Abstract
Purpose of review:
The purpose of this review is to discuss the most up to date research on endometriosis and chronic disease risk, highlighting the role treatments for endometriosis may play in these associations.
Recent findings:
Previous studies have shown a consistent association between endometriosis and risk for epithelial ovarian cancer but the association with other cancers is less clear. Current research indicates that endometriosis may in be associated with risk of systemic lupus erythematosus, and potentially other autoimmune diseases. Limited evidence is also present for the association between endometriosis and cardiovascular disease and related conditions (e.g,. hypertension, hypercholesterolemia). A potential explanation for a portion of the increased risk of chronic diseases among women with endometriosis may relate to treatments for endometriosis impacting these outcomes.
Summary:
Given the prevalence of endometriosis, understanding the relation between endometriosis and other chronic diseases has the potential to impact the health of many women. However, few high-quality studies with limited biases and adequate follow-up currently exist. Future multi-disciplinary research in prospective cohorts, with ample follow-up time, and detailed information on endometriosis characteristics and treatment is critical to advancing our understanding of this disease and its consequences.
Keywords: endometriosis, chronic disease, cancer, autoimmune disease, cardiovascular disease
Introduction
Endometriosis is a common, chronic, gynecologic condition characterized by the presence of endometrial-like tissue outside of the uterine cavity. It affects approximately 10% of reproductive age women resulting in significant health care costs and morbidity. Women with endometriosis may experience many different symptoms and facets of pain including chronic pelvic pain (CPP), dysmenorrhea, dyspareunia, dysuria, and dyschezia. In addition, a significant portion of women with endometriosis also suffer from infertility. These symptoms result in substantial individual and societal level costs.
There are several hypotheses to explain the pathophysiology of endometriosis. The two most accepted hypotheses for endometriosis development include (i) Sampson’s theory of retrograde menstruation, which proposes that implantation and growth of endometrial-like tissue on extra-uterine structures leads to the development of the disease, and (ii) that plaques, particularly those involving the ovaries, are generated by monoclonal tumors that arise from a somatic mutation of ovarian epithelium or pelvic peritoneal mesothelium. Pathways to initiation of coelomic metaplasia remain undetermined, although it is hypothesized that inflammation may play a key role.
Once endometriosis has developed, inflammation may contribute to scarring on peritoneal surfaces, which increases production of prostaglandins, metalloproteinases, cytokines, and chemokines. These inflammatory processes are thought to be responsible for fibrin deposition and adhesion formation. Inflammation, coupled with immune dysregulation, is also thought to contribute to the survival of endometrial tissue outside the uterus contributing to disease progression. Studies showing elevated inflammatory cytokines and dysregulation of mononuclear cells in the peritoneal fluid surrounding endometriotic lesions lend support to inflammation and aberrant immune function as important factors in endometriosis progression.
Recently, it has been recognized that women with endometriosis may be at higher risk for other chronic diseases. However, research in this area has been complicated by methodologic challenges. These challenges have been discussed in detail in previous reviews (1, 2) and include challenges with the endometriosis case definition, temporality and overlap of disease onset and diagnosis, impact of endometriosis treatment on risk for other chronic diseases, confounding from shared risk factors, and propensity for biases (e.g., detection bias, recall bias). In this review, we will discuss the most up to date research on endometriosis and chronic disease risk. We will discuss how the underlying pathophysiology of endometriosis may influence these associations and we will highlight the role treatments for endometriosis may play in these associations. While there is no universal cure for women with endometriosis, many of the commonly utilized treatment options (e.g. hormonal medications, non-steroidal anti-inflammatory drugs (NSAIDs), hysterectomy, oophorectomy) may alter risk of chronic diseases later in life.
Mechanisms of association
There are several potential pathways of the association between endometriosis and chronic disease which vary by disease state (Figure 1), including altered milieu/aberrant environment, shared risk factors, and treatments for endometriosis.
Figure 1.
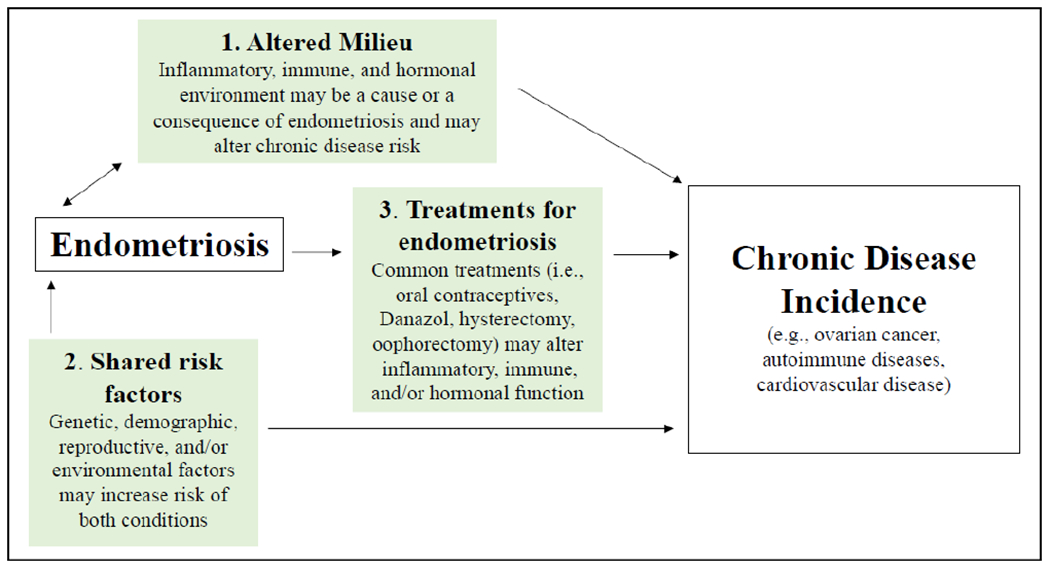
Potential pathways of association between endometriosis and chronic diseases
Aberrant environment/altered milieu
Inflammation:
Women with endometriosis have a hyper-inflammatory milieu both locally (in the peritoneal cavity) and systemically. Several inflammatory markers are elevated in women with endometriosis, such as intracellular adhesion molecule 1 (ICAM-1), C-reactive protein (CRP), interleukin-1 and 6 (IL-1 and IL-6), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF). Elevation of these markers may influence the increased risk of CVD among women with endometriosis. In addition, inflammation has been suggested to play a role in ovarian cancer carcinogenesis, including as a mechanism for the association with endometriosis.
Altered hormonal environment:
Certain hormonal alterations, particularly a hyperestrogenic environment, are associated with the development of endometriotic tissue. Endometriosis plaques have estrogen, progesterone, and androgen receptors and grow in the presence of estrogen, but atrophy when exposed to androgens. This altered environment may place women with endometriosis at risk for hormonally driven cancers. Treatments for endometriosis, that reduce estrogen levels, could reduce risk of some cancer types, while progesterone dominant treatments may decrease endometrial cancer risk, while potentially increasing breast cancer risk (3).
Immune Response:
Although the etiology of endometriosis remains unclear, a leading hypothesis is that aberrant immune system surveillance of tissue fragments from retrograde menstruation promotes the implantation of endometriotic lesions. Thus, endometriosis may be a signal of immune dysregulation that is detectable early in life. Women with endometriosis have been found to have decreased NK cell cytotoxicity and increased macrophage numbers and activation. Macrophages, lymphocytes, and pro-inflammatory proteins in the peritoneal space have been associated with endometriosis lesion survival (4–6). Compared to women without endometriosis, women with endometriosis have depressed cell-mediated immunity but increased humoral immune response (7–9) — abnormalities that may contribute to risk of multiple autoimmune conditions.
Shared risk factors
It is important to recognize that when researching associations between comorbid conditions, a thorough understanding of overlapping risk factors shared between the two conditions is crucial. For example, early age at first menstrual period, short menstrual cycle length, and nulliparity are risk factors for both endometriosis and for some chronic disease endpoints (e.g. ovarian cancer, breast cancer). Thus, without the appropriate study design and statistical confounding strategy, observed associations could be driven by outside factors that increase risk of both disease states.
Cancer
While endometriosis lesions are considered non-malignant, a great deal of research has investigated the relationship between endometriosis and risk for malignancies. The strongest and most consistent association has been between endometriosis and risk for epithelial ovarian cancer. While the association between endometriosis and risk of other cancers is less well-studied, with less robust and primarily unclear results (10).
Ovarian cancer
Ovarian carcinomas are the 17th most common cancer in U.S. women, resulting in lifetime risk of ovarian cancer of 1.3% for a woman in the general population.(11) The term ovarian cancer often encompasses cancers of ovarian, tubal, and peritoneal origin, of which there are five major histotypes. High-grade serous is the most common, accounting for approximately 70-74% of cases, followed by clear cell (10-26%), endometrioid (7-24%), mucinous (2-6%), and low-grade serous (3-5%) (12). It has recently been recognized that most high-grade serous tumors likely originate from fallopian tube epithelial cells, while endometrioid and clear cell tumors show a strong association with endometriosis. Women with endometriosis have consistently been shown to be at an increased risk of the endometrioid and clear cell histotypes, with more recent consortia analyses also reporting increased risk for low-grade serous (13, 14). In an international pooled analysis of cohort studies, the Ovarian Cancer Cohort Consortium (OC3) reported hazard ratios (HR) of 2.32 (95% confidence interval [CI]=1.36-3.95) and 2.87 (95% CI=1.53-5.39) for the associations between endometriosis and the endometrioid and clear cell histotypes, respectively. In addition, the OC3 also reported an increased risk of well-differentiated serous carcinoma (i.e. low-grade serous) (HR=3.77 [95% CI=1.24-11.48])(14). Consistent with these results, the Ovarian Cancer Association Consortium (OCAC), combined 13 case-control studies of ovarian cancer and observed increased risks with the endometrioid (odds ratio [OR]=2.04 [95% CI=1.67-2.48]), clear cell (OR=3.05 [95% CI=2.43-3.84]), and low grade serous (OR=2.11 [95% CI=1.39-3.20]) histotypes (13). While these are robust epidemiologic associations, the absolute risk of ovarian cancer among women with endometriosis is still quite low, with a lifetime risk of approximately 1.8%, slightly higher than the general population.
In addition to differences in risk by ovarian cancer histotypes, prior research has also suggested that there is heterogeneity in the relationship by endometriosis lesion type (15). While ovarian endometriosis (e.g., endometrioma) is the primary type of endometriosis lesion associated with ovarian cancer, peritoneal endometriosis has also been associated with ovarian cancer risk (16). A Finnish registry based reported a standardized incidence ratio (SIR)=4.72 (95% CI=2.75-7.56) for the association between ovarian endometriosis and risk of the endometrioid histotype, and an SIR of 2.03 (95% CI=1.05-3.54) for peritoneal endometriosis with a similar pattern observed for the clear cell estimates. In addition, cancer driver mutations have been recently identified in some, but not all, deep infiltrating endometriosis lesions, however, these type of lesions are not thought to transform into ovarian cancer (17). Thus, there is currently little evidence to help identify which endometriosis lesions will progress to ovarian cancer. Further, there has been great interest in understanding the role of excision surgery for women with endometriosis and ovarian cancer risk. Research has suggested that excision of ovarian endometriomas does not always reduce future development of endometriosis-associated ovarian cancer (18, 19), while the complete removal of endometriosis tissue in women without involvement of the ovaries has been demonstrated to reduce risk of ovarian cancer (20). Varying mechanisms have been proposed to explain these observations and have been discussed in detail in previous reviews (21, 22). One hypothesis is that excision of endometriomas may not remove all cells as some become trapped in ovarian follicles and then accumulating exposure to inflammation due to repeated ovulatory cycles (the “incessant ovulation” hypothesis described below) (21–24) ultimately can result in ovarian cancer.
It is important to note that as the absolute risk of ovarian cancer among women with endometriosis is low (~1.8%) (25), the risk of ovarian cancer must be balanced with the risk of adverse outcomes from endometriosis surgery. Beyond surgery, endometriosis treatments have the potential to impact ovarian cancer risk. However, research on epidemiologic factors, including treatments, that could predict the endometriosis to ovarian cancer transition has been sparse. Oral contraceptive use (a first-line medical therapy for endometriosis), has demonstrated the strongest reduction in ovarian cancer risk among women with endometriosis (26). The mechanism behind this reduction in risk is not clear. It may occur through reducing lifetime number of ovulatory cycles (“incessant ovulation hypothesis” of ovarian cancer development)(23, 24) and/or through reduction in endometrial thickness which would in turn decrease retrograde menstruation; more research is needed in this area.
Skin Cancer
Skin cancer is the most common type of cancer globally (27, 28). Cutaneous melanoma has a low incidence, but higher metastatic potential, while non-melanoma skin cancers (NMSCs) including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are more common. Prior research on endometriosis has suggested an association between phenotypic traits that are associated with sensitivity to sun exposure (red hair, freckling, moles, propensity to sunburn) and endometriosis incidence. Additionally, there is increasing evidence that melanoma may be influenced by hormonal exposures. Thus, researchers have been interested in investigating the association between endometriosis and risk of skin cancer. A recent meta-analysis of nine studies investigating the association between endometriosis and risk of melanoma indicated a 15% increased risk of melanoma (SRR [standardized rate ratio]=1.15 (95% CI=0.97-1.36)) (10). However, this finding did not meet the threshold of statistical significance. Far fewer studies have examined the association with endometriosis and risk of non-melanoma skin cancers (10, 29–33). The most recent study, based on Finnish registry data, reported an increased risk of basal cell carcinoma (BCC) with a standardized incidence ratio (SIR) of 1.18 (95% CI=1.10-1.25) but no association with other non-melanoma skin cancers (SIR=1.08 [95% CI=0.83-1.38]) (32). A similar effect estimate for BCC was observed by Farland, et al., (HR=1.16 [95% CI=0.91-1.48]), although the results were not statistically significant. In addition, Farland, et al. observed a stronger association between endometriosis and BCC among women who had never used premenopausal progestogens, a treatment for endometriosis symptoms. The authors hypothesized that this could indicate that endometriosis stage or severity could modify BCC risk (30), however, additional research is needed to confirm these findings.
The pathophysiology supporting a relationship between endometriosis and skin cancer is not confirmed and likely differs between melanoma and non-melanoma skin cancers. It has been suggested that hormonal factors (i.e. age at menarche, menstrual regularity, oral contraceptives, hormone replacement therapy), may be associated with risk of melanoma (34–36), thus a hormonal pathway may be implicated for both endometriosis and melanoma risk. Additionally shared environmental (e.g. sun exposure, chemical exposure) and genetic factors (e.g. red hair (37–40), freckling (41, 42), number of naevi (40, 41, 43, 44), skin sensitivity to sun exposure (41, 42, 44, 45), and eye color (42, 46)) may increase risk of both conditions. Research related to endometriosis and skin cancer should be replicated and future research should emphasize heterogeneity in endometriosis diagnosis and skin cancer (e.g. location of neoplasm, age at onset).
Breast Cancer
Multiple studies have examined the association between endometriosis and breast cancer risk with mixed results, with studies reporting increased, decreased, and null associations (10, 47). In a 2018 meta-analysis Gandini et al., calculated a SRR of 1.04 (95% CI=0.99-1.09) for the association between endometriosis and risk of breast cancer among 18 studies; however, the between study heterogeneity was high (I2=59%). Multiple hormonal hypotheses have been put forth to support an association between endometriosis and breast cancer, as there are many overlapping risk factors (e.g. earlier age at menarche, shorter menstrual cycle length, leaner body size, nulliparity). Additionally, earlier age at menopause, that may be induced by oophorectomy, may increase breast cancer risk. While most cases of breast cancer are not of a genetic origin, carriers of BRCA1/2 (breast cancer susceptibility genes 1 and 2) mutations are estimated to have a 60-80% lifetime risk of developing breast cancer. While the research related to BRCA and infertility have been conflicting (48), there has been no evidence of an association between endometriosis and BRCA 1/2 mutations. Progesterone-dominant treatments for endometriosis may also contribute to an increased risk of breast cancer.
Breast cancer is increasingly being understood as a heterogeneous disease. Therefore, an important limitation of previous work has been the examination of all breast cancer cases together, despite the recognition that risk factors and biology may different between different breast cancer subtypes (e.g. hormone receptor status, menopausal status at cancer onset, histologic type) (49, 50). Future work examining the association by tumor hormone receptor status, molecular subtype, and menopausal status may help clarify these mixed findings.
Endometrial cancer
At least sixteen studies have examined the association between endometriosis and endometrial cancer. A meta-analysis performed by Gandini et al., reported a SRR of 1.38 (95% CI=1.10-1.74) for the association with endometrial cancer, however large between study heterogeneity was reported (I2=79%) thus this summary result should be interpreted with caution (10). Many of the case-control and cross-sectional studies (51–55) included in this meta-analysis reported that women with endometriosis were at an increased risk of endometrial cancer, while the cohort studies (16, 29, 56–59) tended to report null or modest positive associations that did not reach the threshold of statistical significance. This pattern may suggest that the non-prospective studies on this topic may have a higher potential for recall bias which would over-estimate associations. With respect to endometriosis treatment, higher hysterectomy rates and utilization of progesterone-dominant treatments among women with endometriosis could impact this association if this potential mediator was not properly accounted for in the analysis.
Autoimmune diseases
Immunologic abnormalities have been previously reported among women with endometriosis suggesting an aberrant immunologic response may play a role in its pathogenesis (4). Many autoimmune diseases (e.g., rheumatoid arthritis [RA], systemic lupus erythematosus [SLE]), are more common in women (60), consequently sex-specific hormones likely play a role in their etiology (60, 61). In addition, most autoimmune diseases have a strong genetic component with an estimated heritability of 66% for SLE and 66-68% for RA (62) while endometriosis is estimated to have a heritability of approximately 52% (63). However, to our knowledge, no studies have examined the shared genetics of these conditions.
In regards to observational studies, A survey of a national endometriosis patient group indicated that women with endometriosis also experienced a variety of comorbid autoimmune conditions (64, 65). Recently, a systematic review and meta-analysis examined the current literature in regards to the association between endometriosis and autoimmune diseases (SLE, Sjogren’s syndrome [SS], RA, autoimmune thyroid disorder, celiac disease [CD], multiple sclerosis [MS], inflammatory bowel disease [IBD], and Addison’s disease) in population-based studies (66). Of the 26 studies included in the review, the quality of evidence based on the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) criteria (67) was judged as “Low or Very Low” due to issues with study design (i.e. cross-sectional), risk of bias, unrepresentative study populations, and inadequate adjustment for confounding. Given this low quality of evidence in many studies, our evaluation of the autoimmune disease literature will focus on articles with only limited methodologic issues.
In the recent meta-analysis conducted by Shigesi, et al., three studies of SLE were evaluated as high quality (66). The effect estimates (95% confidence intervals [CI]) for a higher risk of SLE among women with endometriosis were 1.4 (95% CI 1.1-1.8) (68), 1.6 (95% CI=1.2-2.1) (69), and 2.0 (95% CI=1.2-3.5) (70). Similar associations were seen in the two high quality studies (70, 71) of RA, however the summary effect estimate was not statistically significant (1.5 [95% CI=0.7-3.0]) (66). High-quality studies that have been published on the association between endometriosis and other autoimmune conditions have been extremely limited; however, Shigesi et al., concluded that associations with CD, MS, and IBD were present in at least one high quality study.
In the context of the sparse published literature is it difficult to disentangle whether autoimmune diseases and endometriosis are associated primarily due to a common influence of immunological abnormalities and hormonal factors, or whether a portion of the increased risk of autoimmune diseases among women with endometriosis may relate to treatments for endometriosis impacting these outcomes (1). In an analysis in the Nurses’ Health Study II (NHSII), a prospective cohort study of over 116,000 women, adjustment for hysterectomy and oophorectomy attenuated the observed associations between endometriosis and both SLE and RA (70). Twenty three percent and 22% of the association between endometriosis and SLE were attributable to hysterectomy and oophorectomy, respectively; the corresponding estimates for RA were 35% and 43%. Hysterectomy is sometimes used to alleviate symptoms associated with endometriosis, and this may increase autoimmune disease risk. Supporting this, surgical menopause has been associated with risk of SLE and RA (72, 73). Further, in analyses using a case-control design with Swedish registry data, the association between endometriosis and SLE was strongest when restricted to endometriosis diagnosed at the same time as hysterectomy (OR=2.26 [95% CI=1.47-3.64]) (68), which may point to at least a partial role for hysterectomy in modifying risk of SLE among women with endometriosis.
Cardiovascular Disease
Women with endometriosis have been shown in prior research to have a higher risk of hypertension (74), hypercholesterolemia (74–77), and subclinical atherosclerosis (78), suggesting an adverse vascular profile (79, 80). A recent meta-analysis (81) highlighted prior research that has investigated the relationship between endometriosis and dyslipidemia (75, 76, 82–87). While there have been differences across studies in parameters measured and timing of those measurements (e.g. before vs. after endometriosis surgery), two case-control studies found that women with endometriosis had higher levels of low-density lipoprotein (LDL) (75, 76). LDL is sometimes referred to as “bad cholesterol” and can lead to cholesterol build-up in arteries. These case-control findings support the recent finding of the only prospective cohort study to investigate whether women with endometriosis were at increased risk for experiencing hypercholesterolemia. The NHSII found that women with endometriosis were at a 25% increased risk of developing (95% CI=21-30) hypercholesterolemia later in life (74). The risk was strongest among younger women (≤ 40) (p-value interaction=<0.001). Similarly, the Japan Nurses’ Health Study (n=49,927), using cross-sectional data, found that women with endometriosis were at a 30% increased odds of prevalent hypercholesterolemia (95% CI=15-47), although they were unable to adjust for any confounding factors (54).
Both the Japan Nurses’ Health study and the NHSII have also found an increased risk of hypertension for women with endometriosis. The NHSII found that women with endometriosis had a 14% higher risk of developing hypertension (95% CI=9-18]) compared to women without endometriosis, with the greatest risk among younger women (P-value:<0.0001)(74). The Japan Nurses’ Health Study found also reported an increased risk among women with endometriosis (odds ratio [OR]=1.26 [95% CI: 1.07-1,47])(54).
Risk of early-onset hypertension and hypercholesterolemia is concerning because it may increase risk for cardiovascular disease later in life. Recent research from the NHSII has suggested that women with endometriosis had a 62% greater risk of coronary heart disease overall (95% CI=39-89) (88). They also reported a greater risk of myocardial infarction (HR [hazard ratio]=1.52 [95% CI=1.17-1.98]), angiographically confirmed angina (HR=1.91 [95% CI=1.59-2.29]), and coronary artery bypass graft surgery/coronary angioplasty procedure/stent (HR=1.35 [95% CI=1.08-1,69)(89). The Japan Nurses’ Health Study supported these finding and reported a two-fold risk of transient ischemic attack (HR=1.91 [95% CI=1.26-2.90]) and cerebral infarction (HR=2.10 [95% CI=1.15-3.85]) for women with endometriosis compared to women without endometriosis (54).
There are many hypothesized mechanisms between endometriosis and cardiovascular disease, including overlapping pathophysiology and risk factors, as well as treatments for endometriosis modifying risk. As discussed previously, women with endometriosis may also have higher levels of inflammation, and inflammation is an important risk factor of cardiovascular disease risk. It has been suggested that treatments for endometriosis may impact risk of CVD. For example, it is well established that early age at menopause (induced by oophorectomy) can substantially modify cardiometabolic disease onset (90, 91). Meta-analyses have found a 40% greater risk of CVD (92), a 23% greater risk for stroke, and a 20% greater risk of CVD mortality with early age of menopause (93). In the NHSII, they reported that the relationship between endometriosis and CVD was significantly mediated by endometriosis treatment. Of the increased risk for coronary heart disease among women with endometriosis, 42% (95% CI=25-60) could be attributed to hysterectomy/oophorectomy and age at surgery, 31% (95% CI=17-44) could be attributed to postmenopausal hormone use and duration, and 55% (33-77%) could be attributed to all treatment factors (i.e. hysterectomy/oophorectomy/age at surgery, postmenopausal hormone use and duration, and analgesic use)(88). Thus, surgical treatments that are associated with endometriosis, especially hysterectomy and oophorectomy, may have an important role in understanding the association between endometriosis and cardiovascular disease risk. Moreover, hormonal medications may directly and indirectly (e.g. through weight gain, modifying lipid profile) influence CVD risk. Progestin dominant therapies and synthetic androgens (e.g. Danazol) have been found to adversely affect women’s lipid profile (94–99), which may increase risk of cardiovascular disease. However, prior research on CVD risk has not been able to account for the role of these treatment types (74).While, obesity is an established risk factor for cardiovascular disease, the relationship with endometriosis is complex, as most research suggests that obesity and large body size are associated with a reduced risk of endometriosis development (100, 101). However, hormonal treatments for endometriosis have been shown to influence weight gain (97, 102, 103). Clinicians and patients considering these therapies may benefit from counseling around potential cardiovascular disease risk.
Conclusions
Given the prevalence of endometriosis, understanding the relation between endometriosis and other chronic diseases has the potential to impact the health of many women. However, few high-quality studies with limited biases and adequate follow-up currently exist. Thus, our ability to fully understand the underlying mechanisms of the relationship between endometriosis and chronic diseases is limited. Future multi-disciplinary research in prospective cohorts, with ample follow-up time, and detailed information on endometriosis characteristics and treatment is critical to advancing our understanding of this disease and its consequences.
Acknowledgments
Support: HRH is supported by the National Cancer Institute, National Institutes of Health (K22 CA193860).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest: Dr. Farland reports personal fees from Merck & Co., outside the submitted work. Dr. Harris has nothing to disclose.
References
- 1.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21(4):500–16. Epub 2015/03/15. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.**.Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA. Risk for and consequences of endometriosis: A critical epidemiologic review. Best practice & research Clinical obstetrics & gynaecology. 2018;51:1–15. Epub 2018/07/19. doi: 10.1016/j.bpobgyn.2018.06.001. [DOI] [PubMed] [Google Scholar]; This article addresses the critical epidemiologic methodologic complexities in endometriosis research.
- 3.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine reviews. 2013;34(1):130–62. Epub 2013/01/11. doi: 10.1210/er.2012-1043.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 5.Podgaec S, Dias Junior JA, Chapron C, Oliveira RM, Baracat EC, Abrao MS. Th1 and Th2 ummune responses related to pelvic endometriosis. Revista da Associacao Medica Brasileira (1992). 2010;56(1):92–8. Epub 2010/03/27. [DOI] [PubMed] [Google Scholar]
- 6.Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Frontiers in immunology. 2013;4:9 Epub 2013/02/02. doi: 10.3389/fimmu.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nothnick WB. Treating endometriosis as an autoimmune disease. Fertil Steril. 2001;76(2):223–31. Epub 2001/07/31. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmunity reviews. 2012;11(11):806–14. Epub 2012/02/15. doi: 10.1016/j.autrev.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Harris HR, Costenbader KH, Mu F, Kvaskoff M, Malspeis S, Karlson EW, Missmer SA. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann Rheum Dis. 2016;75(7):1279–84. Epub 2015/08/05. doi: 10.1136/annrheumdis-2015-207704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandini S, Lazzeroni M, Peccatori FA, Bendinelli B, Saieva C, Palli D, Masala G, Caini S. The risk of extra-ovarian malignancies among women with endometriosis: A systematic literature review and meta-analysis. Crit Rev Oncol Hematol. 2019;134:72–81. Epub 2019/02/18. doi: 10.1016/j.critrevonc.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Institute NC. SEER cancer statistics review (CSR). 2017.
- 12.Research CotSotSiOC. Summary. Ovarian Cancers Evolving Paradigms in Research and Care. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 13.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–94. Epub 2012/03/01. doi: 10.1016/s1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, Setiawan VW, Visvanathan K, Weiderpass E, Adami HO, Black A, Bernstein L, Brinton LA, Buring J, Butler LM, Chamosa S, Clendenen TV, Dossus L, Fortner R, Gapstur SM, Gaudet MM, Gram IT, Hartge P, Hoffman-Bolton J, Idahl A, Jones M, Kaaks R, Kirsh V, Koh WP, Lacey JV Jr., Lee IM, Lundin E, Merritt MA, Onland-Moret NC, Peters U, Poynter JN, Rinaldi S, Robien K, Rohan T, Sandler DP, Schairer C, Schouten LJ, Sjoholm LK, Sieri S, Swerdlow A, Tjonneland A, Travis R, Trichopoulou A, van den Brandt PA, Wilkens L, Wolk A, Yang HP, Zeleniuch-Jacquotte A, Tworoger SS. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888–98. Epub 2016/06/22. doi: 10.1200/jco.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. The American journal of pathology. 2016;186(4):733–47. Epub 2016/03/26. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saavalainen L, Lassus H, But A, Tiitinen A, Harkki P, Gissler M, Pukkala E, Heikinheimo O. Risk of Gynecologic Cancer According to the Type of Endometriosis. Obstet Gynecol. 2018;131(6):1095–102. Epub 2018/05/10. doi: 10.1097/aog.0000000000002624 [DOI] [PubMed] [Google Scholar]
- 17.Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM. Cancer-Associated Mutations in Endometriosis without Cancer. N Engl J Med. 2017;376(19):1835–48. Epub 2017/05/11. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang WH, Wang KC, Lee WL, Huang N, Chou YJ, Feng RC, Yen MS, Huang BS, Guo CY, Wang PH. Endometriosis and the subsequent risk of epithelial ovarian cancer. Taiwan J Obstet Gynecol. 2014;53(4):530–5. Epub 2014/12/17. doi: 10.1016/j.tjog.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Haraguchi H, Koga K, Takamura M, Makabe T, Sue F, Miyashita M, Urata Y, Izumi G, Harada M, Hirata T, Hirota Y, Wada-Hiraike O, Oda K, Kawana K, Fujii T, Osuga Y. Development of ovarian cancer after excision of endometrioma. Fertil Steril. 2016;106(6):1432–7.e2. Epub 2016/08/21. doi: 10.1016/j.fertnstert.2016.07.1077. [DOI] [PubMed] [Google Scholar]
- 20.Melin AS, Lundholm C, Malki N, Swahn ML, Sparen P, Bergqvist A. Hormonal and surgical treatments for endometriosis and risk of epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2013;92(5):546–54. Epub 2013/04/09. doi: 10.1111/aogs.12123. [DOI] [PubMed] [Google Scholar]
- 21.Bulun SE, Wan Y, Matei D. Epithelial Mutations in Endometriosis: Link to Ovarian Cancer. Endocrinology. 2019;160(3):626–38. Epub 2019/01/19. doi: 10.1210/en.2018-00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herreros-Villanueva M, Chen CC, Tsai EM, Er TK. Endometriosis-associated ovarian cancer: What have we learned so far? Clin Chim Acta. 2019;493:63–72. Epub 2019/02/19. doi: 10.1016/j.cca.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet (London, England). 1971;2(7716):163 Epub 1971/07/17. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 24.Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts Views Vis Obgyn. 2013;5(4):292–7. Epub 2013/01/01. [PMC free article] [PubMed] [Google Scholar]
- 25.Kvaskoff M, Horne AW, Missmer SA. Informing women with endometriosis about ovarian cancer risk. Lancet (London, England). 2017;390(10111):2433–4. Epub 2017/12/07. doi: 10.1016/s0140-6736(17)33049-0. [DOI] [PubMed] [Google Scholar]
- 26.Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am J Obstet Gynecol. 2004;191(3):733–40. Epub 2004/10/07. doi: 10.1016/j.ajog.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 27.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279–82. Epub 2010/03/17. doi: 146/3/279 [pii] 10.1001/archdermatol.2010.4 [doi]. [DOI] [PubMed] [Google Scholar]
- 28.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–6. Epub 2015/05/01. doi: 2281227 [pii] 10.1001/jamadermatol.2015.1187 [doi]. [DOI] [PubMed] [Google Scholar]
- 29.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176(3):572–9. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 30.Farland LV, Lorrain S, Missmer SA, Dartois L, Cervenka I, Savoye I, Mesrine S, Boutron-Ruault MC, Kvaskoff M. Endometriosis and the risk of skin cancer: a prospective cohort study. Cancer Causes Control. 2017;28(10):1011–9. Epub 2017/08/12. doi: 10.1007/s10552-017-0939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melin A, Sparen P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21(5):1237–42. doi: 10.1093/humrep/dei462. [DOI] [PubMed] [Google Scholar]
- 32.Saavalainen L, Lassus H, But A, Tiitinen A, Harkki P, Gissler M, Heikinheimo O, Pukkala E. A Nationwide Cohort Study on the risk of non-gynecological cancers in women with surgically verified endometriosis. Int J Cancer. 2018;143(11):2725–31. Epub 2018/07/08. doi: 10.1002/ijc.31721. [DOI] [PubMed] [Google Scholar]
- 33.Wyshak G, Frisch RE, Albright NL, Albright TE, Schiff I. Reproductive factors and melanoma of the skin among women. Int J Dermatol. 1989;28(8):527–30. Epub 1989/10/01. [DOI] [PubMed] [Google Scholar]
- 34.Kvaskoff M, Bijon A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Cutaneous melanoma and endogenous hormonal factors: a large French prospective study. Am J Epidemiol. 2011;173(10):1192–202. doi: 10.1093/aje/kwq503. [DOI] [PubMed] [Google Scholar]
- 35.Cervenka I, Al Rahmoun M, Mahamat-Saleh Y, Fournier A, Boutron-Ruault M-C, Severi G, Caini S, Palli D, Ghiasvand R, Veierod MB, Botteri E, Tjønneland A, Olsen A, Fortner RT, Kaaks R, Schulze MB, Panico S, Trichopoulou A, Dessinioti C, Niforou K, Sieri S, Tumino R, Sacerdote C, Bueno-de-Mesquita B, Sandanger TM, Colorado-Yohar S, Sanchez MJ, Gil Majuelo L, Lujan-Barroso L, Ardanaz E, Merino S, Isaksson K, Butt S, Ljuslinder I, Jansson M, Travis RC, Khaw K-T, Weiderpass E, Dossus L, Rinaldi S, Kvaskoff M. Exogenous hormone use and cutaneous melanoma risk in women: The European Prospective Investigation into Cancer and Nutrition. International journal of cancer. 2019:10.1002/ijc.32674. doi: 10.1002/ijc.32674. [DOI] [PubMed] [Google Scholar]
- 36.Cervenka I, Mahamat-Saleh Y, Savoye I, Dartois L, Boutron-Ruault MC, Fournier A, Kvaskoff M. Oral contraceptive use and cutaneous melanoma risk: a French prospective cohort study. International journal of cancer. 2018;143(10):2390–9. Epub 2018/09/13. doi: 10.1002/ijc.31644. [DOI] [PubMed] [Google Scholar]
- 37.Missmer SA, Spiegelman D, Hankinson SE, Malspeis S, Barbieri RL, Hunter DJ. Natural hair color and the incidence of endometriosis. Fertil Steril. 2006;85(4):866–70. Epub 2006/04/04. doi: S0015-0282(05)04323-2 [pii] 10.1016/j.fertnstert.2005.12.008 [doi]. [DOI] [PubMed] [Google Scholar]
- 38.Woodworth SH, Singh M, Yussman MA, Sanfilippo JS, Cook CL, Lincoln SR. A prospective study on the association between red hair color and endometriosis in infertile patients. Fertil Steril. 1995;64(3):651–2. Epub 1995/09/01. [DOI] [PubMed] [Google Scholar]
- 39.Wyshak G, Frisch RE. Red hair color, melanoma, and endometriosis: suggestive associations. Int J Dermatol. 2000;39(10):798 Epub 2000/11/30. [DOI] [PubMed] [Google Scholar]
- 40.Frisch RE, Wyshak G, Albert LS, Sober AJ. Dysplastic nevi, cutaneous melanoma, and gynecologic disorders. Int J Dermatol. 1992;31(5):331–5. Epub 1992/05/01. [DOI] [PubMed] [Google Scholar]
- 41.Kvaskoff M, Mesrine S, Clavel-Chapelon F, Boutron-Ruault MC. Endometriosis risk in relation to naevi, freckles and skin sensitivity to sun exposure: the French E3N cohort. Int J Epidemiol. 2009;38(4):1143–53. Epub 2009/04/09. doi: 10.1093/ije/dyp175. [DOI] [PubMed] [Google Scholar]
- 42.Somigliana E, Vigano P, Abbiati A, Gentilini D, Parazzini F, Benaglia L, Vercellini P, Fedele L. ‘Here comes the sun’: pigmentary traits and sun habits in women with endometriosis. Hum Reprod. 2010;25(3):728–33. Epub 2010/01/20. doi: dep453 [pii] 10.1093/humrep/dep453 [doi]. [DOI] [PubMed] [Google Scholar]
- 43.Hornstein MD, Thomas PP, Sober AJ, Wyshak G, Albright NL, Frisch RE. Association between endometriosis, dysplastic naevi and history of melanoma in women of reproductive age. Hum Reprod. 1997;12(1):143–5. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 44.Kvaskoff M, Han J, Qureshi AA, Missmer SA. Pigmentary traits, family history of melanoma and the risk of endometriosis: a cohort study of US women. Int J Epidemiol. 2014;43(1):255–63. Epub 2013/12/18. doi: dyt235 [pii] 10.1093/ije/dyt235 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigano P, Somigliana E, Panina P, Rabellotti E, Vercellini P, Candiani M. Principles of phenomics in endometriosis. Hum Reprod Update. 2012;18(3):248–59. Epub 2012/03/01. doi: dms001 [pii] 10.1093/humupd/dms001 [doi]. [DOI] [PubMed] [Google Scholar]
- 46.Vercellini P, Buggio L, Somigliana E, Dridi D, Marchese MA, Vigano P. ‘Behind blue eyes’dagger: the association between eye colour and deep infiltrating endometriosis. Hum Reprod. 2014;29(10):2171–5. Epub 2014/07/10. doi: deu169 [pii] 10.1093/humrep/deu169 [doi]. [DOI] [PubMed] [Google Scholar]
- 47.Munksgaard PS, Blaakaer J. The association between endometriosis and gynecological cancers and breast cancer: a review of epidemiological data. Gynecol Oncol. 2011;123(1):157–63. Epub 2011/07/12. doi: 10.1016/j.ygyno.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Daum H, Peretz T, Laufer N. BRCA mutations and reproduction. Fertility and sterility. 2018;109(1):33–8. doi: 10.1016/j.fertnstert.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Farland LV, Tamimi RM, Eliassen AH, Spiegelman D, Hankinson SE, Chen WY, Missmer SA. Laparoscopically Confirmed Endometriosis and Breast Cancer in the Nurses’ Health Study II. Obstet Gynecol. 2016;128(5):1025–31. Epub 2016/10/26. doi: 10.1097/aog.0000000000001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosner B, Glynn RJ, Tamimi RM, Chen WY, Colditz GA, Willett WC, Hankinson SE. Breast cancer risk prediction with heterogeneous risk profiles according to breast cancer tumor markers. Am J Epidemiol. 2013;178(2):296–308. Epub 2013/05/07. doi: kws457 [pii] 10.1093/aje/kws457 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burghaus S, Haberle L, Schrauder MG, Heusinger K, Thiel FC, Hein A, Wachter D, Strehl J, Hartmann A, Ekici AB, Renner SP, Beckmann MW, Fasching PA. Endometriosis as a risk factor for ovarian or endometrial cancer - results of a hospital-based case-control study. BMC Cancer. 2015;15:751 Epub 2015/10/22. doi: 10.1186/s12885-015-1821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, Zauber AG, Olson SH. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(5):1448–56. Epub 2009/04/23. doi: 10.1158/1055-9965.Epi-08-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mogensen JB, Kjaer SK, Mellemkjaer L, Jensen A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol Oncol. 2016;143(1):87–92. Epub 2016/07/20. doi: 10.1016/j.ygyno.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 54.Nagai K, Hayashi K, Yasui T, Katanoda K, Iso H, Kiyohara Y, Wakatsuki A, Kubota T, Mizunuma H. Disease history and risk of comorbidity in women’s life course: a comprehensive analysis of the Japan Nurses’ Health Study baseline survey. BMJ open. 2015;5(3):e006360 Epub 2015/03/13. doi: 10.1136/bmjopen-2014-006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zucchetto A, Serraino D, Polesel J, Negri E, De Paoli A, Dal Maso L, Montella M, La Vecchia C, Franceschi S, Talamini R. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev. 2009;18(4):316–21. Epub 2009/06/26. doi: 10.1097/cej.0b013e328329d830 [DOI] [PubMed] [Google Scholar]
- 56.Melin A, Sparen P, Bergqvist A. The risk of cancer and the role of parity among women with endometriosis. Hum Reprod. 2007;22(11):3021–6. Epub 2007/09/15. doi: 10.1093/humrep/dem209. [DOI] [PubMed] [Google Scholar]
- 57.Poole EM, Lin WT, Kvaskoff M, De Vivo I, Terry KL, Missmer SA. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control. 2017;28(5):437–45. Epub 2017/03/17. doi: 10.1007/s10552-017-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saraswat L, Ayansina D, Cooper KG, Bhattacharya S, Horne AW, Bhattacharya S. Impact of endometriosis on risk of further gynaecological surgery and cancer: a national cohort study. BJOG : an international journal of obstetrics and gynaecology. 2018;125(1):64–72. Epub 2017/09/28. doi: 10.1111/1471-0528.14793. [DOI] [PubMed] [Google Scholar]
- 59.Williams CL, Jones ME, Swerdlow AJ, Botting BJ, Davies MC, Jacobs I, Bunch KJ, Murphy MFG, Sutcliffe AG. Risks of ovarian, breast, and corpus uteri cancer in women treated with assisted reproductive technology in Great Britain, 1991-2010: data linkage study including 2.2 million person years of observation. Bmj. 2018;362:k2644 Epub 2018/07/13. doi: 10.1136/bmj.k2644. [DOI] [PMC free article] [PubMed] [Google Scholar]; PMCID: PMC6039832 at www.icmje.org/coi_disclosure.pdf and declare: support for this study from Cancer Research UK and NIHR; MEJ additionally received funding from Breast Cancer Now, and KJB and MFGM received funding from the UK Department of Health and Children with Cancer UK during the study; IJ reports personal fees from Abcodia and Women’s Health Specialists, and receives royalties as co-inventor of the ROCA algorithm; MCD reports personal fees from the Centre for Reproductive and Genetic Health; the authors declare no other relationships or activities that could appear to have influenced the submitted work.
- 60.Tiniakou E, Costenbader KH, Kriegel MA. Sex-specific environmental influences on the development of autoimmune diseases. Clin Immunol. 2013;149(2):182–91. doi: 10.1016/i.clim.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masi A, Kaslow R. Sex effects in systemic lupus erythematosus: a clue to pathogenesis. Arthritis Rheum. 1978;21(4):480–4. [DOI] [PubMed] [Google Scholar]
- 62.Gutierrez-Arcelus M, Rich SS, Raychaudhuri S. Autoimmune diseases - connecting risk alleles with molecular traits of the immune system. Nat Rev Genet. 2016;17(3):160–74. Epub 2016/02/26. doi: 10.1038/nrg.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Treloar S, O’Connor D, O’Connor V, Martin N. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71(4):701–10. [DOI] [PubMed] [Google Scholar]
- 64.Gemmill JA, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Cancers, infections, and endocrine diseases in women with endometriosis. Fertil Steril. 2010;94(5):1627–31. Epub 2009/12/01. doi: 10.1016/j.fertnstert.2009.07.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 66.*.Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, Missmer SA, Rahmioglu N, Zondervan KT, Becker CM. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(4):486–503. Epub 2019/07/02. doi: 10.1093/humupd/dmz014. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article comprehensively reviews the literature on endometreiosis and autoimmune disease with an emphasis on assessing the quality of the evidence.
- 67.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. Epub 2010/12/28. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Harris HR, Simard JF, Arkema EV. Endometriosis and systemic lupus erythematosus: a population-based case-control study. Lupus. 2016;25(9):1045–9. Epub 2016/02/09. doi: 10.1177/0961203316631635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nielsen NM, Jørgensen KT, Pedersen BV, Rostgaard K, Frisch M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum Reprod. 2011;26(6):1555–9. doi: 10.1093/humrep/der105. [DOI] [PubMed] [Google Scholar]
- 70.Harris H, Costenbader K, Mu F, Kvaskoff M, Malspeis S, Karlson E, Missmer S. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann Rheum Dis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merlino LA, Cerhan JR, Criswell LA, Mikuls TR, Saag KG. Estrogen and other female reproductive risk factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Seminars in arthritis and rheumatism. 2003;33(2):72–82. Epub 2003/11/20. doi: 10.1016/s0049-0172(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 72.Bengtsson C, Malspeis S, Orellana C, Sparks JA, Costenbader KH, Karlson EW. Association Between Menopausal Factors and the Risk of Seronegative and Seropositive Rheumatoid Arthritis: Results From the Nurses’ Health Studies. Arthritis Care Res (Hoboken). 2017;69(11):1676–84. Epub 2017/01/14. doi: 10.1002/acr.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56(4):1251–62. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 74.*.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association Between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension (Dallas, Tex : 1979). 2017;70(1):59–65. Epub 2017/06/01. doi: 10.1161/hypertensionaha.117.09056. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows an association between endometriosis and hypercholesterolemia and hypertension that is partically accounted for by endometriosis treatment factors.
- 75.Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC, Poli-Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril.93(7):2433–6. Epub 2009/12/09. doi: S0015-0282(09)03521-3 [pii] 10.1016/j.fertnstert.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 76.Verit FF, Erel O, Celik N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum Reprod. 2008;23(1):100–4. Epub 2007/11/15. doi: dem340 [pii] 10.1093/humrep/dem340. [DOI] [PubMed] [Google Scholar]
- 77.Turgut A, Ozler A, Goruk NY, Tunc SY, Evliyaoglu O, Gul T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur Rev Med Pharmacol Sci. 2013;17(11):1472–8. Epub 2013/06/19. [PubMed] [Google Scholar]
- 78.Pretta S, Remorgida V, Abbamonte LH, Anserini P, Ragni N, Del Sette M, Gandolfo C, Ferrero S. Atherosclerosis in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132(2):226–31. Epub 2006/05/10. doi: 10.1016/j.ejogrb.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 79.Yu E, Rimm E, Qi L, Rexrode K, Albert CM, Sun Q, Willett WC, Hu FB, Manson JE. Diet, Lifestyle, Biomarkers, Genetic Factors, and Risk of Cardiovascular Disease in the Nurses’ Health Studies. Am J Public Health. 2016;106(9):1616–23. Epub 2016/07/28. doi: 10.2105/ajph.2016.303316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taskin O, Rikhraj K, Tan J, Sedlak T, Rowe TC, Bedaiwy MA. Link between Endometriosis, Atherosclerotic Cardiovascular Disease, and the Health of Women Midlife. Journal of minimally invasive gynecology. 2019;26(5):781–4. Epub 2019/04/25. doi: 10.1016/j.jmig.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Tan J, Taskin O, Iews M, Lee AJ, Kan A, Rowe T, Bedaiwy MA. Atherosclerotic cardiovascular disease in women with endometriosis: a systematic review of risk factors and prospects for early surveillance. Reproductive biomedicine online. 2019;39(6):1007–16. Epub 2019/06/08. doi: 10.1016/j.rbmo.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 82.Crook D, Howell R, Sidhu M, Edmonds DK, Stevenson JC. Elevated serum lipoprotein(a) levels in young women with endometriosis. Metabolism: clinical and experimental. 1997;46(7):735–9. doi: 10.1016/s0026-0495(97)90115-3. [DOI] [PubMed] [Google Scholar]
- 83.Hopeman MM, Riley JK, Frolova AI, Jiang H, Jungheim ES. Serum Polyunsaturated Fatty Acids and Endometriosis. Reproductive sciences (Thousand Oaks, Calif). 2015;22(9):1083–7. Epub 2014/12/23. doi: 10.1177/1933719114565030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kinugasa S, Shinohara K, Wakatsuki A. Increased asymmetric dimethylarginine and enhanced inflammation are associated with impaired vascular reactivity in women with endometriosis. Atherosclerosis. 2011;219(2):784–8. Epub 2011/08/10. doi: 10.1016/j.atherosclerosis.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Santoro L, D’Onofrio F, Campo S, Ferraro PM, Tondi P, Campo V, Flex A, Gasbarrini A, Santoliquido A. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Human reproduction (Oxford, England). 2012;27(5):1320–6. Epub 2012/03/12. doi: 10.1093/humrep/des062. [DOI] [PubMed] [Google Scholar]
- 86.Anderson PJ. Neuropsychological outcomes of children born very preterm. Seminars in fetal & neonatal medicine. 2014;19(2):90–6. doi: 10.1016/j.siny.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Tani A, Yamamoto S, Maegawa M, Kunimi K, Matsui S, Keyama K, Kato T, Uemura H, Kuwahara A, Matsuzaki T, Yasui T, Kamada M, Soeki T, Sata M, Irahara M. Arterial stiffness is increased in young women with endometriosis. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2015;35(7):711–5. Epub 2014/12/27. doi: 10.3109/01443615.2014.992871. [DOI] [PubMed] [Google Scholar]
- 88.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and Risk of Coronary Heart Disease. Circulation Cardiovascular quality and outcomes. 2016;9(3):257–64. Epub 2016/03/31. doi: 10.1161/circoutcomes.115.002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Madsen TE, Howard VJ, Jimenez M, Rexrode KM, Acelajado MC, Kleindorfer D, Chaturvedi S. Impact of Conventional Stroke Risk Factors on Stroke in Women: An Update. Stroke. 2018;49(3):536–42. Epub 2018/02/14. doi: 10.1161/strokeaha.117.018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113(5):1027–37. Epub 2009/04/23. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, Van Horn L, Stefanick ML, Trevisan M. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111(12):1462–70. Epub 2005/03/23. doi: 10.1161/01.Cir.0000159344.21672.Fd. [DOI] [PubMed] [Google Scholar]
- 92.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause (New York, NY). 2006;13(2):265–79. Epub 2006/04/29. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 93.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA cardiology. 2016;1(7):767–76. Epub 2016/10/21. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 94.Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobson J. Administration of nasal nafarelin as compared with oral danazol for endometriosis. A multicenter double-blind comparative clinical trial. The New England journal of medicine. 1988;318(8):485–9. doi: 10.1056/NEJM198802253180805. [DOI] [PubMed] [Google Scholar]
- 95.Szeplaki G, Varga L, Valentin S, Kleiber M, Karadi I, Romics L, FOst G, Farkas H. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. J Allergy Clin Immunol. 2005;115(4):864–9. doi: 10.1016/j.jaci.2004.12.1130. [DOI] [PubMed] [Google Scholar]
- 96.Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertility and sterility. 2005;84(5):1375–87. doi: 10.1016/j.fertnstert.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 97.Farquhar C, Prentice A, Singla AA, Selak V. Danazol for pelvic pain associated with endometriosis. Cochrane Database of Systematic Reviews. 2007(4). doi: 10.1002/14651858.CD000068.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohler G, Goretzlehner G, Brachmann K. Lipid metabolism during treatment of endometriosis with the progestin dienogest. Acta obstetricia et gynecologica Scandinavica. 1989;68(7):633–5. doi: 10.3109/00016348909013283. [DOI] [PubMed] [Google Scholar]
- 99.Teichmann AT, Cremer P, Wieland H, Kuhn W, Seidel D. Lipid metabolic changes during hormonal treatment of endometriosis. Maturitas. 1988;10(1):27–33. doi: 10.1016/0378-5122(88)90128-4. [DOI] [PubMed] [Google Scholar]
- 100.Farland LV, Missmer SA, Bijon A, Gusto G, Gelot A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault MC, Kvaskoff M. Associations among body size across the life course, adult height and endometriosis. Hum Reprod. 2017;32(8):1732–42. Epub 2017/06/08. doi: 10.1093/humrep/dex207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum Reprod. 2013;28(7):1783–92. Epub 2013/05/16. doi: det120 [pii] 10.1093/humrep/det120 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2001(4):CD000068-CD. doi: 10.1002/14651858.CD000068. [DOI] [PubMed] [Google Scholar]
- 103.Vercellini P, Fedele L, Pietropaolo G, Frontino G, Somigliana E, Crosignani PG. Progestogens for endometriosis: forward to the past. Human reproduction update. 2003;9(4):387–96. doi: 10.1093/humupd/dmg030. [DOI] [PubMed] [Google Scholar]


