Abstract
Respiratory rate (RR) predicts in-hospital and short-term mortality in patients with a variety of pathophysiological conditions, but its predictive value for long-term cardiovascular (CV) and all-cause mortality in the general population is unknown. Here, we investigated the relationship between mean nocturnal RR and mortality in community-dwelling older men and women.
We measured mean nocturnal RR during sleep from overnight polysomnography in 2686 men participating in the Osteoporotic Fractures in Men Study (MrOS) sleep study and 406 women participating in the Study of Osteoporotic Fractures (SOF) to investigate the relationship between mean nocturnal RR with long-term CV and all-cause mortality.
One hundred sixty-six men (6.1%) in the MrOS cohort (8.9±2.6 years follow-up) and 46 women (11.2%) in the SOF cohort (6.4±1.6 years follow-up) died from CV disease. All-cause mortality was 51.2% and 26.1% during 13.7±3.7 and 6.4±1.6 years follow-up in the MrOS Sleep and the SOF cohort, respectively. Multivariable Cox regression analysis adjusted for significant covariates demonstrated that RR dichotomized at 16 breaths per minute was independently associated with CV mortality (MrOS: HR=1.57 [1.14–2.15], p=0.005; SOF: HR=2.58 [1.41–4.76]), p=0.002) and all-cause mortality (MrOS: HR=1.18 [1.04 – 1.32], p=0.007; SOF: HR=1.50 [1.02 – 2.20], p=0.04).
In community-dwelling older men and women, polysomnography-derived mean nocturnal RR≥16 breaths per minute is an independent predictor of long-term CV and all-cause mortality. Whether nocturnal mean RR can be used as a risk marker warrants further prospective studies.
Keywords: respiratory rate, sleep, cardiovascular mortality
Introduction
Respiratory rate (RR) is one of the four vital signs along with heart rate, arterial blood pressure, and core temperature. In particular in older people [1], RR strongly predicts short-term in-hospital mortality [2, 3] in patients in the setting of pneumonia [4], trauma [5], stroke [6], cardiac arrest [7], and acute heart failure [8]. RR is typically measured by counting the number of breaths for one minute. In adults at rest, any RR between 12 and 20 breathes per minute (bpm) is considered normal; tachypnea is defined as RR greater than 20 bpm [9]. RR may increase with acute pathological conditions like fever, compensation for diabetic ketoacidosis or decompensated acute heart failure. Chronic pathophysiological conditions like decompensated kidney disease, heart failure or chronic obstructive pulmonary disease may also result in RR changes.
While elevated blood pressure [10] and increased heart rate [11] are well-recognized risk factors for long-term CV morbidity and mortality in the general population, the association between RR and long-term CV outcomes is unclear. Schmidt at al. reported an association between nocturnal RR and non-sudden cardiac death in survivors of myocardial infarction using Holter ECG [12] and showed the independent predictive value of daytime RR for long-term outcomes in patients with acute myocardial infarction [13].
Here, we determined the prognostic value of RR for long-term CV and all-cause mortality in two large independent cohorts of community-dwelling older women and men. Since cardiorespiratory variables such as RR vary broadly across the day, adapting to changes in metabolic demand, emotional valences and arousal [14], we measured RR during sleep, a state in which the body is in equilibrium, yielding highly standardized measurement conditions. We analyzed RR derived from overnight home-polysomnograms of 2686 older men participating in the Osteoporotic Fractures in Men Study (MrOS) sleep study and 406 older women participating in the Study of Osteoporotic Fractures (SOF). We hypothesized that nocturnal mean RR predicts CV and all-cause mortality independent from established risk factors.
Methods
Study populations
Osteoporotic Fractures in Men Study (MrOS) sleep
The Osteoporotic Fractures in Men Study (MrOS) was designed to describe the epidemiology of osteoporosis and fractures in older men, including the identification of risk factors for fracture and bone loss. Between March 2000 and April 2002, 5995 community-dwelling men aged 65 years and older were enrolled [15]. Participants were recruited from six U.S. centres. Subjects considered for enrolment in MrOS had to be able to walk without the assistance of another person and not have a bilateral hip replacement [16].
A total of 3135 men from the MrOS cohort were recruited for participation in the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) (ClinicalTrials.gov Identifier: NCT00070681). All men provided written informed consent, and the study was approved by the Institutional Review Board at each site. The men were screened for use of mechanical devices during sleep including pressure mask for sleep apnea, a mouthpiece for snoring or sleep apnea or oxygen therapy. In general, those who reported the nightly use of any of these devices were excluded from the MrOS sleep study. However, 17 men who reported the use of one of these devices but could forego use during the night of the sleep study were included. The 3135 men completed an exam conducted between December 2003 and March 2005 that included a clinic visit and overnight in-home polysomnography (PSG). Of these men, 2911 had technically adequate PSG.
Study of Osteoporotic Fractures
The Study of Osteoporotic Fractures (SOF) is a multisite, prospective, observational study of community-dwelling women aged 65 and older [17]. The 9,704 Caucasian participants constituting the original cohort were residents of four metropolitan areas (Baltimore, MD; Minneapolis, MN; Portland, OR; Pittsburgh/Monongahela Valley, PA). Initial enrollment took place between September 1986 and October 1988, and participants were reassessed at biannual follow-up visits. Six hundred sixty-two African-American women recruited between February 1997 and February 1998 were added to the study.
As part of visit 8, which occurred between January 2002 and February 2004, unattended overnight 12-channel in-home PSG was completed in a convenience subset of 461 women recruited from two of the four clinical centres (Minnesota and Pittsburgh) [18].
Follow-up
MrOS sleep participants were surveyed for potential incident CV or clinically-relevant arrhythmia events by postcard and/or phone contact every four months with > 99% response rate. Relevant medical records and supporting documentation from any potential incident clinical events were obtained by the clinical centre and forwarded to the coordinating centre for centralized adjudication by a board-certified cardiologist using a pre-specified protocol, as previously described [19]. For fatal events, the death certificate and hospital records from the time of death were collected. For fatal events that occurred when participants were not hospitalized, a proxy interview with next of kin and hospital records from the most recent hospitalization in the last 12 months were obtained. Only events confirmed by the adjudicator are included for analysis.
SOF deaths were centrally adjudicated using a State Registered Certificate of Death which was submitted to the Coordinating Center. The Principal Investigator at each of four clinical sites indicated the initial diagnosis for the cause of death. Final classification of cause-specific mortality was centrally adjudicated at the Coordinating Center by a trained physician adjudicator, using the International Classification of Diseases 9th Revision Clinical Modification (ICD-9-CM).
In-Home Overnight Polysomnography
Unattended polysomnography was performed over one night at the participant’s residence using the Compumedics (Abbotsford, Australia) Safiro sleep monitoring system for MrOS Sleep and the Compumedics Siesta system for SOF.
Before the participant’s bedtime, two trained staff members visited the participants to attach sensors and initiate data recording, including two central electroencephalograms, bilateral electrooculograms, bilateral chin electromyogram, a bipolar electrocardiogram, nasal-oral thermistor, nasal flow via pressure transducer and nasal cannula, abdominal and respiratory inductance plethysmography, finger pulse oximetry, bilateral leg movements by piezoelectric sensors, and body position.
Quantification of respiratory rate
Overnight PSG comprising raw data stored in the European Data Format (EDF) as well as scoring information stored in Compumedics output files in Extensible Markup Language (XML) were retrieved for further processing within MATLAB environment using the signal processing toolbox. Breathing effort during sleep extracted from the thoracic respiratory inductance plethysmography belt signal of the PSG data was used for RR analysis during sleep. During preprocessing, signal offsets were removed and a low-pass forward and reverse Butterworth filter (1 Hz) was applied. Expiratory and inspiratory onsets were determined from the respiratory signal by identifying the peaks and valleys using the first-order derivative. The inspiratory onset of artifact-free breaths was used to compute a breath-by-breath measure of the respiratory interval which were then averaged within each subject. The mean respiratory interval of each subject was used to compute RR in breaths per minute.
Other Measures
All participants completed a questionnaire at the time of the sleep visit, which included questions about medical history, specifically history of physician diagnosis of diabetes, hypertension and CV disease (coronary artery disease (CAD) myocardial infarction (MI), stroke, and heart failure (HF), and sleep-disordered breathing (SDB, quantified by the apnea-hypopnea index (AHI)). For MrOS participants, additional information was collected pertaining to chronic obstructive pulmonary disease (COPD), chronic kidney disease, and liver disease. Participants were also asked about their smoking status and alcohol use.
Statistical analysis
Values of RR were divided into quartiles for Kaplan-Meier curve survival analysis and log-rank testing. Anthropometric data, lifestyle metrics, and medical history were compared using dichotomized variables and student’s t-test and chi2 test, respectively. Cox proportional hazard models were constructed for continuous and dichotomized variables. Dichotomization was performed based on the highest quartile in the Kaplan Meier curve. Adjustments were performed for those variables in Table 1 that differed significantly between dichotomized groups in any of the comparisons. Correlations were assessed using Spearman’s rank coefficient.
Table 1:
Study cohort characteristics.
| MrOS | SOF | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | RR | RR <16 bpm |
RR ≥16 bpm |
p-value | RR | RR <16 bpm |
RR ≥16 bpm |
p-value |
| N=2686 | N=1959 | N=727 | N=406 | N=241 | N=165 | |||
| Anthropometric data | ||||||||
| Age at PSG [years] | 76.2±5.4 | 75.9±5.40 | 77.0±5.64 | <0.001 | 83.13±2.94 | 82.9±2.74 | 83.4±3.16 | 0.05 |
| Ethnicity/ race | ||||||||
| White | 2435 (90.7%) | 1773 (90.5%) | 662 (91.1%) | 0.51 | 241 (100%) | 165 (100%) | - | |
| African American | 93 (3.5%) | 64 (3.3%) | 29 (4.0%) | |||||
| Asian | 83 (3.1%) | 64 (3.3%) | 19 (2.6%) | |||||
| Other | 75 (2.8%) | 58 (3.0%) | 17 (2.3%) | |||||
| Body weight | ||||||||
| BMI [kg/m2] | 27.1±3.73 | 26.8±3.64 | 27.7±3.92 | 0.001 | 27.38±4.5 | 27.52±4.58 | 27.22±4.59 | 0.5 |
| Overweight | 1332 (49.6%) | 957 (48.9%) | 375 (51.7%) | 159 (39.8%) | 91 (38.6%) | 68 (41.5%) | 0.8 | |
| Obese | 533 (19.9%) | 363 (18.5%) | 170 (23.4%) | 110 (27.5%) | 66 (28.0%) | 44 (26.8%) | ||
| Systolic blood pressure | 126.9±16.1 | 126.3±15.9 | 128.6±16.7 | 0.001 | ||||
| AHI | 20.02±12.7 | 19.9±12.4 | 20.4±13.6 | 0.3 | 28.23±18.7 | 27.978.71±18.8 | 28.5±18.8 | 0.7 |
| Lifestyle | ||||||||
| Currently smoking | 53 (2.0%) | 32 (1.6%) | 21 (3.0%) | 0.015 | 5 (1.2%) | 3 (1.2%) | 2 (1.3%) | 1.0 |
| Smokers in the past | 1566 (58.3%) | 1124 (57.4%) | 442 (60.9%) | |||||
| Currently consuming alcohol | 1750 (65.5%) | 1319 (67.7%) | 431 (59.7%) | <0.001 | 147 (36.5%) | 90 (37.2%) | 57 (34.5%) | 0.6 |
| Medical history | ||||||||
| Diabetes | 353 (13.2%) | 237 (12.1%) | 116 (16.0%) | 0.008 | 51 (12.5%) | 28 (11.6%) | 23 (13.9%) | 0.5 |
| CAD/MI | 455 (17.0%) | 329 (16.8%) | 126 (17.4%) | 0.7 | 51 (12.7%) | 24 (10.0%) | 27 (16.4%) | 0.06 |
| CHF | 140 (5.2%) | 93 (4.7%) | 47 (6.5%) | 0.07 | 32 (7.9%) | 18 (7.5%) | 14 (8.5%) | 0.7 |
| Hypertension | 1322 (49.3%) | 949 (48.4%) | 373 (51.4%) | 0.17 | 239 (58.9%) | 148 (61.4%) | 91 (55.2%) | 0.2 |
| Stroke | 100 (3.7%) | 68 (3.5%) | 32 (4.4%) | 0.25 | 55 (13.5%) | 26 (10.8%) | 29 (17.6%) | 0.056 |
| Asthma | 211 (7.9%) | 141 (7.2%) | 70 (9.6%) | 0.04 | 49 (12.1%) | 29 (12.0%) | 20 (12.1%) | 1.0 |
| COPD | 137 (5.1%) | 83 (4.2%) | 54 (7.4%) | 0.001 | ||||
AHI – Apnea-Hypopnea index, CAD – coronary artery disease, MI – myocardial infarction, HF – heart failure, COPD – chronic obstructive pulmonary disease
Results
Participant characteristics
Flowcharts of participants included in the study are shown in Figure 1. Characteristics of the MrOS Sleep and SOF cohorts are summarized in Table 1.
Figure 1.
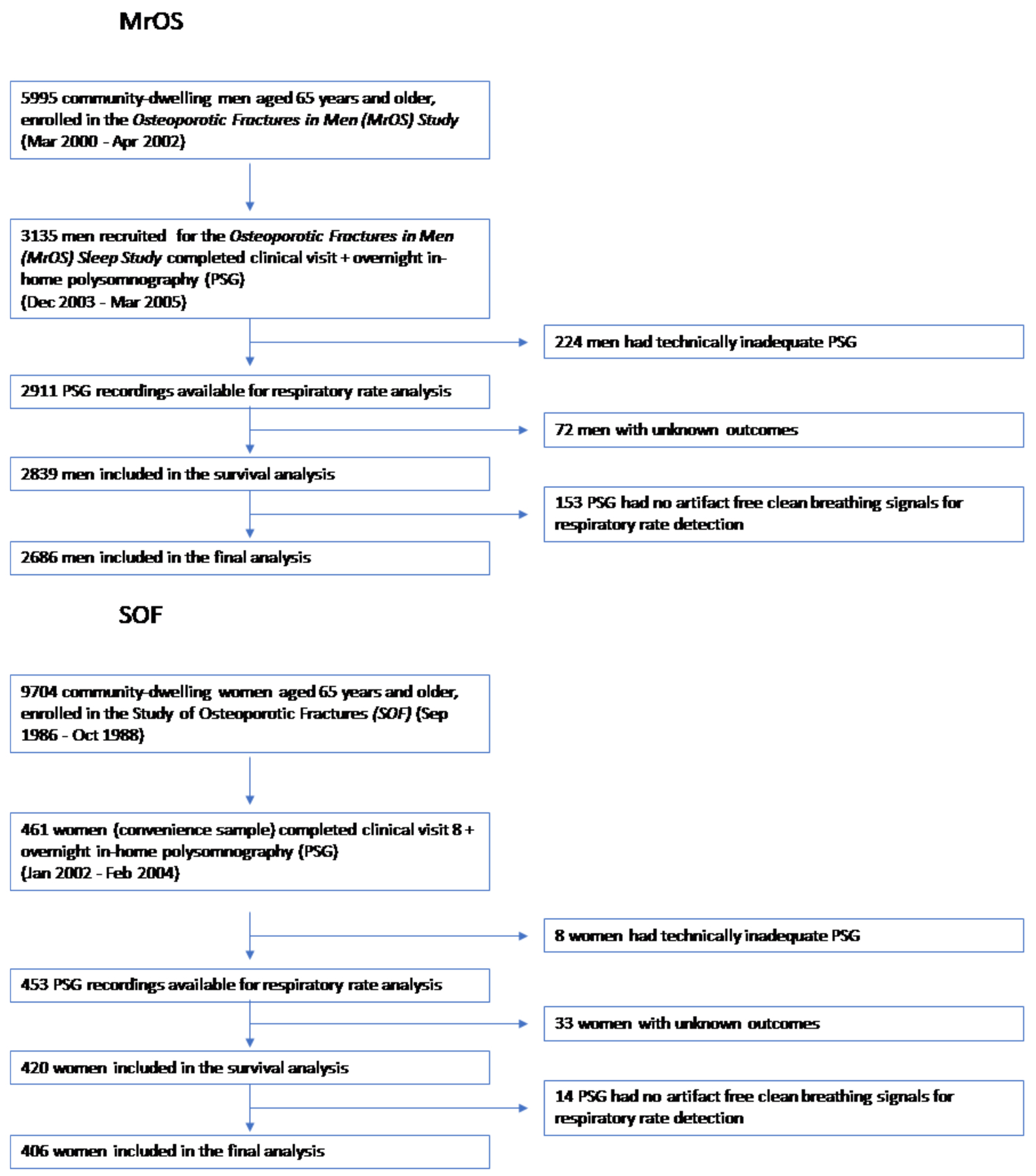
Flow charts of participants included in the analysis of respiratory rate for the MrOS cohort (top) and SOF (bottom).
At the baseline visit, participants in the MrOS cohort were predominately white, aged 76±2 years and their body mass index (BMI) was 27.1±3.7 kg/m2; 50% of men were overweight and 20% obese. Sixty-six per cent of men were consuming alcohol; 2% were current smokers. Nearly 50% of men reported a history of hypertension, 13.2% had diabetes, 17.0% had a history of coronary artery disease/myocardial infarction, and 5.2% had HF. Histories of asthma and COPD were reported by 7.8% and 5.3% of participants, respectively. Incidences of liver disease (2.2%), kidney disease (1.1%) and stroke (3.7%) were low. The average AHI obtained from overnight polysomnography was 20.0±12.7.
The SOF cohort comprised only white women, was older at baseline (81±3 years), but the BMI was comparable (27.4±4.5 kg/m2). Forty per cent of women were overweight and 27% were obese. All the women were white. Similar to the male cohort, the percentage is current smokers was low (1.2%), but the portion of women who consume alcohol was notably lower (37%). Frequencies of diabetes (13%), CAD/MI (13%), HF (7.8%) and asthma (12%) were largely comparable, while the incidence of hypertension (59%) and stroke (14%) was ten per cent higher than in the male cohort. Histories of kidney disease, liver disease, COPD and thyroid function were not reported in SOF.
Mean nocturnal RR in the MrOS and SOF cohorts were 14.8 ±1.8 bpm and 15.5±1.7 bpm, respectively.
Cardiovascular and all-cause mortality
In the MrOS cohort, outcome data on CV mortality were available for 2686 participants. During the follow-up period of 8.9±2.6 years on average, 166 men (6.1%) died from CV disease. Among those were 94 confirmed coronary heart disease deaths, 31 cerebrovascular deaths, 21 heart failure deaths, 4 peripheral vascular disease deaths and 4 arrhythmic deaths. The follow-up for all-cause mortality continued beyond the detailed tracking of CV events. During a period of 13.7±3.7 years on average, 1312 out of 2560 men (51.2%) died from any cause.
In the SOF cohort, outcome data on CV mortality were available for 406 participants. During the 6.4±1.6 years of follow-up, 46 women (11.2%) died from CV disease as defined by the ICD9 code. Of those, 17 deaths were attributed to ischemic heart disease, 18 cases to sudden death post coronary heart disease, 15 deaths to stroke, and 29 deaths to atherosclerosis. 106 women (26.1%) died from any cause.
Univariate survival analysis
The Kaplan-Meier curves of nocturnal RR quartiles of both cohorts (Figures 2) illustrate significantly elevated CV mortality in participants whose RR was in the highest quartile (MrOS: 15.9–18.6 bpm; SOF: 16.6–18.6 bpm) versus those in the lower three quartiles, in whom mortality was largely comparable (10-year mortality in MrOS 8.2% vs. 5.3%, p=0.023; 8-year mortality in SOF: 9.1% vs. 17.6%, p=0.031).
Figure 2.

Mortality analysis of nocturnal respiratory rate. Kaplan-Meier curves for quartiles of respiratory rate in the MrOS sleep cohort (A) and the SOF cohort (B) and log-rank test results. Panels C (MrOS cohort) and D (SOF cohort) show mortality for respiratory rates dichotomized at 16 breaths per minute.
Considering the increased CV mortality of participants in the highest RR quartile, we dichotomized both cohorts into subgroups of participants with RR <16 vs. ≥16 bpm (Table 1) and explored the association between RR and participant characteristics in the MrOS and SOF cohorts. MrOS participants with high RR were marginally, yet significantly older, while the age difference between RR-dichotomized subgroups was not significant in the SOF cohort. Likewise, men with high RR had marginally but significantly higher BMI, whereas no significant BMI difference between RR subgroups was seen between women. MrOS participants with high RR were more likely to smoke and less likely to consume alcohol, but this effect was not observed in women. Systolic Blood pressure, diabetes, asthma and COPD (not reported in SOF) were positively associated with RR in men, while hypertension was weakly negatively associated with RR in women. There was no significant association with the prevalence of respiratory events counted by AHI or cardiac disease and high RR in either men or women.
Kaplan Maier curves of all-cause mortality show a significantly higher mortality for men as well as women with ≥16 bpm (see online supplement).
Cox-proportional hazard analysis
Unadjusted Cox-proportional hazard analysis shows significant associations with nocturnal RR (as a continuous variable) and CV mortality (Table 2). Nocturnal RR≥16 bpm represented a hazard ratio (HR) of 1.91 (p<0.001) in the MrOS cohort and an HR of 2.64 (p=0.002) in the SOF cohort.
Table 2:
Association of respiratory rate with cardiovascular and all-cause mortality by Cox regression.
| cardiovascular mortality | all-cause mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable Analysis* | Univariate analysis | Multivariable Analysis* | |||||
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| MrOS sleep | ||||||||
| Respiratory Rate; bpm | 1.13 [1.03 – 1.23] | 0.007 | 1.07 [0.98 – 1.17] | 0.14 | 1.10 [1.06 – 1.14] | <0.001 | 1.04 [1.01 – 1.08] | 0.005 |
| Respiratory Rate (SD) | 1.24 [1.06 – 1.45] | 0.007 | 1.13 [0.96 – 1.32] | 0.14 | 1.19 [1.13 – 1.26] | <0.001 | 1.08 [1.02 – 1.14] | 0.005 |
| Respiratory Rate ≥16 bpm | 1.91 [1.40 – 2.61] | <0.001 | 1.57 [1.14 – 2.15] | 0.005 | 1.44 [1.28 – 1.62] | <0.001 | 1.18 [1.04 – 1.32] | 0.007 |
| SOF | ||||||||
| Respiratory Rate; bpm | 1.36 [1.11 – 1.66] | 0.002 | 1.34 [1.10 – 1.65] | 0.005 | 1.20 [1.06 – 1.37] | 0.004 | 1.20 [1.06 – 1.37] | 0.005 |
| Respiratory Rate (SD) | 1.66 [1.19 – 2.31] | 0.002 | 1.62 [1.16 – 2.27] | 0.005 | 1.35 [1.10 – 1.66] | 0.004 | 1.35 [1.10 – 1.67] | 0.005 |
| Respiratory Rate ≥16 bpm | 2.64 [1.44 – 4.83] | 0.002 | 2.58 [1.41 – 4.76] | 0.002 | 1.50 [1.02 – 2.19] | 0.03 | 1.50 [1.02 – 2.20] | 0.04 |
adjusted for age, BMI category, smoking, alcohol consumption, diabetes, asthma, systolic blood pressure, apnea-hypopnea index and COPD (MrOS), and hypertension, apnea-hypopnea index and stroke, respectively (SOF).
After adjusting the Cox regression model of nocturnal RR in MrOS for age, BMI category, smoking, alcohol consumption, diabetes, asthma, systolic blood pressure, apnea-hypopnea index and COPD, RR≥16 bpm remained a significant predictor of CV mortality (HR=1.57; p=0.005). In the SOF cohort, nocturnal RR remained a significant predictor of CV mortality after adjusting for hypertension, apnea-hypopnea index and stroke respectively (continuous RR: HR=1.34, p=0.005; dichotomized RR: HR=2.58, p=0.002).
When considering all-cause mortality as outcome, univariate as well as adjusted Cox regression models show significant predictive value of RR when included in the model as continuous or dichotomized variable (Table 2).
Discussion
This study is the first to demonstrate the prognostic value of nocturnal RR for long-term CV and all-cause mortality in older community-dwelling individuals. In two large, independent cohorts of older men and women, nocturnal RR ≥16 bpm was significantly and independently associated with 9-year (MrOS) and 6-year (SOF) CV mortality as well as 14-year (MrOS) and 6-year (SOF) all-cause mortality.
The prognostic value of nocturnal mean RR for long-term mortality may derive from its ability to capture a composite of different factors, including dysfunctional central respiratory control associated with neural disease, and/ or compensatory mechanisms for respiratory or metabolic impairments due to diabetes or chronic renal, pulmonary or cardiac failure. In our data, nocturnal mean RR≥16 bpm was associated with diabetes, asthma, COPD, but importantly, demonstrated predictive value beyond these factors. Correlation analysis of nocturnal mean RR and spirometry, both of which were conducted as part of a MrOS follow-up sleep visit, showed a weak inverse relation between nocturnal mean RR and forced expiratory volume in one second and forced vital capacity, but not with the ratio of the latter (data not shown). This suggests that nocturnal mean RR is in part reflective of daytime respiratory function.
A history of cardiac disease and the severity of concomitant SDB determined by the AHI were not significantly associated with RR ≥16 bpm, suggesting that our findings are not solely explained by preexisting heart failure or SDB as defined by the AHI. While pathophysiological insights cannot be gained and causal relationships cannot be established from cohort studies, elevated RR is likely to reflect an attempt to restore homeostasis/ eucapnia in the presence of impaired lung or airway mechanics [20]. In extreme cases, elevated RR may cause hypocapnia that can lead to electrolyte abnormalities such as hypokalemia, cardiac arrhythmias and conduction abnormalities [21], thereby providing a mechanism by which RR captures CV mortality risk. Various RR-related and -unrelated organ-specific or more systemic compensatory mechanism may contribute to increased all-cause mortality in patients with nocturnal RR ≥16 bpm. Additionally, RR during night may also represent a risk marker in a patient group with increased all-cause mortality.
Comparing the MrOS and SOF cohorts, the associations between participant characteristics and nocturnal mean RR were stronger in the MrOS cohort than in the SOF cohort, but the effect size was generally small. Sex-specific differences in ventilatory control may account for the more pronounced findings in men versus women. For example, hypoxic ventilatory responses are more robust in men than in women [22], which may, therefore, make them more prone to have an elevated RR. The discrepancy in associations between men and women which might also be partly attributed to the higher statistical power of the MrOS cohort, where the sample size was more than six times bigger. The higher age in SOF may also have had an impact and possibly also contributed to the higher HR observed in women compared to men. Considering the predictive value of RR for CV versus all-cause mortality in both cohorts, HR were consistently numerically higher for the former.
In the clinical setting, assessment of cardiorespiratory vital parameters, including RR, forms an important part of a standard physiological examination. Though often omitted from anamnesis, our findings make a case for reconsideration of RR. While simple, visual inspection of chest movements yields reliable RR measurement with the high inter-observer agreement [23], alternatively, breathing-related chest movements can be readily automatically obtained from impedance changes derived from ECG Holter recordings [24], or respiratory inductance plethysmography during overnight sleep studies; contact-free motion measurement is also available via radiofrequency electronics [25, 26]. Wearable health gadgets and smart watches [27], also allow for simple automated measurement of RR in large parts of the population.
From a measurement point of view, assessing RR during sleep, as performed here, appears advantageous, because the body is in the supine position at rest in a state of equilibrium, which represents a standardized condition, reducing disturbing factors and noise levels. Future studies need to establish the relationship between daytime RR and nocturnal RR. During sleep, tidal volume is reduced, the breathing pattern is shallower, but RR is slightly increased [28]. Although nocturnal RR varies between rapid-eye-movement (REM) and non-REM sleep, our analysis yielded similar results (data not shown).
Limitations
Our observations are based on predominantly white old men and women and cannot be extrapolated to other populations. The age at baseline for men was quite high (76±6 years) and even more so for women (81±3 years), which may have introduced a ‘survivor’ bias. Baseline exposure to various conditions was self-reported rather than systematically ascertained through medical records or direct measurement. We did not take into consideration possible confounding effects of medications.
Since nocturnal RR may be affected by SDB, we excluded episodes of apnea and hypopnea from our initial RR measurement, but the main findings remained unaffected when the entire dataset was reanalysed without keeping out various scored (respiratory) events. Our findings are based on single-night overnight polysomnography; further studies must address intra-individual variability. Additionally, the most effective recording technique (e.g. full PSG or simple ECG) needs to be determined. Prospective studies are warranted to confirm the prognostic value of nocturnal mean RR as a risk marker in the general population; mechanistic and outcome studies should test, whether interventions lowering RR during the night could reduce CV mortality and whether changes in RR can help to guide treatment of the underlying disease.
Conclusions
In community-dwelling older men and women, nocturnal RR is an independent predictor of long-term CV and all-cause mortality. Whether RR could be used as a risk marker for mortality in the clinical setting warrants further prospective studies.
Supplementary Material
Acknowledgements
This study was supported through a grant from the Australian Research Council (DP0663345). The MrOS Study and the SOF Study are supported by National Institutes of Health funding. The following institutes provided support: The National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research (U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128). The National Heart, Lung, and Blood Institute (NHLBI) provided funding for the MrOS Sleep ancillary study (R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, R01 HL070839) and the National Sleep Research Resource (R24-HL-114473). The SOF sleep study was supported by grant AG021918, AG026720, AG05394, AG05407, AG08415, AR35582, AR35583, AR35584, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, 2 R01 AG027574-22A1, HL40489, and T32 AG000212-14. Dr. Redline was supported in part by NIH R35HL135818.
Role of funding sources
The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Declaration of interests
Dr Linz reports having served on the advisory board of LivaNova. Dr Linz reports having received lecture and/or consulting fees from LivaNova, and ResMed.
Data sharing
All of the individual participant data generated during this study will be made available at the MrOS Online (https://mrosdata.sfcc-cpmc.net/) and SOF Online (https://sofonline.epi-ucsf.org/interface/) websites.
References
- 1.Wilson AH, Kidd AC, Skinner J, Musonda P, Pai Y, Lunt CJ, Butchart C, Soiza RL, Potter JF, Myint PK. A simple 5-point scoring system, NaURSE (Na+, urea, respiratory rate and shock index in the elderly), predicts in-hospital mortality in oldest old. Age and ageing 2014: 43(3): 352–357. [DOI] [PubMed] [Google Scholar]
- 2.Shappell C, Snyder A, Edelson DP, Churpek MM, American Heart Association’s Get With The Guidelines-Resuscitation I. Predictors of In-Hospital Mortality After Rapid Response Team Calls in a 274 Hospital Nationwide Sample. Critical care medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkes R. Rate of respiration: the forgotten vital sign. Emergency nurse : the journal of the RCN Accident and Emergency Nursing Association 2011: 19(2): 12–17; quiz 18. [DOI] [PubMed] [Google Scholar]
- 4.Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax 2000: 55(3): 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata I, Abe T, Uchida M, Saitoh D, Tamiya N. Ten-year inhospital mortality trends for patients with trauma in Japan: a multicentre observational study. BMJ open 2018: 8(2): e018635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah B, Bartaula B, Adhikari J, Neupane HS, Shah BP, Poudel G. Predictors of In-hospital Mortality of Acute Ischemic Stroke in Adult Population. Journal of neurosciences in rural practice 2017: 8(4): 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen LW, Kim WY, Chase M, Berg KM, Mortensen SJ, Moskowitz A, Novack V, Cocchi MN, Donnino MW, American Heart Association’s Get With the Guidelines - Resuscitation I. The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation 2016: 98: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metra M, Cotter G, El-Khorazaty J, Davison BA, Milo O, Carubelli V, Bourge RC, Cleland JG, Jondeau G, Krum H, O’Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Rainisio M, Kobrin I, McMurray JJ, Teerlink JR. Acute heart failure in the elderly: differences in clinical characteristics, outcomes, and prognostic factors in the VERITAS Study. Journal of cardiac failure 2015: 21(3): 179–188. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Molinero A, Narvaiza L, Ruiz J, Galvez-Barron C. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. Journal of the American Geriatrics Society 2013: 61(12): 2238–2240. [DOI] [PubMed] [Google Scholar]
- 10.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008: 51(1): 55–61. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. American heart journal 1987: 113(6): 1489–1494. [DOI] [PubMed] [Google Scholar]
- 12.Dommasch M, Sinnecker D, Barthel P, Muller A, Dirschinger RJ, Hapfelmeier A, Huster KM, Laugwitz KL, Malik M, Schmidt G. Nocturnal respiratory rate predicts non-sudden cardiac death in survivors of acute myocardial infarction. J Am Coll Cardiol 2014: 63(22): 2432–2433. [DOI] [PubMed] [Google Scholar]
- 13.Barthel P, Wensel R, Bauer A, Muller A, Wolf P, Ulm K, Huster KM, Francis DP, Malik M, Schmidt G. Respiratory rate predicts outcome after acute myocardial infarction: a prospective cohort study. Eur Heart J 2013: 34(22): 1644–1650. [DOI] [PubMed] [Google Scholar]
- 14.Heckmann C, van Leeuwen P, Engelke P, Kesting G, Dittrich F, Kümmell H. Circadian variations of heart rate, respiratory rate and pulse respiration ratio in routinely examined hospital patients. 1990.
- 15.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials 2005: 26(5): 569–585. [DOI] [PubMed] [Google Scholar]
- 16.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemporary clinical trials 2005: 26(5): 557–568. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. Jama 1990: 263(5): 665–668. [PubMed] [Google Scholar]
- 18.Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, Yaffe K. Sleep-disordered breathing and cognition in older women. Journal of the American Geriatrics Society 2008: 56(1): 45–50. [DOI] [PubMed] [Google Scholar]
- 19.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S, Osteoporotic Fractures in Men Study G. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation 2011: 124(11): 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henke KG, Dempsey JA, Kowitz JM, Skatrud JB. Effects of sleep-induced increases in upper airway resistance on ventilation. J Appl Physiol (1985) 1990: 69(2): 617–624. [DOI] [PubMed] [Google Scholar]
- 21.Javaheri S, Corbett W. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med 1998: 128(3): 204–207. [DOI] [PubMed] [Google Scholar]
- 22.Douglas NJ. Control of ventilation during sleep. Clinics in chest medicine 1985: 6(4): 563–575. [PubMed] [Google Scholar]
- 23.Nielsen LG, Folkestad L, Brodersen JB, Brabrand M. Inter-Observer Agreement in Measuring Respiratory Rate. PLoS One 2015: 10(6): e0129493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinnecker D, Dommasch M, Barthel P, Muller A, Dirschinger RJ, Hapfelmeier A, Huster KM, Laugwitz KL, Malik M, Schmidt G. Assessment of mean respiratory rate from ECG recordings for risk stratification after myocardial infarction. J Electrocardiol 2014: 47(5): 700–704. [DOI] [PubMed] [Google Scholar]
- 25.Droitcour A, Lubecke V, Lin J, Boric-Lubecke O. A microwave radio for Doppler radar sensing of vital signs. In: Microwave Symposium Digest, 2001 IEEE MTT-S International; 2001: IEEE; 2001. p. 175–178. [Google Scholar]
- 26.Ballal T, Heneghan C, Zaffaroni A, Boyle P, De Chazal P, Shouldice R, McNicholas WT, Donnelly SC. A pilot study of the nocturnal respiration rates in COPD patients in the home environment using a non-contact biomotion sensor. Physiological measurement 2014: 35(12): 2513. [DOI] [PubMed] [Google Scholar]
- 27.Appelboom G, Camacho E, Abraham ME, Bruce SS, Dumont EL, Zacharia BE, D’Amico R, Slomian J, Reginster JY, Bruyère O. Smart wearable body sensors for patient self-assessment and monitoring. Archives of Public Health 2014: 72(1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax 1982: 37(11): 840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


