criteria and were excluded from the analysis. Data from the remaining pigs (5 CON-N, 4 CON-P, 5 HFF-N, and 3 HFF-P) were log-transformed, and Spearman’s correlations were assessed for pairwise correlations by the rcorr() function in the Hmisc R package (24). P values were extracted so that metabolites in FC constituted the source node and those in colon the target nodes. Data were adjusted for multiple comparisons using the false discovery rate (FDR) procedure (5), and correlations with FDR ≤ 0.01 were used to develop a network in Cytoscape version 3.7.1 (63) to aid in visualizing the strongest relationships.
RESULTS
Results are discussed for HFF-N, HFF-P, CON-N, and CON-P groups if the interaction between diet and probiotics was significant (P ≤ 0.05); otherwise, only the effect of diet (HFF versus CON) and/or probiotic (N versus P) is reported.
NAFLD pigs developed neuronal loss and astrogliosis without changes in cognitive function and physical activity.
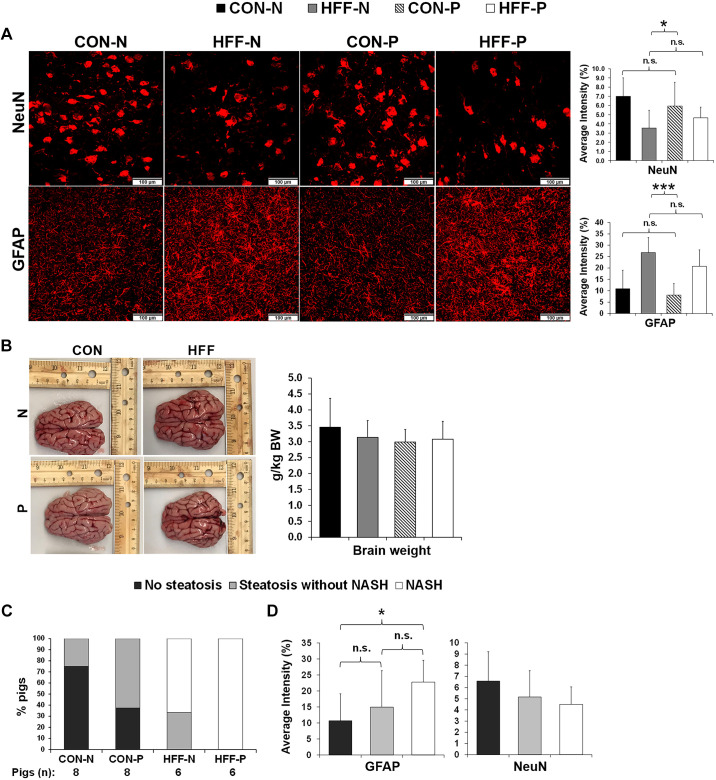
To investigate whether diet-induced NAFLD was associated with neurodegeneration in juvenile Iberian pigs, we analyzed cytokines and markers of glial activation and neuronal loss in FC tissue. Immunostaining of NeuN to identify mature neurons was significantly lower in FC of HFF-fed pigs compared with CON (P ≤ 0.05; Fig. 1A), whereas relative brain weight (Fig. 1B) and neurogenesis marker doublecortin (DCX; Supplemental Fig. 1B; see https://doi.org/10.6084/m9.figshare.12462566) did not differ between groups. We also observed a marked increase in immunostaining intensity for astrocyte reactivity marker GFAP in HFF compared with CON (P ≤ 0.001; Fig. 1A) and in NASH pigs compared with those without steatosis (P ≤ 0.05; Fig. 1, C and D) despite the lack of changes in cytokines and microglial activation protein Iba-1 (Supplemental Fig. S1, B and C). Interestingly, myelin basic protein, which has been shown to increase in AD cortex following myelin damage (74), was higher in HFF-P compared with both HFF-N and CON-P (P ≤ 0.05; Supplemental Fig. S1B).
Fig. 1.
High-fructose high-fat (HFF) diet induced neuronal loss and astrogliosis in frontal cortex of juvenile Iberian pigs. A: immunofluorescence staining of neuronal nuclei (NeuN) and glial fibrillary acidic protein (GFAP) in frontal cortex. Images of representative stains (left) and quantification of staining intensity (right) (n = 10–12 images/animal, with 4–6 animals/treatment) expressed as %total area. B: representative brain images (left) from control (CON-N)-, CON + 6.2 × 104 cfu/mL probiotics (CON-P)-, high-fructose high-fat (HFF-N)-, and HFF + 6.2 × 104 cfu/mL probiotics (HFF-P)-fed pigs taken immediately after euthanasia on day 70 of the study and relative brain weight (right) expressed as grams of brain tissue per kilogram of body weight. C: %pigs that developed steatosis and NASH in each treatment group with CON-N (n = 8), CON-P (n = 8), HFF-N (n = 6), and HFF-P (n = 6). D: %GFAP and NeuN staining intensity indicating that astrogliosis increased with the severity of liver disease. All values are means ± SD. P values were adjusted for multiple testing with Tukey’s post hoc test. *P ≤ 0.05; ***P ≤ 0.001.
Next, we assessed protein expression of neuropathological hallmarks of AD and changes in cognitive functioning and physical activity. Western blotting analysis revealed no diet effect on amyloid-β precursor (APP), amyloid-β peptide (Aβ), or neurofibrillary tangles of hyperphosphorylated (p)Tau, whereas Aβ increased (P ≤ 0.05) and APP had an increasing trend (P ≤ 0.1) in HFF-P compared with HFF-N and CON-P groups (Fig. 2A). The RI in the novel object recognition test was >0.5 for CON-N in week 8 (P ≤ 0.001), for HFF-N in weeks 7 (P ≤ 0.01), 8 (P ≤ 0.001), and 9 (P ≤ 0.01), and for CON-P in weeks 8 and 9 (P ≤ 0.01; Fig. 2C). Furthermore, the mixed-model ANOVA indicated a significant increase in RI for CON-P and HFF-N as compared with HFF-P in week 9 (P ≤ 0.05). There were no differences in daily and overall activity levels between days 60 and 70 of the study across treatment groups (Fig. 2D).
HFF-induced dysregulation of one-carbon metabolites in frontal cortex was associated with markers of neurodegeneration.
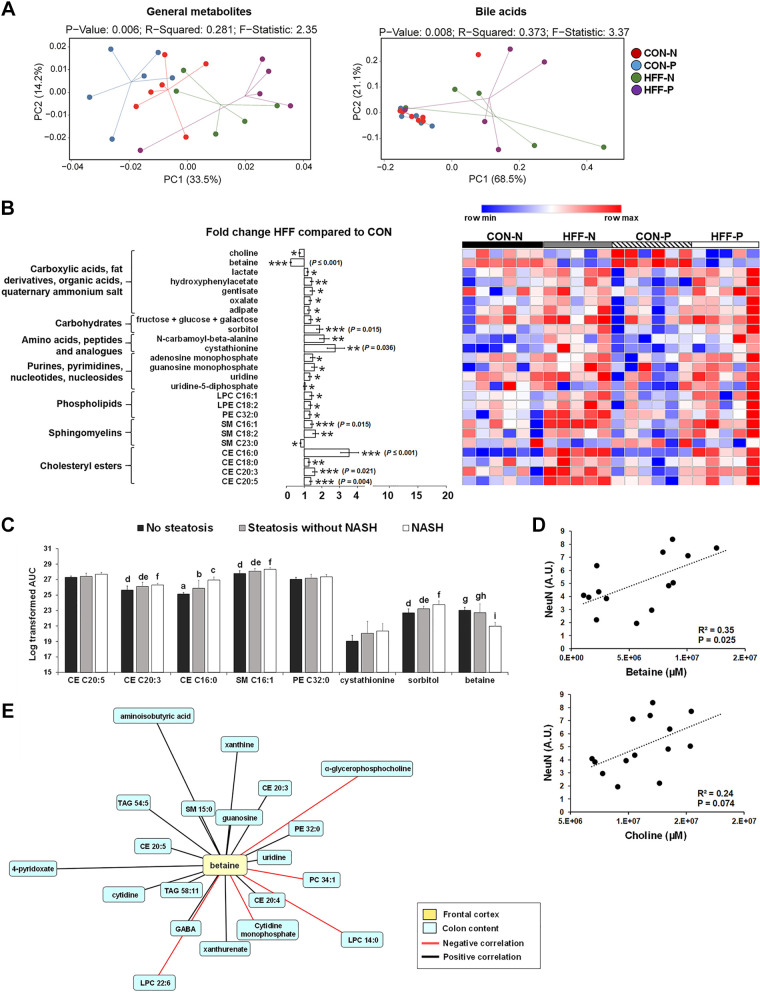
To investigate the potential mechanisms of neurodegeneration in NAFLD pigs, we examined changes in lipid, protein, and carbohydrate metabolism in FC tissue using untargeted metabolomics. A total of 207 metabolites were detected in FC, of which 28 changed between HFF and CON groups. Multivariate analysis showed no differences between probiotic and nonprobiotic animals (Fig. 3A, left). Choline (P ≤ 0.05) and betaine (P ≤ 0.001) levels decreased in FC of HFF-fed pigs compared with CON, whereas other carboxylic acids, including hydroxyphenylacetate, gentisate, oxalate, and adipate, were higher (P ≤ 0.05) in HFF (Fig. 3B). HFF also increased FC levels of lactate (P ≤ 0.05), monosaccharides (P ≤ 0.05), and sorbitol (P ≤ 0.001), the amino acid derivatives N-carbamoyl-β-alanine and cystathionine (P ≤ 0.05), and the purines/pyrimidines adenosine monophosphate, guanosine monophosphate, uridine, and uridine-5-diphosphate (P ≤ 0.05) compared with CON pigs (Fig. 3B). Among lipid species, sphingomyelins 16:1 and 18:2 (P ≤ 0.01), lysophosphatidylcholine 16:1, lysophosphatidylethanolamine 18:2, and phosphatidylethanolamine 32:0 (P ≤ 0.05) and cholesteryl esters 16:0, 18:0, 20:3, and 20:5 (P ≤ 0.001) were all higher for HFF as compared with CON. FC levels of ceramides, acetylcholine, and phosphatidylcholines did not differ between HFF and CON groups (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.12462560). Further analysis of metabolites with FDR ≤ 0.05 showed an increase in cholesteryl ester 16:0 in a stepwise manner from no steatosis to steatosis to NASH (P ≤ 0.05), whereas cholesteryl ester 20:3 (P ≤ 0.01), sphingomyelin 16:1 (P ≤ 0.01), and sorbitol (P ≤ 0.01) were higher in NASH compared with pigs without steatosis (Fig. 3C). Conversely, betaine decreased in NASH compared with pigs without steatosis (P ≤ 0.001; Fig. 3C) and was positively correlated with NeuN (P ≤ 0.01; Fig. 3D).
Fig. 3.
High-fructose high-fat (HFF) diet altered the metabolomic profile in frontal cortex of juvenile Iberian pigs. A: principal component analysis (PCA) of metabolites (left) and bile acids (right) in frontal cortex (FC) discriminated HFF- and control (CON)-fed pigs along first component. Data were scaled to unit variance before PCA assessment. Two-dimensional visualization of PCA scores are projected from their group centroid along components 1 and 2. P value, R-squared, and F-statistic are derived from ANOVA assessed on first principal component. Each point represents an individual pig (n = 5–6 animals/treatment), and color of point denotes diet. B: heatmap of relative abundance of metabolites significantly altered by the diet in FC of 83-day-old pigs, with fold change and significance levels by HFF compared with CON. Columns are individual pigs and rows are log-transformed metabolite data. Blue and red represent the row minimum and maximum values. P values for each metabolite were calculated by a 2-way ANOVA with a mixed model and adjusted for false discovery rate (FDR) with Benjamin-Hochberg procedure. Significant P values for t tests are expressed as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, whereas P values adjusted by FDR are presented in brackets. C: FDR-passing metabolites increased in a stepwise manner from no steatosis to steatosis to NASH. Values are means ± SD. Significant P values adjusted for multiple testing with Tukey’s post hoc test are expressed as follows: a,b,cP ≤ 0.05, d,e,fP ≤ 0.01, and g,h,iP ≤ 0.001. D: relative abundance of choline and betaine in FC was positively correlated (Pearson) with no. of neuronal nuclei (NeuN)-positive cells. E: associations between betaine in frontal cortex and colon content metabolites in 83-day-old pigs fed control (CON-N) (n = 6), CON + 6.2 × 104 cfu/mL probiotics (CON-P; n = 5), high-fructose high-fat (HFF-N; n = 6), and HFF + 6.2 × 104 cfu/mL probiotics (HFF-P; n = 5). Network displays Spearman’s correlations with FDR ≤ 0.01; red and black edges represent positive and negative correlations, respectively. Longer edges correspond to lower P values. Node colors: yellow boxes represent betaine in FC; light blue boxes represent metabolites in colon content. Centered log ratio transformations were applied to relative abundance data before Spearman’s correlations were performed. Only metabolites altered by diet were considered for correlation network.
We have previously shown an increase in relative abundance of choline, α-glycerophosphocholine, and multiple phosphatidylcholines and lysophosphatidylcholines in colon content of NAFLD pigs, possibly due to the saturation of choline absorptive capacity in the gut in response to the HFF diet (25). Because most of brain choline is absorbed in the small intestine and then transported across the blood-brain barrier (35, 73), we next investigated whether changes in one-carbon metabolites in FC were associated with the relative abundance of choline-containing phospholipids in the colon (Fig. 3E). No significant correlations were found for brain choline. Conversely, betaine constituted a major cluster inside the network and was negatively correlated with colonic levels α-glycerophosphocholine, phosphatidylcholine 34:1, and lysophosphatidylcholines C22:6 and C14:0 (increased in HFF) and positively correlated with 4-pyridoxate, nucleotide and nucleoside derivatives, and several triacylglycerides, sphingomyelins, and cholesteryl esters (decreased in HFF). The full correlation network analysis between FC tissue and metabolites in colon digesta is presented in Supplemental Fig. S3 (see https://doi.org/10.6084/m9.figshare.12462569).
Primary BAs increased in frontal cortex of HFF-fed pigs and were associated with the severity of liver disease.
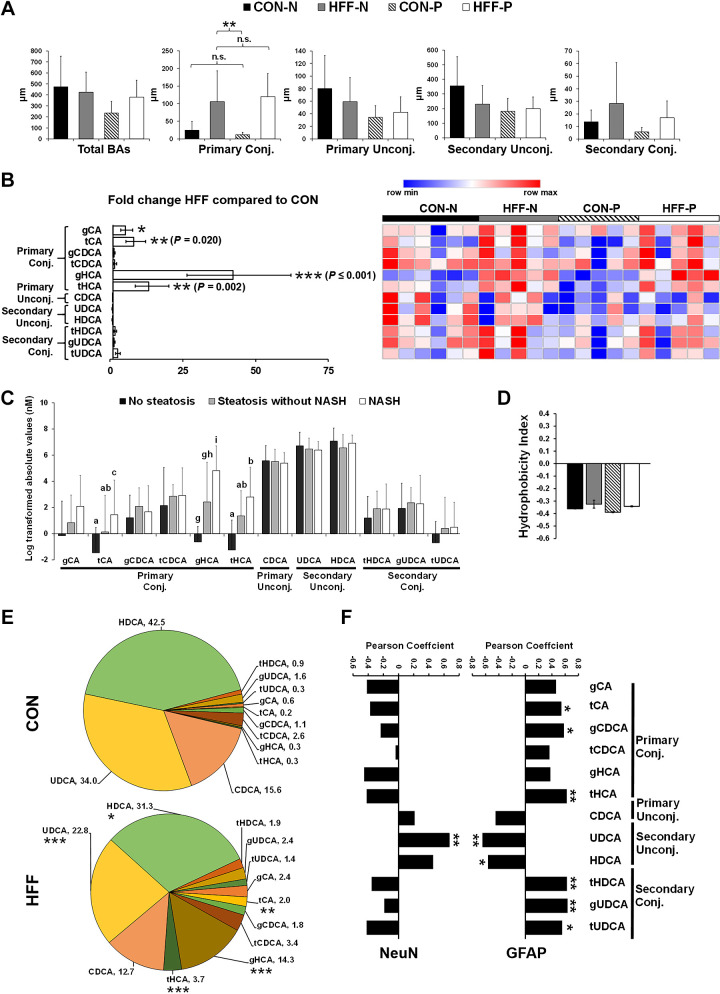
Because changes in microbial-deconjugated BAs have been associated with cognitive decline and brain atrophy in AD patients (40, 50), we next investigated BA levels in FC tissue using a targeted metabolomic assay. Multivariate analysis revealed significant differences between CON and HFF diets, but not a probiotic effect (Fig. 3A, right). We found a large increase in primary conjugated BAs in the FC of HFF-fed pigs (P ≤ 0.01) driven by glycocholic (gCA; P ≤ 0.05), taurocholic (tCA; P ≤ 0.01), glycohyocholic (gHCA; P ≤ 0.001), and taurohyocholic acids (tHCA; P ≤ 0.01; Fig. 4, A and B). Conversely, secondary unconjugated BAs hyodeoxycholic (HDCA) and ursodeoxycholic acids (UDCA) and secondary conjugated BAs taurohyodeoxycholic (tHDCA) and tauro- and glycoursodeoxycholic acids (tUDCA and gUDCA) did not differ between groups (Fig. 4C). Further analysis showed an increase in tCA (P ≤ 0.05), gHCA (P ≤ 0.001), and tHCA (P ≤ 0.05) in pigs with NASH compared with those without steatosis (Fig. 4C). Nonetheless, the hydrophobicity index, which is a quantitative measure of BA cytotoxicity (27), did not differ between groups (Fig. 4D). Interestingly, when values were standardized to relative composition so that sample totals were 100%, the proportion of tCA (P ≤ 0.01), gHCA (P ≤ 0.001), and tHCA (P ≤ 0.001; Fig. 4E) increased in HFF group, whereas HDCA (P ≤ 0.05) and UDCA (P ≤ 0.001) decreased in HFF compared with CON as a result of a dilution effect. Moreover, the relative abundance of tCA, gCDCA, tHCA, tHDCA, gUDCA, and tUDCA was positively correlated with GFAP (P ≤ 0.05; Fig. 4F), whereas UDCA was correlated with the number of NeuN (positive; P ≤ 0.01) and GFAP (negative; P ≤ 0.01) stained cells.
Fig. 4.
Frontal cortex (FC) levels of primary bile acids were increased in high-fructose high-fat (HFF) diet-fed pigs and associated with the severity of liver disease. A: total, primary, and secondary levels of bile acids in frontal cortex (FC) of control (CON-N; n = 6), CON + 6.2 × 104 cfu/mL probiotics (CON-P; n = 5), high-fructose high-fat (HFF-N; n = 5), and HFF + 6.2 × 104 cfu/mL probiotics (HFF-P; n = 5). B: heatmap of absolute abundance of bile acids in frontal cortex of 83-day-old pigs, with fold change and significance levels by HFF compared with CON. Columns are individual pigs, and rows are log-transformed metabolites. Blue and red represent the row minimum and maximum values. P values for each metabolite were calculated by a 2-way ANOVA with a mixed model and adjusted for false discovery rate (FDR) with Benjamin-Hochberg procedure. C: primary conjugated bile acids increased in a stepwise manner from no steatosis to steatosis to NASH. D: hydrophobicity index was calculated according to Heuman, 1989 (27), and did not differ between treatments. E: pie charts of relative abundance of individual bile acids in FC samples of 83-day-old pigs, with fold change and significance levels by HFF compared with CON. F: Perason correlation between relative abundance of bile acids and both neuronal nuclei (NeuN) and glial fibrillary acidic protein (GFAP) staining intensity. Values are means ± SD. Significant P values for Tukey’s post hoc tests are expressed as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, and a,b,cP ≤ 0.05, d,e,fP ≤ 0.01, and g,h,iP ≤ 0.001. Significant FDR-adjusted P values are presented in brackets. CDCA, chenodeoxycholic acid; gCA, glial cholic acid; gCDCA, glial chenodeoxycholic acid; gHCA, glial hyocholic acid; gUDCA, glial ursodeoxycholic acid; HDCA, hyodeoxycholic acid; tCA, total cholic acid; tCDCA, total chenodeoxycholic acid; tHCA, total glial hyocholic acid; tHDCA, total hyodeoxycholic acid; tUDCA, total ursodeoxycholic acid; UDCA, ursodeoxycholic acid.
Kynurenine-to-tryptophan ratio increased in plasma, liver, and frontal cortex of HFF-fed pigs.
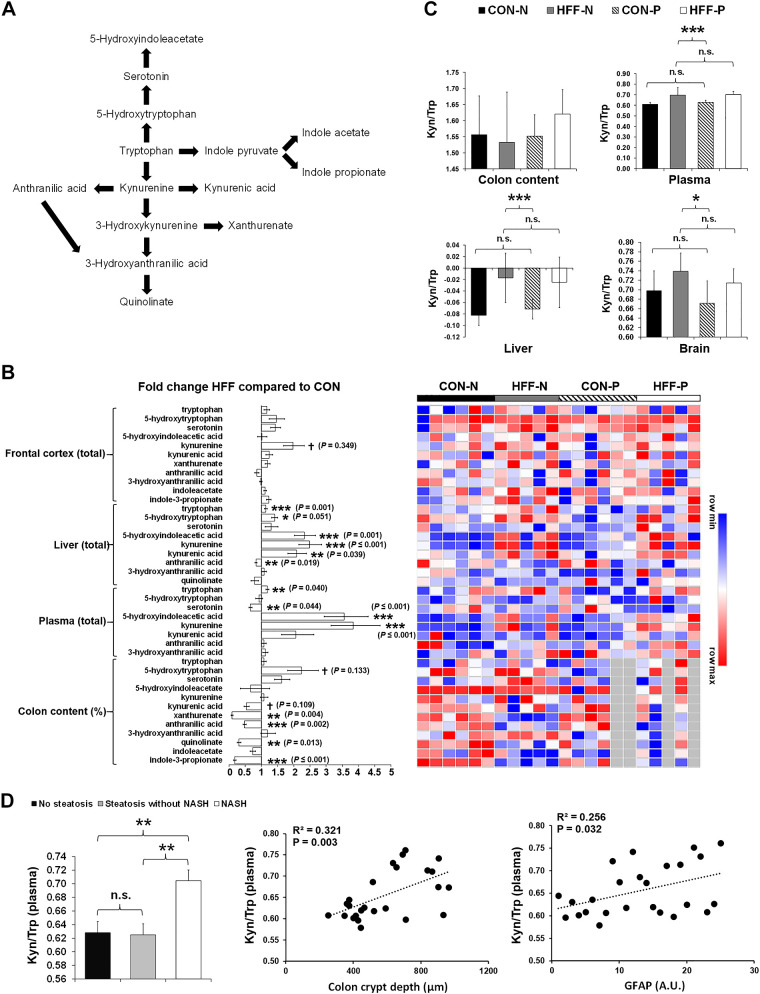
Perturbations in the kynurenine pathway of tryptophan catabolism (Fig. 5A) have been linked to the pathogenesis of neurodegenerative disorders (7). Activation of indoleamine 2,3-dioxygenase (IDO-1) in serum and brain of AD patients increased tryptophan degradation, which in turn may have decreased serotonin availability and promoted the synthesis of neurotoxic metabolites (23, 68). To investigate whether neurodegeneration was associated with a dysregulation of kynurenine pathway in NAFLD pigs, we analyzed markers of tryptophan metabolism in colon, plasma, liver, and FC and calculated the kynurenine-to-tryptophan quotient as a surrogate marker of IDO-1 activity (3). Results are presented in Fig. 5B. In the colon, we found a decrease in relative abundance of kynurenic acid (P ≤ 0.1), xanthurenate (P ≤ 0.05), anthranilic acid (P ≤ 0.001), quinolinate (P ≤ 0.01), and indole-3-propionate (P ≤ 0.001) in HFF compared with CON. Conversely, plasma levels of tryptophan (P ≤ 0.01) and kynurenine (P ≤ 0.001) increased in HFF-fed pigs, whereas serotonin decreased (P ≤ 0.01) and 5-hydroxyindoleacetate (P ≤ 0.001) increased in HFF compared with CON. Similarly, HFF feeding increased hepatic content of tryptophan (P ≤ 0.001), kynurenine (P ≤ 0.001), kynurenic acid (P ≤ 0.01), 5-hydroxytryptophan (P ≤ 0.05), and 5-hydroxyindoleacetate (P ≤ 0.001), whereas it decreased anthranilic acid (P ≤ 0.01). We also observed a tendency to increase kynurenine levels in FC of HFF-fed pigs compared with CON (P ≤ 0.1), whereas tryptophan and serotonin levels did not change between groups. Compared with CON, the kynurenine-to-tryptophan ratio increased in liver (P ≤ 0.001), FC (P ≤ 0.05), and plasma (P ≤ 0.001) of HFF group (Fig. 5C). In addition, the plasma kynurenine-to-tryptophan ratio was higher in NASH pigs compared with those with and without steatosis (P ≤ 0.01) and was positively correlated with crypt depth in colon (P ≤ 0.01) and GFAP in FC (P ≤ 0.05; Fig. 5D).
Fig. 5.
Tryptophan metabolism in colon content, plasma, liver, and frontal cortex (FC) is altered in high-fructose high-fat (HFF)-fed pigs. A: pathways of tryptophan metabolism. Approximately 95% of the tryptophan is degraded to kynurenine through the kynurenine pathway. Kynurenine is metabolized to 3-hydroxykynurenine by kynurenine hydroxylase, to anthranilic acid by kynureninase, and to kynurenic acid by kynurenine aminotransferase. In addition, 3-hydroxykynurenine is degraded to 3-hydroxyanthranilic acid by kynureninase and to xanthurenic acid by kynurenine aminotransferase. In turn, 3-hydroxyanthranilic acid is converted to quinolinate; 1–2% of ingested tryptophan is also converted to 5-hydroxytryptophan and then serotonin by tryptophan hydroxylase and aromatic amino acid decarboxylase. Finally, 3–4% of tryptophan is metabolized to indole acid derivatives by the gut microbiota. B: heatmap of relative abundance of metabolites significantly altered by the diet in colon content, plasma, liver, and frontal cortex of 83-day-old pigs (n = 5–6 animals/treatment), with fold change and significance levels by HFF compared with CON. Columns are individual pigs, and rows are log-transformed metabolite data. Blue and red represent the row minimum and maximum values. Samples not analyzed in a specific tissue are represented in gray. P values for each metabolite were calculated by a 2-way ANOVA with a mixed model and adjusted for false discovery rate (FDR) with Benjamin-Hochberg procedure. Significant P values for t tests are expressed as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, whereas P values adjusted by FDR are presented in brackets. C: kynurenine-to-tryptophan ratio in colon content, plasma, liver, and frontal cortex after 10 wk of HFF and control CON feeding. D: plasma kynurenine-to-tryptophan ratio increased in animals with NASH (left) and was positively correlated (Pearson) with crypt depth in colon and glial fibrillary acidic protein (GFAP) expression in FC (right). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. NS, not significant. CON-N, control; CON-P, CON + 6.2 × 104 cfu/mL probiotics; HFF-N, high-fructose high-fat; HFF-P, HFF + 6.2 × 104 cfu/mL probiotics.
HFF-induced dysbiosis was correlated with neuronal loss and astrogliosis.
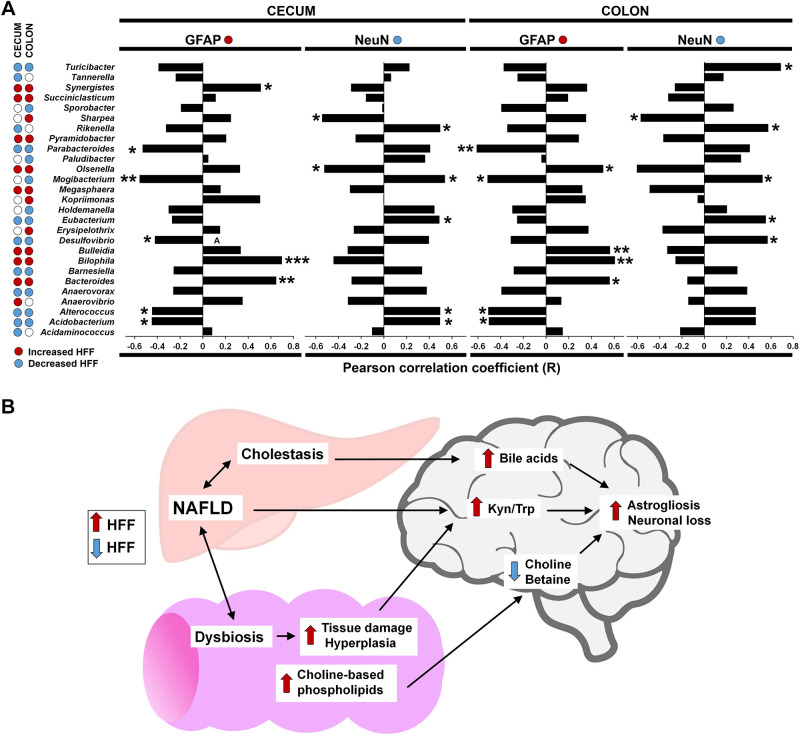
Compositional shifts in intestinal microbiota have been linked to cognitive decline and brain inflammation in patients with hepatic encephalopathy (4) and neurodegenerative disorders (64). Therefore, we next examined whether diet-induced changes in gut microbiota were correlated with FC markers of neuronal loss and astrogliosis. Results are presented in Fig. 6A. For genera that increased in HFF-fed pigs, GFAP was positively correlated in cecum and colon with Bacteroides (P ≤ 0.01 and P ≤ 0.05, respectively) and Bilophila (P ≤ 0.001 and P ≤ 0.01), with Synergistes and Kopriimonas in cecum (P ≤ 0.05), and with Bulleida (P ≤ 0.01) and Olsonella (P ≤ 0.05) in colon. We also observed significant negative correlations for NeuN with Sharpea and Olsonella in both cecum and colon (P ≤ 0.05). Among genera that decreased in HFF compared with CON pigs, GFAP was negatively correlated in cecum and colon with Mogibacterium (P ≤ 0.01 and P ≤ 0.05), Parabacteroides (P ≤ 0.05 and P ≤ 0.01), Acidobacterium (P ≤ 0.05), and Alterococcus (P ≤ 0.05) and with Desulfovibrio in cecum (P ≤ 0.05). NeuN was negatively associated with Eubacterium in cecum and colon (P ≤ 0.05) and positively correlated with Rickenella and Mogibacterium in cecum and colon (P ≤ 0.05), with Acidobacterium and Alterococcus in cecum (P ≤ 0.05), and with Turicibacter and Desulfovibrio in colon (P ≤ 0.05).
Fig. 6.
Diet-induced changes in cecum and colon microbiota were correlated with markers of neurodegeneration. A: Pearson correlation coefficient between relative counts of bacteria in cecum and colon and immunostaining for glial fibrillary acidic protein (GFAP) and neuronal nuclei (NeuN) in frontal cortex of juvenile Iberian pigs (n = 4–6 animals/treatment). Only genera that were significantly different between control (CON) and high-fructose high-fat (HFF) groups were included in the analysis. Blue and red dots represent bacteria increased and decreased in HFF-fed pigs compared with CON. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. B: graphical abstract summarizing the proposed mechanisms linking diet-induced nonalcoholic fatty liver disease (NAFLD) with neurodegeneration in juvenile Iberian pigs. Hepatic injury, cholestasis, colonic hyperplasia and dysbiosis may have dysregulated 1-carbon, bile acid, and tryptophan metabolism in frontal cortex, causing astrogliosis and neuronal loss.
DISCUSSION
Although a connection between liver and brain disease has been established in adults (49, 62, 66) and sexually mature animals (34, 45), the effect of NAFLD in neurodegeneration has not been investigated in pediatric populations. Here, we demonstrate a decrease in the number of neurons and an increase in reactive astrocytes in 12-wk-old Iberian pigs with diet-induced NAFLD. Consistent with metabolic changes in neurodegenerative disorders, we also found a dysregulation of one-carbon metabolites (12) and a marked accumulation of BAs (52), cholesteryl esters (44), and polyol-pathway intermediates (72) in FC of HFF-fed pigs, which were correlated with markers of neurodegeneration and accentuated with the severity of liver disease. However, recognition memory and AD biomarkers Aβ and pTau did not differ between HFF and CON groups, suggesting that neuronal loss and metabolic abnormalities may precede more severe functional or histopathological changes in the cortex. In this regard, mice fed a high-fat diet for 16 wk developed brain atrophy in the absence of Aβ plaques or pTau (45), whereas NAFLD mice without genetic AD predisposition showed tauopathy and amyloid plaques first after 1 yr of high-fat feeding (34).
Our results provide evidence of a link between diet-induced NAFLD and neurodegeneration in juvenile pigs through dysregulation of one-carbon, BA, and tryptophan metabolism (Fig. 6B). Most choline in the brain originates from absorption in the small intestine and then transport across the blood-brain barrier (35, 73). Thus, we postulate that the colonic excretion of choline-containing phospholipids as a result of HFF diet and increase in bile secretory rate (25) may have depleted choline and betaine in FC, causing the degradation of membrane phospholipids and subsequent neuronal loss (70, 71). In addition, the disproportionate decrease in betaine in HFF-fed pigs may have dysregulated homocysteine and gene methylation levels in FC, with potential neurotoxic effects (51, 75). In agreement with these results, choline deficiency impaired brain development in young pigs during the perinatal period (46). Likewise, deterioration of cholinergic neurons of the basal forebrain and choline acetyltransferase activity within the cortex are thought to account for much of the memory loss seen in AD patients (2, 69).
Elevated BAs in the brain have been associated with neurodegenerative conditions in mouse models of liver failure and in patients with AD (40, 41, 49). Here, we demonstrate increased concentrations of primary conjugated BAs in brain tissue, which are consistent with our previous findings in liver, plasma, and luminal contents of HFF-fed pigs (25). Primary conjugated BAs at high concentrations are cytotoxic and can disrupt the blood-brain barrier (22, 65). In addition, the dilution of UDCA in the FC of HFF-fed pigs as a result of the increased levels of primary conjugated BAs may have impacted the viability of neuronal cells and activation of neuroglia (1, 32). Furthermore, UDCA is a farnesoid X receptor antagonist (47), and inhibition of farnesoid X signaling in FC protected against cholesterol accumulation in a mouse model of hepatic encephalopathy (42).
Our results also suggest a link between NAFLD and neurodegeneration through an increase in kynurenine synthesis, which might be further metabolized into neurotoxic metabolites in the brain (7, 68). Because inflammatory conditions in liver (48) and gut (9) induce IDO-1, an intracellular enzyme that catalyzes the rate-limiting step in the conversion of tryptophan to kynurenine (3), it is likely that hepatic injury and gut dysbiosis contributed to the increase in kynurenine-to-tryptophan ratio in plasma and FC of HFF-fed pigs. Whereas hepatic inflammation can be ascribed directly to the macronutrients in the HFF diet (i.e., saturated fat, cholesterol, and fructose), gut dysbiosis may have caused injury to the colonic mucosa, promoting intestinal epithelial proliferation (11, 15) and IDO-1 activity (9). Consistent with the microbiome analysis on fecal samples from AD patients (64) and children with NAFLD (31), we found a significant increase in Bacteroides and Bilophila in cecum and colon content of HFF-fed pigs, which, by increasing the synthesis of microbially derived BAs and hydrogen sulfide, can disrupt the gut barrier function causing inflammation. Colonic hyperplasia may also have been caused by the proliferation of Synergistes, a group of bile-tolerant bacteria capable of hydrogen sulfide production (56), as well as by the decrease in microbial indolic acid derivatives in the digesta, which promote intestinal epithelial barrier function (20).
The evidence that probiotics can modify gut microbial populations has prompted a growing interest in their use as a therapeutic strategy to manage NAFLD (36) and AD (57). Notably, we show here that feeding a probiotic bacteria impaired cognitive skills and increased both Aβ levels and myelin basic protein in FC of HFF-fed pigs. Because the severity of liver disease was also accentuated in the HFF-P group (25), it is possible that probiotic translocation across the intestinal mucosa caused systemic injury in the juvenile pigs (39). In this regard, we identified genes encoding patatin-like phospholipase and lysophospholipases in the probiotic formula, which have been associated with the translocation of Lactobacillus rhamnosus in mice (58).
In conclusion, our results show histological and metabolic evidence of neurodegeneration in FC of juvenile pigs and provide a novel pediatric model to investigate the role of the gut-liver-brain axis in diet-induced NAFLD. Given that dysregulation of brain BAs and one-carbon metabolites increased with the severity of liver disease, interventions targeting BA signaling and choline availability may ameliorate the neurodegenerative processes in NAFLD patients with cholestasis.
GRANTS
This work was supported by the California State University Agriculture Research Institute (Grants 58873 and 58913), Cal Poly internal funding programs Baker/Koob, RSCA, FROST, and STRIDE, the USDA, Agricultural Research Service Grant 3092-51000-060-01, grants from the National Institutes of Health (NIH; Grant DK-094616), and the Texas Medical Center Digestive Diseases Center (NIH Grant P30-DK-56338), BiOWiSH Technologies, Hilmar Ingredients and Acorn Seekers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M. and R.M. conceived and designed research; N.Z., M.R.L., M.M., I.M., G.V.H., M.J.T., and C.R.S. performed experiments; N.Z., M.R.L., R.M., M.M., I.M., G.V.H., M.J.T., R.K.F., M.A-I., and H.G. analyzed data; N.Z., M.R.L., R.M., M.M., I.M., G.V.H., M.J.T., M.A-I., and D.B. interpreted results of experiments; R.M. and M.A-I. prepared figures; N.Z. and I.M. drafted manuscript; N.Z., M.R.L., R.M., M.M., I.M., G.V.H., M.J.T., R.K.F., M.A-I., H.G., C.R.S., and D.B. edited and revised manuscript; N.Z., M.R.L., R.M., M.M., I.M., G.V.H., M.J.T., R.K.F., M.A-I., H.G., C.R.S., and D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Center for Applications in Biotechnology for providing laboratory space, Dr. Lily Laiho for making available the confocal microscope, the Animal Science Department for making available the Veterinary Clinic and Animal Facilities, Susan Tonik, Manuel Murga, Sergio Marsal, and Beth Reynolds for their technical assistance, and Christian Terkatz, Hannah Onat, William Gallo, and Ishmam Nawar for their help during data collection.
REFERENCES
- 1.Abdelkader NF, Safar MM, Salem HA. Ursodeoxycholic acid ameliorates apoptotic cascade in the rotenone model of parkinson’s disease: modulation of mitochondrial perturbations. Mol Neurobiol 53: 810–817, 2016. doi: 10.1007/s12035-014-9043-8. [DOI] [PubMed] [Google Scholar]
- 2.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68: 209–245, 2002. doi: 10.1016/S0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 3.Badawy AAB, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: Time for appraisal. Int J Tryptophan Res 12: 1178646919868978, 2019. doi: 10.1177/1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 303: G675–G685, 2012. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 6.Carpenter RS, Huff EW, Kapur A. Compositions and Methods for Improving Human Health and Nutrition. US Patent 10022409 January 1, 2020.
- 7.Breda C, Sathyasaikumar KV, Sograte Idrissi S, Notarangelo FM, Estranero JG, Moore GG, Green EW, Kyriacou CP, Schwarcz R, Giorgini F. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci USA 113: 5435–5440, 2016. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB; INDIA-FBP Group . Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49: 60–68, 2017. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol 3: 90, 2012. doi: 10.3389/fphar.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad MS, Dilger RN, Johnson RW. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev Neurosci 34: 291–298, 2012. doi: 10.1159/000339311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craven PA, Pfanstiel J, Saito R, DeRubertis FR. Relationship between loss of rat colonic surface epithelium induced by deoxycholate and initiation of the subsequent proliferative response. Cancer Res 46: 5754–5759, 1986. [PubMed] [Google Scholar]
- 12.Dayon L, Guiraud SP, Corthésy J, Da Silva L, Migliavacca E, Tautvydaitė D, Oikonomidi A, Moullet B, Henry H, Métairon S, Marquis J, Descombes P, Collino S, Martin FJ, Montoliu I, Kussmann M, Wojcik J, Bowman GL, Popp J. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: homocysteine and beyond. Alzheimers Res Ther 9: 43, 2017. doi: 10.1186/s13195-017-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Monte SM Triangulated mal-signaling in Alzheimer’s disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis 30, Suppl 2: S231–S249, 2012. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873, 2006. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 15.Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kühl AA. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 7: 4557–4576, 2014. [PMC free article] [PubMed] [Google Scholar]
- 16.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67: 123–133, 2018. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrada LD, Ahumada P, Cabrera D, Arab JP. Liver dysfunction as a novel player in Alzheimer’s progression: Looking outside the brain. Front Aging Neurosci 11: 174, 2019. doi: 10.3389/fnagi.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming SA, Dilger RN. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav Brain Res 321: 50–60, 2017. doi: 10.1016/j.bbr.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7: 1325–1330, 2016. doi: 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- 20.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 8: 13, 2018. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gifford AK, Cloutier S, Newberry RC. Objects as enrichment: Effects of object exposure time and delay interval on object recognition memory of the domestic pig. Appl Anim Behav Sci 107: 206–217, 2007. doi: 10.1016/j.applanim.2006.10.019. [DOI] [Google Scholar]
- 22.Greenwood J, Adu J, Davey AJ, Abbott NJ, Bradbury MWB. The effect of bile salts on the permeability and ultrastructure of the perfused, energy-depleted, rat blood-brain barrier. J Cereb Blood Flow Metab 11: 644–654, 1991. doi: 10.1038/jcbfm.1991.116. [DOI] [PubMed] [Google Scholar]
- 23.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol 31: 395–404, 2005. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE, Dupont C. Hmisc: Harrell Miscellaneous (Online) https://CRAN.R-project.org/package=Hmisc [13 January 2020].
- 25.Hernandez GV, Smith VA, Melnyk M, Burd MA, Sprayberry KA, Edwards MS, Peterson DG, Bennet DC, Fanter RK, Columbus DA, Steibel JP, Glanz H, Immoos C, Rice MS, Santiago-Rodriguez TM, Blank J, VanderKelen JJ, Kitts CL, Piccolo BD, La Frano MR, Burrin DG, Maj M, Manjarin R. Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am J Physiol Gastrointest Liver Physiol 318: G582–G609, 2020. doi: 10.1152/ajpgi.00344.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Carretero A, Weber N, La Frano MR, Ying W, Lantero Rodriguez J, Sears DD, Wallenius V, Börgeson E, Newman JW, Osborn O. Obesity-induced changes in lipid mediators persist after weight loss. Int J Obes 42: 728–736, 2018. doi: 10.1038/ijo.2017.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heuman DM Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res 30: 719–730, 1989. [PubMed] [Google Scholar]
- 28.Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol 301: 44–54, 1990. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- 29.Jelsing J, Nielsen R, Olsen AK, Grand N, Hemmingsen R, Pakkenberg B. The postnatal development of neocortical neurons and glial cells in the Göttingen minipig and the domestic pig brain. J Exp Biol 209: 1454–1462, 2006. doi: 10.1242/jeb.02141. [DOI] [PubMed] [Google Scholar]
- 30.Jernigan TL, Baaré WFC, Stiles J, Madsen KS. Postnatal brain development: structural imaging of dynamic neurodevelopmental processes. Prog Brain Res 189: 77–92, 2011. doi: 10.1016/B978-0-444-53884-0.00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67: 1881–1891, 2018. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 32.Joo SS, Kang HC, Won TJ, Lee DI. Ursodeoxycholic acid inhibits pro-inflammatory repertoires, IL-1 beta and nitric oxide in rat microglia. Arch Pharm Res 26: 1067–1073, 2003. doi: 10.1007/BF02994760. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 57: 1357–1365, 2013. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DG, Krenz A, Toussaint LE, Maurer KJ, Robinson SA, Yan A, Torres L, Bynoe MS. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation 13: 1, 2016. doi: 10.1186/s12974-015-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein J, Gonzalez R, Köppen A, Löffelholz K. Free choline and choline metabolites in rat brain and body fluids: sensitive determination and implications for choline supply to the brain. Neurochem Int 22: 293–300, 1993. doi: 10.1016/0197-0186(93)90058-D. [DOI] [PubMed] [Google Scholar]
- 36.Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res 2012: 1–14, 2012. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36: 1065–1073, 2007. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev 31: 728–751, 2007. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Liong MT Safety of probiotics: translocation and infection. Nutr Rev 66: 192–202, 2008. doi: 10.1111/j.1753-4887.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 40.MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, Louie G, Kueider-Paisley A, Moseley MA, Thompson JW, St John Williams L, Tenenbaum JD, Blach C, Baillie R, Han X, Bhattacharyya S, Toledo JB, Schafferer S, Klein S, Koal T, Risacher SL, Kling MA, Motsinger-Reif A, Rotroff DM, Jack J, Hankemeier T, Bennett DA, De Jager PL, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, van Duijn CM, Saykin AJ, Kastenmüller G, Kaddurah-Daouk R; Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium . Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement 15: 76–92, 2019. [Erratum in Alzheimers Dement 15: 604, 2019.] doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillin M, Frampton G, Quinn M, Ashfaq S, de los Santos M III, Grant S, DeMorrow S. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. Am J Pathol 186: 312–323, 2016. doi: 10.1016/j.ajpath.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMillin M, Grant S, Frampton G, Petrescu AD, Kain J, Williams E, Haines R, Canady L, DeMorrow S. FXR-mediated cortical cholesterol accumulation contributes to the pathogenesis of type A hepatic encephalopathy. Cell Mol Gastroenterol Hepatol 6: 47–63, 2018. doi: 10.1016/j.jcmgh.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan MY The treatment of chronic hepatic encephalopathy. Hepatogastroenterology 38: 377–387, 1991. [PubMed] [Google Scholar]
- 44.Mori T, Paris D, Town T, Rojiani AM, Sparks DL, Delledonne A, Crawford F, Abdullah LI, Humphrey JA, Dickson DW, Mullan MJ. Cholesterol accumulates in senile plaques of Alzheimer disease patients and in transgenic APP(SW) mice. J Neuropathol Exp Neurol 60: 778–785, 2001. doi: 10.1093/jnen/60.8.778. [DOI] [PubMed] [Google Scholar]
- 45.Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J Alzheimers Dis 15: 29–44, 2008. doi: 10.3233/JAD-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mudd AT, Getty CM, Sutton BP, Dilger RN. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr Neurosci 19: 425–433, 2016. doi: 10.1179/1476830515Y.0000000031. [DOI] [PubMed] [Google Scholar]
- 47.Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, Hoesel B, Fauler G, Stojakovic T, Einarsson C, Marschall HU, Trauner M. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol 62: 1398–1404, 2015. doi: 10.1016/j.jhep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, Nakamura N, Ohtaki H, Ito H, Tanaka T, Tsurumi H, Saito K, Seishima M, Moriwaki H. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PLoS One 8: e73404, 2013. doi: 10.1371/journal.pone.0073404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nho K, Kueider-Paisley A, Ahmad S, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, van Duijn C, Saykin AJ, Kaddurah-Daouk R; Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium . Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open 2: e197978, 2019. doi: 10.1001/jamanetworkopen.2019.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, Jia W, Xie G, Ahmad S, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R; Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium . Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimers Dement 15: 232–244, 2019. doi: 10.1016/j.jalz.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J 20: 43–49, 2006. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan X, Elliott CT, McGuinness B, Passmore P, Kehoe PG, Hölscher C, McClean PL, Graham SF, Green BD. Metabolomic profiling of bile acids in clinical and experimental samples of Alzheimer’s disease. Metabolites 7: 28, 2017. doi: 10.3390/metabo7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab 32: 403–414, 2006. doi: 10.1016/S1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- 54.Patidar KR, Bajaj JS. Antibiotics for the treatment of hepatic encephalopathy. Metab Brain Dis 28: 307–312, 2013. doi: 10.1007/s11011-013-9383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piepho HP Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 101: 865–869, 2009. doi: 10.2134/agronj2008.0226x. [DOI] [Google Scholar]
- 56.Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38: 996–1047, 2014. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rezaeiasl Z, Salami M, Sepehri G. The effects of probiotic Lactobacillus and Bifidobacterium strains on memory and learning behavior, long-term potentiation (LTP), and some biochemical parameters in β-amyloid-induced rat’s model of Alzheimer’s disease. Prev Nutr Food Sci 24: 265–273, 2019. doi: 10.3746/pnf.2019.24.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez AV, Baigorí MD, Alvarez S, Castro GR, Oliver G. Phosphatidylinositol-specific phospholipase C activity in Lactobacillus rhamnosus with capacity to translocate. FEMS Microbiol Lett 204: 33–38, 2001. doi: 10.1111/j.1574-6968.2001.tb10858.x. [DOI] [PubMed] [Google Scholar]
- 59.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 54: 344–353, 2011. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 118: 1388–1393, 2006. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 62.Seo SW, Gottesman RF, Clark JM, Hernaez R, Chang Y, Kim C, Ha KH, Guallar E, Lazo M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 86: 1136–1142, 2016. doi: 10.1212/WNL.0000000000002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7: 13537, 2017. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahler JB, Swain MG, Carson R, Bergasa NV, Jones EA. Blood-brain barrier permeability is markedly decreased in cholestasis in the rat. Hepatology 17: 1103–1108, 1993. doi: 10.1002/hep.1840170625. [DOI] [PubMed] [Google Scholar]
- 66.Weinstein G, Zelber-Sagi S, Preis SR, Beiser AS, DeCarli C, Speliotes EK, Satizabal CL, Vasan RS, Seshadri S. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham Study. JAMA Neurol 75: 97–104, 2018. doi: 10.1001/jamaneurol.2017.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 162: 496–500.e1, 2013. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm (Vienna) 107: 343–353, 2000. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 69.Wilcock GK, Esiri MM, Bowen DM, Smith CCT. Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci 57: 407–417, 1982. doi: 10.1016/0022-510X(82)90045-4. [DOI] [PubMed] [Google Scholar]
- 70.Wurtman RJ, Blusztajn JK, Maire JC. “Autocannibalism” of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s disease-A hypothesis. Neurochem Int 7: 369–372, 1985. doi: 10.1016/0197-0186(85)90127-5. [DOI] [PubMed] [Google Scholar]
- 71.Wurtman RJ Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci 15: 117–122, 1992. doi: 10.1016/0166-2236(92)90351-8. [DOI] [PubMed] [Google Scholar]
- 72.Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KAB, Hooper NM, Vardy ERLC, Wu D, Unwin RD, Faull RLM, Dowsey AW, Cooper GJS. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Sci Rep 6: 27524, 2016. doi: 10.1038/srep27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeisel SH Choline: an important nutrient in brain development, liver function and carcinogenesis. J Am Coll Nutr 11: 473–481, 1992. doi: 10.1080/07315724.1992.10718251. [DOI] [PubMed] [Google Scholar]
- 74.Zhan X, Jickling GC, Ander BP, Stamova B, Liu D, Kao PF, Zelin MA, Jin LW, DeCarli C, Sharp FR. Myelin basic protein associates with AβPP, Aβ1-42, and amyloid plaques in cortex of Alzheimer’s disease brain. J Alzheimers Dis 44: 1213–1229, 2015. doi: 10.3233/JAD-142013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuo JM, Portugal GS, Kruger WD, Wang H, Gould TJ, Pratico D. Diet-induced hyperhomocysteinemia increases amyloid-beta formation and deposition in a mouse model of Alzheimer’s disease. Curr Alzheimer Res 7: 140–149, 2010. doi: 10.2174/156720510790691326. [DOI] [PMC free article] [PubMed] [Google Scholar]