Abstract
To investigate if the anxiety associated with coronavirus disease 2019 (COVID-19) is a promoting factor to tinnitus. A retrospective research design collected from 188 tinnitus patients, was used to compare the clinical characteristics of tinnitus between the patients in 2020 under pandemic pressure and those from the matching period in 2019. While anxiety was quantified using the Zung’s Self-rating Anxiety Scale (SAS), tinnitus severity was evaluated using the Tinnitus Handicap Inventory (THI) questionnaire and the test of tinnitus loudness (TL). The assessments were repeated after the sound therapy plus educational counselling (STEC) for 38 patients in 2020 and 58 patients in 2019 and compared with EC alone therapy for 42 patients in 2020 and 17 patients in 2019. A large increase in anxiety was evident in 2020 in both case rate and SAS. The treatment of both methods was less effective in 2020. SAS, THI and TL were all deteriorated after the EC alone treatment in 2020, while an improvement was seen in 2019. This suggests that EC alone could not counteract the stress by COVID-19 at all, and the stress, if not managed well, can significantly increase the severity of tinnitus and associated anxiety. By using the EC subgroup in virtual control, we conclude that anxiety can serve as a promoting factor to tinnitus. We believe that this is the first study report that confirm the causative/promotive role of anxiety on tinnitus during COVID-19 pandemic.
Introduction
The spread of coronavirus disease 2019 (COVID-19) has already reached pandemic proportions, affecting the majority of countries, areas, and territories across the world [1]. By the end of June 2020, over nine million people had tested positive for COVID-19 with the death toll increasing to more than 484,000 globally [2]. Decisive containment measures in China have reduced new cases and the spread of infection [3]. However, worries about the spread of the disease, living difficulties, and financial burden related to the pandemic are likely to have had negative psychosocial impacts on residents, as reported by many recent studies [4–6]. It would be reasonable, therefore, to expect an increase in the incidence of disorders that are associated with psychological issues.
Tinnitus is typically referred to as the perception of sound in the absence of an acoustic stimulus or that is only generated by structures in the ear, commonly described as ringing in one or both ears [7]. While the exact mechanisms of tinnitus remain unclear, many risk or promoting factors have been identified, including sensorineural hearing loss, noise exposure, vestibular schwannoma, ototoxic medications, and emotional stress [8, 9]. Tinnitus has been linked to stress and related disorders in many previous studies. This link has been thoroughly reviewed, repeatedly, by different authors (e.g., [10–17]). The direction and causality of this link remain unclear, as pointed out in many previous studies, although individuals’ emotional states appear to be an important factor mediating the effects of tinnitus loudness on tinnitus-related distress [18–20]; anxiety, somatization, and in particular depression have also been identified as possible mediators of tinnitus-related distress [21–24].
The clinicians in our department noticed that the tinnitus patients seen since the hospital was reopened after COVID-19 had more emotional complaints than before. We thought that this might be related to the various pressures experienced by the patients during the pandemic event and the lockdown. Therefore, the COVID-19 pandemic and lockdown might provide a good opportunity to investigate whether anxiety impacts tinnitus as a promoting or enhancing factor. The present study explored whether anxiety was increased by the COVID-19 pandemic in subjects with tinnitus, and if so whether the increased anxiety affected the severity of tinnitus and the outcomes of tinnitus treatments.
Materials and methods
Study design
In this retrospective study, clinical data from outpatients visiting our department (the Hearing Center of Otolaryngology Department of the Sichuan Provincial People’s Hospital and Sichuan Academy of Medical Sciences, Chengdu, Sichuan, People’s Republic of China) were collected over the same periods, from March 1 to April 14, in both 2020 and 2019. This period in 2020 was the first 6 weeks of the reopening of our department to non-emergency visits after the nationwide lockdown for COVID-19 in China (from January 23 to February 29, 2020) that coincided with the deceleration phase of the pandemic and the resumption of economic activities. In this period, there were concerns about a resurgence of COVID-19 [25].
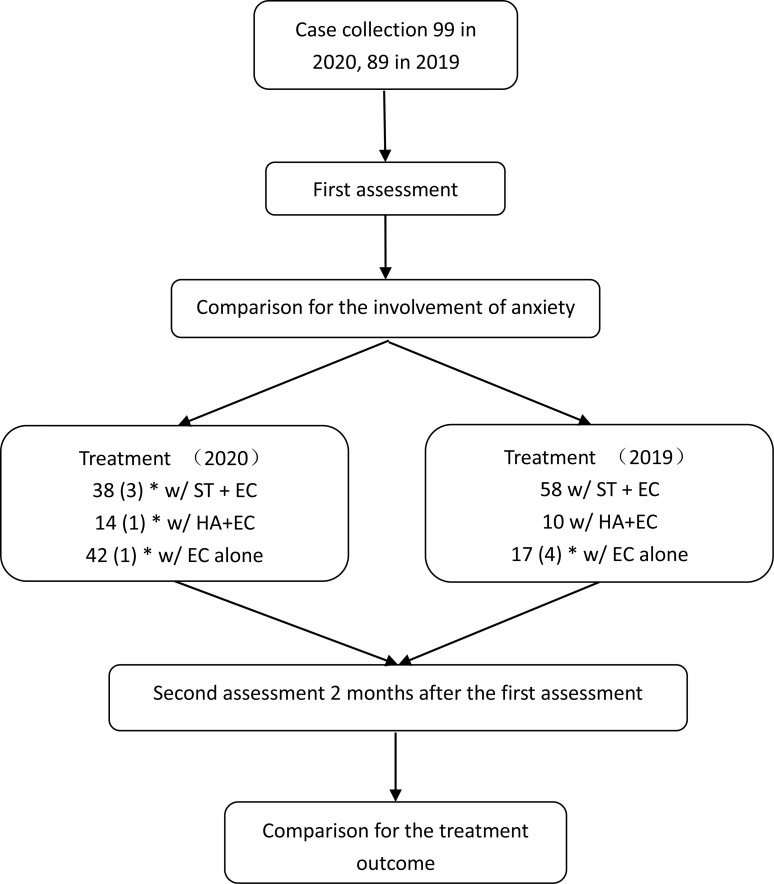
The same protocol was followed for the treatment of patients during both years. On the initial visit, after collecting their history, every patient received a comprehensive audiological and psychological assessment. After the assessment, they were treated with one of three methods based on reported efficacy, financial cost, and the patient’s preference: sound therapy (ST) with educational counseling (EC) or relaxation therapy, sound amplification with EC and relaxation therapy, or EC and relaxation therapy without further treatment. Two months after the initial appointment, every participant was examined in a second assessment. Fig 1 shows a flowchart of the major procedures of this study. Although no procedure was experimental, we sought and received approval for the study from the Ethics Review Board of the Sichuan Provincial People’s Hospital and Sichuan Academy of Medical Sciences (permit number: 2020–355) which was approved in June 2020 and the data were collected after the approval was granted. All data were fully anonymized. This study was conducted according to the principles expressed in the Declaration of Helsinki [26].
Fig 1. Flowchart of the major procedures in this study.
*numbers in parentheses are those of cases that were lost to the study. THI: tinnitus handicap inventory, SAS: Zung’s Self-Rating Anxiety Sale, ST: sound therapy, HA: hearing aid, EC: educational counseling.
Audiological tests and tinnitus evaluation
The procedures for all tests were explained to the patients before they were conducted. All patients were examined using monocular otoscopy to identify any sign of blockage or inflammation in ear canals or perforation in the tympanic membrane. Tympanometry was tested at the most common 226 Hz probe tone, using an AT235 impedance meter (Interacoustics, Assens, Denmark); the type of tympanogram was determined for each ear (with type A as normal). Those who were abnormal in those tests were not included in this study.
The hearing status was tested with pure-tone audiometry (AC40, Interacoustics) in a soundproofed room. The air conduction threshold was examined for frequencies ranging from 250 Hz to 8 kHz using TDH 39 headphones (Telephonics, NY, USA) and bone conduction hearing was examined from 500 Hz to 4 kHz using a B-72 bone-conduction vibrator (Radioear, PA, USA), each in octave steps. The hearing thresholds were determined at each frequency using the standard Hughson–Westlake up–down procedure. Thresholds of 20 dB HL or lower were considered normal. Pitch matching was conducted using a table-top sound generator (BTD01, BetterLife Medical Technology Co., Ltd., Jiangsu, China) to produce pure tones for tonal tinnitus or narrow-band noise for non-tonal tinnitus. The match was established by adjusting the central frequency and bandwidth, which could be changed from 100 Hz to 1 kHz, around the center frequency. In loudness matching, the matched tone or noise was presented continuously to the ear without or less intense tinnitus, and the level of the matching signal was adjusted from low to high in 1 dB steps until the tinnitus loudness (TL) could be matched [27]. In this report, loudness matching results are presented in dB SL.
Educational counseling and relaxation therapy
The counseling was performed by the audiologists for each patient with tinnitus to acknowledge the patient’s suffering, and to help the patient understand tinnitus, demystify the condition, and correct any false preconceptions (duration 1 h) [28]. Relaxation therapy consisted of home-based exercises, such as listening to music, avoiding unnecessary tension, and tai chi [29–31]. Patients were advised to execute this for two sessions of 30 min per day over a period of 8 weeks.
Sound therapy
The first step of the ST was to identify the nature of the tinnitus in pitch and minimum masking level (MML). Pitch matching was conducted using the same sound generator (BTD01) to produce pure tones for tonal tinnitus or narrow-band noise for non-tonal tinnitus to the ear with tinnitus [32]. In MML test, the matched tone or noise was presented continuously, and the level of the masking signal was adjusted from low to high until the tinnitus could hardly be heard [27, 33]. Using the pitch matching and MML data, a sound file was generated for each individual to produce a sound matching their tinnitus in frequency and maskability level. This sound file was the uploaded to an ear level sound generator (BTM-N6, BetterLife Medical Technology Co., Ltd.) that was dispensed to the patient. The patients were instructed to listen to the sound file for 30 min each time, and to gradually increase from once to 3–6 times per day, every day, during the whole course of home-based therapy, which lasted for 2 months.
Questionnaires
All participants involved in this study completed two questionnaires at the initial visit and again during the follow-up, two months later, regardless of their inclusion. The Chinese version of the Tinnitus Handicap Inventory (THI) [34] questionnaire was used in this study, consisting of 25 questions to assess the difficulty caused by tinnitus with respect to its functional, emotional, and catastrophic aspects [35, 36].
A Chinese version of Zung’s Self-rating Anxiety Scale (SAS) questionnaire was used, which was adapted from a previous report [37, 38]. The raw scores were multiplied by 1.25 to generate the index scores [38]. We used a value of 45 as the cut-off for anxiety, instead of 50, since the cut-off of 45 provided a higher sensitivity as reported in the most recent publication [39]. Based on the cut-off, the tinnitus patients were divided into subgroups with and without anxiety in each year or pooled across two years.
Statistical analyses
All parametric data are presented as mean ± standard deviation unless otherwise specified. When the parameters of participants were compared between two groups, the t-test was used or, if among multiple groups, analysis of variance (ANOVA) was used for continuous variables and the chi-square test for categorical variables, including sex, age, and site of tinnitus, and for risk factors among groups. Treatment outcomes were evaluated by comparing the scores of THI and SAS before and after the treatments, using a paired t-test or ANOVA. All analyses were performed using the SPSS 19.0.0 software at a significance level of 0.05. In figures, the significant level was indicated by the number of symbols (e.g., *), with 1, 2 or 3 representing ρ <0.05, 0.01, and 0.001 respectively.
Results
A total of 99 cases were collected between March 1 and April 14, 2020, and 89 in the same period in 2019 (Fig 1). Table 1 compares the demographics and tinnitus characteristics between the subjects in the different years. The case load for tinnitus appeared to be higher in 2020 than in the same period in 2019 (99 vs. 89, or an increase of 11.2%). Such an increase could be largely attributed to the accumulation of cases when all the non-emergency visits were suspended during the lockdown between January and February 2020. The two groups of different years were matched by all clinical characteristics except the incidence of anxiety.
Table 1. Comparison of initial clinical characteristics of patients between 2020 and 2019.
| March-April 2020 | March-April 2019 | ρ value | |
|---|---|---|---|
| Sex (M:F) | 43:56 | 43:46 | .502 |
| Age (year old, M ± SD) | 50.8 ± 15.1 | 52.6 ± 14.7 | .487 |
| Educational background | .812 | ||
| Bachelor and superior | 54 | 47 | |
| Inferior to bachelor | 45 | 42 | |
| Duration (month) | 25 ± 53.6 | 31.3 ± 50.4 | .108 |
| Site | .177 | ||
| Bilateral | 36 | 41 | |
| Unilateral | 63 | 48 | |
| Anxiety involved/total# | 74/99 (74%) | 53/89 (59%) | .026 |
| Risk factors | |||
| Sensorineural hearing loss | 69 | 65 | .614 |
| Noise exposure | 1 | 0 | 1 |
| Hypertension | 3 | 6 | .179 |
| Hyperthyroidism | 1 | 0 | 1 |
| Head/neck trauma | 1 | 0 | 1 |
Chi-square test was used for the between-group comparison, sex, educational background, site, anxiety and the risk factor of sensorineural hearing loss using, t-test on age, Mann-Whitney Rank Sum Test on Duration, Fisher’s exact test on the risk factors of noise exposure, hypertension, hyperthyroidism and head/neck trauma.
The increase in anxiety in 2020 and its impact on THI and TL
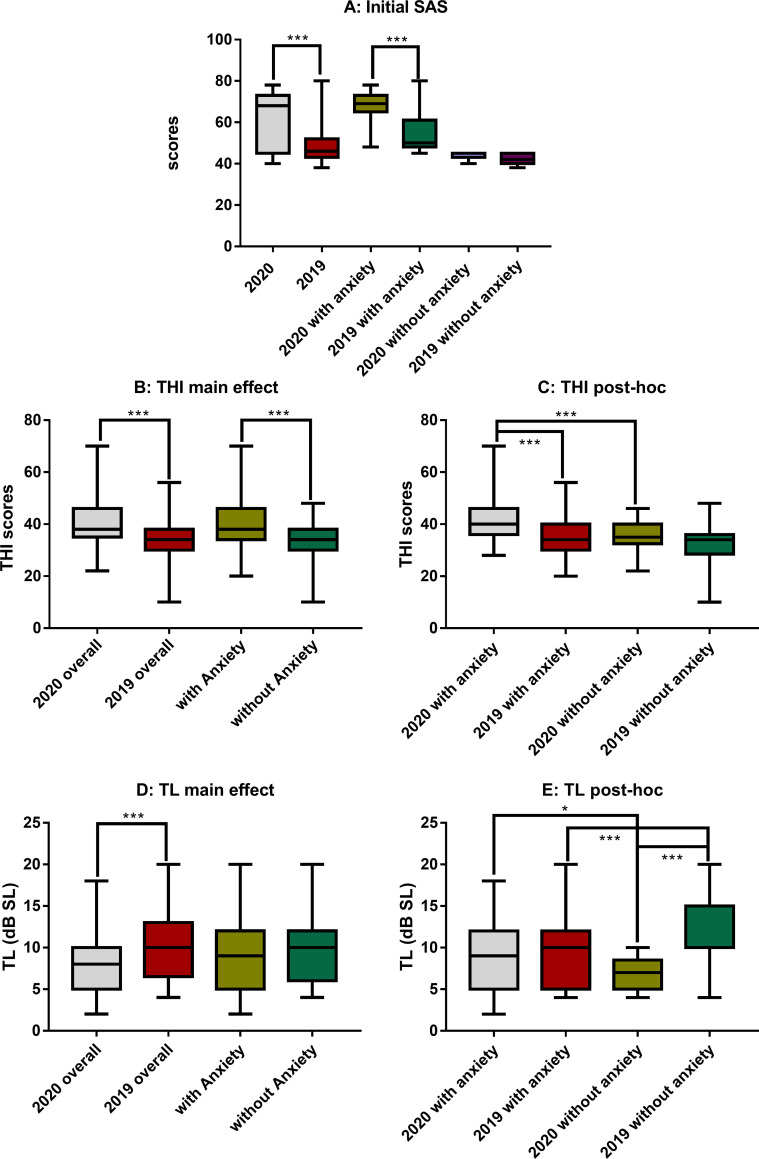
In the 2020 group, 74 out of 99 (or 74.7%) subjects had an SAS higher than 45 (the criterion for anxiety), which was significantly higher than that in the 2019 group (53/89, or 59%, χ2 = 4.938, ρ = 0.026 via chi-square test). Overall, the SAS score in 2020 group was significantly higher than that of 2019 group (61.9 ± 11.9 in 2020 versus 49.1 ± 8.6 in 2019; U = 6867 via Mann-Whitney Rank Sum Test, ρ < 0.001, Fig 2A), which was fully due to the difference in the anxiety subgroups (68.0 ± 6.4 in 2020 vs. 54 ± 8 in 2019; U = 3550 via Mann-Whitney Rank Sum Test, ρ < 0.001, Fig 2A). Therefore, the higher SAS in 2020 was not simply due to the higher incidence of subjects with anxiety, but also the higher level of anxiety in the involved subjects.
Fig 2. Comparisons of initial SAS, THI scores and TL between years and subjects with and without anxiety.
A: SAS showing a significant difference between years and between the subgroups within the two years. B and D: The differences in THI and TL as the result of the two main factors—year and anxiety. C and E: Post-hoc comparison on THI and TL showing the difference within the factors of year and anxiety respectively. Within 2020, subjects with anxiety appeared to have a significantly higher THI and TL; no difference was seen in THI between anxiety and non-anxiety subgroups within 2019, while a higher TL was seen in non-anxiety subgroup within 2019. THI: tinnitus handicap inventory, SAS: Zung’s Self-Rating Anxiety Sale, TL: tinnitus loudness.
The THI score in the 2020 group was (40.1 ± 6.9), which was significantly higher than that in the 2019 group (34 ± 8.3) as shown by the group effect in a two-way ANOVA against year group and anxiety (F1, 184 = 16.278, ρ < 0.001). The ANOVA also demonstrated a significant effect of anxiety: (38.8 ± 8.6) for subjects with anxiety and (33.8 ± 7.5) for those without (F1, 184 = 11.628, ρ < 0.001, Fig 2B). However, there was not a significant interaction between two factors (F1, 184 = 2.3, ρ = 0.131). Post-hoc pairwise comparisons showed that the THI score of anxiety subgroup in 2020 was (41.7 ± 7.7), which was significantly higher than the corresponding subgroup in 2019 (34.8 ± 8.1; q = 6.904, ρ < 0.001), and that of non-anxiety subgroup in 2020 (35.6 ± 5; q = 4.766, ρ < 0.001, Fig 2C). Interestingly, the THI of non-anxiety subgroup in 2020 was (almost) same as that of the anxiety subgroup in 2019 (via t-test, t0.05/76 = 0.432, ρ = 0.667). However, there was no significant difference in THI score across the non-anxiety subgroups between years (Fig 2C).
The between-year difference in THI was further analyzed using a breakdown of the scores in the emotional, functional, and catastrophic questionnaire sections. A significant between-year difference was demonstrated in the emotional score (14.636 ± 3.7 in 2020 and 12.3 ± 3.3 in 2019; the Mann–Whitney rank-sum test, U = 5942.5, ρ < 0.001), in the functional score (18.515 ± 3.6 in 2020 and 15.5 ± 4.2 in 2019, U = 5211.5, ρ < 0.001) and in the catastrophic scores (7.0 ± 2.5 vs. 6.1 ± 2.6, U = 5173, ρ = 0.035). This result suggests that the higher THI in 2020 could be partially related to the increase in anxiety.
A two-way ANOVA similar to that for THI showed a significant year effect with subjects in 2020 had significantly lower TLs (8.3 ± 3.5 dB SL) as compared to those in the 2019 group (10.4 ± 4.3 dB SL; F1, 184 = 21.745, ρ < 0.001). However, the effect of anxiety was not significant (F1, 184 = 0, ρ = 0.977; Fig 2D). The higher TL in 2019 could be largely attributed to the high TL in the non-anxiety subgroup this year as demonstrated by the Post-hoc pairwise test, which showed that the non-anxiety subgroups had a higher TL (11.7 ± 4.1 dB SL) in 2019 than the patients with anxiety in 2019 (9.5 ± 4.3 dB SL, q = 3.627, ρ < 0.001, Fig 2E). Within 2020, however, the anxiety subgroup had an TL of (8.9 ± 3.7) dB SL, which was slightly but significantly higher than the non-anxiety subgroup this year (6.7 ± 2.0 dB SL, q = 3.441, ρ = 0.015; Fig 2E). The result suggests that there is no clear indication whether anxiety played a role in the loudness of tinnitus.
Pearson correlation was conducted between SAS and THI and TL respectively in each year. In 2020, a weak positive correlation was seen between SAS and catastrophic THI (r = 0.319, p = 0.001), but not to another two subscales of THI. In this year there is also a moderate correlation between SAS and TL (r = 0.337, ρ < 0.001). In 2019, however, the significant correlation was seen in any pair of measurement (ρ > 0.05).
Anxiety and treatment outcomes
The 94 patients in the 2020 group completed their face-to-face follow-up 2 months after the first assessment, while this number was 85 in the 2019 group (Fig 1). The numbers of patients who received ST with EC (STEC), hearing aids with EC (HAEC), or EC alone were 38, 14, and 42, respectively in the 2020 group, while the respective numbers were 58, 10, and 17 in the 2019 group. Due to the small sample sizes in patients receiving hearing aids in 2020, we only analyzed the treatment outcomes of STEC and EC alone. No between-year differences were seen in basic demographic features, risk factors and duration of tinnitus between the years in subjects treated with STEC (Table 2) and EC alone (Table 3). The incidence of anxiety in the patients receiving STEC was higher in the 2020 group (Table 2), but not such year difference was seen in patients received EC alone (Table 3).
Table 2. Between-year match in the demographic and selected clinic features in tinnitus patients treated with STEC.
| May-June 2020 | May-June 2019 | ρ-value | |
|---|---|---|---|
| Sex (M:F) | 16:22 | 30:28 | .356 |
| Age (year old, mean ± standard deviation) | 48.2 ± 15.7 | 50.2 ± 14.1 | .629 |
| Educational background | .507 | ||
| Bachelor and superior | 21 | 36 | |
| Inferior to bachelor | 17 | 22 | |
| Duration of tinnitus (month) | 25.5 ± 43.7 | 31.8 ± 54.3 | .428 |
| Site | .454 | ||
| Bilateral | 18 | 32 | |
| Unilateral | 20 | 26 | |
| Anxiety involved/total # | 29/38 (76%) | 32/58 (55%) | .035 |
| Risk factors | |||
| Sensorineural hearing loss | 24 | 40 | .555 |
| Noise exposure | 0 | 0 | \ |
| Hypertension | 1 | 6 | .396 |
| Hyperthyroidism | 0 | 0 | \ |
| Head/neck trauma | 0 | 0 | \ |
Chi-square test was used for the between-group comparisons on sex, educational background, site, anxiety and the risk factor of sensorineural hearing loss using, t-test on age and duration, Fisher’s exact test on the risk factors hypertension.
Table 3. Between-year match in the demographic and selected clinic features in tinnitus patients treated with EC alone.
| May-June 2020 | May-June 2019 | ρ-value | |
|---|---|---|---|
| Sex (M:F) | 19:23 | 5:12 | .262 |
| Age (year old, mean ± standard deviation) | 49.3 ± 15.3 | 56.4 ± 12.9 | .097 |
| Educational background | .149 | ||
| Bachelor and superior | 21 | 5 | |
| Inferior to bachelor | 21 | 12 | |
| Duration of tinnitus (month) | 20.6 ± 33.4 | 27.5 ± 45.7 | .66 |
| Site | .222 | ||
| Bilateral | 15 | 9 | |
| Unilateral | 27 | 8 | |
| Anxiety involved/total # | 29/42 (69%) | 13/17 (76%) | .753 |
| Risk factors | |||
| Sensorineural hearing loss | 27 | 11 | .976 |
| Noise exposure | 0 | 0 | \ |
| Hypertension | 1 | 0 | 1 |
| Hyperthyroidism | 0 | 0 | \ |
| Head/neck trauma | 0 | 0 | \ |
Chi-square test was used for the between-group comparisons on sex, educational background, site and the risk factor of sensorineural hearing loss, t-test on age and tinnitus duration, Fisher’s exact test on anxiety and the risk factors of hypertension.
The effect of treatment on SAS
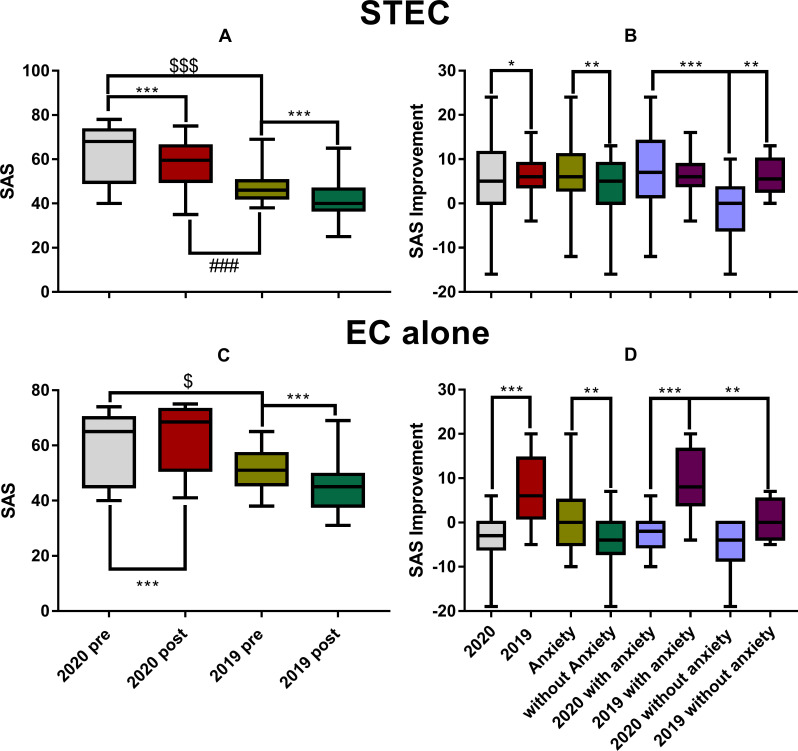
Fig 3 summarized the effect of the two treatments on SAS. In consistency with the data of whole sample (Fig 2A), the pre-treatment SAS was much higher in 2020 than in 2019 for the subjects treated with both STEC (Mann-Whitney Rank Sum Test, U = 411, ρ < 0.001, Fig 3A) and EC alone (U = 460.5, ρ = 0.031, Fig 3C). However, the effect of EC alone on SAS appeared to be qualitatively different from that of STEC in that the SAS was not decreased (improved) but increased in 2020 group after the treatment (Fig 3C), so that the post-treatment SAS in the 2020 group (63 ± 11) was even significantly higher than the before-treatment SAS in the 2019 group (52.9 ± 10, Mann–Whitney rank-sum test, U = 527, ρ < 0.004). This raised the question whether and how the number of subjects qualified as having anxiety changed after each treatment. Such changes were summarized in Table 4. In 2019, a large portion of subjects who had anxiety changed to non-anxiety status after either of the two treatments. In 2020, however, the number of cases with anxiety was increased, slightly after STEC, but largely after EC alone. In each method, there was a significant difference between years in the % change of cases with anxiety.
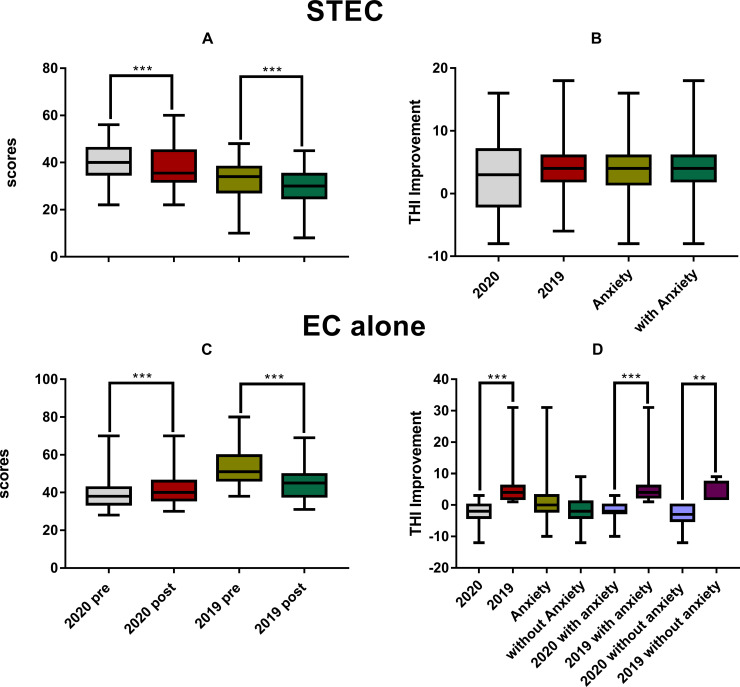
Fig 3.
The SAS difference before and after the treatment of STEC (upper panels) and EC alone (the lower panels). A and C: The pre- and post-SAS. B and D: the pre-post difference of SAS score. STEC treatment reduced SAS in both years (A). However, EC alone did not improve SAS in 2020, instead the SAS was increased significantly in the 2nd assessment (C). Correspondingly, STEC produced a slightly better improvement in SAS in 2019 than in 2020, but improvement by EC alone was much better in 2019 than in 2020, in which SAS was deteriorated. The number of symbols (*, $ or #) represents the level of significance, with 1, 2 or 3 symbols for ρ < 0.05, 0.01, or 0.001 respectively. STEC: sound therapy + educational counseling, EC: educational counseling.
Table 4. Changes of cases with anxiety after the treatments of STEC and EC alone.
| total cases | initial anxiety | # to non-anxiety | # to anxiety | final anxiety | Change % | ρ pre-post treatment | ρ between year within method | ρ between method within year | ||
|---|---|---|---|---|---|---|---|---|---|---|
| STEC | 2020 | 38 | 29 | 1 | 3 | 31 | 6.89% # | .574 | .001 | .488* |
| 2019 | 58 | 32 | 17 | 0 | 15 | -53.10% | .001 | .638 | ||
| EC alone | 2020 | 42 | 29 | 0 | 7 | 36 | 24.1% # | .068 | < .001 * | \ |
| 2019 | 17 | 13 | 6 | 0 | 7 | -46.2% | .037 | \ |
#: a positive change means an increase in cases with anxiety
*: the ρ values were the results of Fisher’s Exact Test, other cells using chi-square tests.
The SAS was significantly reduced in both years after the STEC treatment (Mann-Whitney Rank Sum Test, ρ < 0.001). However, due to the large initial difference, the post-treatment SAS score in the 2020 group (58.0 ± 10.6) was still significantly higher than the pre-treatment SAS in the 2019 group (48.3 ± 8.5, Mann–Whitney rank-sum test, U = 534, ρ < 0.001). These results suggest that the anxiety associated with COVID-19 was not been fully counteracted by the treatment.
To further evaluate the effect of STEC on anxiety, a two-way ANOVA was performed on the pre-post SAS difference against the factor of year group and anxiety (Fig 3B). A significant year difference was seen since the SAS improvement appeared to be slightly but significantly smaller in 2020 (5.0 ± 8.6) than in 2019 (6.1 ± 3.8, F1, 92 = 6.046, ρ = 0.016). However, this is conflicted with the fact that the subjects with anxiety gained more reduction in SAS after STEC as compared with non-anxiety in both years (6.6 ± 6.2 in the subjects with anxiety vs. 4.0 ± 5.9 in the non-anxiety subjects; effect of anxiety: F1, 92 = 10.447, ρ = 0.002). Furthermore, the post-hoc test within 2020 revealed a larger SAS reduction (7.0 ± 8.0) in the anxiety subgroup this year than the non-anxiety subgroup in which the SAS was increased (negative improvement: -1.5 ± 7.2, post-hoc test within 2020, Tukey Method; q = 5.364, ρ < 0.001). This result was in sharp contrast with the null difference in the SAS improvement between the anxiety subgroup (6.6 ± 6.2) and the non-anxiety subgroup (6.0 ± 3.5) in 2019 (via Tukey Method, q = 0.281, ρ = 0.843; Fig 3B).
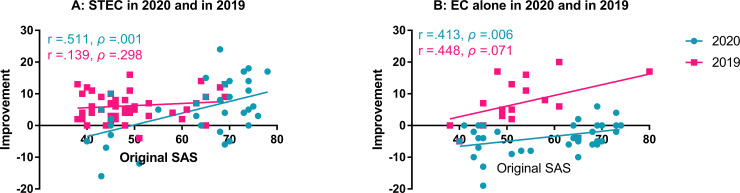
A two-way ANOVA similar to the STEC was done for EC alone and showed a significant effect of year group: the pre-post difference in SAS in 2020 was negative (-3.4 ± 4.6, for an worse SAS) as compared with the large improvement in 2019 (7.1 ± 7.5; F1, 55 = 26.022, ρ < 0.001). Since the initial SAS in the subgroup in 2020 receiving STEC was not significantly different from that in the subgroup receiving EC alone this year (63 ± 12 versus 59.5 ± 12.1; Mann–Whitney rank-sum test, U = 640.5, ρ = 0.129), the deteriorated SAS after EC alone suggests that the subjects in the EC subgroup in 2020 had experienced an increased stress after the first assessment, and the stress largely increased anxiety, which was not counteracted by the EC alone treatment. A significant effect of anxiety was also seen in subjects treated with EC alone: the SAS change after EC was (1 ± 7.4) in patients with anxiety before EC and (-4 ± 6) in those without (via a two-way ANOVA, F1, 55 = 11.038, ρ = 0.002) in years. There was no significant interaction between the two factors (F1, 55 = 2.773, ρ = 0.102). The large deterioration in SAS in the non-anxiety subjects received EC is obviously due to such change in 2020 in which the SAS changes in the non-anxiety subjects was (-5.4 ± 5.7), although this value was not significantly different from the change in non-anxiety subgroup in 2019 (0.5 ± 4.9; post-hoc test, q = 2.813, ρ = 0.052; Fig 3D). In both years, SAS improvement was smaller in the non-anxiety subgroups, and in 2020, SAS was deteriorated, instead of improved, in both anxiety and non-anxiety subgroups. In 2019, the SAS improvement in the anxiety subgroup (9.1 ± 7.1), which was significantly higher than the non-anxiety subgroup (0.5 ± 4.9) (post hoc test, via Tukey Method, q = 4.084, ρ = 0.006). In 2020, the SAS change in the anxiety subgroup was (-2.5 ± 3.8), and that in the non-anxiety subgroup was (-5.4 ± 5.7). However, the difference was not significant (post hoc test, Tukey Method, q = 2.324, ρ = 0.106). To further evaluate the impact of anxiety on clinic features of tinnitus, Pearson product moment correlation was calculated between the initial SAS score and the changes after the treatment. There was a moderate, positive, linear relationship between the initial SAS score and the change in patients receiving STEC in 2020 (r = 0.511, ρ = 0.001), but no significant correlation was found in 2019 (Fig 4A). In addition, a moderate and positive linear relationship was also seen between the initial SAS score and the change in patients receiving EC alone in 2020 (r = 0.413, p = 0.006; Fig 4B) but not in 2019 (r = 0.488, ρ = 0.071). These results suggest that the treatment was more effective for mitigating anxiety in subjects with higher SAS scores in 2020, which was associated with the COVID-19 pandemic.
Fig 4. Correlations between the initial SAS score and the improvement in SAS score.
A: The correlation of STEC by year. B: The correlation of EC alone by year. Significant, moderate correlations were seen in STEC group and EC alone groups in 2020 in which the average initial SAS scores were much higher. SAS: Zung’s Self-rating Anxiety Scale, ST: sound therapy, EC: educational counseling.
The effect of treatments on THI and TL
The effect of the treatments was first examined by self-reported improvement (reduction) of tinnitus loudness. As expected, the case number and rate reporting an improvement were higher in subjects treated with STEC than in those with EC alone in both years. More importantly, the case number with improvement was significantly lower in 2020 group than in 2019 in subjects treated with both methods (Table 5). However, there were no significant differences in the case rate improvements between subgroups with and without anxiety in both STEC group (ρ = 0.76, chi-square test) and EC-alone group (ρ = 0.753, Fisher’s Exact Test).
Table 5. Self-reported improvement of tinnitus loudness in the follow-ups of treatment groups between years.
| STEC group | EC alone group | ρ between methods | |
| 2020 | 27/38 (71%) | 8/42 (19%) | < .001* |
| 2019 | 51/58 (88%) | 9/17 (53%) | .004** |
| ρ between year | .038* | .024** |
*: chi-square test
**: Fisher’s Exact Test.
STEC significantly reduced the THI scores in both 2020 group from (40.7 ± 6.7) to (37.7 ± 8.0) (via paired t-tests, t0.05/37 = 3.253, ρ = 0.002) and 2019 group from (32.7 ± 8.3) to (28.7 ± 7.6) (via Wilcoxon Signed Rank Test, W = -1590, ρ < 0.001) as shown in Fig 5A. Fig 5B summarized the result of a two-way ANOVA on the improvement of THI (the pre-THI minus post-THI) by STEC against the factor of year group and anxiety. There was no significant effect for both factors (year effect: F1, 92 = 2.104, ρ = 0.15; anxiety effect: F1, 92 = 0.09, ρ = 0.759).
Fig 5.
The difference in THI before and after the treatment of STEC (upper panels) and EC alone (the lower panels). A and C: The pre- and post-THI scores. B and D: the pre-post difference of THI score. STEC resulted in a significant THI reduction in both years (A), but there was no significant difference in the amount of reduction between years and between subjects with and without anxiety (B). EC alone reduced THI in 2019, but opposite in 2020 (C and D). The THI got deteriorated in 2020 and worse than 2019 in both subgroups with and without anxiety (D). Therefore, within subjects with or without anxiety, the treatment resulted in a better THI in year 2019. STEC: sound therapy + educational counseling, EC: educational counseling.
Surprisingly, the THI scores in 2020 rose from (39.8 ± 8.9) to (42.1 ± 9.1) after EC alone treatment (Wilcoxon Signed Rank Test, W = 426, ρ < 0.001), while an improvement was seen in 2019 from (35.7 ± 5.2) to (30.2 ± 6.3) (Wilcoxon Signed Rank Test, W = -153, ρ < 0.001, Fig 5C). Therefore, the change in THI by EC alone was (-2.2 ± 2.9) in 2020, but (5.4 ± 6.9) in 2019, as shown by the significant year effect in the two-way ANOVA (F1, 55 = 25.73, ρ < 0.001). In this ANOVA, the effect of anxiety was not significant (Fig 5D). Correspondingly, the THI improvement was larger in 2019 than in 2020 in both anxiety and non-anxiety subgroups: the improvement in the anxiety subgroups were 5.9 ± 7.7 in 2019 but negative (no improvement, -1.7 ± 2.4) in 2020 (q = 7.323, ρ < 0.001 in the post-hoc test between year within anxiety); the improvements in non-anxiety subgroups were 3.7 ± 3.5 in 2019 and -3.4 ± 3.5 in 2020 (q = 4.031, ρ = 0.006 in the post-hoc test between year within non-anxiety).
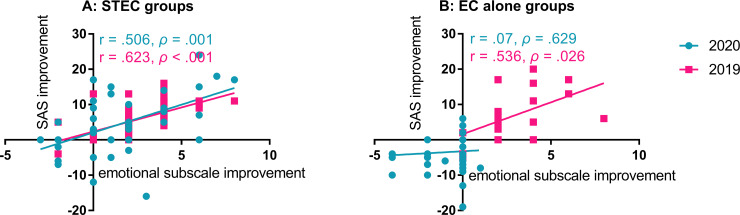
Correlation analysis showed a moderate and positive linear relationship between the improvements of THI in the emotional subscale and the SAS improvement in the subjects treated with STEC in both 2020 (r = 0.506, ρ = 0.001) and 2019 (r = 0.623, ρ < 0.001; Fig 6A). In subjects treated with EC alone, significant correlation was seen only in 2019 group (r = 0.536, ρ < 0.026) but not in 2020 group (Fig 6B). However, there was no significant relationship between the improvements of THI and the SAS changes in the subjects with EC alone in both years (ρ > 0.05).
Fig 6. Correlations between the improvements of emotional section in THI and SAS in 2020 and 2019.
A: The correlation in ST-EC group. B: The correlation in EC alone group. Significant, moderate correlations were seen in ST with EC group in both years and EC alone groups in 2019. SAS: Zung’s Self-rating Anxiety Scale, ST: sound therapy, EC: educational counseling.
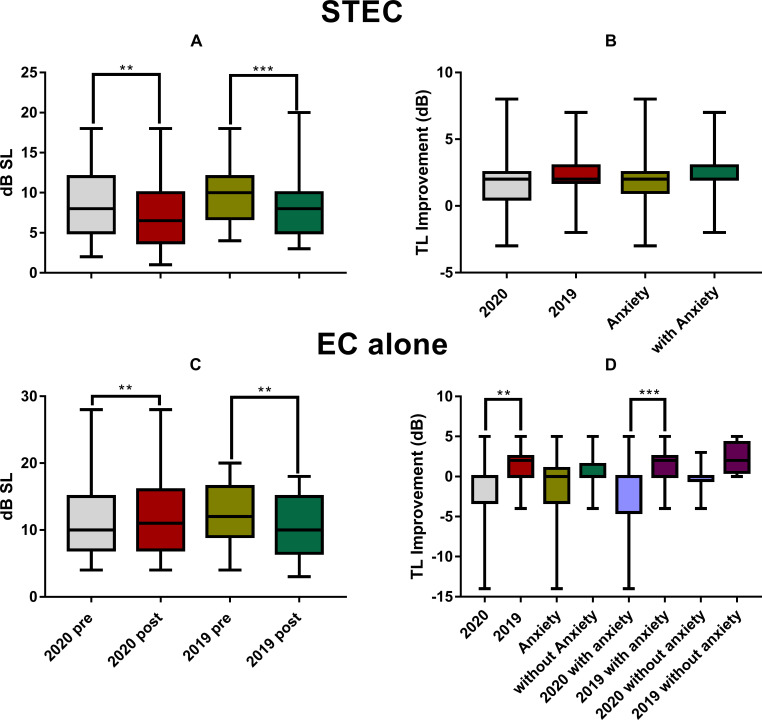
TL was reduced by STEC in 2020 group (from 9 ± 4.4 dB SL to 7.3 ± 4.2 dB SL; Wilcoxon Signed Rank Test, W = -391, ρ = 0.003) and 2019 group (from 10.0 ± 3.8 dB SL to 7.9 ± 3.8 dB SL; W = -1525, ρ < 0.001; Fig 7A). The improvement (2.1 ± 1.7 dB) was slightly higher in 2019 than in 2020 (1.6 ± 2.7 dB), but the difference was not statistically significant as shown by the main effect of year in a two-way ANOVA (F1, 92 = 1.513, ρ = 0.222, Fig 7B). Neither a significant effect of anxiety was seen in this ANOVA (F1, 92 = 0.006, ρ = 0.935).
Fig 7.
The changes in TL between the two assessments before and after the treatment of STEC (upper panels) and EC alone (the lower panels). A and C: The pre- and post-TL. B and D: the pre-post difference of TL. The TL got deteriorated in 2020 and worse than 2019 in the subgroup with anxiety (D). Significance: ** ρ < 0.01, *** ρ < 0.001 in ANOVA. STEC: sound therapy + educational counseling, EC: educational counseling, TL: tinnitus loudness.
Like THI, EC alone treatment in 2020 did not reduced TL, but increased it from (10.9 ± 4.9) dB SL to (12.4 ± 5.8) dB SL (Wilcoxon Signed Rank Test, W = 172, ρ = 0.003), yielding an increase of (1.5 ± 3.1) (Fig 7D). This was opposite to the decrease in TL from (12.4 ± 4.9) dB SL to (10.3 ± 4.7) in 2019 (Wilcoxon Signed Rank Test, W = -91, ρ < 0.001; Fig 7C). Correspondingly, a significant year effect was seen in a two-way ANOVA (-1.5 ± 3.1 in 2020 versus 1.6 ± 2.1 in 2019; F1, 55 = 10.036, ρ = 0.003), which did not show a significant effect of anxiety (F1, 55 = 1.944, ρ = 0.169). However, the year difference was mainly due to the between-year difference in the anxiety subjects in the post-hoc test (-2.1 ± 3.5 in 2020 versus 1.4 ± 2.2 in 2019; Tukey Method, q = 5.24, ρ < 0.001), since no significant difference was seen in non-anxiety subjects between years (q = 2.129, ρ > 0.05, Fig 7D). Moreover, correlation analyses did not show any significant correlation between initial SAS and the change of TL after both treatment in each of the two years. Those results suggest that high anxiety in 2020 made EC alone treatment ineffective in mitigating loudness of tinnitus. The overall correlations between SAS improvements and THI (with subscale THI), TL improvements by two treatment methods in two years were seen in Table 6.
Table 6. Correlation between SAS improvements and those in THI and MML.
| r | ρ-value | r | ρ-value | |
|---|---|---|---|---|
| Target | (A) STEC in 2020 | (B) STEC in 2019 | ||
| THI Total | .459 | .003 | .193 | .146 |
| THI Functional | .17 | .307 | -.379 | .003 |
| THI Emotional | .506 | .001 | .623 | < .001 |
| THI Catastrophic | .313 | .055 | .149 | .265 |
| MML | .134 | .424 | .143 | .286 |
| (C) EC in 2020 | (D) EC in 2019 | |||
| THI Total | .3 | .053 | -.008 | .975 |
| THI Functional | .313 | .04 | -.347 | .172 |
| THI Emotional | .07 | .629 | .536 | .026 |
| THI Catastrophic | .112 | .481 | -.04 | .856 |
| MML | .117 | .461 | .222 | .392 |
r: Person correlation coefficient.
Discussion
Several interesting findings were seen in this retrospective study. (1) We demonstrated a significantly increased anxiety in the tinnitus subjects seen in 2020 in terms of the incidence of subjects with anxiety (Table 1) and the averaged SAS (Fig 2A). Based upon the significant between-year difference, this increase in anxiety is clearly associated with COVID-19 pandemic. (2) The high SAS was associated with a high THI score, especially in the emotional subscale in 2020 as compared with the values of 2019 (Fig 2B and 2C), suggesting that the increased psychological stress in 2020 does enhance tinnitus. (3) However, the increased anxiety was not clearly linked to measure of tinnitus loudness by TL (Fig 2D and 2E). (4) Overall, the treatments of both STEC and EC alone were less effective in 2020 in anxiety reduction (Table 4 and Fig 3B and 3D) and in the self-reported mitigation of tinnitus (Table 5). In fact, the anxiety was even worse after the treatment in 2020, especially in those who received EC alone. This suggested that an increased stress was experienced by the subjects in 2020 group after the first assessment, which could not be counteracted by the therapy. (5) There was no significant difference between years for the reduction of tinnitus severity as measured by THI and TL by STEC (Figs 4B and 5B). (6) However, the treatment of EC alone was much less effective in reducing THI and TL, and in 2020 it resulted in a deterioration increase in anxiety (Table 4, Figs 3D and 4D), THI (Fig 5D) and in TL (Fig 7D). Since EC alone did show benefit in 2019, the deterioration in 2020 suggests that the anxiety in 2020 largely enhanced tinnitus, and made it difficult to be managed.
A significant psychological stress was developed because of the COVID-19 pandemic. Many people had to stressfully grapple with how the pandemic and the lockdown were impacting their way of life by means of frustration, inadequate supplies, financial loss and even stigma [4, 40]. Many recently published articles have revealed the high prevalence of anxiety across China during the COVID-19 pandemic, from 28.8% to 35.1% [41, 42], as compared to the previously reported prevalence of 5.6% and 7.6% for the years of 2009 and 2019, respectively [43, 44]. A cross-sectional survey, using the same anxiety questionnaire as adapted in the present study, reported an average SAS score of (45.89 ± 1.1) among front-line clinical staff during the pandemic [45]. This value was located between the scores for our subjects with and without anxiety (68.0 ± 6.0 vs. 43.9 ± 1.5), and lower than the average for all subjects in the 2020 group (61.9 ± 11.9). This implies that our tinnitus patients seen in 2020 have experienced extremely high psychological pressure, even higher than those medical doctors who were in the most challenging job during the pandemic. The number of tinnitus subjects seen in the 6-week period in 2020 was higher than that last year. However, this increase may be largely attributable to the accumulation of patients during the hospital closure in the national lockdown.
The association between tinnitus and anxiety has been investigated in many previous studies and has been well reviewed [10–17]. However, no information is available on the direction and causality between the two ends of the link [14, 16, 46–48], although many studies have implied that psychological states, such as those related to common stressors, influence perception of, or coping with tinnitus [49, 50]. In this regard, two related systems are involved in tinnitus: (1) the brain regions along the hypothalamic–pituitary–adrenal axis (see reviews [10, 14]), which is the main neuroendocrine system involved in stress response, and (2) the limbic system including the hippocampus and amygdala, which regulates the perception of tinnitus and the adaptation (thereby, the ability to cope with stress) [51–56]. While the data from the previous studies have indicated the possible role of emotional factors in tinnitus via those systems, the relationship was mostly investigated in animal models, or in cross-sectional comparisons across subjects with different levels of tinnitus and those without, with focus on establishing the connection, rather than on the directional nature of the link.
The COVID-19 pandemic provides a good opportunity to investigate whether stress or anxiety could enhance tinnitus as a causative or promotive factor, by clearing some clouds. For example, in many of the previous studies, the effect of anxiety on tinnitus were evaluated in a special population, such as those in veterans [57], in elderly [47], in those with headache [46], and those with sleeping disorders [58]. In other extreme, the link was investigated in cross-sectional studies in which the anxiety cases of different causes was included [59]. Moreover, the anxiety has been evaluated with many different methods, including Hospital Anxiety and Depression Scale [60], Beck Anxiety Index [61], as well as SAS [62]. All those variations make it difficult to generalize a finding, if reported, for the directional nature of the link between anxiety and tinnitus. Although large variation existed across different individuals in relationship to their jobs and financial situations, as well as their closeness to COVID-19 patients, the stress factor associated with this study was much more homogeneous than those that had been examined in previous studies. Moreover, it has been shared by general population rather than impacting on small groups. In addition, the same methodologies were used over the two years, which ensured a valid comparison for verifying the impact of anxiety associated with COVID-10. We therefore think that the between-year differences in the tinnitus clinic afforded a good chance to verify whether anxiety plays a causative or promotive role for tinnitus.
In the present study, at least three lines of evidence pinpointed the causative/promotive role of anxiety on tinnitus. Firstly, the high anxiety (in both the case% and SAS) was associated with the higher THI in all three subscales in 2020. Secondly, the high anxiety reduced the effectiveness of the tinnitus treatment in 2020 as compared with 2019 result, in the change of SAS (Fig 3B and 3D), the case% of subjects with anxiety (Table 4), self-reported improvement in tinnitus loudness (Table 5) and THI (Fig 4D). The results in Table 4 indicate a sharp contrast in the changes of cases with anxiety after the treatments between years: an increase of 6.89% by STEC in 2020 versus a decrease of 53.1% in 2019, an increase of 24.1% by EC alone in 2020 versus a decline of 46.2% in 2019. The between-year differences indicate that the higher-level stress in 2020 affected the efficacy of the two treatments in mitigating anxiety. Furthermore, the self-reported improvement in tinnitus loudness (Table 5) was also significantly less in 2020 in both treatments. Thirdly, the promoting/enhancing effect of anxiety on tinnitus was indicated by the significant difference in the treatment effectiveness between STEC and EC alone. To evaluate the full impact of the stress on tinnitus, an untreated control group would be ideally used. Unfortunately, we do not have such control. However, the EC alone treatment was given for only one time of 30–60 minutes session over the whole 2 months. This was not a comprehensive therapy by any means. Therefore, the EC subgroup could be used as a virtually no-treatment control, although this method exerted a “better than nothing” effect in 2019. The SAS improvement in the STEC group should be largely attributed to the treatment, rather than the natural released from the stress in 2020, since such improvement was not seen in the EC-alone group. Instead, there was a larger increase in SAS score at the end of two months of inventory in this group (5.0 ± 8.6), as compared with (-3.4 ± 4.6) in EC-alone group. Since the intervention in the EC-alone group was minimal, this group could be used as a virtual control for the evaluation of the natural anxiety change over the inventory period. Since there was no significant difference in the initial SAS scores between the subjects treated with different methods in 2020 (Mann–Whitney rank-sum test, ρ = 0.129), the increased SAS score in the EC alone group suggests that the stress and the resulted anxiety were accumulated and increased. This rules out the possibility that the larger improvement in SAS by STEC in the subject is due to the natural release of the anxiety. There were no significant between-year differences in the change of THI and TL by STEC. However, the THI and TL got worse in 2020 EC alone subgroup in association with a large increase in SAS, while the same treatment somehow improved both THI and TL in 2019. These results suggest that the increased stress, if not treated effectively, have significantly enhanced the tinnitus in 2020.
EC is a psychological treatment that was often recommended in combination with other treatments, like sound therapy or hearing aid fitting [63, 64]. However, different effectiveness of EC alone was also reported in some studies. For instance, an early study reported a successful ratio of 18% in tinnitus release [65]; while another study reported a significant THI reduction from (46.11 ± 22.74) to (31.94 ± 20.41) [66]. In the present study, the THI was reduced by (5.4 ± 6.9) after EC alone treatment in 2019. This result demonstrates the effectiveness of our EC treatment, while the quantitative difference between our data and others may reflect the detail difference in EC procedures and other factors such as subject variables. Anyway, the EC alone treatment reduced SAS (Fig 3D), THI (Fig 5D) and TL (Fig 7D) in 2019. However, the change of SAS, THI and TL occurred in the opposite direction after the EC in 2020. The between-year difference validates the use of EC alone as a virtual control because it is obvious that this treatment was not sufficient to counteract the effect of anxiety.
In addition, both customized sound therapy and broadband noise performed effectiveness in reducing the loudness and annoyance of tinnitus [67–69], some studies illustrated the advantages of customized sound therapy compared with broadband noise as lower acoustic energy and more tinnitus loudness improvement [61]. On the other hand, while some studies showed broadband noise induced a reduction in anxiety levels [70], other researches indicated superiority of anxiety reduction of customized sound therapy over broadband noise [61]. The choice of the stimuli of sound therapy for tinnitus patients with anxiety needs more exploration.
Limitations
There were several limitations to our study. Firstly, this was a retrospective study in which only the SAS was used to evaluate anxiety. This makes it difficult to compare our study with previous ones. Secondly, STEC was compared with EC alone without the use of wait-list control, making it difficult to fully evaluate the impact of anxiety on tinnitus. Thirdly, more patients in 2020 selected EC alone treatment probably due to the financial constraints, which may have produced some bias in comparison with 2019 subgroup. Last but not least, the overall sample size in the present study was small as the data were collected only from one hospital within a limited period. Although the data and conclusion are solid in the present study, further investigation would be helpful to verify the conclusion with a larger sample.
Some open-ended questions/questionnaires might help understand the effect of tinnitus in a multidimensional manner through a biopsychosocial perspective [71]. THI conducted various body functions associated with tinnitus though, this measure may not be sensitive enough and explore all the dimensions of tinnitus impact, such as interference with hearing and health effects (drug use, pain) [33, 72]. The introduction of open-end questions and more sensitive questionnaires can harvest more flexible and informative responses from participants to highlight wider issues caused by tinnitus [73].
Currently, the link between anxiety and tinnitus was more evaluated in the direction of how tinnitus, as a stressor, can interact with (pre-existing) psychological disorders and change the subjects responses to them [74], but was not emphasized on the direction whether other stressors would enhance tinnitus. This has been reflected in evaluation tools. For example, the THI questions for the emotional subscale (e.g., Question 22: Does your tinnitus make you feel anxious) obviously ask the impact of tinnitus on emotion, but only one question focuses on the impact of stress on tinnitus (e.g., Question 24: Does your tinnitus get worse when you are under stress) [75]. This bias appears to be a limitation for investigating the causative role of anxiety or stressor on tinnitus, and is likely one of the reasons why there was only a weak correlation between the large increase in SAS in 2020 and the THI in the initial assessment. In future investigation, THI questionnaire should be revised accordingly.
Conclusions
A substantial increase in anxiety was seen in tinnitus subjects in 2020 in association with COVID-19 pandemic and was evident as a promoting factor to tinnitus. The increase in SAS was associated with a smaller increase of THI in 2020, but not by the difference in TL. However, the difference in treatment effect between STEC and EC alone suggested that, the tinnitus severity was increased (in both THI and TL) when it was not comprehensively treated (such as by EC alone). Therefore, the present study provided clear evidence for the promoting effect of anxiety on tinnitus.
Acknowledgments
The authors acknowledge the colleagues for participating in this study. We are deeply indebted to the families who participated in the study.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was funded by Health and Family Planning Commission of Sichuan Province of China [18PJ078] for C.D., the National Natural Science Foundation of China [81770998] for Z.C. and Natural Science & Engineering Research Council of Canada [RGPIN 2017-04493] for J.W. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet (London, England). 2020;395(10231):1225–8. 10.1016/S0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report– 158 2020 [2020 June 26]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200626-covid-19-sitrep-158.pdf?sfvrsn=1d1aae8a_2.
- 3.Liu NN, Tan JC, Li J, Li S, Cai Y, Wang H. COVID-19 Pandemic: Experiences in China and implications for its prevention and treatment worldwide. Current cancer drug targets [Internet]. 2020. April 14 Available from: http://www.eurekaselect.com/180883/article. 10.2174/1568009620666200414151419 [DOI] [PubMed] [Google Scholar]
- 4.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet (London, England). 2020;395(10227):912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W, Wang H, Lin Y, Li L. Psychological status of medical workforce during the COVID-19 pandemic: A cross-sectional study. Psychiatry Res. 2020;288:112936 10.1016/j.psychres.2020.112936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Zhang Y, Zhao J, Zhang J, Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet (London, England). 2020;395(10228):945–7. 10.1016/S0140-6736(20)30547-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer CA. Tinnitus. N Engl J Med. 2018;378(13):1224–31. 10.1056/NEJMcp1506631 [DOI] [PubMed] [Google Scholar]
- 8.Baguley D, McFerran D, Hall D. Tinnitus. Lancet (London, England). 2013;382(9904):1600–7. [DOI] [PubMed] [Google Scholar]
- 9.Tyler RS, Baker LJ. Difficulties experienced by tinnitus sufferers. J Speech Hear Disord. 1983;48(2):150–4. 10.1044/jshd.4802.150 [DOI] [PubMed] [Google Scholar]
- 10.Ziai K, Moshtaghi O, Mahboubi H, Djalilian HR. Tinnitus Patients Suffering from Anxiety and Depression: A Review. Int Tinnitus J. 2017;21(1):68–73. 10.5935/0946-5448.20170013 [DOI] [PubMed] [Google Scholar]
- 11.Pattyn T, Van Den Eede F, Vanneste S, Cassiers L, Veltman DJ, Van De Heyning P, et al. Tinnitus and anxiety disorders: A review. Hear Res. 2016;333:255–65. 10.1016/j.heares.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 12.Durai M, Searchfield G. Anxiety and depression, personality traits relevant to tinnitus: A scoping review. Int J Audiol. 2016;55(11):605–15. 10.1080/14992027.2016.1198966 [DOI] [PubMed] [Google Scholar]
- 13.Malouff JM, Schutte NS, Zucker LA. Tinnitus-related distress: A review of recent findings. Curr Psychiatry Rep. 2011;13(1):31–6. 10.1007/s11920-010-0163-1 [DOI] [PubMed] [Google Scholar]
- 14.Mazurek B, Boecking B, Brueggemann P. Association Between Stress and Tinnitus-New Aspects. Otol Neurotol. 2019;40(4):e467–e73. 10.1097/MAO.0000000000002180 [DOI] [PubMed] [Google Scholar]
- 15.Zirke N, Seydel C, Szczepek AJ, Olze H, Haupt H, Mazurek B. Psychological comorbidity in patients with chronic tinnitus: analysis and comparison with chronic pain, asthma or atopic dermatitis patients. Qual Life Res. 2013;22(2):263–72. 10.1007/s11136-012-0156-0 [DOI] [PubMed] [Google Scholar]
- 16.Wallhausser-Franke E, Brade J, Balkenhol T, D’Amelio R, Seegmuller A, Delb W. Tinnitus: distinguishing between subjectively perceived loudness and tinnitus-related distress. PLoS One. 2012;7(4):e34583 10.1371/journal.pone.0034583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyler RS, Aran JM, Dauman R. Recent Advances in Tinnitus. Am J Audiol. 1992;1(4):36–44. 10.1044/1059-0889.0104.36 [DOI] [PubMed] [Google Scholar]
- 18.Schlee W, Pryss RC, Probst T, Schobel J, Bachmeier A, Reichert M, et al. Measuring the Moment-to-Moment Variability of Tinnitus: The TrackYourTinnitus Smart Phone App. Front Aging Neurosci. 2016;8:294 10.3389/fnagi.2016.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Probst T, Pryss R, Langguth B, Schlee W. Emotional states as mediators between tinnitus loudness and tinnitus distress in daily life: Results from the "TrackYourTinnitus" application. Sci Rep. 2016;6:20382 10.1038/srep20382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Probst T, Pryss R, Langguth B, Schlee W. Emotion dynamics and tinnitus: Daily life data from the "TrackYourTinnitus" application. Sci Rep. 2016;6:31166 10.1038/srep31166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartels H, Middel B, Pedersen SS, Staal MJ, Albers FW. The distressed (Type D) personality is independently associated with tinnitus: a case-control study. Psychosomatics. 2010;51(1):29–38. 10.1176/appi.psy.51.1.29 [DOI] [PubMed] [Google Scholar]
- 22.Bartels H, Pedersen SS, van der Laan BF, Staal MJ, Albers FW, Middel B. The impact of Type D personality on health-related quality of life in tinnitus patients is mainly mediated by anxiety and depression. Otol Neurotol. 2010;31(1):11–8. 10.1097/MAO.0b013e3181bc3dd1 [DOI] [PubMed] [Google Scholar]
- 23.Trevis KJ, McLachlan NM, Wilson SJ. Cognitive Mechanisms in Chronic Tinnitus: Psychological Markers of a Failure to Switch Attention. Front Psychol. 2016;7:1262 10.3389/fpsyg.2016.01262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevis KJ, McLachlan NM, Wilson SJ. Psychological mediators of chronic tinnitus: The critical role of depression. J Affect Disord. 2016;204:234–40. 10.1016/j.jad.2016.06.055 [DOI] [PubMed] [Google Scholar]
- 25.Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, et al. COVID-19: towards controlling of a pandemic. Lancet (London, England). 2020;395(10229):1015–8. 10.1016/S0140-6736(20)30673-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Medical Association. WMA declaration of Helsinki: Ethical principles for medical research involving human subjects 2018. [2020 June 26]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. [Google Scholar]
- 27.Henry JA, Meikle MB. Psychoacoustic measures of tinnitus. J Am Acad Audiol. 2000;11(3):138–55. [PubMed] [Google Scholar]
- 28.Langguth B. Treatment of tinnitus. Curr Opin Otolaryngol Head Neck Surg. 2015;23(5):361–8. 10.1097/MOO.0000000000000185 [DOI] [PubMed] [Google Scholar]
- 29.Tyler RS. Mindfulness-Based Therapy: New Study Underscores Tinnitus Treatment Options. The Hearing Journal. 2014;67(1). 10.1097/01.HJ.0000442741.34475.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arif M, Sadlier M, Rajenderkumar D, James J, Tahir T. A randomised controlled study of mindfulness meditation versus relaxation therapy in the management of tinnitus. J Laryngol Otol. 2017;131(6):501–7. 10.1017/S002221511700069X [DOI] [PubMed] [Google Scholar]
- 31.Tyler RS, Coelho C, Noble W. Tinnitus: standard of care, personality differences, genetic factors. ORL J Otorhinolaryngol Relat Spec. 2006;68(1):14–9; discussion 20–2. 10.1159/000090486 [DOI] [PubMed] [Google Scholar]
- 32.Pan T, Tyler RS, Ji H, Coelho C, Gehringer AK, Gogel SA. The relationship between tinnitus pitch and the audiogram. Int J Audiol. 2009;48(5):277–94. 10.1080/14992020802581974 [DOI] [PubMed] [Google Scholar]
- 33.Tyler RS, Oleson J, Noble W, Coelho C, Ji H. Clinical trials for tinnitus: study populations, designs, measurement variables, and data analysis. Prog Brain Res. 2007;166:499–509. 10.1016/S0079-6123(07)66048-8 [DOI] [PubMed] [Google Scholar]
- 34.Kam AC, Cheung AP, Chan PY, Leung EK, Wong TK, van Hasselt CA, et al. Psychometric properties of the Chinese (Cantonese) Tinnitus Handicap Inventory. Clin Otolaryngol. 2009;34(4):309–15. 10.1111/j.1749-4486.2009.01946.x [DOI] [PubMed] [Google Scholar]
- 35.Meng Z, Zheng Y, Liu S, Wang K, Kong X, Tao Y, et al. Reliability and validity of the chinese (mandarin) tinnitus handicap inventory. Clin Exp Otorhinolaryngol. 2012;5(1):10–6. 10.3342/ceo.2012.5.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–8. 10.1001/archotol.1996.01890140029007 [DOI] [PubMed] [Google Scholar]
- 37.Gao LL, Ip WY, Sun K. Validation of the short form of the Chinese Childbirth Self-Efficacy Inventory in Mainland China. Res Nurs Health. 2011;34(1):49–59. 10.1002/nur.20400 [DOI] [PubMed] [Google Scholar]
- 38.Zung WW. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 39.Dunstan DA, Scott N. Norms for Zung’s Self-rating Anxiety Scale. BMC Psychiatry. 2020;20(1):90 10.1186/s12888-019-2427-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakovljevic M, Bjedov S, Jaksic N, Jakovljevic I. COVID-19 Pandemia and Public and Global Mental Health from the Perspective of Global Health Securit. Psychiatr Danub. 2020;32(1):6–14. 10.24869/psyd.2020.6 [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954 10.1016/j.psychres.2020.112954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. 2020;17(5):1729 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. The Lancet Psychiatry. 2019;6(3):211–24. 10.1016/S2215-0366(18)30511-X [DOI] [PubMed] [Google Scholar]
- 44.Phillips MR, Zhang J, Shi Q, Song Z, Ding Z, Pang S, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. Lancet (London, England). 2009;373(9680):2041–53. [DOI] [PubMed] [Google Scholar]
- 45.Wu K, Wei X. Analysis of Psychological and Sleep Status and Exercise Rehabilitation of Front-Line Clinical Staff in the Fight Against COVID-19 in China. Med Sci Monit Basic Res. 2020;26:e924085–e. 10.12659/MSMBR.924085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lugo A, Edvall NK, Lazar A, Mehraei G, Lopez-Escamez JA, Bulla J, et al. Relationship between headaches and tinnitus in a Swedish study. Sci Rep. 2020;10(1):8494 10.1038/s41598-020-65395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danioth L, Brotschi G, Croy I, Friedrich H, Caversaccio MD, Negoias S. Multisensory environmental sensitivity in patients with chronic tinnitus. J Psychosom Res. 2020;135:110155 10.1016/j.jpsychores.2020.110155 [DOI] [PubMed] [Google Scholar]
- 48.Park E, Kim H, Choi IH, Han HM, Han K, Jung HH, et al. Psychiatric Distress as a Common Risk Factor for Tinnitus and Joint Pain: A National Population-Based Survey. Clin Exp Otorhinolaryngol [Internet]. 2019. December 18 Available from: https://www.ncbi.nlm.nih.gov/pubmed/31842535. https://www.e-ceo.org/upload/pdf/ceo-2019-00563.pdf. 10.21053/ceo.2019.00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarus RS. Toward better research on stress and coping. Am Psychol. 2000;55(6):665–73. 10.1037//0003-066x.55.6.665 [DOI] [PubMed] [Google Scholar]
- 50.Lazarus RS. Coping theory and research: past, present, and future. Psychosom Med. 1993;55(3):234–47. 10.1097/00006842-199305000-00002 [DOI] [PubMed] [Google Scholar]
- 51.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50(1):114–20. 10.1212/wnl.50.1.114 [DOI] [PubMed] [Google Scholar]
- 52.Kapolowicz MR, Thompson LT. Plasticity in Limbic Regions at Early Time Points in Experimental Models of Tinnitus. Front Syst Neurosci. 2019;13:88 10.3389/fnsys.2019.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raghavan P, Steven A, Rath T, Gandhi D. Advanced Neuroimaging of Tinnitus. Neuroimaging Clin N Am. 2016;26(2):301–12. 10.1016/j.nic.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 54.Leaver AM, Seydell-Greenwald A, Rauschecker JP. Auditory-limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear Res. 2016;334:49–57. 10.1016/j.heares.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YC, Xia W, Chen H, Feng Y, Xu JJ, Gu JP, et al. Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384–97. 10.1002/hbm.23525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Chen YC, Feng X, Yang M, Liu B, Qian C, et al. Impairments of thalamic resting-state functional connectivity in patients with chronic tinnitus. European journal of radiology. 2015;84(7):1277–84. 10.1016/j.ejrad.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 57.Hu J, Xu J, Streelman M, Xu H, Guthrie O. The Correlation of the Tinnitus Handicap Inventory with Depression and Anxiety in Veterans with Tinnitus. Int J Otolaryngol. 2015;2015:689375 10.1155/2015/689375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Yao J, Zhang Z, Wang W. Association between sleep quality and psychiatric disorders in patients with subjective tinnitus in China. Eur Arch Otorhinolaryngol. 2016;273(10):3063–72. 10.1007/s00405-016-3906-8 [DOI] [PubMed] [Google Scholar]
- 59.Park E, Kim H, Choi IH, Han HM, Han K, Jung HH, et al. Psychiatric Distress as a Common Risk Factor for Tinnitus and Joint Pain: A National Population-Based Survey. Clin Exp Otorhinolaryngol. 2019. 10.21053/ceo.2019.00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKenna L, Marks EM, Hallsworth CA, Schaette R. Mindfulness-Based Cognitive Therapy as a Treatment for Chronic Tinnitus: A Randomized Controlled Trial. Psychother Psychosom. 2017;86(6):351–61. 10.1159/000478267 [DOI] [PubMed] [Google Scholar]
- 61.Mahboubi H, Haidar YM, Kiumehr S, Ziai K, Djalilian HR. Customized Versus Noncustomized Sound Therapy for Treatment of Tinnitus: A Randomized Crossover Clinical Trial. Ann Otol Rhinol Laryngol. 2017;126(10):681–7. 10.1177/0003489417725093 [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Yao J, Zhang Z, Wang W. Association between sleep quality and psychiatric disorders in patients with subjective tinnitus in China. European Archives of Oto-Rhino-Laryngology. 2016;273(10):3063–72. 10.1007/s00405-016-3906-8 [DOI] [PubMed] [Google Scholar]
- 63.Jastreboff PJ, Jastreboff MM. Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol. 2000;11(3):162–77. [PubMed] [Google Scholar]
- 64.Brennan-Jones CG, Thomas A, Hoare DJ, Sereda M. Cochrane corner: Sound therapy (using amplification devices and/or sound generators) for tinnitus. Int J Audiol. 2020;59(3):161–5. 10.1080/14992027.2019.1643503 [DOI] [PubMed] [Google Scholar]
- 65.Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236–40. [PubMed] [Google Scholar]
- 66.Liu YQ, Chen ZJ, Li G, Lai D, Liu P, Zheng Y. Effects of Educational Counseling as Solitary Therapy for Chronic Primary Tinnitus and Related Problems. Biomed Res Int. 2018;2018:6032525 10.1155/2018/6032525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahboubi H, Ziai K, Djalilian HR. Customized web-based sound therapy for tinnitus. Int Tinnitus J. 2012;17(1):26–30. [PubMed] [Google Scholar]
- 68.Park JM, Kim WJ, Ha JB, Han JJ, Park SY, Park SN. Effect of sound generator on tinnitus and hyperacusis. Acta Otolaryngol. 2018;138(2):135–9. 10.1080/00016489.2017.1386801 [DOI] [PubMed] [Google Scholar]
- 69.Tyler RS, Perreau A, Powers T, Watts A, Owen R, Ji H, et al. Tinnitus Sound Therapy Trial Shows Effectiveness for Those with Tinnitus. J Am Acad Audiol. 2020;31(1):6–16. 10.3766/jaaa.18027 [DOI] [PubMed] [Google Scholar]
- 70.Ilkkaya NK, Ustun FE, Sener EB, Kaya C, Ustun YB, Koksal E, et al. The effects of music, white noise, and ambient noise on sedation and anxiety in patients under spinal anesthesia during surgery. J Perianesth Nurs. 2014;29(5):418–26. 10.1016/j.jopan.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 71.Manchaiah V, Beukes EW, Granberg S, Durisala N, Baguley DM, Allen PM, et al. Problems and Life Effects Experienced by Tinnitus Research Study Volunteers: An Exploratory Study Using the ICF Classification. J Am Acad Audiol. 2018;29(10):936–47. 10.3766/jaaa.17094 [DOI] [PubMed] [Google Scholar]
- 72.Tyler RS, Noble W, Coelho C. Considerations for the design of clinical trials for tinnitus. Acta Otolaryngol Suppl. 2006(556):44–9. 10.1080/03655230600895424 [DOI] [PubMed] [Google Scholar]
- 73.Tyler R, Ji H, Perreau A, Witt S, Noble W, Coelho C. Development and validation of the tinnitus primary function questionnaire. Am J Audiol. 2014;23(3):260–72. 10.1044/2014_AJA-13-0014 [DOI] [PubMed] [Google Scholar]
- 74.Kröner-Herwig B, Zachriat C, Weigand D. Do patient characteristics predict outcome in the outpatient treatment of chronic tinnitus? Psychosoc Med. 2006;3:Doc07 [PMC free article] [PubMed] [Google Scholar]
- 75.Newman CW, Sandridge SA, Bolek L. Development and psychometric adequacy of the screening version of the tinnitus handicap inventory. Otol Neurotol. 2008;29(3):276–81. 10.1097/MAO.0b013e31816569c4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.