Abstract
SARS-CoV-2 is a recently emerged human coronavirus that escalated to a pandemic. There are currently no approved vaccines for SARS-CoV-2, which causes severe respiratory illness or death. Defining the antibody response to SARS-CoV-2 will be essential for understanding disease progression, long-term immunity, and vaccine efficacy. Here we describe two methods for evaluating the neutralization capacity of SARS-CoV-2 antibodies. The basic protocol is a focus reduction neutralization test (FRNT), which involves immunostaining infected cells with a chromogen deposit readout. The alternate protocol is a modification of the FRNT that uses an infectious clone derived SARS-CoV-2 virus expressing a fluorescent reporter. These protocols are adapted for use in a high throughput setting and are compatible with large-scale vaccine studies or clinical testing.
Keywords: SARS-Cov-2, Antibody Neutralization, serological assay, neutralizing antibodies, high throughput neutralization assay
INTRODUCTION
In December 2019, a cluster of individuals presented with an unusual pneumonia in Wuhan, China (Commission, 2019). The causative agent of which was found to be the novel human coronavirus, SARS-CoV-2 (Commission, 2019; N. Zhu et al., 2020). In the following months, SARS-CoV-2 spread rapidly around the globe with a total of 21 million cases and 770,000 deaths (Organization, 2020). Studies defining the immune response against SARS-CoV-2 are critical for vaccine clinical trial design. Analyses of the humoral response to SARS-CoV-2 demonstrated that SARS-CoV-2 infection induces IgG and IgM antibodies directed against the receptor binding domain (RBD) of the spike protein (S) and the total spike protein (Ni et al., 2020; Okba et al., 2020; Pinto et al., 2020; Rogers et al., 2020; Suthar et al., 2020). These antibodies are capable of neutralizing SARS-CoV-2, and most patients develop neutralizing titers within 6 days of diagnosis (Suthar et al., 2020). Neutralization activity likely occurs through antibody blockade of the SARS-CoV-2 RBD with the cognate ACE-2 receptor (Ju et al., 2020; Shi et al., 2020). Recent studies have shown that passive transfer of monoclonal antibodies specific for the RBD are protective against SARS-CoV-2 in mouse, hamster, and rhesus macaque challenge models (Alsoussi et al., 2020; Rogers et al., 2020; Shi et al., 2020). Thus, neutralizing antibodies are an important correlate of protection against SARS-CoV-2 infection, and a potential therapeutic for severe disease.
Fast and reliable serology testing is essential for both tracking the pandemic and vaccine design. However, tools and reagents to study SARS-CoV-2 are highly limited. Many ELISA assays for measuring SARS-CoV-2 specific antibody levels have been described (Amanat et al.; Beavis et al., 2020; Liu et al., 2020; Okba et al., 2020; Stadlbauer et al., 2020). However, these assays are not able to directly assess the functional capacity of SARS-CoV-2 specific antibodies. Traditional neutralization assays include plaque or cytopathic effect-based assays which require long incubation times, are technically challenging and are not amenable to rapidly screening hundreds to thousands of samples. However, recent work has demonstrated the feasibility of performing high-throughput neutralization assays for SARS-CoV-2 (Muruato et al., 2020). Here, we describe the detailed protocol for performing a focus reduction neutralization test (FRNT) to measure the neutralization ability of SARS-CoV-2 antibodies. We developed two optimized FRNT protocols for either plasma or serum, which can be performed in a 96 well plate format in 3 days. In the basic FRNT protocol, SARS-CoV-2 infected cells are visualized through an immunostaining procedure with a primary monoclonal antibody directed against the spike protein. In the alternate FRNT-mNG protocol, a mNeonGreen expressing SARS-CoV-2 virus is utilized to directly image infected cells (Xie et al., 2020). These assays provide rapid high-throughput methods to measure SARS-CoV-2 antibody neutralization.
Basic protocol 1: Focus Reduction Neutralization Test
Plaque assays are a classic methodology for detecting infectious viruses, including Betacoronaviruses. However, this method is labor-intensive, costly, low throughput, and is not efficient for performing large-scale neutralization assays on patient specimens or monoclonal antibodies. We recently developed a focus-forming assay (FFA) platform for SARS-CoV-2 (Suthar et al., 2020; Vanderheiden et al., 2020). This basic protocol provides a step-by-step instruction for performing neutralization assays using this FFA platform. This protocol applies to performing a SARS-CoV-2 neutralization assay on human plasma/serum.
Materials
Vero C1008 (clone E6, ATCC, #CRL-1586)
icSARS-CoV-2 (infectious cloned virus, UTMB, (Xie et al., 2020))
Horseradish peroxidase Avidin D (Vector laboratories, Inc #A-2004)
Biotin labelled anti-SARS-CoV spike antibody (CR3022, Emory University, (Suthar et al., 2020))
Dulbecco’s Modified Eagle Medium (VWR, #45000–304)
Dulbecco’s Phosphate-Buffered Saline (DPBS) (VWR, #4500–436)
Fetal Bovine serum (Biotechne)
Non-essential amino acids (Fisher Scientific, #11140050)
L-glutamine (VWR, 25005Cl)
HEPES buffer (VWR, #4500–690)
Sodium pyruvate (VWR, #45000–710)
Antibiotics (VWR, #45000–616)
Trypan blue (Beta South Technologies, #T8154)
Trypsin-EDTA 0.25% (Thermo Fisher Scientific, #25200072)
96 well Flat bottom cell culture treated plate (Grenier Bio-One, #655180)
Falcon 96 well U-bottom plate (Corning, 353077)
Methylcellulose (Sigma, #M0512–250)
Paraformaldehyde (PFA), 20% (Fisher Scientific, #47340–9M)
Saponin (Sigma, 47036-250G-F)
Bovine Serum Albumin (BSA) (VWR, #0332–100g)
KPL TrueBlue Peroxidase Substrate (VWR, # 95059–168)
Serological pipettes, sterile (VWR or equivalent)
15 mL conicals VWR (89039–664 or equivalent)
50 mL conicals (89039–664 or equivalent)
Tips for Micropipettes, sterile (Rainin LTS or equivalent)
1.5 mL RNA / DNase free tubes (VWR 10160–142 or equivalent)
Vacuum filter system polyethersulfone (PES) membrane 0.22 μM, sterile (Genesee Scientific, #25–227)
20% Formaldehyde solution (Electron Microscopy Sciences, #15713-S)
Hyclone Powder Tissue Culture Media (VWR, #1677–047)
96-well non-skirted PCR Plate or similar (Thermo Fisher Scientific, #AB0600)
Equipment
Laminar flow hood (Labconco Purifier BSC Class II, or equivalent)
Heat block or Water Bath (set to 56°C)
Tissue Culture Incubator (set to 37°C with 5% CO2)
Refrigerator (2–8°C)
Freezer (−60°C to –90°C)
Tissue Culture Microscope (Nikon or equivalent)
Autoclave
Multichannel Pipette (200 μl and 20 μl)
Analytical scale
Labnet Rocker 25 (Model S2025-XLD-B)
Benchmark Scientific Everlast Rocker 247
Special Equipment
ELI-SPOT reader (CTL Analyzers or equivalent)
Human samples
The plasma samples used for this study were collected at Emory University Hospital and Emory University Hospital Midtown in Atlanta. Patients provided informed consent for collection, and archiving of samples as approved by the Emory University Institutional Review Board (IRB 00022371). Post isolation plasma or serum should be stored at −80°C and must be minimally freeze thawed not exceeding twice. A minimum of 18 μl plasma/serum is needed to run one assay in duplicate. 25 μl per sample is ideal considering fluid loss during storage and transfer.
Biosafety statement:
All blood samples should be handled and processed according to Institutional Biosafety Guidelines. This procedure should be performed in accordance with all applicable safety procedures.
Investigators should seek guidance from their local institutional environmental health and safety office to determine the appropriate protocols for working in BSL3 and removal of samples from BSL3.
Cell culture and seeding 96-well plates (Day 1)
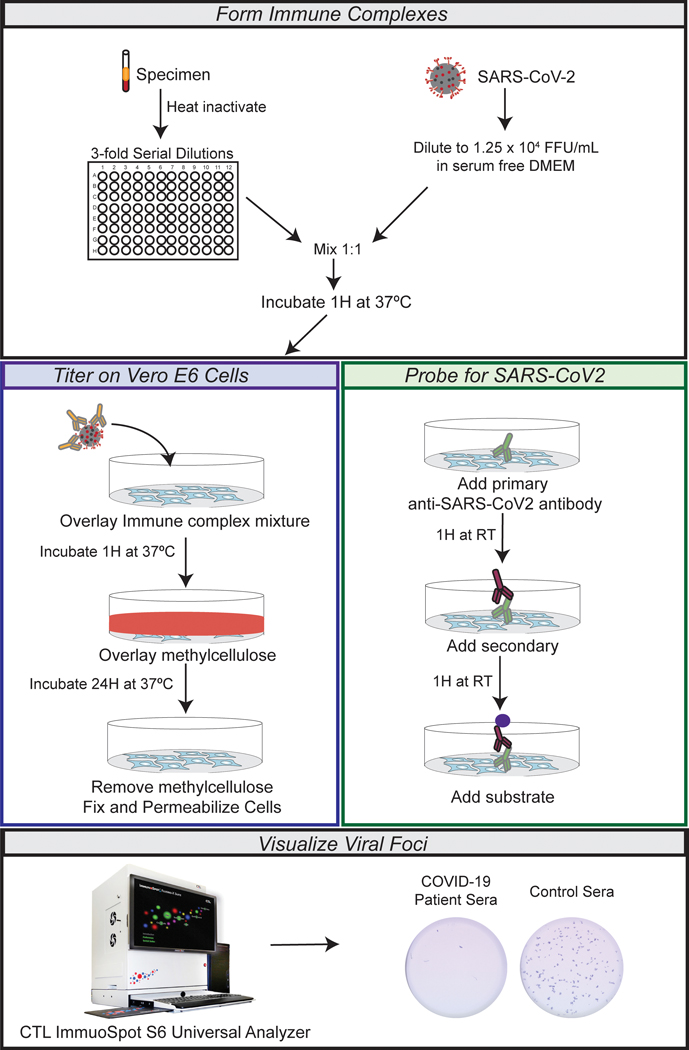
For an overview of the procedure, see Figure 1.
Figure 1. Overview of high-throughput FRNT assay for measuring SARS CoV-2 neutralizing antibodies.
Flow chart demonstrating the experimental outline. In brief, the specimens are serially diluted and mixed with equal parts of icSARS-CoV-2 following 1hour incubation at 37° C and 5% CO2. Then the immune complex mixture is overlaid on top of Vero-E6 cells and incubated at 37° C and 5% CO2 for 1 hour. The viral inoculum is removed and replaced by 0.85% methyl cellulose. Infected cultures are incubated for 24 hour at 37° C and 5% CO2. After which, the cells are fixed with paraformaldehyde and probed for SARS-CoV-2 spike protein. Foci are visualized via chromogen deposit, recorded using CTL ImmunoSpot S6 Universal Analyzer, and quantified using Viridot.
-
1. Produce a healthy culture of Vero E6 cells.
Note: If reviving cells from a freezer stock see ATCC Cell Culture Guide for complete instructions on reviving and culturing cells (“How to Revive Cultures,” 2020).
2. Grow the cells to confluence and split into the needed number of T175 flasks. Flasks can be seeded at 1:4 for confluence 2 days later. The ideal confluence will have media that is a reddish orange color. A confluent flask should contain approximately 2.0×107 cells.
3. Remove media from a confluent T175 cm2 flask of VeroE6 cells.
4. Wash the cells with 5 ml of DPBS.
5. Add 5 ml trypsin and incubate at either room temperature or 37°C for 10–15 minutes.
6. Add 5 ml of cDMEM and mix thoroughly. Remove an aliquot for counting using trypan blue.
7. Resuspend cells at 2.75 × 105 cells/ ml in cDMEM.
8. Using a multichannel pipette, place 150 μl of cell mixture into each well of a 96-well tissue cultured treated plate (4.125 × 104 cells/well).
9. Incubate at 37°C for approximately 24 hours or until the cell monolayer reaches 100% confluency.
Neutralization Assay (Day 2–3)
Note:
Vero E6 cells should be 100% confluent at the time of infection.
All work with live, infectious SARS-CoV-2 must take place in a BSL-3 containment facility.
Before starting, warm methylcellulose overlay to 370 C.
Diluting serum/plasma for neutralization assay
For a schematic of serial dilutions, see Figure 2.
Figure 2. FRNT-Assay plate layout and specimen dilution.
The specimens are heat inactivated and serially diluted at threefold with serum free DMEM with a 1/20 starting dilution. Initially 9 μl of the specimen is mixed with 81 μl of DMEM (becomes 1/20 after adding the viral inoculum) a total of 90 μl in row-1 of a 96 well U-bottomed plate. The specimens are further diluted three-fold by transferring 30 μl from row-1 to row-2. This three-fold serial dilution is carried all the way to the last row of the plate. The last two columns on a plate are assigned for the controls. The controls include virus alone layered on top of Vero-E6 cells, with no specimen and mock (no virus) that does not have either virus or specimen.
10. Thaw the serum/plasma samples at room temperature (~ 25 μL per tube). Once thawed, perform a quick spin (10,000 RPM for 20 sec at 4°C).
11. Place serum/plasma samples in either dry heat block (pre-warmed to 56°C) or water bath (pre-warmed to 56°C) for 30 minutes to heat inactivate the complement.
12. Spin down serum/plasma samples at 10,000 RPM for 5 min at 4°C, and collect the resulting supernatant.
13. Aliquot samples into 96-well sterile PCR plate, a minimum of 12 μl per well in duplicates next to each other.
14. Using a 96-well U-bottom plate, aliquot 81 μl of serum-free DMEM into row A. In remaining rows, add 60 μl of serum-free DMEM.
15. Add 9 μl of plasma from the PCR plate to each column with a multichannel pipette. Mix thoroughly by pipetting at a minimum 5 μl, at least 5 times. Each sample should be run in duplicate. The neutralization titers from these duplicate samples will be averaged to generate the final neutralization titer for a given sample.
16. Using a multichannel pipette, remove 30 μl from Row A and add to Row B.
17. With a new set of tips, pipette up and down 3–5 times. Remove 30 μl and add to Row C.
18. Repeat for Rows C-H. On the last row, remove 30 μl and dispense into a waste container.
Forming immune complexes with icSARS-CoV-2 and infecting VeroE6 cells
-
19. Dilute icSARS-CoV-2 (basic protocol) or icSARS-CoV-2-mNeonGreen (alternate protocol) to a concentration of 750 focus-forming units (FFU) in 60 μl (1.25 × 104 FFU/ml). For 1 × 96-well plate, make 6 ml of diluted virus.
Note: icSARS-CoV-2 is a SARS-CoV-2 virus derived from an infectious clone generated in Xie et al., 2020. Stock solutions should be at 1 × 106 FFU/ml or higher.
20. Dilute 1% complete methylcellulose to 0.85% methylcellulose with serum-free DMEM. Mix thoroughly. For 1 × 96-well plate, prepare approximately 15 ml of diluted methylcellulose.
21. Add 60 μl of diluted virus into every row containing 60 μl of serum dilution from steps 14–16. Mix thoroughly. Note that the serum dilutions are now diluted 2-fold (e.g. 1:10 dilution is now a 1:20 dilution).
22. Incubate at 37°C for 1 hour.
23. Remove the media from the tissue culture plate with Vero E6 cells and add 100 μl of the virus-plasma mixture to the cells with a multichannel pipette. Discard the tips after dispensing mixture into each row of the tissue culture plate.
24. Incubate at 37°C for 1 hour.
25. Remove inoculum. Using a multichannel pipette and wide-mouth pipette tips, add 100 μl of warm methylcellulose overlay to each well.
-
26. Incubate at 37°C with 5% CO2 for 24–30 hours.
Note: Incubation times can vary depending on how large you would like the foci to appear. Foci are visible as early as 18 hours. We have found that 24 hours provides ideal resolution, longer than 30 hours will cause foci to become large and overlapping (Figure 4).
Figure 4. Visualization and quantification of a representative FRNT-mNG assay.
A) Representative FRNT-mNG assay results show a comparison between the viral foci of a convalescent patient versus a healthy control at three different time points, 24, 48 and 72 hours along with a mock infection control. Antibody neutralization is quantified by counting the number of foci for each sample using the Viridot program (Katzelnick et al., 2018). B) The percentage neutralization of a convalescent patient is shown here. Each specimen is tested in two independent assays performed at different times. The FRNT50 titer is interpolated using a 4-parameter nonlinear regression in GraphPad Prism 8.4.3. C) The dot plot depicted here shows FRNT50 titers of 9 healthy control individuals versus 10 convalescent patients.
Focus-forming Assay
-
27. Carefully aspirate the methylcellulose overlay using fine-tipped pipettes.
Note: Take care not to touch the bottom of the well as this will disrupt the monolayer.
28. Wash the cells by adding 150 μl DPBS to each well. Repeat 2–3X. Discard DPBS.
29. Add 100 μl of 2% PFA (diluted in DBPS) to each well.
-
30. Incubate at room temperature for 30 minutes.
Note: We have shown that this fixation step eliminates infectious SARS-CoV-2. Investigators should seek guidance from their local institutional biosafety office to determine the appropriate protocols for decontaminating and removing plates from the BSL3. The remaining steps can be performed in a BSL2 laboratory setting.
31. Remove the fixation buffer by inverting plate and flicking out buffer. Remove any remaining drops by tapping against a stack of paper towels 2–3 times.
32. Wash the cells by adding 150 μl 1X DPBS to each well. This step helps in removing any residual PFA from the wells.
Blocking and staining (Day 3)
33. Remove DPBS from each well and then add 100 μl Permeabilization buffer to each well. Incubate at room temperature for 20 minutes.
34. Remove the Permeabilization buffer by inverting plate and flicking out buffer. Remove any remaining drops by tapping against a stack of paper towels 2–3 times.
35. Dilute biotin-tagged anti-SARS-CoV-2 Spike antibody (CR3022) to a concentration of 0.2 μg/ml in Perm/Wash buffer. Add 50 μl of diluted antibody to each well.
-
36. Incubate on a rocker at room temperature for 1–2 hours.
Note: Primary antibody can be incubated overnight at 4°C (up to 24 hours).
37. Remove primary antibody by inverting plate and flicking out buffer. Remove any remaining drops by tapping against a stack of paper towels 2–3 times.
38. Wash cells 3 times with 1X DPBS. Each wash requires 150 μl/well of 1X DPBS.
39. Dilute secondary antibody (Avidin-HRP) at 1:2000 in Perm/Wash buffer. Add 50 μl of diluted antibody to each well.
40. Incubate on a plate rocker at room temperature for 1 hour.
41. Remove secondary antibody by inverting plate and flicking out buffer. Remove any remaining drops by tapping against a stack of paper towels 2–3 times.
42. Wash cells 4 times with 1X DPBS. Each wash requires 150 μl/well of 1X DPBS.
43. Add 50 μL of TrueBlue Peroxidase Substrate to each well of the plate.
-
44. Incubate at room temperature for 5–30 minutes.
Note: Spots will be visible by 5 minutes post-incubation with substrate. The spots will get darker with longer incubation times. However, background may also increase with longer incubation times.
45. Remove substrate by inverting plate and flicking out buffer. Remove any remaining drops by tapping against a stack of paper towels 2–3 times.
46. Wash the cells by adding 150 μl DPBS to each well.
47. At this point, the assay plates could be stored at 4° C for up to one week, or proceed immediately to the following steps.
Visualizing viral foci (Day 3)
48. Discard DPBS and remove any remaining 1X DPBS by tapping against a stack of paper towels 2–3 times.
49. Image the plates using ELISPOT reader (CTL ImmunoSpot S6 Universal Analyzer).
50. The plates are visualized under the ELISPOT configuration using the following settings: Visible light source (bottom), Auto exposure, Lens control set to position 6196, Range set to 600, Precision set to 10, and Zoom set to 1.6X.
Alternate protocol 1:
An mNeonGreen-based Focus Reduction Neutralization Test (FRNT-mNG)
The alternate protocol is similar to the basic protocol except this protocol utilizes a SARS-CoV-2 virus that expresses mNeonGreen (Xie et al., 2020). The protocol for cell seeding, serum dilutions, immune complex formation, and the focus forming assay are all the same as the basic protocol except for the use of SARS-CoV-2-mNG. However, the FRNT-mNG does not require blocking, probing the cells with primary and secondary antibodies, or visualizing the foci with a peroxidase substrate. Instead, the foci can be directly visualized after fixation using a florescent ELI-SPOT reader. The elimination of multiple probing and washing steps after fixation significantly reduces bench time.
Additional Materials
icSARS-CoV-2-mNG
Cellstar μClear blackout tissue culture treated plate (USA Scientific, #655090)
Vero C1008 (clone E6, ATCC, #CRL-1586)
Dulbecco’s Modified Eagle Medium (VWR, #45000–304)
Dulbecco’s Phosphate-Buffered Saline (DPBS) (VWR, #4500–436)
Fetal Bovine serum (Biotechne)
Non-essential amino acids (Fisher Scientific, #11140050)
L-glutamine (VWR, 25005Cl)
HEPES buffer (VWR, #4500–690)
Sodium pyruvate (VWR, #45000–710)
Antibiotics (VWR, #45000–616)
Trypan blue (Beta South Technologies, #T8154)
Trypsin-EDTA 0.25% (Thermo Fisher Scientific, #25200072)
96 well Flat bottom cell culture treated plate (Grenier Bio-One, #655180)
Falcon 96 well U-bottom plate (Corning, #353077)
Methylcellulose (Sigma, #M0512–250)
Paraformaldehyde (PFA), 20% (Fisher Scientific, #47340–9M)
Serological pipettes, sterile (VWR or equivalent)
15 mL conicals VWR (89039–664 or equivalent)
50 mL conicals (89039–664 or equivalent)
Tips for Micropipettes, sterile (RaininLTS or equivalent)
1.5 mL RNA / DNase free tubes (VWR 10160–142 or equivalent)
Vacuum filter system polyethersulfone (PES) membrane 0.22 μM, sterile (Genesee Scientific, #25–227)
20% Formaldehyde solution (Electron Microscopy Sciences, #15713-S)
Hyclone Powder Tissue Culture Media (VWR, #1677–047)
96-well non-skirted PCR Plate or similar (Thermo Fisher Scientific, #AB0600)
Equipment
Laminar flow hood (Labconco Purifier BSC Class II, or equivalent)
Heat block or Water Bath (set to 56°C)
Tissue Culture Incubator (set to 37°C with 5% CO2)
Refrigerator (2–8°C)
Freezer (–60°C to –90°C)
Tissue Culture Microscope (Nikon or equivalent)
Autoclave
Multichannel Pipette (200 μl and 20 μl)
Analytical scale
Labnet Rocker 25 (Model S2025-XLD-B)
Benchmark Scientific Everlast Rocker 247
Special Equipment
ELI-SPOT reader (CTL Analyzers or equivalent)
Note:
For the alternate protocol a variant of SARS-CoV-2, icSARS-CoV-2-mNeonGreen is used instead of the WT icSARS-CoV-2.
Dilute icSARS-CoV-2-mNeonGreen (alternate protocol) to a concentration of 100 focus-forming units (FFU) in 60 μl (1.67 × 103 FFU/ml). For 1 × 96-well plate, make 6 ml of diluted virus.
It is important to use the blackout 96 well tissue culture clear bottom plates for the FRNT-mNG assay. The blackout plates are important for visualizing the mNG-fluorescence signal and reducing background.
Visualizing the viral foci (Day 3)
Perform Basic protocol steps 1–30.
Remove the fixation buffer inverting plate and flicking out buffer. Remove any remaining drops by tapping against a stack of paper towels 2–3 times.
Wash cells 1 time with 1X PBS. This step helps in removing any residual PFA from the wells.
Add 35 μl of 1X PBS to each well and image the plate on ELISPOT reader (CTL ImmunoSpot S6 Universal Analyzer) under a FITC channel. Visualize plates under the Flourospot plate configuration using an excitation band of 480 nm, Gain setting of 28.0, Exposure setting of 2800ms, Lens control set to position 6196, Range set to 600, Precision set to 10, and Zoom set to 1.8X.
REAGENTS AND SOLUTIONS
Complete DMEM (cDMEM)
Combine 500 ml DMEM, 10% Heat inactivated-FBS, HEPES (25 mM), Sodium pyruvate (10 mM), Antibiotics (10 mM), Non-essential amino acids (10mM), L-glutamine (10 mM).
Filter through the vacuum filter system (500 ml, 0.22 μM PES).
Store at 4°C for up to 3 months.
Methylcellulose overlay (1.0% final concentration)
Weigh out 10 g of methylcellulose and autoclave.
Allow methylcellulose to cool and mix with 500 ml of warm ddH2O. Stir using a magnetic stirrer overnight at 4°C or until methylcellulose has gone into solution.
Make 2X complete DMEM (500 mL; pH 7.4) by mixing 13.4 g DMEM powder into 420 ml ddH2O and adjust pH of the solution to 7.4.
Add antibiotics/antimycotic (10 mM), 25mM HEPES, 10 mM NEAA. Filter through the vacuum filter system (500 ml, 0.22 μM PES).
Mix the dissolved methylcellulose with the 2X DMEM. Final concentration is 1.0% methylcellulose overlay. Place in 37°C water bath until there is a homogenous mixture.
Fixation Buffer
Dilute 20% paraformaldehyde in sterile DPBS to final concentration of 2% paraformaldehyde. Store in a foil-wrapped container for no more than 7 days.
Permeabilization/Antibody diluent buffer (Perm/Ab Buffer)
To 500 ml DPBS add 0.1% saponin and 0.1% BSA. Filter through the vacuum filter system (500 ml, 0.22 μM PES). Aliquot and freeze at −20°C for up to 1 year or store at 4°C for up to 1 week.
COMMENTARY
Background Information
Antibodies perform several key functions during viral infections, including complement recruitment, opsonization, and neutralization. Pathogen-specific antibodies can form immune complexes that inactivate the virus and are cleared by phagocytic cells (Corti & Lanzavecchia, 2013). However, this neutralizing ability can have a wide range of effectivity based on the pathogen or even the patient. The plaque reduction neutralization test (PRNT) is considered to be a classic method for measuring neutralization against Betacoronaviruses (Suthar et al., 2010; Z. Zhu et al., 2007). In PRNT, sera containing the antibodies of interest are serially diluted and incubated with the target virus to form immune complexes. These complexes are then overlaid on a monolayer of susceptible cells and virus diffusion is limited by overlaying agar. Over a period of several days, the monolayer is examined for cytopathic effect, which will result in plaque formation. The neutralization ability of the antibodies is quantified by comparing the number of plaques between cells treated with sera and an untreated control. This method is reliable, well established, and requires few specific reagents. However, PRNTs take several days to perform and to provide adequate plaque resolution, a PRNT must be performed in a 6- or 12-well plate format. This results in the need of large quantities of cells, plates, and reagents to analyze a large number of samples. Thus, while highly sensitive, PRNTs are not ideal for a high throughput setting.
Focus reduction neutralization (FRNT) assays have been developed for several viruses as an alternative to PRNTs (Friedrich & Beasley, 2016; Quicke et al., 2016; Vaidya et al., 2010). This assay uses a similar principle to the PRNT, however instead of visualizing viral growth with plaques, virus is detected using antibodies. In an FRNT assay, the immune complexes are overlaid on a cell monolayer and virus diffusion is immobilized using methylcellulose. The cells are fixed, and virus-specific antibodies conjugated to HRP are used to visualize foci of infected cells. This method provides several advantages to a traditional PRNT assay; 1) FRNTs can be performed in a 96-well plate format, making sample processing much quicker and requiring fewer reagents and cells. 2) FRNTs take 3 days to perform instead of the 5–6 days that a PRNT requires. 3) Fixation of the cells allows for more flexibility in processing, as samples can be stored at 4°C for a few days and visualized in a BSL-2 setting. The major drawback of a FRNT assay compared to the PRNT assay is the requirement of a virus-specific antibody to visualize the foci.
In this protocol, we describe a second method to detect virus neutralization, termed the FRNT-mNG assay that utilizes an mNeonGreen expressing SARS-CoV-2 virus (Xie et al., 2020). Here, antibodies are complexed with SARS-CoV-2-mNG and then overlaid on a monolayer of cells similarly to the FRNT. Infected foci are directly visualized using a fluorescent ELISPOT reader. This method is advantageous for high-throughput applications, as it reduces bench-time compared to the FRNT assay by the omission of the permeabilization, primary and secondary antibody incubations, and washing steps. However, a drawback for the FRNT-mNG is that it requires a virus that expresses an mNeonGreen protein and a method to visualize the foci such as an ELISPOT reader with fluorescence capability.
Parameters and Troubleshooting
For both basic and alternate protocols the following considerations are applicable. It is important to use a low passage sequence-verified SARS-CoV-2 isolate, as cell culture adaptions may influence neutralization titers. It is also essential to use specifically the E6 Vero clone to obtain robust infection. The multiplicity of infection (MOI) may need to be carefully titrated for optimal foci resolution. If the MOI is too low, there will not be enough foci to accurately quantify neutralization activity, if the MOI is too high, there will be too many foci to count. When optimizing the size of the foci, the incubation time post infection is the most important factor. SARS-CoV-2 needs at least 18 hours to develop visible foci in Vero E6 cells. We have observed that longer incubation times leads to larger sized foci making it difficult to distinguish individual foci (Panels A in Figures 3 and 4). In this method, the optimal incubation time was found to be 24–30 hours post infection, however longer or shorter incubation times may work better in a different environment. For the basic protocol, in order to obtain good staining of the foci, it is important to precisely perform the described washes. We have found that reducing the number of washes or volume of PBS per wash can significantly increase the background signal. Additionally, overnight incubation with the primary antibody generally increases the robustness of the staining. For the alternate protocol, if the cells are fixed, it is important to fix cells with paraformaldehyde, not a methanol-based fixation method, which will quench the GFP fluorescence.
Figure 3. Visualization and quantification of a representative FRNT assay.
A) Representative FRNT assay results show a comparison between the viral foci of a convalescent patient versus a healthy control at three different time points, 24, 48 and 72 hours along with a mock infection control. Antibody neutralization is quantified by counting the number of foci for each sample using the Viridot program (Katzelnick et al., 2018). B) The percentage neutralization of a convalescent patient is shown here. Each specimen is tested in two independent assays performed at different times. The FRNT50 titer is interpolated using a 4-parameter nonlinear regression in GraphPad Prism 8.4.3. C) The dot plot depicted here shows FRNT50 titers of 9 healthy control individuals versus 10 convalescent patients.
Understanding Results
The basic and alternate protocols describe methods to measure the neutralization ability of SARS-CoV-2 specific antibodies in a given biological sample. To correctly interpret the results of each assay, it is important to include the following controls: 1) uninfected, untreated mocks, 2) SARS-CoV-2 infected, untreated controls, 3) serum from healthy, SARS-CoV-2 naïve individuals (Fig. 2). Representative images of the foci for the experimental and control samples for an FRNT and FRNT-mNG are shown in Figure 3 and 4. To quantify antibody neutralization, the number of foci is counted for each sample using the Viridot program (Katzelnick et al., 2018). To calculate percentage neutralization, foci numbers are compared between an untreated, infected control and serum treated, infected wells (Fig. 3, 4). The average number of foci in the virus-only sample were used to calculate the neutralization curves: 1 - (ratio of mean number of foci in the presence of sera and foci at the highest dilution of respective sera serum). Each specimen is tested in two independent assays performed at different times. The FRNT50 titer, the dilution at which the serum or plasma sample reaches 50% neutralization capability, is interpolated using a 4-parameter nonlinear regression in GraphPad Prism 8.4.3. The FRNT50 provides a quantitative measure of neutralization capacity and can be used to compare antibody effectiveness between groups or patients. Overall, both the FRNT and FRNT-mNG assays provide methods to quickly and accurately quantify the neutralizing antibodies in a sample and compare between groups.
Time Considerations
Basic and alternate protocol require the establishment of a Vero E6 culture, which is not taken into the time considerations in this protocol. It will take several days to revive a stock from liquid nitrogen and to reach confluency. Both basic and alternate protocols require 3 days to complete, with equal time required for both on day 1 and day 2. However, on day 3, the basic protocol takes about 6 hours to complete, while the alternate protocol requires only 1–2 hours of work.
Acknowledgements
Thanks to Kathy Stephens and Laila Hussaini for their assistance in recruiting these patients. The research reported in this publication was supported in part by an Emory EVPHA Synergy Fund award (M.S.S. and J.W.), COVID-Catalyst-I3 Funds from the Woodruff Health Sciences Center (M.S.S), Center for Childhood Infections and Vaccines (M.S.S), Children’s Healthcare of Atlanta (M.S.S), Woodruff Health Sciences Center 2020 COVID-19 CURE Award (M.S.S), and by the National Institutes of Health (NIH) through the National Institute for Allergy and Infectious Diseases under the award numbers ORIP/OD P51OD011132 (M.S.S), 3U19AI057266-17S1 (M.S.S.), HIPC COVID-19 Supplement U19AI090023 (M.S.S), R01AI127799 (M.S.S.), R01AI148378 (M.S.S.) and the Infectious Diseases Clinical Research Consortium UM1AI148684 (E.A., N.R., J.W., M.S.S.), R00AG049092 (V.D.M) and the World Reference Center for Emerging Viruses and Arboviruses R24AI120942 (V.D.M). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors declare no competing interests.
Literature Cited:
- Alsoussi WB, Turner JS, Case JB, Zhao H, Schmitz AJ, Zhou JQ, . . . Ellebedy AH (2020). A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J Immunol. doi: 10.4049/jimmunol.2000583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, . . . Krammer F A serological assay to detect SARS-CoV-2 seroconversion in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis KG, Matushek SM, Abeleda APF, Bethel C, Hunt C, Gillen S, . . . Tesic V. (2020). Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J Clin Virol, 129, 104468. doi: 10.1016/j.jcv.2020.104468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission WMH (2019). Report of clustering pneumonia of unknown etiology in Wuhan City. [Google Scholar]
- Corti D, & Lanzavecchia A. (2013). Broadly Neutralizing Antiviral Antibodies. Annual Review of Immunology, 31(1), 705–742. doi: 10.1146/annurev-immunol-032712-095916 [DOI] [PubMed] [Google Scholar]
- Friedrich BM, & Beasley DW (2016). ELISA and Neutralization Methods to Measure Anti-West Nile Virus Antibody Responses. Methods Mol Biol, 1435, 129–141. doi: 10.1007/978-1-4939-3670-0_11 [DOI] [PubMed] [Google Scholar]
- How to Revive Cultures. (2020). Retrieved from https://www.atcc.org/how_to_revive_cultures.aspx
- Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, . . . Zhang L. (2020). Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. doi: 10.1038/s41586-020-2380-z [DOI] [PubMed] [Google Scholar]
- Katzelnick LC, Coello Escoto A, McElvany BD, Chávez C, Salje H, Luo W, . . . Cummings DAT (2018). Viridot: An automated virus plaque (immunofocus) counter for the measurement of serological neutralizing responses with application to dengue virus. PLOS Neglected Tropical Diseases, 12(10), e0006862. doi: 10.1371/journal.pntd.0006862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, . . . Zheng S. (2020). Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. Journal of Clinical Microbiology, 58(6), e00461–00420. doi: 10.1128/JCM.00461-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruato AE, Fontes-Garfias CR, Ren P, Garcia-Blanco MA, Menachery VD, Xie X, & Shi PY (2020). A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun, 11(1), 4059. doi: 10.1038/s41467-020-17892-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, . . . Dong C. (2020). Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity, 52(6), 971–977.e973. doi: 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, . . . Haagmans BL (2020). Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis, 26(7), 1478–1488. doi: 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH (2020). Coronavirus disease 2019. Situation Report- 77. Retrieved from [Google Scholar]
- Pinto D, Park Y-J, Beltramello M, Walls AC, Tortorici MA, Bianchi S, . . . Corti D. (2020). Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature, 583(7815), 290–295. doi: 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, . . . Suthar MS (2016). Zika Virus Infects Human Placental Macrophages. Cell Host Microbe, 20(1), 83–90. doi: 10.1016/j.chom.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He W. t., . . . Burton DR (2020). Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science, eabc7520. doi: 10.1126/science.abc7520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, . . . Yan J. (2020). A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. doi: 10.1038/s41586-020-2381-y [DOI] [PubMed] [Google Scholar]
- Stadlbauer D, Amanat F, Chromikova V, Jiang K, Strohmeier S, Arunkumar GA, . . . Krammer F. (2020). SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Current Protocols in Microbiology, 57(1), e100. doi: 10.1002/cpmc.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, . . . Gale M Jr. (2010). IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog, 6(2), e1000757. doi: 10.1371/journal.ppat.1000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, . . . Wrammert J. (2020). Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep Med, 1(3), 100040. doi: 10.1016/j.xcrm.2020.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya SR, Brown DW, Jin L, Samuel D, Andrews N, & Brown KE (2010). Development of a focus reduction neutralization test (FRNT) for detection of mumps virus neutralizing antibodies. J Virol Methods, 163(1), 153–156. doi: 10.1016/j.jviromet.2009.09.006 [DOI] [PubMed] [Google Scholar]
- Vanderheiden A, Ralfs P, Chirkova T, Upadhyay AA, Zimmerman MG, Bedoya S, . . . Suthar MS (2020). Type I and Type III IFN Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J Virol. doi: 10.1128/jvi.00985-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Muruato A, Lokugamage KG, Narayanan K, Zhang X, Zou J, . . . Shi PY (2020). An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe, 27(5), 841–848.e843. doi: 10.1016/j.chom.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, . . . Tan W. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. 382(8), 727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chakraborti S, He Y, Roberts A, Sheahan T, Xiao X, . . . Dimitrov DS (2007). Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci U S A, 104(29), 12123–12128. doi: 10.1073/pnas.0701000104 [DOI] [PMC free article] [PubMed] [Google Scholar]