SUMMARY
Background
We sought to determine whether a community-based intervention to identify and rapidly treat persons living with HIV (PLWH) and support male circumcision (MC) could increase population levels of HIV diagnosis, treatment, viral suppression, and MC in Botswana.
Methods
The Ya Tsie study was a pair-matched community-randomized trial conducted in 30 communities across Botswana from 2013–2018; 15 communities were randomized to receive HIV prevention and treatment interventions (including enhanced HIV testing, earlier antiretroviral treatment [ART], and strengthened MC services) and 15 received standard-of-care. We enrolled a cohort of residents aged 16–64 years from a random ~20% sample of households to assess baseline uptake of the following outcomes: (1) proportion known to be HIV-positive or tested HIV-negative in the preceding 12 months; (2) proportion of PLWH diagnosed and on ART; (3) proportion of PLWH on ART with viral suppression; and (4) proportion of HIV-negative men circumcised. In six communities all residents not previously enrolled in the longitudinal cohort completed an end-of-study survey to provide study-end coverage estimates. Differences in intervention uptake over time by arm were tested via paired student’s t-test.
Findings
At baseline we enrolled 2,625 (n=805 PLWH; 403/402 in standard-of-care/intervention communities) residents from the six communities also participating in the end-of-study survey. An additional 10,791 (n=2,691 PLWH; 1,338/1,353 in standard-of-care/intervention communities) residents completed an end-of-study survey in these communities. After accounting for baseline differences, at study-end, the proportion of PLWH who were diagnosed was significantly higher in the intervention arm (absolute increase of 9% to 93% [n=1,254 of 1,353]) compared to standard-of-care (absolute increase of 2% to 88% [n=1,184 of 1,338]) (P=0·03). Population levels of ART, viral suppression, and MC increased from baseline in both arms, with greater increases in intervention communities (ART P=0·02; viral suppression P=0·02; MC P=0·03). At study-end, in intervention communities, 1,228 PLWH (91% of n=1,353) were on ART; 1,166 PLWH (88% of n=1,321 with available viral load) were virally suppressed, and 673 HIV-negative men (40% of n=1,673) were circumcised in intervention communities. The study (NCT01965470) has been completed.
Interpretation
It is possible to achieve very high population levels of HIV testing and treatment in a high-prevalence setting. Maintaining these coverage levels over the next decade could substantially reduce HIV transmission and potentially eliminate the epidemic in these areas.
Funding
US President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention.
Early initiation of antiretroviral therapy (ART) in persons living with HIV (PLWH) can reduce transmission to sexual partners by at least 96%.1–4 Additional, compelling evidence from two randomized clinical trials demonstrated that starting ART at CD4 cell counts >500/μL improves health outcomes, including survival.5,6 In 2015, based on these reports and mounting observational evidence,7,8 the World Health Organization (WHO) recommended ART for all PLWH regardless of CD4 cell count or clinical stage.9 At the same time, the Joint United Nations Programme on HIV/AIDS (UNAIDS) announced ambitious HIV testing and treatment targets aimed at disrupting HIV transmission by substantially increasing viral suppression levels among PLWH.10 Specifically, the strategy aims to have 90% of PLWH diagnosed; 90% of persons diagnosed on treatment; and 90% of persons on treatment virally suppressed for a combined overall target of 73% (i.e. 90%×90%×90%) of all PLWH on treatment and virally suppressed. By 2030, the targets increase to 95% each, resulting in a combined overall target of 86% virally suppressed (i.e. 95%×95%×95%). However, it is unknown whether it is feasible to increase HIV diagnosis levels to ≥90%, nor whether PLWH with relatively high CD4 cell counts will be motivated to initiate and adhere to ART without noticeable declines in health or quality of life. Additionally, initiating and maintaining large numbers of persons on treatment may be difficult in remote settings where clinic staffing and medication/laboratory testing supply may be unreliable and individuals may be more mobile in search of economic opportunity.
Botswana has a generalized, predominantly heterosexually transmitted, HIV epidemic in which one in five adults are living with HIV.11 In 2002, Botswana became the first African country to provide ART free-of-charge to clinically eligible citizens living with HIV. In 2009 the country rolled out male circumcision (MC) services for adult men nationwide (adding routine neonatal MC in 2013), and most recently, in 2016, adopted universal treatment irrespective of CD4 cell count or clinical staging. Despite these efforts, Botswana remained one of only three countries worldwide with an estimated annual HIV incidence >1% in 2016.12
We sought to determine whether (and to what extent) a community-based intervention aimed at identifying and rapidly treating PLWH and supporting MC could increase population levels of HIV diagnosis, treatment, viral suppression, and adult MC in Botswana.
METHODS
Study design
The “Ya Tsie” study, also known as the Botswana Combination Prevention Project (BCPP), was a pair-matched community-randomized trial conducted in 30 rural and peri-urban communities across Botswana with a total population of 180,000 people.13,14 Communities were matched according to size, age structure, access to health services, and proximity to major urban centers and then randomized within pairs to the intervention or standard-of-care arms (15 communities/arm).
Interventions in the 15 intervention communities included the following (after an initial period of community mobilization [public loudspeaker announcements, early and frequent community leadership engagement, and door-to-door canvassing]): (a) home-based and mobile HIV testing campaigns (approximately two consecutive months per community implemented during first two years) aimed at finding all undiagnosed and/or untreated PLWH, strengthened routine HIV testing in health facilities, and subsequent targeted outreach for men and youth (including HIV testing at workplaces, bars, cattle posts/farms, community events, and multi-disease health fairs); (b) active linkage to care at local clinics for PLWH not on ART (including appointment scheduling within one week, text reminders prior to appointment, and active tracing for missed appointment); (c) expanded ART (beyond national guidelines) for persons with HIV-1 ribonucleic acid (RNA) ≥10,000 copies/mL (if CD4>350 cells/μL) until August 2015; from August 2015–May 2016, ART for PLWH with CD4<500 cells/mm3 and for those with CD4>500 cells/μL if HIV-1 RNA≥10,000 copies/mL; and, starting June 2016, universal ART initiated at first clinic visit; and (d) strengthened MC services (mobilization campaigns, mobile clinics, and peer linkage with scheduled appointments, reminders, and transport). Community residents aged 16–64 years who were Botswana citizens were eligible for the interventions free of charge (non-citizens were eligible for HIV testing).
In standard-of-care communities, PLWH with CD4<350 cells/μL, WHO HIV/AIDS clinical stage III/IV disease, or pregnancy/breastfeeding were eligible for ART from study initiation until June 2016, when universal ART became standard in both arms. The above-described enhanced HIV testing, active linkage-to-care, and MC services were not implemented in standard-of-care communities.
Outcomes
The study had three primary objectives: (1) To determine whether implementation of a combination prevention package can significantly reduce population-level HIV incidence in 16–64-year-old residents in Botswana over a period of approximately 30 months; (2) To estimate population-level uptake of HIV testing, ART, viral suppression and MC services and compare uptake between standard-of-care and intervention communities at baseline and study-end; (3) To estimate the cost per additional infection averted in intervention communities compared with standard-of-care communities. The HIV incidence findings were previously presented elsewhere.15 Cost-effectiveness analyses are underway.
The current manuscript presents the results for the second primary objective. The following outcomes were used to evaluate this objective:
Proportion of community residents aged 16–64 years who report knowing that they are HIV-positive, or report testing HIV-negative in the preceding 12 months;
Proportion of HIV-positive community residents aged 16–64 years who know they are HIV-positive and are receiving ART;
Proportion of HIV-positive community residents aged 16–64 years who know they are HIV-positive, are receiving ART, and have HIV-1 RNA ≤ 400 copies/mL;
Proportion of HIV-negative male community residents aged 16–49 years who are circumcised; and
Proportion of eligible, HIV-positive pregnant female community residents who remained in care and on treatment 12 months post-delivery after initiating ART for Option B+.
The study (NCT01965470) was approved by IRBs at the Botswana Ministry of Health and Wellness and the United States Centers for Disease Control and Prevention. The study protocol is provided in the Appendix.
Participants
We assessed intervention uptake by enrolling two groups of participants: (1) longitudinal cohort comprising a random sample of residents in all 30 communities and; (2) cross-sectional end-of-study community-wide survey in the remaining 80% of residents who did not participate in the longitudinal cohort in six communities. Participants provided written informed consent. Participants aged 16–17 years provided written assent (with parent/guardian written permission). The end-of-study survey communities were purposively selected according to region (one matched pair each in the southeast, central, and northern regions) but without knowledge of HIV incidence or intervention uptake. The end-of-study survey provided the primary assessment of by-arm uptake of intervention services for two reasons: (1) because all participants in the longitudinal cohort underwent HIV testing, we could not compare HIV testing uptake over time in the cohort alone and; (2) repeated contact between study staff and cohort members was expected to affect participant behavior (e.g. by increasing their likelihood of linking to care).
Procedures
Between October 30, 2013 and November 24, 2015, participants were identified and recruited into the longitudinal cohort using a household-based, probabilistic-sampling strategy. Within each of the 30 communities taking part in BCPP, a simple random ~20% sample of plots containing household-like structures was selected using satellite imagery captured between 2012 and 2015 (Google Earth, Mountain View, CA).16 In every household situated on selected plots, a household representative (aged ≥18 years) was asked to list all household members and each potentially eligible member was invited to participate in the study. Eligibility criteria included being 16–64 years of age, spending on average ≥3 nights/month in the household, providing documentation of Botswana citizenship or marriage to a citizen, and providing informed consent (or assent if aged 16–17 years). Households were visited up to three times for enumeration; up to three additional visits were made to enroll each potentially eligible enumerated household member. At study-end, for each of the six communities participating in the end-of-study survey, plot lists were updated with the most recently-captured satellite images available, thus allowing for the identification and inclusion of any newly built homes (and their inhabitants).
Figure 1 summarizes the frequency and timing of data collection activities to assess uptake of intervention services in the two survey groups. All longitudinal cohort participants completed a structured questionnaire on socio-demographics, co-morbid conditions/illnesses, access to and receipt of healthcare, and HIV-related risk behaviors. At each study visit, information about HIV testing history (including date, location, and result) was collected and participants without documented HIV-positive status (e.g., written test result, documented ART) were offered counseling and parallel HIV rapid tests (KHB, Shanghai Kehua Bio-Engineering Co Ltd, Shanghai, China; and Unigold, Trinity Biotech Plc, Bray, Ireland). HIV-1 RNA was tested in all PLWH at the baseline and final visits, irrespective of ART use (Abbott RealTime HIV-1 Assay, Wiesbaden, Germany). Viral suppression was defined as HIV-1 RNA≤400 copies/mL. Point-of-care CD4 count (Pima, Alere Inc, Waltham, MA, USA) was obtained on PLWH not on ART. Documentation of ART (e.g., prescriptions or pills, clinical notes) was required for classifying a participant as on ART. Circumcision status was self-reported and did not distinguish between medical and traditional circumcision. Cohort participants were re-contacted at approximately 12- and 29-months post-baseline (Figure 1); HIV-negative participants underwent repeat HIV testing at all visits.
Figure 1.
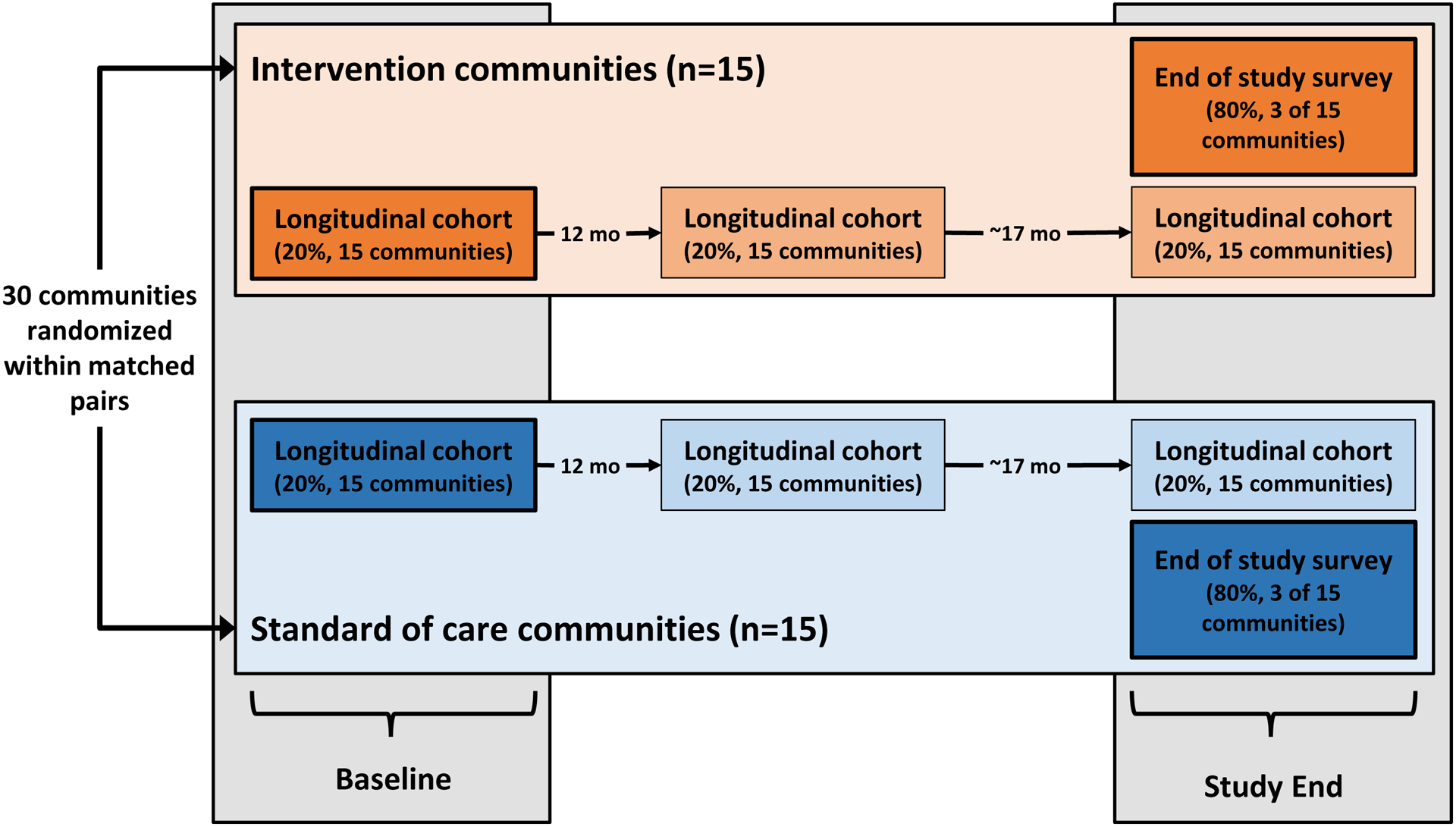
Study schema summarizing data collection activities related to assessment of intervention coverage at baseline and study end according to randomization arm in the Ya Tsie study.
At the same time as the final cohort visit, all community residents not previously enrolled in the longitudinal cohort (i.e. remaining ~80%) from six communities (three pairs, one per geographic region) were asked to participate in a one-time end-of-study survey to assess intervention uptake by arm (Figure 1). We carried out the same study procedures among the end-of-study survey participants as were performed with the longitudinal cohort, including in-home HIV rapid testing, point-of-care CD4 count, HIV-1 RNA testing, and administration of a structured questionnaire.
Statistical analyses
All pre-specified analyses were performed per the protocol and statistical analysis plan using SAS Version 9·4 (SAS Institute, Cary, NC, USA). Uptake of services was reported in terms of frequency and percentages, separately for intervention and standard-of-care communities at baseline and study-end. Baseline coverage for the primary analysis came from the longitudinal cohort in the six communities participating in the end-of-study survey. Intervention uptake at study-end was obtained from the ~80% of residents participating in the end-of-study survey in the same six communities (Figure 1; dark orange and blue boxes at study-end). We also report on change in population levels of ART, viral suppression, and MC by arm over time within the longitudinal cohort only (Figure 1; light orange and blue boxes at study-end), noting that viral loads were not universally obtained (per protocol at the time) in the first two community pairs. Population uptake of interventions at baseline and study-end were assessed as follows:
% HIV-tested or diagnosed as HIV-positive = (HIV-negative participants with documented negative test result within past 12 months or participating PLWH with knowledge of positive status) / (Enrolled participants)
% diagnosed HIV-positive = (HIV-positive participants with knowledge of positive status) / (all participating PLWH)
% on treatment = (participating PLWH with knowledge of positive status receiving ART) / (all participating PLWH)
% virally suppressed (i.e. combined overall UNAIDS target) = (participating PLWH with knowledge of positive status receiving ART with HIV-1 RNA ≤400 copies per mL) / (all participating PLWH)
% circumcised = (HIV-negative male participants aged 16–49 years self-reported to be circumcised) / (HIV-negative male participants aged 16–49 years)
The number of communities participating in the end-of-study survey and the corresponding power to detect differences in intervention uptake at baseline and study-end was determined using formulae from Hayes and Moulton,17 appropriate for a pair-matched cluster randomized trial. In the standard-of-care arm, the anticipated coverage at study-end for % HIV-tested or diagnosed HIV-positive, % on treatment, % virally suppressed, and % circumcised was 48%, 80%, 76% and 50%, respectively. In the intervention arm, anticipated coverage for each of these indicators was 90%, 93%, 95% and 60%, respectively. The intervention arm coverage values used for power and sample size calculations reflected targets set by the implementation team as clinically meaningful. Assuming a coefficient of variation of 0·1 for all coverage indicators, power was estimated to be ≥96% to detect these differences at study-end based on a sample size of 5,931 per arm.
The null hypothesis of no difference in change in uptake of intervention services according to randomization arm was tested via a paired student’s T test with statistic defined as the inverse-variance weighted average of the pair-specific difference (i.e. between standard-of-care and intervention) in prevalence differences (i.e. between baseline and study end). P-values were two-sided and based on cluster-level summary data as the preferred approach for analyses of cluster-randomized trials with small numbers of clusters.17 Individual-level data was used to estimate prevalence ratios (PR) and 95% confidence intervals (CI) from log-linear Poisson regression models with fixed effects for matched pair, time, and matched pair-by-time interaction. All analyses considered the pattern of change in coverage over time and whether that pattern of change differed according to randomization arm. Separate analyses were undertaken for each of the five indicators of population uptake of intervention services.
In post-hoc analyses, we examined potential heterogeneity in the intervention effect according to sex and age (16–24 and 25–64 years) in the same above-described study populations using log-linear Poisson regression with fixed effects for matched pair, time, randomization arm, and their interactions. Because the statistical analysis plan did not include corrections for multiplicity when analyzing post-hoc outcomes, we report these as point estimates and 95% CIs. CI widths were not adjusted for multiplicity, so intervals should not be used to infer definitive subgroup treatment effects. Our pre-specified endpoints for HIV diagnosis, treatment, and viral suppression used a denominator of all PLWH. In a post-hoc analysis, we re-calculated coverage at baseline and study end by randomized arm using UNAIDS “95-95-95” denominators:
“First 95”: % diagnosed PLWH = (PLWH with knowledge of positive status) / (all participating PLWH)
“Second 95”: % on treatment = (participating PLWH with knowledge of positive status currently receiving ART) / (participating PLWH with knowledge of positive status)
“Third 95”: % virally suppressed = (participating PLWH with knowledge of positive status receiving ART who have HIV-1 RNA ≤400 copies/mL) / (participating PLWH with knowledge of positive status receiving ART)
Role of the funding source
The funders played no role in study design; in the collection, analysis, or interpretation of data; in the writing of this report; or in the decision to submit it for publication. The corresponding author, K. Wirth, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
At baseline, in the six communities participating in the end-of-study-survey, a total of 2,625 residents (n=1,304 and n=1,321 from standard-of-care and intervention communities, respectively) enrolled in the 20% longitudinal cohort (Appendix Figure S1) from January 19, 2015, to September 8, 2015. In these same communities, 86% (n=10,791) of 12,489 eligible enumerated residents not previously enrolled in the longitudinal cohort participated in the end-of-study survey from March 30, 2017, to February 25, 2018 (Figure 2; 93% of eligible enumerated residents in standard-of-care and 82% in intervention communities).
Figure 2.
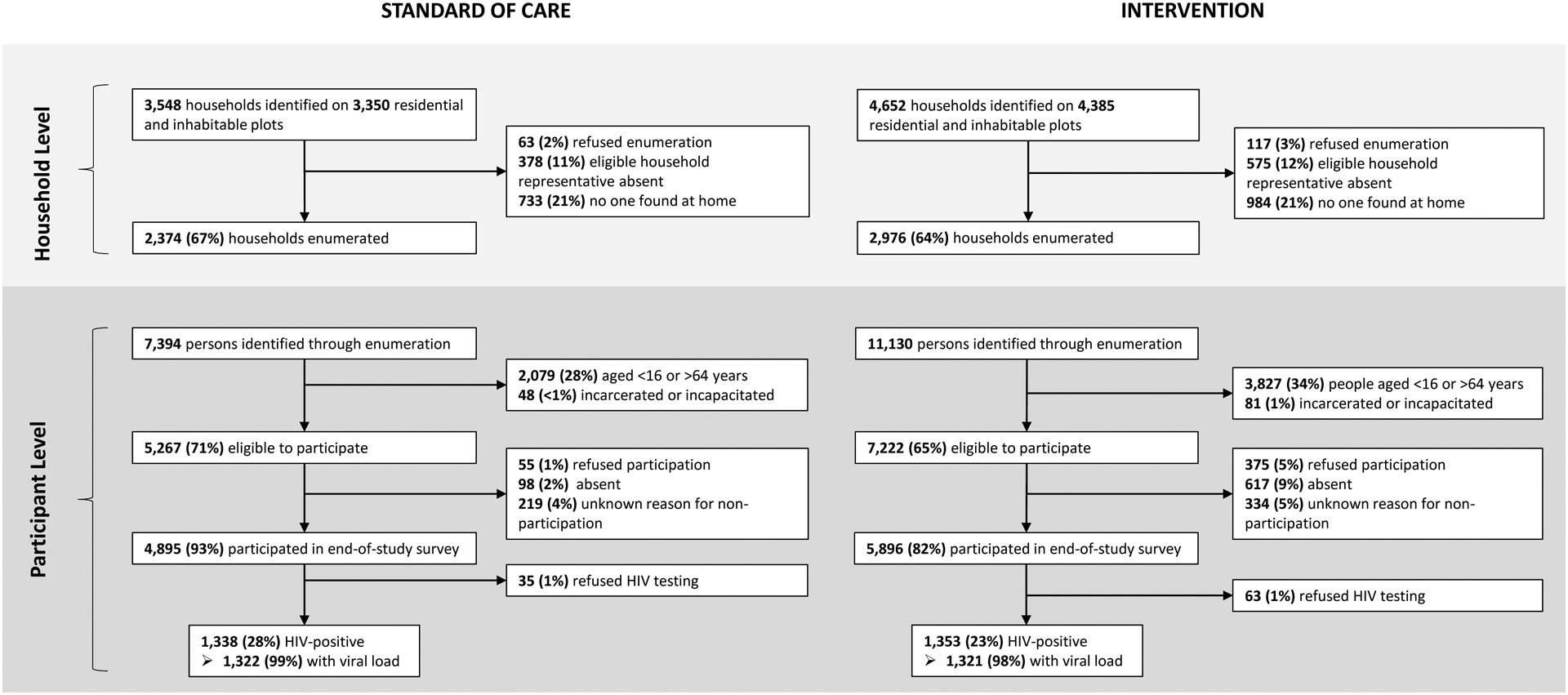
Recruitment, eligibility, and enrollment of participants for the one-time, end-of-study survey in six communities in the Ya Tsie study at the household and participant level by randomization arm.
Table 1 provides enrollment characteristics of community residents participating in the longitudinal cohort or end-of-study survey in the six communities by randomization arm. Among cohort and end-of-study survey participants, 35% and 38% were male (respectively). Median age was 33 years. Very few (<1%) participants refused in-home HIV testing and HIV-1 RNA was obtained on >98% of PLWH (Figure 2).
Table 1.
Summary of enrollment characteristics of community residents participating in either the longitudinal cohort or the end-of-study survey in six communities for the Ya Tsie study according to randomization arm.
| Longitudinal cohort | End-of-study survey | |||||
|---|---|---|---|---|---|---|
| Characteristic | Overall (n=2,625) |
Standard of care (n=1,304) |
Intervention (n=1,321) |
Overall (n=10,791)a |
Standard of care (n=4,895) |
Intervention (n=5,896)a |
| Age at enrollment | ||||||
| 16 to 24 years | 662 (25%) | 332 (25%) | 330 (25%) | 3,017 (28%) | 1,381 (28%) | 1,636 (28%) |
| 25 to 34 years | 780 (30%) | 397 (30%) | 383 (29%) | 3,034 (28%) | 1,352 (28%) | 1,682 (29%) |
| 35 to 44 years | 551 (21%) | 253 (19%) | 298 (23%) | 2,224 (21%) | 1,015 (21%) | 1,209 (21%) |
| 45 to 54 years | 343 (13%) | 171 (13%) | 172 (13%) | 1,406 (13%) | 631 (13%) | 775 (13%) |
| 55 to 64 years | 289 (11%) | 151 (12%) | 138 (10%) | 1,109 (10%) | 516 (11%) | 593 (10%) |
| Median (IQR) | 33 (25, 45) | 32.8 (25–45) | 34 (25–44) | 33 (24, 44) | 33 (24, 44) | 33 (24, 44) |
| Male sex | 930 (35%) | 457 (35%) | 473 (36%) | 4,114 (38%) | 1,875 (38%) | 2,239 (38%) |
| Relationship status | ||||||
| Single or cohabitating | 2,146 (82%) | 1,089 (84%) | 1,057 (80%) | 8,878 (82%) | 4,094 (84%) | 4,784 (82%) |
| Marriedb | 385 (15%) | 177 (14%) | 208 (16%) | 1,547 (14%) | 641 (13%) | 906 (16%) |
| Widowed, divorced, or separated | 93 (4%) | 38 (3%) | 55 (4%) | 350 (3%) | 148 (3%) | 202 (3%) |
| Education | ||||||
| Primary or less | 830 (32%) | 442 (34%) | 388 (29%) | 2,899 (27%) | 1,418 (29%) | 1,481 (25%) |
| Junior secondary | 1,067 (41%) | 533 (41%) | 534 (41%) | 4,344 (40%) | 1,986 (41%) | 2,358 (40%) |
| Senior secondary | 400 (15%) | 194 (15%) | 206 (16%) | 2,117 (20%) | 920 (19%) | 1,197 (20%) |
| Higher than senior secondary | 319 (12%) | 129 (10%) | 190 (14%) | 1,394 (13%) | 549 (11%) | 845 (14%) |
| Incomec | ||||||
| None | 1,615 (62%) | 925 (71%) | 690 (52%) | 7,622 (71%) | 3,617 (74%) | 4,005 (68%) |
| <$96 per month | 389 (15%) | 168 (13%) | 221 (17%) | 707 (7%) | 320 (7%) | 387 (7%) |
| $96 to $477 per month | 480 (18%) | 168 (13%) | 312 (24%) | 1,824 (17%) | 697 (14%) | 1,127 (19%) |
| >$477 per month | 135 (5%) | 41 (3%) | 94 (7%) | 610 (6%) | 246 (5%) | 364 (6%) |
| Number of partners, past 12 monthsd | ||||||
| None | 310 (14%) | 158 (14%) | 152 (13%) | 1,390 (15%) | 687 (16%) | 703 (13%) |
| 1 partner | 1,370 (60%) | 636 (56%) | 734 (63%) | 6,531 (68%) | 2,906 (67%) | 3,625 (69%) |
| 2 or more partners | 611 (27%) | 340 (30%) | 271 (23%) | 1,665 (17%) | 739 (17%) | 926 (18%) |
| Any concurrent sexual partners, past 12 monthse | 593 (30%) | 333 (34%) | 260 (26%) | 1,557 (19%) | 710 (19%) | 847 (19%) |
| HIV prevalencef | 805 (31%) | 403 (31%) | 402 (31%) | 2,691 (25%) | 1,338 (28%) | 1,353 (23%) |
Interquartile range, IQR
N=1 end-of-study survey participants were determined to be eligible for enrollment, but date of birth was inadvertently not recorded.
Relationship status of married includes civil, customary, and/or traditional marriages; unmarried, cohabitating persons are included as single, never married.
Botswana pula (BWP) were converted to US$ based on a rate of BWP10.49 per $1.
Percentages calculated among participants who report ever engaging in sexual activity.
Percentages calculated among participants who reported engaging in sexual activity during the past 12 months.
N=5 longitudinal cohort and N=98 end-of-study survey participants without documentation of a prior positive test result refused HIV testing.
No study-related adverse events were detected. Figure 3 presents the absolute coverage levels (including number of events and evaluable sample sizes) at baseline (from the 20% longitudinal cohort prior to intervention start) and study-end (from 80% end-of-study survey sample) in the three standard-of-care and three intervention communities, for each of the five prespecified indicators of intervention uptake overall and according to sex and age group. Table 2 presents the corresponding PRs and 95% CIs for intervention (vs. standard-of-care) coverage at baseline and study-end. A complete summary of these models is provided in Appendix Tables S1–S5. We note that although the proportion of HIV-positive pregnant women who remain in care and on ART 12 months post-delivery was a prespecified outcome, we had insufficient power to analyze by-arm differences in coverage. Based on an a priori estimated sample size of n=270 per arm (n=540 in total), power to detect differences in coverage according to randomization arm was anticipated to be <60%. Indeed, among the 1,964 HIV-positive women who participated in the end-of-study survey, only 359 (n=218 and 141 in intervention and standard-of-care communities, respectively) had given birth to a child during study follow-up. However, these women were included in the by-arm evaluation of the ART and viral suppression outcomes.
Figure 3. Coverage (number of events / evaluable sample size) at baseline and end of study in standard-of-care and intervention communities participating in the end of study survey for the Ya Tsie study, overall and by sex and age.
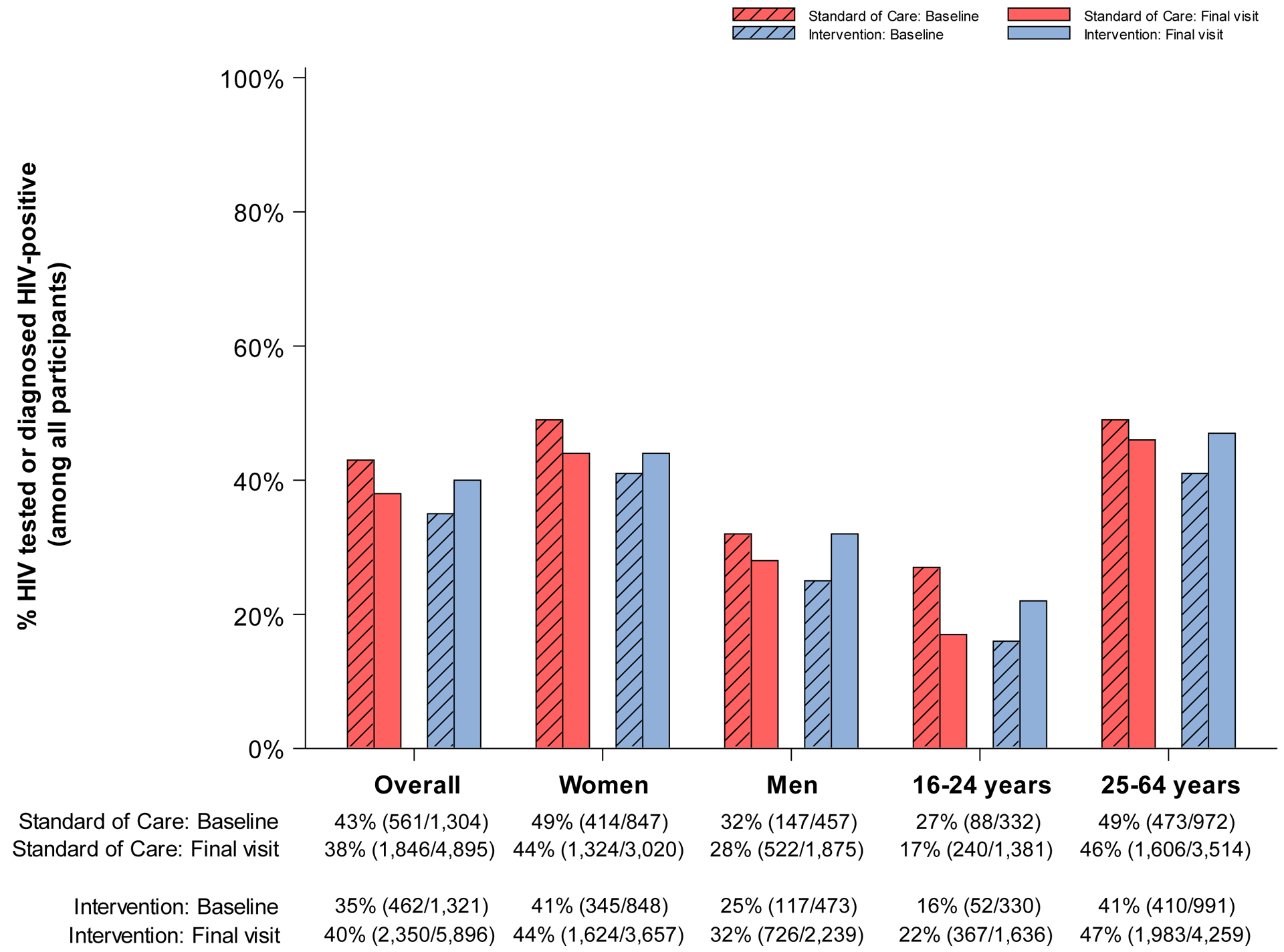
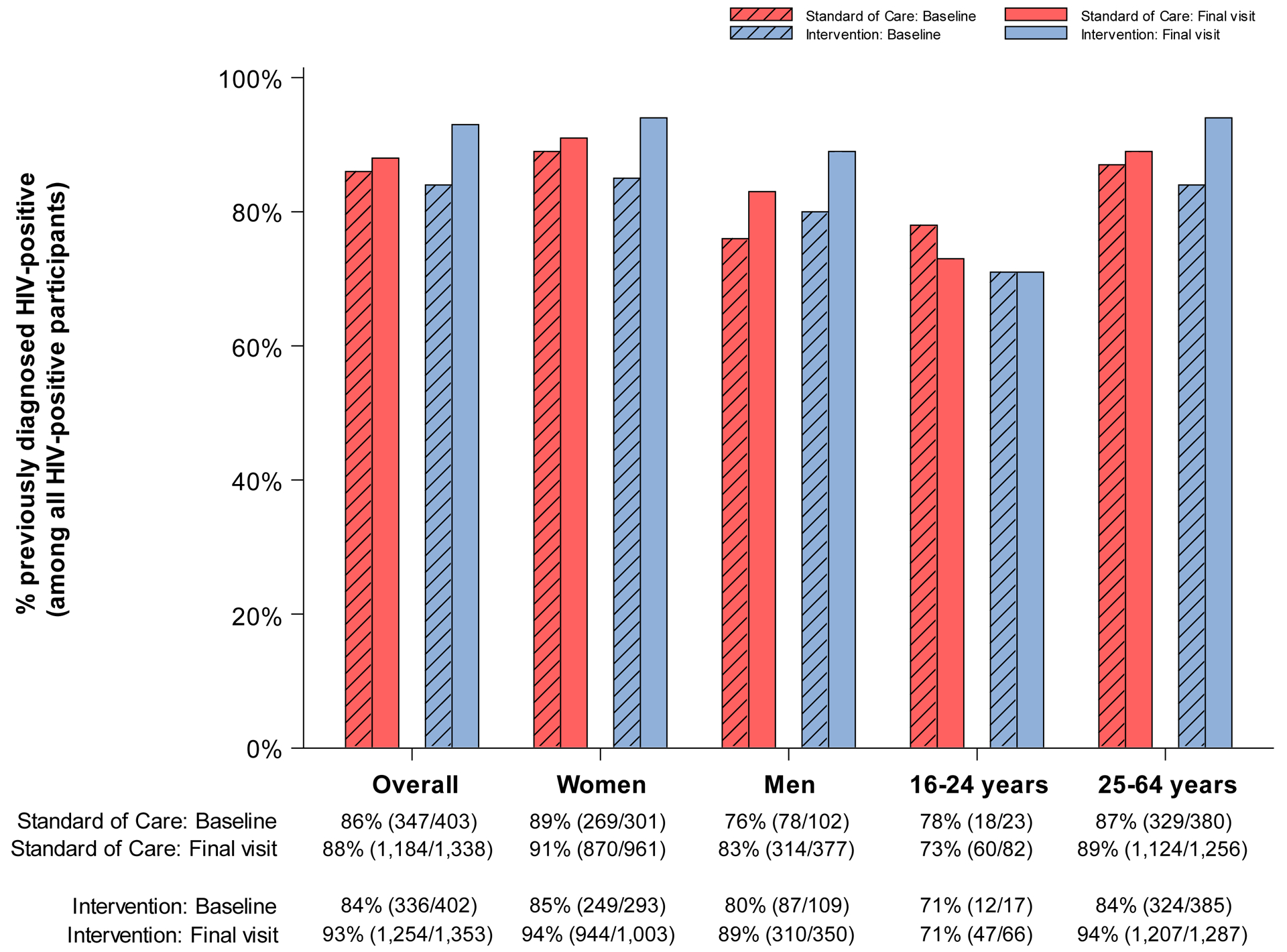
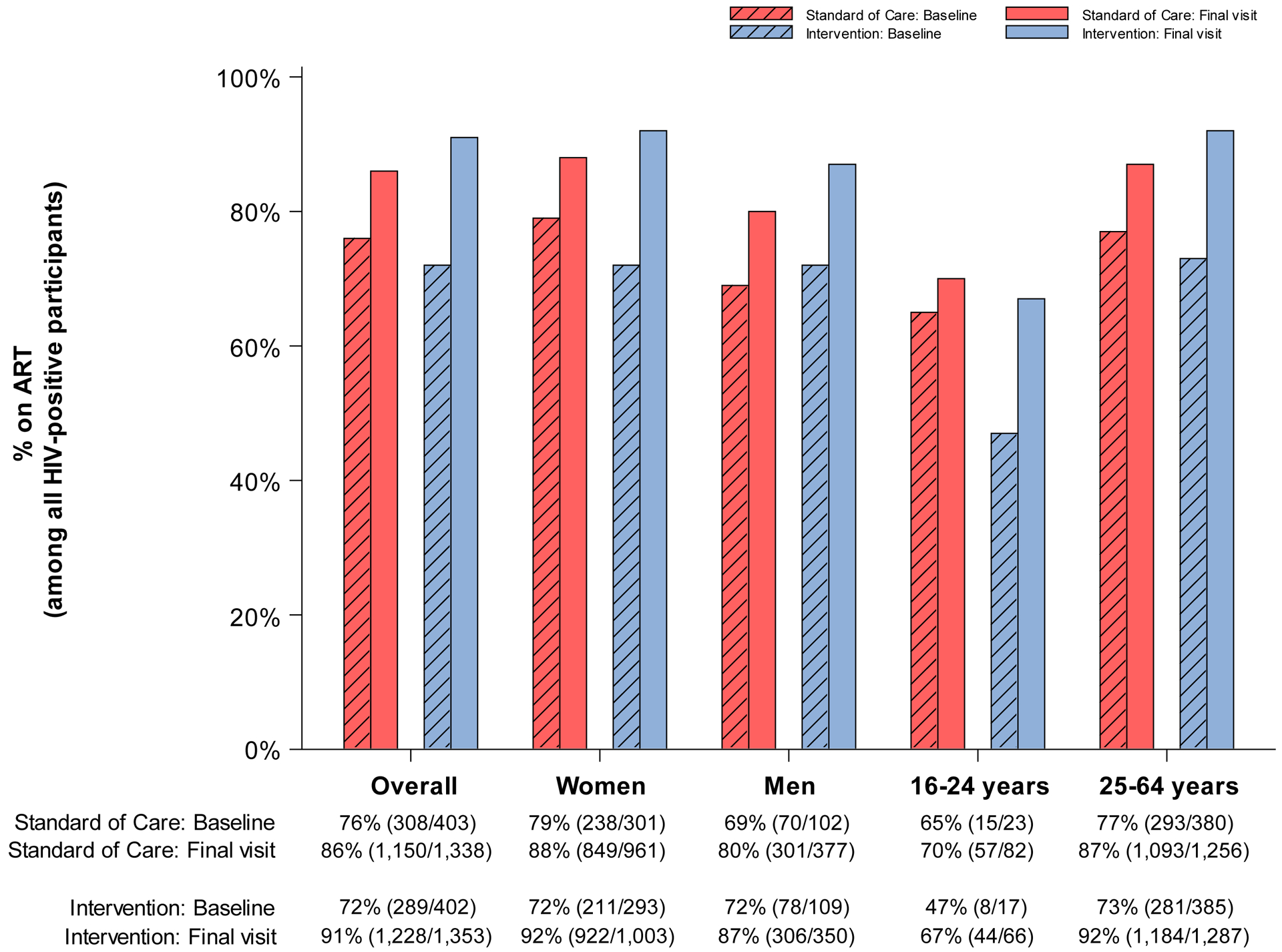
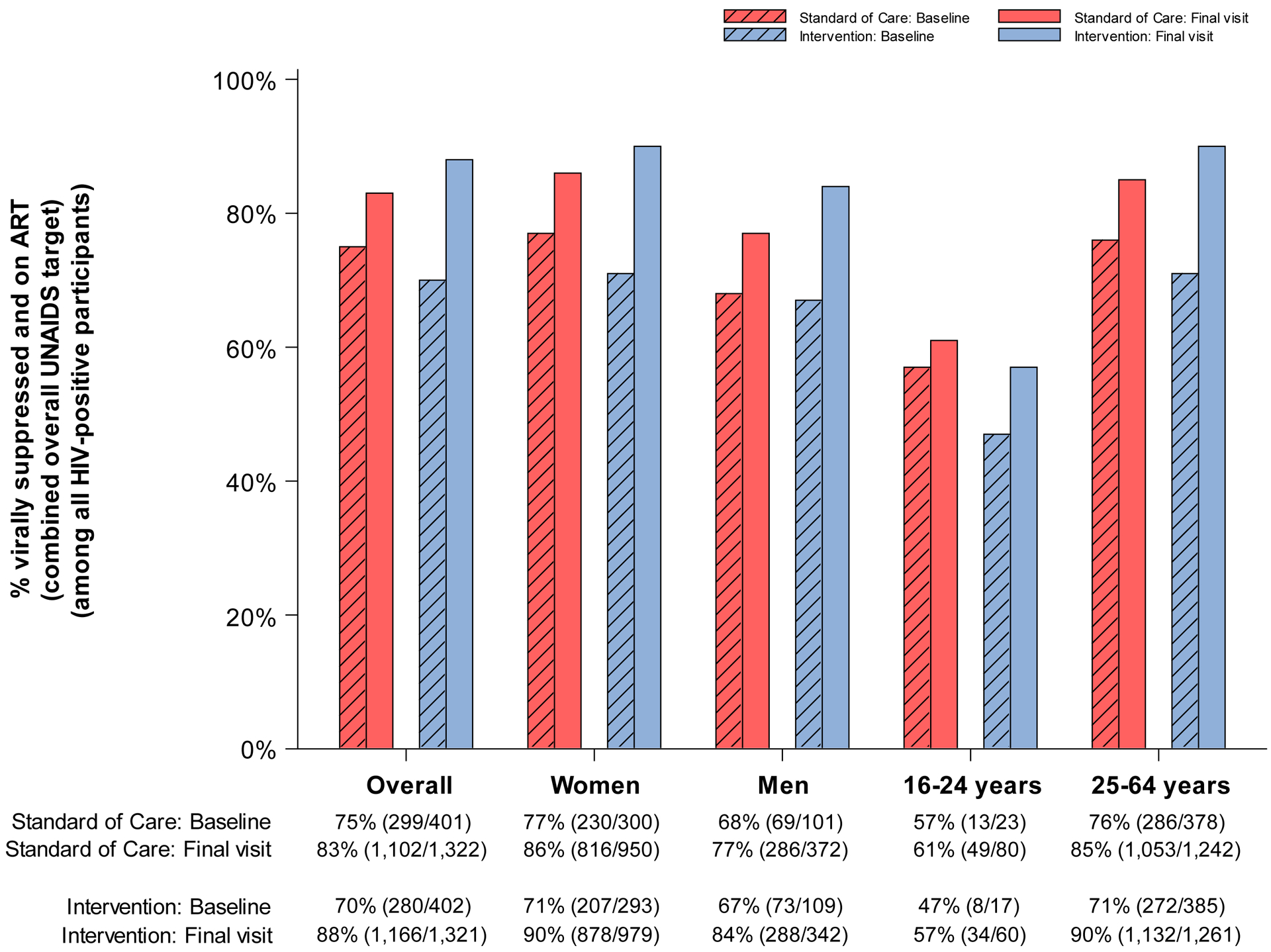
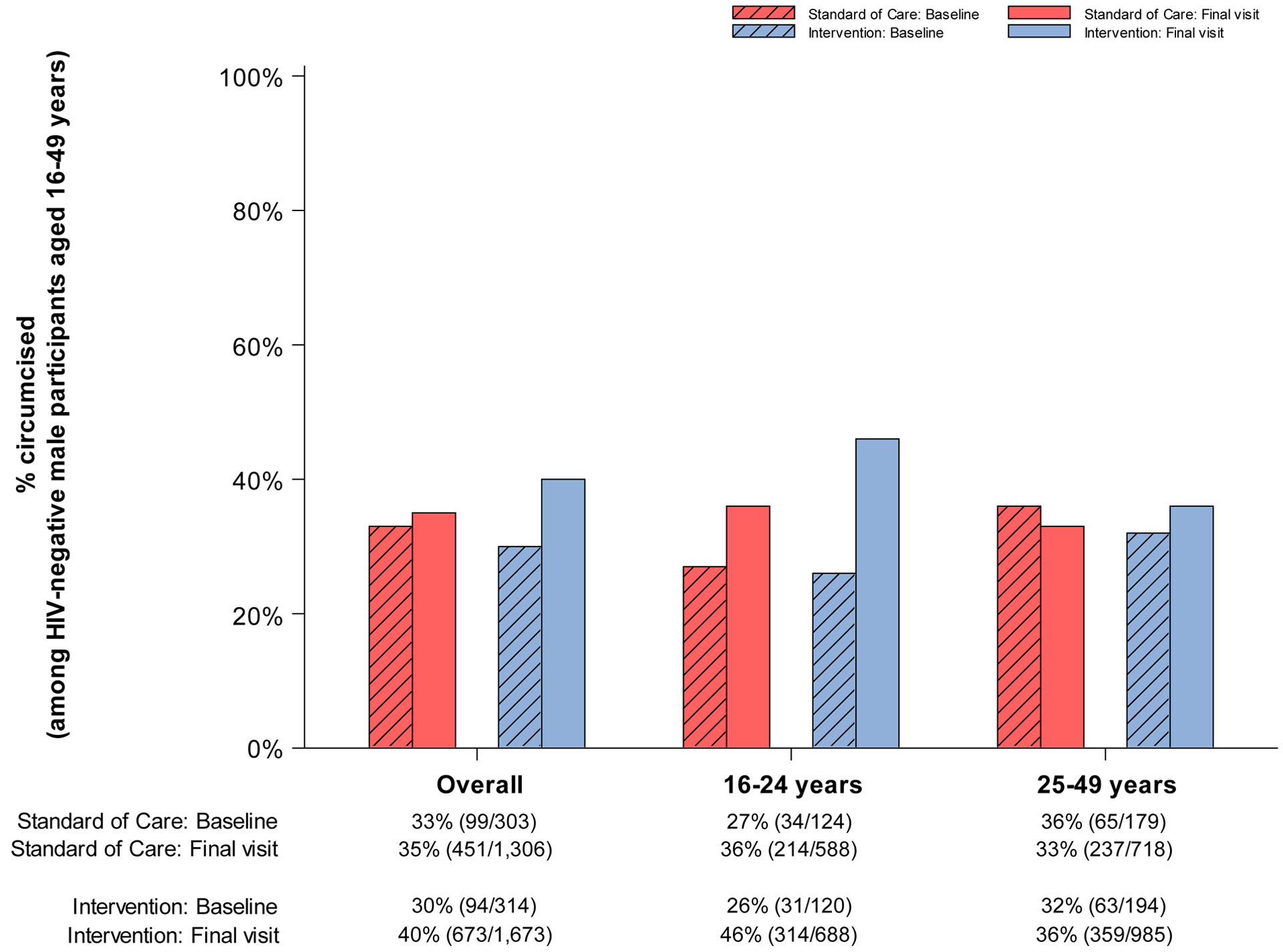
A. % HIV-tested or previously diagnosed HIV-positive (among all participants)
B. % previously diagnosed HIV-positive (among all HIV-positive participants)
C. % on ART (among all HIV-positive participants)
D. % virally suppressed and on ART (combined overall UNAIDS target) (among all HIV-positive participants)
E. % circumcised (among HIV-negative male participants aged 16–49 years)
Table 2.
Prevalence ratios (PR) and 95% confidence intervals (CI) for intervention coverage in six communities participating in the end-of-study survey for the Ya Tsie study.
| Intervention vs. standard-of-care PR (95% CI)a,b |
||
|---|---|---|
| Endpoint | Baseline | Study end |
| HIV-tested (prior 12 months) or previously diagnosed HIV-positive (among all participants) | 0·82 (0·69, 0·98) |
1·29 (1·17, 1·43) |
| Previously diagnosed HIV-positive (among all HIV-positive participants) | 0·97 (0·94, 1·00) |
1·08 (1·02, 1·14) |
| Current receipt of ART (among all HIV-positive participants) | 0·94 (0·93, 0·95) |
1·12 (1·07, 1·17) |
| Virally suppressed on ART (among all HIV-positive participants) | 0·94 (0·93, 0·94) |
1·13 (1·09, 1·17) |
| Self-reported circumcised (among HIV-negative men aged 16–49 years) | 0·92 (0·86, 0·99) |
1·26 (1·17, 1·35) |
All models adjusted for matched pair and matched pair by time interaction.
95% CIs were computed using robust variance accounting for clustering at the community level.
At baseline, a smaller proportion of participants in intervention communities (35%; n=462 of 1,321) had either tested HIV-negative during the prior 12 months or were previously diagnosed as HIV-positive compared to those in standard-of-care communities (43%; n=561 of 1,304) (Table 2; PR for baseline: 0·82; 95% CI: 0·69, 0·98). However, at study-end the proportion reporting prior HIV testing or diagnosis was 5% lower in standard-of-care communities (40%; n=2,350 of 5,896) whereas coverage in the intervention arm was 5% higher (38%; n=1,846 of 4,895) (Figure 3.A.). This difference in change in coverage over time by randomized arm was statistically significant (paired t-test P=0·02). In post-hoc subgroup analyses by age, at baseline prior HIV testing or diagnosis was lowest among participants aged 16–24 years in both arms (27% [n=88 of 332] and 16% [n=52 of 330] in standard-of-care and intervention arms, respectively). At study-end, coverage decreased by 10% (to 17%; n=240 of 1,381) in communities randomized to standard-of-care whereas coverage in the intervention arm was 6% higher (to 22%; n=367 of 1,636).
Among PLWH only, 86% (n=347 of 403) and 84% (n=336 of 402) of participants in standard-of-care and intervention communities were diagnosed at baseline respectively (Figure 3.B.). By study-end, diagnosis of HIV infection increased in both arms, but by a significantly larger amount in communities randomized to intervention (absolute 9% increase to 93% [n=1,254 of 1,353]) compared with standard-of-care communities (absolute 2% increase to 88% [n=1,184 of 1,338]) (paired t-test P=0·03) (Table 2; PR for study\-end: 1·08; 95% CI: 1·02, 1·14).
ART coverage (in all PLWH regardless of prior HIV diagnosis) increased in both arms during the study. However, we observed a significantly larger absolute increase in communities randomized to intervention: By study-end, the proportion of PLWH on ART in intervention communities was 91% (n=1,228 of 1,353) (19% absolute increase from 72% [n=289 of 402] at baseline) compared with 86% (n=1,150 of 1,338) (10% absolute increase from 76% [n=308 of 403]) in standard-of-care communities. This difference in change in treatment coverage over time by randomized arm was statistically significant (paired t-test P=0·02) (Table 2; PR at study-end: 1·12; 95% CI: 1·07, 1·17).
Population-level viral suppression levels followed similar patterns (Figure 3.D.). Among all PLWH (regardless of diagnosis or treatment status), the proportion on ART with viral suppression was higher at study-end across all communities. However, a significantly larger increase in viral suppression was observed in intervention communities (18% absolute increase from 70% [n=280 of 402] at baseline to 88% [n=1,166 of 1,321] at study end) compared with standard-of-care (8% absolute increase from 75% [n=299 of 401] at baseline to 83% [n=1,102 of 1,322] at study end]) (paired t-test P=0·02) (Table 2; PR at study-end: 1·13; 95% CI: 1·09, 1·17). Among young PLWH residing in intervention communities, only 57% (n=34 of 60) were virally suppressed at study-end (Figure 3.D.; 10% absolute increase from 47% [n=8 of 17] at baseline) compared with 90% (n=1,132 of 1,261) of their older counterparts (19% absolute increase from 71% [n=272 of 385] at baseline).
Figure 3.E. summarizes self-reported receipt of MC by randomization arm at baseline and study-end among HIV-negative male participants aged 16–49 years. At baseline, 33% (n=99 of 303) of eligible men reported already being circumcised in standard-of-care communities compared to 30% (n=94 of 314) in those randomized to intervention. At study-end, the proportion of eligible male participants reporting being circumcised increased by 2% (in absolute terms) in the standard-of-care arm (to 35%; n=451 of 1,306) and 10% (to 40%; n=673 of 1,673) in the intervention arm. The difference in uptake over time was statistically significant (paired t-test P=0·03) (Table 2; PR at study-end: 1·26; 95% CI: 1·17, 1·35). In post-hoc subgroup analyses by age, population levels of circumcision were highest among men aged 16–24 years: By study-end, 46% (n=314 of 688) of young men reported being circumcised in the intervention arm (20% absolute increase from 26% [n=31 of 120] at baseline).
For the ART, viral suppression, and MC endpoints, we assessed change in uptake over time by randomization arm in the 20% longitudinal cohort (Figures S2.A–S2.C; Tables S6–S8). We observed a similar pattern of uptake over time among cohort participants: the proportion of PLWH cohort participants on ART and virally suppressed increased over time in both arms with a significantly greater absolute difference observed in the intervention arm (paired t-test for ART and viral suppression: P=0.02 and P=0.01, respectively). At study-end, 96% (n=1,357 of 1,409) of all PLWH cohort members in intervention communities were on ART and virally suppressed (26% absolute increase from 70% [n=1,168 of 1,665] at baseline) (Figure S2.B). In standard-of-care communities, 92% (n=1,311 of 1,432) of PLWH were virally suppressed by study-end, (20% absolute increase from 72% [n=1,168 of 1,631] at baseline). Likewise, MC coverage among HIV-negative male cohort members aged 16–49 years rose in both arms: by study-end, 41% (n=560 of 1,351) and 55% (n=716 of 1,311) of eligible male participants in standard-of-care and intervention communities (respectively) reported being circumcised, an absolute increase of 11% (from 32%; n=492 of 548) and 16% (from 39%; n=596 of 1,545) from baseline levels.
In post-hoc analyses examining population levels of HIV diagnosis, treatment, and viral suppression using the UNAIDS “95-95-95” targets in the six communities taking part in the end-of-study survey, uptake increased in both arms during the study (Figures S3.A–S3.C). At baseline, in intervention communities, 84% (n=336 of 402) of PLWH knew their positive status (1st 95), 85% (n=289 of 340) of these individuals were receiving ART (2nd 95), and 97% (n=280 of 289) of participants receiving ART were also virally suppressed (3rd 95). At study-end, coverage in these communities was 93% (n=1,254 of 1,353), 97% (n=1,228 of 1,262), and 97% (n=1,166 of 1,202) for the 1st, 2nd, and 3rd UNAIDS targets, respectively. In comparison, coverage in standard-of-care communities at baseline was 86% (n=347 of 403), 87% (n=308 of 353), and 97% (n=299 of 308) and 88% (n=1,184 of 1,338), 96% (n=1,150 of 1,196), and 97% (n=1,102 of 1,136) at study end for the 1st, 2nd, and 3rd UNAIDS targets, respectively.
DISCUSSION
In this large community-randomized trial, population levels of HIV treatment and viral suppression increased in all communities during the study period. However, significantly larger increases in coverage were observed in communities randomized to the intervention arm. By study-end, 88% of all PLWH in intervention communities surveyed at study-end were virally suppressed (regardless of prior diagnosis or treatment). This is one of the highest population levels of viral suppression described to date and exceeds the UNAIDS “95-95-95” overall target for viral suppression among all PLWH of 86%. Although MC coverage increased from baseline levels in both arms (and to a significantly greater extent in the intervention arm), overall uptake was lower than expected. According to mathematical modeling studies, achieving these coverage levels is anticipated to markedly reduce HIV incidence and improve the health and survival of PLWH.10
Global progress towards the “90-90-90” and “95-95-95” targets has accelerated since their introduction in 2014. According to UNAIDS, in 2017, 70% of PLWH were diagnosed; 77% of diagnosed persons were receiving treatment and; 82% of those on treatment were virally suppressed.18 However, progress has been uneven. Botswana, Eswatini, and Namibia are the only African countries to achieve or approach achievement of overall targets for viral suppression among all PLWH. Coverage results from other community-randomized trials of combination HIV prevention strategies have been reported. In HPTN 071 (PopART), conducted in 21 communities in Zambia and South Africa, 72% of all PLWH residing in communities randomized to receive universal test-and-treat were virally suppressed after 24 months.19 In the SEARCH trial, conducted in 32 communities in Uganda and Kenya, population viral suppression in intervention communities was 79%.20
The foundation of our intervention strategy was an initial community-wide HIV testing campaign that attempted to reach all residents in the home or at mobile testing venues followed by targeted activities to reach those missed during the first phase (i.e. men, youth). The community-wide campaign allowed for rapid identification of many undiagnosed and/or untreated PLWH. The cost of the community-wide campaign was $99,847 per community with an average cost of $44 per person tested and $671 per person testing positive.21,22 Although the cost per person tested in the home ($53) was higher than those offered in mobile venues ($34), the number of newly identified PLWH was higher with home-based testing. The community-wide campaign, albeit resource-intensive, would not need to be repeated on a regular or even semi-regular basis, assuming a large proportion of the community was reached. After an initial campaign, high-risk and underrepresented populations could be strategically targeted for testing. Our linkage-to-care intervention was effective, resource-efficient, and readily replicable in other resource-constrained settings. Of note, our testing, linkage, and retention strategies relied upon being able to trace individuals through the care cascade with a unique patient identifier. Although Botswana is a middle-income country, the lessons learned from our study are transferrable. Like other countries in the region including Malawi, South Africa, and Zambia among others, Botswana employs a vertical approach to its HIV epidemic. Prevention, care, and treatment strategies are determined by the Ministry of Health and Wellness and delivered within a highly decentralized system of facilities by low-level cadres of health workers (i.e. lay counselors, nurses). Indeed, all our intervention services were administered through existing infrastructure by usual teams of healthcare workers.
In both arms, uptake of HIV testing and treatment services was lowest among youth. However, greater uptake by youth was achieved in the 20% longitudinal cohort. In the intervention arm, 87% (Figure S2.B.) of young PLWH cohort members were virally suppressed by study-end, compared with 57% (Figure 3.D.) in the end-of-study survey population. These differences in intervention uptake between the 20% longitudinal cohort and the end-of-study survey sample suggest that youth living with HIV may benefit from more intensive and/or multiple interventions to successfully engage in care (e.g. during follow-up study staff traced and contacted all cohort members not found at home including those who had moved outside the community, and referred them for care).23,24 In contrast, MC coverage was highest among youth (Figure 3.E.). Although the intervention did not explicitly target youth, this result parallels general trends in Botswana.25 In 2017, 66% of circumcisions performed were among boys aged >15 years.26
Our study has several limitations. First, due to budgetary constraints, the end-of-study survey was only conducted in six communities. Baseline coverage for all endpoints was lower in the end-of-study survey communities randomized to intervention compared to those randomized to standard-of-care. These differences are likely due to random variability resulting from sampling too few communities for the end-of-study survey; previous analyses of baseline data from all 30 communities demonstrate no by-arm imbalances.15 Similarly, fewer persons were enumerated per household in standard-of-care communities compared to intervention. This difference likely resulted from smaller underlying populations as the enumeration procedures were identical across arms. According to the 2011 Botswana Census, the total population of the three standard-of-care communities was approximately 3,000 persons smaller compared to intervention communities.16
Additionally, only 65% of households were enumerated for the end-of-study survey despite multiple contact attempts by research staff at various times and days of the week. It is possible that testing and treatment coverage in unenumerated households systematically differed from enumerated households. During the baseline enumeration activities carried out for the longitudinal cohort, if research staff encountered no one at home, occupancy information was collected from a neighbor or representative at the Village Development Committee (an exercise we lacked the resources for the end-of-study survey). These data showed 79% of missed households were in fact rarely or never occupied and therefore ineligible for study participation.13 If we assume a similar proportion of such households encountered in the end-of-study survey were rarely or never occupied, the proportion enumerated of regularly-occupied households would be 78%. Additionally, as noted above, we were unable to evaluate by-arm differences in uptake of ART among HIV-positive pregnant women accessing PMTCT services due to limited power resulting from small sample sizes. Future studies assessing service uptake in this population could consider planned over-sampling of women of reproductive age in the household and/or recruitment of pregnant women newly presenting for care at antenatal clinics located within the community. Lastly, circumcision status was self-reported and men may misreport circumcision status.27,28
Despite observed imbalances at baseline, the end-of-study survey sample provides the best assessment of intervention service uptake during the study period, as these community members were not affected by participating in a cohort. Indeed, repeated contact between study staff and 20% longitudinal cohort members appears to have affected participant behavior (as anticipated prior to study start): the absolute level of engagement in care by the 20% longitudinal cohort was higher in both arms at study-end. For example, within the three end-of-study survey communities randomized to standard-of-care, 90% of all PLWH enrolled in the cohort were virally suppressed by study-end compared to 83% of PLWH who completed the end-of-study survey.
In conclusion, population levels of HIV diagnosis, treatment, viral suppression, and MC increased in both arms over time with significantly greater increases in intervention communities. By study-end, 88% of all HIV-positive end-of-study survey participants (and 96% of cohort participants) in the intervention arm were virally suppressed, surpassing the UNAIDS’ “95-95-95” target of 86% population viral suppression.10,13 Our findings demonstrate that it is possible to further increase uptake of intervention services in the context of a high-prevalence generalized epidemic, even with high baseline coverage.
Supplementary Material
Acknowledgements
The authors thank study participants, Dikgosi and other community leaders, members of the Ya Tsie Community Advisory Board and the clinic staff and District Health Management Teams at all study sites; the Ya Tsie Study Team at the Botswana Harvard AIDS Institute Partnership, the Harvard T.H. Chan School of Public Health, the CDC, and the Botswana Ministry of Health and Wellness; Ria Madison, Lars Colson, and Lendsey Melton for administrative support; our implementing partners, Tebelopele Voluntary Counseling and Testing Centers, and Jhpiego - Botswana; and the National Institute of Allergy and Infectious Diseases Prevention-Africa Data and Safety Monitoring Board.
Footnotes
Declaration of interests
Grant support was provided during the conduct of this study by the United States National Institutes of Health to VDG, RW, and KEW (R37 AI051164); RW (R01 AI136947); SDP (K23 AI091434); SM (D43 TW000004, D43 TW009610, and D43 TW010543); SL (K24 AI131928 and P30 AI060354); KMP (K23 HD070774); EJTT (R01 CA222147); and EJTT and KEW (R01 AI104459 and R01 AI127271). SM reports grant support from the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (DEL-15-006) funded by the African Academy of Sciences’ Alliance for Accelerating Excellence in Science in Africa, with support from the New Partnership for Africa’s Development Planning and Coordinating Agency and the Wellcome Trust (107752/Z/15/Z). All other authors declare no competing interests.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375: 830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 2018; 5: e438–e47. [DOI] [PubMed] [Google Scholar]
- 4.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316: 171–81. [DOI] [PubMed] [Google Scholar]
- 5.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danel C, Moh R, Gabillard D, et al. A Trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373: 808–22. [DOI] [PubMed] [Google Scholar]
- 7.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339: 966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375: 2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 10.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 11.UNAIDS. Country factsheets: Botswana. 2017. [accessed 2018 August 23]; http://www.unaids.org/en/regionscountries/countries/botswana.
- 12.GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3: e361–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3: e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perriat D, Balzer L, Hayes R, et al. Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc 2018; 21: e25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019; 381: 230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botswana Central Statistics Office. Botswana 2011 population and housing census. Gaborone, Botswana: Botswana Central Statistics Office; 2011. [Google Scholar]
- 17.Hayes RJ, Moulton LH. Cluster randomised trials. 2nd ed. Boca Raton: CRC Press; 2017. [Google Scholar]
- 18.UNAIDS. Ending AIDS: Progress Towards the 90-90-90 Targets. Geneva, Switzerland: UNAIDS; 2017. [Google Scholar]
- 19.Hayes RJ, Donnell D, Floyd S, et al. Effect of universal testing and treatment on HIV incidence - HPTN 071 (PopART). N Engl J Med 2019; 381: 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havlir DV, Balzer LB, Charlebois ED, et al. HIV Testing and treatment with the use of a community health approach in rural Africa. N Engl J Med 2019; 381: 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasry A, Bachanas P, Suraratdecha C, et al. Cost of community-based HIV testing activities to reach saturation in Botswana. AIDS Behav 2019; 23: 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alwano MG, Bachanas P, Block L, et al. Increasing knowledge of HIV status in a country with high HIV testing coverage: Results from the Botswana Combination Prevention Project. PLoS One 2019; 14: e0225076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanoni BC, Elliott RJ, Neilan AM, Haberer JE. Screening for HIV and linkage to care in adolescents: insights from a systematic review of recent interventions in high-versus low- and middle-income settings. Adolesc Health Med Ther 2018; 9: 211–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buttolph J, Inwani I, Agot K, et al. Gender-specific combination HIV prevention for youth in high-burden settings: The MP3 youth observational pilot study protocol. JMIR Res Protoc 2017; 6: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hines JZ, Ntsuape OC, Malaba K, et al. Scale-up of voluntary medical male circumcision services for HIV prevention: 12 countries in southern and eastern Africa, 2013–2016. MMWR Morb Mortal Wkly Rep 2017; 66: 1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis SM, Hines JZ, Habel M, et al. Progress in voluntary medical male circumcision for HIV prevention supported by the US President’s Emergency Plan for AIDS Relief through 2017: longitudinal and recent cross-sectional programme data. BMJ Open 2018; 8: e021835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lissouba P, Taljaard D, Rech D, et al. Adult male circumcision as an intervention against HIV: an operational study of uptake in a South African community (ANRS 12126). BMC Infect Dis 2011; 11: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas AG, Tran BR, Cranston M, Brown MC, Kumar R, Tlelai M. Voluntary medical male circumcision: a cross-sectional study comparing circumcision self-report and physical examination findings in Lesotho. PLoS One 2011; 6: e27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


