Abstract
Purpose of Review
This review will provide an in-depth coverage of the epidemiological and pre-clinical literature surrounding the role of dietary protein in hypertension, with a special emphasis on the history of our work on the Dahl salt-sensitive rat.
Recent Findings
Our studies have dedicated much effort into understanding the relationship between dietary protein and its effect on the development of salt-sensitive hypertension and renal injury. Our evidence over the last 15 years have demonstrated that both the source and amount of dietary protein can influence the severity of disease, where we have determined mechanisms related to immunity, the maternal environment during pregnancy, and more recently the gut microbiota, which significantly contribute to these diet-induced effects.
Summary
Deeper understanding of these dietary protein-related mechanisms may provide insight on the plausibility of dietary modifications as future therapeutic avenues for hypertension and renal disease.
Keywords: Dietary protein, Hypertension, Salt sensitivity, Inflammation, Microbiome, Pregnancy
Introduction
According to the latest American College of Cardiology/American Heart Association guidelines on the levels of systolic and diastolic blood pressure used to define hypertension [1], 46% of the US population can be characterized as hypertensive. While genetic predisposition is the first listed cause of hypertension within the guidelines, environmental exposures comprise the majority of all other risk factors, with those factors being nearly exclusively related to diet (i.e., saturated fat, sodium, potassium, alcohol). This highlights the potential power of dietary modifications in the prevention and treatment of high blood pressure. Studies from our laboratory have shown the influence of such dietary interventions, in particular changes in the non-sodium components of the diet, in determining the severity of salt-induced hypertension and renal damage. Our more recent areas of exploration within the Dahl salt-sensitive (SS) rat model have revealed important contributions of the immune system, the in utero environment, and the gut microbiota to dietary protein-induced modulation of hypertension (Fig. 1). This review examines the potential interplay between these novel mechanisms as potential links between dietary changes and hypertension.
Fig. 1.
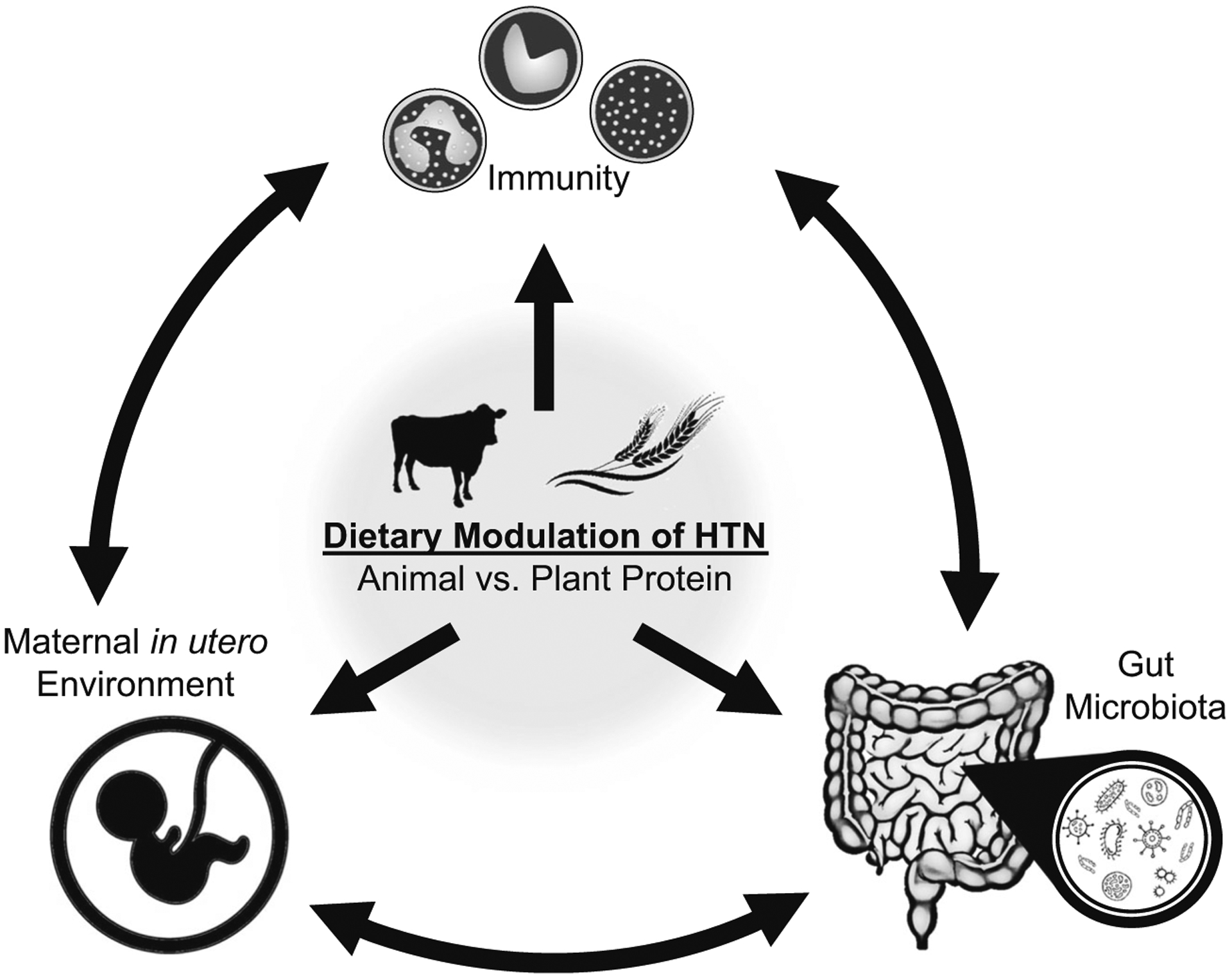
Visual schematic representing the interplay between the immune system, gut microbiome, and maternal environment in the dietary modulation of salt-sensitive hypertension and renal damage
Dietary Protein and Hypertension
Epidemiological studies have highlighted the importance of nutrition in the regulation of blood pressure, where consumption of high salt, carbohydrate, saturated fat, and cholesterol diets is correlated with hypertension [2–4]. In contrast, the effects of a diet high in protein are considered to be somewhat controversial, where evidence supports both protein-induced decreases [4, 5] and increases [6] in blood pressure. Yet in humans with preexisting renal insufficiency, the evidence is quite clear that a high protein diet accelerates the decline in renal function [7–9], where the consumption of a reduced protein diet has been shown to improve total kidney failure outcomes [10]. An important point of consideration is not just the amount, but the source of dietary protein, since varying sources have been associated with different degrees of disease susceptibility. There is strong observational data surrounding the comparison of animal versus plant protein consumption and its effects on overall cardiovascular health, where vegetarians have been shown to have lower blood pressure than omnivores [11] and vegetable protein intake inversely correlates with blood pressure [12]. Importantly, the interventional Dietary Approaches to Stop Hypertension (DASH) trial and the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart) overall confirmed the blood pressure benefit in a diet rich in plant protein consumption [13, 14]. However, the very general animal versus plant distinction is likely oversimplified with the concomitant contribution of nonprotein components [15] and recent evidence suggesting that it could perhaps ultimately be driven by genetics [16].
In various animal models of hypertension, blood pressure has been shown to be modulated by the fat [17–20], carbohydrate [20–22], and protein [23] components of the diet. Our investigations have focused on the utilization of the Dahl SS rat as a model of high blood pressure and chronic kidney disease in response to a high-salt challenge, since it recapitulates many of the hallmarks typically observed in humans with salt-sensitive hypertension [24]. An initial study demonstrated that parental SS rats fed an animal, casein-based purified diet (AIN-76A, Dyets Inc.) led to a significant increase in blood pressure in the offspring compared with grain-fed parental SS rats, regardless of offspring diet, providing the first indication that modulation of non-sodium components of the diet could in turn affect Dahl SS hypertension and renal damage [25]. Over a decade’s worth of additional studies have since explored this in depth, attributing the effect of the purified versus grain diet specifically to the difference in dietary protein source (casein versus wheat gluten) [26], and revealing a distinct contribution for both the immune system [27, 28] and a heritable component [29]. More recent parallel studies have examined purified diet-fed Dahl SS rats from the Medical College of Wisconsin (SS/MCW) and grain-fed Dahl SS rats from Charles River Laboratories (SS/CRL), which have reinforced the blood pressure-lowering nature of this plant versus animal protein chow due to differences in maternal environment [30] and epigenetics in T lymphocytes [31, 32]. Together, there is compelling evidence that dietary protein is an important determinant of Dahl SS hypertension and renal damage.
Dietary Protein and Immunity
Modifying dietary habits is an attractive method for modulating inflammatory disease due to the relative ease and cost of intervention. The Metabolic Syndrome Reduction in Navarra (RESMENA) project compared the RESMENA diet (30% energy from protein) to a diet based on American Heart Association guidelines (15% of energy from protein). This randomized interventional study revealed a direct relationship between dietary protein intake and inflammation. Furthermore, this relationship was specific for increases in animal/meat protein intake but not fish or vegetable protein intake [33]. There is also evidence that increased dietary protein enhances the incidence of inflammatory bowel disease specific to ingestion of animal by-products [34] and that increased dietary protein, specifically of red meats and processed meats, increases relapse of ulcerative colitis [35]. There is no doubt that dietary protein influences the inflammatory state of the gut, but less is known on the connection between the gut and other organ systems.
Education of the immune system in the gut provides an essential role in preventing inappropriate immune responses to the food we eat through a mechanism termed oral tolerance. Mohammad et al. demonstrated this phenomenon using an ovalbumin (OVA)+ alum adjuvant-induced inflammatory model. Mice that were pre-tolerized to OVA orally have an attenuated inflammatory response measured by reduced serum IgE titer. Maintaining these mice on a high-protein diet increased IgE titers, enhanced splenocyte production of Th2-driving IL-4 and Th1-driving IL-12, and enhanced LPS-induced proliferation [36]. This study not only illustrated that tolerization to dietary proteins is important in regulating systemic immune responses but also that increased dietary protein enhances the systemic response. To further highlight the importance of oral tolerance, Kim et al. demonstrated that germ-free mice fed an “antigen-free” diet have lower levels of serum immunoglobulins and fewer intestinal lymphocytes primarily due to a depression in CD4+ memory Tcells accompanied by a reduction in Foxp3+CD4+ T regulatory cells (Tregs). A restoration of these populations was possible when mice were switched to a solid food chow containing whole proteins but not when switched to an amino acid diet [37•]. This study demonstrated that dietary antigens, in the form of whole proteins, are necessary for the proper production of intestinal Tregs. This is an important mechanism to consider when investigating the relationship between dietary protein, oral tolerance, and systemic inflammation.
The Dahl SS animal model shows robust inflammatory activation when subjected to a high-salt challenge exhibiting pronounced infiltration of immune cells into target organs including the vasculature and the kidney. The salt-sensitive increase in blood pressure and renal damage is exacerbated when these animals are on a high-protein diet, accompanied by an increase in infiltrating T cells in the kidney [27]. To counteract this immune activation, treatment with the immunosuppressive drug mycophenolate mofetil completely abrogated the effect of a high-protein diet [27]. Utilization of RAG1−/− Dahl SS rats, lacking T and B cells, demonstrated that the high-protein diet-induced enhanced salt sensitivity is mediated by adaptive immunity [28]. Further work investigated whether the protein source, rather than the amount of protein, in the diet may influence these phenotypes. Compared with Dahl SS rats maintained on the animal protein-based chow (AIN-76A, Dyets), commercially available SS/CRL rats maintained on a whole grain protein-based chow demonstrate a dramatic reduction in salt-induced renal immune cell infiltration as well as a dramatic shift in the transcriptional and methylation profile of infiltrating renal T cells [31, 32], suggesting the involvement of epigenetic mechanisms. Moreover, substituting the animal-based protein source in our standard chow for a specific plant-based protein source (wheat gluten) reduces the infiltration of immune cells into the kidney [29] creating a connection between dietary protein source and salt-induced renal inflammation.
The series of events connecting changes in protein source in the diet and renal inflammation is an area of active investigation, where we believe that antigenic aspects of dietary protein produce an inflammatory response in the gut which is transmitted to the systemic circulation. This systemic inflammation provides an enhanced response to the renal damage accumulating in the kidney due to the salt-sensitive increases in perfusion pressure. To this end, unpublished histological analysis of the intestinal tract of the Dahl SS rat shows striking hypertrophy of the Peyer’s patches and inflammation of the gut epithelium when fed a diet comprised of animal-based protein which is much less pronounced when rats are fed a plant-based protein diet.
Dietary Protein and the Microbiome
The relationship between the diet and the microbiome is certainly twofold, where different dietary habits drive the composition of the gut microbiota, and microbial metabolism dictates the ultimate systemic effects of the diet. To the first point, the fecal microbiota of Western diet-fed European children was compared with that of rural African children fed a diet high in fiber, which clearly demonstrated an improved Firmicutes-to-Bacteroidetes ratio, unique bacterial speciation, and more fecal short-chain fatty acids in the African children fed the rural diet [38]. Furthermore, a dietary interventional study comparing adults placed on either an exclusive plant- or animal-based diet for 5 days revealed the rapidity of the human microbiome to respond and switch between an herbivorous and carnivorous profile [39]. While these pieces of evidence highlight the impact of diet on driving microbiotal differences, the microbiota itself is also a major determinant of the physiologic effects of the diet. In terms of dietary protein specifically, the microbiota contributes directly to proteolysis [40] and the ability of the microbiota to properly ferment dietary proteins is essential for amino acid balance, utilization, and bioavailability [41, 42]; therefore, the composition of both the gut and the diet plays equally important and reciprocal roles critical for maintenance of host health. Well summarized in this recent review by Diether et al., the process of proteolytic fermentation involves a multitude of metabolic pathways and results in the production of diverse metabolites like short-and branch-chained fatty acids, ammonia, amines, hydrogen sulfide, phenols, and indoles, with little known about how a pathological microbiota affects these by-products [43•]. An additional variable of consideration is the source of dietary protein, which has been shown to influence protein digestion itself as well as microbiota composition [44–46]. Interestingly, casein has directly been shown to increase microbial density and decrease microbial diversity, leading to worsened inflammatory bowel disease in a mouse model of DSS-induced colitis [47•]. This reemerging role of immunity and inflammation is a probable, causal link between the diet, microbiota dysbiosis, and the progression of a number of chronic pathologies.
Emerging human and preclinical data provide evidence for strong links between the microbiome and the development of hypertension, where both hypertensive humans and animal models exhibit gut dysbiosis, decreases in microbial diversity, and the hallmark deleterious increase in Firmicutes-to-Bacteroidetes ratio compared with normotensive controls [48, 49]. Some pathogenic factor is derived directly from the hypertensive microbiota, since normotensive WKY rats receiving cecal transplant from spontaneously hypertensive stroke-prone rats also developed hypertension and increased the Firmicutes-to-Bacteroidetes ratio [50]. Furthermore, transplantation of feces from hypertensive humans elevated blood pressure in germ-free mice [51]. More recent studies demonstrate that immune mechanisms appear to be involved between the microbiome and the development of disease. Wilck et al. have attributed the salt-sensitive hypertensive effects in a model of autoimmune encephalomyelitis to a depletion in Lactobacillus murinus, which occurs in a T helper 17 (TH17) cell-dependent manner [51]. The gut dysbiosis observed in both hypertensive human subjects and experimental mice has been associated with increased intestinal inflammation and activation of antigen-presenting cells [52••]. Given the known contribution of immune mechanisms in our model of salt-sensitive hypertension, this new and compelling evidence of how the microbiota shifts in hypertension as well as in response to dietary protein changes provides us with the rationale to explore these mechanisms in our purified casein-fed versus grain- or glutenfed Dahl SS rats. Our preliminary work has determined that these various diets induce massive shifts in the fecal microbiota composition of the SS rats, with a causative, pathogenic factor or species that drives hypertension and renal disease being specifically derived from SS/MCW microbiota [53].
Dietary Protein and Pregnancy
Preeclampsia (PE) is a pregnancy-specific disorder that is characterized by new-onset hypertension accompanied by proteinuria and affects 3–5% of nulliparous pregnant women [54]. Despite a better understanding of the risk factors involved in the pathology, the only cure to alleviate symptoms is to deliver the fetus and placenta. While it is well known that PE has long-term effects on the health of both the mother and child, less is known about how both the health of the mother during pregnancy and her risk of developing PE can be modulated by dietary protein, microbiome, and the immune system.
There are multiple reports that the source of dietary protein can impact the risk of developing preeclampsia. The Norwegian Mother and Child Cohort Study (MoBa) reported that in women, higher dietary intake of vegetables and plant foods was associated with a lower risk of preeclampsia compared with higher intake of processed meats [55]. Furthermore, the type and amount of dietary fiber have also been shown to influence the development of PE. Based upon a food frequency questionnaire during preconception and early pregnancy, Qiu et al. found that the relative risk of developing PE was lowest among the women that fell in the highest quartile for dietary total fiber intake versus those that were in the lowest quartile [56]. Moreover, this association was also observed when water-soluble and insoluble fiber were examined separately. To our knowledge, there are no preclinical studies published investigating how different protein sources contribute to the pathogenesis of PE; however, we have ongoing studies investigating the role of maternal dietary protein intake on the development of a maternal syndrome. Casein-fed Dahl SS rats develop a pregnancy-specific increase in MAP and protein excretion that is absent in dams maintained on the modified AIN-76A wheat gluten diet [57]. One potential mechanism on how dietary intake could play a protective role in PE is through modification of the microbiota composition.
The microbiome is an emerging topic in the pregnancy field, and there is great interest in understanding how alterations of the maternal microbiome can lead to disease in offspring; however, little is known about the role of the maternal microbiome in the pathogenesis of PE. There are reports of differences in gut microbiota composition between healthy and PE women [58], and these differences persist even until 6 weeks postpartum [59]. While there is importance in being able to make such observational associations, to date, there are no studies demonstrating a causal relationship between changes in the microbiota and PE. However, it has been shown that if women are treated with a probiotic during late pregnancy, the risk for PE was reduced [60]. Even in a healthy pregnancy, it has been demonstrated that the composition of the gut microbiome significantly remodels from the first trimester to the third trimester [61•]. Interestingly, when microbiota from the third trimester were transferred into germ-free mice, there was a greater adiposity and inflammation in these mice relative to mice that received microbiota transfer from the first trimester. In regard to the offspring microbiota, infants are born widely undifferentiated and uncolonized [62], with the inoculation process being primarily driven by mode of delivery and type of milk [63, 64]. Thus, the dietary and PE effects on the maternal microbiome and how its transmission to the infant leads to future disease susceptibility will be an important area of future study. More investigations into how the microbiome could be leading to PE and altering the health of the mother and child are necessary to better understand these associations, but one potential mechanism that is plausible to link alterations in the gut microbiota with the risk of PE is the immune system. Normal immune adaptions occur for the maintenance of a healthy pregnancy, but immune dys-function is a clinical manifestation during the onset of PE [65]. In particular, T cells have been linked to the development of PE with increased levels of circulating CD4+ and CD8+ cells [66] and various elevated proinflammatory cytokines such as IL-6, TNF-alpha, and IL-17 [67]. These findings have been recapitulated in preclinical models of PE, such as the Reduced Uterine Perfusion Pressure (RUPP) model, to help understand their roles in the disease process [68]. Our unpublished work shows that Dahl SS rats lacking T cells (SSCD247−/− rats) are protected from developing maternal syndrome and again highlight the importance of the T cell in PE [69].
Our group has previously demonstrated that the severity of salt-induced hypertension and renal damage can be impacted by the protein source in both parental and offspring diets. The initial observations that parental dietary protein can influence offspring’s sensitivity were performed in SS/MCW rats where breeders were maintained on either the casein- or grain-based diet with their sequential litters weaned to either casein- or grain-based diets. It was determined that offspring fed a grain-based diet starting at weaning exhibited an attenuation in salt-induced hypertension and renal disease relative to the offspring fed a casein-based diet [25]. The importance of diet during gestation and lactation was further emphasized when Geurts et al. performed an embryo transfer experiment utilizing SS/MCWand SS/CRL rats. Similarly, it was the diet of the surrogate dam that predicted the severity of hypertension and renal disease in the offspring [30]. While these colonies are essentially genetically identical (0.000001% single nucleotide variants), a colony of SS/MCW rats fed a modified AIN-76A with the protein source being wheat gluten instead of casein was developed in an attempt to eliminate any possible genetic contribution to this phenotype. In a more recent publication, the SS/MCW rats fed wheat gluten chow displayed attenuation in their salt-sensitive phenotype relative to offspring from casein-fed rats [29]. Furthermore, offspring born to parents that were born and bred on the modified wheat gluten AIN-76A diet saw a further protection compared with their casein-fed counterparts. These reports highlight the importance of diet on the severity of the disease phenotype; however, our recently generated unpublished work shows that these diets can also impact the health of the mom during pregnancy [57]. Our work has revealed that dietary protein source can greatly impact both pregnancy and offspring’s susceptibility to salt-sensitive hypertension and renal disease.
Conclusion
Taken together, the current literature supports the view that manipulations to the diet can have drastic effects on the development and severity of disease, namely hypertension. While wheat gluten consumption is generally associated with a negative connotation due to increased public awareness of Celiac disease, it is important to note that gluten is the major protein component of whole grain. For those without gluten intolerances, balanced diets should incorporate whole grains, and in terms of salt-sensitive hypertension, our work highlights the benefit of greater consumption of this plant-based protein versus animal protein. The microbiota undoubtedly plays a contributory role in how various dietary components become systemically exposed to the host, where it then becomes important in how the host responds immunologically, both in the gut as well as in other target organs. Furthermore, understanding how changes to the maternal microbiota shapes the in utero environment and how it impacts offspring immunity and disease susceptibility provides another important area of future investigation and potential intervention.
Acknowledgments
We kindly thank Dr. David Mattson for his continued support and guidance. We also acknowledge funding sources: 19CDA34660184.
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary in-takes: the NHANES-III data. J Nutr. 2003;133(1):211–4. 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 3.Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension. 1988;12(6):589–93. 10.1161/01.hyp.12.6.589. [DOI] [PubMed] [Google Scholar]
- 4.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macro-nutrients. Findings of the Multiple Risk Factor Intervention Trial (MRFIT). Circulation. 1996;94(10):2417–23. 10.1161/01.cir.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 5.Vasdev S, Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int J Angiol. 2010;19(1):e7–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med. 2001;161(4):589–93. 10.1001/archinte.161.4.589. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra R, Lipworth L, Cavanaugh KL, Young BA, Tucker KL, Carithers TC, et al. Protein intake and long-term change in glomerular filtration rate in the Jackson Heart Study. J Ren Nutr. 2018;28(4):245–50. 10.1053/j.jrn.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe D, Machida S, Matsumoto N, Shibagaki Y, Sakurada T. Age modifies the association of dietary protein intake with all-cause mortality in patients with chronic kidney disease. Nutrients. 2018;10(11). 10.3390/nu10111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentine K, Wrone EM. New insights into protein intake and progression of renal disease. Curr Opin Nephrol Hypertens. 2004;13(3):333–6. 10.1097/00041552-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev. 2009;3: CD001892 10.1002/14651858.CD001892.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol. 1974;100(5):390–8. 10.1093/oxfordjournals.aje.a112050. [DOI] [PubMed] [Google Scholar]
- 12.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, et al. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med. 2006;166(1):79–87. 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–64. 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 15.Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr. 2015;6(6):712–28. 10.3945/an.115.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D, Zhou T, Li X, Heianza Y, Liang Z, Bray GA, et al. Genetic susceptibility, dietary protein intake, and changes of blood pressure: the pounds lost trial. Hypertension. 2019. 10.1161/HYPERTENSIONAHA.119.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in Dahl salt-sensitive rats. Clin Exp Hypertens. 2009;31(5):451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimokawa T, Moriuchi A, Hori T, Saito M, Naito Y, Kabasawa H, et al. Effect of dietary alpha-linolenate/linoleate balance on mean survival time, incidence of stroke and blood pressure of spontaneously hypertensive rats. Life Sci. 1988;43(25):2067–75. 10.1016/0024-3205(88)90356-6. [DOI] [PubMed] [Google Scholar]
- 19.Spradley FT, De Miguel C, Hobbs J, Pollock DM, Pollock JS. Mycophenolate mofetil prevents high-fat diet-induced hypertension and renal glomerular injury in Dahl SS rats. Phys Rep. 2013;1(6): e00137 10.1002/phy2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the Dahl salt sensitive rat. Am J Hypertens. 1999;12(2 Pt 1):183–7. 10.1016/s0895-7061(98)00238-6. [DOI] [PubMed] [Google Scholar]
- 21.Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am J Hypertens. 1992;5(9): 585–91. 10.1093/ajh/5.9.585. [DOI] [PubMed] [Google Scholar]
- 22.Young JB, Landsberg L. Effect of oral sucrose on blood pressure in the spontaneously hypertensive rat. Metabolism. 1981;30(5):421–4. 10.1016/0026-0495(81)90173-6. [DOI] [PubMed] [Google Scholar]
- 23.Nevala R, Vaskonen T, Vehniainen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci. 2000;66(2):115–24. 10.1016/s0024-3205(99)00569-x. [DOI] [PubMed] [Google Scholar]
- 24.Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994;23(2):195–9. 10.1161/01.hyp.23.2.195. [DOI] [PubMed] [Google Scholar]
- 25.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, et al. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics. 2004;16(2):194–203. 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 26.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2005;45(4):736–41. 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 27.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57(2): 269–74. 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Phys Regul Integr Comp Phys. 2018;315(1):R28–35. 10.1152/ajpregu.00201.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh AJ, Mattson DL. Parental dietary protein source and the role of CMKLR1 in determining the severity of Dahl salt-sensitive hypertension. Hypertension. 2019;73(2):440–8. 10.1161/HYPERTENSIONAHA.118.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, et al. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65(2):447–55. 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, et al. Dietary effects on Dahl salt-sensitive hypertension, renal damage, and the T lymphocyte transcriptome. Hypertension. 2019;74(4):854–63. 10.1161/HYPERTENSIONAHA.119.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, et al. Epigenetic modifications in T cells: the role of DNA methylation in salt-sensitive hypertension. Hypertension. 2019. 10.1161/HYPERTENSIONAHA.119.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Legarrea P, de la Iglesia R, Abete I, Navas-Carretero S, Martinez JA, Zulet MA. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition. 2014;30(4):424–9. 10.1016/j.nut.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am J Gastroenterol. 2010;105(10):2195–201. 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 35.Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53(10):1479–84. 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammad A, Ota F, Kassu A, Sorayya K, Sakai T. Modulation of oral tolerance to ovalbumin by dietary protein in mice. J Nutr Sci Vitaminol (Tokyo). 2006;52(2):113–20. 10.3177/jnsv.52.113. [DOI] [PubMed] [Google Scholar]
- 37.•.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351(6275):858–63. 10.1126/science.aac5560 [DOI] [PubMed] [Google Scholar]; This study demonstrated that dietary antigens, in the form of whole proteins, are necessary for proper production of intestinal Tregs.
- 38.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986;132(6):1647–56. 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- 41.Dai ZL, Zhang J, Wu G, Zhu WY. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010;39(5): 1201–15. 10.1007/s00726-010-0556-9. [DOI] [PubMed] [Google Scholar]
- 42.Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, et al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res. 2013;68(1):95–107. 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 43.•.Diether NE, Willing BP. Microbial fermentation of dietary protein: an important factor in diet(−)microbe(−)host interaction. Microorganisms. 2019;7(1). 10.3390/microorganisms7010019 [DOI] [PMC free article] [PubMed] [Google Scholar]; In-depth review on the reciprocal relationship between gut microbiota and proteolytic fermentation byproducts.
- 44.Wen S, Zhou G, Song S, Xu X, Voglmeir J, Liu L, et al. Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish. Proteomics. 2015;15(21):3688–98. 10.1002/pmic.201500179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kar SK, Jansman AJM, Benis N, Ramiro-Garcia J, Schokker D, Kruijt L, et al. Dietary protein sources differentially affect microbiota, mTOR activity and transcription of mTOR signaling pathways in the small intestine. PLoS One. 2017;12(11):e0188282 10.1371/journal.pone.0188282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Shi X, Lin X, Ye K, Xu X, Li C, et al. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. 2017;8:1395 10.3389/fmicb.2017.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.•.Kim E, Kim DB, Park JY. Changes of mouse gut microbiota diversity and composition by modulating dietary protein and carbohydrate contents: a pilot study. Prev Nutr Food Sci. 2016;21(1):57–61. 10.3746/pnf.2016.21.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides clear evidence for the adverse effects of a high casein diet on the microbiota composition and intestinal health of mice.
- 48.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–97. 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96–104. 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585–9. 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.••.Ferguson JF, Aden LA, Barbaro NR, Van Beusecum JP, Xiao L, Simmons AJ, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;5 10.1172/jci.insight.126241 [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent publication demonstrating the interplay between increased salt consumption, antigen presenting cell activation, and changes to the gut microbiota.
- 53.Abais-Battad JM, Saravia FL, Lund H, Dasinger JH, Fehrenbach DJ, Alsheikh AJ, et al. Role of gut microbiota and immunity in the dietary modulation of Dahl salt-sensitive hypertension. FASEB J. 2019;33:866.9. [Google Scholar]
- 54.Kenny LC, Black MA, Poston L, Taylor R, Myers JE, Baker PN, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014;64(3):644–52. 10.1161/HYPERTENSIONAHA.114.03578. [DOI] [PubMed] [Google Scholar]
- 55.Brantsaeter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, Alexander J, et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139(6):1162–8. 10.3945/jn.109.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu C, Coughlin KB, Frederick IO, Sorensen TK, Williams MA. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am J Hypertens. 2008;21(8):903–9. 10.1038/ajh.2008.209. [DOI] [PubMed] [Google Scholar]
- 57.Dasinger JH, Abais-Battad JM, Fehrenbach DJ, Lund H, Zemaj J, Alsheikh AJ, et al. Downregulation of placental genes are associated with the development of maternal syndrome in Dahl salt-sensitive rats. Hypertension. 2019;74:A152. [Google Scholar]
- 58.Liu J, Yang H, Yin Z, Jiang X, Zhong H, Qiu D, et al. Remodeling of the gut microbiota and structural shifts in preeclampsia patients in South China. Eur J Clin Microbiol Infect Dis. 2017;36(4):713–9. 10.1007/s10096-016-2853-z. [DOI] [PubMed] [Google Scholar]
- 59.Lv LJ, Li SH, Li SC, Zhong ZC, Duan HL, Tian C, et al. Early-onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front Cell Infect Microbiol. 2019;9:224 10.3389/fcimb.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nordqvist M, Jacobsson B, Brantsaeter AL, Myhre R, Nilsson S, Sengpiel V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open. 2018;8(1):e018021 10.1136/bmjopen-2017-018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.•.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–80. 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the vast microbiotal changes that occur throughout pregnancy and impacts host metabolism, inflammation, and adiposity.
- 62.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warren MF, Hallowell HA, Higgins KV, Liles MR, Hood WR. Maternal dietary protein intake influences milk and offspring gut microbial diversity in a rat (Rattus norvegicus) model. Nutrients. 2019;11(9). 10.3390/nu11092257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS One. 2016;11(4):e0152751 10.1371/journal.pone.0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salazar Garcia MD, Mobley Y, Henson J, Davies M, Skariah A, Dambaeva S, et al. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reprod Immunol. 2018;125: 25–31. 10.1016/j.jri.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 66.Darmochwal-Kolarz D, Saito S, Rolinski J, Tabarkiewicz J, Kolarz B, Leszczynska-Gorzelak B, et al. Activated T lymphocytes in preeclampsia. Am J Reprod Immunol. 2007;58(1):39–45. 10.1111/j.1600-0897.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 67.Jafri S, Ormiston ML. Immune regulation of systemic hypertension, pulmonary arterial hypertension, and preeclampsia: shared disease mechanisms and translational opportunities. Am J Phys Regul Integr Comp Phys. 2017;313(6):R693–705. 10.1152/ajpregu.00259.2017. [DOI] [PubMed] [Google Scholar]
- 68.Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, et al. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Phys Regul Integr Comp Phys. 2016;311(6):R1192–R9. 10.1152/ajpregu.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dasinger JH, Abais-Battad JM, Lund H, Fehrenbach DJ, Alsheikh AJ, Mattson DL. T lymphocytes contribute to the development of maternal syndrome in Dahl SS rats maintained on a low salt diet. FASEB J. 2018;32:911.2. [Google Scholar]


