Abstract
BACKGROUND:
Age-related macular degeneration (AMD) is a common cause of blindness worldwide. Neovascular AMD (nAMD) is an advanced form of the disease, in which excess vascular endothelial growth factor (VEGF) induces growth of new blood vessels that leak fluid, accounting for 90% of vision loss in AMD. Dysfunction of the retinal pigment epithelium likely initiates AMD. Retinal pigment epithelial cells express a G protein-coupled receptor, GPR143, which downregulates VEGF in response to levodopa. Anti-VEGF therapy effectively treats nAMD, suggesting that excessive VEGF activity drives the pathology.
METHODS:
In an open-label pilot study, in patients with newly diagnosed nAMD and naïve to anti-VEGF injections (Cohort-1), the effects of carbidopa-levodopa on vision and anatomic outcomes were evaluated for 4 weeks. Then patients were followed 5 months further with ascending levodopa doses. Patients previously treated with anti-VEGF injection therapy (Cohort-2) were also treated with ascending levodopa doses and evaluated for 6 months.
RESULTS:
Levodopa was safe, well tolerated, and delayed anti-VEGF injection therapy while improving visual outcomes. In the first month, retinal fluid decreased by 29% (P = .02, n = 12) without anti-VEGF treatment. Through 6 months the decrease in retinal fluid was sustained, with a mean frequency of 0.38 injections/month. At month 6, mean visual acuity improved by 4.7 letters in Cohort-1 (P = .004, n = 15) and by 4.8 letters in Cohort-2 (P = .02, n = 11). Additionally, there was a 52% reduction in the need for anti-VEGF injections in Cohort-2 (P = .002).
CONCLUSIONS:
Our findings suggest efficacy and support the pharmacological targeting of GPR143 with levodopa for the treatment of nAMD in future studies.
Keywords: Age-related macular degeneration (AMD), Carbidopa-levodopa, GPR143, L-DOPA, nAMD, Neovascular AMD, Prospective study, Retinal pigment epithelium, Wet AMD
INTRODUCTION
Age-related macular degeneration (AMD) is a common cause of blindness in developed countries, with a rising prevalence as life expectancy increases.1–3 The disease is characterized by the degeneration of the central part of the retina, the macula, which is responsible for high-acuity vision. In the United States, >15% of the population past the age of 70 years has AMD, for which we lack adequate prevention or treatment.3,4 Neovascular age-related macular degeneration (nAMD) is characterized by the abnormal ingrowth of new blood vessels from the choriocapillaris into the sub-retinal pigment epithelial or subretinal space, which can cause fluid and blood to leak.5 The observed angiogenesis is due to excessive vascular endothelial growth factor (VEGF).6 nAMD represents only 10%−15% of all AMD cases, however, it accounts for 90% of the vision loss attributed to the disease.7
One of the strongest risk factors associated with AMD is race, where vision loss from the disease is most prevalent in the white population.8,9 We interpret the racial bias to suggest that ocular pigmentation protects from AMD, because those with the least pigmentation are particularly susceptible. We discovered the ligand for a G protein-coupled receptor that is part of the pigmentation pathway, GPR143.10 The ligand for GPR143 is levodopa, an intermediate product of pigment synthesis.11 GPR143 is found on the apical surface of the retinal pigment epithelium, a monolayer of pigmented cells located between the choroid and neural retina, likely the primary tissue that initiates AMD pathobiology. In retinal pigment epithelial cells, activation of GPR143 by levodopa significantly upregulates pigment epithelium-derived factor (PEDF),12 a potent anti-angiogenesis factor,13 and simultaneously downregulates the production of VEGF.10,12 VEGF is the target of current nAMD therapies to slow angiogenesis. This strategy is successful, but requires frequent intravitreal injections.6 The efficacy of this treatment indicates that nAMD pathobiology is caused by an overabundance of VEGF. Levodopa is commonly used to treat movement disorders, such as Parkinson disease, as the supplemental levodopa is converted to dopamine in neurons.14,15 Brilliant et al16 sought to evaluate whether patients taking levodopa for movement disorders were protected from AMD, likely from activation of GPR143 in the retinal pigment epithelium. They developed a retrospective analysis to determine if there is a possible link between levodopa and AMD; it examined clinical data encompassing >87 million individuals. People with a history of levodopa prescriptions were significantly less likely to develop any type of AMD. Further, if they did develop the disease, age of onset was significantly delayed, by 8 years. This led to the conclusion that levodopa supplementation may protect from AMD, and a prospective study was warranted.
Currently there are limited preclinical models to study AMD. Patients with nAMD require significant ophthalmology care, most notably, frequent anti-VEGF injections to stop angiogenesis. Here we present a study of patients with nAMD to determine whether levodopa has a positive effect on vision and anatomic outcomes, to evaluate the safety and tolerability of levodopa, and determine whether levodopa supplementation can reduce necessary anti-VEGF injections.
METHODS
Study-1 Design: Carbidopa-Levodopa in Neovascular Age-Related Macular Degeneration
This study was a proof-of-concept phase 2 clinical trial conducted in a private ophthalmology practice (ClinicalTrials.gov identifier NCT03022318) between September 2017 and December 2019. Participant recruitment started September 2017. Institutional Review Board approval was obtained prior to study initiation and all patients provided written informed consent. Study-1 enrolled 20 patients who met the inclusion criteria (Supplementary Figure, A). After baseline measurements were obtained, the patients in Cohort 1 (Supplementary Table 1) were assigned to carbidopa-levodopa 25–100 mg (hereinafter termed levodopa) 1 tablet at bedtime (quaque hora somni [QHS]) or 1 tablet 3 times a day (TID). The patients were evaluated weekly for 1 month and continued to be under the care of their referring retina specialist. After each follow-up visit, the retina specialist was notified of the patient’s best-corrected visual acuity (BCVA) and anatomic changes determined by spectral domain-optical coherence tomography (SD-OCT). The patient’s retina specialist ultimately determined whether anti-VEGF therapy was required. At the end of Study-1, patients were enrolled in the 12-month study (Study-2; ClinicalTrials.gov identifier NCT03023059/NCT03197493).
Study-2 Design: Dose Ranging of Carbidopa-Levodopa
This open-label proof-of-concept study conducted in a private ophthalmology practice (ClinicalTrials.gov identifier NCT03023059) started participant recruitment in May 2017. Institutional Review Board approval was obtained prior to study initiation and all patients provided written informed consent. In this trial, patients who had completed Study-1 (Cohort 1, n = 17), or who had been receiving anti-VEGF injections for at least 3 months (Cohort 2, n = 14), were enrolled for 3 months and evaluated monthly. Patients received escalating doses of levodopa; 1 tablet QHS the first month, followed by 1 tablet TID the second month and then 2 tablets TID from the next month on (Supplementary Figure, B). The open-label extension (ClinicalTrials.gov identifier NCT03197493) continued to evaluate the tolerability and efficacy of the highest dose of levodopa (2 tablets TID). After baseline measurements were obtained (Supplementary Table 2), the patients were evaluated monthly and continued to be under the care of their referring retina specialist. After each follow-up visit, the retina specialist was notified of the patient’s BCVA and anatomic changes. The patient’s retina specialist determined whether the patient required anti-VEGF therapy. Although Study-2 is still ongoing, here we report the results of levodopa at the 1- and 6-month time points.
Study Drug: Carbidopa-Levodopa
Levodopa was given as a combination therapy with carbidopa, as is standard for treatment of movement disorders. Inclusion of carbidopa reduces the peripheral conversion of levodopa to dopamine, thus, nausea and other peripheral effects are decreased by approximately 80% and the bio-availability of levodopa in the central nervous system is increased.17,18 An Investigational New Drug Application 132078 was submitted for this indication.
Inclusion/Exclusion Criteria
To be included in the studies, patients had to be at least 50 years old and have a clinical diagnosis of AMD with choroidal neovascularization in one eye, among other criteria (for a complete list of eligibility criteria, see Supplementary Table 3).
Outcome Measures
The primary efficacy endpoint was the mean change in BCVA, which was evaluated by the same 2 investigators. Secondary endpoints were retinal fluid and central retinal thickness (CRT), evaluated by SD-OCT using a Spectralis OCT device (Heidelberg Engineering, Heidelberg, Germany). SD-OCT examinations were performed by the same 2 technicians and comprised a 25-line volume scan over the central 1-mm area. CRT was calculated automatically using the OCT software, and retinal fluid was determined by semi-automated macular segmentation of SD-OCT images at 240-μm intervals, with summation of the retinal area with visible fluid. In Cohort 2, an additional measure was the change in the frequency of anti-VEGF injections during 6 months of treatment, compared with the frequency of injections prior to study enrollment. Safety outcome measures included the incidence and severity of ocular and systemic adverse events.
Statistical Analysis
Sample size was based on practical recruitment considerations, not on a formal power calculation for this exploratory study. We verified the distribution of data prior to analyses with the Shapiro-Wilk test for normal distribution. Data meeting this criterion were compared with Student’s t test. In case of nonparametric testing, Wilcoxon matched-pairs signed rank tests were applied. All statistical analyses were 2-sided, and Holm-Bonferroni adjustments were included. One missing data point was imputed using the last-observation-carried-forward method. Analyses were performed using SPSS (version 25; SPSS Inc, Chicago, Ill) and GraphPad Prism (version 8.3.1; GraphPad Software; San Diego, Calif).
RESULTS
One-Month Evaluation of Outcome Measures in Cohort 1
The primary and secondary results at 1 month are summarized in Figure 1. Twelve patients did not require anti-VEGF intervention; in these patients, the mean improvement in visual acuity was evident 7 days after taking levodopa, with a BCVA increase of 2.9 letters (P = .03). At 4 weeks of taking levodopa, the mean BCVA increased by 5.0 letters (P = 0.03) (Figure 1A). At week 4, the measured mean CRT had a modest but significant decrease of 4.8 μm; P = .02 (Figure 1B). We observed a 29% mean decrease in retinal fluid at week 4; P = .01 (Figure 1C). Ten of the 12 patients in this group responded positively to treatment with a decrease in retinal fluid (Figure 1D). An example of a patient’s retinal fluid response to levodopa treatment through month 3, at the same macular segmentation line, is illustrated in Figure 2.
Figure 1.
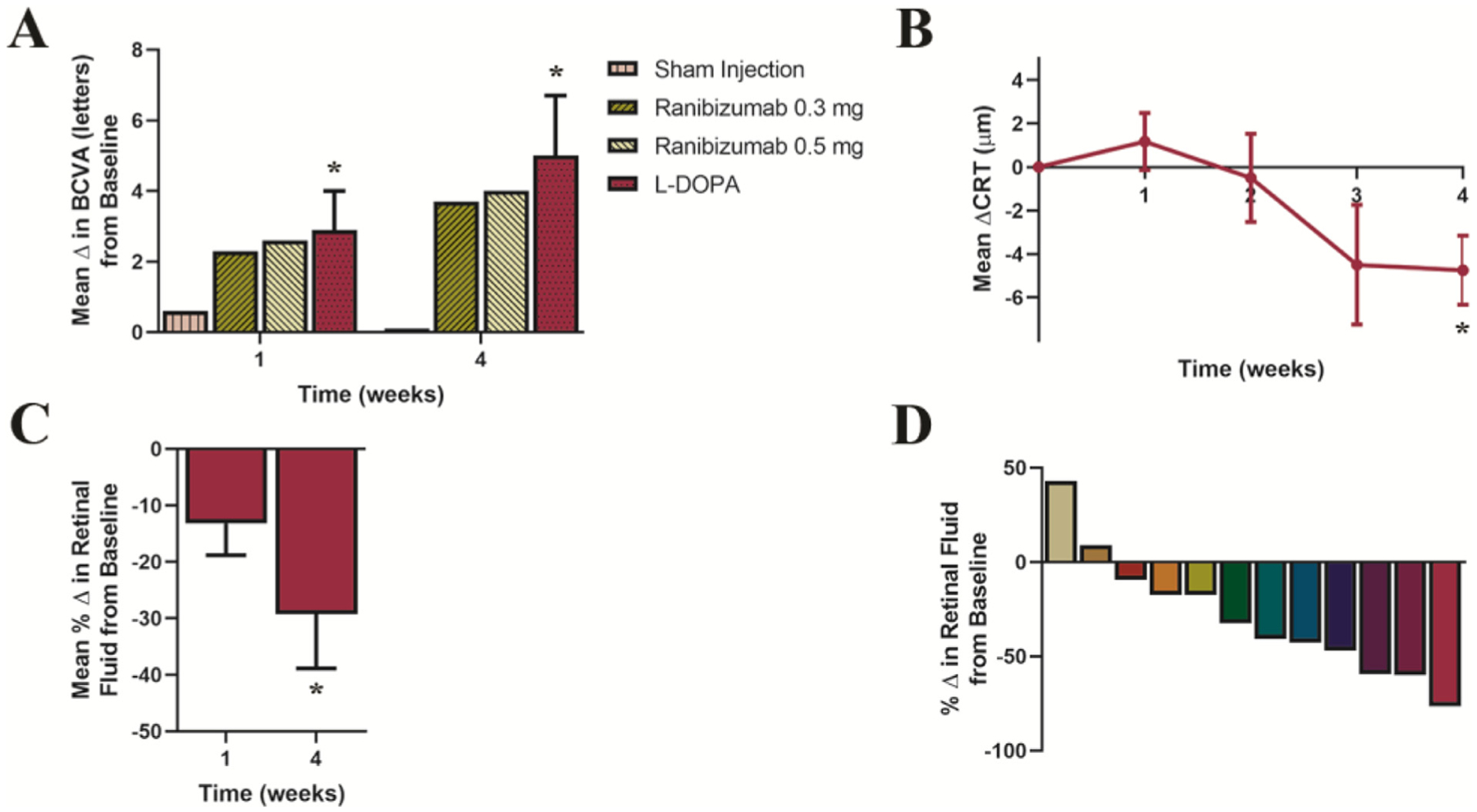
Changes from baseline in best-corrected visual acuity (BCVA), central retinal thickness (CRT), and retinal fluid in patients naïve to intravitreal anti-vascular endothelial growth factor therapy (Cohort 1). (A) Mean change from baseline in BCVA ± SE during a 1-month period of levodopa treatment and for comparison, the reported changes in visual acuity adapted from a multicenter, 24-month, sham-controlled study in patients receiving intravitreal injections of ranibizumab (0.3 mg or 0.5 mg).28 Starting from a baseline mean of 43.4 letters (20/40), at week 1, BCVA increased by 2.9 letters, P = .03; and at week 4, BCVA increased by 5.0 letters with levodopa treatment, P = .03. (B) Mean change from baseline in CRT ± SE over time, CRT decreased by 4.8 μm, P = .02. Wilcoxon-matched pairs signed rank analyses were used to assess changes in BCVA, and paired-sample t tests were used for changes in CRT. (C) Mean percentage change from baseline in retinal fluid ± SE at 1 and 4 weeks. Mean retinal fluid decreased 13% by week 1, P = .07 and by 29% at week 4 (P = .01; 95% CI, −50.3% to −8.2%). To evaluate percentage change in retinal fluid at weeks 1 and 4, from a theoretical mean of no observed change (retinal fluid = 0.0), one-sample t tests were used. (D) Percent change in retinal fluid at 4 weeks in individual patients. Statistical tests in this figure were 2-sided and adjusted for multiple comparisons with Holm-Bonferroni correction, n = 12.
Figure 2.
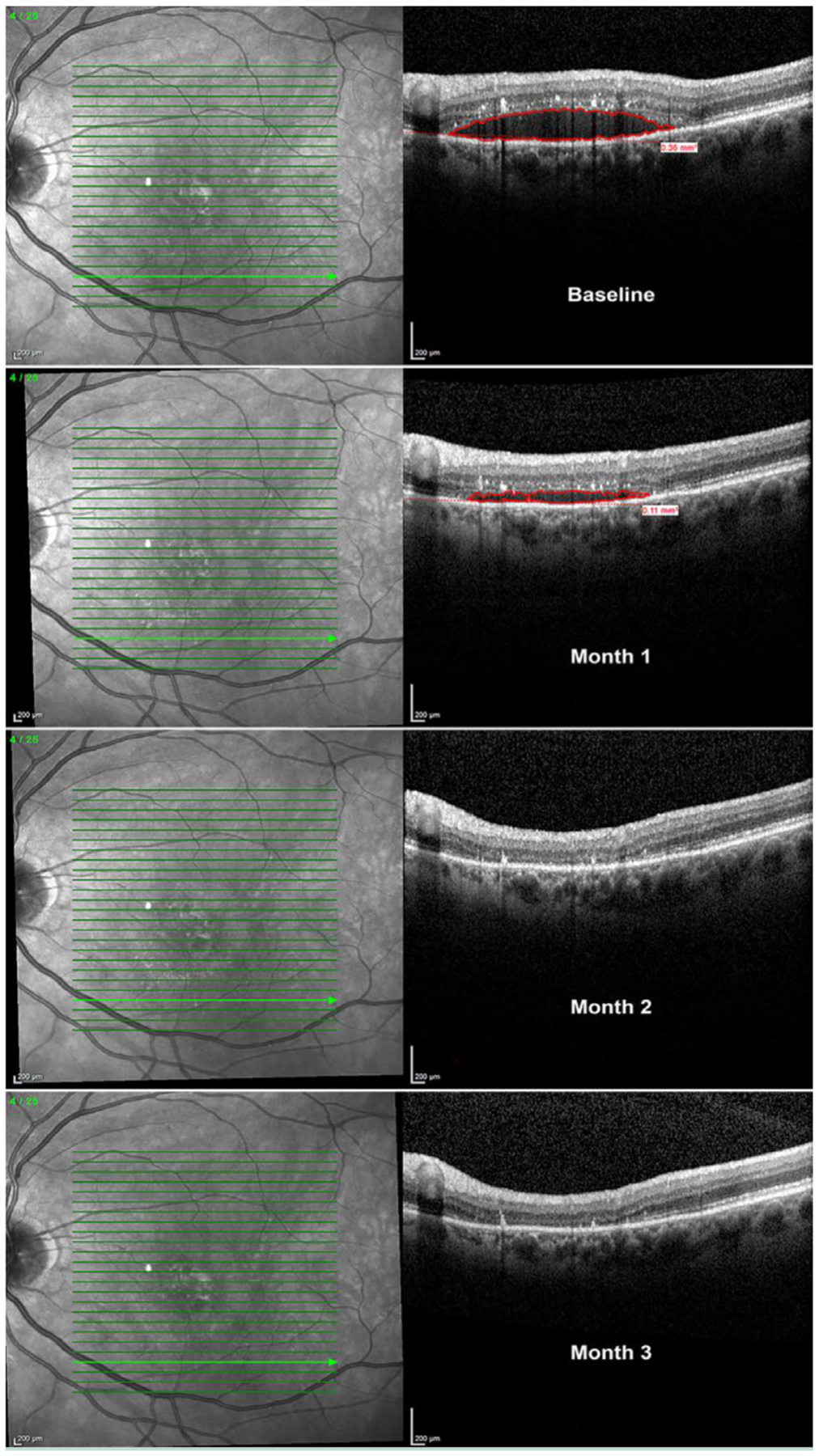
Spectral domain-optical coherence tomography images of the same macular segmentation line at baseline and monthly follow-up visits in a patient naı¨ve to intravitreal anti-vascular endothelial growth factor (VEGF) therapy. There was a 59% reduction in retinal fluid at 1 month; retinal fluid completely resolved at the same macular segmentation line at month 2, and fluid remained stable up to month 3 without anti-VEGF injections. Collectively, all macular segmentation lines revealed a 92% total retinal fluid decrease at 3 months.
Six-Month Evaluation of Outcome Measures in Cohort 1
Over 5 additional months, these patients continued with escalating doses of levodopa treatment. The observed mean BCVA at month 6 illustrated a continued improvement of 4.7 letters (P = .004). In this cohort, 2 patients gained 13 letters, and none lost more than 4 letters. The overall improvement in visual outcome was supported by SD-OCT measurements; mean CRT decreased by 51.4 μm (P = .01), and the mean percentage decrease in retinal fluid was 41% (P = .01). The results are summarized in Table 1. In this group, during the 6 months of levodopa treatment; 4 patients did not require any anti-VEGF injections and the mean anti-VEGF injection frequency was 0.38 injections/month.
Table 1.
Primary and Secondary Endpoints of Eyes with Neovascular Age-Related Macular Degeneration Treated with an Escalating Dose of Levodopa Through 6 Months
| Change from Baseline at Month-6 | Cohort 1 (n = 15) | Cohort 2 (n = 11) |
|---|---|---|
| Change in BCVA (letters) | ||
| Mean ± SE | 4.7 ± 1.4 | 4.8 ± 1.5 |
| 95% CI for mean | (1.8–7.7) | (1.4–8.2) |
| Median | 5.0 | 5.0 |
| P value | .004* | .02† |
| Change in CRT (μm) | ||
| Mean ± SE | −51.4 ± 19.2 | 4.0 ± 9.5 |
| 95% CI for mean | (−92.7 to −10.1) | (−17.1–25.1) |
| Median | −31.0 | −3.0 |
| P value | .01* | ns* |
| Change in retinal fluid (%) | ||
| Mean ± SE | −41 ± 13.4 | 3.7 ± 35.5 |
| 95% CI for mean | (−69.6 to −12) | (−75.3–82.7) |
| Median | −43.5 | −12.8 |
| P value | .01‡ | ns§ |
BCVA = best-corrected visual acuity, measured with the use of the Early Treatment Diabetic Retinopathy Study protocol at 7 m; CI = confidence interval; CRT = central retinal thickness; SE = standard error.
Wilcoxon matched-pairs signed rank analysis.
Paired-sample t test.
One-sample t test.
One-sample Wilcoxon signed rank test. Data meeting the criterion of a normal distribution were compared with parametric Student’s t tests. In case of non-parametric testing, Wilcoxon matched-pairs signed rank tests were applied.
Six-Month Evaluation of Outcome Measures in Cohort 2
The results of Cohort 2 at 6 months are summarized in Figure 3 and Table 1. Noteworthy outcomes include an improvement in visual acuity, with a continued increase in mean BCVA of 4.8 letters (P = .02) (Figure 3A). Nine of the 11 patients in this group gained letters, 3 gained 11 letters, and none lost more than 4 (Figure 3B). Retinal fluid and CRT remained stable; 9 of the 11 patients in Cohort 2 responded positively and were associated with a decrease in retinal fluid at 6 months. Figure 3C illustrates individual percentage change in retinal fluid. During the 6 months of levodopa treatment, 3 patients did not require any anti-VEGF injections, and the mean anti-VEGF injection frequency for the cohort was 0.41 injections/month (Figure 3D), a mean reduction of 52% compared with pre-study (P = .002).
Figure 3.
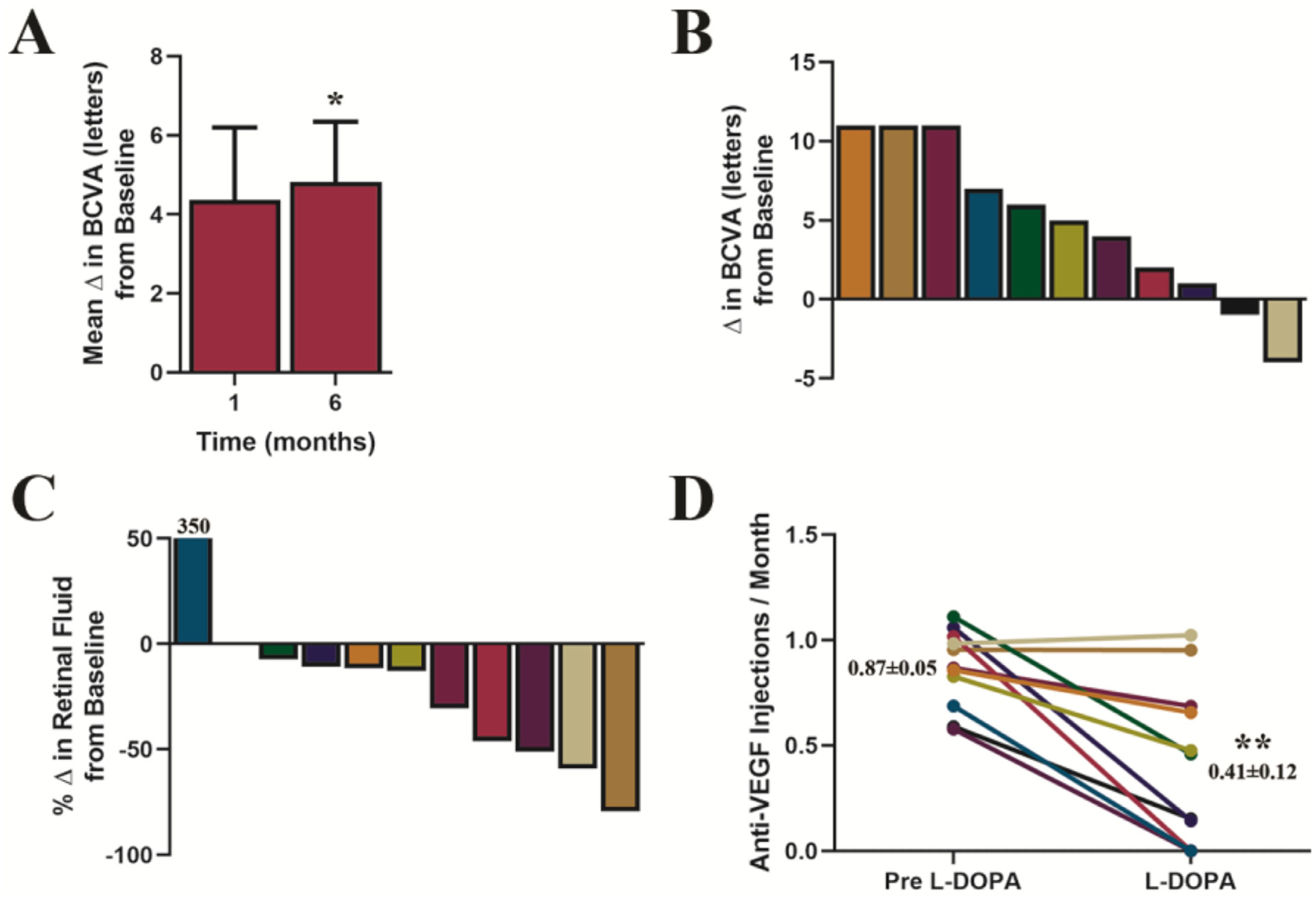
Changes in best-corrected visual acuity (BCVA) and reduction in anti-vascular endothelial growth factor injections during levodopa treatment in Cohort 2. (A) mean changes from baseline in BCVA ± SE in response to levodopa treatment. Starting from a baseline mean of 41 letters (20/40), BCVA increased by 4.4 letters at month 1, P = .06; and continued to improve through month 6, BCVA increased by 4.8 letters, P = .02; paired-sample t test. (B) Illustrates individual patient changes in BCVA at month 6, (C) shows individual patient percentage changes in retinal fluid at month 6, and (D) shows individual patient anti-VEGF injection rates prior to and during levodopa treatment; insets show mean injections per month ± SE. Overall, there was a 52% decrease in the rate of required intravitreal injections, compared with injection frequency prior to levodopa (P = .002; 95% CI, 16%−67%, paired-sample t test). Statistical tests in this figure were 2-sided and adjusted for multiple comparisons with Holm-Bonferroni correction, n = 11.
Ocular and Systemic Adverse Events
Both studies demonstrated an acceptable safety profile in 28 patients treated with levodopa for 6 months. One patient reported an ocular serious adverse event due to an intraocular lens displacement in the study eye (not study drug related) and 1 patient reported an ocular adverse event involving blurred vision. There were 3 serious adverse events that were not study drug related: total knee replacement, pelvic fracture, and hospitalization due to resection of recurrent ovarian cancer. A portion of the non-ocular systemic adverse events reported were generally consistent with those described during treatment with levodopa,18,19 and are summarized in Supplementary Table 4.
DISCUSSION
AMD is a leading cause of irreversible blindness. It affects individuals of all ethnicities; however, vision loss due to AMD is most common in whites and least common in patients of African descent.20,21 While nAMD occurs in only 10%−15% of AMD cases, it is responsible for most of the vision loss,7,22 and is a serious and expensive medical problem. Currently, the accepted treatment for those affected with nAMD involves frequent intravitreal anti-VEGF injections to block VEGF activity.6 Although effective, these injections are very expensive. According to the Centers for Medicare and Medicaid Services, the total cost of the medications in the United States (aflibercept, ranibizumab, and bevacizumab) in 2017 was $4.8 billion, and the total payments for the injection procedure were $3.9 billion, totaling $8.7 billion. Increased life expectancy will further escalate the prevalence of AMD; 8.7% of the global population has AMD, and the number of cases is projected to increase to approximately 288 million in 2040.23 This means the cost of anti-VEGF injection therapy will increase substantially.
The retinal pigment epithelium is the most likely initial tissue affected in AMD; it has a G protein-coupled receptor, GPR143, which is activated by levodopa.10 GPR143 is a component of the pigmentation pathway; the source of levodopa, such that the retinal pigment epithelium both synthesizes and responds to levodopa. The retinal pigment epithelium loses melanin as part of normal aging,24 while also exhibiting decreased secretion of the neurotrophic factor PEDF.25 Our past studies have shown that signaling through GPR143 simultaneously increases PEDF while decreasing VEGF secretion;10,12 both activities may benefit the aging retina. Further, Brilliant et al16 showed that patients with a history of levodopa for movement disorders had a lower risk of developing AMD, and if they did, the age of onset was significantly delayed. This led to the idea that GPR143 signaling can be pharmacologically targeted with levodopa to prevent or treat AMD.
In these proof-of-concept studies, we tested whether levodopa treatment improves visual acuity and the anatomical changes caused by nAMD. We also evaluated the safety and tolerability of escalating doses of levodopa and tested whether levodopa reduces or delays anti-VEGF therapy. Patients with nAMD require frequent evaluations; their vision can deteriorate quickly without anti-VEGF injections, and a delay in anti-VEGF treatment can affect long-term vision preservation.26 With this in mind, we expected that convincing evidence of any levodopa effect, positive or negative, assessed by both investigators and the patient’s referring retina specialist, would be observed quickly and with a limited number of patients, representing minimal risk. Also, because the effects of anti-VEGF injections on nAMD can be seen in days,27 we decided to measure endpoints (BCVA, CRT, and retinal fluid) that allow the identification of early signs of effectiveness of levodopa treatment.
Study-1 evaluated the immediate effects of levodopa in patients with newly diagnosed nAMD. We were able to establish a beneficial visual acuity effect; by 7 days of treatment, the mean BCVA increase was 2.9 letters. In a multicenter sham-controlled study (MARINA) in patients receiving injections of 0.3 or 0.5 mg of ranibizumab, the mean BCVA increases at 1 week were 2.3 and 2.6 letters, respectively, and the sham-injection group increased by 0.6 letters.28 At 4 weeks of levodopa treatment, the mean BCVA significantly increased by 5.0 letters (Figure 1A). In a multicenter randomized clinical trial (CATT) that evaluated the effects of ranibizumab or bevacizumab, at 4 weeks the mean BCVA increase was 3.6 letters and 4.3 letters, respectively.29 The observed early visual acuity improvements with levodopa are comparable with the early visual acuity improvements reported with ranibizumab and bevacizumab. Further, anatomical measures of nAMD pathology, such as increased CRT and retinal fluid, significantly improved after 1 month of levodopa treatment (Figures 1B and 1C). While the natural history of the disease includes fluctuations in retinal fluid, we would not expect the consistent reductions in patients naı¨ve to anti-VEGF injections that we observed, without our intervention (Figure 1D). This retinal fluid observation is consistent with the mechanism of action of levodopa, namely, decreased VEGF activity. Furthermore, the results of Cohort 1 at 6 months showed a significantly improved effect across all endpoints (Table 1). However, levodopa may be unlikely as a stand-alone therapy in patients with newly diagnosed nAMD, as 11 of the 15 patients still required anti-VEGF injections. However, levodopa did result in fewer required anti-VEGF injections (0.38 injections/month) to improve visual function and stabilize nAMD retinal changes, compared with a monthly injection regimen. Similarly, in Cohort 2, we observed a significant improvement in visual acuity with a mean increase of 4.8 letters at 6 months (Figure 3A). The observed effect in our study was comparable with the 5.6- and 6.5-letter gain at 6 months in patients treated monthly with 0.3 mg or 0.5 mg of ranibizumab, respectively.28 The improvement in our study is particularly impressive because the patients were already receiving anti-VEGF injections, which had almost certainly already improved visual acuity and anatomical features prior to enrollment (Table 1). The reported positive results, in conjunction with 3 of the 11 patients not requiring further anti-VEGF injections in 6 months, and a 52% mean decrease in necessary anti-VEGF injections, are particularly significant (Figure 3D).
The major limitations of our study include the small sample size and limited patient racial diversity. As previously mentioned, we designed these initial clinical trials as limited proof-of-concept studies. There are effective treatments for nAMD, and any trial withholding treatment carries risk and may raise ethical concerns. To reduce this, we did not include a placebo-control group, which limits interpretation. However, our results suggest that levodopa treatment had a beneficial effect on nAMD.
While levodopa has a well-established safety profile with known adverse events, these events can be more frequent in an older group of patients, such as those with nAMD.15,17 In this study, levodopa was well tolerated and accompanied by limited adverse events (Supplementary Table 4). Collectively, our observations indicate that this oral, systemic medication with a well-established safety history has the potential to stabilize and improve vision while reducing the pain, risks, and financial burden of frequent anti-VEGF injections. Using levodopa as an adjuvant therapy for nAMD could well alter the course of disease progression and save billions of dollars without sacrificing vision.
Supplementary Material
CLINICAL SIGNIFICANCE.
Levodopa treatment improved visual outcomes and stabilized neovascular age-related macular degeneration-related retinal changes.
In patients taking levodopa, anti-vascular endothelial growth factor (VEGF) injection frequency was reduced.
Oral levodopa was safe and well tolerated.
Levodopa may be effective as an adjuvant to anti-VEGF injection therapy.
ACKNOWLEDGMENT
The authors thank G. Smits for contributions to the statistical analysis.
Funding: Snyder Biomedical Corporation (RWS) financially supported the clinical trials. NEI RO1-EY026544 (BSM) supported other aspects of the study.
Footnotes
Conflict of Interest: The authors TCF, RWS, BSM, and CGJ certify that they have affiliation or involvement in an organization or entity with a financial interest in the subject matter or materials discussed in this manuscript. Snyder Biomedical Corporation has licensed patent (No. US9173862 B2, inventors: Brian S. McKay and John A. Martens) entitled “Methods and Compositions for Treating and Identifying Compounds to Treat Age-Related Macular Degeneration.” A patent (application no. 62/799,444) is pending, entitled “Compositions and Methods for Treating or Limiting Development of Age-Related Macular Degeneration,” filed January 31, 2020 (inventors: RWS and BSM). RWS is founder and president of Snyder Biomedical Corporation. RWS, TCF, and CGJ are shareholders in Snyder Biomedical Corporation. The authors BMB, CAC, and AGF certify that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2020.05.038.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82(11):844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358(24):2606–17. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Chou C-F, Klein BEK, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthamol 2011;129(1):75–80. [DOI] [PubMed] [Google Scholar]
- 4.Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The incidence and progression of age-related macular degeneration over 15 years: the Blue Mountains Eye Study. Ophthalmology 2015;122(12):2482–9. [DOI] [PubMed] [Google Scholar]
- 5.Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest 2014;124(4):1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015;29(6):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration-emerging pathogenetic and therapeutic concepts. Ann Med 2006;38(7):450–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein R, Klein BEK, Knudtson MD, et al. Subclinical atherosclerotic cardiovascular disease and early age-related macular degeneration in a multiracial cohort: The multiethnic study of atherosclerosis. Arch Ophthalmol 2007;125(4):534–43. [DOI] [PubMed] [Google Scholar]
- 9.Fisher DE, Klein BEK, Wong TY, et al. Incidence of age-related macular degeneration in a multi-ethnic United States population the multi-ethnic study of atherosclerosis. Ophthalmology 2016;123(6):1297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol 2008;6(9):e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Mello SAN, Finlay GJ, Baguley BC, Askarian-Amiri ME. Signaling pathways in melanogenesis. Int J Mol Sci 2016;17(7):1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falk T, Congrove NR, Zhang S, McCourt AD, Sherman SJ, McKay BS. PEDF and VEGF-A output from human retinal pigment epithelial cells grown on novel microcarriers. J Biomed Biotechnol 2012; 2012:278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehm BO, Lang G, Volpert O, et al. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia 2003;46(3):394–400. [DOI] [PubMed] [Google Scholar]
- 14.Bastide MF, Meissner WG, Picconi B, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 2015;132:96–168. [DOI] [PubMed] [Google Scholar]
- 15.Barbeau A L-dopa therapy in Parkinson’s disease: a critical review of nine years’ experience. Can Med Assoc J 1969;101(13):59–68. [PMC free article] [PubMed] [Google Scholar]
- 16.Brilliant MH, Vaziri K, Connor TB, et al. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Am J Med 2016;129(3):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunton L, Chabner B, Knollman B. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill Education; 2011. [12th ed]. [Google Scholar]
- 18.Gracies J-M, Olanow W, Davis KL, Charney D, Coyle JT, Nemeroff C. Current and experimental therapeutics of Parkinson’s disease. Neuropsychopharmacology: The Fifth Generation of Progress: An Official Publication of the American Collage of Neeuropsychopharmacology 2002:1795–816. [Google Scholar]
- 19.Sinemet. Drug Ther Bull 1974;12(21):83–4. [PubMed] [Google Scholar]
- 20.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48(3):257–93. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BEK, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006;113(3):373–80. [DOI] [PubMed] [Google Scholar]
- 22.Maguire MG. Comparing treatments for age-related macular degeneration: safety, effectiveness and cost. LDI Issue Brief 2012;17(8):1–4. [PubMed] [Google Scholar]
- 23.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health 2014;2(2):e106–16. [DOI] [PubMed] [Google Scholar]
- 24.Sarna T, Burke JM, Korytowski W, et al. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res 2003;76(1):89–98. [DOI] [PubMed] [Google Scholar]
- 25.Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci 1995;15(7 Pt 1):4992–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muether PS, Hermann MM, Koch K, Fauser S. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefes Arch Clin Exp Ophthalmol 2011;249(5):633–7. [DOI] [PubMed] [Google Scholar]
- 27.Fong AHC, Lai TYY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clin Interv Aging 2013;8:467–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355(14):1419–31. [DOI] [PubMed] [Google Scholar]
- 29.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364(20):1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


