Letter to the Editor
GATA2 is a master transcription factor that governs hematopoietic stem cell (HSC) generation in the mammalian embryo and confers multi-lineage differentiation of hematopoietic stem and progenitor cells [1–4]. Previously, we demonstrated that Gata2 expression in fetal liver myeloid progenitors (MPs) is controlled by its upstream −77 enhancer [5, 6]. While −77−/− fetal liver HSCs exhibited normal or greater than normal long-term repopulating activity, myeloid progenitor (MP) differentiation potential was impaired, thus causing anemia and embryonic lethality. However, whether the −77 enhancer has essential functions to regulate adult hematopoiesis has not been established.
We analyzed adult hematopoiesis in 6-week old wild-type (WT) control, −77+/−, and Gata2+/− mice. Gata2+/− mice served as a positive control for reducing Gata2 expression in adult hematopoietic stem and progenitor cells (HSPCs) [7, 8]. By contrast to normal fetal liver hematopoiesis in −77+/− embryos [5], the HSC compartment was enlarged in −77+/− bone marrow (BM) and spleen (SP), while the multipotent progenitor (MPP) compartment was elevated only in SP (Fig. 1A). −77+/− HSCs and MPPs exhibited decreased apoptosis (Fig. 1B and 1C) and increased quiescence (Fig. 1D and 1E). Our data suggest that increased −77+/− MPPs in SP may result from increased HSC numbers, decreased apoptosis, and/or increased quiescence. By contrast, HSC and MPP compartments were reduced in Gata2+/− mice (Fig. 1A), and these cells exhibited increased apoptosis (Fig. 1B) and quiescence (Fig. 1C). Quantification of Lin− Sca1+ cKit+ (LSK) cells and MPs revealed that similar to HSCs and MPPs, −77+/− LSK cells expanded in SP (Fig. S1A) and exhibited decreased apoptosis (Fig. S1C) and increased quiescence (Fig. S1D). Gata2+/− LSKs were reduced in BM and exhibited increased apoptosis and quiescence, consistent with the previous report [8]. The MP compartment was largely normal in −77+/− and Gata2+/− mice (Fig. S1B). These analyses revealed unexpected phenotypes in −77+/− adult HSCs and MPPs that were not predictable from our analysis of −77+/− embryos [5] nor from Gata2+/− mice [8].
Figure 1. Increased HSC survival in Gata2 −77+/− mice.
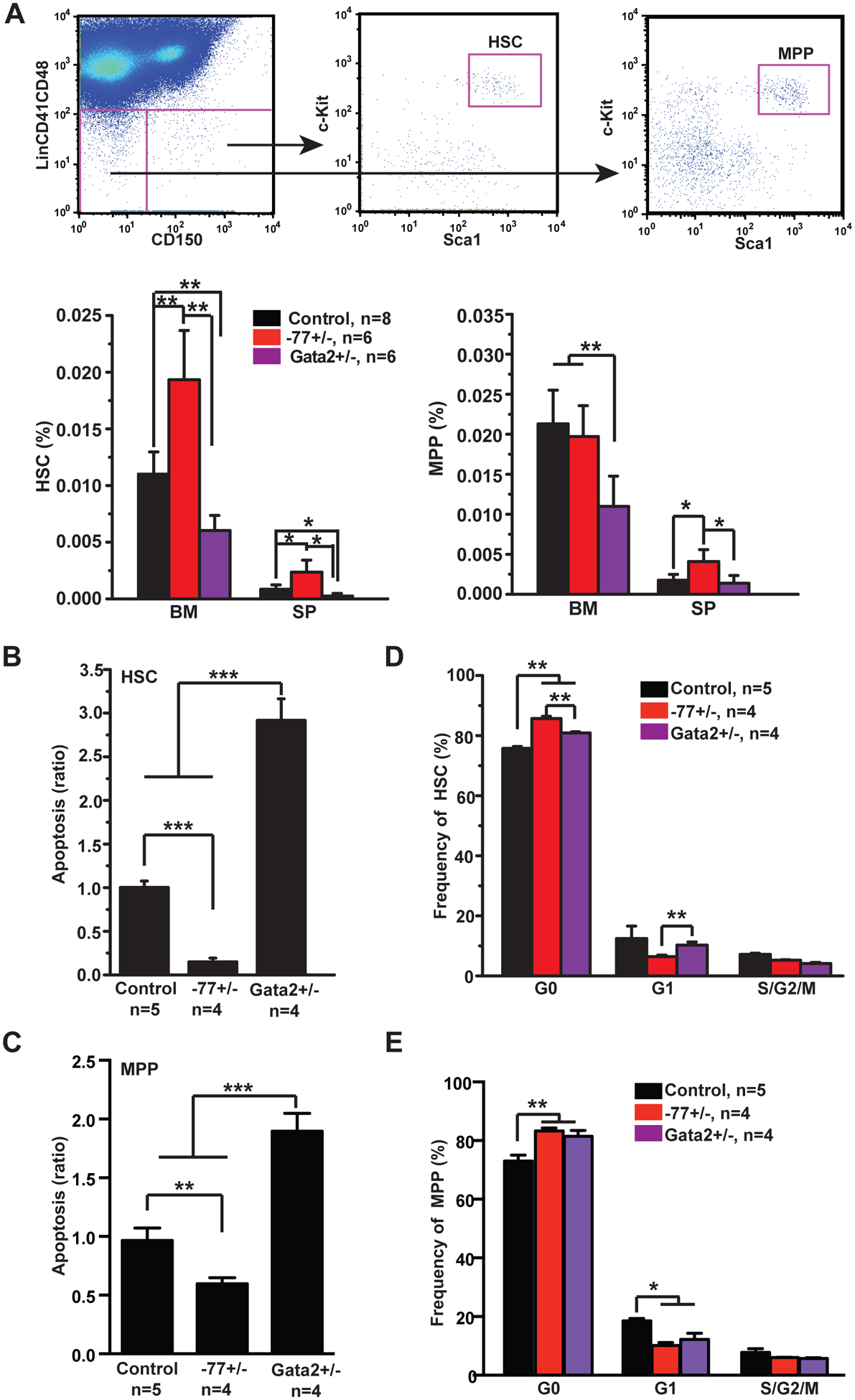
Control (wild-type C57BL/6), Gata2 −77+/− (−77) and Gata2+/− (Gata2+/−) mice were sacrificed at 6-weeks of age. (A) Gating strategy for hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs). HSCs are defined as Lin− Sca1+ c-Kit+ CD41− CD48− CD150+ cells and MPPs are defined as Lin− Sca1+ c-Kit+ CD41− CD48− CD150− cells. HSCs and MPPs were quantified in bone marrow (BM) and spleen (SP). (B, C) Quantification of apoptotic HSCs (B) and MPPs (C) from BM using Annexin V and DAPI. (D, E) Cell cycle analysis of BM HSCs (D) and MPPs (E) using Ki67 and DAPI. The results are presented as means + SD. * P<0.05, ** P<0.01; *** P<0.001.
We conducted a serial competitive reconstitution analysis of −77+/− HSC functions in vivo. WT, −77+/−, and Gata2+/− BM cells (CD45.2+) were mixed with competitor cells (CD45.1+) at 1:1 ratio and transplanted into lethally irradiated recipients (CD45.1+). Consistent with what we found with −77+/− mice, −77+/− BM cells displayed an increase (p<0.001) in multi-lineage reconstitution of primary recipients in comparison to WT BM cells (Fig. 2A). At 16-week post-transplant, recipients were sacrificed, and donor-derived hematopoiesis was analyzed. All −77+/−-derived cells were overrepresented in the primary recipient BM (Fig. 2B), including myeloid, T- and B-lineage cells (Fig. 2C), as well as HSCs, MPPs, common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), megakaryocyte-erythroid progenitors (MEPs), and common lymphoid progenitors (CLPs) (Fig. 2D). In secondary recipients, the reconstitution capacity of −77+/− BM cells was comparable to that of WT BM cells (Fig. S2A). Although expansion of donor-derived HSC and MPP compartments persisted in −77+/− recipients, −77+/−-derived progenitor compartments were indistinguishable from those of WT recipients (Fig. S2B). These results suggest a defect in stem to progenitor cell transition, which was not detected in −77+/− embryos [5]. Consistent with prior reports [7, 8], Gata2+/− BM cells exhibited decreased reconstitution (p <0.01) in primary and secondary recipients (Fig. 2 and S2). In aggregate, our data demonstrate a unique −77 enhancer function to regulate adult HSC activity that was not predicted from analysis of 77+/− embryos or Gata2+/− mice.
Figure 2. Gata2 −77+/− bone marrow cells exhibit increased long-term reconstitution activity in primary recipients.
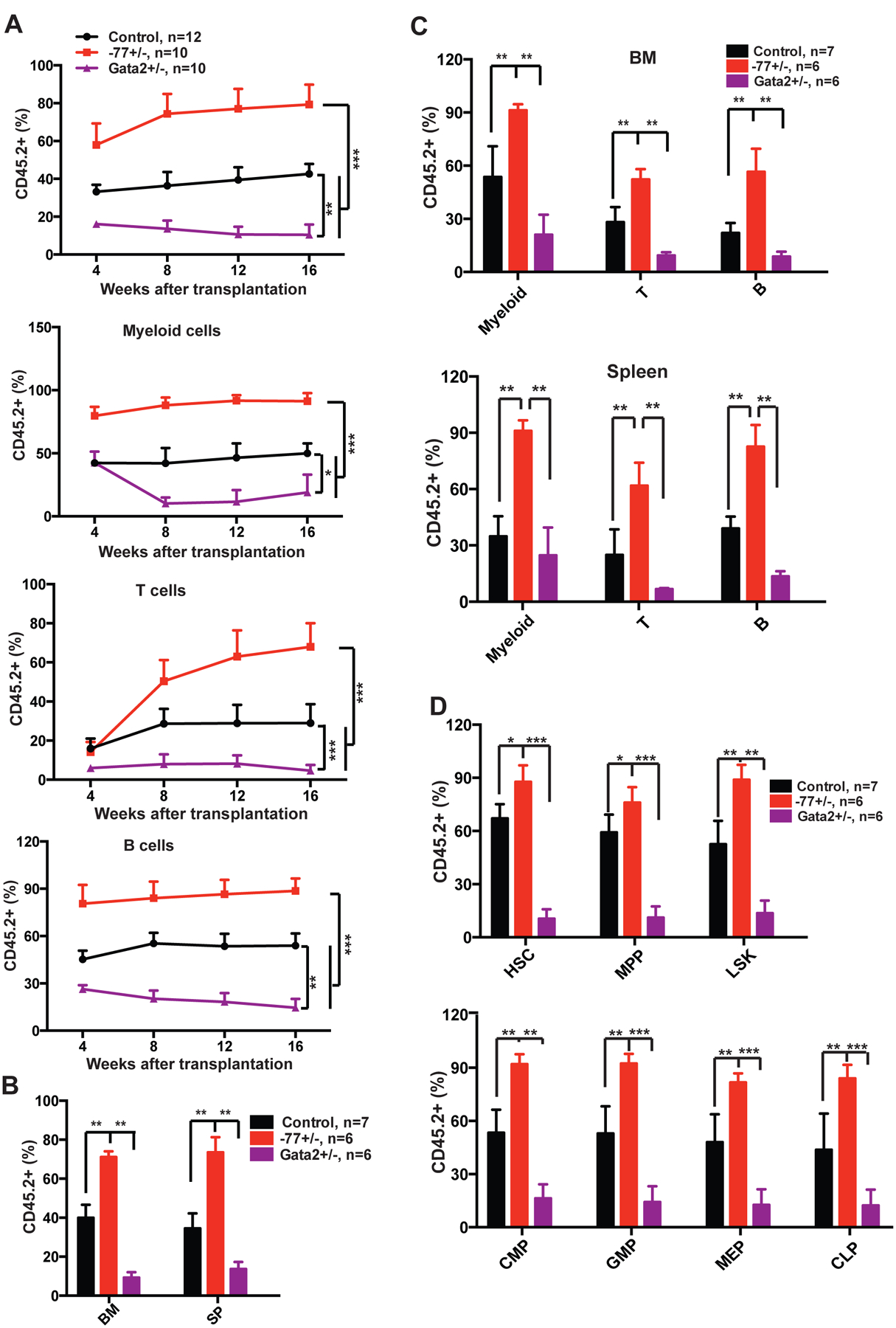
1×106 bone marrow (BM) cells (CD45.2+) from 6-week old control (wild-type C57BL/6), Gata2 −77+/− (−77), and Gata2+/− (Gata2+/−) mice were mixed with 1×106 competitor BM cells (CD45.1+) and injected into lethally irradiated CD45.1+ recipients. (A) Quantification of donor-derived myeloid, T- and B-cells in peripheral blood of primary recipients 4, 8, 12 and 16 weeks after transplantation. (B-D) Primary recipients were sacrificed 16 weeks posttransplant for terminal evaluation. (B) Quantification of donor-derived cells in BM and spleen (SP) of primary recipients. (C) Quantification of donor-derived myeloid, T- and B-cells in BM and SP of primary recipients. (D) Quantification of donor-derived hematopoietic stem cells (HSCs), multipotent progenitors (MPPs), Lin− Sca1+ c-Kit+ (LSKs), common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), megakaryocyte-erythroid progenitors (MEPs), and common lymphoid progenitors (CLPs) in BM of primary recipients. The results are presented as means + SD. * P<0.05, ** P<0.01; *** P<0.001.
To determine whether the −77 enhancer regulates hematopoietic stem and progenitor cells (HSPCs) by conferring Gata2 expression, its only known activity thus far, Gata2 mRNA levels were quantified in WT, −77+/−, and Gata2+/− LSK and MP cell populations using qRT-PCR. Consistent with the prior report [8], Gata2 expression was reduced in Gata2+/− LSKs and MPs. Surprisingly, Gata2 expression in −77+/− LSKs and MP populations was also lower than that of WT cells (Fig. S3). We analyzed transcript levels of genes localized adjacent to Gata2, Eefsec, Rab7, and Rpn1. These genes are known not to be regulated by −77 in fetal liver cells [5]. The mRNA levels of these genes in −77+/− and Gata2+/− LSK and MPs were indistinguishable from those in WT cells (Fig. S3). Furthermore, we sorted WT and −77+/− HSCs and MPPs and quantified mRNA levels of HSC stemness genes and apoptosis genes using qRT-PCR (Fig. S4). We found that c-Kit, Myb, Mpl, and Gata2 were upregulated in −77+/− HSCs (Fig. S4A), while c-Kit, Myb, and Mpl were upregulated and Gata2 was downregulated in −77+/− MPPs (Fig. S4B). For the apoptosis genes, genes promoting cell survival were upregulated and genes promoting apoptosis were downregulated in −77+/− HSCs and MPPs. Our data suggest that the qualitatively different phenotypes of −77+/− and Gata2+/− HSCs is associated with the opposite regulation of Gata2 expression in HSCs. This may reflect downregulation of Gata2 expression in some cells of the −77+/− mice vs. in all cells of the Gata2+/− mutants. Alternatively, this phenotypic difference may reflect a previously unrecognized −77 enhancer function in HSCs.
In addition to their roles in normal hematopoiesis, both −77 and Gata2 itself are linked to oncogenic RAS-driven cancers. In 2% of human AML with poor prognosis, a chromosomal inversion [inv(3)] repositions GATA2 −77 enhancer next to MECOM, increasing MECOM expression, which encodes EVI1, and lowers GATA2 expression, inducing AML [6, 9, 10]. Activating receptor tyrosine kinase (RTK)/Ras mutations characterize ~98% of inv(3) patients [11]. Synergism between Gata2+/− and Evi1 overexpression [12], and synergism between oncogenic Nras and Evi1 overexpression [13] were reported in leukemogenesis. Moreover, in non-small cell lung cancer, GATA2 promotes the survival of oncogenic KRAS-dependent cancer cells, and thus its downregulation constitutes a synthetic lethality in these cells [14]. It remains unknown, however, if −77+/− or Gata2+/− cooperates with oncogenic Kras to promote leukemogenesis. We tested this possibility by generating 4 cohorts of mice, Mx1-Cre (control), KrasLSL G12D/+; Mx1-Cre (Kras), KrasLSL G12D/+; −77+/−; Mx1-Cre (Kras;−77), and KrasLSL G12D/+; Gata2+/−; Mx1-Cre (Kras;Gata2). At 6-weeks of age, Kras;−77 and Kras;Gata2 mice displayed largely similar myeloproliferative neoplasm (MPN) phenotypes as Kras mice, including splenomegaly (Fig. S5A), increased white blood cell count and decreased platelet number in peripheral blood (PB) (Fig. S5B), and increased monocytes and/or neutrophils in various hematopoietic tissues (BM, SP, and PB) (Fig. S5C). At the moribund stage, all the Kras, Kras;−77, and Kras;Gata2 mice developed MPN (Fig. S5D and S5E). They also developed acute T-cell lymphoblastic leukemia (T-ALL) (defined as thymus weight >150 mg as we reported previously [15]) with various penetrances (Fig. S5F). All of these mice had a comparable survival (Fig. S5G). Upon transplantation, Kras, Kras;−77, and Kras;Gata2 BM cells reconstituted recipients indistinguishably (Fig. S6A). The recipients developed a high-incidence of T-ALL and MPN with a lower frequency (Fig. S6B–S6E). Again, no significant difference was detected in their survival (Fig. S6F). Our data demonstrate that neither −77+/− nor Gata2+/− heterozygous mutants significantly impact oncogenic Kras-induced leukemogenesis in these mouse models.
In summary, our study evaluated Gata2 −77 enhancer function in adult hematopoiesis and oncogenic Kras-driven leukemia. We identified a role of −77 enhancer to regulate adult HSC survival. These −77+/− adult hematopoietic phenotypes did not impact survival of oncogenic Kras mice.
Sincerely,
Supplementary Material
Acknowledgements
We are grateful to Dr. Stuart Orkin for generously providing Gata2+/− mice. We thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its Shared Services (Flow Cytometry Laboratory, Genome Editing and Animal Models Shared Resource, and Experimental Pathology Laboratory). The Flow Cytometry Laboratory was supported by NIH Shared Instrument Grants 1S10RR025483-01 (BD FACS AriaII BSL-2 Cell Sorter) and 1S100OD018202-01 (BD LSR Fortessa). This work was supported by NIH grants CA152108 to J.Z and DK69634 to E.H.B and NIH/NCI P30 CA014520-UW-Comprehensive Cancer Center (UWCCC) Support.
Footnotes
Conflict of Interest Disclosures
We declare no competing financial interests.
Methods are described in Supplementary Information.
References
- 1.Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 1994. September 15; 371(6494): 221–226. [DOI] [PubMed] [Google Scholar]
- 2.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 1997. May 15; 89(10): 3636–3643. [PubMed] [Google Scholar]
- 3.de Pater E, Kaimakis P, Vink CS, Yokomizo T, Yamada-Inagawa T, van der Linden R, et al. Gata2 is required for HSC generation and survival. J Exp Med 2013. December 16; 210(13): 2843–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Johnson KD, Chang YI, Boyer ME, Dewey CN, Zhang J, et al. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J Exp Med 2013. December 16; 210(13): 2833–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson KD, Kong G, Gao X, Chang YI, Hewitt KJ, Sanalkumar R, et al. Cis-regulatory mechanisms governing stem and progenitor cell transitions. Sci Adv 2015. September; 1(8): e1500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta C, Johnson KD, Gao X, Ong IM, Katsumura KR, McIver SC, et al. Integrating Enhancer Mechanisms to Establish a Hierarchical Blood Development Program. Cell reports 2017. September 19; 20(12): 2966–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med 2004. October 4; 200(7): 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 2005. July 15; 106(2): 477–484. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki H, Suzuki M, Otsuki A, Shimizu R, Bresnick EH, Engel JD, et al. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell 2014. April 14; 25(4): 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groschel S, Sanders MA, Hoogenboezem R, de Wit E, Bouwman BAM, Erpelinck C, et al. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell 2014. April 10; 157(2): 369–381. [DOI] [PubMed] [Google Scholar]
- 11.Groschel S, Sanders MA, Hoogenboezem R, Zeilemaker A, Havermans M, Erpelinck C, et al. Mutational spectrum of myeloid malignancies with inv(3)/t(3;3) reveals a predominant involvement of RAS/RTK signaling pathways. Blood 2015. January 1; 125(1): 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katayama S, Suzuki M, Yamaoka A, Keleku-Lukwete N, Katsuoka F, Otsuki A, et al. GATA2 haploinsufficiency accelerates EVI1-driven leukemogenesis. Blood 2017. August 17; 130(7): 908–919. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Haigis KM, McDaniel A, Harding-Theobald E, Kogan SC, Akagi K, et al. Hematopoiesis and leukemogenesis in mice expressing oncogenic NrasG12D from the endogenous locus. Blood 2011. February 10; 117(6): 2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar MS, Hancock DC, Molina-Arcas M, Steckel M, East P, Diefenbacher M, et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell 2012. April 27; 149(3): 642–655. [DOI] [PubMed] [Google Scholar]
- 15.Kong G, Du J, Liu Y, Meline B, Chang YI, Ranheim EA, et al. Notch1 gene mutations target KRAS G12D-expressing CD8+ cells and contribute to their leukemogenic transformation. J Biol Chem 2013. June 21; 288(25): 18219–18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


