Summary
Background
Bruton tyrosine kinase is a clinically validated target in mantle cell lymphoma. Acalabrutinib (ACP-196) is a highly selective, potent Bruton tyrosine kinase inhibitor developed to minimise off-target activity.
Methods
In this open-label, phase 2 study, oral acalabrutinib (100 mg twice per day) was given to patients with relapsed or refractory mantle cell lymphoma, until disease progression or unacceptable toxicity. The primary endpoint was overall response assessed according to the Lugano classification, and safety analyses were done in all participants. This trial is registered with ClinicalTrials.gov, number NCT02213926.
Findings
From March 12, 2015, to Jan 5, 2016, 124 patients with relapsed or refractory mantle cell lymphoma were enrolled and all patients received treatment; median age 68 years. Patients received a median of two (IQR 1–2) previous therapies. At a median follow-up of 15·2 months, 100 (81%) patients achieved an overall response and 49 (40%) patients achieved a complete response. The Kaplan-Meier estimated medians for duration of response, progression-free survival, and overall survival were not reached; the 12-month rates were 72% (95% CI 62–80), 67% (58–75), and 87% (79–92%), respectively. The most common adverse events were primarily grade 1 or 2 and were headache (47 [38%]), diarrhoea (38 [31%]), fatigue (34 [27%]), and myalgia (26 [21%]). The most common grade 3 or worse adverse events were neutropenia (13 [10%]), anaemia (11 [9%]), and pneumonia (six [5%]). There were no cases of atrial fibrillation and one case of grade 3 or worse haemorrhage. The median duration of treatment was 13·8 months. Treatment was discontinued in 54 (44%) patients, primarily due to progressive disease (39 [31%]) and adverse events (seven [6%]).
Interpretation
Acalabrutinib treatment provided a high rate of durable responses and a favourable safety profile in patients with relapsed or refractory mantle cell lymphoma. These findings suggest an important role for acalabrutinib in the treatment of this disease population.
Funding
Acerta Pharma, a member of the AstraZeneca Group.
Introduction
Mantle cell lymphoma is an aggressive B-cell non-Hodgkin lymphoma with a poor prognosis.1–3 Almost all patients relapse after frontline therapy, and relapsed or refractory mantle cell lymphoma is incurable.1,3 B-cell receptor (BCR) signalling is central to the survival and proliferation of malignant B cells, and Bruton tyrosine kinase (BTK), an integral member of the BCR pathway, is a clinically validated target in mantle cell lymphoma.4,5
Treatment with the BTK inhibitor ibrutinib produced a high response rate in patients with relapsed or refractory mantle cell lymphoma, changing the treatment paradigm for this population.4 However, ibrutinib has been associated with notable grade 3 or worse toxicities, including atrial fibrillation (6–9% of patients), infection (14–29%), and bleeding (up to 6%).6 Because these side-effects are not characteristic of germline BTK deficiency,7,8 the off-target activity of ibrutinib against other kinases, including tyrosine-protein kinase Tec and interleukin-2-inducible T-cell kinase, has been postulated to be involved in these specific toxicities.9–11
Acalabrutinib (ACP-196) is a highly selective, potent BTK inhibitor developed to minimise off-target activity.10 Findings from in-vitro studies showed that acalabrutinib has more selective BTK inhibition and higher in-vivo potency than ibrutinib.12,13 The phase 1/2 ACE-CL-001 trial10 of acalabrutinib monotherapy in patients with relapsed chronic lymphocytic leukaemia showed that acalabrutinib has rapid oral absorption and a short plasma half-life. The trial10 also showed that 100 mg acalabrutinib given twice per day maintained complete and continuous BTK inhibition over the 24-h dosing interval, with more than 90% BTK occupancy in all patients. This 100 mg twice-daily dose and schedule resulted in high response rates, durable remissions, and a favourable safety profile in patients with relapsed chronic lymphocytic leukaemia.
Based on the promising results with acalabrutinib in the ACE-CL-001 trial, and given that BTK is an established target in mantle cell lymphoma, we initiated the phase 2 ACE-LY-004 trial investigating acalabrutinib 100 mg twice daily in patients with relapsed or refractory mantle cell lymphoma.
Methods
Study design and participants
In this phase 2, single-arm, multicentre, open-label study, patients were enrolled at 40 sites in ten countries (Australia, Belgium, Czech Republic, France, Italy, the Netherlands, Poland, Spain, the UK, and the USA). Eligible patients had confirmed mantle cell lymphoma with translocation t(11;14)(q13;q32), overexpressed cyclin D1, or both, and measurable disease (one or more lesions measuring ≥2 cm in the longest diameter). Patients had relapsed after, or were refractory to, one to five previous therapies. We defined refractory disease as achieving less than partial response with the most recent treatment before study entry. Other eligibility criteria included age 18 years or older and Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower. Exclusion criteria included absolute neutrophil count less than 0·75 × 109 per L or platelet count less than 50 × 109 per L (or neutrophil count <0·50 × 109 per L or platelet count <30 × 109 per L for patients with bone marrow involvement), and creatinine level more than 2·5-times the upper limit of normal. Patients with significant cardiovascular disease (uncontrolled or symptomatic arrhythmias, congestive heart failure, or myocardial infarction) within 6 months of screening, any class 3 or 4 cardiac disease as defined by the New York Heart Association Functional Classification, or corrected QT interval more than 480 ms were excluded. Concomitant treatment with warfarin or equivalent vitamin K antagonists was prohibited. We also excluded patients who previously received BCR inhibitors (BTK, PI3K, or SYK inhibitors) or BCL-2 inhibitors.
The institutional review board at each site approved the protocol. The study was done according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent.
Procedures
Acalabrutinib (100 mg) was given orally twice per day in 28-day cycles until progressive disease or unacceptable toxicity. Dose modification guidelines (appendix) were defined in the study protocol.
We did physical examinations, bone marrow assessments, and image evaluations to assess disease progression. CT scans with contrast were done at the end of cycles 2, 4, and 6 and every three cycles thereafter, and PET-CT scans were done at the end of cycles 2 and 6 and as clinically indicated or to confirm a complete response. A bone marrow aspirate or biopsy was required to confirm a complete response for patients with bone marrow disease involvement at baseline. Gastrointestinal endoscopy was required to confirm a complete response for patients with a history of gastrointestinal involvement as documented by the investigator at baseline. An independent review committee (IRC) also evaluated responses based on CT and PET scans, bone-marrow biopsy specimens, endoscopy results, and clinical data.
We did pharmacokinetic and pharmacodynamic testing in cycles 1 and 2 (appendix). We used the European Organisation for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30) to assess health-related quality of life. The questionnaire was collected at screening, at the end of cycles 2, 4, and 6, and then every three cycles thereafter until progressive disease or use of alternative anticancer therapy. The standardised scores were derived (ranging from 0 to 100) as recommended in the EORTC user manual.
Outcomes
The primary endpoint was overall response, defined as the proportion of patients achieving either a partial response or a complete response at any time during the treatment period based on investigator assessment according to the 2014 Lugano classification.14 Secondary endpoints included investigator-assessed duration of response, progression-free survival, overall survival, safety, pharmacokinetics, and pharmacodynamics. Other secondary endpoints were IRC-assessed overall response, duration of response, and progression-free survival using the Lugano classification. IRC-assessed overall response, duration of response, and progression-free survival based on the criteria established by the International Harmonization Project in 2007,15 as well as the assessment of patient-reported outcomes, were exploratory endpoints.
Safety assessments included the frequency and severity of adverse events, clinical laboratory tests (haematology, clinical chemistry, and urinalysis), vital sign measurements, physical examinations, and ECOG performance status. We graded adverse event severity according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.03).
Statistical analysis
The original protocol consisted of two parallel cohorts (bortezomib-naive and bortezomib-exposed) using a Simon’s two-stage design.16 We did an interim futility analysis using the stopping rules for Simon’s two-stage design. Based on this analysis, enrolment to both cohorts could continue without interruption.
Results from the phase 2 study of ibrutinib in relapsed or refractory mantle cell lymphoma indicated that previous bortezomib exposure does not affect response to BTK inhibitor therapy;4 therefore, the two cohorts were merged. The original planned sample size was retained to provide adequate estimation utility for safety and other secondary analyses. A one-sample χ2 test was used to test the null hypothesis that 20% or fewer patients would achieve an overall response (not considered clinically meaningful). The planned sample size of 117 patients provides more than 99% power to test a difference of 20% versus 40% in overall response at a one-sided significance level of 0·025. The probability of observing one or more instances of a specific adverse event with a true incidence rate of 1%, 2%, or 5% was 69·1%, 90·6%, or 99·8%, respectively, which provided reasonable assurance that events occurring at more than 1% frequency could be detected in this study.
The final analysis was planned to occur approximately 14 months after the last patient was enrolled. Results are presented as of Feb 28, 2017. We included all patients receiving at least one dose of acalabrutinib in efficacy and safety analyses. We applied the same analysis methods for investigator-assessed overall response to IRC-assessed overall response. We did subgroup analyses for the proportion of patients achieving an overall response and complete response using prespecified baseline and disease characteristic variables. We estimated duration of response, progression-free survival, and overall survival using the Kaplan-Meier method. Progression-free survival and duration of response were censored at the last adequate disease assessment before the initiation of subsequent anticancer therapy. Non-compartmental pharmacokinetic analysis of individual plasma acalabrutinib concentration-time data was done using Phoenix WinNonlin (version 6.4). This trial is registered with ClinicalTrials.gov, number NCT02213926.
Role of the funding source
The study protocol and statistical analysis plan were designed by the authors with the sponsor. The sponsor had no role in data collection. Data verification and statistical analyses were done by the sponsor. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From March 12, 2015, to Jan 5, 2016, 124 patients with relapsed or refractory mantle cell lymphoma were enrolled and all patients received treatment. The median age was 68 years (IQR 61–75); 80 (65%) of 124 patients were aged 65 years or older (table 1). At baseline, 93 (75%) had Ann Arbor stage IV disease and 90 (73%) patients had extranodal disease; the most common extranodal sites were bone marrow, gastrointestinal tract, and lung. The simplified Mantle Cell Lymphoma International Prognostic Index scores at baseline were intermediate in 54 (44%) patients and high in 21 (17%) patients. The median number of previous therapies was two (IQR 1–2); 28 (23%) patients received three or more previous therapies, and 30 (24%) were refractory to the most recent treatment. Previous treatment with bortezomib or carfilzomib was reported in 24 (19%), and nine (7%) patients had previously been given lenalidomide. 22 (18%) patients had undergone high-dose chemotherapy with stem-cell transplantation. At a median follow-up period of 15·2 months (IQR 14·2–17·0), 70 patients (56%) were receiving treatment and 54 patients (44%) had discontinued. The median relative dose intensity was 98·5%. The reasons for treatment discontinuation were progressive disease in 39 (31%) patients; adverse events in seven (6%) patients (appendix); initiation of subsequent anticancer therapy in five (4%) patients, all of whom received allogeneic stem-cell transplants; loss to follow-up (one [1%]); patient withdrawal (one [1%]); and patient’s decision to stop treatment (one [1%]). Of the 54 patients who discontinued treatment, subsequent therapy data were available for 38 patients, 29 of whom discontinued due to progressive disease. The most common therapy after progression was a bendamustine-rituximab based regimen or other chemotherapeutic combinations. Four patients received ibrutinib either in combination with rituximab or a proteasome inhibitor. Two patients received chimeric antigen receptors (CAR) T-cell therapy.
Table 1:
Baseline characteristics
| All treated patients (n=124) | |
|---|---|
| Age (years) | 68 (61–75) |
| ≥65 | 80 (65%) |
| Sex | |
| Men | 99 (80%) |
| Women | 25 (20%) |
| ECOG performance status | |
| 0 | 71 (57%) |
| 1 | 44 (35%) |
| 2* | 9 (7%) |
| Simplified MIPI score | |
| Low risk (0–3) | 48 (39%) |
| Intermediate risk (4–5) | 54 (44%) |
| High risk (6–11) | 21 (17%) |
| Missing | 1 (1%) |
| Tumour bulk (cm) | |
| ≥5 | 46 (37%) |
| ≥10 | 10 (8%) |
| Extranodal disease | 90 (73%) |
| Bone marrow | 63 (51%) |
| Gastrointestinal | 13 (10%) |
| Lung | 12 (10%) |
| Ann Arbor stage IV disease | 93 (75%) |
| Number of previous therapies | 2 (1–2) |
| Refractory disease† | 30 (24%) |
| Previous therapy | |
| Rituximab‡ | 118 (95%) |
| CHOP-based regimen | 64 (52%) |
| Bendamustine and rituximab-based regimen | 27 (22%) |
| Hyper-CVAD | 26 (21%) |
| Bortezomib or carfilzomib | 24 (19%) |
| Stem-cell transplant | 22 (18%) |
| Lenalidomide | 9 (7%) |
Data are median (IQR) or n (%). ECOG=Eastern Cooperative Oncology Group performance status. MIPI=Mantle Cell Lymphoma International Prognostic Index. CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone. CVAD=cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
One patient with an ECOG performance status of 1 at screening had an ECOG performance status of 3 at the baseline assessment (cycle 1, day 1).
Refractory disease was defined as an absence of at least a partial response to the last therapy before study entry.4
Alone or as part of a combination regimen.
Pharmacokinetic parameters were evaluated on days 1 and 8 (cycle 1) from plasma acalabrutinib concentrations measured from predose to 6 h after dose (figure 1A, appendix). Overall, acalabrutinib pharmacokinetic parameters indicated relatively rapid absorption and elimination, with low potential for accumulation.
Figure 1: Pharmacokinetics and pharmacodynamics of acalabrutinib in mantle cell lymphoma.
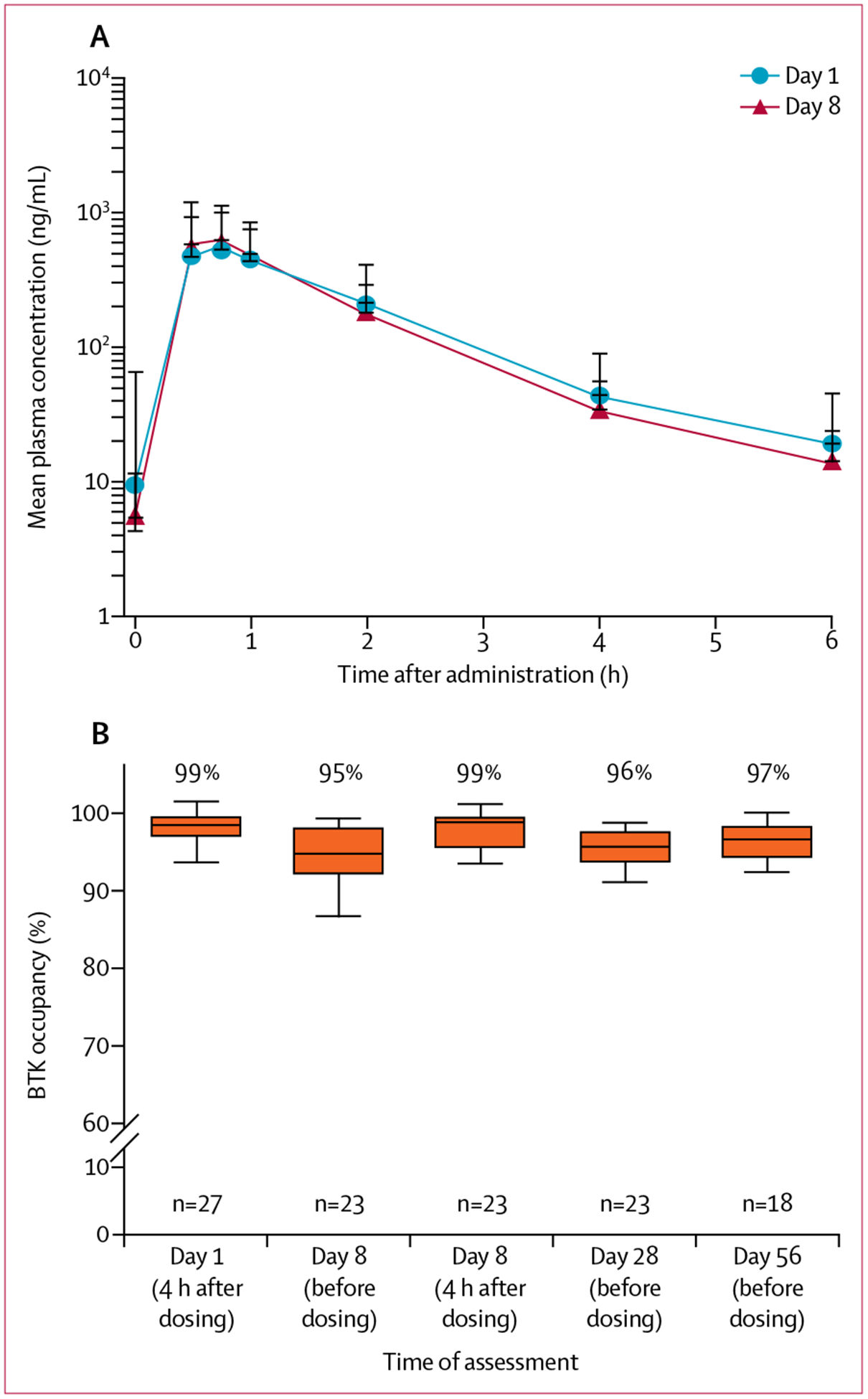
(A) Mean acalabrutinib plasma concentrations over time on day 1 and day 8 after a 100 mg dose. Three (7%) of 44 patients had anomalous initial concentrations on day 1 that were not below the limit of quantification. (B) BTK occupancy on day 1, and predose and post dose during steady-state. Box and whisker plots show median and IQR (Tukey method). BTK=Bruton tyrosine kinase.
Binding of acalabrutinib to BTK (target occupancy) was measured in peripheral blood (figure 1B). Acalabrutinib administered at 100 mg twice per day resulted in a median BTK occupancy of 99% 4 h after dosing (days 1 and 8), and 95–97% at the drug trough timepoints (before next dose) on days 8, 28, and 56. After 28 days of treatment, plasma levels decreased for tumour necrosis factor alpha, CXCL13 (both p<0·0001), and other cytokines involved in inflammation and cell trafficking (appendix). An increase in CD8+ T cells (p=0·01) from baseline was observed in cycle 2, day 28 (appendix).
A reduction in lymphadenopathy was observed in 111 (94%) of 118 patients (figure 2A). 100 (81%) patients achieved an investigator-assessed overall response per the Lugano classification14 and 49 (40%) patients achieved a complete response (table 2). Efficacy was further evaluated by an IRC using the same criteria, which showed that 99 (80%) patients achieved an overall response and 49 (40%) patients achieved a complete response. High concordance was observed between investigator-assessed and IRC-assessed overall response (91%) and complete response (94%).
Figure 2: Efficacy of acalabrutinib in mantle cell lymphoma.
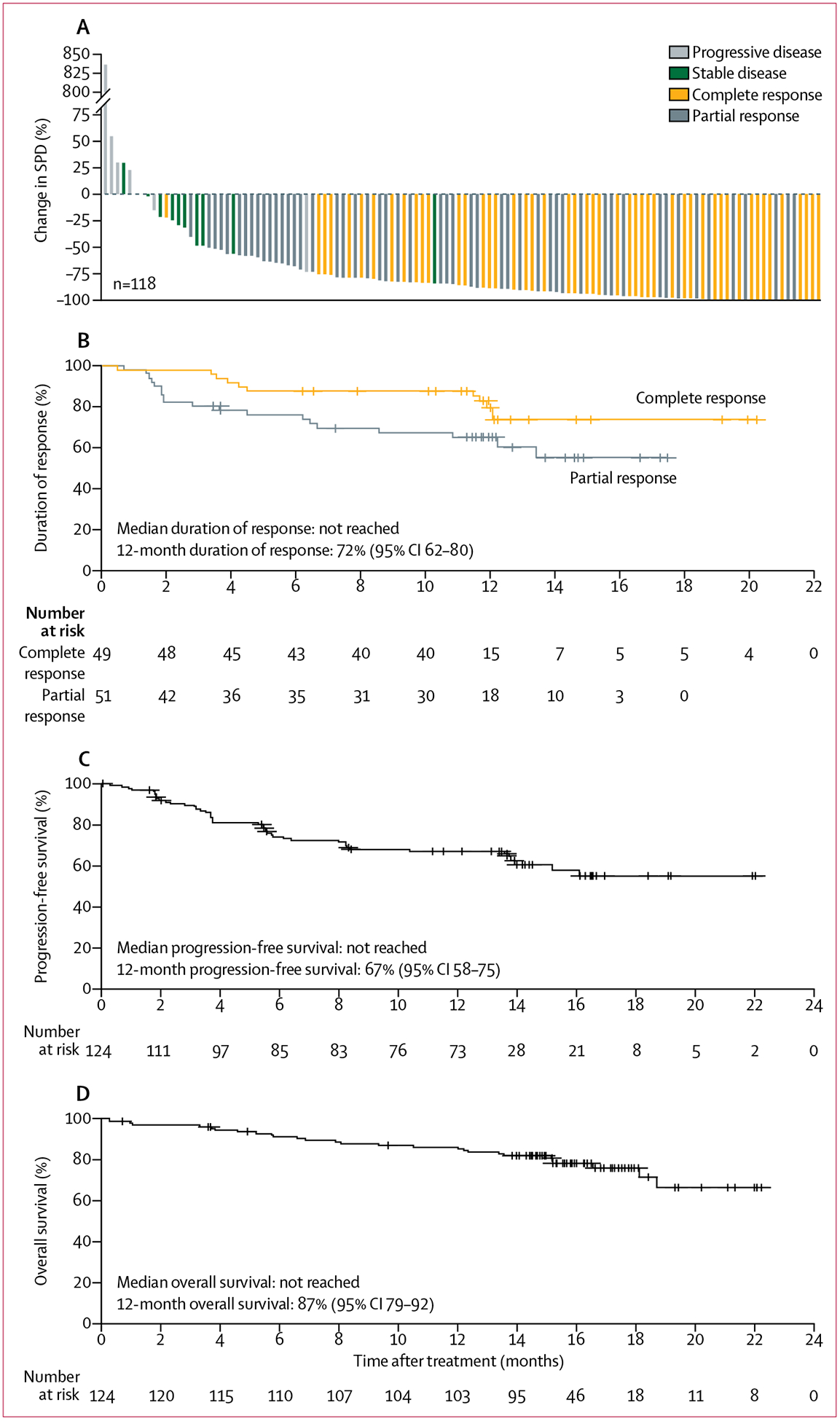
(A) Maximum change from baseline in the SPD of target lesions for all treated patients with baseline and one or more post-baseline lesion measurements. Percentage change in SPD is shown by best response achieved in each patient. (B) Kaplan-Meier curve for duration of response in patients with a complete response versus those with a partial response. Kaplan-Meier curves are shown for progression-free survival (C) and overall survival (D). SPD=sum of the product diameters.
Table 2:
Investigator-assessed and IRC-assessed responses
| Investigator-assessed response | IRC-assessed response | |
|---|---|---|
| Overall response (complete response + partial response) | 100 (81%; 73–87) | 99 (80%; 72–87) |
| Best response | ||
| Complete response | 49 (40%; 31–49) | 49 (40%; 31–49) |
| Partial response | 51 (41%; 32–50) | 50 (40%; 32–50) |
| Stable disease | 11 (9%; 5–15) | 9 (7%; 3–13) |
| Progressive disease | 10 (8%; 4–14) | 11 (9%; 5–15) |
| Not evaluable | 3 (2%; 1–7) | 5 (4%; 1–9) |
Data are n (%; 95% CI). Overall response and best response according to the Lugano Classification14 based on assessment by the investigator (primary endpoint) and an independent review committee (secondary endpoint). Patients without post-baseline disease assessment were not evaluable. IRC=independent review committee.
Investigator-assessed overall response was consistent across prespecified subgroups, though the number of patients who achieved a complete response was lower in patients with Ann Arbor stage IV disease (27 [29%] of 93 patients), bone marrow involvement (nine [14%] of 64), and extranodal disease (25 [28%] of 90; figure 3, appendix). More patients enrolled in the USA achieved a complete response than those outside of the USA (24 [56%] of 45 vs 24 [30%] of 79), which might be due to fewer patients in the USA with bone marrow involvement (12 [27%] of 45 vs 50 [63%] of 79). 92 (92%) of 100 responders responded by the first assessment (end of cycle 2). A response to treatment occurred in 36 (78%) of 46 patients with lymph nodes 5 cm in diameter or larger (figure 3, appendix). The median time to best response was 1·9 months (IQR 1·8–3·7), and the median time to complete response was 3·4 months (1·9–5·5).
Figure 3: Subgroup analysis of overall response.
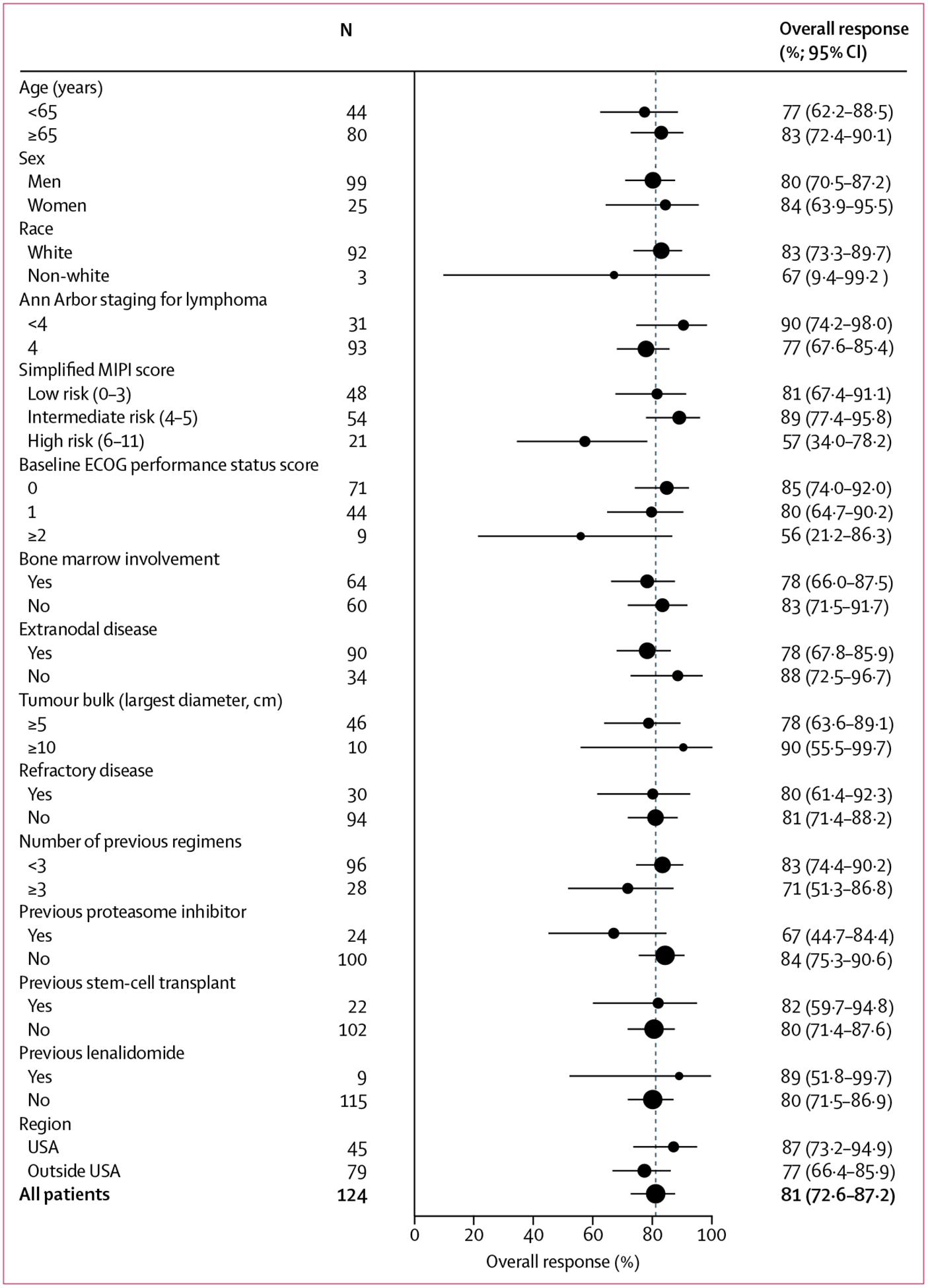
Forest plot containing overall response by prespecified subgroups according to baseline demographic and clinical characteristics. 95% CIs were based on exact binomial distribution. MIPI=Mantle Cell Lymphoma International Prognostic Index. ECOG=Eastern Cooperative Oncology Group.
The Kaplan-Meier estimated medians for duration of response, progression-free survival, and overall survival have not been reached based on investigator assessment (figures 2B–D). At 12 months, the estimated duration of response rate was 72% (95% CI 62–80) and the estimated proportion of patients achieving progression-free survival was 67% (58–75). The estimated percentage of patients achieving 12-month overall survival was 87% (79–92%; figure 2B–D).
The adverse events observed were mostly grade 1 or 2. The most common adverse events of any grade were headache (47 [38%]), diarrhoea (38 [31%]), fatigue (34 [27%]), and myalgia (26 [21%]; table 3). Headache events were mostly grade 1 (30 [64%] of 47 patients); median time to onset was 5 days. The median duration of headache events was 11 days, and most patients (36 [77%] of 47) had only one event. Headache led to withholding of study treatment in two (4%) of 47 patients; none discontinued treatment due to headache. The median time to onset of diarrhoea was 50 days and the median time to resolution was 7 days; most patients (28 [74%] of 38) had only one event. Grade 3 or worse adverse events were infrequent and included primarily neutropenia (13 [10%]), anaemia (11 [9%]), and pneumonia (six [5%]). Serious adverse events were reported in 48 patients (39%; appendix). Serious adverse events were considered treatment-related in 13 (10%) patients; none were reported in more than one patient.
Table 3:
Adverse events
| All grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5* | |
|---|---|---|---|---|---|---|
| Most common events† | ||||||
| Headache | 47 (38%) | 30 (24%) | 15 (12%) | 2 (2%) | 0 | 0 |
| Diarrhoea | 38 (31%) | 21 (17%) | 13 (10%) | 4 (3%) | 0 | 0 |
| Fatigue | 34 (27%)‡ | 24 (19%) | 8 (6%) | 1 (1%) | 0 | 0 |
| Myalgia | 26 (21%) | 19 (15%) | 6 (5%) | 1 (1%) | 0 | 0 |
| Cough | 24 (19%) | 21 (17%) | 3 (2%) | 0 | 0 | 0 |
| Nausea | 22 (18%) | 12 (10%) | 9 (7%) | 1 (1%) | 0 | 0 |
| Pyrexia | 19 (15%) | 14 (11%) | 5 (4%) | 0 | 0 | 0 |
| Most common grade 3 or worse events§ | ||||||
| Anaemia | 15 (12%) | 1 (1%) | 3 (2%) | 10 (8%) | 1 (1%) | 0 |
| Neutropenia | 13 (10%) | 0 | 0 | 6 (5%) | 7 (6%) | 0 |
| Pneumonia | 7 (6%) | 0 | 1 (1%) | 6 (5%) | 0 | 0 |
Data are n (%).
Only one grade 5 event (aortic stenosis) was reported.
Reported in ≥15% of all treated patients.
Includes one case of fatigue without grading.
Reported in ≥5% of all treated patients.
Selected adverse events reported with ibrutinib treatment were reviewed to assess potential BTK inhibitor class effects.4 In this study, there were no cases of atrial fibrillation; three (2%) grade 3 or 4 cardiac adverse events were reported in one patient each and were grade 3 acute coronary syndrome (regarded as treatment-related), grade 3 acute myocardial infarction, and grade 4 cardiorespiratory arrest (both not treatment-related; appendix). Infections occurred in 66 (53%) patients; 50 (40%) had grade 1 or 2 infections (appendix). There was one case of grade 2 Pneumocystis jirovecii pneumonia and one case of grade 2 cytomegalovirus viraemia. Bleeding events, the most frequent of which were contusion and petechiae, occurred in 39 (31%) of patients and were all grade 1 or 2 except for one grade 3 gastrointestinal haemorrhage in one patient with a history of gastrointestinal ulcer (appendix). Anticoagulant use was reported in 53 (43%) patients while on study, but there was no reported use of concurrent anticoagulants in the patient who had the grade 3 gastrointestinal haemorrhage during the time of the event. Of 39 patients who had bleeding events, four had concurrent use of either aspirin or clopidogrel at the time of the event. These four patients had grade 1 or 2 petechiae, contusion, or purpura. One patient had grade 3 hypertension (not treatment-related). Seven (6%) patients discontinued treatment due to adverse events (appendix). Adverse events leading to discontinuation occurred in one patient each and were aortic stenosis, diffuse large B-cell lymphoma, blood blister and petechiae (both in one patient with grade 3 acute coronary syndrome treated with clopidogrel), dyspnoea and leukostasis syndrome (both in one patient), non-cardiac chest pain, pulmonary fibrosis, and thrombocytopenia.
Of 27 deaths, 22 were due to progressive disease; five (4%) patients died within 30 days of receiving the last dose of acalabrutinib (all due to progressive disease). One death was due to grade 5 aortic stenosis in a patient with a history of aortic stenosis (not considered treatment-related). Two deaths were reported as due to other reasons, which included secondary acute myeloid leukaemia and intestinal obstruction caused by complications after removal of a colorectal tumour and stroma, both occurring more than 60 days after the last dose of acalabrutinib. Two deaths were reported with unknown cause at the time of data cutoff; both occurred more than 100 days after the last dose of acalabrutinib.
Lymphocytosis occurred in 38 (31%) of 123 patients (95% CI 23–40). Median time to first post-baseline absolute lymphocyte count meeting the lymphocytosis criteria was 1·1 weeks (IQR 1·1–2·1; appendix). Lymphocytosis resolved for 30 (79%) of 38 patients. Median duration of lymphocytosis was 5·6 weeks (2·3–14·9).
117 (94%) of 124 patients completed the patient-reported outcome assessments at screening, and compliance remained high at subsequent visits, ranging from 78% to 100%. The mean of global health status/quality-of-life score at screening was 68·2. A small numerical improvement in global health status/quality-of-life was observed at cycle 2 compared with that at screening (appendix). The improvement remained stable until cycle 15, after which the small number of patients precluded additional conclusions. The numerical improvement compared with screening, however, did not reach the minimal important difference of 10.17
As an exploratory endpoint, response was also assessed by an IRC using the criteria established by the International Harmonization Project in 2007,15 showing that an overall response was achieved by 93 (75%) patients and a complete response was achieved by 37 (30%; appendix).
Discussion
Relapsed or refractory mantle cell lymphoma remains incurable with standard therapy. Approved agents include bortezomib, lenalidomide, and ibrutinib. Allogenic transplantation could offer long-term remissions to some patients but is limited by treatment-related morbidity and the difficulty of finding a suitable donor.2 In this study in relapsed or refractory mantle cell lymphoma, patients given single-agent acalabrutinib achieved a high overall response and complete response, and these responses were durable. Additionally, overall response was consistent across all prespecified subgroups analysed, including subgroups with a different number of previous regimens. Although the proportion of patients achieving an overall response was consistent with the overall population, fewer patients with extranodal or bone marrow involvement achieved a complete response. The 2014 Lugano Classification14 response criteria, which expanded and standardised the interpretation of PET-CT scans to improve response evaluation, were used in this study and reflect the current standard of care in mantle cell lymphoma. Response was also assessed by an IRC using the criteria established by the International Harmonization Project in 2007 as an exploratory endpoint, resulting in high overall response and complete response.
Acalabrutinib yielded a favourable safety profile in patients with relapsed or refractory mantle cell lymphoma, with few grade 3 or worse adverse events and treatment discontinuations due to adverse events. Previous studies with ibrutinib reported grade 3 or worse atrial fibrillation (6–9% of patients), infection (14–29%), and bleeding (up to 6%).6 Although longer follow-up for this study and other randomised studies is needed to understand the differences in safety profiles between acalabrutinib and ibrutinib, atrial fibrillation was not observed in this study and the frequency of grade 3 or worse infection and haemorrhage were low. Although headache was common in our study, only two patients had grade 3 headache. Headaches tended to occur early in treatment and without reoccurrence, and no patient discontinued due to headache. Overall, treatment with acalabrutinib demonstrated a favourable benefit–risk profile and represents a promising treatment option for patients with relapsed or refractory mantle cell lymphoma. Data from the ongoing ACE-CL-006 trial directly comparing acalabrutinib with ibrutinib in previously treated patients with high-risk chronic lymphocytic leukaemia will further differentiate the safety profiles of the two treatments.
The higher selectivity of acalabrutinib to BTK potentially results in less off-target activity and thus an improved tolerability profile, even with twice-daily dosing.13 More frequent dosing might contribute to improved efficacy with covalent inhibitors. Because covalent binding leads to permanent BTK inactivation, the duration of the pharmacodynamic effect is a function of BTK de-novo synthesis rate, which might be faster in malignant cells.13 Indeed, twice-daily dosing of acalabrutinib resulted in complete and continuous inhibition of BTK signalling over 24 h in patients with relapsed or refractory mantle cell lymphoma and in patients with chronic lymphocytic leukaemia.10
The overall decrease in plasma cytokines or chemokines after 1 month of treatment is consistent with previous findings in patients with chronic lymphocytic leukaemia treated with acalabrutinib10 and in patients with mantle cell lymphoma receiving ibrutinib.18 These findings add to the growing body of evidence indicating that BTK inhibition disrupts the tumour microenvironment, limiting the supply of cytokines and chemokines necessary for complex interactions with stromal and accessory cells important for tumour growth and survival.18–22 This disruption might contribute to lymphocytosis, also observed with ibrutinib, probably due to decreased cell adhesion and egress of cells out of the tissues.20,23,24 As acalabrutinib is a highly selective BTK inhibitor, these data suggest that the effect on cytokine concentrations might have been specific to BTK inhibition.
Given the single-arm nature of this study, comparisons with the safety and efficacy reported in the single arm ibrutinib study are limited.4 The patient population enrolled in each trial differed; the ibrutinib study enrolled patients that were more heavily pretreated with a median number of three previous therapies (compared with two in this study) and only 14% of patients had a low risk simplified Mantle Cell Lymphoma International Prognostic Index score (compared with 39%). Because BTK is a validated target in mantle cell lymphoma, physicians might have been more willing to enrol less advanced patients into this trial, rather than choosing approved therapies or stem-cell transplant. Longer follow-up will add more certainty to the response duration and the toxicity profile. Finally, data on Ki67 expression and blastoid and pleomorphic histologic variants, and correlation with efficacy, could provide further information on the usefulness of this agent in patient subsets.
In conclusion, the results of this phase 2 study in relapsed or refractory mantle cell lymphoma showed that twice-daily acalabrutinib monotherapy resulted in high proportions of patients achieving an overall response and complete response, with responses that were durable and clinically meaningful. An alternative BTK inhibitor with greater target selectivity and potency, compelling efficacy, and a differentiated safety profile provides an attractive new therapeutic option for patients with relapsed or refractory mantle cell lymphoma.13 As such, these data have the potential to change the current practice for relapsed or refractory mantle cell lymphoma. Further studies of acalabrutinib are underway, including an ongoing global, phase 3, double-blind, randomised trial of bendamustine and rituximab with acalabrutinib versus placebo in first-line treatment of mantle cell lymphoma.
Supplementary Material
Research in context.
Evidence before this study
Bruton tyrosine kinase (BTK) inhibition with ibrutinib is the current standard of care for patients with relapsed or refractory mantle cell lymphoma. However, tolerability can be a problem leading to treatment disruption and discontinuation. To better understand the usefulness and the limitations of ibrutinib treatment in this patient population, we searched PubMed for all clinical trial publications using the search terms “mantle cell lymphoma” AND “ibrutinib” AND (“relapsed” OR “refractory”) AND “trial”. Existing evidence for the activity of BTK inhibition with ibrutinib in relapsed or refractory mantle cell lymphoma includes a single-arm, phase 2 trial, the findings of which showed substantial efficacy. Additional evidence from a randomised phase 3 trial showed that ibrutinib was superior to temsirolimus, and supportive evidence was provided in a phase 1/1b combination trial of ibrutinib with rituximab and bendamustine. Although promising activity has been shown with ibrutinib, it has also been associated with grade 3 or worse toxicities, notably atrial fibrillation, infection, and bleeding. These side-effects are not characteristic of germline BTK deficiency, suggesting that the off-target activity of ibrutinib against other kinases may be involved in some of the observed toxicities. Promising activity and tolerability with another BTK inhibitor acalabrutinib was reported in patients with relapsed or refractory chronic lymphocytic leukaemia. Acalabrutinib is a highly selective, potent BTK inhibitor developed to minimise off-target activity. In-vitro studies have shown that acalabrutinib has more selective BTK inhibition, less off-target kinase activity, and higher in-vivo potency than ibrutinib. At the time this trial was initiated, no articles for acalabrutinib had been published.
Added value of this study
To our knowledge, this is the first study evaluating the efficacy and safety of acalabrutinib in mantle cell lymphoma. Using the Lugano classification, investigator-assessed overall response was achieved in 81% of patients, and 40% of patients achieved a complete response, and responses were durable after a median follow-up of 15·2 months. Compared with previous results in the existing literature, the responses reported in this study are among the highest rates reported for a single drug in patients with relapsed or refractory mantle cell lymphoma. Acalabrutinib also yielded a favourable safety profile, with few grade 3 or worse adverse events and treatment discontinuations due to adverse events. Atrial fibrillation was not observed in this study, and grade 3 or worse infection and haemorrhage were infrequent.
Implications of all the available evidence
The results of our study suggest that acalabrutinib has positive activity in relapsed or refractory mantle cell lymphoma. Moreover, acalabrutinib has a differentiated safety profile compared with that previously reported with BTK inhibition. As such, these data have the potential to change the current practice for relapsed or refractory mantle cell lymphoma.
Acknowledgments
The study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. We thank the investigators and coordinators at each of the clinical sites; the patients who participated in this trial and their families; Maria Badillo (The University of Texas MD Anderson Cancer Center); employees of Acerta Pharma who contributed to this trial, including Elena Bibikova, Jean Cheung, Tracy Clevenger, Michael Gulrajani, Soma Jallegowda, Feng Jin, Allard Kaptein, Willem Klaassen, Phillip Liu, Diana Mittag, Terry Podoll, Archie Thurston, Yan Xu, and Christine Zhu; and Stephanie Morgan (Team9Science) for medical writing assistance funded by Acerta Pharma.
Footnotes
Declaration of interests
MW reports grants and personal fees from Pharmacyclics, Janssen, and Acerta Pharma, outside the submitted work. SR reports personal fees from AstraZeneca and Acerta Pharma, outside the submitted work. PLZ reports personal fees from Celgene, Roche, Janssen, Gilead, Takeda, Servier, Bristol-Myers Squibb, and Merck, outside the submitted work. AG reports non-financial support from Celgene, Genentech, and Pharmacyclics, during the conduct of the study; and personal fees from Celgene, Pharmacyclics, Acerta Pharma, and Takeda outside the submitted work. OC reports grants, personal fees, and non-financial support from Roche, Gilead, and Takeda; personal fees and non-financial support from Bristol-Myers Squibb, MSD, and Celgene; and personal fees from AbbVie, outside the submitted work. SDS reports grants from Acerta Pharma, during the conduct of the study; and grants from Seattle Genetics, Merck Sharpe and Dohme, Janssen Research and Development, Pharmacyclics, Genentech, and Portola Pharmaceuticals, outside the submitted work. FM reports personal fees from Roche Genetech; and personal fees from Celgene, Bristol-Myers Squibb, Janssen, and Gilead, outside the submitted work. CP reports personal fees and non-financial support from MundiPharma; grants, personal fees, and non-financial support from Roche Pharma; grants and personal fees from Janssen, Bristol-Myers Squibb; personal fees from Takeda; and grants from Acerta Pharma, during the conduct of the study. BS reports personal fees from Celgene, Bayer, Amgen, Baxalta, Jazz, and Pfizer; grants from Rosetta Genomics, Clonoseq, DeBartolo Institute for Personalized Medicine, and Incyte, outside the submitted work. AD reports grants from Acerta Pharma during the conduct of the study; grants, personal fees, and non-financial support from F Hoffmann-La Roche, Celgene, Takeda Pharma, Gilead Sciences; personal fees and non-financial support from CTI BioPharma; grants from Bayer and GlaxoSmithKline; grants and personal fees from Janssen, Pfizer, and Karyopharma; personal fees and non-financial support from Mundipharma; and personal fees from Kite Pharma, outside the submitted work. JDu reports personal fees from AbbVie and Roche, outside the submitted work. EJ reports personal fees from Merck, Seattle Genetics, Spectrum, and Pharmacyclics, outside the submitted work. SLG reports personal fees and non-financial support from Roche; grants and personal fees from Janssen-Cilag; and personal fees from Celgene, outside the submitted work. TR reports grants from Acerta Pharma and Pharmacyclics, and grants and personal fees from Janssen, during the conduct of the study. WR reports personal fees and non-financial support from Acerta Pharma, is an investor in Acerta at Quogue BioVentures II, outside the submitted work, and is an inventor for acalabrutinib patents pending or issued. TC is currently employed by Acerta Pharma and is a shareholder of AstraZeneca. AH is currently an employee of Acerta Pharma; and has a patent pending for acalabrutinib. RI is currently an employee of Acerta Pharma; and has a patent pending for acalabrutinib. JGS is currently an employee of Acerta Pharma with stock options in the company. WJ reports grants from Acerta Pharma during the conduct of the study; and grants from Janssen, Merck, Gilead, TG Therapeutics, Sandoz, Pfizer, and Takeda, outside the submitted work. JDo, RD, AH, XH, RI, PP, WR, and JGS are employees of Acerta Pharma. GD, TL, RE, APK, and LO declare no competing interests.
References
- 1.Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol 2016; 34: 1256–69. [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Ferrero S. The role of targeted treatment in mantle cell lymphoma: is transplant dead or alive? Haematologica 2016; 101: 104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JB, Zain JM, Kahl BS. Current approaches to mantle cell lymphoma: diagnosis, prognosis, and therapies. Am Soc Clin Oncol Educ Book 2017; 37: 512–25. [DOI] [PubMed] [Google Scholar]
- 4.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369: 507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol 2012; 31: 119–32. [DOI] [PubMed] [Google Scholar]
- 6.Pharmacyclics. Imbruvica package insert. Sunnyvale, CA: Pharmacyclics, 2017. [Google Scholar]
- 7.Saffran DC, Parolini O, Fitch-Hilgenberg ME, et al. Brief report: a point mutation in the SH2 domain of Bruton’s tyrosine kinase in atypical X-linked agammaglobulinemia. N Engl J Med 1994; 330: 1488–91. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Q, Zhang M, Winkelstein J, Chen SH, Ochs HD. Unique mutations of Bruton’s tyrosine kinase in fourteen unrelated X-linked agammaglobulinemia families. Hum Mol Genet 1994; 3: 1899–900. [DOI] [PubMed] [Google Scholar]
- 9.Stephens DM, Spurgeon SE. Ibrutinib in mantle cell lymphoma patients: glass half full? Evidence and opinion. Ther Adv Hematol 2015; 6: 242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia 2015; 29: 783–87. [DOI] [PubMed] [Google Scholar]
- 12.Covey T, Gulrajan M, Krantz F, et al. ACP-196: a novel covalent Bruton’s tyrosine kinase (Btk) inhibitor with improved selectivity and in vivo target coverage in chronic lymphocytic leukemia (CLL) patients. Cancer Res 2015; 75 (suppl): 2596. [Google Scholar]
- 13.Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase (BTK) inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther 2017; 363: 240–52. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32: 3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–86. [DOI] [PubMed] [Google Scholar]
- 16.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 17.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–44. [DOI] [PubMed] [Google Scholar]
- 18.Chang BY, Francesco M, De Rooij MF, et al. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood 2013; 122: 2412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davids MS, Burger JA. Cell trafficking in chronic lymphocytic leukemia. Open J Hematol 2012; 3 (suppl 1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiestner A The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015; 100: 1495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood 2007; 110: 3316–25. [DOI] [PubMed] [Google Scholar]
- 22.Jayappa KD, Portell CA, Gordon VL, et al. Microenvironmental agonists generate de novo phenotypic resistance to combined ibrutinib plus venetoclax in CLL and MCL. Blood Adv 2017; 1: 933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119: 1182–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012; 119: 2590–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


