Abstract
In the healthy, untrained young adult, a case is made for a respiratory system (airways, pulmonary vasculature, lung parenchyma, respiratory muscles, and neural ventilatory control system) that is near ideally designed to ensure a highly efficient, homeostatic response to exercise of varying intensities and durations. Our aim was then to consider circumstances in which the intra/extrathoracic airways, pulmonary vasculature, respiratory muscles, and/or blood-gas distribution are underbuilt or inadequately regulated relative to the demands imposed by the cardiovascular system. In these instances, the respiratory system presents a significant limitation to O2 transport and contributes to the occurrence of locomotor muscle fatigue, inhibition of central locomotor output, and exercise performance. Most prominent in these examples of an “underbuilt” respiratory system are highly trained endurance athletes, with additional influences of sex, aging, hypoxic environments, and the highly inbred equine. We summarize by evaluating the relative influences of these respiratory system limitations on exercise performance and their impact on pathophysiology and provide recommendations for future investigation.
Keywords: airways, diaphragm, gas exchange, pulmonary vasculature, ventilation
INTRODUCTION
Four to five decades ago, when the study of exercise physiology was in its infancy, debate over the “limiting factors” to maximum oxygen transport and exercise performance centered solely on cardiovascular responses and muscle metabolism. The respiratory system, including the airways, lung parenchyma, pulmonary vasculature, and respiratory muscles, was viewed as overbuilt and unmalleable in response to endurance training. In the ensuing decades, we have witnessed a gradual accumulation of evidence that has enriched our knowledge of the remarkable precision and capacity of the healthy respiratory system’s response to the substantial and varied demands of exercise. Additionally, research has also revealed several circumstances in which one or more links in this system was shown to be imperfect, anatomically underbuilt, and/or incurred excessive biological costs. Indeed, select components of the respiratory system structure and/or function have even been shown to be negatively impacted via intense exercise training. Our synthesis aims to provide a comprehensive examination of evidence obtained across this broad spectrum of successes and failures experienced by the healthy respiratory system.
KEY FEATURES OF THE JUST RIGHT/OVERBUILT RESPIRATORY SYSTEM RESPONSE TO EXERCISE IN THE UNTRAINED HEALTHY YOUNG ADULT MALE
Ventilation
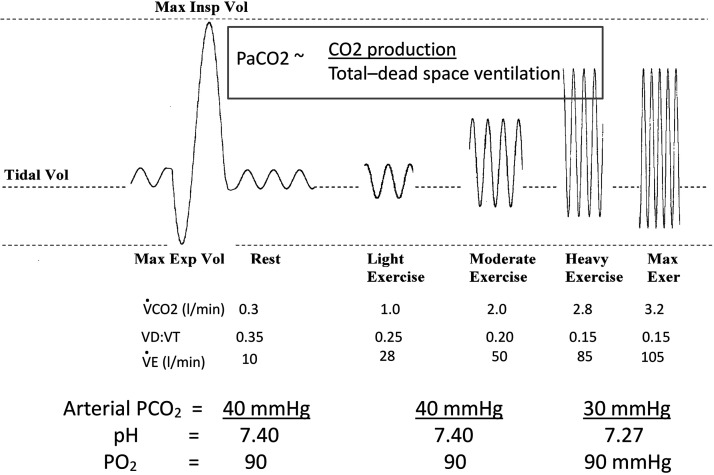
A key response is the near isocapnic hyperpnea during mild to moderate exercise intensities (see Fig. 1). Remarkably, this proportional match of increasing alveolar ventilation to increasing CO2 production occurs even in the face of a falling dead space to tidal volume ratio (Vd/Vt) from rest through increasing exercise intensity. In the late Brian Whipp’s words, “… the system seems to ‘know’ that when Vd/Vt is reduced, V̇e ‘needs’ to increase less per unit V̇co2 to affect its regulatory function,” i.e., to maintain the ratio of alveolar ventilation to carbon dioxide production (V̇a/V̇co2) and arterial partial pressure of carbon dioxide () near resting values (212). This remarkable tracking of V̇a to V̇co2 is also evidenced with healthy aging, during which the ratio of alveolar ventilation to perfusion (V̇a/Q̇)·nonuniformity and Vd/Vt increase in an aging lung with loss of elastic recoil and airway narrowing, yet the ratio of minute ventilation to carbon dioxide production (V̇e/V̇co2) increases during exercise so the remains precisely regulated (92). Thus, the correlative data linking V̇a to V̇co2 are undeniable, and some ingenious experiments in animals at rest have used manipulation of “CO2 flow” to the lung to demonstrate a causative proportional response of V̇a to V̇co2 in the absence of muscle contraction, at least over a limited range of ΔV̇co2 (152). However, the underlying mechanisms through which V̇co2 acts as its own controller remain a mystery, i.e., without identifiable stimuli or receptor sites. Theoretically, an argument has been made for an “internal stored memory model” adapted by the respiratory controller over time (perhaps through trial and error) to somehow track metabolic CO2 production and/or its exchange at the lung, resulting in an energetically cost-effective elimination of CO2 in the steady state of exercise (158). In addition to this underlying V̇co2-V̇a link, feedback from group III-IV muscle afferents and feedforward from central command to locomotor muscles are also likely to contribute significantly to both exercise hyperpnea and to the hyperventilation of heavy intensity exercise (53).
Fig. 1.
Depiction of typical ventilatory and arterial blood gas responses to steady-state, incremental exercise in untrained young adult healthy subjects [maximal oxygen consumption (V̇o2max) ~35–50 mL·kg−1·min−1; age 20–35 yr). Note: a) the incremental rise in tidal volume (Vt) and then breathing frequency (fB) with inspiratory time remaining slightly < expiratory time and the reduction in end-expiratory lung volume; b) the fall in the dead space to tidal volume ratio (Vd/Vt) and ratio of minute ventilation to carbon dioxide production (V̇e/V̇co2), which maintain the ratio of alveolar ventilation to carbon dioxide production (V̇a/V̇co2) and partial pressure of carbon dioxide () near resting values throughout moderate intensity exercise; and c) the hyperventilatory response to heavy and maximum exercise intensities commensurate with the onset of metabolic acidosis.
Breathing Mechanics
The mechanical efficiency of the “just right” ventilatory response to exercise ensures little or no symptoms of a conscious “awareness” of hyperpnea during exercise. In Fig. 1 note the tradeoff between increasing breathing frequency and tidal volume and the reduction in end-expiratory lung volume with increasing exercise intensity. These highly reproducible responses ensure minimal increases in absolute dead space ventilation because breathing frequency is constrained. Furthermore, the elastic work of breathing is minimized by constraining the increase in Vt to the linear portion of the pressure-volume relationship. This is achieved by both limiting the increase in Vt and reducing end-expiratory lung volume below functional residual capacity via active expiration (70). One potential mechanism, as yet untested in the human for the Vt/breathing frequency (fb) tradeoff with increasing V̇e, is the vagally mediated inhibitory feedback from lung stretch as Vt rises (86). That the resistive work of breathing is also minimized with even maximum exercise intensities is ensured by the structure and precise neural control of both the intra- and extrathoracic airway calibers.
Although the upper extrathoracic airway from nares to glottis may potentially present a major site of resistance to flow during exercise, this circumstance is avoided at least over a range of five- to sevenfold increases in flows experienced in the untrained subject by the precise distribution of efferent respiratory motor output over cranial nerves, which activate pharyngeal and laryngeal airway dilator muscles just milliseconds before phrenic nerve activation. This precise coordination of upper airway versus pump muscle activation with each inspiration ensures that the upper airway is stiffened and widened, thereby resisting progressively rising collapsing transmural pressures generated by the chest wall pump (48). The smaller intrathoracic airways <2 mm diameter and under smooth muscle control also undergo maximum bronchodilation during exercise as regulated by feedback from group III-IV muscle afferents, lung stretch, and increasing circulating catecholamines (96).
Respiratory Muscles
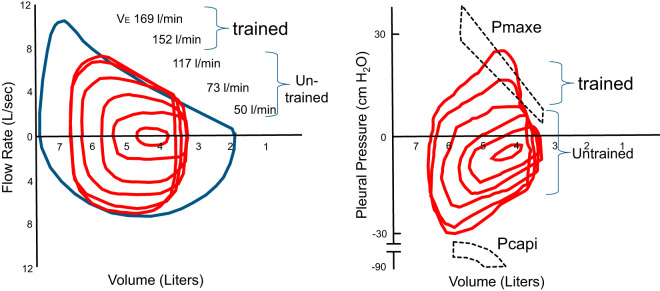
The diaphragm and accessory inspiratory and expiratory respiratory muscles, in contrast to limb locomotor muscles of similar mixed fast-twitch oxidative and glycolytic and slow-twitch oxidative fiber type, also appear to be ideally suited in many ways for the increased ventilatory demands of even heavy intensity endurance exercise: 1) the mitochondrial volume, capillary density, and aerobic capacity of the diaphragm substantially exceed that of the limbs (155); 2) use of supramaximal motor nerve stimulation showed that diaphragm or expiratory muscle fatigue did not occur as a result of either incremental exercise to maximum or endurance exercise to exhaustion at <80% maximal oxygen consumption (V̇o2max) (91, 176); 3) in vitro evidence has shown rodent diaphragm arterioles to be relatively resistant to norepinephrine-induced vasoconstriction as compared with feed vessels from predominantly red or white limb locomotor muscles (1), and these data imply a relatively protective effect against sympathetically mediated vasoconstriction during whole body exercise, i.e., enhanced sympatholysis, in the diaphragm; and 4) ventilatory requirements of exercise are met by the combined recruitment not only of the diaphragm and of abdominal expiratory muscles but also up to nearly 50–60 “accessory” respiratory muscles of the trunk, neck, and upper torso during heavy exercise (4, 154), i.e., the “load” is widely distributed. In addition to these ideal characteristics and recruitment of respiratory muscles during exercise, the resistive and elastic loads placed on these muscles are minimized by the aforementioned regulation of airway caliber as well as end-expiratory and tidal volumes. Furthermore, the reduced end-expiratory lung volume and increased intra-abdominal pressures ensure optimal lengthening of the inspiratory muscles in meeting rising demands for greater force production (94). Consequently, in untrained subjects exercising at V̇o2max and generating 100–110 L/min V̇e, respiratory muscle oxygen consumption and blood flow averaged 8–10% of V̇o2max and cardiac output (2, 67). Furthermore, inspiratory muscles are required to generate only 40–50% of their dynamic capacity, and the maximum flow-volume envelope readily handles the required volumes and flow rates without generating intrathoracic pressures during expiration in excess of those producing “effective” flows (see Fig. 2, values for untrained subjects).
Fig. 2.
Mean values for tidal flow-volume and pressure-volume loops at rest and with incremental treadmill running in untrained and trained subjects [aged 25 ± 1 yr, maximal oxygen consumption (V̇o2max) 40 ± 2 (untrained) and 73 ± 1 mL·kg−1·min−1 (trained)]. Both panels are plotted relative to group mean total lung capacity and residual lung volumes. The average maximum effective pleural pressure on expiration (Pmaxe) indicates the pressures at which further increases in expiratory pressure elicit no further increments in flow rate. The maximum dynamic inspiratory pressures available (Pcapi) are also shown across all lung volumes and flow rates achieved during exercise. These values for dynamic inspiratory capacity were determined before exercise via body plethysmography (94). The width of Pmaxe and Pcapi at a given lung volume indicate 95% confidence interval. Note that: a) untrained subjects throughout exercise showed no or little intersection of tidal with maximum flow-volume loops, expiratory pressures remained below Pmaxe, and peak tidal inspiratory pressures remained <50–60% of maximum dynamic pressure; and b) all trained subjects reached Pmaxe at 90–100% of V̇o2max and 85–100% of Pcapi at V̇o2max, showed dynamic hyperinflation at the highest exercise intensities and at V̇o2max, and showed no further increase in minute ventilation (V̇e) with added fraction of inspired carbon dioxide or reduced fraction of inspired oxygen [Adapted from Johnson et al. (94)].
Seven decades ago, Arthur Otis, Wallace Fenn, and Hermann Rahn provided underlying principles governing optimal responses to ensure minimization of the elastic, viscous, and turbulent elements of the work of breathing with changing ventilatory requirements (149). These pioneers of respiratory science surely must have had exercise hyperpnea in mind as the ultimate example of the “just right” response.
Pulmonary Vasculature
The structure of the pulmonary (vs. systemic) vasculature seems to be ideally suited for its role in accommodating the entire cardiac output because it is thin-walled, highly compliant and distensible, and relatively protected from the vasoconstrictive effects of high sympathetic nerve activity (139, 167). At rest, the mean Ppa ~15 mmHg is sufficient to maintain flow through to the left atrium at a modest perfusion pressure of ~7 mmHg (vs. 90 mmHg in the systemic circulation). At 15–20 L/min cardiac output, pulmonary vascular distensibility in vivo equals that determined in vitro in isolated vessels, resistance decreases ~50–60% below rest, and pulmonary arterial pressure increases to 20–25 mmHg, probably due exclusively to increasing left atrial pressure that is transmitted upstream across the pulmonary vasculature (166, 167). This high distensibility of the pulmonary vasculature also allows vessel recruitment to provide a maximum pulmonary capillary blood volume of about three times resting levels at maximum exercise. The expanded capillary volume ensures a sufficiently long average red cell transit time in the pulmonary capillaries, i.e., from ~1.0 s at rest to 0.4–0.6 s at 15–20 L/min cardiac output (CO). Thus, an alveolar to capillary O2 equilibrium is readily achieved despite a falling mixed venous Po2 and rising cardiac output (mean red blood cell transit time = pulmonary capillary volume/blood flow). Furthermore, because distensibility is independent of vessel diameter and therefore similar across lung regions, this ensures a fairly homogeneous distribution of blood flow.
Gas Exchange
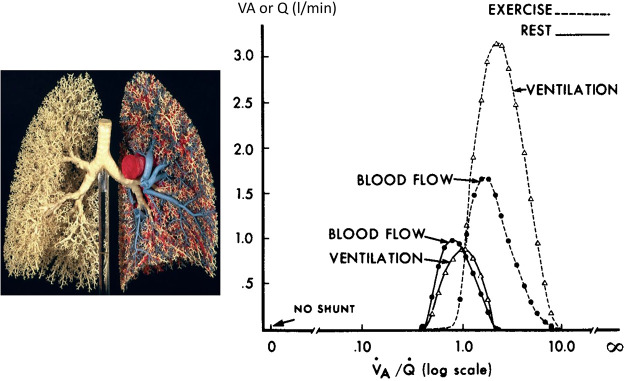
There are some inefficiencies in gas exchange with exercise, as evidenced by two- to threefold increases in the alveolar to arterial Po2 difference above resting values at V̇o2max in the 3–3.5 L/min range. Most of this inefficiency appears to be due to increases in nonuniformity in V̇a/Q̇ distribution within isogravitational lung regions from rest to exercise (see Fig. 3) (59, 204). Despite this increased nonuniformity, during exercise overall V̇a increases out of proportion to cardiac output, thereby ensuring high overall V̇a/Q̇ and avoiding areas of low V̇a/Q̇ during exercise. The hyperventilatory response to heavy exercise raises alveolar Po2 sufficiently to offset the widened alveolar-arterial partial pressure difference (A-aDO2) and prevent arterial hypoxemia. Thus, arterial oxyhemoglobin (HbO2) saturation is typically reduced 2–4% below rest during heavy intensity exercise due solely to a reduced HbO2 affinity induced via metabolic acidosis and increased temperature (Bohr effect). Finally, plasma water turnover is increased into the lung’s interstitial fluid space during exercise, but extraordinary drainage capacity of the interstitial fluid via the thoracic lymphatic system provides protection against movement from the interstitial into the alveolar spaces (100).
Fig. 3.
Ratio of ventilation to perfusion (V̇a/Q̇) at rest and during moderate-intensity steady-state exercise [oxygen consumption (V̇o2) 2.2 L/min, cardiac output (CO) 18 L/min, alveolar-arterial partial pressure difference (A-aDO2) 22 mmHg] in healthy male subjects, as determined by the multiple inert gas elimination technique (MIGET). MIGET estimates the “continuous” distribution of V̇a/Q̇·based on the retention and elimination of 6 infused inert gases of varying solubility (204, 205). Note that V̇a/Q̇ distribution (log scale x-axis) is very narrow at rest, centered around an overall V̇a/Q̇ of ~0.9 and spanning units with V̇a/Q̇ ~0.5 to 3.0. During exercise, the nonuniformity of V̇a/Q̇ increases > rest, but overall V̇a increases out of proportion to Q̇ so that the mean V̇a/Q̇ has more than doubled from rest to exercise. Thus, the lowest V̇a/Q̇ ratios at rest no longer exist during exercise, and pulmonary capillary blood flow is now exposed to higher alveolar (and therefore end-capillary) Po2. Given the exercise-induced reduction in mixed venous O2 content, the widened V̇a/Q̇ distribution accounted for about one-half the widened A-aDO2 during exercise. The authors could attribute the remainder of the increase in A-aDO2 during this moderate-intensity exercise to an extrapulmonary shunt of deoxygenated mixed venous blood (Thebesian and bronchial venous drainage) amounting to ~1% of the prevailing CO, which would result in a significant end-capillary to arterial Po2 difference. An alternate explanation attributes the extra widening of the A-aDO2 (beyond that accounted for by V̇a/Q̇ nonuniformity) to alveolar-capillary diffusion limitation (204). Given the more uniform topographical distribution of V̇a/Q̇ reported from rest to exercise (13, 20), the increased dispersion of V̇a/Q̇ quantified via MIGET in this study was attributed to an increased nonuniformity of V̇a and Q̇ distribution within isogravitational lung regions (59) [Based on findings reported in Gledhill et al. (58)].
Summary: Just Right/Overbuilt: Optimal Design?
Ewald Weibel (1929–2019), the late, pioneering lung morphologist envisioned “what makes a good lung,” fit to provide our organs with the O2 they need at rest and exercise, according to the principles of optimal design (209). He emphasized the very large yet extraordinarily thin and delicate surface area of contact between air and blood, maintained by an exceedingly small amount of supportive tissue arranged in a hierarchical design of elastin and collagen fibers woven into the capillaries together with a fine layer of stabilizing surfactant at the air-liquid interface. This ingenious architectural design observed rules of fractal geometry in packing this huge surface area into the limited space of the chest cavity as well as providing a system of airways and blood vessels reaching all points evenly on the gas exchange surface.
As discussed in the preceding sections, optimal design principles also appear to extend to the entire healthy respiratory system when faced with the enhanced demand for gas transport during exercise. Thus, the remarkable architecture of the “good lung” and its vasculature is complemented by the design of the highly aerobic capacity of the respiratory musculature and the precision and efficiency with which the feedback and feedforward components of the neural respiratory control network mysteriously controls its activity and coordination to drive alveolar ventilation precisely in proportion to tissue CO2 production while minimizing elastic and resistive loads on the respiratory muscles and preserving adequate O2 transport. Accordingly, it seems highly unlikely that any aspect of the ventilation, gas exchange, pulmonary vasculature, or respiratory muscle response to progressive or sustained exercise presents a significant limitation to exercise performance, at least in the young untrained male adult exercising in a normoxic environment. We now address whether this optimal design also applies to highly trained individuals, the elderly, females as well as males, in hypoxic environments, and in non-human mammalian species selected for their extraordinary exercise capability.
DEMAND VERSUS CAPACITY: RELATIVE MALLEABILITY IN O2 TRANSPORT SYSTEMS
Many of the physiological variables contributing to maximum O2 transport undergo substantial levels of phenotypic plasticity in response to intense, long-term physical training. For example, increases in V̇o2max are accompanied by enhanced circulating blood volume and Hb mass, increased left ventricular compliance and volume, reduced vascular resistance, and increased muscle mitochondrial volume and capillary density and therefore O2 extraction (118). Should one also expect the lung parenchyma, airways, respiratory muscles, pulmonary vasculature, and ventilatory control system to undergo the appropriate phenotypic plasticity to match the demands for greater O2 transport imposed by the higher metabolic rates achieved in the endurance-trained individual? After all, these functions and structures appear to be overbuilt and/or regulated to a near-perfect extent in the untrained, healthy, young adult.
First, lung volumes and diffusion surface areas in mammalian species are capable of undergoing phenotypic plasticity in response to several types of chronic stressors, at least during maturation. These include long-term exposure to hypoxic environments (23, 80), partial pneumonectomy (80), and even severe starvation and refeeding (23, 123). Furthermore, in some mammals, such as the pronged-horn antelope with V̇o2max three- to fourfold greater than that of sedentary species of similar body mass, the lung’s diffusion capacity rises in proportion to other enhanced cardiovascular and muscle metabolic capacities (193, 209). Genetic influences were also revealed via artificial selection for running endurance in rodents. Even though 7 generations of selective breeding yielded no pulmonary changes, 15 generations provided lung volumes and diffusion capacities that were increased out of proportion to body mass (99). However, this plasticity is not forthcoming in the healthy lung’s response to physical training, either during or following maturation (168, 177, 180). Even human endurance-trained athletes with superior V̇o2max may or may not have marginally enhanced diffusion capabilities, lung volumes, and maximal flow-volume capacities (30, 33, 168, 194). The absence of training effects on lung morphology is especially surprising given the adaptive role of chronic lung stretch (for example, with pneumonectomy) and metabolism (for example, with nutritional changes) in altering lung volume and diffusion surface (164). In contrast, the aerobic capacity of respiratory muscles, is, like limb locomotor muscle, uniformly increased in response to intense training (187). Thus, given the limited plasticity in the lung with training and even via genetic endowment in the Olympian, the airways, diffusion surface, pulmonary vasculature, and respiratory muscles need a greater “safety margin” to accommodate the increased demand driven by the more malleable controllers of O2 transport and utilization. We now discuss the accumulated evidence to date, which documents successes and failures and highlights those groups that are most susceptible to exercise-induced respiratory system limitations.
UNDERBUILT/MALADAPTED AIRWAYS IN HIGHLY TRAINED ENDURANCE ATHLETE
Capacity for Flow-Volume Less Than Demand
Figure 2 shows mean values in a group of highly trained runners in whom the requirements for ventilation during heavy to maximum exercise meet or exceed their maximum volitional flow-volume loop. The corresponding esophageal pressure-volume loops demonstrate that the tidal expiratory pressures at these high work rates often exceed maximum “effective” pressure, where further increases in expiratory muscle effort provide no additional flow because of positive, collapsing transmural airway pressures. The inspiratory muscles are required to produce forces within 5–10% of their maximum dynamic pressure available for velocity of shortening and force production at the flows and volumes achieved at maximum exercise. Peak intrathoracic pressures (ITPs) are in the ± 30–35 cmH2O range and gastric pressures in the ± 15–25 cmH2O range, as active expiration is initiated during even mild exercise with subsequent progressive recruitment of rib cage and abdominal expiratory muscles at rising exercise intensities.
The consequences of the “excessive” ventilatory demand and associated intrathoracic and abdominal pressures during heavy to maximum exercise in trained subjects include the following:
-
•
Hyperinflation, which allows increased flow rates but places the inspiratory muscles at a shorter and less optimal length for force production and requires tidal volume to be generated on a less linear, more inefficient part of the pressure-volume relationship. Dynamic hyperinflation does not always occur, even in the presence of complete expiratory flow limitation, although this circumstance also results in excessive expiratory intrathoracic and abdominal pressures.
-
•
Excessive inspiratory and expiratory work of breathing leading to respiratory muscle metabolite accumulation and fatigue eliciting associated reflex effects on limb vascular resistance (see respiratory muscle demand greater than capacity).
-
•
Constrained hyperventilatory response to heavy exercise, as demonstrated by the negligible or reduced ventilatory response to superimposed inspired CO2 and/or hypoxia (vs. those responses obtained at lower exercise intensities) (29, 94, 126). Increases in the hyperventilatory response to exercise and/or superimposed chemical stimuli were observed when the maximum flow-volume envelope was enlarged and expiratory flow limitation reduced via low-density He:O2 inhalation (39, 126, 133).
-
•
Limited numbers of studies have manipulated intrathoracic and abdominal pressures in exercising humans and canines and observed the following cardiovascular consequences. During inspiration, reducing the negativity of ITP reduced stroke volume (67), reflecting the importance of preload, and raising abdominal pressure increased resistance and impeded the return of blood flow in the femoral vein (130). During expiration, with small superimposed increments in ITP, stroke volume is reduced presumably because a decreased transmural pressure across the ventricles impedes diastolic filling (130, 190). Together, these findings point to a significant respiratory constraint of venous return and stroke volume in heavy exercise secondary to a combination of positive intra-abdominal pressures and especially positive intrathoracic pressures during expiration: effects that would be exacerbated via expiratory flow limitation.
Using the overlap metric of tidal to maximum flow-volume (62),1 a significant prevalence of underbuilt maximum flow-volume envelopes in response to the demands of ventilation during heavy-maximum intensity exercise has been reported in endurance-trained athletes of all ages and especially in the master athlete and in females (see exceptional susceptibility to respiratory system limitations). In addition to excessive ventilatory demand driven by high work rates in the highly trained subject, exercise-induced flow limitation is also precipitated (even at relatively low levels of ventilation) by compromised airway structure, as observed in females versus males and in the lungs of elderly master athletes (see below). Alternatively, those athletes (e.g., swimmers) with especially large lung volumes and airways and very large maximum flow-volume envelopes have excessive safety margins in lung morphology and will avoid expiratory flow limitation and its sequelae even at high ventilatory demands (8). The large lung volumes seen in competitive swimmers may act to provide selective advantage, with studies indicating that lung volume appears superior even at a young age and that growth is not enhanced by subsequent training exposure (19).
Underbuilt Intrathoracic Airways
We refer here to airways extending from the main bronchi beginning with generation eight of the tracheal bronchial tree with 2 mm or less diameter, lacking cartilaginous support, and lined with smooth muscle and surfactant. In most healthy untrained subjects, these airways maximally bronchodilate with exercise (see above). Furthermore, moderate-intensity exercise training regimens commonly reduce airway inflammatory markers and airway hyperresponsiveness in mildly asthmatic individuals (128, 202). Training has also been shown to prevent cigarette smoking-induced chronic obstructive pulmonary disease in rodents by inhibiting local oxidative stress and proinflammatory cytokine release in the airways combined with release of anti-inflammatory cytokines from contracting skeletal muscle (196).
In contrast, elite endurance-trained athletes have a high prevalence of airway narrowing during or immediately following high-intensity exercise (98, 111, 163, 185). Furthermore, high-intensity training in human cross-country skiers or in horses or sled dog athletes causes disruption and remodeling of the airway epithelial layer, often accompanied by hypersensitized airway reactivity (95). The high ventilatory demands requiring flow rates in excess of ten times resting levels in the endurance-trained athlete appear to induce injury and remodeling of the airway epithelium via two types of stress (7). First, dehydration stress occurs over the first 10–12 airway generations as a high rate of evaporative water loss occurs and the osmolality of the very thin airway surface layer increases. This leads to a sloughing/detachment of the epithelial layer (7, 64). Second, mechanical sheer stress, via repeated stretch and compression of airway epithelium, likely invokes release of cytokines and epidermal growth factors (151). In the elite endurance athlete who trains daily in a cold, dry environment, the repeated release of these molecules forms an integral part of the injury-repair process in the subepithelial environment, promoting a hypersensitization of the contractile properties of the airway smooth muscle (97, 98, 151). This process seems to be at least partially reversible, with studies indicating that airway inflammation and hyperresponsiveness can revert to normal following a period of detraining in elite swimmers. Indeed, this has led some authors to propose that the development of lower airway dysfunction in some groups of elite athletes may be considered an occupational lung disease (74, 162).
Although exercise-induced bronchoconstriction (EIB) is primarily manifested immediately following intense, sustained exercise, airway resistance is also usually compromised, leading to an increased work of breathing during exercise, along with a constrained alveolar ventilation, V̇a/Q̇ nonuniformity (138), and O2 and CO2 exchange (71, 72). Several weeks of inhaled corticosteroid treatment have been shown to improve breathing mechanics and gas exchange in these subjects along with an enhancement of exercise performance (72).
Underbuilt Upper Extrathoracic Airway
The upper or extrathoracic airway, defined anatomically as the airway section lying above or proximal to the thoracic inlet, has generally been overlooked in exercise performance science (163). This is remarkable, given the fact that this airway segment contributes a high proportion of the total resistive load on the respiratory system and indeed is often described as the “bottleneck” of the airway (31, 179). Moreover, it does not function as a simple airflow conduit and has evolved intricate structural and neuromuscular capability to ensure effective and often synchronous delivery of a number of physiological functions, including swallowing, vocalization, and cough (81).
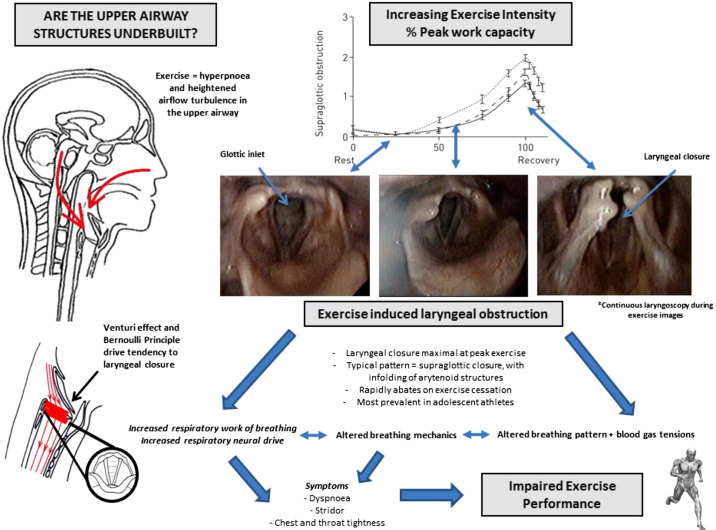
During vigorous exercise and as ventilatory demand progressively increases, there is evidence of a transient dilatation of the laryngeal inlet, likely driven by heightened neural traffic to the primary laryngeal abductor muscles (83). In all subjects, however, the laryngeal inlet remains a distinct “choke” point within the airway conduit. Movement of air across the upper airway is governed by the same laws of aerodynamics that dictate the behavior of any fluid traversing a tubular structure. Specifically, both the Venturi effect (linear movement through a tube causes decrease in lateral pressure) and Bernoulli principle (pressure is least where the velocity is greatest) dictate that as airflow increases, so negative transmural pressure gradients encourage inward collapse of surrounding (i.e., laryngeal) structures. Any compromise in the dimensions of the laryngeal space, even slightly beyond normal parameters, can thus cause a substantial increase in airway resistance, and as the glottic aperture narrows, this encourages any vulnerable structures to approximate medially or collapse inwardly (52) (see Fig. 4).
Fig. 4.
Schematic diagram highlighting the interplay of factors relevant to exercise-induced laryngeal obstruction (EILO). Laryngeal images taken during exercise of increasing intensity (images acquired at rest and moderate- and peak-intensity exercise), showing EILO. Images on schematic approximated to a figure adapted from Olin et al. (146), highlighting the close relationship between exercise-intensity and onset of EILO. [Adapted from Olin et al. (146) with permission of the © ERS 2020.]
At rest or in states of mild to moderate hyperpnoea, the movement of laryngeal structures appears to be of very limited or no immediate relevance. However, in athletic individuals capable of increasing ventilation considerably, the potential for flow-driven pressure changes across the glottic inlet becomes increasingly relevant. Indeed, it has been postulated that the laryngeal structures exist in a state of “force balance,” such that if airflow is modeled to increase indefinitely, then there would come a point in all individuals whereby the forces promoting glottic opening would be overcome and some form of medial or inward laryngeal collapse would occur (15, 37).
Although seemingly hypothetical, exercise-associated upper airway closure is an increasingly well-recognized and highly prevalent issue in athletic individuals. This transient phenomenon, termed exercise-induced laryngeal obstruction (EILO) (although still called exercise-induced vocal cord dysfunction in some centers), is now recognized to affect between 5 and 7% of all adolescents (90), ~7% of randomly selected Northern Europeans under 25 yr old (27), and 15–20% of athletes with apparently unexplained exertional breathlessness (142). There appears to be a female preponderance in most studies, and this appears to reflect the demographic characteristics encountered in real-life clinical practice; however, some epidemiological studies reveal no gender differences (90).
Laryngeal closure during exercise is associated with the development of dyspnea and stridor (82), an increased resistive work of breathing, heightened respiratory neural drive (207), and arterial hypoxemia (69). These deleterious consequences for physiological performance are transient and abate rapidly on exercise cessation (146). In an extreme form, however, the presence of fixed laryngeal narrowing (e.g., with a glottic stenosis) results in significant hypercapnia during exercise and a progressive hypopnoea, driven by the consequent mandatory prolongation of the inspiratory phase of the respiratory cycle (3).
The precise etiology of EILO is currently unknown; however, it seems likely that a relative deficiency in the structural integrity of some laryngeal structures (e.g., the supraglottic structures) is relevant in many cases (144). Studies extrapolating and modeling increasing flow, from axial imaging allied with computational flow dynamics, indicate that glottic narrowing is predictable, based on the aforementioned Venturi principle, and closure has significant up- and downstream consequences for airflow (54) and most likely for the work of breathing. Overall, this would tend to support a viewpoint that the upper airway, and specifically the larynx, could in many cases be considered relatively underbuilt for the very high ventilatory demand state. It is conceivable that extreme adaptation in other physiological systems (e.g., in cardiovascular function) places the larynx in an unfavorable or maladaptive position under states of extreme hyperpnoea in some (susceptible) individuals. In support of this argument is the finding that EILO appears to be most highly prevalent in adolescent female athletes and, indeed, at least in some series appears to regress to a degree with further maturation (119). Indeed, although there are limitations inherent to making accurate flow measurements in this area, it is generally agreed that in the majority of young individuals, EILO arises as the consequence of an imbalance between forces acting to maintain abduction (i.e., structural and neuromuscular support) and the airflow-associated pressure changes acting to promote glottic adduction. Moreover, when athletes are reevaluated following laryngeal corrective surgery (for anatomical excessive tissue in the arytenoid area of the larynx) (50), there is an apparent improvement in symptom burden but also a corresponding reduction in markers of resistive loading and work of breathing (50, 207). Overall, therefore, it is conceivable that in individuals with either a structural or functional predisposition to having an underbuilt upper airway (e.g., as may be seen in some young female athletes), the forces promoting laryngeal abduction become challenged and often overwhelmed by exercise hyperpnoea and the associated physical pressure changes and airway turbulence that ensues. This acts to encourage the upper airway to dynamically adopt behavior that leads to inspiratory flow limitation and, akin to that seen in expiratory flow limitation, it may be possible to establish this by evaluating the influence of further pressure modulation on flow in this region. An analogous physiological scenario is that of obstructive sleep apnea, whereby for afflicted individuals there is typically structural upper airway abnormalities that then predispose an individual to airway collapse during a state of reduced tonic input to the airway dilator musculature; i.e., at the onset of sleep.
Despite significant progress in our understanding and awareness of the role of the upper airway in exercise physiology, over the past decade, the field still remains at a somewhat embryonic stage. A considerable amount of further work is needed now to better decipher the pathophysiology underpinning the laryngeal structural deficiencies seen in so many young individuals with EILO, i.e., why it is that the upper airway in some athletic individuals is underbuilt. We also need to progress our understanding of the factors dictating airflow, turbulence, and pressure change in the upper airway (55) and start to routinely employ objective visualization techniques (114) to ensure physiologists and clinicians have an accepted and standardized approach in assessing this problem; only then will we start to better appreciate whether the extrathoracic airway is over or underbuilt for exercise.
UNDERBUILT PULMONARY VASCULATURE
Effects of High Cardiac Output Combined with Limited Vasodilation
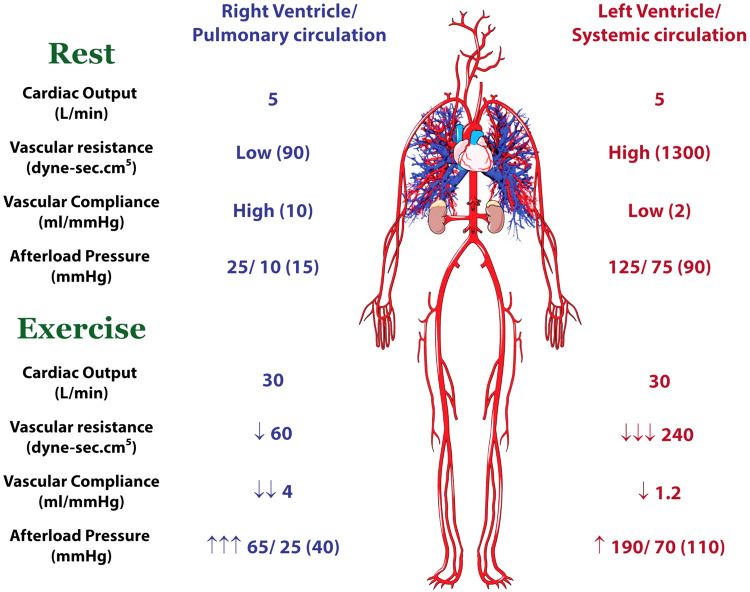
The benefit of the low resistance, high compliance pulmonary vascular circuit is that it reduces right ventricular (RV) work. The work, and oxygen demand, of the right ventricle is highly dependent on afterload (pulmonary arterial pressures) and to a lesser extent on heart rate, inotropy, and preload. Thus, low pulmonary artery pressures at rest enable the RV to do very little work, and the heart has adapted to these requirements such that the RV has approximately one-quarter the mass and contractile reserve of the left ventricle (LV) (108). In other words, the RV is built to deal with low pressures and the LV is built for moderate to high pressures. As outlined above, this arrangement works beautifully under resting conditions and during mild to moderate activities with modest increases in cardiac output.
Pulmonary artery pressures increase in a near-linear manner with exercise. Multiple studies using echocardiographic estimates of pulmonary artery pressures and direct invasive measures have demonstrated that mean pulmonary artery pressures increase by ~1 mmHg for every liter of cardiac output (113). Thus, in a well-trained athlete who increases cardiac output from 5 L/min to more than 30 L/min, the mean pulmonary artery pressure would be expected to increase nearly threefold, as compared with the systemic mean blood pressure that typically increases 30–40% to maximal exercise intensity. As summarized in Fig. 5, the work demands of the RV during exercise increase disproportionately to those of the LV. This concept was interrogated by La Gerche et al. (106), who used a combination of cardiac imaging and direct invasive pressure measurements at rest and at peak exercise intensity in athletes and nonathletes. RV end-systolic wall stress increased in athletes more than in nonathletes, but in both cohorts the increase was far greater than for the LV (125% vs. 14%, P < 0.0001). At rest, the systolic wall stress in the RV was less than half that of the LV and was therefore matched to its lesser mass and contractile reserve. However, at peak exercise the contractile forces, work, and oxygen demands of the RV would seem to exhaust the reserve given its lesser myocyte mass. This disproportionate RV work during exercise has also been elegantly demonstrated in exercising dogs, in which the venous effluent from the RV and LV can be sampled separately. Although coronary blood flow to the two ventricles is similar, the coronary venous Po2 falls considerably more in the RV, reflecting the greater increase in work (68, 199).
Fig. 5.
Comparison of the presystemic and systemic circulation demands during exercise as compared with rest. The low resistance, high compliance pulmonary circulation imposes little work on the right ventricle (RV) at rest, but during exercise there is limited capacity to reduce resistance further. With the distension of the vasculature during exercise, compliance reduces. Thus, the pulmonary and RV pressures increase 2- to 3-fold. In the systemic circulation, the vascular resistance falls markedly, leading to a marked attenuation of the increase in ventricular work. Figure is based on data and concepts from La Gerche et al. (106).
A logical extension of the physiological constraints of the pulmonary circulation during intense prolonged exercise is that one might expect to observe RV dysfunction or fatigue. That is exactly what has been observed. La Gerche et al. (104) were the first to observe marked dilation and dysfunction of the RV after intense endurance exercise, and this has been validated in numerous subsequent studies using both echocardiography and cardiac MRI (28, 102, 136, 148, 150, 198). The degree of dysfunction appears related to both the intensity and duration of exercise as might be expected from the physiology: the intensity mandating high RV load and the duration exhausting the metabolic reserves and possibly resulting in some secondary inflammatory damage and interstitial damage (47). In the longer term, the RV tends to partially adapt by increasing its myocardial mass (and contractile reserve). In athletes, the RV-to-LV mass has been observed to be greater than in nonathletes (17, 106).
Clinical Consequences of Right Heart Remodeling
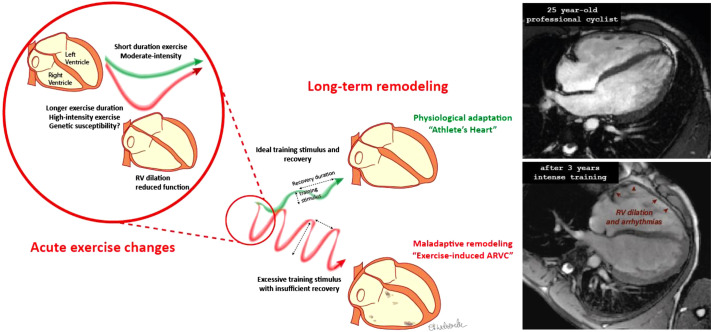
There are important clinical consequences of the underbuilt pulmonary vasculature and overstressed RV in endurance athletes. The repeated physiological stressors and resultant acute myocardial damage can result in chronic adverse remodeling that predisposes some athletes to potentially life-threatening arrhythmias (73, 103, 201). It has been well established that endurance exercise is associated with an increase in RV arrhythmias in people with a genetic predisposition to certain cardiomyopathies, suggesting that there is a potential environmental-genetic interaction in which intense exercise can exacerbate abnormalities in athletes with a weakness in cardiac structure and function (87, 178, 208). Increasingly accepted is the concept that a similar process may develop in some endurance athletes who have no identifiable genetic predisposition (109, 181) in a condition coined “exercise-induced right ventricular cardiomyopathy” by Hein Heidbuchel in 2003 (73) (see Fig. 6). The concept of exercise-induced RV fatigue raises another intriguing hypothesis: could this limit cardiac performance during prolonged exercise? Could it explain the “cardiac drift” in which heart rate and perceived exertion tend to increase and output plateaus or decreases? This has seldom been interrogated, but in a small cohort of athletes, Claessen et al. (28) studied biventricular function during exercise at the completion of an endurance cycle race and observed a relative failure of RV augmentation. This deserves further attention and may provide novel insights into central fatigue during intense prolonged exercise.
Fig. 6.
Acute right ventricular (RV) load during exercise leading to chronic remodeling and arrhythmias. The disproportionate load on the RV during exercise can predispose to chronic remodeling that may be adaptive or maladaptive. These different responses may be related to training, genetics, or other unknown factors. The maladaptive athlete’s heart is predisposed to arrhythmias that predominantly arise from the remodeled RV. ARVC, arrhythmogenic RV cardiomyopathy. [Adapted from La Gerche and Heidbuchel. (105) with permission from Wolters Kluwer Health.]
Thus, a picture emerges of a degree of central limitation during intense exercise with potentially severe complications in a minority of athletes. However, as opposed to earlier descriptions of exercise physiology focusing on the systemic circulation and LV, research over the past few decades has narrowed much of the constraint to the pulmonary circulation and its perfusing pump, the RV. There are, however, several ways in which the body attempts to attenuate the pulmonary vascular limitations during exercise. As discussed in the earlier sections, pulmonary blood flow is not uniform; rather, there are zones of relative hypoperfusion at rest: those of greater ventilation and others of greater perfusion. Flow down larger diameter capillaries (such as the subpleural capillaries) is prioritized during exercise, and this phenomenon has been demonstrated with the novel technique of contrast echocardiography in which bubbles of 15–25 µm are injected into the venous circulation and fail to pass through the lung microvasculature at rest (45, 116). During exercise, these bubbles are observed to pass through the pulmonary circulation, and in those subjects in whom this phenomenon is observed, it is associated with greater reductions in pulmonary vascular resistance, greater cardiac outputs, and enhanced exercise capacity (107, 173). This represents an intriguing way in which the body attenuates the constraint on flow and, to some extent, helps protect the RV against the physiological stresses imposed during strenuous exercise.
This also leads to the sensible hypothesis that pharmacological modification of the pulmonary vasculature could be used to attenuate the limitation imposed by the pulmonary vasculature and protect the RV from damage. There are several medications, such as phosphodiesterase-5 and endothelin inhibitors, that promote vasodilation of the pulmonary arteries in a relatively specific manner. This hypothesis has been addressed in a number of trials of healthy nonathletic and athletic individuals at sea-level, altitude, and normobaric hypoxia with very mixed results (22). A simple explanation for the minimal or absent efficacy of these therapies is that strenuous exercise induces near-maximal pulmonary vasodilation in healthy subjects such that pulmonary vasodilators have minimal additional effect.
In summary, the RV and pulmonary circulation represent something of an Achilles’ heel that constrains central output during intense exercise, particularly when the exercise is prolonged, and may result in RV damage and arrhythmias in a minority of athletes. Although there are some inbuilt mechanisms to attenuate the stress, it stands to reason that the presystemic circulation favors energy preservation and efficiency and that the RV has evolved to be better suited to resting and low/moderate activity than to intense endurance exercise.
UNDERBUILT ALVEOLAR TO ARTERIAL GAS EXCHANGE
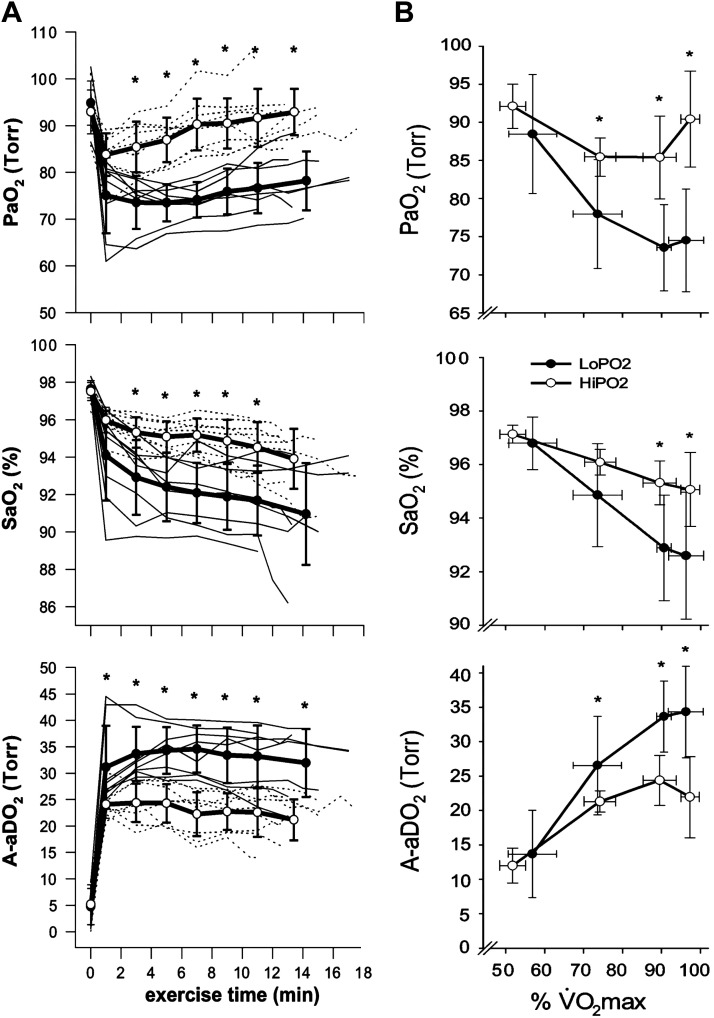
In the endurance-trained athlete (male and female, children, young and older adults), arterial HbO2 desaturation amounting to 4–12% below resting values occurs during heavy intensity incremental or constant load exercise, with a prevalence ranging between 30 and 70% among the various groups studied to date (n = ~20–100), using temperature-corrected measures of arterial blood gases or pulse oximetry (32, 36, 39, 42, 66, 67, 145, 159). In susceptible athletes, the HbO2 desaturation almost always first appears during submaximal steady-state exercise, usually at intensities at or slightly in excess of the lactate threshold and typically reaching a nadir at maximal exercise (see Fig. 7B). It is attributable in about equal amounts to a 10–30 mmHg reduction in below rest combined with a pH and temperature-induced decrease in HbO2 affinity. In turn, the reduced correlates most strongly with excessively widened A-aDO2 (~30–50 mmHg) in most cases and secondarily to a constrained hyperventilatory response ( ~36–43 mmHg) (36). Exercise-induced arterial hypoxemia (EIAH) and its determinants are highly reproducible within subjects upon repeat testing (189). Finally, a consistent observation across studies is the marked variability in EIAH among athletes of equivalent V̇o2max (see Fig. 7).
Fig. 7.
Major features of exercise-induced arterial hypoxemia (EIAH). A: pulmonary gas exchange in a group of 17 female competitive runners during constant load treadmill running at 90% of maximal oxygen consumption (V̇o2max; 14 km/h, 3% grade) to exhaustion (preceded by 3 min at each of 50 and 75% of V̇o2max). Subjects were 27 ± 7 yr old, with V̇o2max of 50 ± 4 (44–56) mL·kg−1·min−1. Subjects were divided into those with >15 vs. <10 mmHg exercise-induced reductions in arterial partial pressure of oxygen (), with no differences in V̇o2max between EIAH and non-EIAH groups. Note the reductions in and raised alveolar-arterial partial pressure differences (A-aDO2s) within the first minute of exercise at 90% V̇o2max, which remained constant through exhaustion. Further reductions in arterial oxygen saturation () were secondary to a Bohr effect rightward shift in oxyhemoglobin HbO2 dissociation as arterial pH fell to 7.26 and esophageal temperature rose 2.3°C. B: mean ± SD values for the EIAH and non-EIAH groups during 3 min of treadmill walking/running at each of 50, 75, and 90% V̇o2max. Note significant EIAH was already present at 75% V̇o2max. Nadirs in and with A-aDO2 widened to maximum values occurred at 90–100% V̇o2max. *Significant difference between groups (P < 0.05) [Adapted from Wetter et al. (211)].
-
•
A few studies have examined potential mechanisms underlying the excessive A-aDO2 by comparing subjects with EIAH versus those without, as summarized below. Application of the multiple inert gas elimination technique (MIGET) to athletes with severe EIAH (169) revealed approximately equal contributions to the widened A-aDO2 from V̇a/Q̇ maldistribution, a constrained hyperventilatory response, and a combination of alveolar capillary diffusion disequilibrium perhaps combined with some degree of small intra/extrapulmonary shunts. The diffusion limitation may be explained by excessive exercise-induced decreases in the compound variable D/βQ, (D is diffusion capacity, β is the shape of the HbO2 dissociation curve in the pulmonary capillary, and Q is blood flow) (153). In the EIAH athlete, a normal or even reduced maximum diffusion capacity combined with a high β (i.e., steeper slope of the HbO2 dissociation curve) secondary to increased O2 extraction and reduced mixed venous partial pressure of oxygen () and a high Q̇ are common. Although exercise-induced intrapulmonary shunts do occur, it remains undecided whether they contribute significantly to EIAH (115).
-
•
Durand et al. (43) compared moderate EIAH versus non-EIAH trained-subjects, reporting higher Ppa (> 42 mmHg) and higher pulmonary vascular resistance, with equivalent diffusing capacity for carbon monoxide (DLco) during maximum exercise. These findings point to contributions to EIAH from V̇a/Q̇ maldistribution secondary to excessive Ppa precipitating interstitial edema, V̇a/Q̇ nonuniformity, and/or intrapulmonary shunt (191, 210). The presence of red cells in bronchoalveolar lavage fluid following intense exercise in trained cyclists was suggestive of a mechanical stress-induced disruption of the blood-gas barrier (77).
-
•
The onset of EIAH during submaximal exercise speaks against the concept of excessive demand (vs. capacity) as the sole determinant of EIAH in the athlete. Wetter et al. (211) tested the possibility that exercise-induced inflammation of the small, peripheral airways associated with chronic, intense physical training (see Underbuilt Intrathoracic Airways) might account for a widened A-aDO2, perhaps via ventilation distribution nonuniformity. Although significant numbers of athletes did show evidence for increased airway reactivity and/or increased small airway resistance, these characteristics did not clearly distinguish those with EIAH. High-resolution imaging of ventilation distribution needs to be applied during exercise to address this question.
-
•
The constrained hyperventilatory response to maximum exercise will contribute to EIAH via a reduced overall V̇a/Q̇ in the face of a nonuniform V̇a/Q̇ distribution and falling concentration of mixed venous oxygen (). The ventilatory constraint is attributable to a significant extent in most athletes to mechanical limitations on both the expiratory (airways) and inspiratory (muscles) phases of respiration. However, there may also be an important constraint of neurally mediated “central drive” to breathe in play, as mechanical limits to V̇e are often approached but not reached in many of these EIAH athletes with a reduced hyperventilatory response (66, 94). This proposed reduction in central respiratory motor output likely reflects a broad interindividual continuum of responsiveness to both the multiple feedback and feedforward excitatory drives to breathe during heavy intensity exercise, in addition to the inhibitory effects of feedback from the lung and chest wall (53).
The consequences of EIAH have been quantified using increments in fraction of inspired oxygen () just sufficient to maintain HbO2 saturation at resting levels. These studies have revealed a threshold of ~3% decrement in arterial oxygen saturation () to begin to detect a significant negative effect on V̇o2max, with a decrement of ~2% in V̇o2max for each ensuing 1% fall in (65, 143, 160). Thus, the maximum decrement in V̇o2max attributable to EIAH is ~15% in humans (at 88% ). Similar studies conducted during cycling time trials showed that preventing the EIAH reduced the level of locomotor muscle fatigue and improved endurance performance (175)2.
RESPIRATORY MUSCLE DEMAND GREATER THAN CAPACITY
At high-intensity exercise, multiple primary and accessory inspiratory and expiratory muscles are recruited, requiring V̇o2s and blood flows amounting to 15–20% of the maximum V̇o2 and CO in trained subjects (2, 67, 121, 182). The use of electrical or magnetic supramaximal stimulation of the motor nerves (1–20 Hz) using transdiaphragmatic pressure (Pdi) or gastric pressure (Pg) as a measure of force output showed that both the diaphragm and expiratory muscles undergo significant fatigue during high-intensity sustained exercise so long as the exercise intensity is in excess of 80% of peak work rate. Respiratory muscle fatigue begins around the midpoint and persists with further exercise to exhaustion and persists for 1–2 h in recovery (91, 206). Highly trained subjects required a higher absolute work rate than less-trained subjects did to experience diaphragm fatigue (11). Additional evidence that O2 transport to the respiratory muscles did not meet metabolic requirements was obtained using near-infrared spectroscopy monitoring, which revealed deoxygenation of accessory respiratory muscles of the trunk during heavy intensity cycling exercise (112, 200).
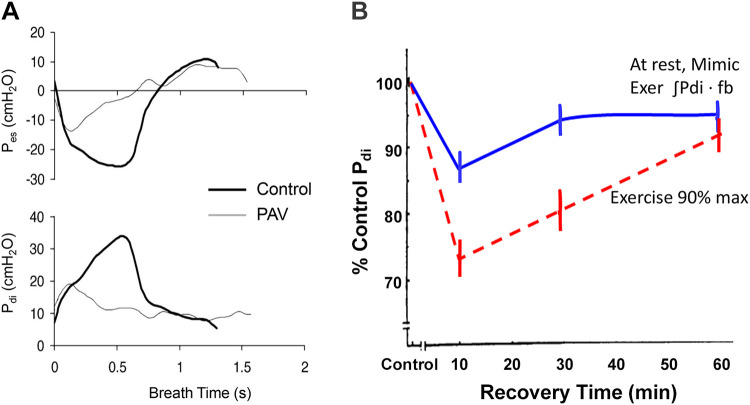
Two lines of evidence in the exercising human have revealed the major factors contributing to exercise-induced metabolite accumulation and fatigue of the diaphragm (see Fig. 8, A and B). First, when a mechanical ventilator was used to reduce the inspiratory work of breathing by 40–60%, exercise-induced diaphragm fatigue did not occur (10). Second, when the level of diaphragmatic work incurred during high-intensity exercise was volitionally reproduced with the subject at rest, the resulting diaphragm fatigue was either nonexistent or greatly reduced and recovery was almost immediate; furthermore, a substantially higher level of voluntary diaphragmatic work was required to elicit diaphragm fatigue comparable to that incurred during the treadmill exercise (see Fig. 8B) (12). These findings reveal that both the absolute level of the work of breathing and the coincident locomotor muscle exercise, per se, contributed significantly to exercise-induced diaphragm fatigue: the former through imposing a high sustained demand for O2 transport, and the latter likely via the effects of limb-exercise-induced sympathoexcitation on curtailing increases in respiratory muscle vascular conductance and blood flow (182). This proposed effect of heavy intensity limb exercise on curtailing diaphragmatic blood flow is analogous to the finding that substantial increases in leg vascular conductance during moderate to heavy intensity cycling were curtailed via increased sympathetically mediated vasoconstriction achieved by superimposing arm exercise (170, 192). Thus, although in vitro findings in rodent muscle vasculature support an attenuated vasoconstrictor responsiveness in the diaphragm versus limb vasculature (1), during high-intensity exercise the respiratory muscle vasculature appears not to be completely resistant to the influence of enhanced sympathetic vasoconstrictor activity emanating from the working limbs (157, 182).
Fig. 8.
Major contributions to exercise-induced diaphragm fatigue from work of the diaphragm (A) and locomotor muscle work (B). A: a proportional assist ventilator (PAV) was used to reduce the peak inspiratory esophageal and transdiaphragmatic pressures by 40–70% during exercise to exhaustion at >80% maximal oxygen consumption (V̇o2max). This reduced work of breathing prevented diaphragm fatigue, presumably a reflection of reduced metabolite accumulation and therefore less stimulation of respiratory muscle group III-IV afferents. The consequences of this respiratory muscle unloading included: a) decreased blood flow to accessory respiratory muscles; b) reductions in both median nerve muscle sympathetic nerve activity (MSNA) in the resting arm and norepinephrine spillover across the working limb; c) increased vascular conductance and blood flow to the working limbs; and d) less limb fatigue and prolonged exercise performance [see summary in Sheel et al. (182)]. B: diaphragm fatigue measured as the transdiaphragmatic pressure (Pdi) response to 1–20 Hz phrenic nerve stimulation before and after treadmill exercise to exhaustion at 90% V̇o2max vs. before and after voluntary mimic at rest of the diaphragmatic work achieved during exercise. The greater diaphragm fatigue and the prolongation of recovery following the treadmill exercise reflects the effects of coincidental locomotor muscle exercise, per se (see text) [Adapted from Babcock et al. (12)]. fb, breathing frequency.
The cardiovascular consequences of the normally occurring exercise-induced inspiratory and expiratory muscle work and metabolite accumulation were revealed through the use of mechanical ventilation (see summary in Fig. 8A). These included reduced sympathetic vasoconstrictor activity and increased blood flow to locomotor muscles, leading to reduced limb fatigue and increased exercise performance. These sympathetic and cardiovascular consequences of respiratory muscle unloading occurred during high-intensity exercise in which diaphragm fatigue was prevented but also during very brief duration high-intensity exercise and even during submaximal exercise, during which diaphragm fatigue was unlikely to have occurred (40, 67). Based on pioneering investigations of phrenic nerve afferents in rodent and canine preparations (76, 85, 88, 174), we propose that these cardiovascular responses in exercising humans resulting from the prevention of metabolite accumulation in the respiratory muscles (via a reduced work of breathing) were secondary to attenuation of the group III-IV metaboreceptor-induced increase in sympathetic vasoconstrictor activity. The reductions in limb fatigue and improvement in endurance exercise performance with respiratory muscle unloading are presumed to reflect the beneficial consequences of preventing reflex effects of metabolite accumulation in the respiratory muscles. This interpretation is consistent with the exacerbation of limb muscle fatigue and reductions in performance elicited by fatiguing the diaphragm (via voluntary hyperpnea at rest) before cycling exercise (213).
EXCEPTIONAL SUSCEPTIBILITY TO RESPIRATORY SYSTEM LIMITATIONS
There is growing evidence that many highly fit humans, the healthy elderly, females, athletes exposed to high altitudes, and select athletically gifted canines, felines, and equines experience an especially high prevalence of respiratory system limitations to exercise performance.
Thoroughbred Horse: Underbuilt and Inadequately Designed
Owing to highly selective breeding over 300 yr and originating in a small gene pool, this species provides the ultimate example of an underbuilt lung structure providing the primary source of exercise limitation (156). On the “demand” side, a massive maximum O2 flux capacity (V̇o2max 140–220 mL/kg) is supported by the sheer mass of active skeletal muscle (55% body mass), mitochondrial volume, and capillary density together with a very large compliant heart (1.5–2.0% body mass) capable of producing up to a 2-L stroke volume and >400 L/min cardiac output. This huge O2 transport system is complemented by an O2 extraction across the active muscle that exceeds 90% at V̇o2max and an exercise-induced splenic contraction that doubles circulating red cell mass and O2 carrying capacity. Despite the prodigious structural capacity of the thoroughbred equine lung, including a 2,400-m exchange surface area, it remains grossly underbuilt and inadequately designed, as demonstrated by the following responses to heavy intensity exercise: 1) Ppa exceeds 120 mmHg secondary to the high cardiac output and downstream effects of a raised left atrial pressure, with right ventricular wall thickness equal to or exceeding that of the left (108); 2) expansion of the pulmonary capillary blood volume is insufficient to maintain red cell transit time >0.3 s; 3) a high dead space ventilation occurs because of the horse’s extreme tracheal length and high breathing frequency coupled to locomotion, yet the resultant need for greater total minute ventilation is opposed by a high resistive work of breathing because of obligate nasal ventilation coupled with a partial collapse of the unsupported nasal airway on inspiration (156). The result is a marked alveolar hypoventilation ( ~50–60 mmHg), i.e., “respiratory failure,” in the face of marked swings in intrapleural pressure; 4) a combination of a short red blood cell transit time plus severe hypoventilation induces severe EIAH in all thoroughbreds studied ( ~60–70 mmHg), and a right-shifted HbO2 dissociation curve via the Bohr effect secondary to a marked respiratory plus metabolic acidosis exacerbates HbO2 arterial desaturation (75–85% ); and 5) the combined high intraluminal pulmonary capillary pressures plus marked intrapleural pressure swings rupture the alveolar capillary interface with “hemorrhage” of red cells into the alveolar space and airways (14, 122, 156).
When EIAH is prevented [via modest increases in (203)] or the high resistive work of breathing is alleviated via nasal stents (156), exercise performance is substantially improved in all thoroughbred equines.
Aging: Does the Age-Dependent Decline in Respiratory System Capacity Parallel or Exceed That in Demand?
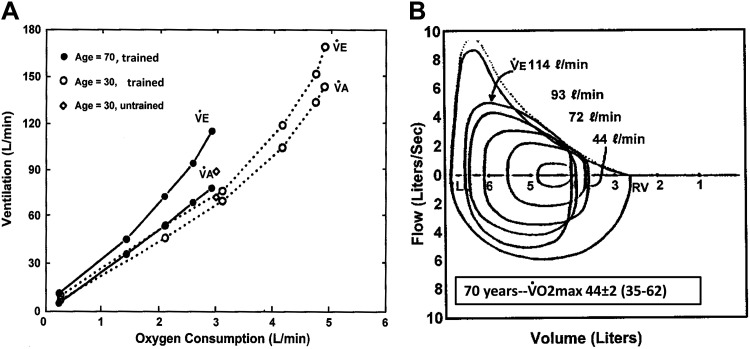
Beginning as early as the second decade, lung structure begins its inexorable decline, including loss of lung elastic recoil, alveolar duct enlargement, and rib cage calcification. Functionally, these changes are manifested in reductions in the maximum effective expiratory pressure and the maximum flow-volume envelope, leading to increased airway closing volume, hyperinflation, and reduced inspiratory and vital capacities as well as a less-compliant chest wall and pulmonary vasculature and a markedly reduced lung diffusion capacity (92). Accordingly, in the sixth through eighth decades, even at rest, both trained and untrained subjects show airway closure at higher lung volumes, hyperinflation, and a moderate widening of the alveolar to arterial Po2 difference with 5–15 mmHg reductions in .
During moderate- through maximum-intensity exercise, an increased V̇e/V̇co2 with elevated breathing frequency and reduced Vt combined with mild hyperinflation results in increased work of breathing and dyspneic sensations, even in the untrained elderly subject with V̇o2max of 25–30 mL/kg and maximum V̇e in the 60–90 L/min range (89, 92, 120, 132) (see Fig. 9). The augmented V̇e/V̇co2 demonstrates a remarkable plasticity within the aging respiratory control system, which ensures homeostasis of V̇a/V̇co2 and during exercise near resting levels. On the other hand, this compensation comes at the substantial expense of an increased work of breathing associated with exertional dyspnea, with some evidence at maximum exercise of ventilatory constraint, as shown by ventilatory increases with He:O2 plus reduced ventilatory responses to added (9, 26). This enhanced perception of breathlessness on exertion likely contributes to a curtailment of an active lifestyle with healthy aging. However, it is doubtful if the respiratory system becomes a major limitation to maximum exercise performance in the untrained elderly because the 35–40% reductions in forced expiratory volume in 1 s (FEV1) and DLco at age 70 versus 25 yr are accompanied by 40% reductions in V̇o2max, i.e., maximal demand. This reduced V̇o2max is primarily driven by age-dependent reductions in ventricular compliance and muscle mitochondrial volume and therefore in maximum cardiac output and the maximum a-V̇o2 difference (75, 134, 135, 165).
Fig. 9.
Two major contributions to increased work of breathing during exercise in healthy elderly trained subjects. A: airway closure and narrowing on expiration occurs at higher lung volumes with aging, causing nonuniform ventilation distribution and increased falling dead space to tidal volume ratio (Vd/Vt) at rest (0.39 old vs. 0.30 young) and during exercise (0.29 vs. 0.15). Thus, at any given exercise carbon dioxide production (V̇co2), total minute ventilation (V̇e) is elevated in the elderly, which produces similar alveolar ventilation (V̇a) and partial pressure of carbon dioxide () as in the young but requires an increased work of breathing. B: maximum expiratory flow at 50% total lung capacity (MEF50) is reduced by 25–35% at age 70 vs. 30 yr and the maximum effective pressure for productive flow is reduced by 50–70%. Combined with the higher V̇e/V̇co2, this limited capacity for effective expiratory flow means that most trained 70-yr-old subjects achieve maximum effective expiratory pressures (and exceed 90% of maximum inspiratory dynamic pressures) during exercise at an oxygen consumption (V̇o2) that is 40% less and at a level of V̇e that is 50–75 L/min below that required in younger trained adults (compare with Fig. 2). At maximum exercise, many highly trained elderly showed little or no ventilatory increase with added chemoreceptor stimuli, and approximately one-third of the group showed alveolar-arterial partial pressure differences (A-aDO2) >30 mmHg and arterial oxygen saturation in the 87–93% range [Figure based on findings published in Miller and Dempsey (129)].
On the other hand, might an imbalance of O2 transport demand versus respiratory system capacity occur in highly trained elderly subjects whose trainable cardiovascular system and muscle metabolic capacities allow a V̇o2max in the 40–60 mL·kg−1·min−1 range, requiring ventilations in the range of 100–130 L/min and cardiac outputs of 15+ L/min (49, 79, 92, 197). In many highly trained 70-yr-old subjects, their maximum capacities for flow-volume and diffusion are still capable of accommodating the levels of maximum ventilations and O2/CO2 exchange achieved by untrained 30-yr-old subjects. This accommodation, even after 40 yr of aging, attests to the substantial reserve available in the healthy young lung. However, as in their younger trained counterparts, significant numbers of master athletes will experience severe expiratory flow limitation during heavy intensity-maximum exercise, with pleural pressures exceeding maximum effective pressure and a high work of breathing with substantial dyspnea. Less frequently, these elderly athletes experience limited hyperventilation, widened A-aDO2 in excess of 30–35 mmHg, 10–25 mmHg, and 4–10% below resting values (9, 63, 92, 129, 161) (see Fig. 9).
A limited number of observations based on invasive catheterization revealed a marked pulmonary hypertension during exercise in healthy elderly subjects (61, 166). Even at cardiac outputs of 10–15 L/min, the pulmonary artery (as well as pulmonary wedge) pressures consistently rose to levels that were twice those in healthy younger subjects, i.e., Ppa ~25–40 mmHg. In young adults, these levels of Ppa are achieved at cardiac outputs in the range of 20–30 L/min (139). These remarkable elevations in pulmonary vascular resistance and pressures in the elderly were attributed to age-related myocardial stiffness, causing reduced diastolic compliance of the ventricular wall and contributing to high left ventricular filling pressures.
Some, but not all, cross-sectional comparisons between trained and untrained elderly subjects show superior lung function in the trained subjects (9, 63, 92, 93, 124). Furthermore, a longitudinal 7-yr study showed that habitual physical training did not provide a protective effect against age-induced declines in lung function at rest and exercise in the sixth and seventh decades (124). Thus, available evidence, although limited, suggests that the lungs of older athletes, like their younger counterparts, are untrainable, whereas the cardiovascular, hematological, and muscular systems remain highly trainable. Thus, significant numbers of the trained elderly experience respiratory system limitations to heavy intensity exercise. The major difference in the master athlete is that these limitations occur at much lower metabolic demands than in their younger counterparts.
Underbuilt Conducting Airways in Females
Based on measures of lung mechanics, Jere Mead hypothesized 40 yr ago that the airway calibers of adult women were of smaller diameter than those of men even when compared at equal lung volumes: a condition that he termed “airway dysanapsis” (127). More recently, high-resolution computed tomography has demonstrated that airway cross-sectional areas are comparable between the sexes throughout maturation through pubescence, but postpubescent females show a 20–30% reduction in the diameter of the trachea and most mainstem bronchi (101, 171, 183). A smaller lung size in adult females accounts for much of these sex differences in airway size, but even when a limited number of comparisons were made at equivalent lung volumes the postpubescent and adult female still had narrowed trachea and bronchi (183). Resting diffusion capacity and lung volumes are also lower in girls and women versus boys and men, even when adjusted for age, height, and Hb concentration (141).
These sex differences in airway morphology are manifested during moderate through heavy intensity exercise:
-
•
Partial expiratory flow limitation with constraint of the hyperpneic response begins to occur at 40–60 L/min V̇e in healthy adult females of all ages and training status. Complete expiratory flow limitation at maximum exercise is observed in trained females at maximum V̇e much lower than in men (62, 125, 132).
-
•
At any given V̇e during exercise, the resistive work and oxygen cost of breathing, dyspneic sensations, and diaphragm fatigue are increased in adult females 20–70 yr of age (41, 57). When young men and women were compared at identical levels of diaphragmatic work (achieved at rest via pressure-threshold loading), diaphragmatic fatigue development was not different between the sexes (57).
-
•
In significant numbers of fit adult women, EIAH occurs secondary to both constrained hyperventilation and widened A-aDO2 during heavy intensity exercise (18, 39, 41, 66, 67, 211) as it does in fit men. The difference between the sexes was that EIAH commonly occurs in women with much lower V̇o2max (45–60 mL/kg). Pulmonary diffusion capacity and capillary blood volume are also lower in fit young women versus men during exercise at comparable cardiac outputs, and these differences were attributable entirely to lower lung volumes in females (18).
-
•
Preventing flow limitation via He:O2 breathing prevents hyperinflation and provides an increased hyperventilatory response (125). Substantially reducing the work of breathing (via mechanical ventilation) redistributes blood flow and reduces limb fatigue in women as it does in men, again at lower absolute exercise intensities in women (38, 41, 42, 57).
We interpret these data to mean that adult women of all ages and over a wide range of fitness levels have hormonally determined trachea, bronchi, and lung sizes that are underbuilt for the flow rates demanded by heavy intensity exercise. Because the consequences of airway dysanapsis are likely to exist in the majority of women and be manifested even in moderately heavy exercise, we would predict that adult women across the fitness range would experience a greater susceptibility to respiratory limitations to exercise than do men.
Respiratory System Underbuilt/Maladapted for Hypoxic Environments
Over five decades ago, the Mexico City Olympics revealed the devastation that even apparently “mild” elevations [2,200 m; barometric pressure ~580 mmHg; inspired partial pressure of oxygen () ~122 mmHg; arterial HbO2 saturation ~93% at rest] rendered the endurance performances of elite sea-level athletes. Subsequent experiments have confirmed the decrements in V̇o2max and endurance performance experienced by those athletes upon acute and especially short-term exposures to even moderate altitudes, beginning in the 600––1,300-m range (58, 60, 188). These performance decrements have been strongly linked with the following maladaptations: 1) the magnitude of exercise-induced arterial O2 desaturation in hypoxic environments, secondary to diffusion limitation [whether these athletes did or did not experience significant EIAH in normoxia (24, 35, 58, 60, 110)]; 2) an excessive work of breathing and its effects on locomotor muscle fatigue and exertional dyspnea secondary to hyper-chemosensitivity induced by the synergistic effects on the drive to breathe of hypoxemia combined with exercise (5, 195) [in sharp contrast to these maladaptive responses to exercise in the sojourner, trained or untrained residents of high altitude have acquired a markedly reduced chemosensitivity plus an enhanced pulmonary diffusion capacity that allows them to protect their arterial Po2 during exercise via a narrowed A-aDO2 without excessive hyperventilation and dyspnea (23, 34, 117)]; and 3) enhanced pulmonary vascular resistance and Ppa secondary to hypoxic pulmonary vasoconstriction combined with the athlete’s high pulmonary blood flows (44, 46, 51, 140). In cases with enhanced heterogeneity of hypoxic-induced pulmonary vasoconstriction, capillary pressures during high-intensity exercise would be sufficiently high in some overperfused regions of the lung to provide excessive mechanical stress, loss of alveolar-capillary barrier integrity, and pulmonary edema, along with a worsening of arterial hypoxemia (44).
In summary, exercise performance in the highly trained athlete during sojourn to what are normally viewed as even “mild” and “moderate” high altitudes is now limited primarily by a pulmonary diffusion capacity, respiratory muscle work and aerobic capacity, neurochemical respiratory control system, and pulmonary vasculature that are underbuilt or excessively sensitive for the combined stresses of hypoxia and high-intensity exercise. In view of these multifaceted negative consequences to endurance performance at high altitude in the athletic sojourner, it appears paradoxical that such large numbers of elite athletes utilize training regimens requiring hypoxic exposure with the intent of enhancing their Hb mass and performance at sea level (131). The efficacy of this practice has recently been questioned (172, 184).
SUMMARY
Relative Importance of the Respiratory System to Exercise Limitation
In almost all healthy subjects, the evidence is clear that it is the cardiovascular system in general and maximum stroke volume specifically, together with circulating blood volume and Hb mass, that are the major gatekeepers regulating O2 transport during exercise. The respiratory system “inadequacies” or constraints we have discussed previously (with some notable exceptions) do not occur in all healthy subjects, and when they do, they account for relatively minor limitations to performance. For example: 1) EIAH occurs to a variable extent in the highly trained subject and at most accounts for ~15% of the V̇o2max and 15–25% of exercise-induced limb fatigue; 2) EIB occurs with high prevalence in the highly trained endurance athletes, but its effects on exercise performance are not well quantified and the underlying mechanisms for exercise limitation remain elusive; 3) EILO elicits severe dyspneic sensations, constrains ventilation and gas exchange, and presents a major limit to exercise performance; however, EILO prevalence among the highly trained subject awaits more precise characterization in larger populations of athletes across the world; 4) we cannot yet predict with certainty the effects of a limited pulmonary vascular recruitment on stroke volume or exercise performance, i.e., is maximum stroke volume really determined by the “weakest ventricle”?; and 5) positive expiratory intrathoracic pressures appear to exert a modulatory but likely relatively small constraint on maximum stroke volume.
Notable exceptions to these inconsistent influences of pulmonary system limitations include the following:
-
•
the dominant role of the lung’s failed gas exchange as a determinant of exercise performance in the equine thoroughbred and in the human athlete in hypoxic environments;
-
•
the consistent effect across healthy trained and untrained subjects of respiratory muscle work on locomotor muscle blood flow, fatigue, and endurance exercise performance carried out at high intensities;
-
•
aging and sex differences in lung morphology and flow-volume capacities offer another consistent yet unquantified respiratory system limitation, primarily via their effects on respiratory muscle work and its sequelae, especially in the highly trained subject.
FUTURE WORK
The uncovering of limits to the healthy respiratory system response to exercise has opened new avenues of exploration to further understand the pathophysiology of exercise performance limitation in chronic cardiovascular and respiratory diseases (6, 25, 56, 130, 137, 147, 186). Related unresolved problems that deserve in-depth scrutiny include the following:
-
•
longitudinal investigation of exercise training effects on the aging lung, conducted with state-of-the-art measures of lung physiology, endocrinology, and lung imaging and analogous to recently conducted training studies on aging with cardiovascular outcome measures (21, 78, 79). It is now well established that exercise training increases antioxidant defenses and anti-inflammatory mediator release from contracting muscle, capable of inhibiting inflammation and oxidative stress (53, 83). It is important that investigation of these exercise-induced mechanisms be extended to include the human lung during healthy aging and in chronic pulmonary diseases.
-
•
sex influences on lung morphology and pulmonary vasculature distensibility.
-
•
neural control of extrathoracic upper airway caliber during exercise and its contribution to EILO.
-
•
long-term pathophysiology of pulmonary vascular remodeling in the highly trained and especially in the master athlete.
-
•
potential negative impacts of long-term high-intensity training on the lung parenchyma: does injury to the alveolar capillary surface impact EIAH and explain its occurrence even during submaximal exercise?
-
•
quantification of influences of the respiratory versus locomotor muscle pumps on venous return and stroke volume in a setting of heavy intensity exercise.
-
•
comparison of respiratory muscle vs. locomotor muscle vascular responsiveness to sympathetic innervation and their effects on blood flow distribution during exercise. Also, does specific inspiratory muscle training influence blood flow distribution during exercise? If so, how?
Progress toward addressing these questions would benefit greatly from advances in methods for imaging the small airways and pulmonary vasculature and for quantifying diaphragmatic blood flow and heart volume dimensions in the human during heavy intensity exercise.
GRANTS
This work was supported in part by a grant from the National Heart, Lung, and Blood Institute (R21 HL137874 to J. Dempsey). A. L. Gerche is supported by a Future Leader Fellowship from the National Heart Foundation of Australia (102206).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.D. conceived and designed research; J.A.D., A.L.G., and J.H.H. prepared figures; J.A.D., A.L.G., and J.H.H. drafted manuscript; J.A.D., A.L.G., and J.H.H. edited and revised manuscript; J.A.D., A.L.G., and J.H.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Professors William Sheel, Rob Boushel, and Barbara Morgan for their insightful discussion of the concepts in this manuscript. Benjamin Dempsey provided important technical support. The authors are especially grateful to the three anonymous reviewers for Journal of Applied Physiology whose critiques led to substantial improvement of the manuscript. J. A. Dempsey expresses his gratitude to the UW Rankin Lab staff and faculty and especially to the 68 pre- and post-doctoral trainees for 55+ years of collaboration and friendship.
Footnotes
The maximum flow-volume envelope as conventionally measured (see Fig. 2) is subject to underestimation of true maximum expiratory flow due to gas compression. Complete expiratory flow limitation is best assessed by determining whether expiratory flow increases in response to a superimposed negative pressure during exercise. Nevertheless, significant overlap of the tidal to (postexercise) maximum flow-volume envelope in heavy intensity exercise has been associated with dynamic airway compression (see Fig. 2) and constrained ventilatory responsiveness to chemoreceptor stimuli (see above). These effects suggest that even partial or “impending” flow limitation has physiological consequences.
The use of partial pharmacological blockade of group III-IV afferents from working limbs has shown that muscle metabolite accumulation negatively impacts time trial cycling performance by both: a) reducing force output for a given central locomotor drive (“peripheral fatigue”); and b) via feedback attenuation of central motor neuronal output (“central fatigue”) (16, 84).
REFERENCES
- 1.Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to α1- adrenergic constriction than gastrocnemius arterioles. J Appl Physiol (1985) 92: 1808–1816, 2002. doi: 10.1152/japplphysiol.01152.2001. [DOI] [PubMed] [Google Scholar]
- 2.Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol (1985) 72: 1818–1825, 1992. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- 3.Al-Bazzaz F, Grillo H, Kazemi H. Response to exercise in upper airway obstruction. Am Rev Respir Dis 111: 631–640, 1975. [DOI] [PubMed] [Google Scholar]
- 4.Aliverti A, Cala SJ, Duranti R, Ferrigno G, Kenyon CM, Pedotti A, Scano G, Sliwinski P, Macklem PT, Yan S. Human respiratory muscle actions and control during exercise. J Appl Physiol (1985) 83: 1256–1269, 1997. doi: 10.1152/jappl.1997.83.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293: R2036–R2045, 2007. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- 6.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299: R314–R324, 2010. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol 122: 225–235, 2008. doi: 10.1016/j.jaci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Armour J, Donnelly PM, Bye PT. The large lungs of elite swimmers: an increased alveolar number? Eur Respir J 6: 237–247, 1993. [PubMed] [Google Scholar]
- 9.Babb TG Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol (1985) 82: 746–754, 1997. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 10.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol (1985) 93: 201–206, 2002. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- 11.Babcock MA, Pegelow DF, Johnson BD, Dempsey JA. Aerobic fitness effects on exercise-induced low-frequency diaphragm fatigue. J Appl Physiol (1985) 81: 2156–2164, 1996. doi: 10.1152/jappl.1996.81.5.2156. [DOI] [PubMed] [Google Scholar]
- 12.Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol (1985) 78: 1710–1719, 1995. doi: 10.1152/jappl.1995.78.5.1710. [DOI] [PubMed] [Google Scholar]
- 13.Bake B, Wood L, Murphy B, Macklem PT, Milic-Emili J. Effect of inspiratory flow rate on regional distribution of inspired gas. J Appl Physiol 37: 8–17, 1974. doi: 10.1152/jappl.1974.37.1.8. [DOI] [PubMed] [Google Scholar]
- 14.Bayly WM, Hodgson DR, Schulz DA, Dempsey JA, Gollnick PD. Exercise-induced hypercapnia in the horse. J Appl Physiol (1985) 67: 1958–1966, 1989. doi: 10.1152/jappl.1989.67.5.1958. [DOI] [PubMed] [Google Scholar]
- 15.Björnsdóttir US, Gudmundsson K, Hjartarson H, Bröndbo K, Magnússon B, Juliusson S. Exercise-induced laryngochalasia: an imitator of exercise-induced bronchospasm. Ann Allergy Asthma Immunol 85: 387–391, 2000. doi: 10.1016/S1081-1206(10)62552-5. [DOI] [PubMed] [Google Scholar]
- 16.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohm P, Schneider G, Linneweber L, Rentzsch A, Krämer N, Abdul-Khaliq H, Kindermann W, Meyer T, Scharhag J. Right and left ventricular function and mass in male elite master athletes: a controlled contrast-enhanced cardiovascular magnetic resonance study. Circulation 133: 1927–1935, 2016. doi: 10.1161/CIRCULATIONAHA.115.020975. [DOI] [PubMed] [Google Scholar]
- 18.Bouwsema MM, Tedjasaputra V, Stickland MK. Are there sex differences in the capillary blood volume and diffusing capacity response to exercise? J Appl Physiol (1985) 122: 460–469, 2017. doi: 10.1152/japplphysiol.00389.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bovard JM, Welch JF, Houghton KM, McKenzie DC, Potts JE, Sheel AW. Does competitive swimming affect lung growth? Physiol Rep 6: e13816, 2018. doi: 10.14814/phy2.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan AC, Bentivoglio LG, Beerel F, MacLeish H, Zidulka A, Bates DV. Factors affecting regional distribution of ventilation and perfusion in the lung. J Appl Physiol 19: 395–402, 1964. doi: 10.1152/jappl.1964.19.3.395. [DOI] [PubMed] [Google Scholar]
- 21.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116: 736–745, 2014. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter EA, Sheel AW, Milsom WK, Koehle MS. Sildenafil does not improve performance in 16.1 km cycle exercise time-trial in acute hypoxia. PLoS One 14: e0210841, 2019. doi: 10.1371/journal.pone.0210841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerny FC, Dempsey JA, Reddan WG. Pulmonary gas exchange in nonnative residents of high altitude. J Clin Invest 52: 2993–2999, 1973. doi: 10.1172/JCI107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman RF, Emery M, Stager JM. Degree of arterial desaturation in normoxia influences O2max decline in mild hypoxia. Med Sci Sports Exerc 31: 658–663, 1999. doi: 10.1097/00005768-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 51: 1663–1671, 2008. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortés-Télles A, Webb KA, O’Donnell DE. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med 187: 1315–1323, 2013. doi: 10.1164/rccm.201211-1970OC. [DOI] [PubMed] [Google Scholar]
- 27.Christensen PM, Thomsen SF, Rasmussen N, Backer V. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol 268: 1313–1319, 2011. doi: 10.1007/s00405-011-1612-0. [DOI] [PubMed] [Google Scholar]
- 28.Claessen G, Claus P, Ghysels S, Vermeersch P, Dymarkowski S, LA Gerche A, Heidbuchel H. Right ventricular fatigue developing during endurance exercise: an exercise cardiac magnetic resonance study. Med Sci Sports Exerc 46: 1717–1726, 2014. doi: 10.1249/MSS.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 29.Clark JM, Sinclair RD, Lenox JB. Chemical and nonchemical components of ventilation during hypercapnic exercise in man. J Appl Physiol 48: 1065–1076, 1980. doi: 10.1152/jappl.1980.48.6.1065. [DOI] [PubMed] [Google Scholar]
- 30.Coffman KE, Carlson AR, Miller AD, Johnson BD, Taylor BJ. The effect of aging and cardiorespiratory fitness on the lung diffusing capacity response to exercise in healthy humans. J Appl Physiol (1985) 122: 1425–1434, 2017. doi: 10.1152/japplphysiol.00694.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole P, Savard P, Miljeteig H, Haight JS. Resistance to respiratory airflow of the extrapulmonary airways. Laryngoscope 103: 447–450, 1993. doi: 10.1002/lary.5541030415. [DOI] [PubMed] [Google Scholar]
- 32.Constantini K, Tanner DA, Gavin TP, Harms CA, Stager JM, Chapman RF. Prevalence of exercise-induced arterial hypoxemia in distance runners at sea level. Med Sci Sports Exerc 49: 948–954, 2017. doi: 10.1249/MSS.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 33.Dempsey JA Is the lung built for exercise? Med Sci Sports Exerc 18: 143–155, 1986. doi: 10.1249/00005768-198604000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Dempsey JA, Forster HV, Birnbaum ML, Reddan WG, Thoden J, Grover RF, Rankin J. Control of exercise hyperpnea under varying durations of exposure to moderate hypoxia. Respir Physiol 16: 213–231, 1972. doi: 10.1016/0034-5687(72)90052-7. [DOI] [PubMed] [Google Scholar]
- 35.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355: 161–175, 1984. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol (1985) 87: 1997–2006, 1999. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- 37.Dion GR, Eller RL, Thomas RF. Diagnosing aerodynamic supraglottic collapse with rest and exercise flexible laryngoscopy. J Voice 26: 779–784, 2012. doi: 10.1016/j.jvoice.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat-Seon Y, Henderson WR, Koehle MS, Boushel R, Sheel AW. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102: 1535–1547, 2017. doi: 10.1113/EP086566. [DOI] [PubMed] [Google Scholar]
- 39.Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC, Sheel AW. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 591: 3017–3034, 2013. doi: 10.1113/jphysiol.2013.252767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominelli PB, Katayama K, Vermeulen TD, Stuckless TJR, Brown CV, Foster GE, Sheel AW. Work of breathing influences muscle sympathetic nerve activity during semi-recumbent cycle exercise. Acta Physiol (Oxf) 225: e13212, 2019. doi: 10.1111/apha.13212. [DOI] [PubMed] [Google Scholar]
- 41.Dominelli PB, Molgat-Seon Y, Sheel AW. Sex differences in the pulmonary system influence the integrative response to exercise. Exerc Sport Sci Rev 47: 142–150, 2019. doi: 10.1249/JES.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 42.Dominelli PB, Sheel AW. Exercise-induced arterial hypoxemia; some answers, more questions. Appl Physiol Nutr Metab 44: 571–579, 2019. doi: 10.1139/apnm-2018-0468. [DOI] [PubMed] [Google Scholar]
- 43.Durand F, Gaston AF, Vicenzi M, Deboeck G, Subirats E, Faoro V. Noninvasive pulmonary hemodynamic evaluation in athletes with exercise-induced hypoxemia. Chest 157: 1568–1578, 2020. doi: 10.1016/j.chest.2020.01.037. [DOI] [PubMed] [Google Scholar]
- 44.Eldridge MW, Braun RK, Yoneda KY, Walby WF. Effects of altitude and exercise on pulmonary capillary integrity: evidence for subclinical high-altitude pulmonary edema. J Appl Physiol (1985) 100: 972–980, 2006. doi: 10.1152/japplphysiol.01048.2005. [DOI] [PubMed] [Google Scholar]
- 45.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol (1985) 97: 797–805, 2004. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- 46.Eldridge MW, Podolsky A, Richardson RS, Johnson DH, Knight DR, Johnson EC, Hopkins SR, Michimata H, Grassi B, Feiner J, Kurdak SS, Bickler PE, Wagner PD, Severinghaus JW. Pulmonary hemodynamic response to exercise in subjects with prior high-altitude pulmonary edema. J Appl Physiol (1985) 81: 911–921, 1996. doi: 10.1152/jappl.1996.81.2.911. [DOI] [PubMed] [Google Scholar]
- 47.Elliott AD, La Gerche A. The right ventricle following prolonged endurance exercise: are we overlooking the more important side of the heart? A meta-analysis. Br J Sports Med 49: 724–729, 2015. doi: 10.1136/bjsports-2014-093895. [DOI] [PubMed] [Google Scholar]
- 48.England SJ, Bartlett D Jr. Changes in respiratory movements of the human vocal cords during hyperpnea. J Appl Physiol 52: 780–785, 1982. doi: 10.1152/jappl.1982.52.3.780. [DOI] [PubMed] [Google Scholar]
- 49.Everman S, Farris JW, Bay RC, Daniels JT. Elite distance runners: a 45-year follow-up. Med Sci Sports Exerc 50: 73–78, 2018. doi: 10.1249/MSS.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 50.Famokunwa B, Sandhu G, Hull JH. Surgical intervention for exercise-induced laryngeal obstruction: A UK perspective. Laryngoscope In press. doi: 10.1002/lary.28497. [DOI] [PubMed] [Google Scholar]
- 51.Faoro V, Boldingh S, Moreels M, Martinez S, Lamotte M, Unger P, Brimioulle S, Huez S, Naeije R. Bosentan decreases pulmonary vascular resistance and improves exercise capacity in acute hypoxia. Chest 135: 1215–1222, 2009. doi: 10.1378/chest.08-2222. [DOI] [PubMed] [Google Scholar]
- 52.Ferris BG Jr, Mead J, Opie LH. Partitioning of respiratory flow resistance in man. J Appl Physiol 19: 653–658, 1964. doi: 10.1152/jappl.1964.19.4.653. [DOI] [PubMed] [Google Scholar]
- 53.Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol 2: 743–777, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Frank-Ito DO, Schulz K, Vess G, Witsell DL. Changes in aerodynamics during vocal cord dysfunction. Comput Biol Med 57: 116–122, 2015. doi: 10.1016/j.compbiomed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Fretheim-Kelly Z, Halvorsen T, Heimdal JH, Strand E, Vollsaeter M, Clemm H, Roksund O. Feasibility and tolerability of measuring translaryngeal pressure during exercise. Laryngoscope 129: 2748–2753, 2019. doi: 10.1002/lary.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 57.Geary CM, Welch JF, McDonald MR, Peters CM, Leahy MG, Reinhard PA, Sheel AW. Diaphragm fatigue and inspiratory muscle metaboreflex in men and women matched for absolute diaphragmatic work during pressure-threshold loading. J Physiol 597: 4797–4808, 2019. doi: 10.1113/JP278380. [DOI] [PubMed] [Google Scholar]
- 58.Gledhill N, Froese A, Dempsey J. Ventilation to perfusion distribution during exercise in health. In: Muscular Exercise and the Lung, edited by Dempsey J, Reed C. Madison, WI: University of Wisconsin Press, 1976, p. 325–344. [Google Scholar]
- 59.Glenny RW, Lamm WJ, Albert RK, Robertson HT. Gravity is a minor determinant of pulmonary blood flow distribution. J Appl Physiol (1985) 71: 620–629, 1991. doi: 10.1152/jappl.1991.71.2.620. [DOI] [PubMed] [Google Scholar]
- 60.Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, Campbell DP, Emonson DL. Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol (1985) 80: 2204–2210, 1996. doi: 10.1152/jappl.1996.80.6.2204. [DOI] [PubMed] [Google Scholar]
- 61.Granath A, Jonsson B, Strandell T. Circulation in healthy old men, studied by right heart catheterization at rest and during exercise in supine and sitting position. Acta Med Scand 176: 425–446, 1964. doi: 10.1111/j.0954-6820.1964.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 62.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagberg JM, Yerg JE II, Seals DR. Pulmonary function in young and older athletes and untrained men. J Appl Physiol (1985) 65: 101–105, 1988. doi: 10.1152/jappl.1988.65.1.101. [DOI] [PubMed] [Google Scholar]
- 64.Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR Jr, Aitken ML. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med 172: 679–686, 2005. doi: 10.1164/rccm.200412-1667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Effect of exercise-induced arterial O2 desaturation on V̇o2max in women. Med Sci Sports Exerc 32: 1101–1108, 2000. doi: 10.1097/00005768-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol 507: 619–628, 1998. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985) 85: 609–618, 1998. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 68.Hart BJ, Bian X, Gwirtz PA, Setty S, Downey HF. Right ventricular oxygen supply/demand balance in exercising dogs. Am J Physiol Heart Circ Physiol 281: H823–H830, 2001. doi: 10.1152/ajpheart.2001.281.2.H823. [DOI] [PubMed] [Google Scholar]
- 69.Haverkamp H, Miller J, Rodman J, Romer L, Pegelow D, Santana M, Dempsey J. Extrathoracic obstruction and hypoxemia occurring during exercise in a competitive female cyclist. Chest 124: 1602–1605, 2003. doi: 10.1378/chest.124.4.1602. [DOI] [PubMed] [Google Scholar]
- 70.Haverkamp HC, Dempsey JA, Miller JD, Romer LM, Eldridge MW. Physiologic responses to exercise. In: Physiologic Basis of Respiratory Disease, edited by Hamid Q, Shannon J, Martin J. Hamilton, Ontario: B.C. Decker, 2005, p. 525–540. [Google Scholar]
- 71.Haverkamp HC, Dempsey JA, Miller JD, Romer LM, Pegelow DF, Rodman JR, Eldridge MW. Gas exchange during exercise in habitually active asthmatic subjects. J Appl Physiol (1985) 99: 1938–1950, 2005. doi: 10.1152/japplphysiol.00041.2005. [DOI] [PubMed] [Google Scholar]
- 72.Haverkamp HC, Dempsey JA, Pegelow DF, Miller JD, Romer LM, Santana M, Eldridge MW. Treatment of airway inflammation improves exercise pulmonary gas exchange and performance in asthmatic subjects. J Allergy Clin Immunol 120: 39–47, 2007. doi: 10.1016/j.jaci.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Heidbüchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R, Van Lierde J. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J 24: 1473–1480, 2003. doi: 10.1016/S0195-668X(03)00282-3. [DOI] [PubMed] [Google Scholar]
- 74.Helenius I, Rytilä P, Sarna S, Lumme A, Helenius M, Remes V, Haahtela T. Effect of continuing or finishing high-level sports on airway inflammation, bronchial hyperresponsiveness, and asthma: a 5-year prospective follow-up study of 42 highly trained swimmers. J Allergy Clin Immunol 109: 962–968, 2002. doi: 10.1067/mai.2002.124769a. [DOI] [PubMed] [Google Scholar]
- 75.Hepple RT Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci 6: 211, 2014. doi: 10.3389/fnagi.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill JM Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res 856: 240–244, 2000. doi: 10.1016/S0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins SR, Schoene RB, Henderson WR, Spragg RG, Martin TR, West JB. Intense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletes. Am J Respir Crit Care Med 155: 1090–1094, 1997. doi: 10.1164/ajrccm.155.3.9116992. [DOI] [PubMed] [Google Scholar]
- 78.Howden EJ, Levine BD. Response by Howden and Levine to Letters Regarding Article, “Reversing the Cardiac Effects of Sedentary Aging in Middle Age—a Randomized Controlled Trial: Implications for Heart Failure Prevention”. Circulation 138: 1759–1760, 2018. doi: 10.1161/CIRCULATIONAHA.118.036249. [DOI] [PubMed] [Google Scholar]
- 79.Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams-Huet B, Levine BD. Reversing the cardiac effects of sedentary aging in middle age—a randomized controlled trial: implications for heart failure prevention. Circulation 137: 1549–1560, 2018. doi: 10.1161/CIRCULATIONAHA.117.030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsia CC Coordinated adaptation of oxygen transport in cardiopulmonary disease. Circulation 104: 963–969, 2001. doi: 10.1161/hc3401.094928. [DOI] [PubMed] [Google Scholar]
- 81.Hull JH, Backer V, Gibson PG, Fowler SJ. Laryngeal dysfunction: assessment and management for the clinician. Am J Respir Crit Care Med 194: 1062–1072, 2016. doi: 10.1164/rccm.201606-1249CI. [DOI] [PubMed] [Google Scholar]
- 82.Hull JH, Godbout K, Boulet LP. Exercise-associated dyspnea and stridor: thinking beyond asthma. J Allergy Clin Immunol Pract 8: 2202–2208, 2020. doi: 10.1016/j.jaip.2020.01.057. [DOI] [PubMed] [Google Scholar]
- 83.Hurbis CG, Schild JA. Laryngeal changes during exercise and exercise-induced asthma. Ann Otol Rhinol Laryngol 100: 34–37, 1991. doi: 10.1177/000348949110000106. [DOI] [PubMed] [Google Scholar]
- 84.Hureau TJ, Weavil JC, Thurston TS, Wan HY, Gifford JR, Jessop JE, Buys MJ, Richardson RS, Amann M. Pharmacological attenuation of group III/IV muscle afferents improves endurance performance when oxygen delivery to locomotor muscles is preserved. J Appl Physiol (1985) 127: 1257–1266, 2019. doi: 10.1152/japplphysiol.00490.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussain SN, Chatillon A, Comtois A, Roussos C, Magder S. Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. J Appl Physiol (1985) 70: 77–86, 1991. doi: 10.1152/jappl.1991.70.1.77. [DOI] [PubMed] [Google Scholar]
- 86.Iber C, Simon P, Skatrud JB, Mahowald MW, Dempsey JA. The Breuer-Hering reflex in humans. Effects of pulmonary denervation and hypocapnia. Am J Respir Crit Care Med 152: 217–224, 1995. doi: 10.1164/ajrccm.152.1.7599827. [DOI] [PubMed] [Google Scholar]
- 87.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 62: 1290–1297, 2013. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jammes Y, Balzamo E. Changes in afferent and efferent phrenic activities with electrically induced diaphragmatic fatigue. J Appl Physiol (1985) 73: 894–902, 1992. doi: 10.1152/jappl.1992.73.3.894. [DOI] [PubMed] [Google Scholar]
- 89.Jensen D, Ofir D, O’Donnell DE. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir Physiol Neurobiol 167: 87–100, 2009. doi: 10.1016/j.resp.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Johansson H, Norlander K, Berglund L, Janson C, Malinovschi A, Nordvall L, Nordang L, Emtner M. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 70: 57–63, 2015. doi: 10.1136/thoraxjnl-2014-205738. [DOI] [PubMed] [Google Scholar]
- 91.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460: 385–405, 1993. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson BD, Badr MS, Dempsey JA. Impact of the aging pulmonary system on the response to exercise. Clin Chest Med 15: 229–246, 1994. [PubMed] [Google Scholar]
- 93.Johnson BD, Reddan WG, Seow KC, Dempsey JA. Mechanical constraints on exercise hyperpnea in a fit aging population. Am Rev Respir Dis 143: 968–977, 1991. doi: 10.1164/ajrccm/143.5_Pt_1.968. [DOI] [PubMed] [Google Scholar]
- 94.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol (1985) 73: 874–886, 1992. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 95.Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med 161: 2086–2091, 2000. doi: 10.1164/ajrccm.161.6.9907025. [DOI] [PubMed] [Google Scholar]
- 96.Kaufman MP, Forster HV. Reflexes controlling the circulatory, ventilatory, and airway responses to exercise. In: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems, edited by Terjung R Bethesda, MD: American Physiological Society, 1996, p. 381–447. Handbook of Physiology. [Google Scholar]
- 97.Kippelen P, Anderson SD. Airway injury during high-level exercise. Br J Sports Med 46: 385–390, 2012. doi: 10.1136/bjsports-2011-090819. [DOI] [PubMed] [Google Scholar]
- 98.Kippelen P, Fitch KD, Anderson SD, Bougault V, Boulet LP, Rundell KW, Sue-Chu M, McKenzie DC. Respiratory health of elite athletes—preventing airway injury: a critical review. Br J Sports Med 46: 471–476, 2012. doi: 10.1136/bjsports-2012-091056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kirkton SD, Howlett RA, Gonzalez NC, Giuliano PG, Britton SL, Koch LG, Wagner HE, Wagner PD. Continued artificial selection for running endurance in rats is associated with improved lung function. J Appl Physiol (1985) 106: 1810–1818, 2009. doi: 10.1152/japplphysiol.90419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koizumi T, Roselli RJ, Parker RE, Hermo-Weiler CI, Banerjee M, Newman JH. Clearance of filtered fluid from the lung during exercise: role of hyperpnea. Am J Respir Crit Care Med 163: 614–618, 2001. doi: 10.1164/ajrccm.163.3.2004205. [DOI] [PubMed] [Google Scholar]
- 101.Kuo W, Ciet P, Andrinopoulou ER, Chen Y, Pullens B, Garcia-Peña P, Fleck RJ, Paoletti M, McCartin M, Vermeulen F, Morana G, Lee EY, Tiddens HA; Normal Chest CT Study Group . Reference values for central airway dimensions on ct images of children and adolescents. AJR Am J Roentgenol 210: 423–430, 2018. doi: 10.2214/AJR.17.18597. [DOI] [PubMed] [Google Scholar]
- 102.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33: 998–1006, 2012. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 103.La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees L, Droogne W, Van Cleemput J, Claus P, Heidbuchel H. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J 36: 1998–2010, 2015. doi: 10.1093/eurheartj/ehv202. [DOI] [PubMed] [Google Scholar]
- 104.La Gerche A, Connelly KA, Mooney DJ, MacIsaac AI, Prior DL. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart 94: 860–866, 2008. doi: 10.1136/hrt.2006.101063. [DOI] [PubMed] [Google Scholar]
- 105.La Gerche A, Heidbuchel H. Can intensive exercise harm the heart? You can get too much of a good thing. Circulation 130: 992–1002, 2014. doi: 10.1161/CIRCULATIONAHA.114.008141. [DOI] [PubMed] [Google Scholar]
- 106.La Gerche A, Heidbüchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc 43: 974–981, 2011. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- 107.La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbüchel H, Prior DL. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol (1985) 109: 1307–1317, 2010. doi: 10.1152/japplphysiol.00457.2010. [DOI] [PubMed] [Google Scholar]
- 108.La Gerche A, Rakhit DJ, Claessen G. Exercise and the right ventricle: a potential Achilles’ heel. Cardiovasc Res 113: 1499–1508, 2017. doi: 10.1093/cvr/cvx156. [DOI] [PubMed] [Google Scholar]
- 109.La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, Matthijs G, Heidbüchel H. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 96: 1268–1274, 2010. doi: 10.1136/hrt.2009.189621. [DOI] [PubMed] [Google Scholar]
- 110.Lawler J, Powers SK, Thompson D. Linear relationship between VO2max and VO2max decrement during exposure to acute hypoxia. J Appl Physiol (1985) 64: 1486–1492, 1988. doi: 10.1152/jappl.1988.64.4.1486. [DOI] [PubMed] [Google Scholar]
- 111.Lee JH, An J, Won HK, Kang Y, Kwon HS, Kim TB, Cho YS, Moon HB, Song WJ, Hull JH. Prevalence and impact of comorbid laryngeal dysfunction in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 145: 1165–1173, 2020. doi: 10.1016/j.jaci.2019.12.906. [DOI] [PubMed] [Google Scholar]
- 112.Legrand R, Marles A, Prieur F, Lazzari S, Blondel N, Mucci P. Related trends in locomotor and respiratory muscle oxygenation during exercise. Med Sci Sports Exerc 39: 91–100, 2007. doi: 10.1249/01.mss.0000241638.90348.67. [DOI] [PubMed] [Google Scholar]
- 113.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 128: 1470–1479, 2013. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 114.Lin J, Walsted ES, Backer V, Hull JH, Elson DS. Quantification and analysis of laryngeal closure from endoscopic videos. IEEE Trans Biomed Eng 66: 1127–1136, 2019. doi: 10.1109/TBME.2018.2867636. [DOI] [PubMed] [Google Scholar]
- 115.Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol (1985) 104: 1418–1425, 2008. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- 116.Lovering AT, Stickland MK. Not hearing is believing: novel insight into cardiopulmonary function using agitated contrast and ultrasound. J Appl Physiol (1985) 109: 1290–1291, 2010. doi: 10.1152/japplphysiol.01083.2010. [DOI] [PubMed] [Google Scholar]
- 117.Lundby C, Calbet JA, van Hall G, Saltin B, Sander M. Pulmonary gas exchange at maximal exercise in Danish lowlanders during 8 wk of acclimatization to 4,100 m and in high-altitude Aymara natives. Am J Physiol Regul Integr Comp Physiol 287: R1202–R1208, 2004. doi: 10.1152/ajpregu.00725.2003. [DOI] [PubMed] [Google Scholar]
- 118.Lundby C, Robach P. Performance enhancement: what are the physiological limits? Physiology (Bethesda) 30: 282–292, 2015. doi: 10.1152/physiol.00052.2014. [DOI] [PubMed] [Google Scholar]
- 119.Maat RC, Hilland M, Røksund OD, Halvorsen T, Olofsson J, Aarstad HJ, Heimdal JH. Exercise-induced laryngeal obstruction: natural history and effect of surgical treatment. Eur Arch Otorhinolaryngol 268: 1485–1492, 2011. doi: 10.1007/s00405-011-1656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mahler DA, Fierro-Carrion G, Baird JC. Evaluation of dyspnea in the elderly. Clin Geriatr Med 19: 19–33, 2003. doi: 10.1016/s0749-0690(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 121.Manohar M Inspiratory and expiratory muscle perfusion in maximally exercised ponies. J Appl Physiol (1985) 68: 544–548, 1990. doi: 10.1152/jappl.1990.68.2.544. [DOI] [PubMed] [Google Scholar]
- 122.Manohar M, Hutchens E, Coney E. Pulmonary haemodynamics in the exercising horse and their relationship to exercise-induced pulmonary haemorrhage. Br Vet J 149: 419–428, 1993. doi: 10.1016/S0007-1935(05)80108-3. [DOI] [PubMed] [Google Scholar]
- 123.Massaro D, Massaro GD. Invited review: pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol 282: L345–L358, 2002. doi: 10.1152/ajplung.00374.2001. [DOI] [PubMed] [Google Scholar]
- 124.McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol (1985) 78: 1957–1968, 1995. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- 125.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol (1985) 84: 1872–1881, 1998. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- 126.McClaran SR, Wetter TJ, Pegelow DF, Dempsey JA. Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol (1985) 86: 1357–1366, 1999. doi: 10.1152/jappl.1999.86.4.1357. [DOI] [PubMed] [Google Scholar]
- 127.Mead J Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. [DOI] [PubMed] [Google Scholar]
- 128.Mendes FA, Almeida FM, Cukier A, Stelmach R, Jacob-Filho W, Martins MA, Carvalho CR. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc 43: 197–203, 2011. doi: 10.1249/MSS.0b013e3181ed0ea3. [DOI] [PubMed] [Google Scholar]
- 129.Miller J, Dempsey J. Pulmonary limitation to exercise performance: the effects of healthy aging and COPD. In: Lung Development and Regeneration, edited by Massaro D New York: Marcel Dekker, 2004. [Google Scholar]
- 130.Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am J Physiol Heart Circ Physiol 292: H580–H592, 2007. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- 131.Millet GP, Brocherie F. Hypoxic training is beneficial in elite athletes. Med Sci Sports Exerc 52: 515–518, 2020. doi: 10.1249/MSS.0000000000002142. [DOI] [PubMed] [Google Scholar]
- 132.Molgat-Seon Y, Dominelli PB, Ramsook AH, Schaeffer MR, Molgat Sereacki S, Foster GE, Romer LM, Road JD, Guenette JA, Sheel AW. The effects of age and sex on mechanical ventilatory constraint and dyspnea during exercise in healthy humans. J Appl Physiol (1985) 124: 1092–1106, 2018. doi: 10.1152/japplphysiol.00608.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Molgat-Seon Y, Ramsook AH, Peters CM, Schaeffer MR, Dominelli PB, Romer LM, Road JD, Guenette JA, Sheel AW. Manipulation of mechanical ventilatory constraint during moderate intensity exercise does not influence dyspnoea in healthy older men and women. J Physiol 597: 1383–1399, 2019. doi: 10.1113/JP277476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Montero D The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. Eur J Prev Cardiol 22: 1200–1211, 2015. doi: 10.1177/2047487314553780. [DOI] [PubMed] [Google Scholar]
- 135.Montero D, Díaz-Cañestro C. Maximal cardiac output in athletes: influence of age. Eur J Prev Cardiol 22: 1588–1600, 2015. doi: 10.1177/2047487314566759. [DOI] [PubMed] [Google Scholar]
- 136.Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han SY, Francis A, Walker JR, Kirkpatrick ID, Neilan TG, Sharma S, Jassal DS. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol 103: 1467–1472, 2009. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- 137.Musch TI Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am J Physiol 265: H1721–H1726, 1993. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- 138.Muñoz PA, Gómez FP, Manrique HA, Roca J, Barberà JA, Young IH, Anderson SD, Rodríguez-Roisin R. Pulmonary gas exchange response to exercise- and mannitol-induced bronchoconstriction in mild asthma. J Appl Physiol (1985) 105: 1477–1485, 2008. doi: 10.1152/japplphysiol.00108.2008. [DOI] [PubMed] [Google Scholar]
- 139.Naeije R, Chesler N. Pulmonary circulation at exercise. Compr Physiol 2: 711–741, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D, Faoro V. Pulmonary artery pressure limits exercise capacity at high altitude. Eur Respir J 36: 1049–1055, 2010. doi: 10.1183/09031936.00024410. [DOI] [PubMed] [Google Scholar]
- 141.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med 153: 656–664, 1996. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 142.Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 45: 2030–2035, 2013. doi: 10.1249/MSS.0b013e318298b19a. [DOI] [PubMed] [Google Scholar]
- 143.Nielsen HB, Madsen P, Svendsen LB, Roach RC, Secher NH. The influence of PaO2, pH and SaO2 on maximal oxygen uptake. Acta Physiol Scand 164: 89–97, 1998. doi: 10.1046/j.1365-201X.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 144.Norlander K, Johansson H, Emtner M, Janson C, Nordvall L, Nordang L. Differences in laryngeal movements during exercise in healthy and dyspnoeic adolescents. Int J Pediatr Otorhinolaryngol 129: 109765, 2020. doi: 10.1016/j.ijporl.2019.109765. [DOI] [PubMed] [Google Scholar]
- 145.Nourry C, Fabre C, Bart F, Grosbois JM, Berthoin S, Mucci P. Evidence of exercise-induced arterial hypoxemia in prepubescent trained children. Pediatr Res 55: 674–681, 2004. doi: 10.1203/01.PDR.0000114481.58902.FB. [DOI] [PubMed] [Google Scholar]
- 146.Olin JT, Clary MS, Fan EM, Johnston KL, State CM, Strand M, Christopher KL. Continuous laryngoscopy quantitates laryngeal behaviour in exercise and recovery. Eur Respir J 48: 1192–1200, 2016. doi: 10.1183/13993003.00160-2016. [DOI] [PubMed] [Google Scholar]
- 147.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oomah SR, Mousavi N, Bhullar N, Kumar K, Walker JR, Lytwyn M, Colish J, Wassef A, Kirkpatrick ID, Sharma S, Jassal DS. The role of three-dimensional echocardiography in the assessment of right ventricular dysfunction after a half marathon: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr 24: 207–213, 2011. doi: 10.1016/j.echo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 149.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 2: 592–607, 1950. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- 150.Oxborough D, Shave R, Warburton D, Williams K, Oxborough A, Charlesworth S, Foulds H, Hoffman MD, Birch K, George K. Dilatation and dysfunction of the right ventricle immediately after ultraendurance exercise: exploratory insights from conventional two-dimensional and speckle tracking echocardiography. Circ Cardiovasc Imaging 4: 253–263, 2011. doi: 10.1161/CIRCIMAGING.110.961938. [DOI] [PubMed] [Google Scholar]
- 151.Persson CG, Erjefält JS, Andersson M, Greiff L, Svensson C. Extravasation, lamina propria flooding and lumenal entry of bulk plasma exudate in mucosal defence, inflammation and repair. Pulm Pharmacol 9: 129–139, 1996. doi: 10.1006/pulp.1996.0015. [DOI] [PubMed] [Google Scholar]
- 152.Phillipson EA, Bowes G, Townsend ER, Duffin J, Cooper JD. Role of metabolic CO2 production in ventilatory response to steady-state exercise. J Clin Invest 68: 768–774, 1981. doi: 10.1172/JCI110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Piiper J, Scheid P. Model for capillary-alveolar equilibration with special reference to O2 uptake in hypoxia. Respir Physiol 46: 193–208, 1981. doi: 10.1016/0034-5687(81)90121-3. [DOI] [PubMed] [Google Scholar]
- 154.Pilarski JQ, Leiter JC, Fregosi RF. Muscles of breathing: development, function, and patterns of activation. Compr Physiol 9: 1025–1080, 2019. doi: 10.1002/cphy.c180008. [DOI] [PubMed] [Google Scholar]
- 155.Polla B, D’Antona G, Bottinelli R, Reggiani C. Respiratory muscle fibres: specialisation and plasticity. Thorax 59: 808–817, 2004. doi: 10.1136/thx.2003.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poole DC, Erickson HH. Highly athletic terrestrial mammals: horses and dogs. Compr Physiol 1: 1–37, 2011. doi: 10.1002/cphy.c091001. [DOI] [PubMed] [Google Scholar]
- 157.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol (1985) 88: 186–194, 2000. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- 158.Poon CS, Tin C, Yu Y. Homeostasis of exercise hyperpnea and optimal sensorimotor integration: the internal model paradigm. Respir Physiol Neurobiol 159: 1–13, 2007. doi: 10.1016/j.resp.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Powers SK, Dodd S, Lawler J, Landry G, Kirtley M, McKnight T, Grinton S. Incidence of exercise induced hypoxemia in elite endurance athletes at sea level. Eur J Appl Physiol Occup Physiol 58: 298–302, 1988. doi: 10.1007/BF00417266. [DOI] [PubMed] [Google Scholar]
- 160.Powers SK, Lawler J, Dempsey JA, Dodd S, Landry G. Effects of incomplete pulmonary gas exchange on VO2 max. J Appl Physiol (1985) 66: 2491–2495, 1989. doi: 10.1152/jappl.1989.66.6.2491. [DOI] [PubMed] [Google Scholar]
- 161.Prefaut C, Durand F, Mucci P, Caillaud C. Exercise-induced arterial hypoxaemia in athletes: a review. Sports Med 30: 47–61, 2000. doi: 10.2165/00007256-200030010-00005. [DOI] [PubMed] [Google Scholar]
- 162.Price OJ, Ansley L, Menzies-Gow A, Cullinan P, Hull JH. Airway dysfunction in elite athletes–an occupational lung disease? Allergy 68: 1343–1352, 2013. doi: 10.1111/all.12265. [DOI] [PubMed] [Google Scholar]
- 163.Price OJ, Walsted ES, Hull JH. Understanding the total airway response to exercise: Current perspectives and future challenges. Current Opinions in Physiology 10: 185–192, 2019. doi: 10.1016/j.cophys.2019.05.014. [DOI] [Google Scholar]
- 164.Rannels DE Role of physical forces in compensatory growth of the lung. Am J Physiol 257: L179–L189, 1989. doi: 10.1152/ajplung.1989.257.4.L179. [DOI] [PubMed] [Google Scholar]
- 165.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 166.Reeves JT, Dempsey JA, Grover RF. Pulmonary circulation during exercise. In: Pulmonary Vascular Physiology and Pathophysiogy, edited by Weir EK, Reeves JT. New York: Marcel Dekker, 1989, p. 107–133. [Google Scholar]
- 167.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 288: L419–L425, 2005. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 168.Reuschlein PS, Reddan WG, Burpee J, Gee JB, Rankin J. Effect of physical training on the pulmonary diffusing capacity during submaximal work. J Appl Physiol 24: 152–158, 1968. doi: 10.1152/jappl.1968.24.2.152. [DOI] [PubMed] [Google Scholar]
- 169.Rice AJ, Thornton AT, Gore CJ, Scroop GC, Greville HW, Wagner H, Wagner PD, Hopkins SR. Pulmonary gas exchange during exercise in highly trained cyclists with arterial hypoxemia. J Appl Physiol (1985) 87: 1802–1812, 1999. doi: 10.1152/jappl.1999.87.5.1802. [DOI] [PubMed] [Google Scholar]
- 170.Richter EA, Kiens B, Hargreaves M, Kjaer M. Effect of arm-cranking on leg blood flow and noradrenaline spillover during leg exercise in man. Acta Physiol Scand 144: 9–14, 1992. doi: 10.1111/j.1748-1716.1992.tb09261.x. [DOI] [PubMed] [Google Scholar]
- 171.Ripoll JG, Guo W, Andersen KJ, Baker SE, Wiggins CC, Shepherd JRA, Carter RE, Welch BT, Joyner MJ, Dominelli PB. Sex differences in paediatric airway anatomy. Exp Physiol 105: 721–731, 2020. doi: 10.1113/EP088370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Robach P, Hansen J, Pichon A, Meinild Lundby AK, Dandanell S, Slettaløkken Falch G, Hammarström D, Pesta DH, Siebenmann C, Keiser S, Kérivel P, Whist JE, Rønnestad BR, Lundby C. Hypobaric live high-train low does not improve aerobic performance more than live low-train low in cross-country skiers. Scand J Med Sci Sports 28: 1636–1652, 2018. doi: 10.1111/sms.13075. [DOI] [PubMed] [Google Scholar]
- 173.Roberts TJ, Burns AT, MacIsaac RJ, MacIsaac AI, Prior DL, La Gerche A. Diagnosis and significance of pulmonary microvascular disease in diabetes. Diabetes Care 41: 854–861, 2018. doi: 10.2337/dc17-1904. [DOI] [PubMed] [Google Scholar]
- 174.Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol (1985) 95: 1159–1169, 2003. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- 175.Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol 290: R365–R375, 2006. doi: 10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- 176.Romer LM, Miller JD, Haverkamp HC, Pegelow DF, Dempsey JA. Inspiratory muscles do not limit maximal incremental exercise performance in healthy subjects. Respir Physiol Neurobiol 156: 353–361, 2007. doi: 10.1016/j.resp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ross KA, Thurlbeck WM. Lung growth in newborn guinea pigs: effects of endurance exercise. Respir Physiol 89: 353–364, 1992. doi: 10.1016/0034-5687(92)90093-C. [DOI] [PubMed] [Google Scholar]
- 178.Ruwald AC, Marcus F, Estes NA III, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 36: 1735–1743, 2015. doi: 10.1093/eurheartj/ehv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Røksund OD, Heimdal JH, Olofsson J, Maat RC, Halvorsen T. Larynx during exercise: the unexplored bottleneck of the airways. Eur Arch Otorhinolaryngol 272: 2101–2109, 2015. doi: 10.1007/s00405-014-3159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation 38, Suppl: VII1–VII78, 1968. [PubMed] [Google Scholar]
- 181.Sawant AC, Bhonsale A, te Riele AS, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H, James CA. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc 3: e001471, 2014. doi: 10.1161/JAHA.114.001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sheel AW, Boushel R, Dempsey JA. Competition for blood flow distribution between respiratory and locomotor muscles: implications for muscle fatigue. J Appl Physiol (1985) 125: 820–831, 2018. doi: 10.1152/japplphysiol.00189.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985) 107: 1622–1628, 2009. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Siebenmann C, Dempsey JA. Hypoxic training is not beneficial in elite athletes. Med Sci Sports Exerc 52: 519–522, 2020. doi: 10.1249/MSS.0000000000002141. [DOI] [PubMed] [Google Scholar]
- 185.Silvestri M, Crimi E, Oliva S, Senarega D, Tosca MA, Rossi GA, Brusasco V. Pulmonary function and airway responsiveness in young competitive swimmers. Pediatr Pulmonol 48: 74–80, 2013. doi: 10.1002/ppul.22542. [DOI] [PubMed] [Google Scholar]
- 186.Simon M, LeBlanc P, Jobin J, Desmeules M, Sullivan MJ, Maltais F. Limitation of lower limb V̇o2 during cycling exercise in COPD patients. J Appl Physiol (1985) 90: 1013–1019, 2001. doi: 10.1152/jappl.2001.90.3.1013. [DOI] [PubMed] [Google Scholar]
- 187.Sollanek KJ, Burniston JG, Kavazis AN, Morton AB, Wiggs MP, Ahn B, Smuder AJ, Powers SK. Global proteome changes in the rat diaphragm induced by endurance exercise training. PLoS One 12: e0171007, 2017. doi: 10.1371/journal.pone.0171007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Squires RW, Buskirk ER. Aerobic capacity during acute exposure to simulated altitude, 914 to 2286 meters. Med Sci Sports Exerc 14: 36–40, 1982. doi: 10.1249/00005768-198201000-00007. [DOI] [PubMed] [Google Scholar]
- 189.St. Croix CM, Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Effects of prior exercise on exercise-induced arterial hypoxemia in young women. J Appl Physiol (1985) 85: 1556–1563, 1998. doi: 10.1152/jappl.1998.85.4.1556. [DOI] [PubMed] [Google Scholar]
- 190.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol (1985) 96: 1920–1927, 2004. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 191.Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol 561: 321–329, 2004. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Strange S Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol 514: 283–291, 1999. doi: 10.1111/j.1469-7793.1999.283af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Taylor CR Structural and functional limits to oxidative metabolism: insights from scaling. Annu Rev Physiol 49: 135–146, 1987. doi: 10.1146/annurev.ph.49.030187.001031. [DOI] [PubMed] [Google Scholar]
- 194.Tedjasaputra V, Bouwsema MM, Stickland MK. Effect of aerobic fitness on capillary blood volume and diffusing membrane capacity responses to exercise. J Physiol 594: 4359–4370, 2016. doi: 10.1113/JP272037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thoden JS, Dempsey JA, Reddan WG, Birnbaum ML, Forster HV, Grover RF, Rankin J. Ventilatory work during steady-state response to exercise. Fed Proc 28: 1316–1321, 1969. [PubMed] [Google Scholar]
- 196.Toledo-Arruda AC, Vieira RP, Guarnier FA, Suehiro CL, Caleman-Neto A, Olivo CR, Arantes PMM, Almeida FM, Lopes FDTQS, Ramos EMC, Cecchini R, Lin CJ, Martins MA. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J Appl Physiol (1985) 123: 674–683, 2017. doi: 10.1152/japplphysiol.00819.2016. [DOI] [PubMed] [Google Scholar]
- 197.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) 114: 3–10, 2013. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, Colar JM, Haines DE, McCullough PA. Acute cardiac effects of marathon running. J Appl Physiol (1985) 108: 1148–1153, 2010. doi: 10.1152/japplphysiol.01151.2009. [DOI] [PubMed] [Google Scholar]
- 199.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985) 97: 404–415, 2004. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 200.Turner LA, Tecklenburg-Lund S, Chapman RF, Stager JM, Duke JW, Mickleborough TD. Inspiratory loading and limb locomotor and respiratory muscle deoxygenation during cycling exercise. Respir Physiol Neurobiol 185: 506–514, 2013. doi: 10.1016/j.resp.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 201.Venlet J, Piers SR, Jongbloed JD, Androulakis AF, Naruse Y, den Uijl DW, Kapel GF, de Riva M, van Tintelen JP, Barge-Schaapveld DQ, Schalij MJ, Zeppenfeld K. Isolated subepicardial right ventricular outflow tract scar in athletes with ventricular tachycardia. J Am Coll Cardiol 69: 497–507, 2017. doi: 10.1016/j.jacc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 202.Vieira RP, Toledo AC, Ferreira SC, Santos AB, Medeiros MC, Hage M, Mauad T, Martins MA, Dolhnikoff M, Carvalho CR. Airway epithelium mediates the anti-inflammatory effects of exercise on asthma. Respir Physiol Neurobiol 175: 383–389, 2011. doi: 10.1016/j.resp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 203.Wagner PD, Erickson BK, Seaman J, Kubo K, Hiraga A, Kai M, Yamaya Y. Effects of altered FIo2 on maximum Vo2 in the horse. Respir Physiol 105: 123–134, 1996. doi: 10.1016/0034-5687(96)00044-8. [DOI] [PubMed] [Google Scholar]
- 204.Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol (1985) 61: 260–270, 1986. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- 205.Wagner PD, Saltzman HA, West JB. Measurement of continuous distributions of ventilation-perfusion ratios: theory. J Appl Physiol 36: 588–599, 1974. doi: 10.1152/jappl.1974.36.5.588. [DOI] [PubMed] [Google Scholar]
- 206.Walker DJ, Walterspacher S, Schlager D, Ertl T, Roecker K, Windisch W, Kabitz HJ. Characteristics of diaphragmatic fatigue during exhaustive exercise until task failure. Respir Physiol Neurobiol 176: 14–20, 2011. doi: 10.1016/j.resp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 207.Walsted ES, Faisal A, Jolley CJ, Swanton LL, Pavitt MJ, Luo YM, Backer V, Polkey MI, Hull JH. Increased respiratory neural drive and work of breathing in exercise-induced laryngeal obstruction. J Appl Physiol (1985) 124: 356–363, 2018. doi: 10.1152/japplphysiol.00691.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang W, Orgeron G, Tichnell C, Murray B, Crosson J, Monfredi O, Cadrin-Tourigny J, Tandri H, Calkins H, James CA. Impact of exercise restriction on arrhythmic risk among patients with arrhythmogenic right ventricular cardiomyopathy. J Am Heart Assoc 7: e008843, 2018. doi: 10.1161/JAHA.118.008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Weibel ER What makes a good lung? Swiss Med Wkly 139: 375–386, 2009. [DOI] [PubMed] [Google Scholar]
- 210.West JB, Mathieu-Costello O. Stress failure of pulmonary capillaries as a limiting factor for maximal exercise. Eur J Appl Physiol Occup Physiol 70: 99–108, 1995. doi: 10.1007/BF00361536. [DOI] [PubMed] [Google Scholar]
- 211.Wetter TJ, St. Croix CM, Pegelow DF, Sonetti DA, Dempsey JA. Effects of exhaustive endurance exercise on pulmonary gas exchange and airway function in women. J Appl Physiol (1985) 91: 847–858, 2001. doi: 10.1152/jappl.2001.91.2.847. [DOI] [PubMed] [Google Scholar]
- 212.Whipp BJ Control of the exercise hyperpnea: the unanswered question. Adv Exp Med Biol 605: 16–21, 2008. doi: 10.1007/978-0-387-73693-8_3. [DOI] [PubMed] [Google Scholar]
- 213.Wüthrich TU, Notter DA, Spengler CM. Effect of inspiratory muscle fatigue on exercise performance taking into account the fatigue-induced excess respiratory drive. Exp Physiol 98: 1705–1717, 2013. doi: 10.1113/expphysiol.2013.073635. [DOI] [PubMed] [Google Scholar]