Abstract
Anencephaly is a severe malformation of the central nervous system (CNS), being one of the most common types of neural tube defects. It is defined as total or partial absence of the calvarium, with absence of the brain. Anencephaly has an incidence of 1 to 5 in every 1000 births, and the mortality rate is 100% during intrauterine life or within hours or days after birth. The etiology of anencephaly remains unclear, but various maternal-related environmental and genetic risk factors have been reported, which include diabetes, obesity, exposure to different drugs or toxins, genetic polymorphisms and mutations, as well as positive family history for neural tube defects. One of the most important nutritional factors in the development of anencephaly is folate deficiency. Methylenetetrahydrofolate reductase (MTHFR) gene codes the enzyme involved in the intracellular metabolism of folic acid; the 677C-T polymorphism of this gene causes the thermolability of the enzyme and decreased enzymatic activity, which is also dependent of folate plasmatic level. Etiopathogenesis of anencephaly includes several mutations in various other genes, such as: platelet-derived growth factor receptor alpha (PDGFRA), cadherin epidermal growth factor (EGF) laminin G (LAG) seven-pass G-type receptor 1 (CELSR1), Vang-like 1 (VANGL1) and Vang-like 2 (VANGL2), the last two being involved in the process of neurulation. Screening tests include maternal serum alpha-fetoprotein level and ultrasound (US) examination. During the first trimester US screening, anencephaly is now detected in all cases, but in order to decrease the complication rate of pregnancy termination, the diagnosis should be established as soon as possible, during the pregnancy confirmation US. We conclude that given that anencephaly is a severe malformation of the CNS, morphological characterization could improve the screening by US that is mandatory in the first trimester in order to plan the best, safe and early management.
Keywords: anencephaly, folic acid, genetic polymorphism
⧉ Definition
Anencephaly is a severe malformation of the central nervous system (CNS), being the most common type of neural tube defect. The etymology comes from the Greek words “an”, which means “without” and “enkephalos”, which means “encephalon”. It represents the total or partial absence of the calvarium with absence of the brain. The brainstem, cerebellum and diencephalon are usually present [1].
Anencephaly can be classified as meroacrania if foramen magnum is not involved, holoacrania if the defect goes beyond foramen magnum and holoacrania with rachischisis if it is associated with spina bifida [2].
The development of the neural tube is a multistep process. Therefore, the etiology of neural tube defects is indeed an interaction between genes and environmental factors [3].
⧉ Epidemiology
Neural tube defects are frequent among newborns, given that anencephaly occurs in about 1–5 of every 1000 births. In United States, about one in every 4600 babies is born with anencephaly [4]. Geographic variation is a factor to be taken into consideration: countries such as Mexico, China, Turkey or British Isles have a high rate of prevalence [5]. The mortality rate is 100% during intrauterine life or within hours or days after birth and the termination of pregnancy percent is greater than 83% [6]. Other structural anomalies can be associated in 12–25% and also genetic abnormalities can be detected in 1–5% [7].
⧉ Etiopathogenesis
Although the etiology of anencephaly remains unclear, there are cited various environmental and genetic risk factors for mother (Table 1).
Table 1.
Main etiological factors and mechanisms of anencephaly
|
Author(s), year |
Category |
Etiological factor |
Mechanism |
Reference(s) |
|
van Gool et al., 2018; Gunnarsdottir et al., 2019; Sadler, 2011 |
Environmental conditions |
Nutritional factors: folic acid deficiency |
Alternated DNA synthesis and methylation |
|
|
Barron, 2016; Aguilar-Garduño et al., 2010 |
Exposure to nitrates, pesticides, organic solvents |
Alteration of the migration of male gametes Alteration of DNA transfer during meiosis |
||
|
Kashif et al., 2019 |
Anticonvulsant use, excess of vitamin A intake |
Folic acid antagonism |
[13] |
|
|
Aguilar-Garduño et al., 2010 |
Socioeconomic status |
Low nutrient intake Exposure to toxic substances |
[12] |
|
|
Barron, 2016 |
Fever/hyperthermia |
Unknown |
[11] |
|
|
Barron, 2016; Pelizzari et al., 2015; Yaliwal & Desai, 2012 |
Genetics of both population and familial ancestry |
MTHFR 677C-T and 1298A-C polymorphisms PDGFRA frameshift variant and missense variant VANGL1 & 2, Pax3, CELSR1, AMT and GLDC mutations SNPs in PARD3 PCMT1 polymorphisms |
Decrease activity of MTHFR affect DNA synthesis and methylation Alteration of PCP Abnormal down and up-regulation of miRNAs affect cell cycle regulation and cell proliferation |
|
|
Barron, 2016 |
Maternal condition |
Obesity Pre-gestational/gestational diabetes |
Insulin resistance Hyperglycemia Reduced folate levels |
[11] |
|
Maternal Hispanic ethnicity |
Low folate intake Fumonisins contamination 677C-T polymorphism |
|||
|
Agopian et al., 2013 |
Fetal condition |
Female gender |
Unknown |
[16] |
AMT: Aminomethyltransferase; CELSR1: Cadherin epidermal growth factor (EGF) laminin G (LAG) seven-pass G-type receptor 1; DNA: Deoxyribonucleic acid; GLDC: Glycine decarboxylase (dehydrogenase); miRNA: Micro-ribonucleic acid; MTHFR: Methylenetetrahydrofolate reductase; PARD3: Par-3 family cell polarity regulator; Pax3: Paired box 3; PCMT1: Protein carboxyl methyltransferase 1; PCP: Planar cell polarity; PDGFRA: Platelet-derived growth factor receptor alpha; SNP: Single nucleotide polymorphism; VANGL1 & 2: Vang-like 1 & 2.
One of the most important nutritional factors in pathogenesis of anencephaly is folate deficiency. It could have multiple causes and supplementation with folic acid represent a major public health issue, reducing the risk of neural tube defects [8].
Antiepileptic drugs represent one of the most important environmental factors. Kashif et al. state that some recommendation should be taken into account by neurologists to avoid neural tube defects, such as folic acid supplements or monitoring Lamotrigine, Carbamazepine, and Phenytoin levels. Valproate is considered the most teratogenic and therefore should never be given as first line of treatment [13]. More than that, Isotretinoin, which is used as a dermatological drug, and selective serotonin reuptake inhibitors are associated with an increased risk of giving birth to an anencephalic fetus [13]. Exposure to nitrates doubles the odds of neural tube defects in women whose occupations include working with cleaning products, spray paints or paint thinners [11]. Also, nitrate exposure is found in farming communities, where the private wells might be contaminated by fertilizers and animal waste [11]. Exposure to pesticides at home, at work or in the community was associated to higher risk of having a child with neural tube defects, especially when it comes to anencephaly [11]. Organic solvents, such as aliphatic hydrocarbons, aromatic hydrocarbons and their halogenated derivatives, halogenated hydrocarbons, aliphatic alcohols, glycols and methoxyethanol are associated with development of anencephaly in fetuses whose mothers were exposed in the last five years before birth [12]. Moreover, even the father exposure to organic solvents and pesticides during the periconceptional period was associated with an increased risk of having a child with anencephaly, considering the fact that these products have a toxic effect on male gametes, altering the migration and the transfer of deoxyribonucleic acid (DNA) during meiosis [12]. Organic solvents are used the industry of varnishes and paints, production of colorants and explosives, production of natural and synthetic rubber, leather and shoe industry, enamels, adhesives, lacquers and putty, production of polymers and also during the cleaning and degreasing of industrial machinery [12].
Women who use hot tub or sauna in the early stages of pregnancy are at higher risk for neural tube defects. This also applies to women who experienced high fever in the same period of the pregnancy [11].
The fact that Hispanics have pregnancies associated with neural tube defects nearly twice as often as non-Hispanic whites might have several causes [11]. First of all, Hispanics tend to consume products made from corn masa flour (such as tortillas), which is not fortified with folic acid, as cornmeal and corn grits [11]. Secondly, commercial corn supplies can be contaminated with mycotoxins called fumonisins, which is produced by a mold and may cause anencephaly, but whose effect might be lowered by folic acid administration [11].
Diabetes, hyperinsulinemia and a body mass index (BMI) of 30 kg/m2 or higher are associated with higher risk of giving birth to a child with neural tube defects [11]. On the other side, weight loss diets and weights loss products in early stages of the pregnancy and even prior to it doubled the risk of neural tube defects, probably because of the low nutrient intake, including folic acid [11]. The risk of neural tube defects was almost tripled in women who used diuretics during the first trimester, as a part of a weight-loss strategy [11].
Neural tube defects, i.e., anencephaly and spina bifida, are responsible of perinatal, neonatal, infant, and under-five mortality, which is the reason why folic acid fortification of wheat flour and maize flour became mandatory in certain countries such as the United States, 1300 new cases of anencephaly and spina bifida being prevented each year by this method that have been implemented since January 1998 [17]. Besides folic acid intake, weight loss in obese women at reproductive age might be used as an attempt of preventing neural tube defects [16].
However, traditional risk factors are implied in less than 50% of neural tube defects [16]. The risk of having another child with anencephaly in the presence of positive family history varies between 2% to 10%, compared to 0.006% in woman with no prior history. The recurrence risk is high in siblings of affected individuals compared to the general population [16]. Even female infant gender is cited as a risk factor, as most of the anencephalic fetuses are females [16]. Anencephaly was also associated with chromosomal abnormalities, such as Edwards syndrome – trisomy 18 [14]. Also, studies show that many of the anencephalic fetuses present associated abnormalities, such as spina bifida, cleft palate, clubbed foot, clubbed hands and gastroschisis, suggesting the overwhelming presence of genetics in the neural tube defects [18]. These aspects highlight the importance of genetic factors in the etiopathogenetics of neural tube defects [11, 14, 15]. Hence, this is the reason why over the last few years, more and more studies have assessed the involvement of genetic and additional nongenetic risk factors in the development of anencephaly [16].
There are various genes that might play a role in the etiopathogenesis of neural tube defects and thus in anencephaly, such as genes implied in planar cell polarity (PCP) pathways and folate metabolism [19].
Etiopathogenesis of anencephaly definitely includes the interaction between genetic and nutrient factors [20]. Methylenetetrahydrofolate reductase (MTHFR) gene codes for the enzyme, which is involved in the metabolism of folic acid, converting 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the circulating form of folate [11, 15, 20, 21]. In this reaction, vitamin B12 is used as a cofactor and the reaction is catalyzed by methionine synthase [20]. Methionine is implied in complex chemical processes implied in DNA synthesis, cell division and tissue growth, and also in DNA methylation, which is essential in gene expression and chromatin structure [20]. The activity of the enzyme can be reduced by the presence of two common polymorphisms, i.e., 677C-T and 1298A-C [20]. The 677C-T polymorphism causes the thermolability of the enzyme and decrease enzymatic activity, because of an alanine to valine substitution and, as a result, the risk for neural tube defects becomes 2–4 folds higher in homozygous patients [15, 20]. Folic acid levels influence this risk factor [15]. The 1298A-C polymorphism, which is caused by a missense mutation, determines the substitution of an adenine with a cytosine, resulting in changing a glutamate into an alanine residue, but the relative risk is lower in this case compared to 677C-T mutation [15,20]. Hence, it was found that the interaction between MTHFR 677C-T polymorphism and folate status is implied in the pathogenesis of anencephaly, given that a decreased enzyme activity leads to low active folate form, which is needed for normal neural tube development [9, 11, 20]. However, the 677C-T polymorphism is implied in the etiopathogenesis of anencephaly mostly in the population of Mexico [20, 22].
In the vertebrate embryo, there are two types of neuroepithelial cell division, i.e., apicobasal and planar cell divisions [23]. The development of cell polarity plays a crucial role in the process of neurulation, being implied in the neural tube closure [23]. Neurulation represents the process of neural tube formation during early embryogenesis. The neural tube appears from the neural plates. By the end of third week, the neural plates will form the neural folds (at the lateral edges) and the neural groove (in the midline). The neural folds will fuse in the midline and the neural tube is formed. Until fusion is complete the cranial and caudal regions will communicate through cranial and caudal neuropores with amniotic cavity. In the 25th–28th day of gestation these neuropores will close, completing the neurulation [24]. PCP is a molecular mechanism responsible of giving cells a coordinated polarized orientation, which is implied in the transition of the neural plate to neural tube, where cells are polarized along the apical–basal axis [23, 25]. The apical surfaces of the cells are oriented to the neural tube lumen, while the basal surfaces outline the neural tube. This process is possible through the existence of polarity proteins, which mediate cell-to-cell adhesion [23]. Several nonsynonymous mutations and single nucleotide polymorphism (SNPs) were found in the PCP pathway genes, such as Vang-like 1 (VANGL1) and Vang-like 2 (VANGL2) [23]. Also, mutations in other genes, such as platelet-derived growth factor receptor alpha (PDGFRA) and cadherin epidermal growth factor (EGF) laminin G (LAG) seven-pass G-type receptor 1 (CELSR1) that are implied in PCP might be implied in the etiopathogenesis of neural tube defects [17, 19].
Sudiwala et al. state that mutations in paired box 3 (Pax3) gene lead to neural tube defects, Pax3 gene being responsible for neurulation to proceed. Supplements of folic acid restore proliferation in the cranial neuroepithelium, being state that the effect of folic acid to promote cell cycle compensates for the loss of Pax3 and therefore folic acid prevents cranial neural tube defects, such as anencephaly [26].
PDGFRA is a gene encoding for a cell-surface tyrosine kinase receptor, implied in embryonic development and cell proliferation [19]. One study revealed a PDGFRA c.3029_3030delAG frameshift variant, which leads to premature stop codon, in an anencephalic patient [19]. There were identified two other cases of anencephalic fetuses, which presented a rare c.236G>A missense variant of the same gene [19]. The same study also identified variants of aminomethyltransferase (AMT) and glycine decarboxylase (dehydrogenase) (GLDC) genes, which belong to the mitochondrial glycine cleavage system (GCS) [19].
Partitioning defective 3 homolog (PARD3) is a gene encoding a protein implied in cell polarity [23]. Mutations in PARD3 were also associated with increased risk of neural tube defects, six SNPs that constructed five haplotypes being identified in a study performed in a region of China [23]. All SNPs were identified in intronic areas, except rs118153230, which is a SNP located in exon 16 [23]. It encodes for a protein domain that interacts with atypical protein kinase C (aPKC), which is implied in epithelia-specific junctional structures [23]. This mutation leads to the substitution of serine with asparagine within the aPKC binding region [23].
Maternal polymorphisms in protein carboxyl methyltransferase 1 (PCMT1) gene might be a risk factor for anencephaly in Chinese population [21]. The rs4816 SNP might be implied in isolated anencephaly, but the study included a small number of patients [21].
Micro-ribonucleic acids (miRNAs) are small, single-stranded and non-coding RNA molecules, which are implied in messenger RNA activity, having the role of posttranscriptional regulators [27,28]. The miRNAs are implied in cell cycle regulation, apoptosis and cell differentiation and can potentially regulate numerous genes [27,28]. miR-22 targets genes, such as MTHFR, transcobalamin receptor (TCblR), transcobalamin 2 (TCN2), solute carrier family 19 member 1 (SLC19A1), methionine adenosyltransferase 2A (MAT2A) and methylenetetrahydrofolate dehydrogenase [nicotinamide adenine dinucleotide phosphate (NADP+) dependent] 2 (MTHFD2), are implied in processes that include folate and vitamin B12 [27]. In cells that are grown in low folate concentration, miR-22 was found significantly upregulated [27]. The miRNA is implied in the etiopathogenesis of anencephaly through mitogen-activated protein kinase (MAPK) signaling pathway [27]. There are differences between healthy fetal brain tissues and tissues from anencephalic fetuses in terms of down and up-regulation of miRNAs, considering that miRNAs are dynamically regulated during neurodevelopment [28]. In the tissue collected from the fetuses with anencephaly, miRNAs, miR-22, miR-23a, miR-34a, miR-103, miR-125a, miR-126, miR-132, miR-134, miR-138, miR-185, miR-198 and miR-451 were up-regulated and miR-9, miR-212, miR-124, miR-138, miR-103/107 and miR-149 were down-regulated [27,28]. Folate deficiency implies abnormal nucleotide synthesis and biological methylation, which might be involved in the etiopathogenesis of anencephaly [28].
⧉ Embryology
At the beginning of the third week, on day 18, the ectodermal germ layer is disc-shaped, with the cephalic region wider than the caudal region [10, 29]. The thickening of the overlying embryonic ectoderm and thus the formation of an elongated neural plate is induced by the notochord and prechordal mesoderm found below [10, 29,30]. This is a major inductive event taking place during gastrulation [10, 29]. Hence, the ectoderm will differentiate into the neural plate and peripheral surface ectoderm [29]. The process of neural induction is the first step of the neurulation, while the last step will be the closure of the caudal neuropore, by the end of the 4th week [10, 29,30]. Neurulation is the formation of the neural plate and the neural tube and begins in days 22–23, near the 4th to 6th pairs of somites [30]. The neural plate is a slipper-shaped structure found on the posterior aspect of the trilaminar embryo, that appears first in the cranial extremity and expands toward the primitive streak. It is the earliest rudiment of the CNS [10, 29,30]. Since day 19, the presence of indentations in the neural plate delimit the primordia of the three primary brain vesicles, namely the prosencephalon (or forebrain), the mesencephalon (of midbrain) and the rhombencephalon (or hindbrain) [10, 29]. The cranial two thirds of the neural plate and tube, starting from the cranial extremity to the 4th pair of somites, will form the future brain, while the caudal one third will form the future spinal cord [30].
Gradually, the neural plate folds up, such that by the end of the third week (approximately 18th day), its middle region will form a depression called neural groove, while its lateral edges will elevate and form the neural folds [10, 29,30]. The lateral edges of the neural plate in the cranial end of the embryo will become peculiar prominent [30]. After approaching each other, the neural folds will fuse in the midline, first in the cervical region, near the 5th somite, after which the fusion will proceed cranially and caudally [10, 30]. During the 4th week, at the end of this process, the neural tube will be formed and its lumen will become the neural canal [10, 29,30]. The neural tube will be separated from the surface ectoderm, which will differentiate into the epidermis [10, 29,30]. Between the neural tube and the future epidermis, neural crest cells will form the neural crest [30]. Before and immediately after the closure of the neural tube, the neuroepithelial cells which form the wall of the neural tube will divide and determine the occurrence of a pseudostratified columnar epithelium, whose cells are connected by junctional complexes, which will form the neuroepithelium (neuroectoderm). While the neuroprogenitor cells will proliferate, migrate, and differentiate to form the CNS components, the neural canal will form the ventricular system and the central canal of the spinal cord [30].
Via the rostral and caudal neuropores, the cephalic and caudal open ends of the neural tube communicate with the amniotic cavity. Closure of the cranial neuropore takes place at the 18- to 20-somite stage (i.e., approximately 25th day) and starts from the initial closure site in the cervical region, while the closure of the posterior neuro-pore takes place approximately two days later, at the 25-somite stage (i.e., 27th day). A vascular circulation of the neural tube will appear at the time of the closure of the neuropores [30].
The cephalic portion of the new formed tubular structure consists of the primary brain vesicles, while the caudal portion is narrower and forms the spinal cord [10, 29]. The primary brain vesicles are the prosencephalon (or forebrain), the mesencephalon (of midbrain) and the rhombencephalon (or hindbrain) [10, 29,30]. The prosencephalon divides into telencephalon and diencephalon, while the rhombencephalon divides into the metencephalon and myelencephalon, all processes taking place in the 5th week [29]. These four vesicles, along with the mesencephalon, will form five secondary brain vesicles [29]. The cephalic flexure will appear in the midbrain region, while the cervical flexure will form between the hindbrain and the spinal cord, both being ventrally directed, while at the pontine flexure, it will be dorsally directed [10, 29,30].
Birth defects of the CNS might appear when the process of neurulation is altered by different factors [30]. If the rostral neuropore of the neural tube fails to close during the 4th week, exencephaly will appear [10, 30]. This might appear because of disturbance in cell fates, cell adhesion and the mechanisms of neural tube closure [30].
Anencephaly is always associated with acrania and, as the calvaria is absent, the brain will not be protected and it will degenerate, but the brainstem will remain unaffected [10, 30]. More than that, the destructive process also appears because of the abnormal brain structure and vascularization, defined by formation of new blood vessels [30]. On macroscopic evaluation, the remaining part of the brain has a spongy aspect, forming a vascular mass composed almost of hindbrain structures [30].
Formation of neural tube defects might also imply other mechanisms, such as abnormal processes that occur in sites involved in neural tube formation – possibly five [30]. Anencephaly appears if site 2 fails to fuse, while spina bifida cystica results in site 1 closure abnormalities [30]. If sites 2, 4 and 1 fail to close, craniorachischisis will appear. The absence of site 3 fusion is a rare event [30].
⧉ Clinical and morphological features
Imaging diagnosis by US examination is one of the most important steps in detecting prenatal CNS anomalies. During the first trimester screening, between 11–13+6 weeks of gestation, in the second and third trimester, anencephaly can be detected in 100% of cases [31,32]. The current challenge in daily practice is early diagnosis when the complication rate of pregnancy termination is lower. Diagnosis of anencephaly presenting with acrania and varying degrees of cerebral degeneration can be established via transvaginal US. This requires extensive knowledge of all morphological features which are present during early fetal stage, before mechanical and chemical damage due to prolonged exposure to amniotic fluid affect the development of the encephalon.
Specific search for the sonographic markers for this condition is the key for a reliable diagnosis of the anencephaly at the routine 8–10 weeks US scan. The gestational age according to the crown–rump length is significantly reduced in all affected fetuses by an average of two weeks less than expected, due to the absence of the cranium and the degeneration of the brain (Figure 1). The sonographic and pathological findings of exencephaly is a variable amount of recognizable cerebral tissue, but in anencephaly the hemispheres are replaced by masses of connective and vascular tissue with spread islands of neural tissue, called area cerebrovasculosa or angiomatous stroma. In the coronal view of the head, the irregular masses above the fetal orbits floating into the amniotic fluid without cranial structures above were described as the ‘Mickey mouse’ sign [33].
Figure 1.
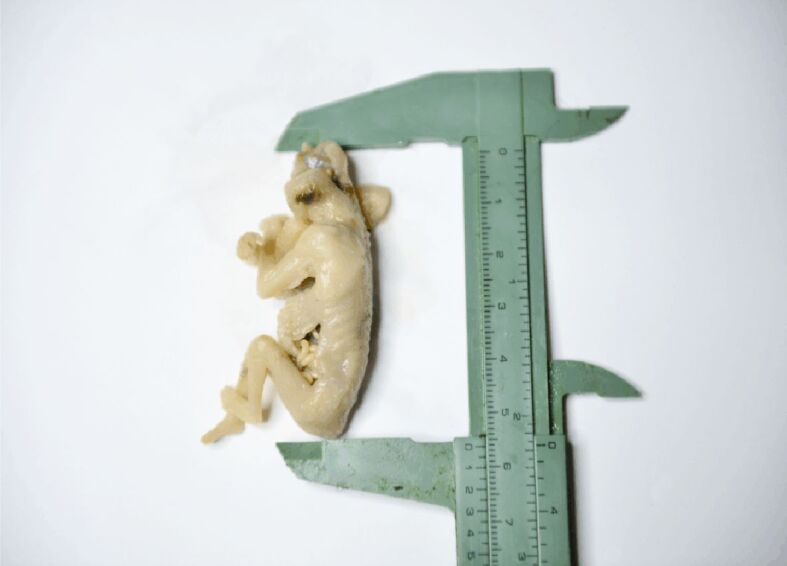
Demonstrating the significantly reduced crown-rump length in a 14-week-old anencephalic embryo – note that due to the absence of cranium and degeneration of brain the crown-rump length is reduced with two weeks than expected. (Collection of Department of Anatomy and Embryology, Carol Davila University of Medicine and Pharmacy)
The clinical correlation of the abnormal brain development is represented by the occurrence of hydramnios in the last two months of pregnancy, considering the failure of the mechanism for swallowing in the fetus and therefore another sonographic feature is polyhydramnios, present in up to 50% of cases, but it develops later in second or third trimester [10, 34].
Anencephaly is connected not only with abnormalities of the CNS, but also other organs and tissues. Associated malformations like cervical rachischisis (failure in fusion of neural arches), cleft palate, cardiac and pulmonary defects, club foot, overlapping fingers, rocker-bottom feet, hexadactyly and other musculoskeletal defects, adrenal and pulmonary hypoplasia, microphthalmia, enlarged pinnae, kidney agenesis and omphalocele are impossible to diagnose early in the first trimester, but single umbilical artery can be suspected as early as week 10. Ossification is visible from the 11th week of gestation and frontal, parietal and major portions of the occipital bones may often be affected [35].
Another US feature to be taken into consideration is represented by the presence of fibrous amniotic bands. ‘Turkish turban’ sign was described by Sepulveda et al. in a study based on transvaginal and three-dimensional (3D) US examination in the first trimester of gestation and represents the result of amniotic band that forms around the external base of the developing calvarium [36]. Kostadinov & Shapiro mentioned an unusual case of amniotic band sequence, in which the pregnancy was terminated in the 18th week and the fetus presented multiple congenital anomalies, including anencephaly due to the result of amniotic band [37].
Although some structural anomalies can be missed, based on the experience of the examiner, the quality of the US machines or maternal features, such as high BMI, anencephaly is an “always detectable” malformation, which means it is mandatory recognizable in the first trimester, as early as possible [38].
As stated above, second trimester ultrasonography detects anencephaly in 100% of cases. Corpus callosum and cerebellum are not sufficiently developed in the first trimester in order to complete the diagnosis, but they can be identified in the second trimester. Anencephaly means that there is a failed closure of the midbrain and forebrain, but there is normal fusion in the cerebellum, pons and medulla oblongata [39]. The “frog eye” sign can be recognized and it means that protruding orbital structures are associated with abnormal development of the cortex [33].
Macroscopic diagnosis can be made immediately post-partum, and the obstetrician can easily establish it using the following criteria: absence of calvarium and scalp, external presence of a hemorrhagic or fibrotic mass and absence of cerebral hemispheres (Figures 2,3,4) [40]. The macroscopic diagnosis can also be confirmed during autopsy, in terminated pregnancies, spontaneous abortions, intrauterine fetal death and liveborn infants. A comprehensive postmortem examination is important in order to confirm the US findings and to offer a more detailed diagnosis [39]. Hypothalamus is typically absent and malformations of cerebellum, brainstem, optic nerves, and spinal cord may be recognizable. The absence or underdevelopment of the pituitary leads to adrenal hypoplasia that is always present.
Figure 2.

The appearance of the viscerocranium of a 9-week-old anencephalic fetus (anterior view). (Collection of Department of Anatomy and Embryology, Carol Davila University of Medicine and Pharmacy)
Figure 3.
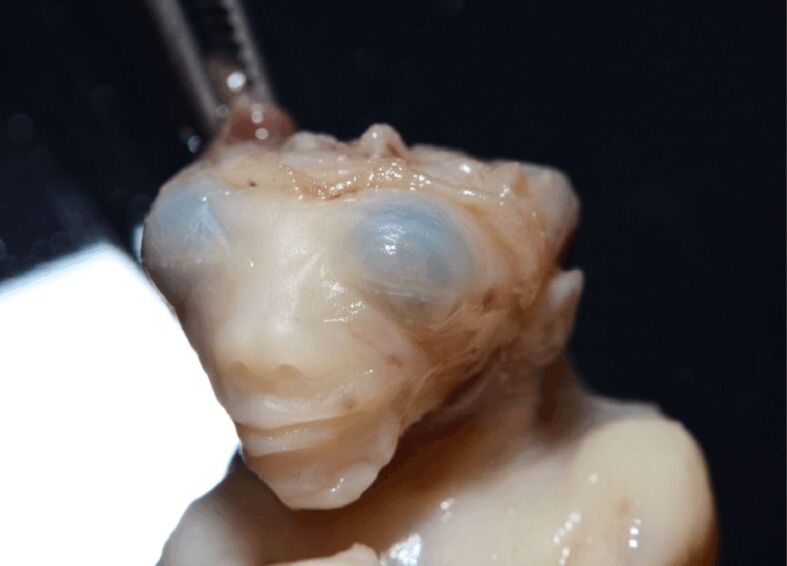
Detail of the cranium in a 9-week-old anencephalic fetus. Note that the cranial defect is not covered by skin. (Collection of Department of Anatomy and Embryology, Carol Davila University of Medicine and Pharmacy)
Figure 4.
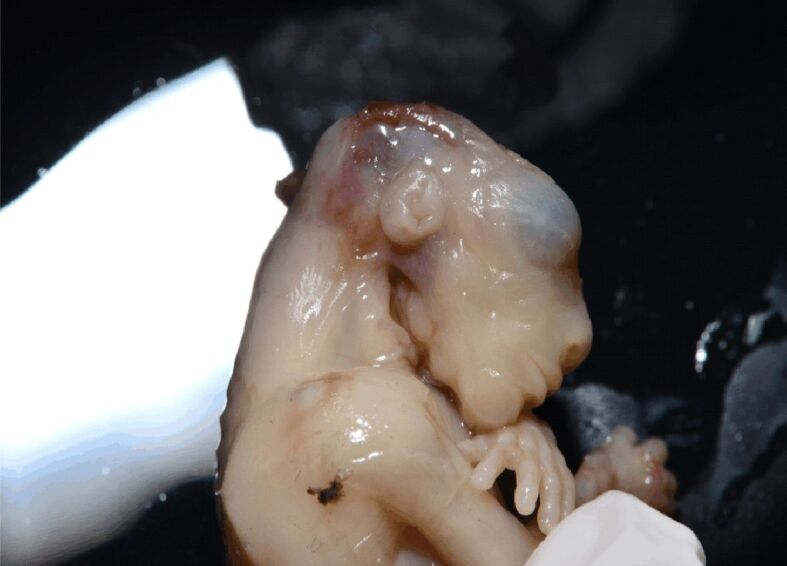
Lateral view of the cephalic extremity in a of a 9-week-old anencephalic fetus. Note the contrast between the normally developed viscerocranium and the absence of the neurocranium. (Collection of Department of Anatomy and Embryology, Carol Davila University of Medicine and Pharmacy)
During autopsy, external examination may reveal any of the following morphological alterations: complete or partial absence of the skull bones, ossification center development delay, orbital prominence and area cerebrovasculosa (section of abnormal hypervascular tissue admixed with neuroglial tissue and disorganized choroid plexuses located at the base of the skull). The skull base usually reveals a superficial sella turcica, underdeveloped hypophysis and hypoplastic brainstem. Examination of the ocular system may reveal that the optic nerves are underdeveloped, and that the retina features a reduced number of ganglion cells or none at all. The lungs may feature aspiration of brain tissue. Microscopic examination of the cephalic region reveals complete absence or abnormal hypothalamic tissue. Neurohypophysis may also be completely absent or present and abnormal. If present, adenohypophysis is usually small and composed of morphologically normal-looking cells. Electron microscopy and immunohistochemistry demonstrate reduced and degenerated corticotrophs.
A peculiar presentation of anencephaly is abnormal attachment of the placenta to the site of the skull defect. These infants may feature other associated malformations including low placement of ears, cleft lip, cleft palate or bilateral congenital talipes equinovarus [41].
It is currently accepted that growth and maturation of the placenta as well as other tissues is based on the transformation of an undifferentiated population of cells. Among those, most evolve to a population of largely differentiated, end-stage cells, that normally do not divide further. However, this process appears to be somewhat affected in the placenta associated with anencephaly. The implication of this observation is highly speculative, but it is that the intact fetus participates in the control of placental maturation. Batson et al. evaluated a series of 12 placentas from anencephalic infants and compared them with 12 control cases from normal pregnancies. They concluded that in all 12 cases, the placenta of anencephalic infants was strikingly less mature when viewed routinely in light microscopy and confirmed by morphometric analysis. This was indicated by the marked persistence of cytotrophoblast with occasional mitoses in the placenta of anencephalic infants [42].
⧉ Current state of knowledge
International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) stated that first trimester ultrasonography is recommended for gestational age, fetus viability and fetal gross anatomy. Therefore, fetal head with third ventricle, choroid plexus, thalamus, posterior fossa, and intracranial translucency should be always identified during the first trimester. The “gold standard” for fetal anatomy is the second trimester ultrasonography, between 18–23 weeks of gestation, but the first trimester is enough for early diagnosis of anencephaly. Santana et al. stated that transvaginal and abdominal US is recommended in improving diagnosis accuracy for different conditions. They also mentioned that 12–25% of the anencephalic fetuses had other structural anomalies associated [35].
Araujo Júnior et al. affirmed that cranial defect can be appreciated better in coronal imaging of the face, but it can also be evaluated in a sagittal plan. In coronal section, the calvaria above the orbit is absent, suggesting the diagnosis of anencephaly [43].
Gonçalves et al. stated that 3D US could identify the precise localization of normal anatomy and pathological structures through evaluation of three anatomical planes at the same time, although no studies proved that 3D US is superior to two-dimensional (2D) US for evaluation of brain anomalies [44].
The dysfunction of the pituitary–adrenal axis encountered in anencephalic pregnancies may alter the duration of the gestation. Thus, pregnancies can be either atypically long, or atypically short [45]. Some authors have also observed an atypical growth pattern of anencephalic newborns characterized by generalized growth restriction. This pattern is caused by hypoplastic adrenals, which secrete insufficient cortisol and manifests as diffuse atrophy of all organs, nonetheless spearing the lymphoid tissue, especially the thymus which may appear even relatively large [46]. Also, these fetuses may appear overweight due to the excessive subcutaneous adiposity caused by abnormal carbohydrate handling. This aspect is like that observed in decapitated rabbit fetuses which have abnormally high insulin levels and can be antagonized by exogenous adrenocorticotropic hormone (ACTH) [47].
A study from 2018 found that children born to women who ate sprouted potatoes four or more times a week while pregnant were more likely to have some health issues, like cleft palates and anencephaly. This effect seemed to be less likely with vitamin B adequacy [48].
Tica et al. stated that some conditions must be taken into consideration for the differential diagnosis, such as amniotic loop syndrome, big encephalocele, microcephaly and iniencephaly [49].
Edwards & Hui affirmed that fetal structural anomalies complicate 2–3% of all pregnancies and the identification of a major defect in first trimester leading to more management options than at a later discovery. They also stated that higher detection rate in the first trimester is mandatory for lethal anomalies, such as anencephaly [50]. Besides this study, various other studies claim that first trimester ultrasonography is truly an essential imaging method involved in the diagnosis of fetal anomalies [50, 51].
Definitive diagnosis during the first trimester will allow counseling of parents and termination of pregnancy [43, 52]. If termination of the pregnancy is not an option, there are some maternal outcomes that should be considered. Ekmekci & Gencdal affirmed that the complications associated with ongoing pregnancies are stillbirth, polyhydramnios, elective Caesarean delivery, redundant Caesarean deliveries, labor induction, shoulder dystocia and ante/postpartum hemorrhage [5].
For those women whom termination of pregnancy is not an option, it is important for the clinician to be informed about the mother’s risk factors, such as obesity, diabetes, hypertension, autoimmune disease and to provide the best method of delivery. Monitoring for the complications stated above is mandatory [53].
Termination of pregnancies in Japan is similar to those in North American countries or European countries and Kondo et al. mentioned in a study developed over two years (2014–2015) including more than 311 000 pregnant women, that half of the pregnancies with neural tube defects were terminated, 80% among those with anencephaly [54].
⧉ Conclusions
Anencephaly is the most frequent type of neural tube defect. The importance of first trimester US screening for this malformation is warranted by its high incidence among newborns and allows the clinician to establish the appropriate management. Although most cases of anencephaly are isolated defects of neural tube closure, we emphasize the importance of a complete fetal examination. Facial clefts which cannot be explained from a developmental perspective, or any other gross facial asymmetry could be related to early amnion rupture sequence.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Santana MVMC, Canêdo FMC, Vecchi AP. Anencephaly: knowledge and opinion of gynecologists, obstetricians and pediatricians in Goiânia. Rev Bioét. 2016;24(2):374–385. [Google Scholar]
- 2.Theofanakis C, Theodora M, Sindos M, Daskalakis G. Prenatal diagnosis of sirenomelia with anencephaly and craniorachischisis totalis: a case report study. Medicine (Baltimore) 2017;96(50):e9020–e9020. doi: 10.1097/MD.0000000000009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom (Kyoto) 2006;46(2):55–67. doi: 10.1111/j.1741-4520.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 4.Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, Lupo PJ, Riehle-Colarusso T, Cho SJ, Aggarwal D, Kirby RS, National Birth Defects Prevention Network National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111(18):1420–1435. doi: 10.1002/bdr2.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekmekci E, Gencdal S. What’s happening when the pregnancies are not terminated in case of anencephalic fetuses. J Clin Med Res. 2019;11(5):332–336. doi: 10.14740/jocmr3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CY, Honein MA, Dana Flanders W, Howards PP, Oakley GP, Rasmussen SA. Pregnancy termination following prenatal diagnosis of anencephaly or spina bifida: a systematic review of the literature. Birth Defect Res A Clin Mol Teratol. 2012;94(11):857–863. doi: 10.1002/bdra.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wertaschnigg D, Reddy M, Ramkrishna J, da Silva Costa F, Sepulveda W, Rolnik DL, Meagher S. Ultrasound appearances of the acrania-anencephaly sequence at 10 to 14 weeks’ gestation. J Ultrasound Med. 2020 doi: 10.1002/jum.15267. [DOI] [PubMed] [Google Scholar]
- 8.van Gool JD, Hirche H, Lax H, De Schaepdrijver L. Folic acid and primary prevention of neural tube defects: a review. Reprod Toxicol. 2018;80:73–84. doi: 10.1016/j.reprotox.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsdottir AB, Thorsteinsdottir SL, Hjartardottir H, Hardardottir H. Pre- and postnatal diagnosis of congenital central nervous system anomalies in Iceland in 1992–2016] Laeknabladid. 2019;105(12):547–553. doi: 10.17992/lbl.2019.12.260. [DOI] [PubMed] [Google Scholar]
- 10.Sadler TW. Langman’s medical embryology. 10. Madison County, Montana, USA: Lippincott Williams & Wilkins; 2011. pp. 65–79.pp. 433–468. [Google Scholar]
- 11.Barron S. Anencephaly: an ongoing investigation in Washington State. Am J Nurs. 2016;116(3):60–66. doi: 10.1097/01.NAJ.0000481286.16720.6e. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Garduño C, Lacasaña M, Blanco-Muñoz J, Borja-Aburto VH, García AM. Parental occupational exposure to organic solvents and anencephaly in Mexico. Occup Environ Med. 2010;67(1):32–37. doi: 10.1136/oem.2008.044743. [DOI] [PubMed] [Google Scholar]
- 13.Kashif T, Fathima N, Usman N, Qaseem A, Jayaraj JS. Women with epilepsy: anti-epileptic drugs and perinatal outcomes. Cureus. 2019;11(9):e5642–e5642. doi: 10.7759/cureus.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelizzari E, Valdez CM, Picetti JS, Cunha AC, Dietrich C, Fell PRK, Targa LV, Zen PRG, Rosa RFM. Characteristics of fetuses evaluated due to suspected anencephaly: a population-based cohort study in southern Brazil. Sao Paulo Med J. 2015;133(2):101–108. doi: 10.1590/1516-3180.2013.8012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaliwal LV, Desai RM. Methylenetetrahydrofolate reductase mutations, a genetic cause for familial recurrent neural tube defects. Indian J Hum Genet. 2012;18(1):122–124. doi: 10.4103/0971-6866.96680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agopian AJ, Tinker SC, Lupo PJ, Canfield MA, Mitchell LE. National Birth Defects Prevention Study. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res A Clin Mol Teratol. 2013;97(1):42–46. doi: 10.1002/bdra.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arth A, Kancherla V, Pachón H, Zimmerman S, Johnson Q, Oakley GP. A 2015 global update on folic acid-preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol. 2016;106(7):520–529. doi: 10.1002/bdra.23529. [DOI] [PubMed] [Google Scholar]
- 18.Gole RA, Meshram PM, Hattangdi SS. Anencephaly and its associated malformations. J Clin Diagn Res. 2014;8(9):AC07–AC09. doi: 10.7860/JCDR/2014/10402.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida M, Cullup T, Boustred C, James C, Docker J, English C, GOSGENE. Lench N, Copp AJ, Moore GE, Greene NDE, Stanier P. A targeted sequencing panel identifies rare damaging variants in multiple genes in the cranial neural tube defect, anencephaly. Clin Genet. 2018;93(4):870–879. doi: 10.1111/cge.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacasaña M, Blanco-Muñoz J, Borja-Aburto VH, Aguilar-Garduño C, Rodríguez-Barranco M, Sierra-Ramirez JA, Galaviz-Hernandez C, Gonzalez-Alzaga B, Garcia-Cavazos R. Effect on risk of anencephaly of gene-nutrient interactions between methylenetetrahydrofolate reductase C677T polymorphism and maternal folate, vitamin B12 and homocysteine profile. Public Health Nutr. 2012;15(8):1419–1428. doi: 10.1017/S136898001100334X. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Wang F, Wang J, Xie H, Guo J, Liu C, Wang L, Lu X, Bao Y, Wang G, Zhong R, Niu B, Zhang T. Maternal PCMT1 gene polymorphisms and the risk of neural tube defects in a Chinese population of Lvliang high-risk area. Gene. 2012;505(2):340–344. doi: 10.1016/j.gene.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Kumble Bhat V, Tiwari A, Kodaganur SG, Tontanahal SJ, Sarda A, Malini KV, Kumar A. A homozygous mutation in TRIM36 causes autosomal recessive anencephaly in an Indian family. Hum Mol Genet. 2017;26(6):1104–1114. doi: 10.1093/hmg/ddx020. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Chen X, Shangguan S, Bao Y, Lu X, Zou J, Guo J, Dai Y, Zhang T. Association study of PARD3 gene polymorphisms with neural tube defects in a Chinese Han population. Reprod Sci. 2012;19(7):764–771. doi: 10.1177/1933719111433886. [DOI] [PubMed] [Google Scholar]
- 24.Sadler TW. Langman’s medical embryology. 10. Madison County, Montana, USA: Lippincott Williams & Wilkins; 2011. pp. 285–315. [Google Scholar]
- 25.Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2012;94(10):824–840. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- 26.Sudiwala S, Palmer A, Massa V, Burns AJ, Dunlevy LPE, de Castro SCP, Savery D, Leung KY, Copp AJ, Greene NDE. Cellular mechanisms underlying Pax3-related neural tube defects and their prevention by folic acid. Dis Models Mech. 2019;12(11):dmm042234–dmm042234. doi: 10.1242/dmm.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WD, Yu X, Fu X, Huang S, Jin SJ, Ning Q, Luo XP. MicroRNAs function primarily in the pathogenesis of human anencephaly via the mitogen-activated protein kinase signaling pathway. Genet Mol Res. 2014;13(1):1015–1029. doi: 10.4238/2014.February.20.3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Chang H, Li Y, Zhang T, Zou J, Zheng X, Wu J. MicroRNAs: potential regulators involved in human anencephaly. Int J Biochem Cell Biol. 2010;42(2):367–374. doi: 10.1016/j.biocel.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Schoenwolf GC, Bleyl SB, Brauer PR, Francis PH. Larsen’s human embryology. 4. New York–Edinburgh: Churchill Livingstone; 2008. pp. 101–120. [Google Scholar]
- 30.Moore KL, Dalley AF II. Clinically oriented anatomy. 5. Philadelphia, USA: Lippincott Williams & Wilkins; 2006. pp. 51–57.pp. 379–411. [Google Scholar]
- 31.Krantz DA, Hallahan TW, Carmichael JB. Screening for open neural tube defects. Clin Lab Med. 2016;36(2):401–406. doi: 10.1016/j.cll.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Katorza E, Gat I, Duvdevani N, Meller N, Pardo N, Barzilay E, Achiron R. Fetal brain anomalies detection during the first trimester: expanding the scope of antenatal sonography. J Matern Fetal Neonatal Med. 2018;31(4):506–512. doi: 10.1080/14767058.2017.1289165. [DOI] [PubMed] [Google Scholar]
- 33.Hall JW, Denne N, Minardi JJ, Williams D, Balcik BJ. Check the head: emergency ultrasound diagnosis of fetal anencephaly. West J Emerg Med. 2016;17(4):460–463. doi: 10.5811/westjem.2016.5.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatzipapas IK, Whitlow BJ, Economides DL. The ‘Mickey Mouse’ sign and the diagnosis of anencephaly in early pregnancy. Ultrasound Obstet Gynecol. 1999;13(3):196–199. doi: 10.1046/j.1469-0705.1999.13030196.x. [DOI] [PubMed] [Google Scholar]
- 35.Santana EFM, Júnior EA, Tonni G, Costa FDS, Meagher S. Acrania–exencephaly–anencephaly sequence phenotypic characterization using two- and three-dimensional ultrasound between 11 and 13 weeks and 6 days of gestation. J Ultrason. 2018;18(74):240–246. doi: 10.15557/JoU.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sepulveda W, De La Maza F, Meagher S. An unusual first-trimester ultrasound presentation of the acrania–anencephaly sequence: the "Turkish turban" sign. J Ultrasound Med. 2020;39(4):829–832. doi: 10.1002/jum.15161. [DOI] [PubMed] [Google Scholar]
- 37.Kostadinov S, Shapiro S. Unusual case of amniotic band sequence. Int J Surg Pathol. 2017;25(5):430–430. doi: 10.1177/1066896916680073. [DOI] [PubMed] [Google Scholar]
- 38.Bardi F, Smith E, Kuilman M, Snijders RJM, Bilardo CM. Early detection of structural anomalies in a primary care setting in the Netherlands. Fetal Diag Ther. 2019;46(1):12–19. doi: 10.1159/000490723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Struksnæs C, Blaas HGK, Vogt C. Autopsy findings of central nervous system anomalies in intact fetuses following termination of pregnancy after prenatal ultrasound diagnosis. Pediatr Dev Pathol. 2019;22(6):546–557. doi: 10.1177/1093526619860385. [DOI] [PubMed] [Google Scholar]
- 40.Tafuri SM, Lui F. Embryology, anencephaly. StatPearls [Internet], StatPearls Publishing, Treasure Island, FL, USA, 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545244/ [PubMed] [Google Scholar]
- 41.David TJ, Nixon A. Congenital malformations associated with anencephaly and iniencephaly. J Med Genet. 1976;13(4):263–265. doi: 10.1136/jmg.13.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batson JL, Winn K, Dubin NH, Parmley TH. Placental immaturity associated with anencephaly. Obstet Gynecol. 1985;65(6):846–847. [PubMed] [Google Scholar]
- 43.Araujo Júnior E, Rolo LC, Tonni G, Haeri S, Ruano R. Assessment of fetal malformations in the first trimester of pregnancy by three-dimensional ultrasonography in the rendering mode. Pictorial essay. Med Ultrason. 2015;17(1):109–114. doi: 10.11152/mu.2013.2066.171.eaj. [DOI] [PubMed] [Google Scholar]
- 44.Gonçalves LF. Three-dimensional ultrasound of the fetus: how does it help. Pediatr Radiol. 2016;46(2):177–189. doi: 10.1007/s00247-015-3441-6. [DOI] [PubMed] [Google Scholar]
- 45.Naeye RL, Blanc WA. Organ and body growth in anencephaly. A quantitative, morphological study. Arch Pathol. 1971;91(2):140–147. [PubMed] [Google Scholar]
- 46.Hayek A, Driscol SG, Warshaw JB. Endocrine studies in anencephaly. J Clin Invest. 1973;52(7):1636–1641. doi: 10.1172/JCI107343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack PM, Milner RD. Effect of decapitation and ACTH on somatic development of the rabbit fetus. Biol Neonate. 1975;26(3–4):195–204. doi: 10.1159/000240730. [DOI] [PubMed] [Google Scholar]
- 48.Ni W, Tian T, Zhang L, Li Z, Wang L, Ren A. Maternal periconceptional consumption of sprouted potato and risks of neural tube defects and orofacial clefts. Nutr J. 2018;17(1):112–112. doi: 10.1186/s12937-018-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tica VI, Beghim M, Tica I, Zaher M, Beghim E. Anencephaly: pitfalls in pregnancy outcome and relevance of the prenatal exam. Rom J Morphol Embryol. 2009;50(2):295–297. [PubMed] [Google Scholar]
- 50.Edwards L, Hui L. First and second trimester screening for fetal structural anomalies. Semin Fetal Neonatal Med. 2018;23(2):102–111. doi: 10.1016/j.siny.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Munteanu O, Cîrstoiu MM, Filipoiu FM, Bohîlţea R, Bulescu IA, Berceanu C. Morphological and ultrasonographic study of fetuses with cervical hygroma. A case series. Rom J Morphol Embryol. 2016;57(4):1421–1427. [PubMed] [Google Scholar]
- 52.Bohîlţea RE, Tufan CF, Cîrstoiu MM, Dumitru AV, Georgescu TA, Sajin M, Bodean OM, Munteanu O, Brătilă E, Ofiţeru AM, Berceanu C. Body stalk anomaly in a monochorionic-diamniotic twin pregnancy – case report and review of the literature. Rom J Morphol Embryol. 2017;58(4):1453–1460. [PubMed] [Google Scholar]
- 53.Cooper S, Williams NS. Best mode of delivery for fetal life-limiting conditions. Obstet Gynecol. 2019;133(2):368–371. doi: 10.1097/AOG.0000000000003065. [DOI] [PubMed] [Google Scholar]
- 54.Kondo A, Akada S, Akiyama K, Arakawa M, Ichi S, Inamoto Y, Ishida T, Ishikawa H, Itoh T, Izumi A, Kimura F, Kondo AS, Matsuoka R, Miyauchi A, Mochizuki J, Momohara Y, Morikawa S, Morioka M, Morota N, Nakabe K, Obayashi S, Oku M, Samura O, Sasahara J, Sase M, Shimamoto K, Shimamura K, Sumigama S, Tada K, Takahashi H, Tani A, Wada S, Wada-HIraike O, Watanabe T, Yamaguchi M, Yasui T, Yokomine M. Real prevalence of neural tube defects in Japan: how many of such pregnancies have been terminated. Congenit Anom (Kyoto) 2019;59(4):118–124. doi: 10.1111/cga.12333. [DOI] [PubMed] [Google Scholar]


