Abstract
Aim: The definition of fetal growth restriction (FGR) refers to the incapability of a fetus to achieve the appropriate estimated growth, with expected fetal weight below the 10th percentile calculated for its gestational age. Placental factors and hypoxemia are considered to be essential elements with influence on intrauterine growth restriction (IUGR) and fetal death. The purpose of the present study was to investigate the macroscopic and microscopic pathological findings regarding the placentas in pregnancies complicated by influence on IUGR. Patients, Materials and Methods: Our study included 42 third-trimester pregnant patients admitted to the Cuza Vodă Hospital of Obstetrics and Gynecology, Iaşi, Romania, in the last three years. Soon after delivery, the 42 placentas were collected and analyzed; 32 placentas came from cases previously diagnosed with influence on IUGR and were included in our study group. Ten other placentas included in the control group were selected from uncomplicated pregnancies. Standard Hematoxylin–Eosin (HE) staining method, as well as Periodic Acid–Schiff (PAS) staining, and immunohistochemical techniques for cluster of differentiation 31 (CD31) and collagen IV were used in order to highlight the morphological features of the studied placentas. Results: Our study revealed that reduced placental dimensions and eccentric umbilical cord insertion are correlated with the birthweight of the fetuses with IUGR (p<0.05). The most common histological finding in our study group was placental infarction later correlated with IUGR, but a certain causality could not be demonstrated, as this finding was also present in normal pregnancies. Other histopathological findings were also present in the influence on IUGR group, such as fibrin deposits, diffuse calcification, chronic villitis, avascular chronical villi, with no significant statistical correlations. CD31 was strongly immunoexpressed in the villous endothelial cells. Collagen IV presented a strong immunoreaction in the basement membrane and mesenchyme of the placental villi. Conclusions: Our study revealed a correlation between the dimensions of the diameters and volume of the maternal placenta and the presence of influence on IUGR. Moreover, it confirms the available data suggesting that the place of insertion of the umbilical cord is correlated with the weight of the fetus. Further studies with extended panel antibodies are needed in order to determine and complete the role of these morphological changes in the development of influence on IUGR.
Keywords: intrauterine growth restriction, placental volume, placental infarction, perivillous fibrin deposits, immunohistochemistry
⧉ Introduction
The definition of fetal growth restriction (FGR) refers to the incapability of a fetus to achieve the appropriate estimated growth, with expected fetal weight below the 10th percentile calculated for its gestational age [1]. Proper development and fetal growth are heavily influenced by maternal supply of oxygen and necessary nutrients through the placenta. The different possible causes that inhibit the fetus to reach its genetically potential weight are still under investigation and several theories have been put forward [2]. Placental factors and hypoxemia are considered to be essential elements with influence on intrauterine growth restriction (IUGR) and fetal death [3]. Different placental structures and functions may be involved and this leads to anatomical, physiological, vascular and other morphological abnormalities [4]. Various gross morphological changes of the placenta have been mentioned, such as placental weight [5,6,7] or fetal/placental weight ratio [7,8], type of umbilical cord insertion in the placenta, and also its length or presence of knots and other anomalies [9,10]. Structural changes, such as chronic villitis, avascular villi, perivillous fibrinoid deposition, cytotrophoblast hyperplasia and basement membrane thickening, have all been associated with FGR [11,12,13]. In order to enhance current clinical knowledge of the etiology of IUGR, anatomopathological examinations of placentas could be extremely revealing [14].
Aim
In our study, we aimed to evaluate the macroscopic and histopathological (HP) features, as well as the immunoexpression of cluster of differentiation 31 (CD31) and collagen IV in placental specimens from pregnancies complicated with IUGR, looking for any clinico-morphological correlations, which could explain the processes that characterize FGR.
⧉ Patients, Materials and Methods
The purpose of our study was to examine the macroscopic and microscopic pathological findings regarding the placentas in pregnancies complicated by IUGR. The main macroscopic parameters that were analyzed in this article included: placental diameters, aspect of membranes, the insertions of the umbilical cord in the placenta, number of umbilical cord vessels, umbilical cord diameter. These parameters where afterwards corroborated with the microscopically HP characteristics.
Our study included 42 third-trimester pregnant patients admitted to the Cuza Vodă Hospital of Obstetrics and Gynecology, Iaşi, Romania, in the last three years. We collected a total of 42 placentas soon after delivery. Of these, 32 samples came from cases previously diagnosed with IUGR and were included in our study group. Ten other placentas were selected from pregnancies without complications and functioned as the control group.
The following histological features of the placentas were systematically assessed: vasculitis, villous infarction, chronic vilitis, villous hypoplasia, intervillous thrombi or hematomas, perivillous and basal plate fibrin deposition, cytotrophoblast proliferation, thickening of trophoblastic basal membrane, stromal fibrosis, multifocal chorangiomatosis [10].
Sections obtained from placental specimens were investigated by routine HP exam, being fixed in buffered formalin for 24 hours and processed for paraffin embedding. For microscopic assessment of the above-mentioned placental morphological aspects, 4–5 μm serial sections were stained with Hematoxylin–Eosin (HE) and Periodic Acid–Schiff (PAS) (both study and control groups), and furthermore prepared for immunohistochemical (IHC) (study group).
IHC exam with type IV collagen mouse monoclonal antibody (CIV22, Cell Marque) and CD31 mouse monoclonal antibody (JC70, Cell Marque) used automated IHC BenchMark ULTRA Protocol with ultraView Universal 3,3’-Diaminobenzidine (DAB) Detection Kit. The counterstaining was performed using Hematoxylin II-Ventana.
For semi-quantitative assessment of the antibodies, we considered the following classification of immunoexpression: 0 for no staining, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining.
In our study, the exclusion criteria regarding fetuses were the existence of a chromosomal or congenital anomaly, hydrops fetalis or multiple pregnancies. The maternal exclusion criteria were diabetes mellitus, pre-eclampsia and eclampsia, genitourinary infections, sepsis, antenatal hemorrhage (vasa praevia and placenta praevia, abruptio placentae) and systemic disorders.
Fetal biometric measurements obtained through ultrasonography (femur length, abdominal circumference, biparietal diameter, head circumference and estimated weight) were used in order to establish the diagnosis of IUGR.
The management of the study was conducted in full compliance with the ethical principles contained in the “Human Rights Declaration” adopted in Helsinki, which follows the Good Practice Rules in the Clinical Study and Legal Regulations and an informed consent has been obtained from every patient
We used Microsoft Excel to create our database and the statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) v. 20.
⧉ Results
Clinical characteristics of subjects included in this study are illustrated in Table 1.
Table 1.
The clinical characteristics in control and study groups (data given as numbers)
|
Clinical characteristics |
Study group |
Control group |
|
Age of mother [years] (mean±SD) |
29.03±5.664 |
29.24±6.030 |
|
Gestational age at birth [weeks] (mean±SD) |
35.75±3.2 |
38.14±0.85 |
|
Birth weight [g] (mean±SD) |
2059.45±669.87 |
3277.94±379.03 |
|
Premature birth (<37 weeks) |
70.2% |
2.77% circulation |
SD: Standard deviation.
There was no significant age difference between the mothers included in the two groups. Gestational ages, fetuses’ weights at birth, and measured placental dimensions were significantly different in the two groups (p<0.05). As expected, we observed a much higher incidence of premature birth, defined as birth before 37 weeks of pregnancy, in the study group (70.2%), while in control group only 2.77% were cases of premature births. We acknowledged a birth weight in the study group of 2059.45±669.87 g, compared to 3277.94±379.03 g in control group. Mean gestational age of the fetuses diagnosed with IUGR was lower at birth (35.75±3.2 weeks) than in the control group (38.14±0.85 weeks). The placentas from pregnancies included in our study group tend to be smaller than those collected for the control group, as shown in Table 2.
Table 2.
Placental dimensions
|
Measured dimensions [cm] |
Study group |
Control group |
p |
|
Length |
15.75±6.22 |
21±2.3 |
<0.05 |
|
Width |
12.12±2.23 |
16±3.6 |
<0.05 |
|
Depth |
1.2±0.38 |
2.3±0.3 |
<0.05 |
Macroscopic findings of placenta and umbilical cord in our study group are presented in Table 3. It is worth mentioning is the higher incidence of umbilical cord anomalies, including velamentous attachment to the chorionic disc (34.2%) or eccentric insertion (19.2%), abnormal number of vessels or even ruptured vessels (3.125%), and torsion of the umbilical cord (6.25%).
Table 3.
Macroscopic findings of placentas/umbilical cords (percentage)
|
Type of umbilical cord |
Study group |
|
Umbilical cord attachment to the chorionic disc |
|
|
Central |
46.6% (15) |
|
Eccentric |
19.2% (6) |
|
Velamentous |
34.2% (11) |
|
No. of umbilical cord vessels |
|
|
Two |
21.87% (7) |
|
Three |
71.13% (25) |
|
Ruptured umbilical cord |
3.125% (1) |
|
Umbilical cord torsion |
6.25% (2) |
The most common HP aspects identified and illustrated by HE and PAS stainings were as follows: villous infarction, parietal vascular thrombosis, perivillous and basal plate fibrin deposition, syncytiotrophoblastic knots, thickening of trophoblastic basal membrane, stromal fibrosis, multi-focal chorangiomatosis, villous hypoplasia (Table 4).
Table 4.
Histopathological features in the study placentas
|
Microscopic findings |
Study group (%) |
Control group (%) |
|
Infarction |
28 (87.5%) |
5 (5%) |
|
Thrombosis |
25 (78.12%) |
4 (40%) |
|
Perivillous fibrin |
32 (100%) |
6 (60%) |
|
Calcifications |
20 (62.5%) |
2 (2%) |
|
Basement membrane thickening |
28 (87.5%) |
3 (3%) |
|
Stromal fibrosis |
28 (87.5%) |
3 (30%) |
|
Chorangiomatosis |
18 (56.25%) |
– |
|
Villous hypoplasia |
26 (81.25%) |
2 (2%) |
|
Placental hematoma |
16 (50%) |
– |
|
Syncytiotrophoblastic knots |
24 (75%) |
2 (2%) |
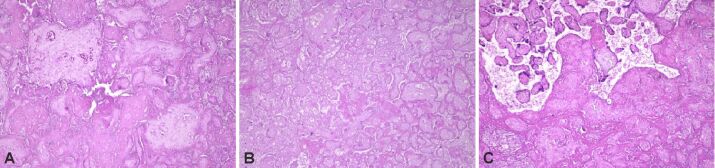
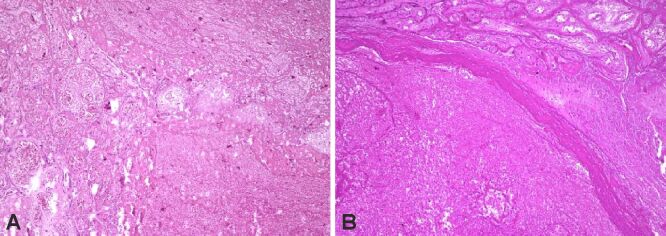
We found infarction, sometimes associated with distal villous hypoplasia (rare, small, sometimes filiform villi), and intervillous thrombosis to be more numerous in placental cotyledons coming from study group fetuses (Figure 1A,1B,1C; Figure 2, A and B). During the examinations, we were able to identify different stages of the process, from new, small infarcted areas, to old, extensive regions. We observed that the incidence of villous infarction was higher in the study group (87.5%) than in the control group (50%). The most frequent HP feature linked with infarction or thrombosis was chronic villitis, among perivillous fibrin deposits.
Figure 1.
Subacute and chronic placental infarction (A and B) with distal villous hypoplasia (C). HE staining: (A–C) ×100
Figure 2.
(A and B) Parietal vascular thrombosis. HE staining: (A and B) ×100
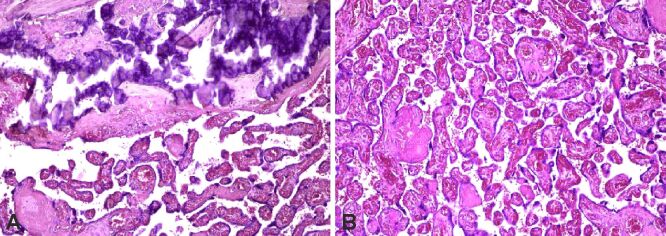
Perivillous fibrin deposition (Figure 3A,3B,3C; Figure 4, A and B) was more frequently present within the IUGR group (93.75%) than in the control group (60%). Placental hematoma (Figure 5, A and B) as well as calcifications (Figure 6A) were also encountered in most cases of the study group (81.25% versus 0%; 62.5% versus 2%), affecting the uteroplacental circulation. Stromal fibrosis, multifocal villous chorangiomatosis, syncytiotrophoblastic knots (Figure 6, A and B; Figure 7, A and B), as well as basement membrane thickening (Figure 8, A and B) were also observed more frequently in the study group than in the control group (Table 4).
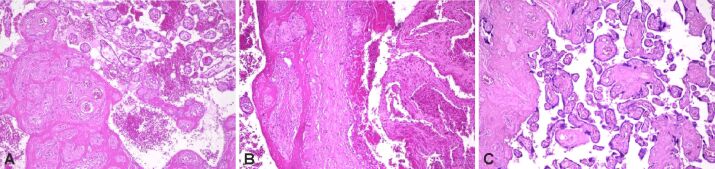
Figure 3.
(A–C) Perivillous fibrin deposits. HE staining: (A–C) ×100
Figure 4.
Massive perivillous fibrin deposition (A) and calcifications (B). PAS staining: (A) ×100; (B) ×200. PAS: Periodic Acid–Schiff
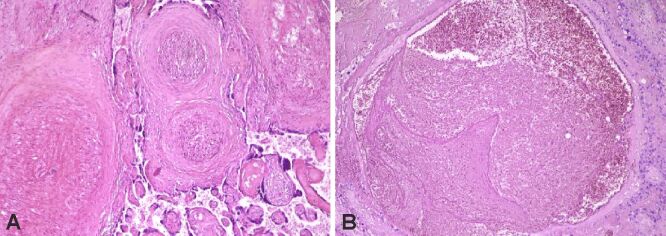
Figure 5.
(A and B) Intraplacental hematoma. HE staining: (A and B) ×100
Figure 6.
Chorangiomatosis (A and B) and extensive calcifications (A). HE staining: (A and B) ×100
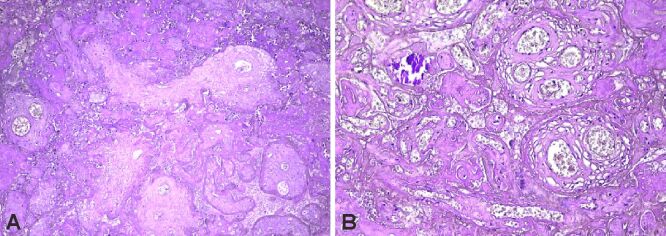
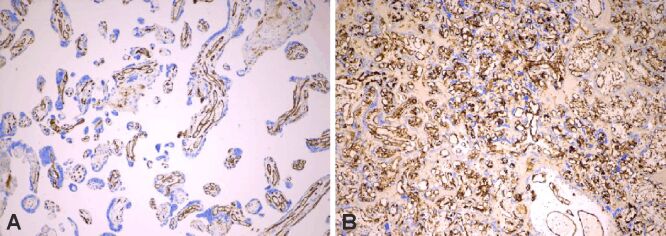
Figure 7.
(A) Strong CD31+ immunoexpression in terminal and stem villi, with numerous syncytiotrophoblastic knots; (B) Strong CD31+ immunoexpression highlighting villous chorangiomatosis. Anti-CD31 antibody immunomarking: (A) ×100; (B) ×40. CD31: Cluster of differentiation 31
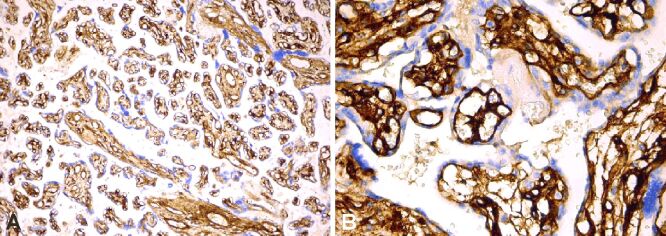
Figure 8.
(A and B) Strong collagen IV+ immunoexpression in the basement membrane and matrix of the terminal and stem villi. Anti-collagen IV antibody immunomarking: (A) ×100; (B) ×400
The IHC assessment of CD31 presented a moderate to strong positive immunoexpression in endothelial cells from villous capillaries (Figure 7, A and B), highlighting also the areas with chorangiomatosis, and a weak to negative immunoexpression in the infarcted areas and those exposed to massive perivillous fibrin deposition (Table 5).
Table 5.
CD31 immunoexpression in the study group placentas
|
CD31 immunointensity |
Study group (%) |
|
0 |
– |
|
1+ |
2 (6.25%) |
|
2+ |
4 (12.5%) |
|
3+ |
26 (81.25%) |
CD31: Cluster of differentiation 31.
Most of the cases from the study group presented a strong immunoreaction of the collagen IV, marking the thickening of the villous basement membrane, as well as the presence of type IV collagen in the mesenchyme of the placental villi (Figure 8, A and B; Table 6).
Table 6.
Collagen IV immunoexpression in the study group placentas
|
Collagen IV immunointensity |
Study group (%) |
|
0 |
4 (12.5%) |
|
1+ |
1 (3.12%) |
|
2+ |
2 (6.25%) |
|
3+ |
25 (78.12%) |
⧉ Discussions
Our study provides a comprehensive analysis of anatomopathological aspects in pregnancies that resulted in IUGR. We focused on both the macroscopic and microscopic and lesions of the collected specimens, as well as the immunoreaction of CD31 endothelial marker and collagen IV, trying to identify any correlation with clinical and ultrasound parameters that could explain the evolution of these complicated pregnancies.
In this study, we managed to show that there is a significant correlation between the placental dimensions, implicitly the placental volume, and the birthweight of the fetuses.
In order to have a homogenous classification, we defined villous infarction as a localized area of ischemic villous necrosis. An intervillous thrombus was characterized as a contained clot found in the intervillous space [15]. We considered chronic villitis an infiltration of the stroma by maternal T-lymphocytes [16] and perivillous fibrin deposition as the presence of fibers with a measured thickness less than 10 mm, and specific cross-striation of the filaments [17]. Trophoblastic basal membrane thickening was defined as an increased thickness of villi, suggestive of an elevated secretion of basal lamina molecules [17].
Previous studies considered that the placental weight presented with the best correlation with FGR [14, 18]. However, Jakó et al. (2019) presented a research in which the calculated volume of the placenta measured at the time of delivery showed a stronger correlation with birthweight, compared to the association between placental weight and birthweight [19]. This encourages the necessity of developing reliable methods for a more accurate examination of the placental dimensions in utero, in order to better evaluate the growth restriction. Furthermore, our study shows significant differences between the smallest placental diameter between the group of patients with IUGR and the control group. This parameter together with the placental volumes are feasible to measure antenatally through echography as prognostic factors. Ultrasound can be used to evaluate the placental diameters and volume more precisely than placental weight [20].
Current studies focus intensely on investigating also the utility of magnetic resonance imaging (MRI) in assessing the placental volume and shape. Consequently, MRI may provide additional information on the morphological characteristics of the placenta. Studies showed that placentas in IUGR pregnancies present more oval and thicker aspect in MRI acquisitions than placentas of normal pregnancies, which have a circular outline [21]. The morphological measurements we obtained are in line with these findings. Isakov et al. (2018) tried to identify and characterize a percentile curve for the placental volume. According to multiple systematic performable measurements, they proposed novel diagnostic parameters so that values could be used in clinical practice [22].
Regarding the trimester in which placental measurements are relevant, three-dimensional ultrasonographical measurements in the first trimester can be moderately correlated with fetal weight. On the other hand, measurements taken in the second trimester are associated with good predictive values (up to 45%) and can be applied in a clinical setting for screening high-risk pregnancies [23].
As for the umbilical cord, our study reveals that almost 2/3 of the specimens that we examined showed either an eccentric or a velamentous insertion and were correlated with IUGR (p<0.05). Similar findings were reported by many other studies [24,25,26], while Brouillet et al. (2014) showed that noncentral cord insertion (paracentral, velamentous) can be correlated with FGR and low birth weight in single fetus pregnancies [27]. Regarding the relationship between peripheral cord insertion and IUGR, we might assume, based on mathematical and geometrical principles, that a central umbilical cord insertion is required in order to provide an equal distribution and exchange of blood.
Although placental infarction can be found in both normal and abnormal pregnancies, this lesion presents clinico-morphological significance when it affects at least an average of 10–20% of the placental volume. The relationship between fetal hypoxia, subsequent IUGR and the presence of multiple placental infarction lesion has already been reported in literature [28]. In the present study, different degrees of placental infarction were detected in 28 (87.5%) cases of the study group, results similar with those previously published [29]. In our study group, we reported 25 cases with villous thrombosis, highlighting the literature results which suggest that vascular fetal thrombotic disorders are commonly found in women with adverse pregnancy outcomes [30].
The HP evaluation of the placenta also reveals in the study group other morphopathological changes in the study group, most of them in a certainly higher proportion than in the control group: intervillous thrombi, diffuse calcifications, avascular terminal villi, chronic villitis, villous hypoplasia, syncytiotrophoblastic knots, thickening of the basement membrane, multifocal chorangiomatosis. Although not specific to IUGR pregnancies, research reveals that these lesions are linked with IUGR, systemic autoimmune disorders, intrauterine infection and sepsis, genetic disorders, toxic substances, abnormal interaction between host and the placenta, and confined placental mosaicism [31,32]. Redline et al. (2004) [33] revealed that the massive deposition of intervillous fibrin presented strong correlation with placental and fetal weight. Based on existing knowledge, it is stated that diffuse calcification often occurs in mature placentas and reflects placental senescence, but there is a relationship between preterm placental calcification and adverse pregnancy outcome [34]. İskender-Mazman et al. (2014) found no relationship between IUGR and diffuse dystrophic calcification [35].
The IHC evaluation of CD31 endothelial markers in the study group revealed a strong immunoreaction in villous endothelial cells, emphasizing its importance as a placental endothelial marker [29], as well as a complementary IHC parameter in the diagnosis of villous chorangiomatosis or other fetal vasculopathy associated with IUGR.
The strong immunoreactivity of the anti-collagen IV antibody in almost all cases of the study group suggests and completes the already published studies, which demonstrate the existence of type IV collagen outside the basement membrane, being described also in the mesenchyme of the placental villi [36]. This accumulation of collagen IV in the villous extracellular matrix could support and explain, besides the invasive character of the trophoblast at the implantation site, the impairment of the maternal–fetal inductive relationships, with possible restriction of nutrient circulation, which can lead to fetal IUGR.
⧉ Conclusions
Our study revealed a correlation between the dimensions of the diameters and volume of the maternal placenta and the presence of IUGR. Furthermore, it confirms the available data suggesting that the place of insertion of the umbilical cord is correlated with the weight of the fetus. The most common histological finding in our study group was placental infarction, which was correlated with IUGR, but a certain causality could not be demonstrated, as this finding was also present in normal pregnancies. CD31-positive immunoexpression of the villous endothelial cells can represent a supplementary parameter in the diagnosis of chorioangiomatosis or other fetal vasculopathy associated with IUGR. The strong immunoexpression of collagen IV inside or outside the basement membrane of the placental villous structures could explain the disturbance of the specific maternal–fetal inductive relationships from IUGR pregnancies. Further larger studies with extended panel immunomarkers are needed in order to determine and complete the role of these morphological changes in the development of IUGR.
Conflict of interests
The authors declare that they have no conflict of interests.
Authors’ contribution
Alexandru Cărăuleanu and Dragoş Nemescu equally contributed to the manuscript.
References
- 1.Committee on Practice Bulletins – Gynecology, American College of Obstetricians and Gynecologists, Washington, DC 20090-6920, USA. Intrauterine growth restriction. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2001;72(1):85–96. [PubMed] [Google Scholar]
- 2.Maulik D, Frances Evans J, Ragolia L. Fetal growth restriction: pathogenic mechanisms. Clin Obstet Gynecol. 2006;49(2):219–227. doi: 10.1097/00003081-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pardi G, Marconi AM, Cetin I. Placental–fetal interrelationship in IUGR fetuses – a review. Placenta. 2002;23(Suppl A):S136–S141. doi: 10.1053/plac.2002.0802. [DOI] [PubMed] [Google Scholar]
- 4.Maulik D. Fetal growth restriction: the etiology. Clin Obstet Gynecol. 2006;49(2):228–235. doi: 10.1097/00003081-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Heinonen S, Taipale P, Saarikoski S. Weights of placentae from small-for-gestational age infants revisited. Placenta. 2001;22(5):399–404. doi: 10.1053/plac.2001.0630. [DOI] [PubMed] [Google Scholar]
- 6.Fox H, Wells M, editors. Haines and Taylor obstetrical and gynaecological pathology. 5. New York: Churchill Livingstone; 2003. pp. 1273–1326. [Google Scholar]
- 7.Biswas S, Ghosh SK. Gross morphological changes of placentas associated with intrauterine growth restriction of fetuses: a case control study. Early Hum Dev. 2008;84(6):357–362. doi: 10.1016/j.earlhumdev.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79(3):157–159. doi: 10.1080/j.1600-0412.2000.079003157.x. [DOI] [PubMed] [Google Scholar]
- 9.Osak R, Webster KM, Bocking AD, Campbell MK, Richardson BS. Nuchal cord evident at birth impacts on fetal size relative to that of the placenta. Early Hum Dev. 1997;49(3):193–202. doi: 10.1016/s0378-3782(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 10.Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: associated placental pathologic features. Am J Obstet Gynecol. 1995;173(4):1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 11.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113(5):580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SH, Perrin E, editors. Pathology of the placenta. 2. New York: Churchill Livingstone; 1999. pp. 89–106. [Google Scholar]
- 13.Park SY, Kim MY, Kim YJ, Chun YK, Kim HS, Kim HS, Hong SR. Placental pathology in intrauterine growth retardation. Korean J Pathol. 2002;36(1):30–37. https://www.jpatholtm.org/journal/view.php?number=2142 [Google Scholar]
- 14.Vedmedovska N, Rezeberga D, Teibe U, Melderis I, Donders GG. Placental pathology in fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):36–40. doi: 10.1016/j.ejogrb.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM. Atlas of Nontumor Pathology, Armed Forces Institute of Pathology (AFIP) 1. Washington, D.C: American Registry of Pathology (ARP); 2004. Placental pathology; pp. 123–127. [Google Scholar]
- 16.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38(10):1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Benirschke K, Kaufmann P. Pathology of the human placenta. 4. New York: Springer-Verlag; 2000. p. 196. [Google Scholar]
- 18.Teasdale F. Idiopathic intrauterine growth retardation: histomorphometry of the human placenta. Placenta. 1984;5(1):83–92. doi: 10.1016/s0143-4004(84)80051-x. [DOI] [PubMed] [Google Scholar]
- 19.Jakó M, Surányi A, Kaizer L, Németh G, Bártfai G. Maternal hematological parameters and placental and umbilical cord histopathology in intrauterine growth restriction. Med Princ Pract. 2019;28(2):101–108. doi: 10.1159/000497240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen S, Bjelland EK, Haavaldsen C, Eskild A. Placental weight in pregnancies with high or low hemoglobin concentrations. Eur J Obstet Gynecol Reprod Biol. 2016;206:48–52. doi: 10.1016/j.ejogrb.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Dahdouh S, Andescavage N, Yewale S, Yarish A, Lanham D, Bulas D, du Plessis AJ, Limperopoulos C. In vivo placental MRI shape and textural features predict fetal growth restriction and postnatal outcome. J Magn Reson Imaging. 2018;47(2):449–458. doi: 10.1002/jmri.25806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isakov KMM, Emerson JW, Campbell KH, Galerneau F, Anders AM, Lee YK, Subramanyam P, Roberts AE, Kliman HJ. Estimated placental volume and gestational age. Am J Perinatol. 2018;35(8):748–757. doi: 10.1055/s-0037-1615285. [DOI] [PubMed] [Google Scholar]
- 23.Quant HS, Sammel MD, Parry S, Schwartz N. Second-trimester 3-dimensional placental sonography as a predictor of small-for-gestational-age birth weight. J Ultrasound Med. 2016;35(8):1693–1702. doi: 10.7863/ultra.15.06077. [DOI] [PubMed] [Google Scholar]
- 24.Cai LY, Izumi S, Koido S, Uchida N, Suzuki T, Matsubayashi H, Sugi T, Shida N, Kikuchi K, Yoshikata K. Abnormal placental cord insertion may induce intrauterine growth restriction in IVF-twin pregnancies. Hum Reprod. 2006;21(5):1285–1290. doi: 10.1093/humrep/dei494. [DOI] [PubMed] [Google Scholar]
- 25.Kent EM, Breathnach FM, Gillan JE, McAuliffe FM, Geary MP, Daly S, Higgins JR, Dornan J, Morrison JJ, Burke G, Higgins S, Carroll S, Dicker P, Manning F, Malone FD. Placental cord insertion and birthweight discordance in twin pregnancies: results of the national prospective ESPRiT Study. Am J Obstet Gynecol. 2011;205(4):376e1–376e7. doi: 10.1016/j.ajog.2011.06.077. [DOI] [PubMed] [Google Scholar]
- 26.Manolea MM, Dijmărescu AL, Popescu FC, Novac MB, Diţescu D. Evaluation of the implantation site morphology in spontaneous abortion. Rom J Morphol Embryol. 2015;56(1):125–131. [PubMed] [Google Scholar]
- 27.Brouillet S, Dufour A, Prot F, Feige JJ, Equy V, Alfaidy N, Gillois P, Hoffmann P. Influence of the umbilical cord insertion site on the optimal individual birth weight achievement. Biomed Res Int. 2014;2014:341251–341251. doi: 10.1155/2014/341251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Günyeli I, Erdemoğlu E, Ceylaner S, Zergeroğlu S, Mungan T. Histopathological analysis of the placental lesions in pregnancies complicated with IUGR and stillbirths in comparison with noncomplicated pregnancies. J Turk Ger Gynecol Assoc. 2011;12(2):75–79. doi: 10.5152/jtgga.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novac MV, Niculescu M, Manolea MM, Dijmărescu AL, Iliescu DG, Novac MB, Rotaru LT, Stoenescu MF, Tabacu MC, Tudorache Ş, Busuioc CJ, Gheonea IA. Placental findings in pregnancies complicated with IUGR – histopathological and immunohistochemical analysis. Rom J Morphol Embryol. 2018;59(3):715–720. [PubMed] [Google Scholar]
- 30.Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in placenta. J Matern Fetal Med. 1998;7(6):277–286. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<277::AID-MFM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol. 2002;5(2):159–164. doi: 10.1007/s10024001-0195-y. [DOI] [PubMed] [Google Scholar]
- 32.Syridou G, Spanakis N, Konstantinidou A, Piperaki ET, Kafetzis D, Patsouris E, Antsaklis A, Tsakris A. Detection of cytomegalovirus, parvovirus B19 and Herpes simplex viruses in cases of intrauterine fetal death: association with pathological findings. J Med Virol. 2008;80(10):1776–1782. doi: 10.1002/jmv.21293. [DOI] [PubMed] [Google Scholar]
- 33.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, Prashner HR, Waters BL, Society for Pediatric Pathology, Perinatal Section, Maternal Vascular Perfusion Nosology Committee Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7(3):237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen KH, Chen LR, Lee YH. Exploring the relationship between preterm placental calcification and adverse maternal and fetal outcome. Ultrasound Obstet Gynecol. 2011;37(3):328–334. doi: 10.1002/uog.7733. [DOI] [PubMed] [Google Scholar]
- 35.İskender-Mazman D, Akçören Z, Yiğit Ş, Kale G, Korkmaz A, Yurdakök M, Durukan T. Placental findings of IUGR and non-IUGR. Turk J Pediatr. 2014;56(4):368–373. [PubMed] [Google Scholar]
- 36.Oefner CM, Sharkey A, Gardner L, Critchley H, Oyen M, Moffett A. Collagen type IV at the fetal–maternal interface. Placenta. 2015;36(1):59–68. doi: 10.1016/j.placenta.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]