Abstract
Binge alcohol consumption elicits acute and robust increases of muscle sympathetic nerve activity (MSNA), yet the impact of evening binge drinking on morning-after MSNA is unknown. The present study examined the effects of evening binge alcohol consumption on polysomnographic sleep and morning-after MSNA. We hypothesized that evening binge drinking (i.e. 4–5 drink equivalent in <2 h) would reduce sleep quality and increase morning-after blood pressure (BP) and MSNA. Following a familiarization night within the sleep laboratory, 22 participants (12 men, 10 women; 25 ± 1 yr) were examined after simulated binge drinking or fluid control (randomized, crossover design). Morning MSNA was successfully recorded across both conditions in 16 participants (8 men, 8 women) during a 10-min baseline and three Valsalva’s maneuvers (VM). Binge drinking reduced rapid eye movement (REM) sleep (15 ± 1 vs. 20 ± 1%, P = 0.003), increased stage II sleep (54 ± 1 vs. 51 ± 1%, P = 0.002), and increased total urine output (2.9 ± 0.2 vs. 2.1 ± 0.1 liters, P < 0.001) but did not alter morning-after urine specific gravity. Binge drinking increased morning-after heart rate [65 (54–72) vs. 58 (51–67) beats/min, P = 0.013] but not resting BP or MSNA. Binge drinking elicited greater sympathoexcitation during VM (38 ± 3 vs. 43 ± 3 bursts/min, P = 0.036). Binge drinking augmented heart rate (P = 0.002), systolic BP (P = 0.022), and diastolic BP (P = 0.037) reactivity to VM phase IV and blunted cardiovagal baroreflex sensitivity during VM phases II (P = 0.028) and IV (P = 0.043). In conclusion, evening binge alcohol consumption disrupted REM sleep and morning-after autonomic function. These findings provide new mechanistic insight into the potential role of binge drinking on cardiovascular risk.
NEW & NOTEWORTHY Chronic binge alcohol consumption is associated with future cardiovascular disease (CVD) risk in both men and women. In addition, binge alcohol consumption is known to disrupt normal sleep quality during the early morning hours, coinciding with the morning sympathetic surge. In the present study, an evening of binge alcohol consumption increased baseline morning heart rate and cardiovascular reactivity during the Valsalva maneuver (VM) strain. Specifically, muscle sympathetic nerve activity and phase IV hemodynamic responses increased during VM the morning after binge alcohol consumption. The autonomic dysfunction and increased cardiovascular reactivity during VM suggests a contributing mechanism to CVD risk present in individuals who binge drink.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/binge-drinking-sleep-and-sympathetic-activity/.
Keywords: autonomic activity, baroreflex sensitivity, ethanol, sleep, hypertension
INTRODUCTION
Nearly 20 million Americans suffer from alcohol-related problems or addiction (1). Binge drinking (i.e., 4–5 drink equivalent in <2 h) is responsible for ∼76% of all alcohol consumed in the U.S., and epidemiological studies report that binge alcohol consumption increases the risk for hypertension (2), stroke (3), and sudden cardiac death (4). Among its many physiological effects, alcohol robustly activates the sympathetic nervous system. Our laboratory has reported that simulated binge alcohol consumption increases muscle sympathetic nerve activity (MSNA) (5), a finding consistent with several other alcohol and microneurographic studies (6–12). Accordingly, alcohol-mediated sympathoexcitation is suspected to be a key contributor to increased cardiovascular risk associated with binge drinking.
Although several studies report that alcohol consumption increases resting MSNA in humans (5–12), all have focused on acute effects (i.e., 1–2 h after alcohol consumption). Moreover, these prior studies have typically administered the binge alcohol dose early in the morning after overnight fast, which is not representative of typical evening binge drinking patterns. The risk for stroke (13), myocardial infarction (14), and sudden cardiac death (15) are highest at night and early morning, coinciding with the “morning surge” of autonomic activity between the hours of 6 AM and noon.
Chronic and heavy consumers of alcohol demonstrate high nocturnal sympathetic activity (estimated by heart rate variability) and high morning blood pressure (16) and a rapid increase in blood pressure just before waking (17). Although important, these cross-sectional studies do not provide direct evidence of alcohol-mediated sympathetic overactivity. Additionally, alcohol consumption can facilitate high sleep propensity initially due to the depressant effect, with slow wave sleep (SWS) being highly prevalent during the first half of the night and highly fragmented sleep the second half of the night (18, 19). This coincides with two recent experimental studies that report evening binge alcohol consumption increased the morning surge in arterial blood pressure (16, 20). Additionally, Ekman et al. (21) reported that evening binge drinking increased nocturnal plasma norepinephrine levels and suggested that this autonomic surge may coincide with sleep disturbances; however, these authors did not have any direct assessment of sleep.
The primary aim of this study was to determine the impact of evening binge alcohol consumption on polysomnography sleep and morning-after blood pressure and MSNA. A secondary aim was to determine the impact of binge drinking on baroreflex sensitivity (BRS) and sympathetic reactivity. This randomized control trial (NCT03567434) combines a pragmatic approach (i.e., evening binge drinking) with state-of-the-art techniques for assessing neural cardiovascular control and sleep in humans (i.e., microneurography, finger plethysmography, and polysomnography). We hypothesized that evening binge drinking would reduce sleep quality, increase morning-after blood pressure and MSNA, and decrease BRS.
METHODS
Participants
Young, healthy adults were recruited from Michigan Technological University and the surrounding community. Inclusion criteria for each participant were ages 21–40 yr, body mass index (BMI) of 18.5–35.0 kg/m2, and at least one binge drinking episode within the last 6 mo (i.e., 4–5 drink equivalent within 2 h). Participants were excluded if they had a history of autonomic dysfunction, cardiovascular disease, asthma, diabetes, or alcohol use disorder or had a current prescription for any cardiovascular or antihypertensive medications. All participants were nonsmokers.
Participants abstained from alcohol for a minimum of 24 h and caffeine and exercise for a minimum of 12 h before laboratory testing. Exclusion criteria specific to women included pregnancy, breast feeding, oral or intrauterine contraceptives, and hormone replacement therapy. Eligible women had regular and consistent menstrual cycles (i.e., 25–32 days) and were tested in either the early follicular (EF; i.e., 2–5 days after onset of menstruation) or mid-luteal (ML; 8–10 days after ovulation) phase. The Michigan Technological University Institutional Review Board approved all testing procedures, which conformed to guidelines contained within the Declaration of Helsinki. Investigators explained these procedures and obtained written, informed consent from all participants.
Screening procedures began with a preliminary phone screen conducted by a laboratory research study coordinator or graduate student on 78 individuals. Criteria included height, weight, age, sex, drinking habits, tobacco use, diabetes and sleep apnea history, use of heart and blood pressure medications, alcohol use disorder questionnaire, pregnancy status, birth control use, and menstrual cycle length. All eligible participants self-identified as binge drinkers (i.e., consumption of 4–5 alcoholic beverages within 2 h within the past six months). A total of 56 potential participants were eligible and invited to Michigan Tech’s Sleep Research Laboratory for an orientation session. Twenty-two individuals either failed to schedule an orientation session, moved out of the area, or were no longer interested. Following a detailed description of the study and consent process, 35 eligible participants finalized their study enrollment by confirming aldehyde dehydrogenase 2 (ALDH-2) function via flushing questionnaire (22) and completing an at-home ApneaLink screen (ResMed, San Diego, CA). Those who reported excessive facial/whole body flushing accompanied by rapid heart rate, nausea, and headache following consumption of one to two alcoholic beverages were assumed to have a deficiency in ALDH-2, which converts acetaldehyde to acetate, and were excluded from the study. Thirteen individuals were withdrawn from the study due to birth control/menstrual cycle irregularities, no longer being identified as binge drinker, loss of interest, or administrative issues (i.e., failure to schedule 2nd visit or preventative measures for potential adverse events related to alcohol consumption). Twenty-two (11 women) participants completed the study, and of this cohort, a stable nerve recording was obtained on 16 participants (8 women; 6 EF and 2 ML).
Measurements
Polysomnography.
A standard polysomnographic (PSG) sleep study (Natus Medical, Middleton, WI) was performed on each participant in the Sleep Research Laboratory. Sleep electroencephalography (EEG) was recorded and scored via 10–20 electrode placements with two frontal, central, and occipital lead referenced electrodes placed at the mastoid process on the opposite side of the head. In accordance with established standards of the American Academy of Sleep Medicine (AASM), electrooculography (EOG) and electromyography (EMG) were recorded via two electrodes near the eyes and three electrodes placed on the chin, respectively. Thorax and abdomen piezoelectric effort belts were used to monitor respiratory effort, and respiratory flow was also measured using a nasal cannula. Blood oxygen saturation via pulse oximetry was recorded for any potential desaturations associated with apneic events. Leg movements were measured via EMG, with surface electrodes placed on the tibialis anterior of each leg. Sleep staging, apneic/respiratory events, limb movements, and arousals were defined and scored according to the AASM. All sleep studies were scored by a board-certified sleep physician (C. A. Smoot).
Blood pressure and heart rate.
Arterial blood pressure was measured in the seated position before instrumentation by an automated sphygmomanometer. During the baseline and the subsequent three VMs, beat-to-beat arterial pressure was recorded using finger plethysmography (NOVA; Finapres Medical Systems, Amsterdam, The Netherlands). Prior to each baseline, supine blood pressures were used to calibrate finger plethysmography. Arterial blood pressures were expressed as systolic (SAP) and diastolic (DAP) arterial pressure. Heart rate (HR) was recorded continuously via a three-lead electrocardiogram, and respiratory rate was continuously measured using a pneumobelt.
Microneurography.
A reference electrode was inserted subcutaneously 2–3 cm from the microneurography electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (total gain of 80,000), where the nerve signal was band-pass filtered (700–2,000 Hz) and integrated (time constant, 0.1 s) to obtain a mean voltage display of nerve activity. Acceptable recordings of MSNA were defined by spontaneous, pulse-synchronous bursts that increased during end-expiratory apnea and remained unchanged during auditory stimulation or stroking of the skin.
Muscle sympathetic nerve activity.
Multifiber recordings of MSNA were obtained by inserting a tungsten microelectrode (Frederick Haer, Bowdoin, ME) into the peroneal nerve of the right leg. Microneurography is currently the only available technique to directly record postganglionic sympathetic nerve activity in humans and is detailed in the following reviews (23, 24). Additional descriptions of the microneurographic technique and analyses, including the sympathetic (25, 26) and cardiovagal (27, 28) spontaneous baroreflex analyses (25, 29), are described below.
Valsalva’s maneuver.
During each VM, participants forcefully exhaled to 40 mmHg for 15 s into a modified pressure manometer. Each of the three VM strains was separated by a 1-min recovery with normal breathing. Investigators ensured that the nose was sealed with a nose clip and verified a small leak in the modified manometer to keep the glottis open during each VM strain.
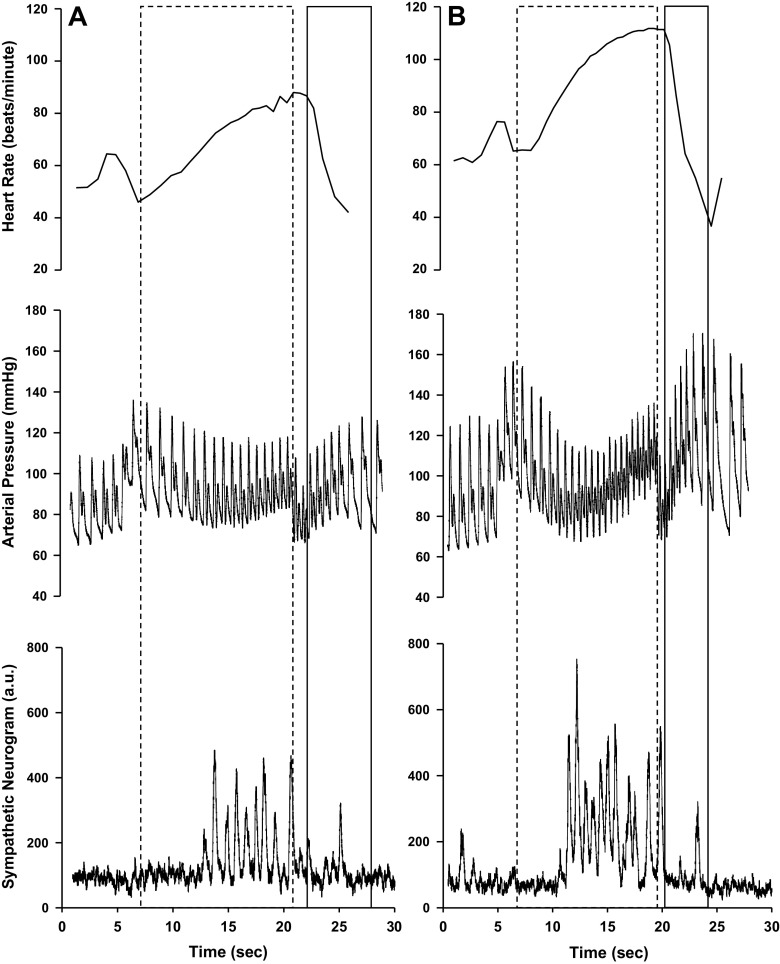
Four VM phases were identified as described by Hamilton et al. (30) and Smith et al. (31) and previously used within our laboratory (32). Figure 1 depicts a representative VM response following fluid control (A) and alcohol (B). Phase I was determined from strain onset to the highest value of arterial blood pressure. This highest-pressure value designated the beginning of phase II. Phase III began directly following the release of the strain and ended after the first blood pressure increased poststrain. Phase IV was determined from first blood pressure increase poststrain to the first decrease in arterial blood pressure following overshoot.
Figure 1.
Tracings of heart rate, blood pressure, and muscle sympathetic nerve activity during the 15-s Valsalva’s maneuver (VM) for 1 representative participant following fluid control (A) and binge alcohol dose (B). Phase II of VM is outlined with a dashed line, which constitutes the first blood pressure decrease from strain onset to strain termination. Phase IV of VM is represented with a solid line and begins with the first blood pressure increase after strain termination to first blood pressure decrease during blood pressure overshoot.
Valsalva’s maneuver sympathetic baroreflex sensitivity was determined by comparing all sympathetic bursts occurring during the 15-s strain period of the VM to the maximum fall of DAP in early phase II. Bursts of MSNA during the 15-s strain were determined after adjusting for calculated burst reflex latencies. For cardiovagal baroreflex sensitivity (cvBRS) during VM, the slope methods reported by Kautzner et al. (33) were utilized. Briefly, VM cvBRS was determined by the linear relationship of SAP and RRI during a hypotensive stimulus (i.e., early phase II) and a hypertensive stimulus (i.e., phase IV, “overshoot”). To be considered a valid sequence, correlation coefficients were set as >0.70, and only VMs with reproducible and appropriate temporal patterns were included in the final analysis (i.e., elimination of “square-wave” VM). Twelve recordings were excluded from analysis. The mean coefficient value for the VM protocol was 0.983 ± 0.004. Valid VM trials were averaged for each subject.
Baroreflex function: spontaneous methodologies.
Cardiovagal and sympathetic baroreflex sensitivities (BRS) were determined using spontaneous approaches during the initial 10-min baseline. Sympathetic BRS was determined by utilizing the spontaneous DAP-MSNA slope method, which related changes in MSNA with fluctuations in DAP during the initial 10-min baseline (25, 26). DAP of each cardiac cycle was quantified into 3-mmHg intervals (bins) during baseline. Burst incidence for each bin was quantified and plotted against each corresponding DAP. The slope between DAP and MSNA burst incidence was generated via a linear regression analysis. This analysis was weighted for the number of cardiac cycles in each DAP bin, and the slope of the regression line was defined as the sympathetic BRS. Inclusion criteria for the sympathetic BRS included negative slopes with a minimum r value of 0.40, which is consistent with prior studies in our laboratory (34, 35). Four participants’ data were excluded with the stated criteria, where the mean coefficient value was 0.79 ± 0.05 for the remaining 12 participants.
Spontaneous cardiovagal BRS was assessed via the sequence method during the initial 10-min baseline (27, 28). In brief, three or more progressive changes of SAP and corresponding R-R interval (RRI; lag 1) were identified as baroreflex sequences. Both up-up (i.e., 3 or more increases in SAP in tandem with lengthening RRI) and down-down (i.e., progressive decreases in SAP in tandem with shortening RRI) were quantified. Criteria for an acceptable sequence included 1 mmHg for SAP and 4 ms for RRI. A linear regression analysis of each sequence of RRI and SAP determined the reported slope. A minimum r value of 0.7 was used as the criteria for accepting sequences. Up-up and down-down sequences within the 10-min baseline were averaged for each subject.
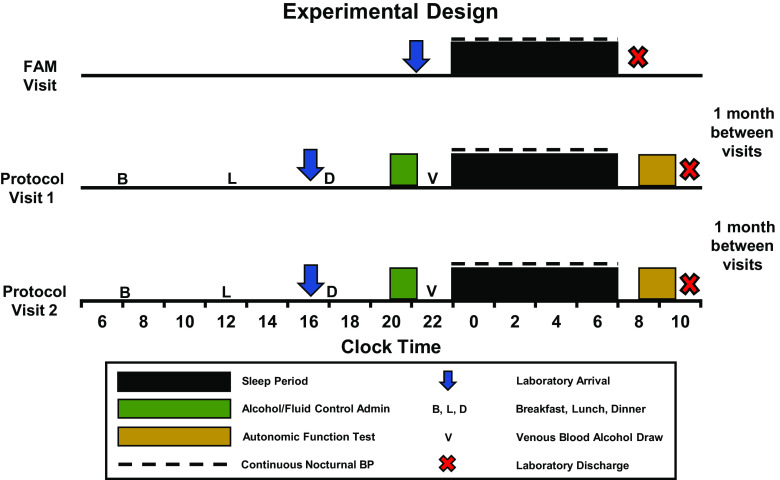
Experimental Design
Participants arrived for laboratory testing three times. During their first visit, participants reported to the Sleep Research Laboratory at 9 PM for a familiarization night. This included a routine PSG sleep study with dual-cuff beat-to-beat finger plethysmography to confirm the absence of obstructive sleep apnea, periodic limb movements, and other sleep disorders. Using a randomized, crossover design, eligible participants returned to the laboratory for two more visits, with 1 mo in between each visit to ensure that all women were captured during the same ovarian cycle phase (36). For each of these experimental visits (alcohol versus fluid control), participants reported to the laboratory at 4 PM for baseline testing that included blood alcohol content via breathalyzer (Intoximeters, Inc., St. Louis, MO) and urine-specific gravity to estimate hydration status (Schmidt Haensch, Berlin, Germany). Ad libitum water consumption was provided throughout the experimental protocol. A standardized breakfast, lunch, and dinner were provided to each participant in consultation with a registered dietitian to ensure similar caloric intake with meal based on each individual’s BMI and activity level (37). Following dinner, participants remained in a semi-recumbent position for approximately 2 h. Multiple validated questionnaires were completed during this time, including the Pittsburgh Sleep Quality Index (PSQI) (38, 39), Epworth Sleepiness Scale (ESS) (40), Insomnia Severity Index (ISI) (41), Center for Epidemiological Studies Depression questionnaire (42), and state and trait anxiety inventories (STAI) (43), to provide demographic data on sleep quality, depression, and anxiety during participants’ first experimental visits to the laboratory. STAI raw scores were determined by summing responses of a Likert scale for each response and extrapolated to a standardized score based upon participants’ ages in accordance to the STAI manual (43).
At 8 and 9 PM, alcohol doses were administered and diluted in a 1:3 ratio of 95% ethanol to desired fruit juice (orange or cranberry juice) to mimic an evening of binge alcohol consumption. The alcohol dose was 1 g/kg dose for men and a 0.85 g/kg dose for women. The fluid control included an exact volume of only fruit juice. Each dose was divided into three portions and given at the top of the hour in 5-min intervals. An oral breathalyzer was administered every 15 min from 8 to 10 PM to track intoxication. At 9:15 PM, PSG and finger plethysmography instrumentation occurred. A venous blood sample was collected at 10 PM for blood alcohol content (BAC). The participant entered bed at 10:45 PM, with lights out at 11 PM. Following an 8-h sleep opportunity, lights were turned on at 7 AM, with an immediate urine sample measurement for urine-specific gravity as well as intravenous blood sample for alcohol content and sex steroids.
At ∼8 AM, three seated blood pressures were taken with an automated sphygmomanometer (Omron HEM-907XL, Kyoto, Japan) after 5 min of quiet rest. At ∼8:15 AM, participants were positioned supine on a cushioned tilt table for hemodynamic and autonomic instrumentation. Once microneurography and hemodynamic parameters were established, a nonrecorded 10-min period was allotted to ensure stable measurements. Upon confirmation of stable recordings, neural and hemodynamic variables were recorded during an official 10-min baseline, followed by three Valsalva’s maneuvers (VM). Figure 2 offers a graphical representation highlighting main experimental events on the familiarization night and each experimental visit.
Figure 2.
Experimental design of the present study consisting of familiarization (FAM) night and two testing protocols where binge alcohol or fluid control were provided. A 1-mo interval was allotted between each laboratory visit.
Statistical Analysis
All data were analyzed statistically using commercial software (SPSS 25.0; IBM SPSS, Armonk, NY). Assumptions of normality tests were conducted on each variable of interest. We utilized paired-samples t tests to compare differences between conditions (i.e., alcohol versus fluid control) when assumptions of normal distribution were met. The Wilcoxon signed-rank test was conducted on nonnormally distributed variables, which were limited to baseline heart rate, SAP-RRI up-up, urine-specific gravity, fluid consumption, and sBRS during VM. Data are presented as means ± SE for data that met assumptions of normality and as median (25th to 75th percentile) if assumptions of normality were violated. Most variables were analyzed via two-tailed statistical tests, whereas one-tailed statistical tests were utilized for directional hypotheses related to impaired sleep, heighted MSNA, elevated blood pressure, and reduced baroreflex function. Pearson correlation was used to examine the relationship between changes in the primary sleep outcome variable (i.e., total sleep time) and the primary autonomic outcome variables (i.e., MSNA at rest and during VM). Significance level was set as α ≤ 0.05.
RESULTS
Participants were 24 ± 1 yr with a BMI of 27 ± 1 kg/m2. Table 1 highlights that our sample reported adequate sleep quality (PSQI), did not report excessive daytime sleepiness (ESS), and did not have insomnia symptoms (ISI) under normal sleep conditions. Participants did not reach the threshold for potential clinical depression or anxiety.
Table 1.
Baseline participant characteristics
| Variable | Value (n = 22) |
|---|---|
| Age, yr | 24 ± 1 |
| Height, cm | 171 ± 2 |
| Weight, kg | 79 ± 3 |
| PSQI | 5 ± 0.4 |
| Epworth sleepiness scale | 6 ± 0.7 |
| Insomnia severity index | 4 ± 0.5 |
| Depression questionnaire | 6 ± 1.1 |
| STAI state, raw | 29 ± 2 |
| STAI state, standard | 43 ± 2 |
| STAI trait, raw | 33 ± 2 |
| STAI trait, standard | 47 ± 2 |
Values are means ± SE. PSQI, Pittsburgh sleep quality index; STAI, state and trait anxiety inventory. Depression, n = 17; STAI state, n = 20; STAI trait, n = 18.
The 2-h binge drinking episode significantly increased blood alcohol content (0 ± 0 vs. 91 ± 5 mg/dL, P < 0.001) and breath alcohol concentration (0.000 ± 0.000 vs. 0.095 ± 0.004%, P < 0.001) compared with fluid control. Blood alcohol content (0 ± 0 mg/dL) and breath alcohol concentration (0 ± 0%) returned to normal the morning after binge drinking.
Table 2 depicts PSG after evening binge alcohol or fluid control. Binge drinking decreased total sleep time (P = 0.006), sleep efficiency (P = 0.004), and the percentage of REM sleep (P = 0.003) compared with fluid control. Binge drinking increased the percentage of stage II sleep compared with fluid control (P = 0.002), whereas all other non-REM sleep stages were comparable across alcohol and fluid control conditions. Total urine output was elevated after binge drinking (2121 ± 192 vs. 2851 ± 231 mL, P < 0.001), but this was largely offset by additional fluid consumption [1,369 (1,027–1,495) vs. 1,541 (1,086–1,782) mL, P = 0.056], which resulted in no change in urine-specific gravity the morning after binge alcohol consumption [1.017 (1.011–1.021) vs. 1.016 (1.011–1.021) a.u., P = 0.650].
Table 2.
Sleep after evening binge alcohol consumption
| Variable | Fluid Control (n = 22) | Alcohol Dose (n = 22) | P Value |
|---|---|---|---|
| Total sleep time, min | 434 ± 5 | 417 ± 7* | 0.006 |
| Sleep efficiency, % | 91 ± 1 | 87 ± 2* | 0.004 |
| Sleep onset latency, min | 9 ± 3 | 8 ± 3 | 0.140 |
| REM latency, min | 118 ± 10 | 132 ± 14 | 0.092 |
| Number of arousals | 58 ± 6 | 57 ± 4 | 0.441 |
| Stage 1 sleep, % | 5 ± 1 | 5 ± 1 | 0.240 |
| Stage 2 sleep, % | 51 ± 1 | 54 ± 1* | 0.002 |
| Stage 3 sleep, % | 25 ± 2 | 25 ± 1 | 0.390 |
| REM sleep, % | 19 ± 1 | 16 ± 1* | 0.003 |
Values are means ± SE. REM, rapid eye movement. *P < 0.05, fluid control vs. corresponding alcohol dose via paired t test.
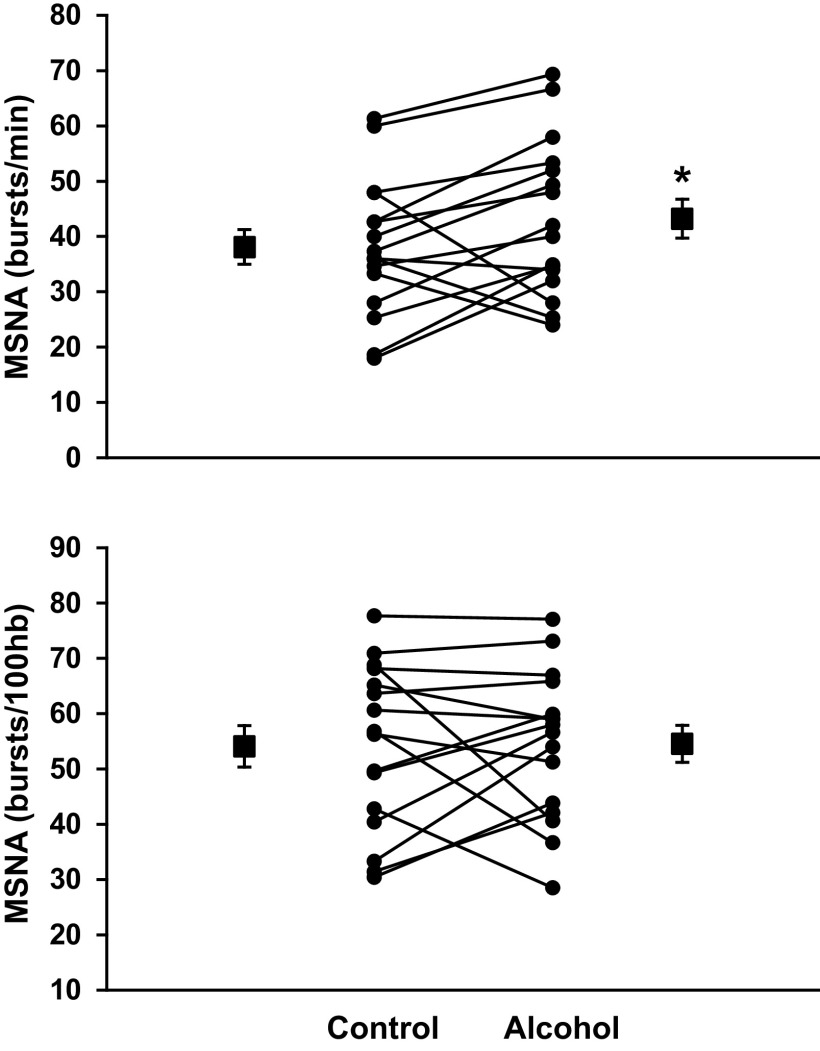
Table 3 demonstrates that binge drinking increased morning-after resting HR (P = 0.013), but not baseline MSNA, blood pressure, or spontaneous cardiovagal BRS. Figure 3 demonstrates that evening binge alcohol elicited a greater increase of morning-after MSNA during VM when expressed as burst frequency (38 ± 3 vs. 43 ± 3 bursts/min, P = 0.036) but not burst incidence (54 ± 4 vs. 55 ± 3 bursts/100hb, P = 0.447). Spontaneous sBRS assessed during baseline was not different between alcohol and fluid control conditions. In contrast, VM sympathetic BRS trended lower following binge drinking compared with fluid control (P = 0.062). Changes in total sleep time between alcohol and fluid control conditions were not associated with changes in MSNA burst frequency at baseline (r = −0.025, P = 0.891) or during VM (r = −0.132, P = 0.471).
Table 3.
Morning hemodynamic and autonomic responses after evening binge drinking
| Variable | Fluid Control (n = 16) | Alcohol Dose (n = 16) | P Value |
|---|---|---|---|
| Systolic arterial pressure, mmHg | 113 ± 3 | 111 ± 3 | 0.292 |
| Diastolic arterial pressure, mmHg | 66 ± 2 | 64 ± 2 | 0.131 |
| Mean arterial pressure, mmHg | 82 ± 2 | 80 ± 2 | 0.153 |
| Heart rate, beats/min | 58 (51–67) | 65 (54–72)† | 0.013 |
| MSNA, bursts/min | 18 ± 2 | 19 ± 2 | 0.389 |
| MSNA, bursts/100hb | 31 ± 4 | 31 ± 3 | 0.870 |
| cvBRS up-up, ms/mmHg | 22 (14–49) | 20 (11– 44) | 0.459 |
| cvBRS down-down, ms/mmHg | 23 ± 2 | 23 ± 3 | 0.858 |
| sBRS, BI/mmHg | −1.60 ± 0.20 | −1.62 ± 0.19 | 0.948 |
| VM sBRS, bursts·15 s−1·mmHg−1 | −0.58 (−0.67 to −0.44) | −0.47 (−0.63 to −0.42) | 0.062 |
Values are means ± SE for normally distributed and median (25th to 75th percentile) for nonnormally distributed variables. BI, burst incidence; cvBRS, cardiovagal baroreflex sensitivity; hb, heart beat; MSNA, muscle sympathetic nerve activity; sBRS, spontaneous baro- reflex sensitivity. †P < 0.05, fluid control vs. corresponding alcohol dose via Wilcoxon signed-rank test.
Figure 3.
Morning muscle sympathetic nerve activity (MSNA) during a 15-s Valsalva maneuver (VM) after fluid control and evening binge alcohol consumption. MSNA burst frequency (P = 0.036), but not burst incidence (P = 0.447), was elevated following binge alcohol consumption during VM. *P < 0.05 between conditions via paired-samples t tests between alcohol and fluid control.
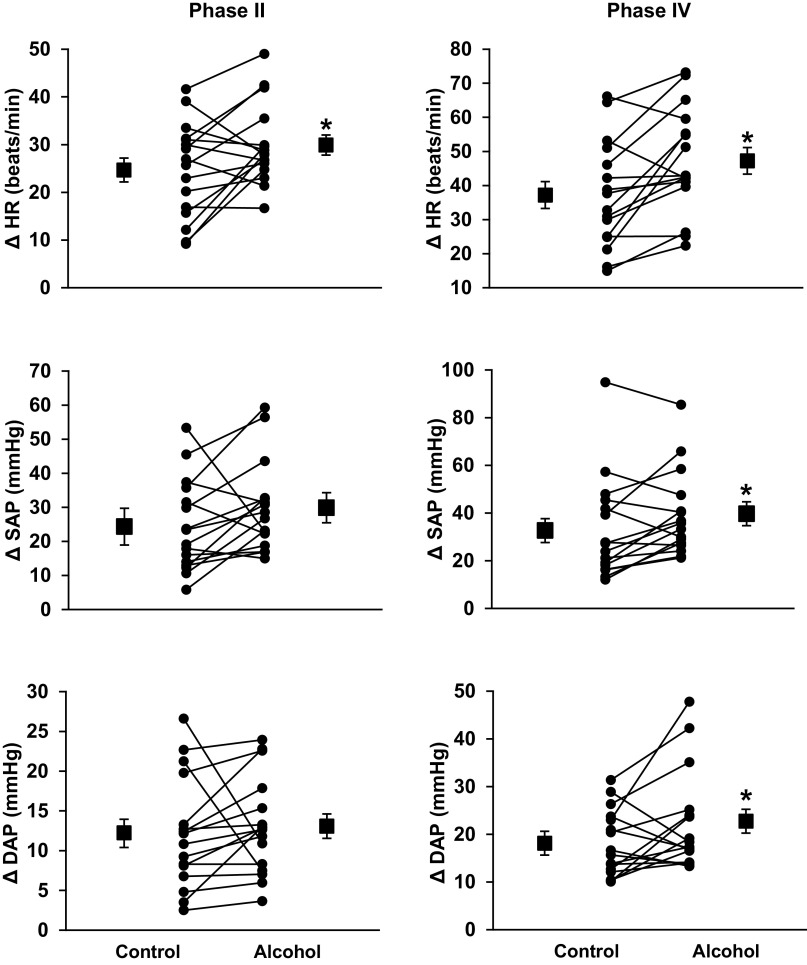
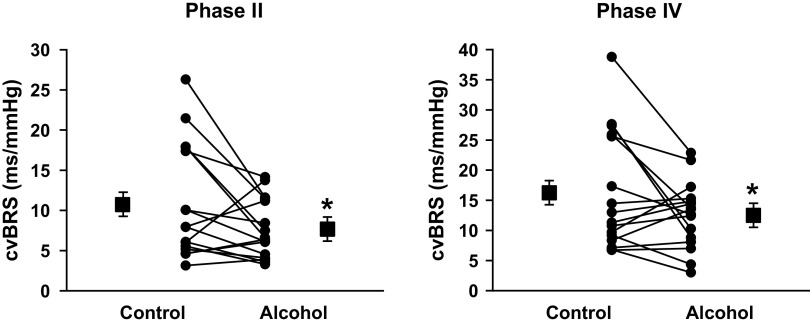
Figure 4 illustrates HR, SAP, and DAP responses during phase II and phase IV of VM. Increases in HR during phase II (Δ25 ± 2 vs. Δ30 ± 3 mmHg, P = 0.018) and phase IV (Δ37 ± 4 vs. Δ47 ± 4 mmHg, P = 0.002) were significantly augmented following a night of binge drinking compared with fluid control. Reductions of SAP (Δ−24 ± 3 vs. Δ−30 ± 3 mmHg, P = 0.056) and DAP (Δ−12 ± 2 vs. Δ−13 ± 2 mmHg, P = 0.301) during VM phase II were not different between conditions. In contrast, increases in SAP (Δ33 ± 5 vs. Δ39 ± 4 mmHg, P = 0.022) and DAP (Δ18 ± 2 vs. Δ22 ± 3 mmHg, P = 0.037) during VM phase IV were significantly augmented following a night of binge drinking compared with fluid control. Figure 5 demonstrates that following a night of binge drinking, VM phase II (11 ± 2 vs. 8 ± 1 ms/mmHg, P = 0.028) and phase IV (16 ± 2 vs. 13 ± 1 ms/mmHg, P = 0.043) cvBRS were significantly blunted compared with fluid control visit.
Figure 4.
Changes in heart rate (HR), systolic arterial pressure (SAP), and diastolic arterial pressure (DAP) during phase II and IV of Valsalva’s maneuver (VM). Increases in HR during VM were augmented during both phase II (P = 0.018) and IV (P = 0.002) after evening binge alcohol consumption. Phase II SAP and DAP drop (expressed as absolute changes for visual purposes) were not different between conditions (P = 0.059 and 0.301, respectively). Morning SAP (P = 0.022) and DAP (P = 0.037) “overshoot” during phase IV VM were augmented following binge alcohol consumption. *P < 0.05 between conditions via paired-samples t tests between alcohol and fluid control.
Figure 5.
Cardiovagal baroreflex sensitivity (cvBRS) during phase II and IV of Valsalva’s maneuver (VM). cvBRS during phase II (P = 0.028) and IV (P = 0.043) of VM were significantly blunted the morning after binge alcohol consumption. *P < 0.05 between conditions via paired-samples t tests between alcohol and fluid control.
DISCUSSION
Whereas numerous experimental studies report consistent increases of MSNA after simulated binge drinking, these prior investigations have focused on the acute effects within minutes to hours and at times of day that are not representative of normal binge drinking habits (i.e., morning after overnight fast). The present study takes a pragmatic yet well-controlled experimental approach to determine the impact of evening binge drinking on both sleep and morning autonomic function. We report four novel findings. First, and in contrast to acute alcohol consumption studies and our initial hypothesis (5, 8), baseline blood pressure and MSNA were not significantly different the morning after binge drinking and fluid control. Second, in contrast to baseline measurements, there were significant HR, blood pressure, and MSNA differences between alcohol and fluid control during the sympathoexcitatory VM. Specifically, the alcohol condition was associated with greater HR reactivity during VM phase II and IV, augmented SAP and DAP reactivity during VM phase IV, blunted cardiovagal BRS during VM phase II and IV, and heightened MSNA burst frequency during VM. Collectively, these changes are indicative of heighted autonomic impairment the morning after a night of binge drinking. Third, binge drinking significantly impaired objective measures of sleep as determined using gold-standard polysomnography. In particular, binge drinking reduced total sleep time, sleep efficiency, and REM, findings that are consistent with prior alcohol/sleep studies (44, 45). Finally, although we anticipated higher urine-specific gravity the morning after binge drinking, this was not observed in the present study, which was perhaps due to allowing ad libitum water during evening and night. In summary, evening binge drinking disrupted sleep and morning sympathetic reactivity and baroreflex function. These findings address a significant gap in our understanding of binge alcohol consumption and neurovascular control of blood pressure and suggest a potential mechanism for increased cardiovascular risk of binge drinking.
Acute alcohol consumption has been shown to augment HR (5, 7–12, 46, 47), mean arterial pressure (MAP) (5, 7, 9, 10, 48), and MSNA (5, 7–12). Prior work from our laboratory demonstrated that acute alcohol ingestion increased MSNA and heart rate but did not alter resting MAP (5). These findings suggest that acute ingestion of alcohol may not necessarily evoke parallel increases in baseline blood pressure and MSNA. In the present study, baseline blood pressure and MSNA were similar between binge alcohol consumption and fluid control. However, there was a significant increase in resting HR the morning after alcohol consumption, a finding that was consistent with acute alcohol consumption. Although speculative, morning epinephrine may have been increased in our sample of participants following a night of binge drinking. Epinephrine is upregulated following acute alcohol consumption and contributes to peripheral vasodilation via β2-receptor (49). It is possible that lingering alcohol-mediated epinephrine reduced sympathetic vascular transduction, contributing to larger fluctuations of blood pressure only during VM but not during baseline. Utilizing ambulatory blood pressure monitoring, Seppä et al. (48) observed similar findings, reporting that evening binge drinking did not alter morning-after blood pressure but increased morning-after heart rate.
The VM is an easily administered and reproducible stressor that provides indices of both sympathetic and vagal regulation of HR and blood pressure (i.e., phases II and IV) (30, 31). In contrast to the baseline measurements, the present study demonstrated augmented sympathetic neural outflow and reduced vagal input the morning following evening binge alcohol consumption during VM. This included heightened MSNA burst frequency to phase II of VM the morning after binge drinking and a number of HR, blood pressure, and BRS differences indicative of reduced baroreflex function the morning after binge alcohol consumption. Notably, spontaneous cvBRS was reduced after evening binge alcohol consumption in addition to a trend for reduced sympathetic BRS during VM. It is important to note that although augmented sympathoexcitation during VM was observed after binge drinking when expressed as burst frequency, VM sympathoexcitation was not different between the alcohol and fluid control conditions when MSNA was expressed as burst incidence. This is likely due to the significant increase in HR reactivity during VM phase II, which resulted in more cardiac cycles and opportunities for MSNA bursts during the 15-s VM strain. Nevertheless, the increased MSNA burst frequency during VM is still relevant, as it still likely equates to more norepinephrine release, which impacts vasoconstriction and cardiovascular risk. Finally, the augmented sympathetic and/or hemodynamic responses to VM after acute binge drinking could have implications related to morning-after nausea and vomit. Specifically, nausea has been shown to be related to autonomic dysfunction in a variety of populations (50, 51). When combined with the sympathetic surge observed during Valsalva strain, the autonomic dysfunction the morning after binge drinking may increase the risk of adverse cardiovascular events.
It is important to note that Howes and Reid (52) conducted a protocol similar to the present study, but utilizing the VM following daily consumption of a 0.8 g/kg dose of ethanol for 1 wk in participants who classified as moderate drinkers when compared with 1 wk of alcohol abstinence. These authors observed no differences in hemodynamic or norepinephrine changes between conditions during the VM. The differences between Howes and Reid (52) and our findings suggest key physiological differences between light to moderate drinking and binge drinking with regard to neural control of blood pressure. However, metabolites of evening alcohol consumption may also have contributed to the sympathoexcitation observed during VM in the present study. Acetate is a key byproduct of ethanol metabolism via alcohol dehydrogenase (53). Chapp et al. (54) observed that direct acetate injection into rats induced sympathoexcitation and a pressor response. Accordingly, morning-after ethanol metabolites may have contributed to differences between Howes and Reid (52) and the present study.
Alcohol has a well-known acute diuretic property, partially facilitated through inhibition of the posterior pituitary hormone arginine vasopressin (AVP) (55, 56). This diuresis results in acute increases in serum osmolality and increased urine excretion, similar to what was observed in our study. Interestingly, we did not observe any differences in urine-specific gravity the morning following either condition, which was indicative of no disparity in hydration status. It is important to note that the impact of alcohol on inhibition of AVP secretion may only be acute (56). Taivainen et al. (56) observed the impact of alcohol on AVP and fluid balance over a period of 14 h, utilizing a concentration of alcohol similar to that used in our study (i.e., 1.2 g/kg). They observed a biphasic response of AVP, showing an acute inhibition, and a delayed antidiuretic response to alcohol consumption overnight (56). This delayed antidiuretic effect, in addition to a trend toward increased water consumption during the binge alcohol condition, may have directly impacted and stabilized morning urine-specific gravity. Increases in osmolality have been shown to be directly related with baseline sympathoexcitation (57, 58) and increased baroreflex sensitivity in humans (59). In our study, we did not observe any differences in baseline MSNA or baroreflex sensitivity, indicating that any potential differences in plasma osmolality did not manifest as baseline sympathoexcitation but could have contributed to heighted VM sympathoexcitation and cardiovascular reactivity.
Our study confirms previous findings that binge alcohol consumption disrupts normal sleep architecture via compromised REM sleep (44, 45). Specifically, decreased REM sleep percentage was offset with increased stage II sleep after binge alcohol consumption. This is believed to be the result of ethanol’s action within the brain, which competitively binds to GABA receptors and contributes to the sedative effect following acute alcohol consumption (60). Although assessed in only a limited number of studies due to technical difficulty of microneurography, REM is the most sympathoexcitatory sleep stage (61–63), even when compared with waking levels (64). It is possible that the selective deprivation of REM contributed to the heighted sympathoexcitation during VM. This is consistent with prior studies, which have demonstrated altered baroreflex gain and/or setpoint that is indicative of the larger blood pressure oscillations and increased blood pressure during REM sleep (61, 65). Although there have been some sleep deprivation studies examining MSNA (34, 35), they have focused on total sleep deprivation and not selective deprivation of sleep stages such as REM. It is possible the disrupted sleep, and not the alcohol consumption, is an underlying mechanism for the observed morning-after autonomic disruption during Valsalva’s Maneuver. Future work in this area appears warranted.
We acknowledge the following limitations. Although our sample of young, healthy individuals is nearly equally sampled between men and women, it is imperative to be attentive to potential sex differences. We do not yet have statistical power to adequately examine the potential impact of sex, but this is a secondary aim of the current project, where data collection is expected for two more years. This is of importance given the known difference in resting MSNA and BP between young men and women (66). Moreover, resting MSNA has been shown to be different during various ovarian phases (i.e., early follicular versus mid luteal) (36). Second, our current experimental approach does not allow us to equivocally differentiate whether the observed autonomic dysfunction the morning after binge drinking is related to alcohol consumption or the compromised sleep. Importantly, changes in total sleep time were not significantly associated with MSNA at rest or during VM. Accordingly, future work will also examine the potential influence of ovarian phase on morning MSNA in female binge drinkers. Finally, 12 VM recordings were excluded from a total of 96 VM trials performed due to abnormal blood pressure response (i.e., square waveform). For the majority of participants, average VM responses consisted of two to three trials. This limitation is minimized, as temporal patterns in VM response are highly reproducible when normal physiological waveform responses are obtained (33).
In summary, there is adequate epidemiological evidence to suggest a relation between binge alcohol consumption and cardiovascular risk (2–4). Alcohol-induced sympathoexcitation is believed to contribute to this relationship, but the morning-after effects of alcohol on sympathetic activity are equivocal. Although evening binge drinking did not alter morning-after blood pressure and MSNA at rest, we observed significant blood pressure and MSNA differences between alcohol and fluid control during the sympathoexcitatory VM. These differences included sympathoexcitation during phase II of VM and reduced baroreflex function using a number of VM analyses. These findings provide new mechanistic insight between the relation between binge drinking and cardiovascular disease.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA-024892.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.S. and J.R.C. conceived and designed research; I.M.G., H.A.C., A.L.T., J.A.B., J.J.D., and J.R.C. performed experiments; I.M.G., H.A.C., A.L.T., J.A.B., C.A.S., J.J.D., and J.R.C. analyzed data; I.M.G., H.A.C., A.L.T., J.A.B., C.A.S., J.J.D., and J.R.C. interpreted results of experiments; I.M.G. prepared figures; I.M.G. drafted manuscript; I.M.G., H.A.C., A.L.T., J.A.B., C.A.S., J.J.D., and J.R.C. edited and revised manuscript; I.M.G., H.A.C., A.L.T., J.A.B., C.A.S., J.J.D., and J.R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our team of undergraduate research assistants, including Maggie Blevins, Abigail Botz, Morgan Colling, Alexa Destrampe, Sarah Dix, Bella Nutini, Grant Thivierge, Alex Rondorf, and Jonathon Worden. We also thank Terry Anderson for administrative support, our sleep technician Jennifer Nicevski, and Kelsae Eliszewski for dietary consultation.
REFERENCES
- 1.National Institutes of Health. Rethinking drinking: alcohol and your health. National Institute on Alcohol Abuse and Alcoholism; No. 10-3770 (Revised April 2010): 1–18, 2010. [Google Scholar]
- 2.Abramson JL, Lewis C, Murrah NV. Relationship of self-reported alcohol consumption to ambulatory blood pressure in a sample of healthy adults. American journal of hypertension 23: 994–999, 2010. doi: 10.1038/ajh.2010.109. [DOI] [PubMed] [Google Scholar]
- 3.Sundell L, Salomaa V, Vartiainen E, Poikolainen K, Laatikainen T. Increased stroke risk is related to a binge-drinking habit. Stroke 39: 3179–3184, 2008. doi: 10.1161/STROKEAHA.108.520817. [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol 50: 1009–1014, 2007. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 5.Carter JR, Stream SF, Durocher JJ, Larson RA. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am J Physiol Endocrinol Metab 300: E771–778, 2011. doi: 10.1152/ajpendo.00674.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade AC, Cesena FH, Consolim-Colombo FM, Coimbra SR, Benjo AM, Krieger EM, Luz PL. Short-term red wine consumption promotes differential effects on plasma levels of high-density lipoprotein cholesterol, sympathetic activity, and endothelial function in hypercholesterolemic, hypertensive, and healthy subjects. Clinics (Sao Paulo) 64: 435–442, 2009. doi: 10.1590/S1807-59322009000500011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: a preliminary report. J Hypertens Suppl 7: S20–S21, 1989. doi: 10.1097/00004872-198900076-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Potentiated sympathetic and hemodynamic responses to alcohol in hypertensive vs. normotensive individuals. J Hypertens 29: 537–541, 2011. doi: 10.1097/HJH.0b013e328342b2a9. [DOI] [PubMed] [Google Scholar]
- 9.Iwase S, Matsukawa T, Ishihara S, Tanaka A, Tanabe K, Danbara A, Matsuo M, Sugiyama Y, Mano T. Effect of oral ethanol intake on muscle sympathetic nerve activity and cardiovascular functions in humans. J Auton Nerv Syst 54: 206–214, 1995. doi: 10.1016/0165-1838(95)00022-P. [DOI] [PubMed] [Google Scholar]
- 10.Randin D, Vollenweider P, Tappy L, Jéquier E, Nicod P, Scherrer U. Suppression of alcohol-induced hypertension by dexamethasone. N Engl J Med 332: 1733–1737, 1995. doi: 10.1056/NEJM199506293322601. [DOI] [PubMed] [Google Scholar]
- 11.Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol 294: H605–612, 2008. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- 12.van de Borne P, Mark AL, Montano N, Mion D, Somers VK. Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension 29: 1278–1283, 1997. doi: 10.1161/01.HYP.29.6.1278. [DOI] [PubMed] [Google Scholar]
- 13.Elliott WJ Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 29: 992–996, 1998. doi: 10.1161/01.STR.29.5.992. [DOI] [PubMed] [Google Scholar]
- 14.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, Braunwald E; the MILIS Study Group*. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313: 1315–1322, 1985. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 15.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. The American journal of cardiology 60: 801–806, 1987. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 16.Ohira T, Tanigawa T, Tabata M, Imano H, Kitamura A, Kiyama M, Sato S, Okamura T, Cui R, Koike KA, Shimamoto T, Iso H. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension 53: 13–19, 2009. doi: 10.1161/HYPERTENSIONAHA.108.114835. [DOI] [PubMed] [Google Scholar]
- 17.Nakashita M, Ohkubo T, Hara A, Metoki H, Kikuya M, Hirose T, Tsubota-Utsugi M, Asayama K, Inoue R, Kanno A, Obara T, Hoshi H, Totsune K, Satoh H, Imai Y. Influence of alcohol intake on circadian blood pressure variation in Japanese men: the Ohasama study. Am J Hypertens 22: 1171–1176, 2009. doi: 10.1038/ajh.2009.160. [DOI] [PubMed] [Google Scholar]
- 18.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev 5: 287–297, 2001. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- 19.Yules RB, Lippman ME, Freedman DX. Alcohol administration prior to sleep. The effect on EEG sleep stages. Arch Gen Psychiatry 16: 94–97, 1967. doi: 10.1001/archpsyc.1967.01730190096012. [DOI] [PubMed] [Google Scholar]
- 20.Hillbom M, Saloheimo P, Juvela S. Alcohol consumption, blood pressure, and the risk of stroke. Curr Hypertens Rep 13: 208–213, 2011. doi: 10.1007/s11906-011-0194-y. [DOI] [PubMed] [Google Scholar]
- 21.Ekman AC, Leppäluoto J, Huttunen P, Aranko K, Vakkuri O. Ethanol inhibits melatonin secretion in healthy volunteers in a dose-dependent randomized double blind cross-over study. J Clin Endocrinol Metab 77: 780–783, 1993. doi: 10.1210/jcem.77.3.8370699. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama A, Muramatsu T, Ohmori T, Kumagai Y, Higuchi S, Ishii H. Reliability of a flushing questionnaire and the ethanol patch test in screening for inactive aldehyde dehydrogenase-2 and alcohol-related cancer risk. Cancer Epidemiol Biomarkers Prev 6: 1105–1107, 1997. [PubMed] [Google Scholar]
- 23.Carter JR Microneurography and sympathetic nerve activity: a decade-by-decade journey across 50 years. Journal of neurophysiology 121: 1183–1194, 2019. doi: 10.1152/jn.00570.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Autonomic Neuroscience 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delius W, Hagbarth KE, Hongell A, Wallin B. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 26.Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol 242: H185–H190, 1982. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- 27.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol 254: H377–H383, 1988. [Erratum in Am J Physiol 1988 Sep;255(3 Pt 2):following H405]. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- 28.Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol 268: H1682–H1687, 1995. doi: 10.1152/ajpheart.1995.268.4.H1682. [DOI] [PubMed] [Google Scholar]
- 29.Sundlöf G, Wallin B. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. The Journal of physiology 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton W, Woodbury R, Harper H. Physiologic relationships between intrathoracic, intraspinal and arterial pressures. JAMA 107: 853–856, 1936. doi: 10.1001/jama.1936.02770370017005. [DOI] [Google Scholar]
- 31.Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva's maneuver revisited: a quantitative method yielding insights into human autonomic control. Am J Physiol 271: H1240–H1249, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Carter JR. Baroreflex sensitivity analysis: spontaneous methodology vs. Valsalva's maneuver. Clin Auton Res 23: 133–139, 2013. doi: 10.1007/s10286-013-0195-9. [DOI] [PubMed] [Google Scholar]
- 33.Kautzner J, Hartikainen JE, Camm AJ, Malik M. Arterial baroreflex sensitivity assessed from phase IV of the Valsalva maneuver. Am J Cardiol 78: 575–579, 1996. doi: 10.1016/S0002-9149(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 34.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol 302: H1991–1997, 2012. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter JR, Fonkoue IT, Greenlund IM, Schwartz CE, Mokhlesi B, Smoot CA. Sympathetic neural responsiveness to sleep deprivation in older adults: sex differences. Am J Physiol Heart Circ Physiol 317: H315–H322, 2019. doi: 10.1152/ajpheart.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241–247, 1990. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 38.Buysse DJ, Reynolds CF 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 14: 331–338, 1991. [Erratum in Sleep 1992 Feb;15(1):83]. [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research 28: 193–213, 1989. . doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Johns MW A new method for measuring daytime sleepiness: the Epworth sleepiness scale. sleep 14: 540–545, 1991. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 41.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine 2: 297–307, 2001. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 42.American Psychological Association. Center for Epidemiological Studies‐Depression. 2012.
- 43.Spielberger CD State‐Trait anxiety inventory. The Corsini encyclopedia of psychology : 1–1, 2010. [Google Scholar]
- 44.Chan JK, Trinder J, Andrewes HE, Colrain IM, Nicholas CL. The acute effects of alcohol on sleep architecture in late adolescence. Alcohol Clin Exp Res 37: 1720–1728, 2013. doi: 10.1111/acer.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feige B, Gann H, Brueck R, Hornyak M, Litsch S, Hohagen F, Riemann D. Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcoholism Clin Exp Res 30: 1527–1537, 2006. doi: 10.1111/j.1530-0277.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 46.Narkiewicz K, Cooley RL, Somers VK. Alcohol potentiates orthostatic hypotension: implications for alcohol-related syncope. Circulation 101: 398–402, 2000. doi: 10.1161/01.CIR.101.4.398. [DOI] [PubMed] [Google Scholar]
- 47.Spaak J, Tomlinson G, McGowan CL, Soleas GJ, Morris BL, Picton P, Notarius CF, Floras JS. Dose-related effects of red wine and alcohol on heart rate variability. Am J Physiol Heart Circ Physiol 298: H2226–H2231, 2010. doi: 10.1152/ajpheart.00700.2009. [DOI] [PubMed] [Google Scholar]
- 48.Seppä K, Sillanaukee P. Binge drinking and ambulatory blood pressure. Hypertension 33: 79–82, 1999. doi: 10.1161/01.HYP.33.1.79. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N, Imai S, Saito F, Suzuki K, Tanaka H, Kushiro T, Yagi H, Hirayama A. Alcohol produces imbalance of adrenal and neuronal sympathetic activity in patients with alcohol-induced neurocardiogenic syncope. Circ J 72: 979–985, 2008. doi: 10.1253/circj.72.979. [DOI] [PubMed] [Google Scholar]
- 50.Bosser G, Caillet G, Gauchard G, Marcon F, Perrin P. Relation between motion sickness susceptibility and vasovagal syncope susceptibility. Brain Res Bull 68: 217–226, 2006. doi: 10.1016/j.brainresbull.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 51.Iwase S, Mano T [Microgravity and autonomic nervous system. Nihon Rinsho 58: 1604–1612, 2000. [PubMed] [Google Scholar]
- 52.Howes LG, Reid JL. Changes in blood pressure and autonomic reflexes following regular, moderate alcohol consumption. J Hypertens 4: 421–425, 1986. doi: 10.1097/00004872-198608000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Maxwell CR, Spangenberg RJ, Hoek JB, Silberstein SD, Oshinsky ML. Acetate causes alcohol hangover headache in rats. PLoS One 5: e15963, 2010. doi: 10.1371/journal.pone.0015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapp AD, Gui L, Huber MJ, Liu J, Larson RA, Zhu J, Carter JR, Chen QH. Sympathoexcitation and pressor responses induced by ethanol in the central nucleus of amygdala involves activation of NMDA receptors in rats. Am J Physiol Heart Circ Physiol 307: H701–709, 2014. doi: 10.1152/ajpheart.00005.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hobson RM, Maughan RJ. Hydration status and the diuretic action of a small dose of alcohol. Alcohol Alcohol 45: 366–373, 2010. doi: 10.1093/alcalc/agq029. [DOI] [PubMed] [Google Scholar]
- 56.Taivainen H, Laitinen K, Tahtela R, Kiianmaa K, Valimaki MJ. Role of plasma vasopressin in changes of water balance accompanying acute alcohol intoxication. Alcoholism Clin Exp Res 19: 759–762, 1995. doi: 10.1111/j.1530-0277.1995.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 57.Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol 289: H2456–H2460, 2005. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- 58.Greaney JL, Ray CA, Prettyman AV, Edwards DG, Farquhar WB. Influence of increased plasma osmolality on sympathetic outflow during apnea. Am J Physiol Regul Integr Comp Physiol 299: R1091–1096, 2010. doi: 10.1152/ajpregu.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol 293: H2313–H2319, 2007. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- 60.Lobo IA, Harris RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav 90: 90–94, 2008. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okada H, Iwase S, Mano T, Sugiyama Y, Watanabe T. Changes in muscle sympathetic nerve activity during sleep in humans. Neurology 41: 1961–1966, 1991. doi: 10.1212/WNL.41.12.1961. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu T, Takahashi Y, Suzuki K, Kogawa S, Tashiro T, Takahasi K, Hishikawa Y. Muscle nerve sympathetic activity during sleep and its change with arousal response. J Sleep Res 1: 178–185, 1992. doi: 10.1111/j.1365-2869.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 63.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 64.Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in man. Brain 114: 1281–1295, 1991. doi: 10.1093/brain/114.3.1281. [DOI] [PubMed] [Google Scholar]
- 65.Berteotti C, Franzini C, Lenzi P, Zoccoli G, Silvani A. Surges of arterial pressure during REM sleep in spontaneously hypertensive rats. Sleep 31: 111–117, 2008. doi: 10.1093/sleep/31.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]