Summary
Background
Emotional stress is associated with increased risk of cardiovascular disease. We imaged the amygdala, a brain region involved in stress, to determine whether its resting metabolic activity predicts risk of subsequent cardiovascular events.
Methods
Individuals aged 30 years or older without known cardiovascular disease or active cancer disorders, who underwent 18F-fluorodexoyglucose PET/CT at Massachusetts General Hospital (Boston, MA, USA) between Jan 1, 2005, and Dec 31, 2008, were studied longitudinally. Amygdalar activity, bone-marrow activity, and arterial inflammation were assessed with validated methods. In a separate cross-sectional study we analysed the relation between perceived stress, amygdalar activity, arterial inflammation, and C-reactive protein. Image analyses and cardiovascular disease event adjudication were done by mutually blinded researchers. Relations between amygdalar activity and cardiovascular disease events were assessed with Cox models, log-rank tests, and mediation (path) analyses.
Findings
293 patients (median age 55 years [IQR 45.0–65.5]) were included in the longitudinal study, 22 of whom had a cardiovascular disease event during median follow-up of 3.7 years (IQR 2.7–4.8). Amygdalar activity was associated with increased bone-marrow activity (r=0.47; p<0.0001), arterial inflammation (r=0.49; p<0.0001), and risk of cardiovascular disease events (standardised hazard ratio 1.59, 95% CI 1.27–1.98; p<0.0001), a finding that remained significant after multivariate adjustments. The association between amygdalar activity and cardiovascular disease events seemed to be mediated by increased bone-marrow activity and arterial inflammation in series. In the separate cross-sectional study of patients who underwent psychometric analysis (n=13), amygdalar activity was significantly associated with arterial inflammation (r=0.70; p=0.0083). Perceived stress was associated with amygdalar activity (r=0.56; p=0.0485), arterial inflammation (r=0.59; p=0.0345), and C-reactive protein (r=0.83; p=0.0210).
Interpretation
In this first study to link regional brain activity to subsequent cardiovascular disease, amygdalar activity independently and robustly predicted cardiovascular disease events. Amygdalar activity is involved partly via a path that includes increased bone-marrow activity and arterial inflammation. These findings provide novel insights into the mechanism through which emotional stressors can lead to cardiovascular disease in human beings.
Introduction
Psychosocial stress is both a byproduct of adversity and an important precipitant of morbidity. Chronic stress is associated with an increased risk of cardiovascular disease,1,2 with an attributable risk that is on par with that of other major cardiovascular risk factors.3–5 However, little is known about the mechanisms that translate stress into cardiovascular events.
Although several factors could account for the risk of cardiovascular disease attributable to stress, the brain’s salience network, an ensemble of interconnected structures involved in complex functions such as cognition and emotion, is thought to have an important role. Activation of this network, which includes the amygdala as a key component,6 leads to hormonal, autonomic, and behavioural changes typically associated with fear and stress.7 The amygdala’s efferent projections to the brainstem participate in the sympathetic responses to stress.8 In murine models, stress increases proliferation of haemopoietic stem cells and progenitor cells in the bone marrow, accelerates innate immune cell output and cytokine production, and potentiates atherosclerosis.9–13 However, whether a homologous pathway exists in human beings is unknown. Furthermore, although amygdalar reactivity is known to be heightened in individuals with pre-existing atherosclerosis,14 neither human nor animal studies have yet shown whether amygdalar activation precedes and predisposes to the subsequent development of cardiovascular events.
Activation of the neural circuitry underlying the perception of fear-related stimuli can be reproducibly imaged using functional MRI (fMRI) and 18F-fluorodeoxyglucose (18F-FDG) PET/CT.15,16 Amygdalar activity is upregulated in conditions marked by stress, such as post-traumatic stress disorder, anxiety, and depression.16–20 By showing cellular glycolysis, 18F-FDG PET/CT can be used to simultaneously quantify not only regional brain metabolism (activity),16–19,21 but also haemopoietic tissue activity and large vessel arterial inflammation,12,22 thereby making it uniquely suitable for investigation of linked activity among these systems and the pathogenesis of atherosclerosis. Accordingly, we used 18F-FDG-PET/CT to test the hypotheses that amygdalar activity is associated with haemopoietic activity and arterial inflammation, and predicts the development of future cardiovascular disease events.
Methods
Study design and participants
We did two complementary imaging studies: a longitudinal outcomes study to assess the relation between resting amygdalar metabolic activity, atherosclerotic inflammation, and subsequent cardiovascular events, and a smaller, cross-sectional perceived stress study to assess the relation between psychometric measures of perceived stress, resting amygdalar metabolic activity, and atherosclerotic inflammation.
Participants were identified from a pool of 6088 patients who underwent 18F-FDG PET/CT for clinical assessment (mainly for cancer screening) at the Massachusetts General Hospital (Boston, MA, USA) between Jan 1, 2005, and Dec 31, 2008. Predefined inclusion criteria included either absence of previous history of cancer or remission from cancer for at least 1 year before imaging (and throughout the follow-up period), absence of cardiovascular disease or acute or chronic inflammatory or autoimmune disease at time of imaging, and age older than 30 years. To ensure adequate information for events adjudication, an additional inclusion criterion was the availability of at least three clinical encounter notes in the medical records a minimum of 1 year apart. A full list of inclusion criteria is included in the appendix. All individuals who were included in a previous study23 of the relation between arterial inflammation and cardiovascular disease events were included in our study if their scans showed the amygdala. The study protocol was approved by the Partners Human Research Committee. No specific informed consent was needed for this study.
Procedures
Two cardiologists (AT and QAT) blinded to PET/CT data used clinical records to adjudicate cardiovascular disease events, which were defined, according to the Framingham Heart Study,24 as coronary death, myocardial infarction, coronary insufficiency, angina, cerebrovascular accidents, revascularisation, peripheral artery disease, and heart failure. Two additional and increasingly stringent subcategories of events were assessed as exploratory endpoints: major adverse cardiovascular events (which excluded angina without evidence of occlusive coronary disease), and atherosclerotic major adverse cardiovascular events (which required identification of potential culprit atherosclerotic plaque in association with the events; appendix).
18F-FDG was given intravenously at a dose of ~370 MBq after an overnight fast. After tracer injection, individuals sat in a quiet waiting room and PET/CT was done around 1 h later with an integrated scanner (Biograph 64, Siemens Healthcare, Erlangen, Germany [or similar]). Non-gated, non-contrast-enhanced CT (120 keV, ~50 mAs) was done for attenuation correction. Image analyses (appendix) were done by a radiologist (AI) who was blinded to the clinical data and used a dedicated workstation. Analysis of amygdalar activity was based on a validated approach that has shown associations with anxious temperament19,25 and clinical manifestations of stress-related disorders.16–19,21 18F-FDG uptake in the amygdala was determined by placing circular regions of interest in the right and left amygdalae and measuring the mean and maximum tracer accumulation (ie, standardised uptake value [SUV]) in each region of interest. Amygdalar activity was corrected for background cerebral (amygdalac, as mean temporal lobe SUV) and cerebellar (amygdalacbl, as mean cerebellar SUV) activity.26
Bone-marrow activity, splenic activity, and arterial inflammation were measured according to previously validated methods12,20 by deriving SUVs from the target tissue (bone marrow, spleen, or aortic and carotid walls) and correcting them for venous blood background activity to calculate target-to-background ratios. Arterial 18F-FDG uptake is a well validated measure of arterial inflammation that relates to atherosclerotic macrophages22 and predicts subsequent cardiovascular disease events.23 18F-FDG uptake in subcutaneous adipose tissue was derived as a control measure of glycolytic activity. Coronary artery calcium score and visceral adipose tissue volume were derived from CT images (appendix).
Perceived stress study
To assess further the role of stress in the associations noted in the longitudinal study, in a separate cross-sectional study we tested the hypotheses that perceived stress is associated with resting amygdalar metabolic activity, arterial inflammation, and inflammatory biomarkers. 13 individuals with an increased burden of chronic stress (ie, history of post-traumatic stress disorder) were recruited from the community, completed the well validated, ten-item Perceived Stress Scale (PSS-10)27 and underwent 18F-FDG PET (Siemens mMR, Erlangen, Germany). Amygdalar activity, C-reactive protein (CRP), and arterial inflammation were measured (appendix). Further details of the perceived stress study are in the appendix.
Statistical analysis
Continuous variables are listed as mean (SD) or, when not normally distributed, median (IQR). We used Pearson product-moment correlation to assess univariate associations for normally distributed variables, and Spearman correlation coefficients for non-normally distributed variables. Cox proportional hazards models were used, with or without the addition of potential confounders as covariates, to calculate hazard ratios (HRs) and 95% CIs. Additionally, we did log-rank tests to generate Kaplan-Meier estimates (and associated curves) of cardiovascular event-free survival, comparing clinical events in patients with higher-versus-lower amygdalar activity. Mediation analysis—which tests a putative causal relation among variables (ie, a path)—was also done to test whether amygdalar activity exerts its effect on cardiovascular disease via postulated mediators (bone-marrow activity and arterial inflammation), either singularly or in series. Statistical significance was determined as p values less than 0.05. We used SPSS (version 23.0) for all statistical analyses (appendix).
Role of the funding source
There was no funding source for this study. AT, AI, RAPT, and RKP had full access to all study data, and AT had final responsibility for the decision to submit for publication.
Results
Imaging and cardiovascular disease events data were available for 293 people in the longitudinal outcomes study (figure 1). Baseline characteristics are listed in table 1. Individuals who developed subsequent cardiovascular events had an increased prevalence of several atherosclerotic risk factors compared with those who did not develop cardiovascular events. Of the atherosclerotic risk factors that were significant on univariate analyses, two remained significantly associated with cardiovascular disease events in multivariate analyses: age (standardised β 0.27; p=0.0003) and smoking (0.22; 0.0028). Furthermore, age and family history of coronary disease were associated with amygdalar activity (appendix).
Figure 1: Study cohort.
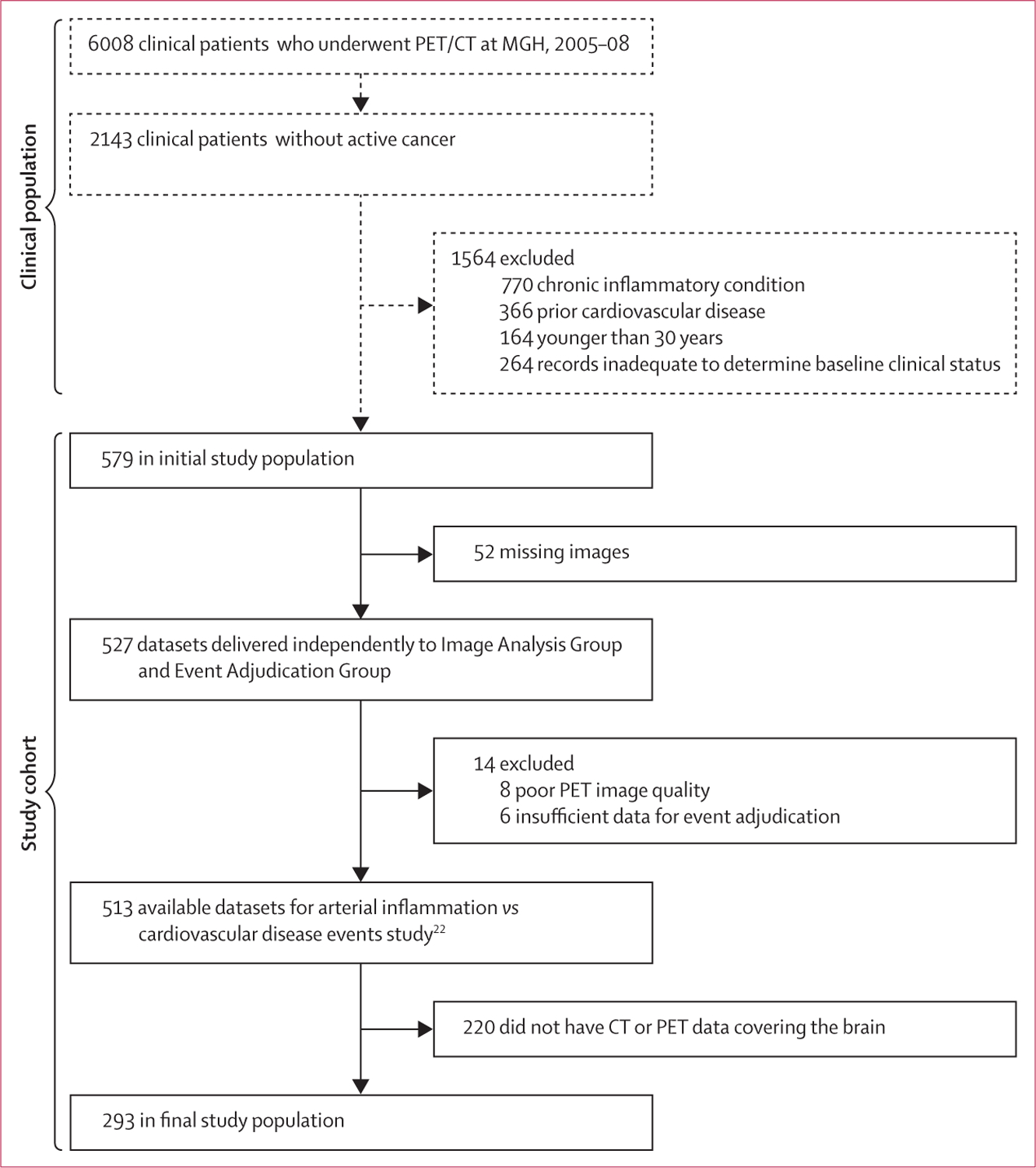
Eligible patients were selected on the basis of pre-defined criteria. All patients meeting these criteria were included. Image analyses and event adjudication were performed by mutually blinded investigators. 18F-FDG=18F-fluorodeoxyglucose. MGH=Massachusetts General Hospital.
Table 1:
Baseline characteristics of participants in outcomes study
| Full cohort (n=293) | No cardiovascular event (n=271) | Cardiovascular event (n=22) | p value (event vs no event) | |
|---|---|---|---|---|
| Median age, years (IQR) | 55.0 (45.0–65.5) | 55.0 (44.0–64.0) | 64.5 (60.0–78.3) | <0.0001 |
| Male | 124 (42%) | 111 (41%) | 13 (59%) | 0.10 |
| White race | 271 (93%) | 243 (89%) | 20 (91%) | 0.85 |
| Current smoker | 27 (9%) | 20 (7%) | 7 (32%) | 0.0001 |
| Hypertension | 103 (35%) | 89 (32%) | 14 (64%) | 0.0035 |
| Diabetes mellitus | 25 (8%) | 19 (7%) | 6 (27%) | 0.0010 |
| Hyperlipidaemia | 85 (29%) | 74 (27%) | 11 (50 %) | 0.0241 |
| Mean total cholesterol (SD) | 190.7 (47.4) | 193.4 (47.9) | 174.6 (42.1) | 0.10 |
| Mean LDL cholesterol (SD) | 109.6 (38.9) | 111.4 (39.2) | 98.6 (36.2) | 0.17 |
| Statin therapy | 61 (21%) | 51 (19%) | 10 (46%) | 0.0030 |
| Mean Framingham risk score (SD) | 5.9 (6.2) | 5.1 (5.9) | 10.6 (5.9) | 0.0002 |
| Median body-mass index (IQR) | 26.8 (23.4–31.0) | 26.6 (23.2–30.9) | 27.0 (24.8–32.6) | 0.36 |
| Coronary artery calcium score | .. | .. | 0.0012 | |
| 0–10 | 204 (70%) | 192 (71%) | 12 (55%) | .. |
| 11–99 | 39 (13%) | 33 (12%) | 6 (27%) | .. |
| ≥100 | 33 (11%) | 29 (11%) | 4 (18 %) | .. |
| History of cancer | 256 (87%) | 241 (89%) | 15 (68%) | 0.0114 |
| Previous chemotherapy | 234 (80%) | 221 (82%) | 13 (59%) | 0.0047 |
| History of depression or anxiety* | 29 (10%) | 25 (9%) | 4 (20%) | 0.13 |
| Antidepressant drug use* | 27 (9%) | 24 (9%) | 3 (15%) | 0.42 |
Data are n (%), unless otherwise specified. The coronary artery calcium score is a measure derived from coronary CT. The presence of calcium in the coronary artery suggests the presence of coronary atherosclerotic disease.
Data for depression and anxiety and for antidepressant drug use were available for 288 participants—268 in the no events group and 20 in the cardiovascular disease events group.
During median follow-up of 3.7 years (IQR 2.7–4.8), 22 individuals experienced 39 cardiovascular disease events. The 22 index events were eight myocardial infarctions, three unstable angina, two peripheral arterial disease, six strokes, one heart failure, and two new onset angina (appendix). Amygdalar activity (ie, amygdalac and amygdalacbl) robustly predicted the risk of developing a subsequent cardiovascular event (figure 2, table 2), yielding adjusted standardised HRs of approximately 1.6 (ie, a 1.6-times increased risk of a cardiovascular event for each increase of one SD in amygdalar signal). However, activity in the background brain structures (cerebral or cerebellar) or control extra-cranial tissue (subcutaneous fat) was not significantly associated with cardiovascular disease (appendix).
Figure 2: Amygdalar, arterial, and bone-marrow uptake of 18F-FDG in individuals with and without subsequent cardiovascular disease events.
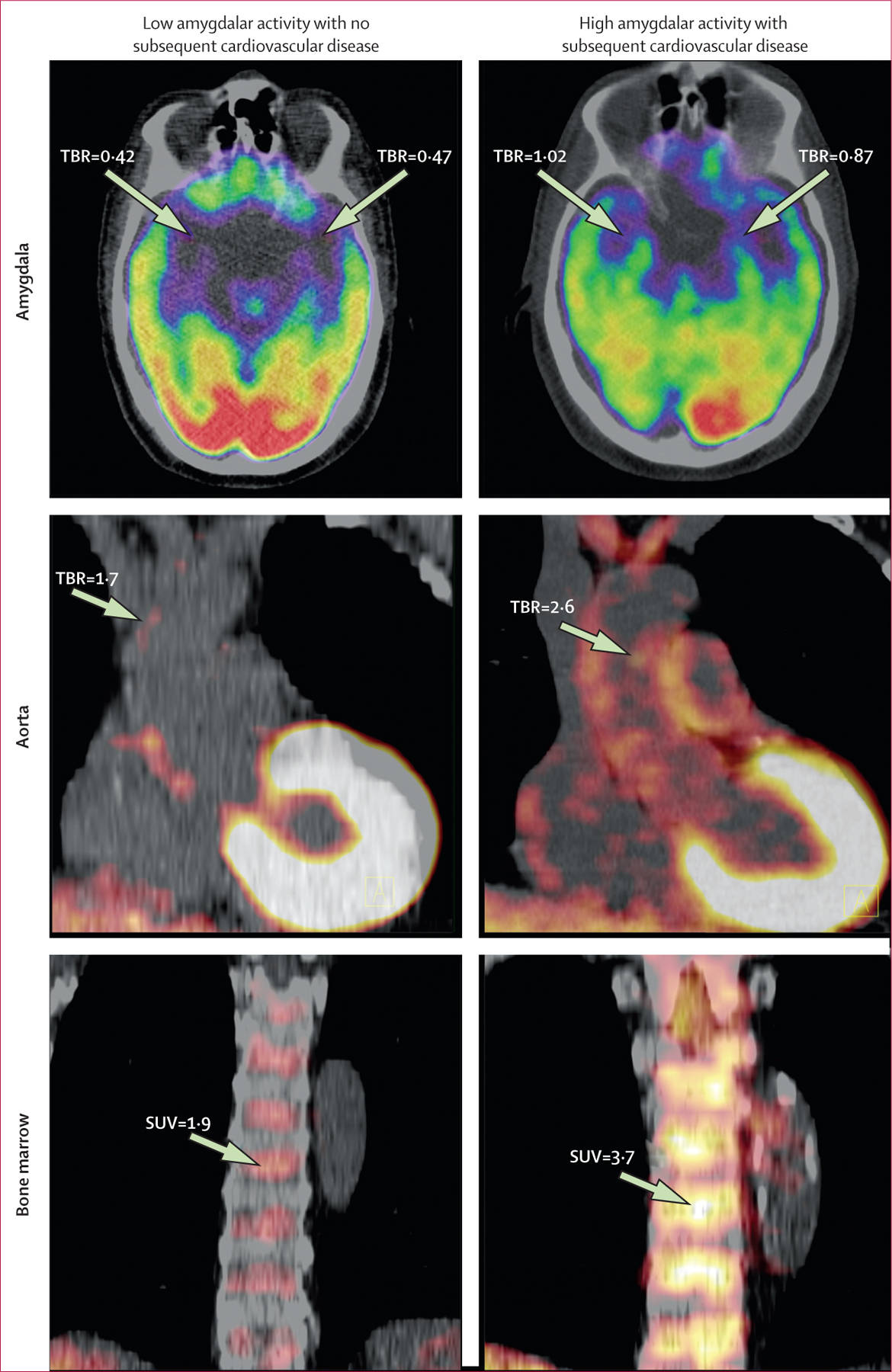
Axial views of amygdala (upper left and right), coronal views of aorta (middle left and right), and coronal views of bone marrow (lower left and right) are shown. 18F-FDG uptake was increased in the amygdala, bone marrow, and arterial wall (aorta), in a patient who experienced an ischaemic stroke during the follow-up period (right) compared with a patient who did not (left). 18F-FDG=18F fluorodeoxyglucose. SUV=standardised uptake value. TBR=target-to-background ratio.
Table 2:
Univariate and multivariate analysis of neural tissue activity vs cardiovascular disease events
| Max max amygdalac (primary measure) |
Mean mean amygdalac (secondary measure) |
Mean max amygdalac (secondary measure) |
Mean L amygdalacbl (post-hoc measure) |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Univariate | ||||||||
| Per unit change | 14.1 (4.0–50.0) | <0.0001 | 41.1 (8.7–194.5) | <0.0001 | 19.4 (4.7–79.9) | <0.0001 | 101.4 (12.5–822.4) | <0.0001 |
| Per SD change | 1.59 (1.27–1.98) | <0.0001 | 1.58 (1.30–1.91) | <0.0001 | 1.57 (1.27–1.95) | <0.0001 | 1.71 (1.33–2.20) | <0.0001 |
| Covariates: age and sex | ||||||||
| Per unit change | 5.0 (1.3–19.1) | 0.0193 | 11.5 (2.0–66.5) | 0.0064 | 5.7 (1.2–25.8) | 0.0246 | 48.0 (6.7–342.0) | 0.0001 |
| Per SD change | 1.32 (1.05–1.68) | 0.0193 | 1.35 (1.09–1.67) | 0.0064 | 1.35 (1.30–1.64) | 0.0246 | 1.58 (1.25–2.00) | 0.0001 |
| Covariate: Framingham risk score | ||||||||
| Per unit change | 4.5 (1.3–15.7) | 0.0192 | 8.8 (1.8–43.7) | 0.0078 | 5.1 (1.2–20.8) | 0.0237 | 29.0 (4.6–183.7) | 0.0003 |
| Per SD change | 1.30 (1.04–1.62) | 0.0192 | 1.31 (1.08–1.67) | 0.0078 | 1.28 (1.03–1.59) | 0.0237 | 1.49 (1.20–1.85) | 0.0003 |
| Covariates: combined cardiac risk factors* | ||||||||
| Per unit change | 7.6 (2.0–28.4) | 0.0027 | 11.0 (1.8–65.5) | 0.0087 | 8.4 (1.8–39.3) | 0.0066 | 26.1 (2.6–260.5) | 0.0054 |
| Per SD change | 1.42 (1.13–1.79) | 0.0027 | 1.34 (1.13–1.79) | 0.0087 | 1.38 (1.09–1.75) | 0.0066 | 1.47 (1.12–1.93) | 0.0054 |
| Covariate: pre-existing atherosclerotic disease (CAC score) | ||||||||
| Per unit change | 10.7 (2.7–42.9) | 0.0008 | 31.6 (2.7–42.9) | <0.0001 | 14.3 (3.0–68.0) | 0.0008 | 55.2 (5.0–614.3) | 0.0011 |
| Per SD change | 1.51 (1.19–1.93) | 0.0008 | 1.53 (1.24–1.88) | <0.0001 | 1.50 (1.08–1.90) | 0.0008 | 1.61 (1.21–2.14) | 0.0011 |
| Covariate: history of depression or anxiety | ||||||||
| Per unit change | 18.1 (5.0–65.5) | <0.0001 | 51.0 (10.3–251.7) | <0.0001 | 25.1 (6.0–105.8) | <0.0001 | 125.0 (14.4–1081.3) | <0.0001 |
| Per SD change | 1.66 (1.32–2.08) | <0.0001 | 1.62 (1.33–1.97) | <0.0001 | 1.63 (1.31–2.03) | <0.0001 | 1.77 (1.37–2.29) | <0.0001 |
| Covariate: antidepressant use | ||||||||
| Per unit change | 17.3 (4.8–62.2) | <0.0001 | 48.5 (10.0–235.5) | <0.0001 | 24.1 (5.8–100.1) | <0.0001 | 118.3 (14.0–997.5) | <0.0001 |
| Per SD change | 1.65 (1.32–2.06) | <0.0001 | 1.61 (1.33–1.95) | <0.0001 | 1.62 (1.31–2.01) | <0.0001 | 1.76 (1.37–2.26) | <0.0001 |
We measured amygdalar activity with several different approaches, providing a primary, two secondary, and one post-hoc measure. Amygdalar activity was corrected for background cerebral (c) or cerebellar (cbl) neural tissue activity. Max max is the maximum SUV for the right and left amygdalae. Mean mean is the mean of the mean SUVs in the right and left amygdalae. Mean max is the mean of the maximum SUVs in the right and left amygdalae. Mean L is the mean SUV for the left amygdala. CAC=coronary artery calcium. HR=hazard ratio. SUV=standardised uptake value.
The cardiac risk factors that were entered into this model were each of the standard cardiovascular risk factors that were significantly associated with the development of events on the basis of univariate analysis (table 1)—age, smoking, hypertension, diabetes, dyslipidaemia, and family history—which were entered as cofactors in a stepwise (backward) conditional manner.
Furthermore, the relation between amygdalar activity and cardiovascular disease events remained significant after multivariate adjustments for cardiovascular risk factors, Framingham risk scores, and pre-existing atherosclerotic disease burden (table 2; appendix), and after correction for coronary artery calcium score or visceral adipose tissue volume (appendix). Notably, amygdalar activity generally remained associated with cardiovascular disease in subgroups with or without pre-clinical evidence of atherosclerosis at baseline (as coronary atherosclerotic calcification), subgroups with or without a high burden of coronary atherosclerotic risk factors, and subgroups with or without a previous history of cancer (table 3).
Table 3:
Subgroup analyses of amygdalar activity vs cardiovascular disease
| Max max amygdalac (primary measure) |
Mean mean amygdalac (secondary measure) |
Mean max amygdalac (secondary measure) |
Mean L amygdalacbl (post-hoc measure) |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Individuals with previous subclinical coronary artery disease (CAC>0) | ||||||||
| Per unit change | 7.1 (1.5–34.2) | 0.0151 | 19.6 (2.1–180.3) | 0.0086 | 7.8 (1.3–45.7) | 0.0236 | 186.0 (3.9–8956.3) | 0.0082 |
| Per SD change | 1.41 (1.07–1.85) | 0.0151 | 1.44 (1.10–1.89) | 0.0086 | 1.37 (1.04–1.79) | 0.0236 | 1.86 (1.17–2.94) | 0.0082 |
| Individuals without previous subclinical coronary artery disease (CAC=0) | ||||||||
| Per unit change | 19.9 (2.7–144.3) | 0.0031 | 49.1 (5.1–473.9) | 0.0008 | 34.5 (3.7–324.4) | 0.0020 | 122.9 (7.9–1903.6) | 0.0006 |
| Per SD change | 1.69 (1.19–144.34) | 0.0031 | 1.61 (1.22–2.13) | 0.0008 | 1.71 (1.22–2.41) | 0.0020 | 1.77 (1.28–2.44) | 0.0006 |
| Individuals with ≥3 coronary risk factors | ||||||||
| Per unit change | 13.3 (3.1–57.4) | 0.0050 | 23.4 (3.7–147.3) | 0.0008 | 19.4 (3.6–105.7) | 0.0006 | 56.7 (7.7–416.2) | <0.0001 |
| Per SD change | 1.57 (1.22–2.03) | 0.0050 | 1.47 (1.18–1.85) | 0.0008 | 1.57 (1.21–2.03) | 0.0006 | 1.61 (1.27–2.04) | <0.0001 |
| Individuals with <3 coronary risk factors | ||||||||
| Per unit change | 12.0 (1.1–128.8) | 0.0401 | 57.8 (2.7–1227.7) | 0.0093 | 15.8 (1.2–212.5) | 0.0378 | 4.2 (0.0–9658.5) | 0.72 |
| Per SD change | 1.54 (1.02–2.34) | 0.0401 | 1.62 (1.13–2.39) | 0.0093 | 1.52 (1.02–2.26) | 0.0378 | 1.18 (0.47–2.96) | 0.72 |
| Individuals with previous cancer | ||||||||
| Per unit change | 9.9 (1.5–64.3) | 0.0164 | 46.5 (4.0–544.9) | 0.0022 | 12.7 (1.6–103.1) | 0.0171 | 330.2 (5.2–21 007.7) | 0.0062 |
| Per SD change | 1.49 (1.08–2.07) | 0.0164 | 1.60 (1.18–2.17) | 0.0022 | 1.47 (1.07–2.02) | 0.0171 | 2.00 (1.22–3.25) | 0.0062 |
| Individuals without previous cancer | ||||||||
| Per unit change | 12.9 (2.2–76.5) | 0.0050 | 18.8 (2.0–174.7) | 0.0098 | 18.2 (2.4–139.8) | 0.0054 | 24.5 (1.5–397.9) | 0.0244 |
| Per SD change | 1.56 (1.15–2.13) | 0.0050 | 1.43 (1.09–1.88) | 0.0098 | 1.55 (1.14–2.12) | 0.0054 | 1.46 (1.05–2.03) | 0.0244 |
We measured amygdalar activity with several different approaches, providing a primary, two secondary, and one post-hoc measure. Amygdalar activity was corrected for background cerebral (c) or cerebellar (cbl) neural tissue activity. No additional adjustments were included in the subgroup analyses. Coronary risk factors include age older than 55 years, male sex, current smoker, hypertension, diabetes, and dyslipidaemia. Max max is the maximum SUV for the right and left amygdalae. Mean mean is the mean of the mean SUVs in the right and left amygdalae. Mean max is the mean of the maximum SUVs in the right and left amygdalae. Mean L is the mean SUV for the left amygdala. CAC=coronary artery calcium. HR=hazard ratio. SUV=standardised uptake value.
Amygdalar activity generally remained associated with cardiovascular events when more stringent definitions of events were used (ie, major adverse cardiovascular events and atherosclerotic major adverse cardiovascular events). The associated HRs increased relative to the stringency of the event definition (table 4). Additionally, amygdalar activity seemed to be associated with the timing of the cardiovascular disease event: individuals with higher resting amygdalar activity experienced subsequent cardiovascular disease events sooner than those with lower resting amygdalar activity (appendix).
Table 4:
Analysis of neural tissue activity vs events—additional event definitions
| Max max amygdalac (primary measure) |
Mean mean amygdalac (secondary measure) |
Mean max amygdalac (secondary measure) |
Mean L amygdalacbl (post-hoc measure) |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Cardiovascular disease | ||||||||
| Per unit change | 14.1 (4.0–50.0) | <0.0001 | 41.1 (8.7–194.5) | <0.0001 | 19.4 (4.7–79.9) | <0.0001 | 101.4 (12.5–822.4) | <0.0001 |
| Per SD change | 1.59 (1.27–1.98) | <0.0001 | 1.58 (1.30–1.91) | <0.0001 | 1.57 (1.27–1.95) | <0.0001 | 1.71 (1.33–2.20) | <0.0001 |
| MACE | ||||||||
| Per unit change | 15.9 (4.4–58.1) | <0.0001 | 45.7 (9.1–228.9) | <0.0001 | 21.7 (5.1–92.8) | <0.0001 | 114.2 (12.9–1014) | <0.0001 |
| Per SD change | 1.62 (1.29–2.03) | <0.0001 | 1.60 (1.31–1.95) | <0.0001 | 1.6 (1.28–1.99) | <0.0001 | 1.75 (1.35–2.27) | <0.0001 |
| AMACE | ||||||||
| Per unit change | 23.7 (1.6–350.0) | 0.0212 | 432.9 (4.2–44499.4) | 0.0102 | 41.0 (1.3–1278.2) | 0.0343 | 315 (3.8–26196.9) | 0.0108 |
| Per SD change | 1.74 (1.09–2.78) | 0.0212 | 2.11 (1.19–3.72) | 0.0102 | 1.76 (1.04–3.00) | 0.0343 | 1.98 (1.17–3.33) | 0.0108 |
We measured amygdalar activity with several different approaches, providing a primary, two secondary, and one post-hoc measure. Amygdalar activity was corrected for background cerebral (c) or cerebellar (cbl) neural tissue activity. Max max is the maximum SUV for the right and left amygdalae. Mean mean is the mean of the mean SUVs in the right and left amygdalae. Mean max is the mean of the maximum SUVs in the right and left amygdalae. Mean L is the mean SUV for the left amygdala. HR=hazard ratio. MACE=major adverse cardiovascular event. AMACE=atherosclerotic major adverse cardiovascular event (requires confirmed presence of an atherosclerotic culprit lesion). SUV=standardised uptake value.
To further explore the relation between amygdalar activity and cardiovascular disease, we compared outcomes for individuals with high amygdalar activity outcomes for those with low amygdalar activity. We determined the threshold values defining high activity by three distinct approaches: receiver operating characteristic analysis, which yielded values with the highest accuracy to identify subsequent cardiovascular disease events; one or more SD above the mean; and as 90th percentile or greater. All remaining individuals were characterised as having low activity.
When the primary amygdalar imaging endpoint was dichotomised into high-activity and low-activity groups, Cox regression analyses yielded significantly increased HRs (table 5) and Kaplan-Meier analyses yielded significant group differences (figure 3). We noted similar findings when the secondary and post-hoc measures of amygdalar activity were similarly dichotomised (table 5). Additionally, amygdalar activity remained robustly predictive of cardiovascular disease when several other means of measuring it were used in sensitivity analyses (appendix).
Table 5:
Adjusted HRs for high vs low amygdalar activity
| Determination of high vs low cutoff | Adjusted HR (95% CI) | p | |
|---|---|---|---|
| Primary amygdalar measure | |||
| Max max amygdalac | Above vs below ROC-optimised threshold | 5.8 (2.1–16.0) | <0.0001 |
| Max max amygdalac | Above vs below mean+1 SD | 4.8 (1.8–12.5) | 0.0013 |
| Max max amygdalac | Above vs below 90th percentile | 4.2 (1.6–11.1) | 0.0030 |
| Secondary amygdalar measure | |||
| Mean mean amygdalac | Above vs below ROC-optimised threshold | 25.3 (9.1–70.5) | <0.0001 |
| Mean max amygdalac | Above vs below ROC-optimised threshold | 9.2 (3.5–23.9) | <0.0001 |
| Post-hoc amygdalar measure | |||
| Mean L amygdalacbl | Above vs below ROC-optimised threshold | 40.5 (15.3–107.1) | <0.0001 |
Amygdalar activity is corrected for background cerebral (c) or cerebellar (cbl) neural tissue activity. Max max is the maximum SUV for the right and left amygdalae. Mean mean is the mean of the mean SUVs in the right and left amygdalae. Mean max is the mean of the maximum SUVs in the right and left amygdalae. HR=hazard ratio. ROC=receiver operating characteristic. SUV=standardised uptake value.
Figure 3: Kaplan-Meier survival curves of low vs high amygdalar activity based on the 90th percentile cutoff (A) or the mean (SD) cutoff (B).
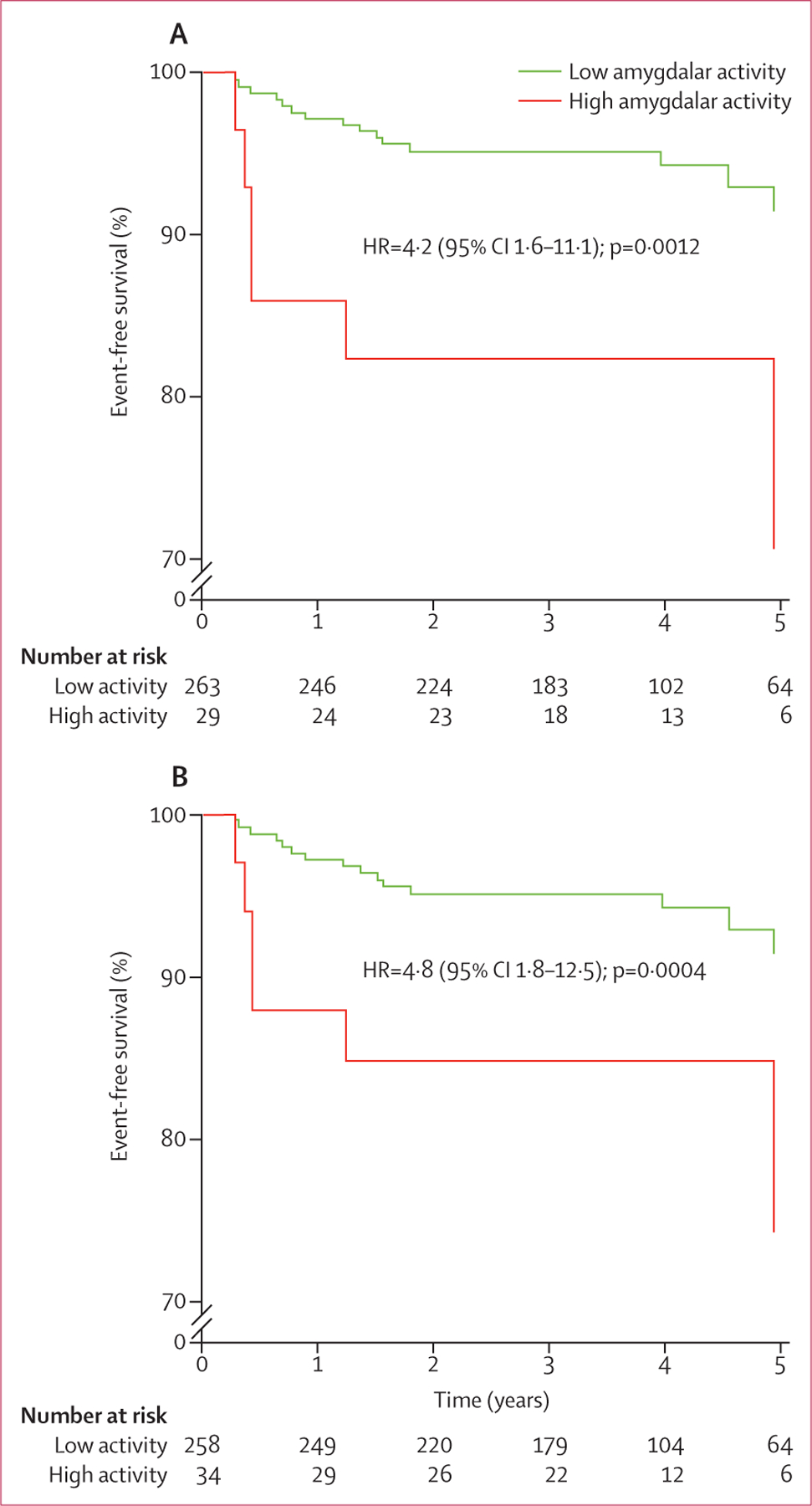
Event-free survival for the primary amygdalar endpoint (max max amygdalac—ie, the maximum standardised uptake value for the right and left amygdalae, corrected for background cerebral tissue activity) are shown. p values were calculated with the log-rank test, and cox regression analyses were done to calculate HRs. HR=hazard ratio.
Amygdalar activity correlated with haemopoietic tissue activity, expressed as 18F-FDG-uptake in the bone marrow and spleen (table 6), and with arterial 18F-FDG uptake, a well validated measure of arterial inflammation.22,28 Moreover, measures of haemopoietic activity, especially in bone marrow, were associated with several measures of circulating blood cells, including total white blood cell count and neutrophil and lymphocyte counts (appendix). However, amygdalar activity did not correlate with 18F-FDG-uptake in control tissue (ie, subcutaneous fat; table 6).
Table 6:
Correlation of amygdalar activity with haemopoietic activity and arterial inflammation
| Mean mean |
Mean max |
Max max |
||||
|---|---|---|---|---|---|---|
| Correlation coefficient | p value | Correlation coefficient | p value | Correlation coefficient | p value | |
| Aortic inflammation | 0.49 | <0.0001 | 0.45 | <0.0001 | 0.41 | <0.0001 |
| Carotid inflammation* | 0.47 | <0.0001 | 0.43 | <0.0001 | 0.40 | <0.0001 |
| Splenic activity | 0.50 | <0.0001 | 0.47 | <0.0001 | 0.46 | <0.0001 |
| Bone-marrow activity | 0.44 | <0.0001 | 0.40 | <0.0001 | 0.40 | <0.0001 |
| Control tissue uptake of 18F-FDG uptake (subcutaneous fat) | 0.02 | 0.73 | 0.02 | 0.80 | 0.02 | 0.79 |
Pearson product-moment correlations. All measures are uncorrected SUVs. Mean mean is the mean of the mean SUVs in the right and left amygdalae. Mean max is the mean of the maximum SUVs in the right and left amygdalae. Max max is the maximum SUV for the right and left amygdalae. 18F-FDG=18F fluorodeoxyglucose. SUV=standardised uptake value.
Mean of the right and left carotid mean maximum SUVs.
Bone-marrow activity was a significant mediator of the relation between amygdalar activity and arterial inflammation, accounting for a substantial 46% of the total effect. Arterial inflammation was a significant mediator of the relation between amygdalar activity and cardiovascular disease events, accounting for 39% of the total effect. Serial two-mediator analysis supported the hypothesised indirect path of increased amygdalar activity leading to increased bone-marrow activity leading to increased arterial inflammation leading to cardiovascular disease events (figure 4). These results suggest that bone-marrow activity and arterial inflammation, in series, have an important role in mediation of the association between amygdalar activity and cardiovascular disease events. Finally, when the path implicating bone-marrow activity was excluded, the residual path of increased amygdalar activity leading to increased arterial inflammation leading to cardiovascular disease events (figure 4) was also significant, suggesting that amygdalar activity also influences arterial inflammation and hence cardiovascular events through means other than haemopoiesis. That the mediators account for less than 100% of the total effects also suggests that amygdalar activity also affects cardiovascular disease events through means other than arterial inflammation.
Figure 4: Serial mediation model for hypothesised pathway to a cardiovascular disease event.
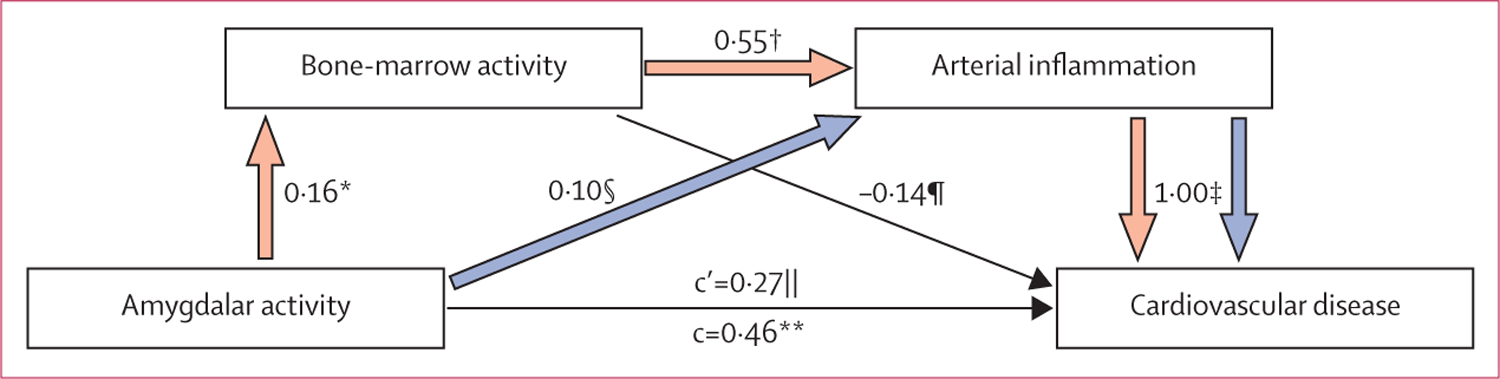
n=266. A single-mediator analysis showed that bone-marrow activity was a significant mediator of the relation between amygdalar activity and arterial inflammation. Another single-mediator analysis showed that arterial inflammation was a significant mediator of the relation between amygdalar activity and cardiovascular disease events. A serial two-mediator analysis testing the hypothesised indirect path of increased resting amygdalar activity leading to increased bone-marrow activity leading to increased arterial inflammation leading to cardiovascular disease events (red arrows) was significant. Additionally, excluding the path through bone-marrow activity, the residual path of increased resting amygdalar activity leading to increased arterial inflammation leading to cardiovascular disease event (blue arrows) was also significant. Amygdalar activity was assessed as the primary measure, max max amygdalac (ie, the maximum standardised uptake value for the right and left amygdalae, corrected for background cerebral tissue activity). Bone-marrow activity was measured by 18F-FDG uptake in vertebral bone marrow corrected for background uptake in the superior vena cava. Arterial inflammation was measured by 18F-FDG uptake in the aortic wall corrected for background uptake in the superior vena cava. In the figure, c represents the total effect of amygdalar activity on cardiovascular disease events, whereas c’ is the residual direct effect of amygdalar activity on cardiovascular disease events (independent of mediated effects). Standardised regression coefficients or log odds ratios are shown; all analyses incorporated age, sex, and baseline coronary artery calcification (as a control for pre-existing atherosclerotic disease burden) as covariates. The appendix contains additional explanation. 18F-FDG=18F fluorodeoxyglucose. *p=0.0073. †p<0.0001. ‡p=0.0044. §p=0.0432. ¶p=0.6409. ||p=0.2196. **p=0.013.
In the cross-sectional study, in congruence with the longitudinal study’s findings, amygdalar activity strongly correlated with arterial inflammation (r=0.70, p=0.0083). Perceived stress was associated with amygdalar activity (0.56; 0.0485; figure 5A), arterial inflammation (0.59; 0.0345, figure 5B) and CRP (0.83; 0.0210; figure 5C). Furthermore, amygdalar activity mediated most of the relation between perceived stress and arterial inflammation (p<0.05; appendix).
Figure 5: Perceived stress associated with amygdalar activity (A), arterial inflammation (B), and CRP (C) in cross-sectional validation sub-study.
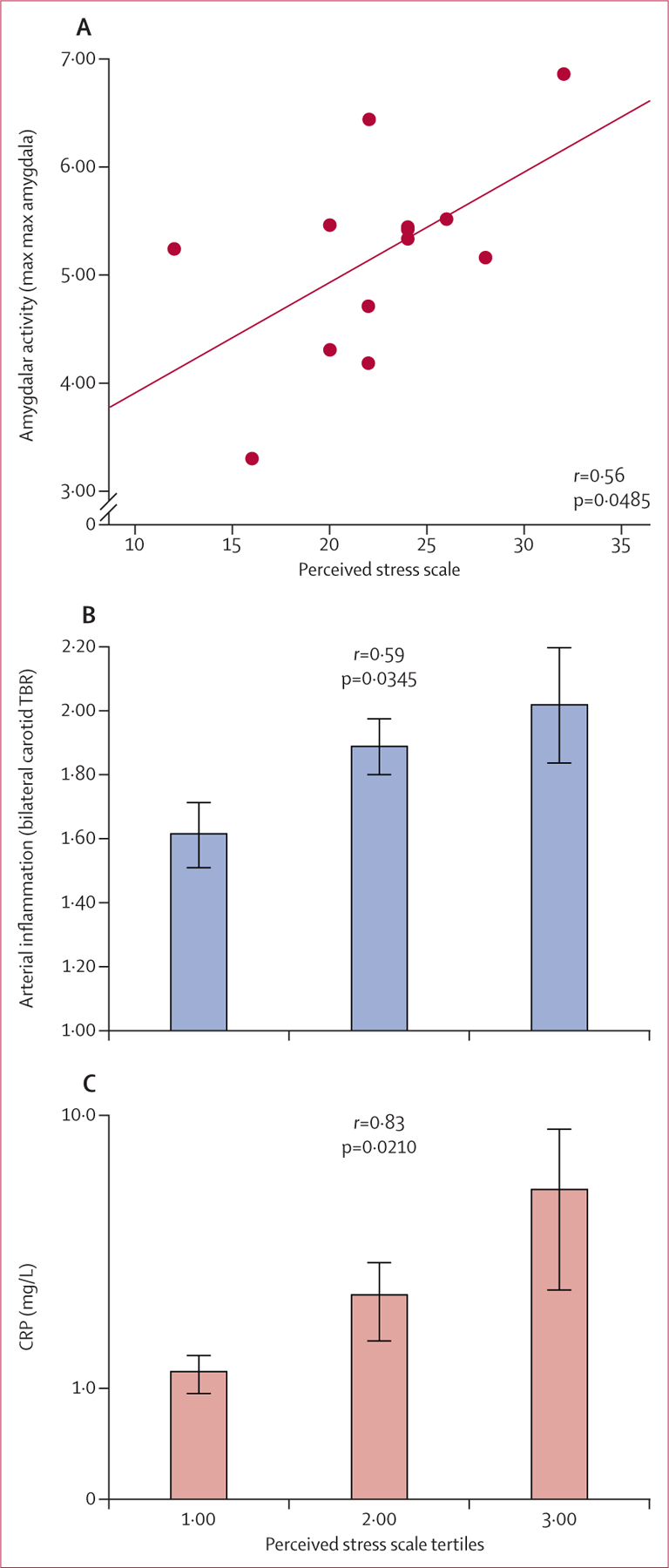
Perceived stress was assessed with a validated questionnaire. Error bars in (B) and (C) represent the standard error of the mean. TBR=target-to-background ratio. CRP=C-reactive protein.
Discussion
Our results show, for the first time in human beings, that resting metabolic activity within the amygdala, a key component of the brain’s salience network involved in stress, significantly predicts the development of cardiovascular disease independently of established cardiovascular risk factors. Furthermore, we showed that amygdalar activity is associated with increased haemopoietic activity and increased arterial inflammation. In the companion cross-sectional study, amygdalar activity was associated with perceived stress. In the longitudinal outcomes study, moreover, the link between amygdalar activity and cardiovascular disease events was substantially mediated by arterial inflammation (which in turn was substantially mediated by upregulated bone-marrow activity). These findings provide new and important insights, specifically that the amygdala could be a key structure in the mechanism linking stress to cardiovascular events, and that upregulation of haemopoietic tissue activity and increased atherosclerotic inflammation are additionally implicated in a neural–haemopoietic–arterial axis.
Psychological stress has long been thought of as an important human malady. Over the past several decades, increasing attention has been paid to the physical manifestations of stress. Despite evidence linking psychological stress and cardiovascular disease,29 cardiovascular risk management has remained focused on other risk factors, possibly partly as a result of poor understanding of the mechanisms underlying stress-associated cardiovascular disease. Our study is clinically important because it advances this understanding and suggests targets for novel therapeutic approaches to reduce cardiovascular disease risk.
Stress prompts activation of both the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis, leading to increases in circulating catecholamines, glucocorticoids, and (eventually) inflammatory cytokines.30–32 Additionally, stress can increase heart rate and blood pressure via the autonomic nervous system, all of which can contribute to endothelial dysfunction.33 However, these mechanisms do not entirely explain the link between stress and cardiovascular disease. Work in animals has yielded important new insights into another (and potentially more important) mechanism by which stress might induce cardiovascular disease. Murine studies show that stress leads to mobilisation and release of neutrophils and monocytes, and increases activity of haemopoietic progenitor cells in the bone marrow.13 Haemopoietic progenitors might accumulate in the circulation and the spleen and are thus poised to potentiate leucocyte supply by establishing extra-medullary haemopoiesis.34–36 In mice, bone-marrow-derived monocytes released in response to variable stress or to stressful events (such as myocardial infarction) migrate to the arterial wall, where they instigate atherosclerotic inflammation.11,13,37 Results from animal studies38 point to increased haemopoiesis and arterial inflammation as important mechanisms in stress. They also prompt two crucial questions that were addressed by our study: is this mechanism relevant to human beings, and how does the brain participate?.
Our results clearly identify the amygdala as a key neural structure associated with future cardiovascular disease events. Previous human imaging studies demonstrated that amygdalar activity is correlated with the inflammatory response to stress39 and the presence of pre-existing preclinical atherosclerosis.14 However, a longitudinal pathogenetic link such as that which we noted, to the best of our knowledge has never been identified. Furthermore, by demonstrating a significant pathway of increased amygdalar activity leading to increased bone-marrow activity leading to increased arterial inflammation leading to cardiovascular disease event (figure 6), our findings are a key addition to the literature. Increased amygdalar activity and its downstream consequences of upregulated haemopoietic and inflammatory activity could also be implicated in other medical conditions in which inflammation has an important role.
Figure 6: A model of stress leading to atherosclerotic inflammation.
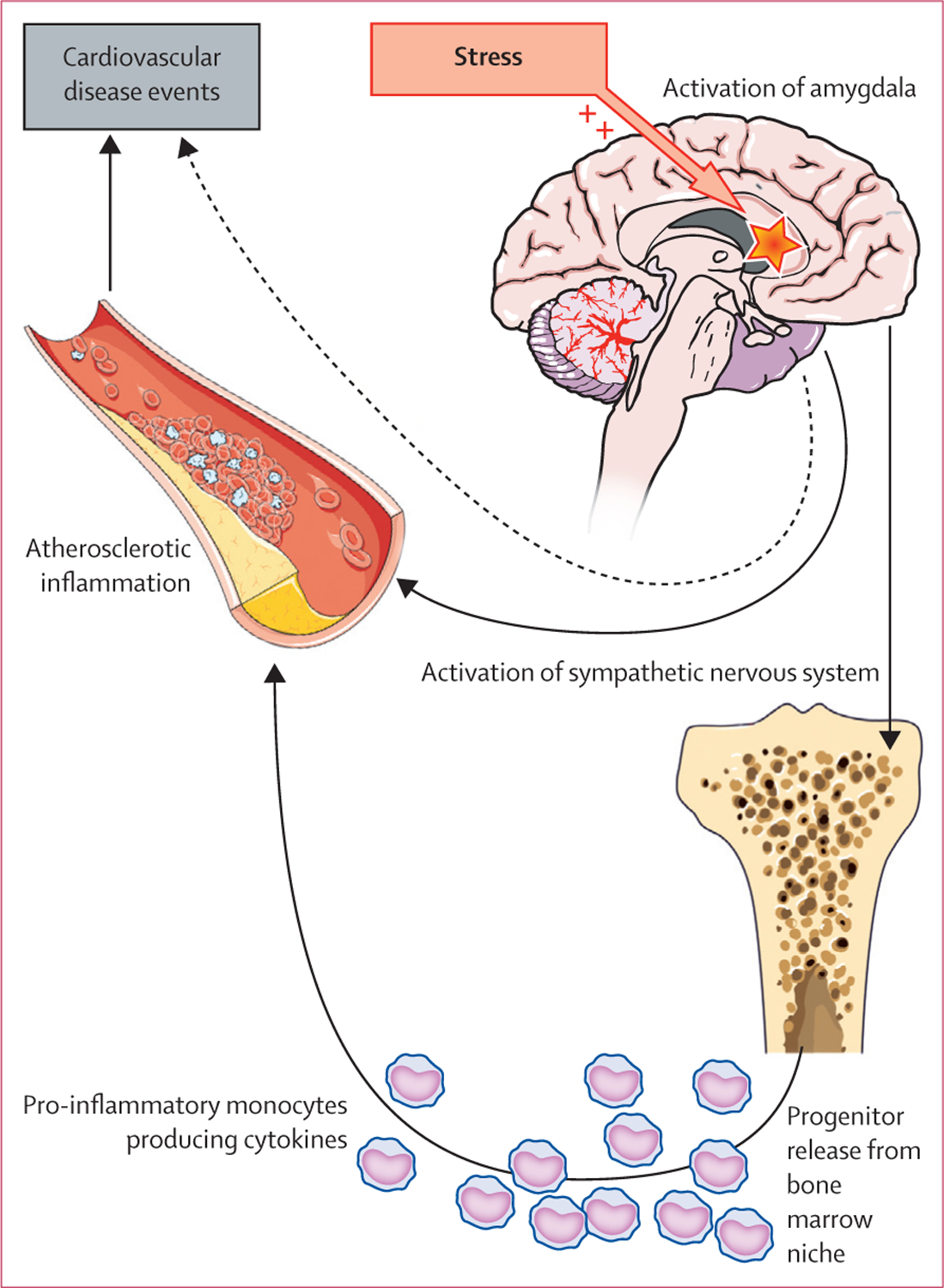
Data suggest that at least two biologically significant pathways link amygdalar activity to cardiovascular disease events in human beings. One of these pathways is sympathetic. The other, which is the object of this study, includes activation of the bone marrow (and release of inflammatory cells), which in turn lead to atherosclerotic inflammation and its atherothrombotic manifestations.
Our study has several limitations. First, the participants in the outcomes study were identified from a clinical database of patients who had undergone 18F-FDG PET/CT for clinical indications (mainly cancer screening), thus possibly limiting the generalisability of our findings. However, the association between amygdalar activity and cardiovascular disease events remained robust in the subgroup of individuals who did not have history of cancer, suggesting that this potential confounder is not responsible for the main observations of this study. Second, 271 (93%) of 293 individuals in the outcomes study were white. However, in the separate cross-sectional perceived stress substudy, most participants were not white and none had a previous history of cancer (data not shown). In that substudy, the relation between amygdalar activity and arterial inflammation remained robust, lending support to the findings of the main study. Third, standard questionnaires were not used in the outcomes study, and thus the relation between perceived stress or mental disorder and cardiovascular disease events was not directly assessed. However, the perceived stress substudy showed a relation between perceived stress and both amygdalar activity and arterial inflammation, thus providing independent validation of the findings. Fourth, a positive mediation analysis, as we report here, is consistent with, but not demonstrative of, causation—particularly when a portion of the data are cross-sectional, as was the case with amygdalar activity, bone-marrow activity, and arterial inflammation. To infer causation, further longitudinal or interventional studies are needed. Additionally, the pathway from amygdalar activity to cardiovascular disease presented herein does not purport to encompass all possible pathological influences on any of its nodes. Candidate influences include, among others, sympathetic activity, hormones (eg, neuropeptide Y, GABAergic neurosteroids), and cytokines. Finally, the sample size of the main study was modest and only 22 participants had a cardiovascular disease event. These limitations are substantially counterbalanced by several important innovations, including the unique, simultaneous quantification of arterial, amygdalar, and bone-marrow activity, and their associations with cardiovascular disease events in human beings.
Acitvation of the brain stress network and its downstream consequences, including haemopoietic tissue activation and increased arterial inflammation, could be targets for therapies designed to interrupt a vicious cycle between stress and cardiovascular events. Our findings should prompt not only further investigation of the mechanisms that regulate this axis, but also studies of how to interrupt pathogenetic transmission along it. One possible future research avenue would be to experimentally induce amygdalar activation (eg, by stressful mental imagery40 or presentation of fear-related stimuli16), and then examine the acute effect on bone-marrow activity and arterial inflammation. In a more ambitious vein, meditation has been shown to reduce amygdalar activity.41 In a study of 226 individuals,42 those randomly assigned to a 12-week stress-reduction course experienced an approximately 50% reduction in cardiovascular disease events compared with individuals who underwent cardiac rehabilitation but not stress management training.
Our findings raise the hypothesis that the benefits noted in that stress-reduction study42 could partly have been due to a therapeutic action on the neural–haemopoietic–arterial axis described herein. In the future, larger studies will be needed to assess modulation of this axis, which could produce a substantial beneficial clinical effect. Such studies should examine the impact of various stress-management strategies (targeting upstream portions of this axis), in addition to pharmacotherapies that target other aspects of this axis. In the meantime, when encountering a patient with a stress syndrome, clinicians could reasonably consider the possibility that alleviation of stress might result in benefits to the cardiovascular system. Eventually, chronic stress could be treated as an important risk factor for cardiovascular disease, one that is routinely screened for and effectively managed, like other major cardiovascular disease risk factors.
Our study shows, for the first time, a relation between neural tissue activity and subsequent cardiovascular events and suggests that the brain’s salience network, bone marrow, and arterial inflammation together form an axis that could accelerate the development of cardiovascular disease. Furthermore, our findings raise the possibility that efforts to attenuate psychosocial stress could produce benefits that extend beyond an improved sense of psychological wellbeing, and could beneficially impact the atherosclerotic milieu. Future studies of this neural–haemopoietic–arterial axis might lead to insights into how to further reduce the burden of cardiovascular disease.
Supplementary Material
Research in context.
Evidence before the study
Chronic stress carries an attributable risk for cardiovascular disease that is on par with other recognised risk factors, such as smoking, increased lipid concentrations, hypertension, and diabetes. Despite the prevalence and potency of this risk factor, little is known about the mechanisms that translate stress into cardiovascular disease events. To assess existing work, we searched PubMed with the terms “psychosocial stress”, “arterial inflammation”, “bone marrow”, “hematopoietic”, “human”, and “cardiovascular disease” for articles published in English before June 18, 2016. Although a link between stress, bone-marrow activity, and arterial inflammation had been identified in animal studies, this link had not previously been assessed in human beings. Furthermore, we found no studies in which the relation between neural tissue activity and cardiovascular disease events was assessed in either animal models or people.
Added value of this study
Our study provides several novel observations that together define a mechanism linking stress to cardiovascular events. We show for the first time in human beings that resting metabolic activity within the amygdala is significantly associated with the risk of developing cardiovascular disease independently of established cardiovascular risk factors. Furthermore, the link between amygdalar activity and cardiovascular disease events was substantially mediated by arterial inflammation (which in turn was substantially mediated by upregulated bone-marrow activity). These observations provide new and important insights, specifically that the amygdala could be a key structure in the mechanism linking stress to cardiovascular disease events, and that upregulation of haemopoietic tissue activity and increased atherosclerotic inflammation are additionally implicated in a neural–haemopoietic–arterial axis.
Implications of all the available evidence
Our results provide unique insights into mechanisms translating stress to cardiovascular disease and raise the possibility that alleviation of psychosocial stress could produce benefits that extend beyond an improved sense of psychological wellbeing, by improving the atherosclerotic milieu. Eventually, chronic stress could be treated as an important risk factor for cardiovascular disease, one that is routinely screened for and effectively managed, similar to other major cardiovascular disease risk factors.
Acknowledgments
The Harvard Catalyst Statistical Consulting Service provided consultation about statistical methods. Amanda Montoya at Ohio State University made helpful suggestions about mediation analysis. We also wish to acknowledge support from the US National Institutes of Health—grants R01HL122177 (AT), R01HL128264 (MN), R01HL071021 (ZAF), and 1P01HL131478 (ZAF).
Funding None.
Footnotes
See Online for appendix
Declaration of interests
AT reports grants from Genentech and Takeda and personal fees from Takeda, Actelion, AstraZeneca, and Amgen during this study for research outside the submitted work. UH reports grants from the National Heart, Lung, and Blood Institute’s Framingham Heart Study, American College of Radiology Imaging Network, Kowa Company, and Heartflow, and personal fees from the American Heart Association during the study. JWM reports a grant from Avanir Pharmaceuticals and Otsuka, personal fees from Janssen Research and Development, ProPhase, Genentech, and Impel Neuropharma, and a pending patent for Neuropeptide Y as a treatment for mood and anxiety disorders outside the submitted work. All other authors declare no competing interests.
References
- 1.Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress and risk of peripheral vascular disease, abdominal aortic aneurysm, and heart failure: pooling of sixteen cohort studies. Atherosclerosis 2014; 236: 385–88. [DOI] [PubMed] [Google Scholar]
- 2.Nabi H, Kivimaki M, Batty GD, et al. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: the Whitehall II prospective cohort study. Eur Heart J 2013; 34: 2697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosengren A, Hawken S, Ôunpuu S, et al. ; for the INTERHEART Investigators. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 953–62. [DOI] [PubMed] [Google Scholar]
- 4.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–89. [PubMed] [Google Scholar]
- 5.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339: 1349–57. [DOI] [PubMed] [Google Scholar]
- 6.Wang SS, Yan XB, Hofman MA, Swaab DF, Zhou JN. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci Bull 2010; 26: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain, Behav Immun 2015; 50: 18–30. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8: 2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang T, Chen Y, Liu H, Zhou Z, Zhai Y, Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb Res 2010; 126: 386–92. [DOI] [PubMed] [Google Scholar]
- 10.Bernberg E, Ulleryd MA, Johansson ME, Bergstrom GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE −/− mice. Atherosclerosis 2012; 221: 359–65. [DOI] [PubMed] [Google Scholar]
- 11.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012; 487: 325–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015; 8: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014; 20: 754–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry 2009; 65: 943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci 2003; 985: 389–410. [DOI] [PubMed] [Google Scholar]
- 16.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62: 273–81. [DOI] [PubMed] [Google Scholar]
- 17.Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry 2002; 7: 234–42. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, Vermetten E, Schmahl C, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 2005; 35: 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox AS, Oler JA, Shelton SE, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci USA 2012; 109: 18108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Du R, Zhu Y, et al. PET mapping of neurofunctional changes in a post-traumatic stress disorder model. J Nucl Med 2016; 57: 1474–77. [DOI] [PubMed] [Google Scholar]
- 21.Wang G-J, Volkow ND, Telang F, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 2009; 106: 1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006; 48: 1818–24. [DOI] [PubMed] [Google Scholar]
- 23.Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging 2013; 6: 1250–59. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–53. [DOI] [PubMed] [Google Scholar]
- 25.Oler JA, Fox AS, Shelton SE, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 2010; 466: 864–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britz-Cunningham SH, Millstine JW, Gerbaudo VH. Improved discrimination of benign and malignant lesions on FDG PET/CT, using comparative activity ratios to brain, basal ganglia, or cerebellum. Clin Nucl Med 2008; 33: 681–87. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav 1983; 24: 385–96. [PubMed] [Google Scholar]
- 28.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammationresults of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 2013; 62: 909–17. [DOI] [PubMed] [Google Scholar]
- 29.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012; 9: 360–70. [DOI] [PubMed] [Google Scholar]
- 30.De Bosscher K, Van Craenenbroeck K, Meijer OC, Haegeman G. Selective transrepression versus transactivation mechanisms by glucocorticoid receptor modulators in stress and immune systems. Eur J Pharmacol 2008; 583: 290–302. [DOI] [PubMed] [Google Scholar]
- 31.Nikkheslat N, Zunszain PA, Horowitz MA, et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun 2015; 48: 8–18. [DOI] [PubMed] [Google Scholar]
- 32.Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Advance Exper Med Biol 1999; 461: 107–16. [DOI] [PubMed] [Google Scholar]
- 33.Harrison NA, Cooper E, Voon V, Miles K, Critchley HD. Central autonomic network mediates cardiovascular responses to acute inflammation: relevance to increased cardiovascular risk in depression? Brain Behav Immun 2013; 31: 189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32: 2045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waterhouse DF, Cahill RA, Sheehan F, McCreery C. Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vasc Health Risk Manage 2008; 4: 177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 2012; 60: 1512–20. [DOI] [PubMed] [Google Scholar]
- 37.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013; 339: 161–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodes GE, Pfau ML, Leboeuf M, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA 2014; 111: 16136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muscatell KA, Dedovic K, Slavich GM, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun 2015; 43: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontalcortex during traumatic imagery in male and female vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61: 168–76. [DOI] [PubMed] [Google Scholar]
- 41.Lutz J, Brühl AB, Scheerer H, Jäncke L, Herwig U. Neural correlates of mindful self-awareness in mindfulness meditators and meditation-naïve subjects revisited. Biol Psychol 2016; 119: 21–30. [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal JA, Sherwood A, Smith PJ, et al. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation 2016; 133: 1341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


