Abstract
Opioids depress minute ventilation primarily by reducing respiratory rate. This results from direct effects on the preBötzinger Complex as well as from depression of the Parabrachial/Kölliker-Fuse Complex, which provides excitatory drive to preBötzinger Complex neurons mediating respiratory phase-switch. Opioids also depress awake drive from the forebrain and chemodrive.
Keywords: awake drive, chemodrive, opioids, Parabrachial Nucleus/Kölliker-Fuse Complex, preBötzinger Complex
Introduction
Respiration is an automatic process that ensures adequate oxygen uptake and carbon dioxide removal from the body. The primary drive to breathe is derived from chemoreception, and respiratory phase duration and pattern are influenced by feedback from lung and airway receptors (110). Automatic breathing is generated in the brain stem and continues during sleep as well as under sedation (FIGURE 1). In the awake state, respiration is heavily influenced by the cortico-limbic system of the forebrain (review in Ref. 44), and respiratory activity changes promptly with arousal (70), emotions, and the level of physical activity. Volitional control allows for breath-holds, hyperventilation, coughing, and complex motor functions like vocalization (72). Loss of “awake drive” during sleep results in a decrease in respiratory minute ventilation (49) and a reduced hypoxic ventilatory response (11, 91, 118).
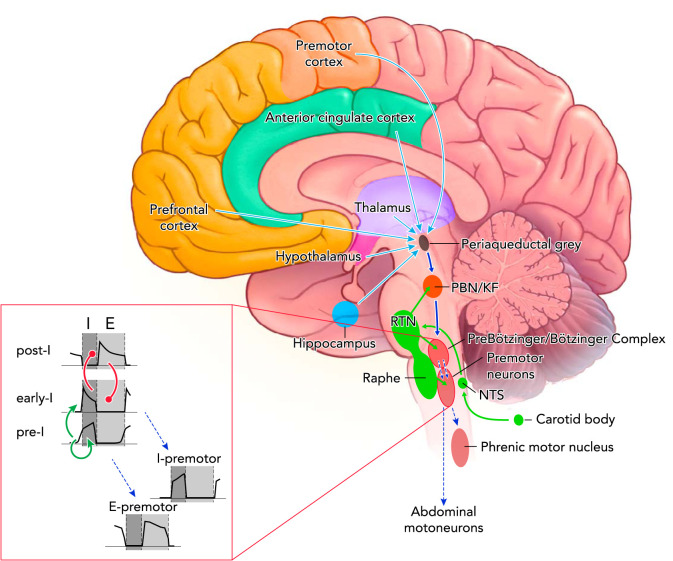
FIGURE 1.
Excitatory connections within the respiratory control center and opioid effects
Tonic chemodrive (green solid arrows) is the main excitatory drive to the medullary rhythm generator. A large component of chemodrive is routed through the Parabrachial Nucleus/Kölliker-Fuse Complex to phase-switching neurons in the preBötzinger Complex and determines respiratory rate (dark blue solid arrow). Phasic inputs from the preBötzinger Complex activate inspiratory (I) and expiratory (E) premotor and motoneurons (blue dotted arrows). Respiratory motor output (tidal volume) depends on direct projections from the retrotrapezoid nucleus to preBötzinger Complex neurons, premotor neurons, and motoneurons. The cortico-limbic system contributes tonic drive to the medullary rhythm generator (light blue solid arrows). Not shown: direct projections from the motor cortex to phrenic motoneurons as a pathway to override automatic rhythm; projections from the hypothalamus and cerebellum to the medullary raphe, which may contribute to the state-dependency of respiratory activity. Opioid effects: Parabrachial Nucleus/Kölliker-Fuse Complex activity was depressed in all studies and at all opioid concentrations. Opioid-induced depression was also shown for the preBötzinger Complex, the nucleus of the solitary tract, and the medullary raphe. Opioid-induced sedation suggests depression of forebrain inputs. Premotor neurons are only directly depressed at very high opioid concentrations. Inset: neuronal subtypes constituting the core of respiratory rhythm generation. Through mutual excitation, network activity of pre-inspiratory neurons (pre-I) in the preBötzinger Complex results in activation of early inspiratory (early-I) neurons (green arrows). Activity of these neurons is terminated through inhibition by post-inspiratory neurons (post-I), which themselves are inhibited by early-I neurons during the inspiratory phase (red circles). Phasic excitation is relayed to inspiratory (I) and expiratory (E) premotor neurons.
Opioid-induced respiratory depression (OIRD) is an important problem in the perioperative period (84) and increasingly in the community (170). The respiratory depressant effect is dose-dependent, and the magnitude of respiratory depression correlates with the level of sedation and analgesia (29, 92). In the clinical setting, opioids can cause mild sedation, hypoventilation, an increase in Pco2 above 50 Torr, and a decrease in oxygen saturation already with standard analgesic doses (20, 53, 74, 116a, 165). At that stage, patients can be prompted to breathe by voice command or touch. With deeper sedation, painful stimuli are still able to arouse the patient and elicit respiration. Pco2 can exceed 60 Torr (20) (53, 74, 165). Very high opioid doses depress respiration to a degree where neither severe hypoxia nor hypercapnia nor pain will be sufficient to generate respiratory efforts (90). These “overdoses” require artificial ventilation or treatment with the opioid antagonist naloxone (20). The gradual decline in minute ventilation and the concomitant increase in sedation suggest that OIRD involves multiple areas in the forebrain and brain stem.
This review describes the mechanism of respiratory rhythm generation in the brain stem, how excitatory drive from chemoreceptors and the forebrain contributes to minute ventilation, and how these areas are affected by opioids. Since opioids mostly depress respiratory rate, and apnea results from an arrest of the respiratory cycle in the expiratory phase rather than from a severe decrease in respiratory tidal volume (48, 83, 97, 127, 137), we particularly highlight the mechanism of respiratory rate control and how it is depressed by opioids. We focus on in vivo studies that examine respiratory mechanisms at physiological levels of respiratory drive, and we present data obtained in human subjects wherever available.
Organization of the Respiratory System
Respiratory Rhythm Generation
Respiratory rhythm originates in the ventrolateral medulla where the neuronal network of the preBötzinger Complex and Bötzinger Complex converts tonic excitatory respiratory drive into a distinct inspiratory and expiratory phase (FIGURE 1). Phasic respiratory activity is relayed via premotor neurons in the caudal medulla to motoneurons in the spinal cord. Phrenic motoneurons excite the main inspiratory muscle, the diaphragm, whereas thoraco-abdominal motoneurons phasically excite abdominal muscle activity during active expiration (34, 88, 130). The neurons of the preBötzinger Complex, the Bötzinger Complex, and the adjacent parafacial respiratory group can be classified by location, discharge pattern, neurotransmitters, genetics, and connectivity (see reviews in Refs. 34, 56, 130, 132). There is significant topical overlap between functionally distinct neuronal populations (5, 140, 172).
Respiratory rhythm generation relies on the activity of pre-inspiratory neurons to start the inspiratory phase (“inspiratory on-switch”; FIGURE 1, INSET). Synchronized activity of a sufficient number of pre-inspiratory neurons (76) activates other inspiratory neurons in the preBötzinger Complex to generate a full inspiratory cycle (2, 34, 50, 104). The level of excitability of preBötzinger Complex inspiratory neurons is modulated by inhibitory inputs, which affect the rate of depolarization and the duration of the refractory period (2, 4) (FIGURE 2). Inspiration is terminated when inspiratory neurons are inhibited by post-inspiratory neurons (“inspiratory off-switch”) (27, 45, 104). Research is ongoing to elicit whether respiratory rate is determined mostly by the depolarization rate of pre-inspiratory and inspiratory neurons in the preBötzinger Complex (71) or whether it equally depends on the activity of expiratory neurons in the Bötzinger Complex (7, 104, 132) that inhibit inspiratory neurons (71). In addition to neurons contributing to respiratory phase switching, the preBötzinger and Bötzinger Complexes also contain inspiratory and expiratory neurons whose discharge prolongs phase duration and contributes to inspiratory and expiratory motor output (tidal volume; FIGURE 1) (88, 141).
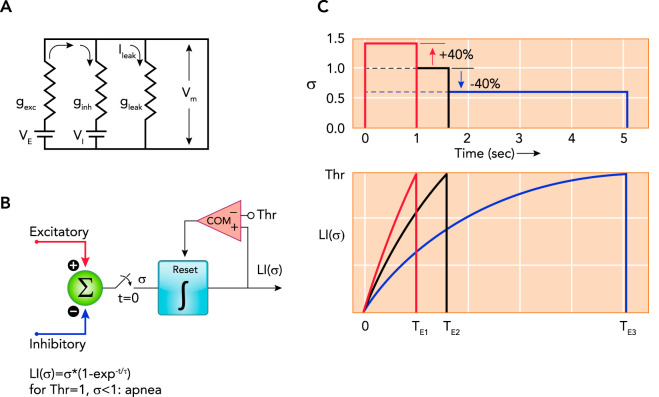
FIGURE 2.
Model of the components determining the membrane potential and illustrating the effects of changes in excitatory and/or inhibitory inputs
A: model of the components determining the membrane potential (Vm) of a preBötzinger Complex phase-switching neuron: , where gexc is excitatory conductance, VE is equilibrium potential for excitatory currents, ginh is inhibitory conductance, VI is equilibrium potential for inhibitory currents, and gleak is leak conductance, e.g., GIRK channels (see text for details). B and C: functional timer model to illustrate the effects of changes in excitatory and/or inhibitory inputs on the neuronal membrane trajectory (see Refs. 28, 115, 171–173). B: the sum of all excitatory and inhibitory inputs (σ) is gated to a leaky integrator (LI) at time, t = 0 s. The magnitude of σ determines the rate of the exponential rise of Vm to a threshold (Thr). Crossing the threshold resets the leaky integrator via a comparator (COM). The time to crossing the threshold determines the phase duration. C: graphic illustration of the timing operation using the example of rhythmogenic pre-inspiratory neurons. Neuronal discharge in pre-inspiratory neurons begins when Thr is reached and results in inspiratory on-switch. Inspiratory on-switch terminates the expiratory phase, i.e., the time to Thr for pre-inspiratory neurons determines expiratory duration (TE). Due to the nonlinear nature of this mechanism, increases and decreases in σ of the same magnitude cause strikingly different changes in phase duration. Shown are three examples for σ (upper) and the corresponding leaky integrator outputs LI (σ) (lower). Setting σ = 1.0 as a baseline reference results in a duration of TE2 = 1.6 s (black lines). Increasing σ by 40% results in TE1 = 1 s (red), whereas decreasing σ by 40% results in TE3 = 5 s (blue). Physiological examples for an increase in σ could be an increase in neuronal activity in the parabrachial nucleus (PBN)/Kölliker-Fuse nucleus (137) or an increase in inhibitory activity during the preceding inspiratory phase, which shortens inspiratory duration and presumably shortens the post-inspiratory refractory period (3). The latter could be due to vagal pulmonary stretch receptor input during lung inflation or increased activity of preBötzinger Complex inhibitory neurons during the inspiratory phase (3). Decreases in σ could be due to a reduction in PBN activity via inhibition of PBN neurons by opioids, by increases in pulmonary stretch receptor activity during the expiratory phase (173), or by increases in preBötzinger Complex GABAergic/glycinergic neuronal activity during the expiratory phase (3).
Using comparative cytoarchitecture and immunohistochemistry, Schwarzacher et al. identified the equivalent of the preBötzinger Complex in the human ventrolateral medulla (139). Functional MRI studies showed inspiratory activity in this area that alternated with expiratory activity in the adjacent caudal ventrolateral pons/parafacial group (63). Interestingly, preBötzinger Complex activity was increased during loaded inspiration in healthy controls, whereas patients with chronic obstructive pulmonary disease, which is associated with impaired airflow during expiration, showed increased activation of the parafacial group (63).
Respiratory Drive
The term “respiratory drive” is used broadly in respiratory control to describe excitatory inputs to brain stem areas that increase respiratory activity. Respiratory chemodrive summarizes the tonic excitatory inputs to the medullary rhythm generator that result from activation of chemoreceptive brain stem areas and the carotid body (56) by Pco2 and hypoxia (FIGURE 1). Hypoxia (12) and hypercapnia (30) cause large increases in minute ventilation. In humans, this was associated with increased activity in the carotid body and the nucleus of the solitary tract (101, 126). Short episodes of mild hypercapnia also increased activity in the thalamic nuclei, the pontine raphe, the Parabrachial Nucleus/Kölliker-Fuse Complex, and the locus coeruleus (126). No additional increase was observed once Pco2 exceeded ~65 Torr (81).
A large component of respiratory chemodrive originates in the retrotrapezoid nucleus, which contains chemosensitive neurons (111, 144, 156), and also integrates the peripheral chemoreceptive inputs from the carotid body (55). Individuals with a genetic lack of chemoreceptive retrotrapezoid nucleus neurons (congenital central hypoventilation syndrome) suffer from severe hypoventilation during sleep (129, 161). The retrotrapezoid nucleus projects glutamatergic excitatory inputs to the Bötzinger Complex, preBötzinger Complex, Parabrachial Nucleus/Kölliker-Fuse Complex, and premotor neurons, and phrenic motoneurons (14, 17). Glutamate receptor antagonism in the preBötzinger Complex results in short, irregular, low-amplitude breaths and ultimately apnea (25, 113), highlighting the importance of glutamatergic drive for respiratory function (FIGURE 3A).
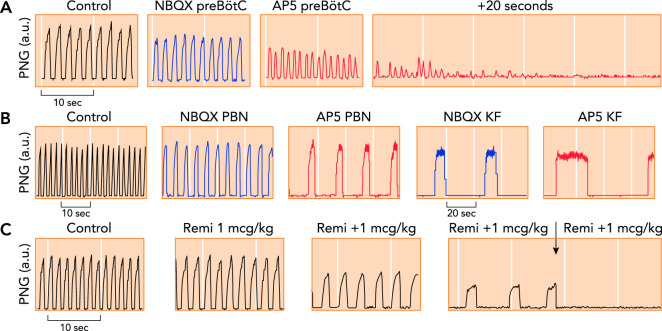
FIGURE 3.
Phrenic neurogram tracings obtained in adult decerebrate rabbits in vivo with Pco2 constant
A: antagonism of glutamate receptor function in the preBötzinger Complex (preBötC) through local microinjection of the AMPA receptor antagonist NBQX (blue) and the NMDA receptor antagonist AP5 (red) caused tachypnea with decreased peak phrenic amplitude, followed by apnea (25). B: antagonism of glutamate receptor function in the parabrachial nucleus (PBN) and Kölliker-Fuse nucleus (KF) through local microinjection of NBQX (blue) and AP5 (red) caused severe bradypnea, whereas peak phrenic amplitude was only little decreased. Complete apnea was never observed (116). Please note the different time scales. C: repeated intravenous boluses of the mu-opioid agonist remifentanil (Remi; 1 mcg · kg–1 bolus–1) caused increasing bradypnea due to prolongation of inspiratory and expiratory phase duration. The fourth bolus resulted in apnea (arrow). Remifentanil also substantially depressed peak phrenic activity (123), suggesting an effect on both the PBN/KF and the preBötC.
A second chemosensitive system consists of the neurons of the medullary raphe (see reviews in Refs. 26, 66). Raphe neurons form serotonergic synapses, at times colocalized with substance P, with neurons in the preBötzinger Complex and the pontine respiratory group (128). Both neurotransmitters cause a decrease in membrane potential of preBötzinger Complex neurons and thus modulate their level of excitability (128). Input from the raphe obscurus modulated the pH response of retrotrapezoid nucleus neurons (162). The raphe obscurus receives multiple synaptic inputs from suprapontine areas (below) and preBötzinger Complex inspiratory neurons (128), suggesting that the raphe contributes to state-dependent modulation of respiratory activity (128).
Additional respiratory drive originates in the cortico-limbic system. In humans, MRI diffusion tractography established prominent connections between the hippocampus and the raphe, locus coeruleus, and nucleus paragigantocellularis lateralis (42), the human equivalent of the preBötzinger Complex (139). This connection may be responsible for increasing respiration as part of the “fight-or-flight” response. Hippocampus and amygdala appear to mediate the “urge to breathe” during hypercarbia-stimulated or inspiratory-resistive loaded breathing (61, 126). In anesthetized rats, stimulation of hypothalamic nuclei increased respiratory rate and tidal volume (Ref. 158 and review in Ref. 77). Many of these efferents are relayed by the Parabrachial Nucleus/Kölliker-Fuse Complex (22), but some directly project to the spinal cord (21). The hypothalamus also sends orexinergic projections to the raphe magnus, raphe obscurus, and retrotrapezoid nucleus, which do not affect baseline respiration but enhance CO2 sensitivity during the nocturnal active phase in rats (review in Ref. 114). Direct connections between the motor cortex and phrenic motoneurons were shown histologically (133) and functionally (8) in cats. In humans, functional MRI showed an activation of the motor cortex with inspiratory loading (Ref. 166, and see review in Ref. 44). Broadly, the activity of these areas contributes tonic “awake drive” to the medullary respiratory center (105, 117). Additional awake drive is produced by the lateral reticular formation of the medulla (117), emphasizing the state dependency of respiratory drive.
The periaqueductal gray lies within the tegmentum of the midbrain, at the juncture between the forebrain and the brain stem. The periaqueductal gray provides an important connection for sensory feedback from the nociceptive areas of the spinal cord and lung mechanoreceptors relaying work of breathing (23, 24) toward the thalamus (95) and, in the opposite direction, for drive from the prefrontal, premotor, motor, and cingular cortex (33, 96) to the locus coeruleus, lateral PBN, nucleus ambiguous, and raphe magnus and pallidus (94). It thus contributes to the integration of the respiratory pattern, vocalization, and upper airway maneuvers with changing emotions and behaviors (Ref. 151, and reviewed in Ref. 153). Stimulation of a subarea of the periaqueductal gray resulted in tachypnea (152, 153). This response was significantly reduced by inhibition of the lateral PBN (62), suggesting that respiratory drive coming from the periaqueductal gray was relayed by the PBN. In cats, activity of the cerebellar fastigial nucleus did not alter baseline respiratory rate but increased the response to severe hypercapnia and hypoxia (163, 164). Anatomical connections suggest that this effect is also mediated by the PBN (154).
Multilevel Opioid Effects
The preBötzinger Complex and Parabrachial Nucleus/Kölliker-Fuse Complex Are Prime Targets for Drugs That Depress Respiratory Rate
The concomitant increase of respiratory rate and tidal volume with hypoxia and hypercapnia may suggest that both parameters are tightly linked. However, the parameters can be “uncoupled” by reducing Parabrachial Nucleus/Kölliker-Fuse Complex activity (39, 41, 47, 89, 116). The Parabrachial Nucleus/Kölliker-Fuse Complex is an important relay station for tonic excitatory inputs from the retrotrapezoid nucleus (142) and the medullary raphe, as well as from the forebrain, the periaqueductal gray, the cerebellum, and the ascending nociceptive inputs (73) to the medullary rhythm generator. In decerebrate rabbits, blockage of glutamatergic inputs to the Parabrachial Nucleus/Kölliker-Fuse Complex depressed respiratory rate by >90% but decreased peak phrenic activity only by <20% (116) (FIGURE 3B). Subsequent exposure to hypoxic hypercapnia during persistent glutamatergic block increased respiratory rate only to 25% of control, whereas peak phrenic activity increased to 160% of control.
Considering that the respiratory rhythm is generated in the preBötzinger Complex, a possible explanation for this differential effect is that excitatory drive to the preBötzinger Complex is not nonspecific, as depicted in some models (54, 132), but that different brain stem areas specifically excite certain neuronal subpopulations (7, 116). Histological and functional studies have demonstrated excitatory projections from the retrotrapezoid nucleus to the Parabrachial Nucleus/Kölliker-Fuse Complex (14, 142) and preBötzinger Complex (14) and also from the Parabrachial Nucleus/Kölliker-Fuse Complex to the preBötzinger Complex (51, 172). The importance of Parabrachial Nucleus/Kölliker-Fuse Complex activity for respiratory rate suggests that the majority of excitatory drive to phase-switching neurons in the preBötzinger Complex/Bötzinger Complex is relayed through the Parabrachial Nucleus/Kölliker-Fuse Complex. On the other hand, inputs that determine respiratory tidal volume likely project directly from the retrotrapezoid nucleus to neurons in the medullary rhythm generator as well as premotor neurons and motoneurons in the spinal cord (FIGURE 1).
As described above, inspiratory on-switch depends on the depolarization rate of pre-inspiratory and inspiratory preBötzinger Complex neurons (4, 5, 18, 19, 171, 172) (FIGURE 2C), whereas inspiratory off-switch is likely regulated by the depolarization rate of post-inspiratory neurons (7, 41, 116). FIGURE 2C illustrates that, when the sum of inputs (σ) is large, i.e., excitatory inputs () to a phase-switching neuron are much higher than inhibitory inputs (), the membrane potential quickly reaches its discharge threshold, and the duration of the preceding respiratory phase (T) is short. In this case, a small decrease in excitatory drive causes only a small increase in phase duration. In contrast, when the sum of inputs is small, time to phase-switch is prolonged. Under these conditions, a decrease in excitatory drive from the Parabrachial Nucleus/Kölliker-Fuse Complex to pre-inspiratory neurons or direct inhibition of pre-inspiratory neurons would substantially prolong the expiratory phase. Furthermore, a small additional decrease in excitatory drive or increase in inhibition would result in a very long expiratory phase (116) and potentially apnea. This explains the observation that respiratory rate variation is greater when excitatory drive to the rhythm generator is low (19).
Opioids hyperpolarized respiratory neurons through a mu-opioid-receptor-coupled G-protein-gated inwardly rectifying potassium (GIRK) conductance (gleak; FIGURE 2A) in the preBötzinger Complex and Kölliker-Fuse nucleus in vitro (85, 108, 160). Opioids thus have the potential to slow depolarization of phase-switching neurons in the preBötzinger Complex either through a direct, inhibitory effect on these neurons or through inhibition of Parabrachial Nucleus/Kölliker-Fuse Complex neurons, which reduces the excitatory drive to the preBötzinger Complex.
Opioid Effects on Respiratory Rate and Motor Output in vivo
Multiple studies point to a direct opioid effect on preBötzinger Complex neurons. In mice in vivo, opioids activated a GIRK channel, and block of GIRK channels in the preBötzinger Complex reduced the respiratory depression from systemic mu-opioid agonists (108). Microinfusion of the opioid antagonist naloxone into the preBötzinger Complex completely prevented the 25% respiratory rate depression from small doses of IV fentanyl in rats (107). In contrast, in in vivo dogs (112) and rabbits (145), naloxone injection into the bilateral preBötzinger Complex did not reverse a 50% respiratory rate depression caused by an intravenous remifentanil infusion; further analysis suggested that the opioid effect on expiratory duration was mediated mostly outside the preBötzinger Complex (145). Interestingly, in vitro bath application of mu-opioid receptor agonists inhibited inspiratory preBötzinger Complex neurons (102, 157) but did not affect pre-inspiratory neurons (102) or expiratory neurons (157).
In the in vivo rabbit preparation, naloxone injection into the parabrachial nucleus partially reversed respiratory rate depression from an intravenous remifentanil infusion from 50% to 20% (103). In the in vivo decerebrate dog preparation, microinjected naloxone into the parabrachial nucleus produced a full reversal of remifentanil-induced bradypnea/apnea (127). In the in situ rat model, injection of the opioid antagonist CTAP into the bilateral Kölliker-Fuse nucleus prevented apnea from systemic fentanyl infusion (137). In rabbits and rats, respiratory rate depression was not fully reversed, with local microinjections of opioid antagonists in the parabrachial nucleus or Kölliker-Fuse nucleus. The quantitative differences in the naloxone effects may be due to species differences.
In freely behaving mice, mu-opioid receptor deletion in the bilateral Kölliker-Fuse nucleus reduced the respiratory rate depression from small (10 mg/kg), intermediate (30 mg/kg), and high (100 mg/kg) morphine doses by ~20% at each dose. Mu-opioid receptor deletion in the bilateral preBötzinger Complex also reduced respiratory rate depression after 10 mg/kg morphine; however, there was no effect at higher morphine doses (160). In a similar model, mu-opioid receptor deletion in the preBötzinger Complex attenuated the respiratory rate depression from 20 mg/kg morphine from 50% to 70% of control rate (3). Additional attenuation of the morphine effect from subsequent mu-opioid receptor deletion in the second area was not statistically significant; however, the study may have been underpowered to show the effect (3) (FIGURE 4). Taken together, these studies show that opioid-induced respiratory rate depression results in a large degree from a combination of direct depression of the preBötzinger Complex and a decrease in excitatory drive from the Parabrachial Nucleus/Kölliker-Fuse Complex to the preBötzinger Complex. The depression of each area appears to be dose-dependent and species-dependent.
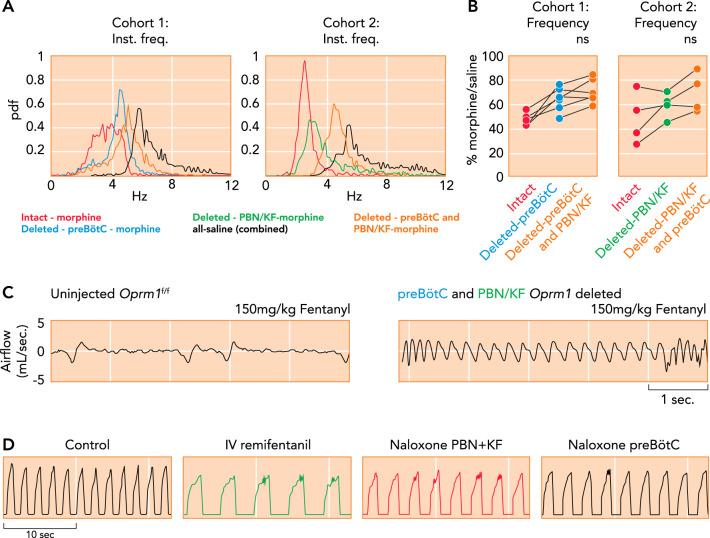
FIGURE 4.
Critical importance of the Parabrachial Nucleus/Kölliker-Fuse complex and preBötzinger Complex for opioid-induced respiratory depression
A–C: μ-opioid receptors (Oprm1) were selectively deleted in Oprm1 f/f mice through Cre-virus injection into the preBötC and PBN/KF in this (cohort 1) or reverse order (cohort 2) with 4–5 wk between injections and plethysmography recordings (data from A–C from Ref 3, and used with permission from eLife). A: probability density function plot of the respiratory rate for a representative animal from cohorts 1 and 2 after intraperitoneal morphine (20 mg/kg) or saline before and after Oprm1 deletion in the PBN/KF and preBötC. B: pooled data showed that morphine-induced respiratory rate depression was significantly attenuated after Oprm1 deletion in the preBötC. Oprm1 deletion in both areas reduced morphine-induced respiratory rate depression from ~50% to ~30%. C: plethysmography recordings in mice show that Oprm1 deletion in the preBötC + PBN/KF prevented respiratory rate depression from a very high intraperitoneal fentanyl dose (150 mg/kg; right) that usually caused lethal apnea before Oprm1 deletion (left). D: consistent with the murine studies, phrenic neurogram tracings obtained in an adult decerebrate rabbit in vivo show that sequential microinjections of the opioid antagonist naloxone into the bilateral PBN + KF and bilateral preBötC reversed the respiratory rate depression from intravenous remifentanil infusion (see Ref. 123).
A recent study in freely behaving rats demonstrated the existence of a non-opioid-sensitive, chemo-insensitive respiratory rhythm that developed after very high opioid doses (300 µg/kg fentanyl) (59). Respiratory activity appeared to be driven primarily by expiratory abdominal efforts (59). In a different rat model, phasic expiratory activity was observed even after inspiratory activity was suppressed by fentanyl (102). Although the severe hypoxia and acidosis associated with the high fentanyl dose (58) makes it unlikely that humans would survive to develop such rhythm, the study raises the interesting question of whether opioids also inhibit inhibitory pathways that normally suppress phasic respiratory activity.
Low doses of systemic opioids that decreased respiratory rate did not directly depress bulbospinal premotor neurons, although mu-opioid receptors were present on these neurons (83, 149). Near-apneic fentanyl doses hyperpolarized premotor neurons, suggesting that high doses directly depress neuronal activity (57, 83). Remifentanil infusion depressed phrenic nerve amplitude more than peak premotor neuronal activity, suggesting an additional, direct depressant effect of opioids on phrenic motoneurons (149). To date, no studies have investigated the effects of opioids on brain stem mechanisms in humans.
Chronic opioid use leads to desensitization and cellular tolerance (86), resulting in increased opioid requirements to maintain the same level of intended analgesia or euphoria. At the same time, higher opioid doses may be tolerated before respiratory depression occurs. In freely behaving mice, chronic exposure to morphine resulted in a decrease in respiratory depression from systemic bolus doses of morphine and fentanyl (64). However, respiratory depression was not reduced at high fentanyl doses, suggesting only a limited cross tolerance between morphine and fentanyl (64). Multiple clinical studies have highlighted that the risk of (fatal) overdose in patient populations was increased at higher total opioid doses, suggesting that chronic opioid use did not eliminate the dose-dependency of respiratory depression (reviewed in Ref. 38). There is no evidence that the locations or network mechanism of OIRD differ between opioid-naive and opioid-tolerant individuals.
Opioid Effects on Respiratory Drive in vivo
In human volunteers, analgesic doses of morphine decreased the hypoxic ventilatory response (12) (FIGURE 5A) as well as the ventilatory response to hypercarbia (30) (FIGURE 5B). This was possibly due to an effect on the nucleus of the solitary tract: In anesthetized rats, injection of the mu-opioid antagonist CTAP into the commissural subnucleus of the nucleus of the solitary tract almost completely reversed the depression of the hypoxic ventilatory response caused by intravenous opioids (169). In the same model, injection of CTAP into the caudal medullary raphe partially reversed the depression of the hypercapnic ventilatory response from systemic opioids (168). In contrast, in in vivo rats, morphine doses that caused apnea did not decrease discharge frequency or CO2 response in chemosensitive retrotrapezoid nucleus neurons (111).
FIGURE 5.
Contributions of chemodrive and “awake drive” to minute ventilation and opioid effects
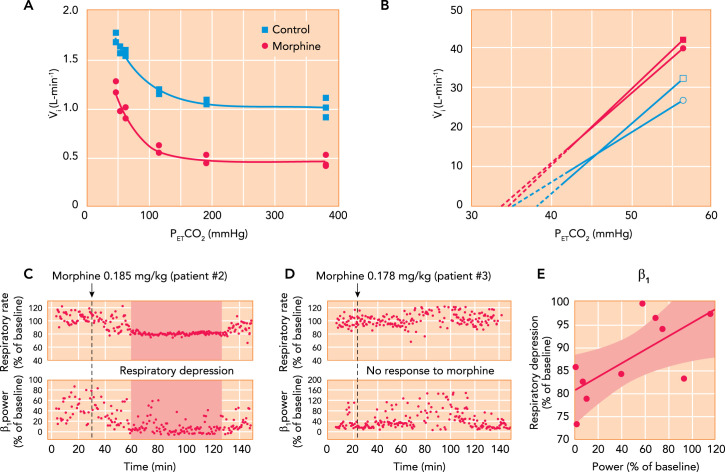
A: hypoxic ventilatory response curves in a chloralose-urethane anesthetized cat during control (solid square) and after administration of 0.15 mg/kg IV morphine. Morphine decreased minute ventilation during hyperoxia but did not change the increase in minute ventilation with hypoxia (see Ref. 12). B: mean ventilatory response to increasing inspiratory carbon dioxide concentrations obtained in 12 male (square) and 12 female (circle) human volunteers during control (filled) and after 0.1 mg/kg IV morphine, followed by 0.03 mg · kg–1 · h–1 (open). The continuous lines are the linear regression lines through the data points, and broken lines are extrapolated to the apneic threshold. In men and women, morphine decreased minute ventilation differently: in men by increasing the apneic threshold but in women by decreasing carbon dioxide sensitivity (see Ref. 30). C–E: association between respiratory depression from analgesic doses of morphine and loss of “awake drive” per electroencephalogram in pediatric patients. C: after 0.185 mg/kg morphine, patient 2 presented substantial respiratory rate depression associated with a decrease in β1 power. D: in patient 3, a similar dose (0.178 mg/kg) did not reduce β1 power or cause notable respiratory depression. E: in 10 patients, the severity of respiratory rate depression correlated with the intensity of the reduction in β1 power (R = 0.715, P = 0.02) (105).
Opioids also dose-dependently cause sedation, and this effect reduces or eliminates excitatory forebrain inputs to the brain stem (16, 29, 92, 135) (FIGURE 1). Functional MRI in human volunteers who received sedative doses of remifentanil showed decreased activity in the prefrontal cortex, anterior cingulate, thalamus, subthalamic nucleus, cerebellum, and periaqueductal gray (125), i.e., in areas that mediate pain and other unpleasant sensations and that contain a high concentration of opioid receptors (9). This may explain why already small opioid doses eliminate the “urge to breathe” during breathholds or with inspiratory CO2 challenges (99, 125). This effect can be clinically exploited to reduce the sensation of air hunger in patients with heart failure (43). It may also explain why the severe hypoxia and hypercapnia after opioid overdoses do not cause patient distress (46).
In anesthetized rats, intravenous morphine inhibited acetylcholine release in the prefrontal cortex and decreased arousal (121). Direct fentanyl injection into hypothalamic subnuclei led to a decrease in respiratory rate for >20 min (159). The importance of the cortical arousal state was shown in patients who received morphine for postoperative pain control. Respiratory rate was decreased on average by 8%, and the decrease correlated with the decrease in beta-1 power in the electroencephalogram (105) (FIGURE 5, C–E). In freely behaving rats, 100 µg/kg fentanyl—approximately equivalent to a strong analgesic dose of 5 µg/kg in humans—caused significant sedation and reduced alpha and beta-2 power in the EEG recording (106). The increase in theta power correlated with respiratory rate depression (106). Interestingly, opioids did not affect the motor cortex (125), i.e., as long as patients remain awake, they are able to breathe on command (84).
The role of respiratory subareas in OIRD depends on the relative contribution of these areas to respiratory activity as well as on their sensitivity to different opioid concentrations. Until now, in vivo studies have not differentiated between these two factors. In addition, more research is needed to determine whether the opioid effect on any individual area is modified by changed inputs from other areas. No study to date has assessed the relative contributions to OIRD of all factors and all affected areas, i.e., chemodrive, awake drive, the Parabrachial Nucleus/Kölliker-Fuse Complex and the preBötzinger Complex, in the same model.
Future Research
Sedative Drugs
Clinically used sedatives like the alpha-2 antagonist dexmedetomidine (78a) and the GABAA-receptor agonist midazolam cause limited respiratory depression even at high doses, whereas the NMDA-receptor antagonist ketamine and the GABAA agonist propofol can cause severe respiratory depression and apnea (see review in Ref. 150). All of these drugs enhance the respiratory depressant effect of opioids (67, 122). Benzodiazepines, for example, are frequently found in overdose victims in the community (75). Alpha-2 receptors have not been identified in the preBötzinger Complex or Parabrachial Nucleus/Kölliker-Fuse Complex, suggesting that any enhancement of OIRD may be due to removal of “awake drive.” NMDA and GABAA receptors have been located in all respiratory-related brain stem areas, providing potential targets for sedative agents (25, 27, 36, 37, 41, 79, 98, 113, 115, 158). We hypothesize that sedatives affect respiratory rate through the same mechanism as opioids, i.e., by decreasing neuronal excitability of phase-switching neurons in the preBötzinger Complex (FIGURE 2A). The main excitatory inputs to preBötzinger Complex neurons are glutamate receptor mediated (25, 113), whereas inhibitory inputs are GABAA or glycine receptor mediated (4, 27). Sedatives that block glutamate-receptor function or enhance GABAA-receptor function thus have the potential to decrease respiratory rate, similarly to opioids, either through direct depression of preBötzinger Complex neurons or through depression of Parabrachial Nucleus/Kölliker-Fuse Complex activity, which lowers glutamatergic drive to the preBötzinger Complex. This would explain how sedative doses that cause only small decreases in membrane excitability and thus respiratory rate when the drug is given by itself can result in severe respiratory slowing or apnea when added after neuronal excitability is already decreased by systemic opioids (FIGURE 2C). In addition, sedatives may depress the activity of other preBötzinger Complex neurons, premotor neurons, and phrenic motoneurons, and thus contribute to a decrease in tidal volume. The exact locations mediating respiratory depression from sedatives have been studied in far less detail than opioids (150), and, just as for opioids, the effects on individual areas may depend on the drug dose (Table 1).
TABLE 1.
Studies of opioid and sedative effects in individual respiratory-related areas
| Brain Region | Mu-Opioid Receptor | NMDA Receptor | GABAA Receptor |
|---|---|---|---|
| Carotid body | 12, 78 | 65 | 65, 167 |
| Nucleus tractus solitarii | 30, 169 | 124 | |
| Retrotrapezoid nucleus | 111 | 109 | 87, 155 |
| Medullary raphe | 168 | 10 | 87 |
| Forebrain | 105, 121, 125 | 93, 120 | 1, 60, 69, 119 |
| Periaqueductal gray | 138 | ||
| Parabrachial Nucleus/Kölliker-Fuse Complex | 3, 85, 103, 137, 160 | 40, 115 | 32, 35, 142 |
| Pre-Bötzinger Complex | 3, 107, 112, 145, 160 | 25, 113 | 15, 27, 98 |
| Premotor neurons | 57, 83, 149 | 80, 134 | 36, 100, 146, 148 |
| Phrenic motoneurons | 68, 149 | 100 | 100 |
Numbers correspond to reference numbers. Bold: where available, we quote “clinically relevant studies,” i.e., in vivo studies that record respiratory neuronal or global respiratory output during systemic drug application and localized antagonist injection or receptor deletion. Italic: if such studies are not available, we present in vivo studies recording respiratory-related neurons or global output during localized application of the sedative agent or the respective receptor agonist or antagonist at clinically relevant or higher concentrations; alternatively, these are descriptive studies using fMRI or EEG activity in humans. Roman: if no in vivo studies are available, we present in vitro studies using localized application of the receptor agonist/antagonist or indirect evidence. Sedatives included are NMDA receptor antagonists (e.g., ketamine) and GABAA receptor agonists (e.g., Propofol and midazolam). Although clinically relevant opioid concentrations have been studied in vivo in many areas, this research still needs to be performed for clinically used sedatives.
Respiratory Stimulants
We have described that opioids depress respiratory activity through effects on multiple areas of the central nervous system. This is of practical relevance for the development of pharmacological agents designed to counteract OIRD (see detailed review in Ref. 31). For example, opioid-induced depression of the Parabrachial Nucleus/Kölliker-Fuse Complex significantly reduces excitatory drive to the preBötzinger Complex. This likely limits the respiratory-stimulating effects of receptor agonists that specifically stimulate neurons in the preBötzinger Complex (82, 97). Similarly, opioids depress areas that relay chemodrive to the respiratory center (168, 169). This likely limits the benefit of drugs that enhance only peripheral chemodrive (136). Most promising are glutamate receptor modulators that may increase neuronal activity in many affected brain stem regions; however, so far, only AMPA receptor modulators have been investigated (116a, 131). Likely, the effectiveness of respiratory stimulants will always be limited at very high opioid doses, and their use has to be balanced with their central nervous and cardiovascular side effects (31, 52).
Acknowledgments
The authors are supported by National Institute of General Medical Sciences Grant R01-GM-112960.
No conflicts of interest, financial or otherwise, are declared by the author(s).
B.P., V.M., E.J.Z., E.A.E.S., and A.G.S. interpreted results of experiments; B.P., V.M., E.J.Z., and A.G.S. prepared figures; B.P., V.M., E.J.Z., E.A.E.S., and A.G.S. edited and revised manuscript; E.J.Z., E.A.E.S., and A.G.S. approved final version of manuscript; A.G.S. conceived and designed research; A.G.S. performed experiments; A.G.S. analyzed data; A.G.S. drafted manuscript.
References
- 1.Antkowiak B Different actions of general anesthetics on the firing patterns of neocortical neurons mediated by the GABA(A) receptor. Anesthesiology 91: 500–511, 1999. doi: 10.1097/00000542-199908000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Ashhad S, Feldman JL. Emergent elements of inspiratory rhythmogenesis: network synchronization and synchrony propagation. Neuron 106: 482–497.e4, 2020. doi: 10.1016/j.neuron.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmutsky I, Wei XP, Kish E, Yackle K. Opioids depress breathing through two small brainstem sites. eLife 9: e52694, 2020. doi: 10.7554/eLife.52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baertsch NA, Baertsch HC, Ramirez JM. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat Commun 9: 843, 2018. doi: 10.1038/s41467-018-03223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baertsch NA, Severs LJ, Anderson TM, Ramirez JM. A spatially dynamic network underlies the generation of inspiratory behaviors. Proc Natl Acad Sci USA 116: 7493–7502, 2019. doi: 10.1073/pnas.1900523116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett WH, Jenkin SEM, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB. The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J Neurophysiol 119: 401–412, 2018. doi: 10.1152/jn.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassal M, Bianchi AL. Inspiratory onset or termination induced by electrical stimulation of the brain. Respir Physiol 50: 23–40, 1982. doi: 10.1016/0034-5687(82)90004-4. [DOI] [PubMed] [Google Scholar]
- 9.Baumgärtner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Höhnemann S, Piel M, Rösch F, Wester HJ, Henriksen G, Stoeter P, Bartenstein P, Treede RD, Schreckenberger M. High opiate receptor binding potential in the human lateral pain system. Neuroimage 30: 692–699, 2006. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Beltrán-Castillo S, Olivares MJ, Contreras RA, Zúñiga G, Llona I, von Bernhardi R, Eugenín JL. D-serine released by astrocytes in brainstem regulates breathing response to CO2 levels. Nat Commun 8: 838, 2017. doi: 10.1038/s41467-017-00960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benarroch EE Control of the cardiovascular and respiratory systems during sleep. Auton Neurosci 218: 54–63, 2019. doi: 10.1016/j.autneu.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Berkenbosch A, Teppema LJ, Olievier CN, Dahan A. Influences of morphine on the ventilatory response to isocapnic hypoxia. Anesthesiology 86: 1342–1349, 1997. doi: 10.1097/00000542-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol 520: 1047–1061, 2012. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Respiratory responses induced by blockades of GABA and glycine receptors within the Bötzinger complex and the pre-Bötzinger complex of the rabbit. Brain Res 1344: 134–147, 2010. doi: 10.1016/j.brainres.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Bouillon T, Bruhn J, Radu-Radulescu L, Andresen C, Cohane C, Shafer SL. A model of the ventilatory depressant potency of remifentanil in the non-steady state. Anesthesiology 99: 779–787, 2003. doi: 10.1097/00000542-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593: 2909–2926, 2015. doi: 10.1113/JP270053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butera RJ Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. J Neurophysiol 82: 382–397, 1999. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- 19.Butera RJ Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations Of coupled pacemaker neurons. J Neurophysiol 82: 398–415, 1999. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- 20.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth 93: 212–223, 2004. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 21.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol 272: 579–604, 1988. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 22.Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240: 153–160, 1985. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Eldridge FL, Wagner PG. Respiratory-associated rhythmic firing of midbrain neurones in cats: relation to level of respiratory drive. J Physiol 437: 305–325, 1991. doi: 10.1113/jphysiol.1991.sp018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Eldridge FL, Wagner PG. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol 90: 99–113, 1992. doi: 10.1016/0034-5687(92)90137-L. [DOI] [PubMed] [Google Scholar]
- 25.Cook-Snyder DR, Miller JR, Navarrete-Opazo AA, Callison JJ, Peterson RC, Hopp FA, Stuth EAE, Zuperku EJ, Stucke AG. The contribution of endogenous glutamatergic input in the ventral respiratory column to respiratory rhythm. Respir Physiol Neurobiol 260: 37–52, 2019. doi: 10.1016/j.resp.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corcoran AE, Richerson GB, Harris MB. Functional link between the hypocretin and serotonin systems in the neural control of breathing and central chemosensitivity. J Neurophysiol 114: 381–389, 2015. doi: 10.1152/jn.00870.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining preBötzinger Complex rhythm- and pattern-generating neural microcircuits in vivo. Neuron 91: 602–614, 2016. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Angelo E Verification of a model for the mechanisms controlling expiratory duration in rabbits under various conditions. Respir Physiol 59: 239–264, 1985. doi: 10.1016/0034-5687(85)90011-8. [DOI] [PubMed] [Google Scholar]
- 29.Dahan A, Romberg R, Teppema L, Sarton E, Bijl H, Olofsen E. Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusion. Anesthesiology 101: 1201–1209, 2004. doi: 10.1097/00000542-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology 88: 903–913, 1998. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M, Niesters M. Averting opioid-induced respiratory depression without affecting anesthesia. Anesthesiology 128: 1027–1037, 2018. doi: 10.1097/ALN.0000000000002184. [DOI] [PubMed] [Google Scholar]
- 32.Damasceno RS, Takakura AC, Moreira TS. Respiratory and sympathetic chemoreflex regulation by Kölliker-Fuse neurons in rats. Pflugers Arch 467: 231–239, 2015. doi: 10.1007/s00424-014-1525-z. [DOI] [PubMed] [Google Scholar]
- 33.Dampney RA, Furlong TM, Horiuchi J, Iigaya K. Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Auton Neurosci 175: 17–25, 2013. doi: 10.1016/j.autneu.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci 19: 351–367, 2018. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhingra RR, Dutschmann M, Galán RF, Dick TE. Kölliker-Fuse nuclei regulate respiratory rhythm variability via a gain-control mechanism. Am J Physiol Regul Integr Comp Physiol 312: R172–R188, 2017. doi: 10.1152/ajpregu.00238.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol 80: 2368–2377, 1998. doi: 10.1152/jn.1998.80.5.2368. [DOI] [PubMed] [Google Scholar]
- 37.Dogas Z, Stuth EAE, Hopp FA, McCrimmon DR, Zuperku EJ. NMDA receptor-mediated transmission of carotid body chemoreceptor input to expiratory bulbospinal neurones in dogs. J Physiol 487: 639–651, 1995. doi: 10.1113/jphysiol.1995.sp020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 315: 1624–1645, 2016. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutschmann M, Herbert H. NMDA and GABAA receptors in the rat Kolliker-Fuse area control cardiorespiratory responses evoked by trigeminal ethmoidal nerve stimulation. J Physiol 510: 793–804, 1998. doi: 10.1111/j.1469-7793.1998.793bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 42.Edlow BL, McNab JA, Witzel T, Kinney HC. The structural connectome of the human central homeostatic network. Brain Connect 6: 187–200, 2016. doi: 10.1089/brain.2015.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellingsrud C, Agewall S. Morphine in the treatment of acute pulmonary oedema–Why? Int J Cardiol 202: 870–873, 2016. doi: 10.1016/j.ijcard.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Evans KC Cortico-limbic circuitry and the airways: insights from functional neuroimaging of respiratory afferents and efferents. Biol Psychol 84: 13–25, 2010. doi: 10.1016/j.biopsycho.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezure K Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol 35: 429–450, 1990. doi: 10.1016/0301-0082(90)90030-K. [DOI] [PubMed] [Google Scholar]
- 46.Faull OK, Jenkinson M, Clare S, Pattinson KT. Functional subdivision of the human periaqueductal grey in respiratory control using 7 tesla fMRI. Neuroimage 113: 356–364, 2015. doi: 10.1016/j.neuroimage.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman JL, Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J Neurophysiol 39: 31–44, 1976. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson LM, Drummond GB. Acute effects of fentanyl on breathing pattern in anaesthetized subjects. Br J Anaesth 96: 384–390, 2006. doi: 10.1093/bja/ael011. [DOI] [PubMed] [Google Scholar]
- 49.Fink BR Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol 16: 15–20, 1961. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- 50.Funk GD, Greer JJ. The rhythmic, transverse medullary slice preparation in respiratory neurobiology: contributions and caveats. Respir Physiol Neurobiol 186: 236–253, 2013. doi: 10.1016/j.resp.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Geerling JC, Yokota S, Rukhadze I, Roe D, Chamberlin NL. Kölliker-Fuse GABAergic and glutamatergic neurons project to distinct targets. J Comp Neurol 525: 1844–1860, 2017. doi: 10.1002/cne.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golder FJ, Hewitt MM, McLeod JF. Respiratory stimulant drugs in the post-operative setting. Respir Physiol Neurobiol 189: 395–402, 2013. doi: 10.1016/j.resp.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Gupta R, Haydock T. Severe hypercapnia caused by acute heroin overdose. Ann Emerg Med 43: 665–666, 2004. doi: 10.1016/j.annemergmed.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Guyenet PG Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guyenet PG, Bayliss DA, Stornetta RL, Kanbar R, Shi Y, Holloway BB, Souza GMPR, Basting TM, Abbott SBG, Wenker IC. Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J Physiol 596: 3029–3042, 2018. doi: 10.1113/JP274357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guyenet PG, Stornetta RL, Souza GMPR, Abbott SBG, Shi Y, Bayliss DA. The retrotrapezoid nucleus: central chemoreceptor and regulator of breathing automaticity. Trends Neurosci 42: 807–824, 2019. doi: 10.1016/j.tins.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haji A, Okazaki M, Ohi Y, Yamazaki H, Takeda R. Biphasic effects of morphine on bulbar respiratory neuronal activities in decerebrate cats. Neuropharmacology 45: 368–379, 2003. doi: 10.1016/S0028-3908(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 58.Haouzi P, Guck D, McCann M, Sternick M, Sonobe T, Tubbs N. Severe hypoxemia prevents spontaneous and naloxone-induced breathing recovery after fentanyl overdose in awake and sedated rats. Anesthesiology 132: 1138–1150, 2020. doi: 10.1097/ALN.0000000000003156. [DOI] [PubMed] [Google Scholar]
- 59.Haouzi P, Mellen N, McCann M, Sternick M, Guck D, Tubbs N. Evidence for the emergence of an opioid-resistant respiratory rhythm following fentanyl overdose. Respir Physiol Neurobiol 277: 103428, 2020. doi: 10.1016/j.resp.2020.103428. [DOI] [PubMed] [Google Scholar]
- 60.Hara M, Kai Y, Ikemoto Y. Enhancement by propofol of the gamma-aminobutyric acidA response in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology 81: 988–994, 1994. doi: 10.1097/00000542-199410000-00026. [DOI] [PubMed] [Google Scholar]
- 61.Hayen A, Wanigasekera V, Faull OK, Campbell SF, Garry PS, Raby SJM, Robertson J, Webster R, Wise RG, Herigstad M, Pattinson KTS. Opioid suppression of conditioned anticipatory brain responses to breathlessness. Neuroimage 150: 383–394, 2017. doi: 10.1016/j.neuroimage.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayward LF, Castellanos M, Davenport PW. Parabrachial neurons mediate dorsal periaqueductal gray evoked respiratory responses in the rat. J Appl Physiol (1985) 96: 1146–1154, 2004. doi: 10.1152/japplphysiol.00903.2003. [DOI] [PubMed] [Google Scholar]
- 63.Hess A, Yu L, Klein I, De Mazancourt M, Jebrak G, Mal H, Brugière O, Fournier M, Courbage M, Dauriat G, Schouman-Clayes E, Clerici C, Mangin L. Neural mechanisms underlying breathing complexity. PLoS One 8: e75740, 2013. doi: 10.1371/journal.pone.0075740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G. Fentanyl depression of respiration: comparison with heroin and morphine. Br J Pharmacol 177: 254–266, 2020. doi: 10.1111/bph.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Hypoxic ventilatory drive in dogs during thiopental, ketamine, or pentobarbital anesthesia. Anesthesiology 43: 628–634, 1975. doi: 10.1097/00000542-197512000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol (1985) 108: 1425–1432, 2010. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann VL, Vermeyen KM, Adriaensen HF, Meert TF. Effects of NMDA receptor antagonists on opioid-induced respiratory depression and acute antinociception in rats. Pharmacol Biochem Behav 74: 933–941, 2003. doi: 10.1016/S0091-3057(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 68.Honda H, Kawasaki Y, Baba H, Kohno T. The mu opioid receptor modulates neurotransmission in the rat spinal ventral horn. Anesth Analg 115: 703–712, 2012. doi: 10.1213/ANE.0b013e318259393d. [DOI] [PubMed] [Google Scholar]
- 69.Horiuchi J, McDowall LM, Dampney RA. Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol 587: 5149–5162, 2009. doi: 10.1113/jphysiol.2009.179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO(2) levels between sleep and wakefulness. J Physiol 534: 881–890, 2001. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huckstepp RT, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. eLife 5: e14203, 2016. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hugelin A Forebrain and midbrain influences on respiration. Compr Physiol: 2011. doi: 10.1002/cphy.cp030202. [DOI] [Google Scholar]
- 73.Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol 143: 215–233, 2004. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 74.Jolley C, Bell J, Rafferty G, Moxham J, Strang J. Understanding heroin overdose: a study of the acute respiratory depressant effects of injected pharmaceutical heroin. PLoS One 10: e0140995, 2015. doi: 10.1371/journal.pone.0140995. . A correction for this article is available at https://doi.org/10.1371/journal.pone.0143672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta K, Nagappa M, Prasad A, Abrahamyan L, Wong J, Weingarten TN, Chung F. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open 8: e024086, 2018. doi: 10.1136/bmjopen-2018-024086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci 33: 9235–9245, 2013. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kc P, Dick TE. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir Physiol Neurobiol 174: 55–64, 2010. doi: 10.1016/j.resp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirby GC, McQueen DS. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Br J Pharmacol 88: 889–898, 1986. doi: 10.1111/j.1476-5381.1986.tb16263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78a.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg 103: 63–67, 2006. doi: 10.1213/01.ANE.0000219592.82598.AA. [DOI] [PubMed] [Google Scholar]
- 79.Krolo M, Stuth EA, Tonkovic-Capin M, Dogas Z, Hopp FA, McCrimmon DR, Zuperku EJ. Differential roles of ionotropic glutamate receptors in canine medullary inspiratory neurons of the ventral respiratory group. J Neurophysiol 82: 60–68, 1999. doi: 10.1152/jn.1999.82.1.60. [DOI] [PubMed] [Google Scholar]
- 80.Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Relative magnitude of tonic and phasic synaptic excitation of medullary inspiratory neurons in dogs. Am J Physiol Regul Integr Comp Physiol 279: R639–R649, 2000. doi: 10.1152/ajpregu.2000.279.2.R639. [DOI] [PubMed] [Google Scholar]
- 81.Lai YL, Tsuya Y, Hildebrandt J. Ventilatory responses to acute CO2 exposure in the rat. J Appl Physiol 45: 611–618, 1978. doi: 10.1152/jappl.1978.45.4.611. [DOI] [PubMed] [Google Scholar]
- 82.Lalley PM D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regul Integr Comp Physiol 289: R45–R51, 2005. doi: 10.1152/ajpregu.00868.2004. [DOI] [PubMed] [Google Scholar]
- 83.Lalley PM Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285: R1287–R1304, 2003. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- 84.Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, Domino KB. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 122: 659–665, 2015. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 85.Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT. μ Opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J Physiol 593: 4453–4469, 2015. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levitt ES, Williams JT. Morphine desensitization and cellular tolerance are distinguished in rat locus ceruleus neurons. Mol Pharmacol 82: 983–992, 2012. doi: 10.1124/mol.112.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577: 307–318, 2006. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindsey BG, Nuding SC, Segers LS, Morris KF. Carotid bodies and the integrated cardiorespiratory response to hypoxia. Physiology (Bethesda) 33: 281–297, 2018. doi: 10.1152/physiol.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ling L, Karius DR, Speck DF. Role of N-methyl-D-aspartate receptors in the pontine pneumotaxic mechanism in the cat. J Appl Physiol (1985) 76: 1138–1143, 1994. doi: 10.1152/jappl.1994.76.3.1138. [DOI] [PubMed] [Google Scholar]
- 90.Lötsch J, Dudziak R, Freynhagen R, Marschner J, Geisslinger G. Fatal respiratory depression after multiple intravenous morphine injections. Clin Pharmacokinet 45: 1051–1060, 2006. doi: 10.2165/00003088-200645110-00001. [DOI] [PubMed] [Google Scholar]
- 91.Lovering AT, Dunin-Barkowski WL, Vidruk EH, Orem JM. Ventilatory response of the cat to hypoxia in sleep and wakefulness. J Appl Physiol (1985) 95: 545–554, 2003. doi: 10.1152/japplphysiol.01051.2002. [DOI] [PubMed] [Google Scholar]
- 92.Luginbühl M, Schumacher PM, Vuilleumier P, Vereecke H, Heyse B, Bouillon TW, Struys MM. Noxious stimulation response index: a novel anesthetic state index based on hypnotic-opioid interaction. Anesthesiology 112: 872–880, 2010. doi: 10.1097/ALN.0b013e3181d40368. [DOI] [PubMed] [Google Scholar]
- 93.MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol 432: 483–508, 1991. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mantyh PW Connections of midbrain periaqueductal gray in the monkey. II. Descending efferent projections. J Neurophysiol 49: 582–594, 1983. doi: 10.1152/jn.1983.49.3.582. [DOI] [PubMed] [Google Scholar]
- 95.Mantyh PW Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J Neurophysiol 49: 567–581, 1983. doi: 10.1152/jn.1983.49.3.567. [DOI] [PubMed] [Google Scholar]
- 96.Mantyh PW Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol 206: 146–158, 1982. doi: 10.1002/cne.902060205. [DOI] [PubMed] [Google Scholar]
- 97.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226–229, 2003. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 98.Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibitions within pre-Bötzinger and Bötzinger complexes. eNeuro 3: ENEURO.0011-16.2016, 2016. doi: 10.1523/ENEURO.0011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Masuda A, Ohyabu Y, Kobayashi T, Yoshino C, Sakakibara Y, Komatsu T, Honda Y. Lack of positive interaction between CO2 and hypoxic stimulation for P(CO2)-VAS response slope in humans. Respir Physiol 126: 173–181, 2001. doi: 10.1016/S0034-5687(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 100.McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CFL, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol 110: 161–176, 1997. doi: 10.1016/S0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 101.McKay LC, Critchley HD, Murphy K, Frackowiak RS, Corfield DR. Sub-cortical and brainstem sites associated with chemo-stimulated increases in ventilation in humans. Neuroimage 49: 2526–2535, 2010. doi: 10.1016/j.neuroimage.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003. doi: 10.1016/S0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, Stucke AG. A subregion of the parabrachial nucleus partially mediates respiratory rate depression from intravenous remifentanil in young and adult rabbits. Anesthesiology 127: 502–514, 2017. doi: 10.1097/ALN.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molkov YI, Rubin JE, Rybak IA, Smith JC. Computational models of the neural control of breathing. Wiley Interdiscip Rev Syst Biol Med 9: e1371, 2017. doi: 10.1002/wsbm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montandon G, Cushing SL, Campbell F, Propst EJ, Horner RL, Narang I. Distinct cortical signatures associated with sedation and respiratory rate depression by morphine in a pediatric population. Anesthesiology 125: 889–903, 2016. doi: 10.1097/ALN.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 106.Montandon G, Horner RL. Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep 9: 14122, 2019. doi: 10.1038/s41598-019-50613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31: 1292–1301, 2011. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124: 641–650, 2016. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2006. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mortola JP How to breathe? Respiratory mechanics and breathing pattern. Respir Physiol Neurobiol 261: 48–54, 2019. doi: 10.1016/j.resp.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 111.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 112.Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol 103: 409–418, 2010. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mutolo D, Bongianni F, Nardone F, Pantaleo T. Respiratory responses evoked by blockades of ionotropic glutamate receptors within the Bötzinger complex and the pre-Bötzinger complex of the rabbit. Eur J Neurosci 21: 122–134, 2005. doi: 10.1111/j.1460-9568.2004.03850.x. [DOI] [PubMed] [Google Scholar]
- 114.Nattie E Julius H. Comroe, Jr., distinguished lecture: central chemoreception: then...and now. J Appl Physiol (1985) 110: 1–8, 2011. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Navarrete-Opazo AA, Cook-Snyder DR, Miller JR, Callison JJ, McCarthy N, Palkovic B, Stuth EAE, Zuperku EJ, Stucke AG. Endogenous glutamatergic inputs to the Parabrachial Nucleus/Kölliker-Fuse Complex determine respiratory rate. Respir Physiol Neurobiol 277: 103401, 2020. doi: 10.1016/j.resp.2020.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Navarrete-Opazo AA, Cook-Snyder DR, Miller JR, Callison JJ, McCarthy N, Palkovic B, Stuth EAE, Zuperku EJ, Stucke AG. Endogenous glutamatergic inputs to the Parabrachial Nucleus/Kölliker-Fuse Complex determine respiratory rate. Respir Physiol Neurobiol 277: 103401, 2020. doi: 10.1016/j.resp.2020.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116a.Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA, Lötsch J. Selective antagonism of opioid-induced ventilatory depression by an Ampakine molecule in humans without loss of opioid analgesia. Clin Pharmacol Ther 87: 204–211, 2010. doi: 10.1038/clpt.2009.194. [DOI] [PubMed] [Google Scholar]
- 117.Orem J, Kubin L. Respiratory physiology: central neural control. In: Principles and Practice of Sleep Medicine, edited by Kryger M, Roth T, Dement W. Philadelphia, PA: Saunders, 2000, p. 205–220. [Google Scholar]
- 118.Orem J, Lovering AT, Dunin-Barkowski W, Vidruk EH. Endogenous excitatory drive to the respiratory system in rapid eye movement sleep in cats. J Physiol 527: 365–376, 2000. doi: 10.1111/j.1469-7793.2000.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orser BA, Bertlik M, Wang LY, MacDonald JF. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol 116: 1761–1768, 1995. doi: 10.1111/j.1476-5381.1995.tb16660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orser BA, Pennefather PS, MacDonald JF. Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology 86: 903–917, 1997. doi: 10.1097/00000542-199704000-00021. [DOI] [PubMed] [Google Scholar]
- 121.Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology 103: 779–787, 2005. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 122.Overdyk FJ, Dowling O, Marino J, Qiu J, Chien HL, Erslon M, Morrison N, Harrison B, Dahan A, Gan TJ. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One 11: e0150214, 2016. doi: 10.1371/journal.pone.0150214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Palkovic B, Callison J, Marchenko V, Stuth E, Zuperku E, Stucke A. Effects of different systemic opioid doses on subareas of the ventral respiratory column. FASEB J 34, Suppl 1: 1, 2020. doi: 10.1096/fasebj.2020.34.s1.02201. [DOI] [Google Scholar]
- 124.Pamenter ME, Nguyen J, Carr JA, Powell FL. The effect of combined glutamate receptor blockade in the NTS on the hypoxic ventilatory response in awake rats differs from the effect of individual glutamate receptor blockade. Physiol Rep 2: e12092, 2014. doi: 10.14814/phy2.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci 29: 8177–8186, 2009. doi: 10.1523/JNEUROSCI.1375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, Rogers R, Tracey I, Wise RG. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage 44: 295–305, 2009. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 127.Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol 108: 2430–2441, 2012. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO(2) chemosensitivity in conditional Phox2b mutants. J Neurosci 31: 12880–12888, 2011. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramirez JM, Baertsch N. Defining the rhythmogenic elements of mammalian breathing. Physiology (Bethesda) 33: 302–316, 2018. doi: 10.1152/physiol.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110: 1364–1370, 2009. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- 132.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 29: 58–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rikard-Bell GC, Bystrzycka EK, Nail BS. Cells of origin of corticospinal projections to phrenic and thoracic respiratory motoneurones in the cat as shown by retrograde transport of HRP. Brain Res Bull 14: 39–47, 1985. doi: 10.1016/0361-9230(85)90175-3. [DOI] [PubMed] [Google Scholar]
- 134.Robinson D, Ellenberger H. Distribution of N-methyl-d-aspartate and non-N-methyl- d-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol 389: 94–116, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 135.Romberg R, Olofsen E, Sarton E, den Hartigh J, Taschner PE, Dahan A. Pharmacokinetic-pharmacodynamic modeling of morphine-6-glucuronide-induced analgesia in healthy volunteers: absence of sex differences. Anesthesiology 100: 120–133, 2004. doi: 10.1097/00000542-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 136.Roozekrans M, van der Schrier R, Okkerse P, Hay J, McLeod JF, Dahan A. Two studies on reversal of opioid-induced respiratory depression by BK-channel blocker GAL021 in human volunteers. Anesthesiology 121: 459–468, 2014. doi: 10.1097/ALN.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 137.Saunders SE, Levitt ES. Kölliker-Fuse/Parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Respir Physiol Neurobiol 275: 103388, 2020. doi: 10.1016/j.resp.2020.103388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schenberg LC, De Aguiar JC, Graeff FG. GABA modulation of the defense reaction induced by brain electrical stimulation. Physiol Behav 31: 429–437, 1983. doi: 10.1016/0031-9384(83)90062-8. [DOI] [PubMed] [Google Scholar]
- 139.Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134: 24–35, 2011. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- 140.Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- 141.Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Silva JN, Lucena EV, Silva TM, Damasceno RS, Takakura AC, Moreira TS. Inhibition of the pontine Kölliker-Fuse nucleus reduces genioglossal activity elicited by stimulation of the retrotrapezoid chemoreceptor neurons. Neuroscience 328: 9–21, 2016. doi: 10.1016/j.neuroscience.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 143.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Souza GMPR, Kanbar R, Stornetta DS, Abbott SBG, Stornetta RL, Guyenet PG. Breathing regulation and blood gas homeostasis after near complete lesions of the retrotrapezoid nucleus in adult rats. J Physiol 596: 2521–2545, 2018. doi: 10.1113/JP275866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stucke AG, Miller JR, Prkic I, Zuperku EJ, Hopp FA, Stuth EA. Opioid-induced respiratory depression is only partially mediated by the preBötzinger complex in young and adult rabbits in vivo. Anesthesiology 122: 1288–1298, 2015. doi: 10.1097/ALN.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stucke AG, Stuth EAE, Tonkovic-Capin V, Tonkovic-Capin M, Hopp FA, Kampine JP, Zuperku EJ. Effects of halothane and sevoflurane on inhibitory neurotransmission to medullary expiratory neurons in a decerebrate dog model. Anesthesiology 96: 955–962, 2002. doi: 10.1097/00000542-200204000-00025. [DOI] [PubMed] [Google Scholar]
- 147.Stucke AG, Stuth EAE, Tonkovic-Capin V, Tonkovic-Capin M, Hopp FA, Kampine JP, Zuperku EJ. Effects of sevoflurane on excitatory neurotransmission to medullary expiratory neurons and on phrenic nerve activity in a decerebrate dog model. Anesthesiology 95: 485–491, 2001. doi: 10.1097/00000542-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 148.Stucke AG, Zuperku EJ, Krolo M, Brandes IF, Hopp FA, Kampine JP, Stuth EA. Sevoflurane enhances gamma-aminobutyric acid type A receptor function and overall inhibition of inspiratory premotor neurons in a decerebrate dog model. Anesthesiology 103: 57–64, 2005. doi: 10.1097/00000542-200507000-00012. [DOI] [PubMed] [Google Scholar]
- 149.Stucke AG, Zuperku EJ, Sanchez A, Tonkovic-Capin M, Tonkovic-Capin V, Mustapic S, Stuth EA. Opioid receptors on bulbospinal respiratory neurons are not activated during neuronal depression by clinically relevant opioid concentrations. J Neurophysiol 100: 2878–2888, 2008. doi: 10.1152/jn.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stuth EA, Stucke AG, Zuperku EJ. Effects of anesthetics, sedatives, and opioids on ventilatory control. Compr Physiol 2: 2281–2367, 2012. doi: 10.1002/cphy.c100061. [DOI] [PubMed] [Google Scholar]
- 151.Subramanian HH Descending control of the respiratory neuronal network by the midbrain periaqueductal grey in the rat in vivo. J Physiol 591: 109–122, 2013. doi: 10.1113/jphysiol.2012.245217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci 28: 12274–12283, 2008. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Subramanian HH, Holstege G. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog Brain Res 212: 351–384, 2014. doi: 10.1016/B978-0-444-63488-7.00017-3. [DOI] [PubMed] [Google Scholar]
- 154.Supple WF Jr, Kapp BS. Anatomical and physiological relationships between the anterior cerebellar vermis and the pontine parabrachial nucleus in the rabbit. Brain Res Bull 33: 561–574, 1994. doi: 10.1016/0361-9230(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 155.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 586: 2975–2991, 2008. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takeda S, Eriksson LI, Yamamoto Y, Joensen H, Onimaru H, Lindahl SGE. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology 95: 740–749, 2001. doi: 10.1097/00000542-200109000-00029. [DOI] [PubMed] [Google Scholar]
- 158.Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 294: R477–R486, 2008. doi: 10.1152/ajpregu.00633.2007. [DOI] [PubMed] [Google Scholar]
- 159.Tsushima H, Mori M, Matsuda T. Effects of fentanyl, injected into the hypothalamic supraoptic and paraventricular nuclei, in a water-loaded and ethanol-anesthetized rat. Neuropharmacology 29: 757–763, 1990. doi: 10.1016/0028-3908(90)90129-F. [DOI] [PubMed] [Google Scholar]
- 160.Varga AG, Reid BT, Kieffer BL, Levitt ES. Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice. J Physiol 598: 189–205, 2020. doi: 10.1113/JP278612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H; ATS Congenital Central Hypoventilation Syndrome Subcommittee . An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 181: 626–644, 2010. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- 162.Wu Y, Proch KL, Teran FA, Lechtenberg RJ, Kothari H, Richerson GB. Chemosensitivity of Phox2b-expressing retrotrapezoid neurons is mediated in part by input from 5-HT neurons. J Physiol 597: 2741–2766, 2019. doi: 10.1113/JP277052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum 1: 35–40, 2002. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- 164.Xu F, Zhou T, Gibson T, Frazier DT. Fastigial nucleus-mediated respiratory responses depend on the medullary gigantocellular nucleus. J Appl Physiol (1985) 91: 1713–1722, 2001. doi: 10.1152/jappl.2001.91.4.1713. [DOI] [PubMed] [Google Scholar]
- 165.Yang C, Xi J, Sheikh S, Hakim A, Nadella S, Dhital S, Meier M, Nnanna O, Meyers D. A scoring system for prediction and risk stratification of CO2 narcosis. Emerg Med 8: 368, 2018. doi: 10.4172/2165-7548.1000368. [DOI] [Google Scholar]
- 166.Yu L, De Mazancourt M, Hess A, Ashadi FR, Klein I, Mal H, Courbage M, Mangin L. Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Hum Brain Mapp 37: 2736–2754, 2016. doi: 10.1002/hbm.23205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang M, Clarke K, Zhong H, Vollmer C, Nurse CA. Postsynaptic action of GABA in modulating sensory transmission in co-cultures of rat carotid body via GABA(A) receptors. J Physiol 587: 329–344, 2009. doi: 10.1113/jphysiol.2008.165035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107: 288–297, 2007. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- 169.Zhang Z, Zhuang J, Zhang C, Xu F. Activation of opioid μ-receptors in the commissural subdivision of the nucleus tractus solitarius abolishes the ventilatory response to hypoxia in anesthetized rats. Anesthesiology 115: 353–363, 2011. doi: 10.1097/ALN.0b013e318224cc1f. [DOI] [PubMed] [Google Scholar]
- 170.Zoorob M Fentanyl shock: the changing geography of overdose in the United States. Int J Drug Policy 70: 40–46, 2019. doi: 10.1016/j.drugpo.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 171.Zuperku EJ, Stucke AG, Hopp FA, Stuth EA. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. J Neurophysiol 117: 1030–1042, 2017. doi: 10.1152/jn.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zuperku EJ, Stucke AG, Krolikowski JG, Tomlinson J, Hopp FA, Stuth EA. Inputs to medullary respiratory neurons from a pontine subregion that controls breathing frequency. Respir Physiol Neurobiol 265: 127–140, 2019. doi: 10.1016/j.resp.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zuperku EJ, Hopp FA, Kampine JP. Central integration of pulmonary stretch receptor input in the control of expiration. J Appl Physiol 52: 1296–1315, 1982. doi: 10.1152/jappl.1982.52.5.1296. [DOI] [PubMed] [Google Scholar]