Abstract
Gulf War Illness (GWI) is a chronic disorder affecting approximately 30% of the veterans who served in the 1991 Gulf War. It is characterised by a constellation of symptoms including musculoskeletal pain, cognitive problems and fatigue. The cause of GWI is not definitively known but exposure to neurotoxicants, the prophylactic use of pyridostigmine bromide (PB) pills, and/or stressors during deployment have all been suspected to play some pathogenic role. Recent animal models of GWI have suggested neuroinflammatory mechanisms may be implicated, including a dysregulated activation of microglia and astrocytes. However, neuroinflammation has not previously been directly observed in veterans with GWI. To measure GWI-related neuroinflammation in GW veterans, we conducted a Positron Emission Tomography (PET) study using [11C]PBR28, which binds to the 18 kDa translocator protein (TSPO), a protein upregulated in activated microglia/macrophages and astrocytes.
GWI (n=15) and healthy controls (HC, n=33, including a subgroup of healthy Gulf War veterans, HCVET, n=8), were examined using integrated [11C]PBR28 PET/MRI. Standardized uptake values normalized by occipital cortex signal (SUVR) were compared across groups and against clinical variables and circulating inflammatory cytokines (TNF-α, IL-6 and IL-1β). SUVR were validated against volume of distribution ratio (n=13).
Whether compared to the whole HC group, or only the HCVET subgroup, veterans with GWI demonstrated widespread cortical elevations in [11C]PBR28 PET signal, in areas including precuneus, prefrontal, primary motor and somatosensory cortices. There were no significant group differences in the plasma levels of the inflammatory cytokines evaluated. There were also no significant correlations between [11C]PBR28 PET signal and clinical variables or circulating inflammatory cytokines.
Our study provides the first direct evidence of brain upregulation of the neuroinflammatory marker TSPO in veterans with GWI and supports the exploration of neuroinflammation as a therapeutic target for this disorder.
1. Introduction
Gulf War Illness (GWI) is a chronic disorder affecting approximately 30% of the nearly 700,000 veterans who served in the 1991 Gulf War (Binns et al., 2008). It is characterised by a constellation of symptoms including musculoskeletal pain, fatigue, and cognitive/affective decrements. GWI has been suspected to be caused by exposure to neurotoxicants (including the nerve gas sarin and/or pesticides used to prevent insect-borne diseases), the prophylactic use of pyridostigmine bromide (PB) pills (an acetylcholinesterase inhibitor commonly used to protect troops from the harmful effects of nerve agents), and/or the experience of physical stressors (e.g., extreme temperature changes, sleep deprivation, physical exertion) during deployment (Binns et al., 2008; Fukuda et al., 1998; Janulewicz et al., 2018; Maule et al., 2018; Steele et al., 2012; Sullivan et al., 2018).
The wide range of symptoms of GWI is indicative of a complex underlying pathophysiology, for which the etiology has remained largely undetermined. Many of the symptoms reported by veterans with GWI are indicative of central nervous system (CNS) dysfunction, and indeed this has been corroborated by structural and functional neuroimaging and biomarker studies (Abou-Donia et al., 2017; White et al., 2016). Dysfunction of the CNS includes alterations in brain white matter [e.g., reduced volume and increased mean diffusivity; (Heaton et al., 2007; Rayhan et al., 2013b)], decreases in metabolite levels [e.g., lower NAA/creatine ratio; (Menon et al., 2004)], decreases in cerebral blood flow (Haley et al., 2009), reduced gray matter volume (Chao et al., 2010; Rayhan et al., 2013a) and altered gray matter activity in response to behavioral, sensory and chemical stimuli (Calley et al., 2010; Gopinath et al., 2012; Haley et al., 2009). There are also reports and reviews documenting neurobehavioral dysfunction such as slower motor function, poorer visual and verbal memory and worse attention in GWI, and studies have shown that these symptoms are associated with inflammatory cytokines and reduced hippocampal volume (Janulewicz et al., 2018; Jeffrey et al., 2019; O’Donovan et al., 2015; Sullivan et al., 2018).
While the exact pathophysiology of GWI remains unknown, recent studies suggest a possible role for neuroinflammation, and dysregulated activation of microglia and astrocytes (Madhu et al., 2019b; Parihar et al., 2013). Microglia are the resident macrophages of the CNS and rapidly activate in response to pathological danger signals (Kreutzberg, 1996). Acutely, this response is essential for survival, as it allows for the identification of a potentially harmful event, limiting its impact and favoring its resolution. However, overactivation of microglia can lead to production of excessive pro-inflammatory cytokines and excitotoxins, which can be deleterious (Mika, 2008). Similarly, astrocytes can enact responses to pathological events that are adaptive in the acute phase, but can sometimes become dysregulated and pathogenic (Pekny and Pekna, 2014; Pekny et al., 2014). It has been proposed that such aberrant activation can be the result of glial cells being ‘primed’ by prior pathological events including systemic infections, toxic environmental exposures, and trauma, making them more vulnerable to subsequent stressors, in a ‘two-hit’ model (Blaylock and Maroon, 2011; Perry et al., 1985; Watkins et al., 2007a). In GWI, multiple neurotoxicant chemical exposures (pesticides, PB, sarin), compounded with the experience of mental or physical stressors, has been suggested to be among potential triggering mechanisms for the chronic symptoms and neuroinflammation in GWI (Binns et al., 2008). For instance, in a recent mouse model investigation, the exposure to a sarin surrogate (DFP) induced widespread neuroinflammation in multiple brain areas such as the frontal cortex, hippocampus, cerebellum and the hypothalamus, and these effects were exacerbated by pre-exposure to corticosterone, the endogenous glucocorticoid typically released in conditions of high physiological stress (O’Callaghan et al., 2015). Despite these animal observations, to our knowledge, GWI-related neuroinflammation has never been demonstrated in humans.
Here we hypothesized that veterans with GWI would demonstrate neuroinflammation in the CNS compared to veterans without GWI and healthy civilians. More specifically, we hypothesized that the pattern of neuroinflammation would be similar to that observed in participants with fibromyalgia (Albrecht et al., 2019). Both fibromyalgia and GWI are in fact accompanied by chronic sickness behavior, with similar hallmarks such as widespread musculoskeletal pain, fatigue, and cognitive difficulties (Arnett and Clark, 2012; Maule et al., 2018; O’Callaghan and Miller, 2019; Wolfe et al., 1990), suggesting the possibility that these conditions present shared mechanisms.
In this study, we used positron emission tomography (PET) and the radioligand [11C]PBR28 to evaluate and document the role of neuroinflammation in veterans with GWI. [11C]PBR28 binds to the 18-kDa translocator protein (TSPO) (Briard et al., 2008; Brown et al., 2007), a mitochondrial protein that is expressed at very low levels in the healthy CNS but becomes dramatically upregulated by activated microglia/macrophages and reactive astrocytes, and is therefore considered a surrogate marker of neuroinflammation (Cagnin et al., 2007; Lacor et al., 1996; Lavisse et al., 2012; Rupprecht et al., 2010).
2. Materials and methods
2.1. Study design
The study was conducted at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital. The institutional review board and the Radioactive Drug Research Committee approved this study. All participants gave written informed consent.
2.2. Participants
Fifteen veterans with GWI (12 males, 51.1 ± 1.3 years old [mean ± SD]), and 33 healthy controls (17 males, 47.9 ± 12.6 years old; HC) were recruited for the study. The HC sample included 8 healthy veterans of the Gulf War (7 males, 51.4 ± 2.1 years old; HCVET) and 25 healthy civilians (10 males, 46.8 ± 2.8 years old; HCCIV). All GWI veterans met the Kansas diagnostic criteria for GWI which requires endorsing at least three out of six symptom domains (fatigue, pain, neurological, skin, gastrointestinal, and respiratory) and either moderate or multiple mild symptoms within each domain (Steele, 2000). Veterans not meeting Kansas GWI or other exclusionary criteria were considered healthy controls. Participants from all groups were excluded for the presence of a history of major psychiatric illness, neurological illness, cardiovascular disease, inability to communicate in English and for contraindication for PET/MR scanning (e.g., pacemaker, metallic implants, pregnancy, etc.).
2.3. Behavioral visit
All participants in the study were asked to complete the Beck Depression Inventory [BDI; (Beck et al., 1961)] and the Brief Pain Inventory (BPI; (Daut et al., 1983)]. In addition, all veterans were asked to complete the 2011 American College of Rheumatology (ACR) self-report survey for the assessment of fibromyalgia symptoms (Wolfe et al., 2011), the Kansas GWI Questionnaire to detect and score the severity of GWI (Steele, 2000) and Conner’s Continuous Performance Test III (CPT3) for the assessment of attention (Conners et al., 2000). During the visit, venous blood was drawn from all participants in order to have all participants genotyped for the Ala147Thr TSPO polymorphism, which predicts binding affinity to the radioligand (Owen et al., 2010; Owen et al., 2012). Subjects exhibiting the Thr/Thr genotype, which predicts low affinity binding status, were excluded from the imaging procedures, whereas participants with the Ala/Ala or Ala/Thr polymorphisms, which are associated with high and mixed affinity binding, respectively, were allowed to proceed.
2.4. Imaging visit
At the beginning of the imaging visit, a subset of subjects (n=32) had venous blood collected to measure the level of circulating inflammatory cytokines, using the Meso Scale Discovery V-Plex Plus Proinflammatory Panel. Cytokine analyses were performed through a third-party vendor (Beantown Biotech, Natick MA). In this study we focused on IL-6, TNF-α and IL-1β because their level is commonly reported as altered in animal models and/or humans with pain disorders (Ji et al., 2013; Loggia et al., 2015) and/or GWI (Johnson et al., 2016; O’Donovan et al., 2015). Brain imaging was then performed with a Siemens PET/MRI scanner, consisting of a dedicated brain avalanche photodiode-based PET scanner in the bore of a Siemens 3T Tim Trio MRI (Kolb et al., 2012). Up to 15 millicurie (mCi) of [11C]PBR28, produced in-house using a procedure modified from the literature (Imaizumi et al., 2007), were injected as an intravenous bolus, and dynamic PET were acquired for 90 min as described previously (Albrecht et al., 2018; Loggia et al., 2015). Because the HCCIV were recruited through a different study protocol, they happened to have a significantly lower injected dose compared to GWI (GWI: 14.307±1.07 mCi [529.4±39.5 Mbq]; HCVET: 14.307±0.98 mCi [529.4±36.2 Mbq]; HCCIV: 12.262±1.42 mCi [453.7±56.1 Mbq; mean±SD]; GWI vs HCCIV: p = 0.002). However, there was no difference in dose between GWI and HCVET (p = 0.99). For anatomical localization, spatial normalization and generation of attenuation correction maps (Izquierdo-Garcia et al., 2014b), a multi-echo MPRAGE (T1-weighted structural MRI) volume was also acquired (TR/TE1/TE2/TE3/TE4 = 2530/1.64/3.5/5.36/7.22 ms, flip angle = 7°, voxel size = 1mm isotropic).
In 17 participants (6 veterans), a radial artery catheter was inserted and blood samples were collected at 6-10s intervals for the first three minutes, followed by samples collected at 5, 10, 20, 30, 50, 70 and 90 minutes post [11C]PBR28 injection. These data were collected for the purpose of creating a radiometabolite-corrected arterial input function to perform full kinetic modelling, in order to validate the semiquantitative ratio metric used in the study (see below). Technical issues were encountered for 4 participants, thus the blood data from these participants were excluded from further analyses. Arterial blood processing was performed as previously described by Albrecht et al. (2018).
2.5. Imaging data preprocessing
From the [11C]PBR28 PET data, standard uptake volume ratio (SUVR) images, from 60-90 min post-injection data, were generated as described previously (Albrecht et al., 2018; Loggia et al., 2015; Zurcher et al., 2015). Essentially, standard uptake volume (SUV) images, computed by normalizing radioactivity by injected dose/body weight, were attenuation corrected using a published MR-based method (Izquierdo-Garcia et al., 2014a). These SUV maps were then nonlinearly transformed to MNI space, smoothed with an 8mm full width half-maximum Gaussian kernel, and then intensity-normalised by dividing them by the mean SUV extracted from the occipital cortex [identified using a label from the AAL atlas available in PMOD (Tzourio-Mazoyer et al., 2002)] to obtain SUVR maps. We have previously utilized this approach for quantification of [11C]PBR28 PET data in patients with chronic low back pain, amyotrophic lateral sclerosis (Albrecht et al., 2018) and, more relevant for the current study, fibromyalgia (Albrecht et al., 2019), a condition with a clinical presentation similar to that of GWI. The lack of significant group differences in [11C]PBR28 SUV signal in the occipital cortex was confirmed in this study when the GWI veterans were compared to all HCs (p = 0.81) and when they were compared with the subset of HCVET (p = 0.87). In order to further support the validity of SUVR as an outcome metric, we compared SUVR against distribution volume (VT) ratio (DVR), in a subset of participants for whom arterial plasma data were available [n = 13; detailed methods described in (Albrecht et al., 2018)]. To this end, VT was computed from “target regions” (ie. regions identified as statistically significant across groups in the voxel-wise analyses in this study; see below) as well as the occipital cortex, using radiometabolite-corrected arterial input function (AIF) and traditional 2-tissue-compartmental modelling. Each target region was divided by occipital cortex VT to obtain DVR. In all evaluated regions, SUVR were strongly correlated with DVR (8.7 x10−7 ≤ p ≤ 8.9 x10−3, 0.69 ≤ r ≤ 0.95; Supplementary Fig. 1). These results provide support for the use of SUVR as a viable PET metric in our study.
2.6. Statistical analysis
Group differences were assessed with Student’s t-tests for continuous variables (age, clinical and cytokine variables) and chi-square tests for categorical variables (sex and genotype), using Statistica (TIBCO Software Inc., v.13). The main group analyses compared the GWI group with the whole HC group, taking advantage of the relatively large sample of controls. While differences in age between these groups did not meet our threshold for statistical significance (p = 0.34), the differences in sex distribution approached significance (p = 0.061). For this reason, comparisons between these groups included sex as a covariate. In addition, because TSPO genotype affects binding affinity (Owen et al., 2010; Owen et al., 2012), all PET analyses were also corrected for genotype. In addition to these main analyses, we compared the GWI group against the subset of healthy controls who were GW veterans (n = 8). These secondary, exploratory analyses were performed to evaluate whether the effects observed in the main analyses could be observed when contrasting groups that were better demographically matched and had comparable GW combat exposure. Because neither sex (p = 0.65) nor age (p = 0.67) were significantly different across these groups, analyses between GWI and HCVET were only corrected for genotype.
Group analyses were performed using two strategies. First, because one of the hypotheses of this study was that GWI patients would demonstrate similar neuroinflammatory patterns as those observed in fibromyalgia patients, we performed ROI analyses using, as our a priori ROIs, statistically significant clusters from our previous study demonstrating increased [11C]PBR28 signal in that patient group: primary motor/somatosensory cortex (M1/S1), dorsolateral prefrontal cortex (dlPFC), precuneus and anterior mid cingulate cortex (aMCC) (Albrecht et al., 2019). Next, a whole brain voxel-wise analysis was performed, in order to evaluate the possible presence of group differences in the [11C]PBR28 signal beyond the boundaries of the a priori ROIs, as well as to localize any effects observed in the ROI analyses with higher spatial accuracy. Because the injected dose was significantly different between GWI and HC, these analyses were repeated including injected dose as a covariate in the analyses. These analyses were performed with FSL’s FEAT GLM tool (www.fmrib.ox.ac.uk/fsl, version 5.0.10). For ease of visualization of the cortical effects and for better comparison with the results of the fibromyalgia study, imaging results are visualized on a surface (FreeSurfer’s fsaverage) in the main manuscript. In addition, results were also overlaid onto MNI volumetric standard brain for visualisation of white matter and subcortical structures (Supplementary Figure 3).
For visualization purposes, as well as for correlation analyses (see below), data were extracted from the significant clusters identified in the voxel-wise analyses comparing GWI and HC, anatomically split using labels from the Harvard-Oxford Cortical Structural Atlas (Centre for Morphometric Analyses, http://www.cma.mgh.harvard.edu/fsl_atlas.html).
In GWI patients, the [11C]PBR28 signal from these ROIs was correlated with clinical variables (Kansas GWI score, fibromyalgia score, score on the fatigue item of the ACR self-report survey for the assessment of fibromyalgia symptoms, CPT3 hit reaction time, BPI pain and BDI), in order to evaluate potential association between neuroinflammation and GWI symptom severity, as well as with levels of circulating cytokines (IL-6, TNF-α and IL-1β), to explore the relationship between central and peripheral inflammation (correcting for genotype). Because these analyses were exploratory, the results in this case were not corrected for multiple comparisons.
3. Results
3.1. Participant characteristics
Demographic and other key characteristics for all participants are displayed in Table 1. As briefly mentioned in the methods section, there was no significant difference in sex or age between the GWI and HC groups, (p = 0.061 and 0.339, respectively). Differences in genotype distributions between these groups approached but did not reach statistical significance (p = 0.051). There was also no significant difference between GWI and HCVET groups in sex, genotype, or age (p = 0.651, 0.435 and 0.919, respectively).
Table 1:
Participant characteristics
| Participant characteristics | |||
|---|---|---|---|
| GWI | HC | GWI vs HC | |
| N | 15 | 33 (all) | - |
| 8 (veterans) | |||
| Sex | 12M; 3F | 17M; 15F (all) | p = 0.06 |
| 7M; 1F (veterans) | p = 0.65 | ||
| Age (years: mean ± sd) | 51.1 ± 5.0 | 47.9 ± 12.6 (all) | p = 0.34 |
| 51.4 ± 6.1 (veterans) | p = 0.92 | ||
| TSPO polymorphism | 5H; 10M | 21H; 12M (all) | p = 0.05 |
| 4H; 4M (veterans) | p = 0.44 | ||
3.2. Behavioral measures and blood cytokine levels
There was a significant difference between groups in all behavioral measures, with the GWI participants demonstrating higher Kansas GWI and fibromyalgia scores, fatigue, pain and depression except for the Conner’s CPT3 HRT test (Table 2). There was no significant elevation in levels of IL-6, IL-1β and TNF-α in veterans with GWI when compared to HC (p’s > 0.05; Table 2).
Table 2:
Participant clinical and cytokine variables
| Group clinical and cytokine variables | |||
|---|---|---|---|
| GWI | HC | GWI vs HC | |
| Kansas GWI score | 45.2 ± 18.86 | 1.75 ± 2.96 (veterans) | p = 5.49 x10−6 |
| ACR Fibromyalgia score | 18.4 ± 6.98 | 1.25 ± 2.12 (veterans) | p = 2.12 x10−6 |
| ACR Fatigue score | 2.1 ± 0.80 | 0 (veterans) | p = 9.69 x10−7 |
| Conner’s CPT3 Hit Reaction Time | 54.4 ± 8.81 | 47.88 ± 4.88 (veterans) | p = 0.103 |
| BPI Pain Intensity | 5.6 ± 1.42 | 0.27 ± 0.58 (all) | p < 0.0001 |
| 0.56 ± 0.95 (veterans) | p = 2.57 x10−8 | ||
| BDI | 17.7 ± 9.92 | 1.62 ± 2.45 (all) | p = 1.12 x10−9 |
| 2.69 ± 3.65 (veterans) | p = 0.0008 | ||
| IL-6 | 1.63 ± 1.05 | 0.76 ± 0.78 (all) | p = 0.0344 |
| 1.26 ± 0.91 (veterans) | p = 0.428 | ||
| TNF-α | 6.16 ± 5.14 | 2.44 ± 1.97 (all) | p = 0.0295 |
| 3.33 ± 2.68 (veterans) | p = 0.2195 | ||
| IL-1β | 0.93 ± 0.39 | 1.02 ± 0.69 (all) | p = 0.6862 |
| 1.04 ± 0.53 (veterans) | p = 0.6104 | ||
3.3. Imaging results: a priori ROI analyses
Compared to the HC group, GWI participants demonstrated significantly elevated [11C]PBR28 PET signal in the dlPFC, precuneus and aMCC, i.e., three out of four of the a priori ROIs. The PET signal elevations in the dlPFC and precuneus remained statistically significant when GWI were compared with HCVET, and additional signal elevations were observed in M1-S1, (Figure 1).
Figure 1: ROI analyses.
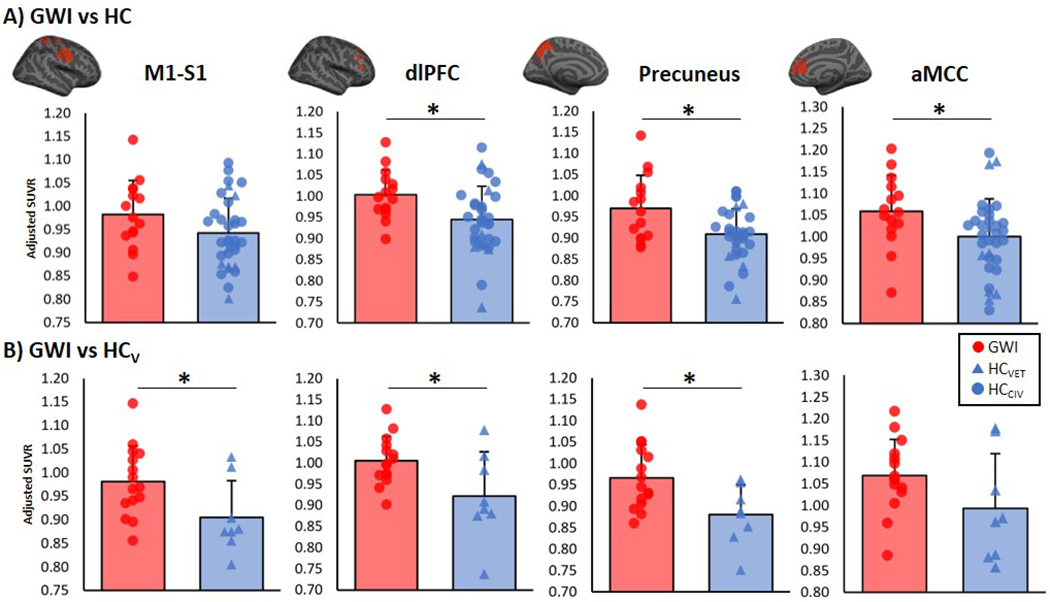
Group differences in [11C]PBR28 standardized volume uptake (SUVR) in a priori ROIs. These regions were selected as they demonstrated [11C]PBR28 PET signal elevations in fibromyalgia patients. Top panel: Average ± standard deviation SUVR extracted showing differences between GWI and HC (adjusted for genotype and sex). Bottom panel: Average ± standard deviation SUVR extracted showing differences between GWI and HCVET (adjusted for genotype). Surface projections of regions are displayed in red above the plots. * significant difference between groups (p < 0.05). M1 = primary motor cortex; S1 = primary somatosensory cortex; dlPFC = dorsolateral prefrontal cortex; aMCC = anterior mid cingulate cortex.
3.4. Imaging results: voxel-wise group differences
The voxel-wise comparison between GWI and HC revealed widespread cortical [11C]PBR28 PET signal elevations (and no regions of PET signal reduction) in GWI. These were observed both within the regions used as a priori ROIs in the analysis previously described (S1, M1, dlPFC, aMCC and precuneus) as well as additional regions (dorsomedial prefrontal cortex [dmPFC], paracingulate cortex, anterior cingulate cortex [ACC], ventral medial PFC [vmPFC] and posterior cingulate cortex [PCC]; Figure 2A). Several of these regions [S1, M1, dlPFC, dmPFC, precuneus and the superior parietal lobule (SPL; Figure 2B)] survived statistical significance when GWI were compared with the HCVET subgroup. For display purposes, the mean SUVR values from a subset of regions are displayed in Figure 2C. In addition, as injected dose was significantly different between groups when veterans with GWI were compared with HC, we ran an exploratory voxel-wise analysis, including injected dose as a covariate. This analysis yielded similar results (Supplementary Figure 2).
Figure 2:
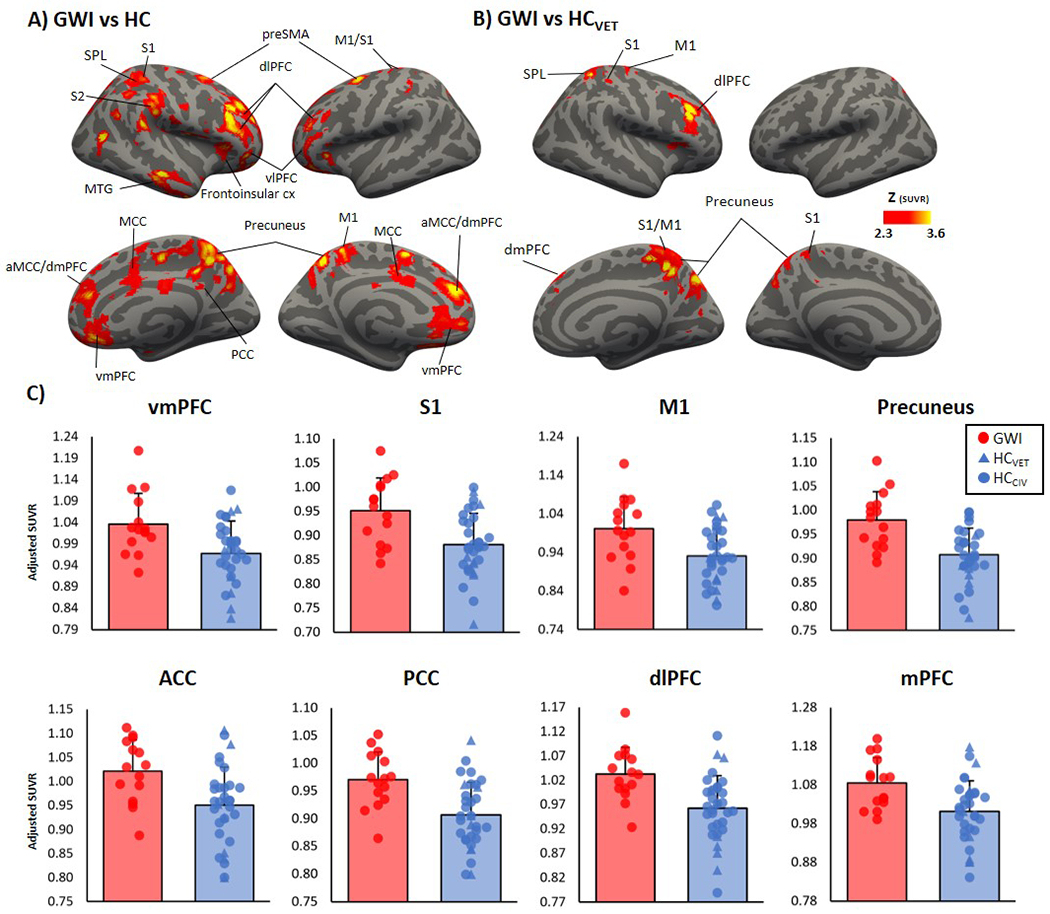
Voxel-wise group difference in [11C]PBR28 standardized volume uptake (SUVR). A. Surface projection maps displaying areas with significantly elevated [11C]PBR28 SUVR in GWI (n=15) compared to HC (n=33), in voxel-wise analyses, data adjusted for sex and genotype. B. Surface projection maps displaying areas with significantly elevated [11C]PBR28 SUVR in GWI (n=15) compared to HCVET (n=8), in voxel-wise analyses, data adjusted for genotype. C. Average ± standard deviation SUVR extracted from several clusters identifies as statistically significant in the voxel-wise SUVR analysis from A. Data plots have been adjusted for sex and genotype. vmPFC = ventral medial prefrontal cortex; S1 = primary somatosensory cortex; M1 = primary motor cortex; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; mPFC = medial prefrontal cortex; aMCC = anterior mid cingulate cortex; SPL = superior parietal cortex; MCC = mid cingulate cortex; vlPFC = ventrolateral prefrontal cortex; S2 = secondary somatosensory cortex; cx = cortex.
3.5. Imaging results: regression analyses
Regions that showed elevations in SUVR in GWI compared to HC were selected as an ROI for correlations with clinical variables and circulating cytokine levels in the GWI group only, in exploratory analyses. In no region was there a significant correlation between SUVR signal in GWI and clinical variables or circulating inflammatory cytokines (Table 3).
Table 3:
GWI [11C]PBR28 correlations with clinical and cytokine variables
| GWI [11C]PBR28 correlations with clinical variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| vmPFC | S1 | M1 | Precuneus | ACC | PCC | dlPFC | mPFC | ||
| Kansas GWI score | r value | −0.3772 | −0.1244 | −0.3110 | −0.1458 | −0.1728 | −0.2780 | −0.3772 | −0.2967 |
| p value | 0.1840 | 0.6720 | 0.2790 | 0.6190 | 0.5550 | 0.3360 | 0.1900 | 0.3030 | |
| ACR Fibromyalgia score | r value | −0.3472 | 0.2181 | −0.1311 | 0.1828 | 0.1263 | 0.0116 | −0.0823 | −0.0474 |
| p value | 0.2240 | 0.4540 | 0.6550 | 0.5320 | 0.6670 | 0.9690 | 0.7800 | 0.8720 | |
| ACR Fatigue score | r value | −0.2340 | 0.2363 | −0.0427 | 0.1678 | 0.1778 | −0.1055 | 0.0398 | 0.0858 |
| p value | 0.4210 | 0.4160 | 0.8850 | 0.5660 | 0.5430 | 0.7200 | 0.8930 | 0.7700 | |
| Conner’s CPT3 Hit Reaction Time | r value | 0.0180 | −0.0412 | −0.0782 | −0.0529 | 0.0350 | 0.1562 | −0.3173 | 0.0941 |
| p value | 0.9510 | 0.8890 | 0.7910 | 0.8570 | 0.9050 | 0.5940 | 0.2690 | 0.7490 | |
| BPI Pain Intensity | r value | −0.4269 | 0.2825 | −0.0211 | 0.2466 | 0.4259 | −0.0009 | 0.3951 | 0.1422 |
| p value | 0.1280 | 0.3280 | 0.9430 | 0.3950 | 0.1290 | 0.9980 | 0.1620 | 0.6280 | |
| BDI | r value | −0.2185 | −0.0712 | −0.1650 | −0.0981 | −0.2404 | −0.2495 | −0.2776 | −0.3849 |
| p value | 0.4530 | 0.8090 | 0.5730 | 0.7390 | 0.4080 | 0.3900 | 0.3370 | 0.1740 | |
| IL-6 | r value | 0.2803 | −0.2696 | −0.1165 | −0.1851 | −0.2864 | −0.1407 | −0.5564 | 0.0936 |
| p value | 0.3320 | 0.3510 | 0.6920 | 0.5260 | 0.3210 | 0.6310 | 0.0750 | 0.7500 | |
| TNF-α | r value | 0.7217 | 0.6942 | 0.6156 | 0.7709 | 0.7747 | 0.7562 | 0.7360 | 0.8292 |
| p value | 0.0120 | 0.0180 | 0.0440 | 0.0050 | 0.0050 | 0.0070 | 0.0100 | 0.0020 | |
| IL-1β | r value | 0.1616 | 0.2283 | 0.2511 | 0.1904 | 0.1601 | 0.4680 | 0.0134 | 0.3483 |
| p value | 0.6350 | 0.5000 | 0.4560 | 0.5750 | 0.6380 | 0.1470 | 0.9690 | 0.2940 | |
4. Discussion
The current study provides in-vivo evidence of neuroinflammation in veterans with GWI. When compared to GW veterans without GWI and healthy civilians, veterans with GWI demonstrated elevated TSPO binding, as measured with [11C]PBR28 PET. This marker of neuroinflammation demonstrated elevated levels throughout cortical areas such as precuneus, prefrontal cortex, and primary motor and somatosensory areas, as well as underlying white matter and the putamen. The neuroinflammatory signal elevations observed in GWI demonstrated spatial similarities to that observed in fibromyalgia (Albrecht et al., 2019), as we had hypothesized given the overlap in the clinical presentation of the two conditions. Fatigue, musculoskeletal pain, disturbed sleep, memory and attention deficits are some of the symptoms that affect both conditions (Binns et al., 2008; Clauw, 2014). In fact, GWI veterans are often diagnosed with fibromyalgia (Blanchard et al., 2019).
While this study represents the first report of in-vivo neuroinflammation imaging in veterans with GWI, these results conform to a body of preclinical research that has shown neuroinflammation in animal models of GWI (White et al., 2016). Such models have shown that the exposure to neurotoxicant chemicals such as irreversible acetylcholinesterase inhibitor (AChEi), organophosphate pesticides, nerve agents and prophylactic treatment with pyridostigmine bromide pills (PB; a reversible AChEi), i.e., the same compounds the veterans had been exposed to during the GW, induces chronic neuroinflammation (Banks and Lein, 2012; Koo et al., 2018; O’Callaghan et al., 2015; Ojo et al., 2014; Parihar et al., 2013). Additionally, pyrethroid pesticides, which were also widely used during the GW, mediate their action by opening voltage gated sodium channels which results in excessive neuronal firing (Hue and Mony, 1987), possibly inducing neurogenic inflammation both centrally (Xanthos and Sandkühler, 2013), as well as peripherally (Chiu et al., 2012; Roosterman et al., 2006). While our study showed that veterans with GWI demonstrated elevations of central (TSPO PET signal) markers of inflammation, we found no evidence of elevations in peripheral (pro inflammatory cytokines) markers of inflammation.
In addition to neurotoxicant exposure, physical and mental stressors can induce neuroinflammation, perhaps compounding its effects. Indeed, some animal models of GWI are produced through the combination of exposure to GW-relevant neurotoxicant AChEi chemicals (sarin, pesticides, PB) and stress (White et al., 2016). In these models of GWI, astrogliosis and/or microglial activation have been reported within the prefrontal cortex, the hippocampus, striatum, hypothalamus, olfactory bulb and the cerebellum (O’Callaghan et al., 2015; Ojo et al., 2014; Parihar et al., 2013). Interestingly, O’Callaghan et al. (2015) found that these neuroinflammatory responses were greatly exacerbated when animals were pre-treated with rodent stress hormone corticosterone (CORT), administered at levels compatible with those observed with high physiological stress (O’Callaghan et al., 2015), possibly at the level that the veterans might have experienced given the harsh conditions that were prevalent during the Gulf War [e.g., extreme heat or cold, or sleep deprivation (Gifford et al., 2006)]. Indeed, studies have shown that sleep disturbances and heat stress can induce neuroinflammation (Chauhan et al., 2017; Zhu et al., 2012). Furthermore, these stressors, along with other brain insults including a history of mild traumatic brain injury (mTBI) can ‘prime’ glial cells for aberrant activation which might lead to chronic neuroinflammation (Blaylock and Maroon, 2011; Burda et al., 2016; Chen et al., 2014; Kuhlmann and Guilarte, 2000; Perry et al., 1985; Watkins et al., 2007a).
In this investigation, we have used [11C]PBR28 to image TSPO binding as a marker of neuroinflammation. Though TSPO is constitutively expressed by many cell types, within the CNS, this protein is upregulated primarily or exclusively in glial cells during neuroinflammatory responses, and hence can be used as a sensitive marker of glial activation (Wei et al., 2013). Indeed, animal models of neuropathic pain have shown increased TSPO expression co-localised with activated microglia and astrocytes (Liu et al., 2016; Wei et al., 2013). Similarly, TSPO expression has been localised with activated astrocyte and microglia in animal models and human investigations of multiple sclerosis, Alzheimer’s disease and HIV encephalitis (Abourbeh et al., 2012; Chen and Guilarte, 2006; Cosenza-Nashat et al., 2009; Gulyas et al., 2009; James et al., 2017) and animal models and human post-mortem studies of ischemia (Cosenza-Nashat et al., 2009; Martin et al., 2010; Rojas et al., 2007). Further, TSPO PET imaging in amyotrophic and primary lateral sclerosis patients demonstrates increased TSPO signal in the primary motor cortex (Alshikho et al., 2016; Alshikho et al., 2018; Paganoni et al., 2018; Zurcher et al., 2015), a region where glial activation can be documented histologically (Hudson et al., 1993; Kawamata et al., 1992 ; Rothstein et al., 1995). Similarly, in Alzheimer’s Disease, glial activation can be observed in amyloid positive regions (Araujo and Cotman, 1992; Rozemuller et al., 1989), and these regions have shown elevated TSPO expression (Kreisl et al., 2013; Parbo et al., 2017). Likewise, glial activation has been reported in the basal ganglia of Huntington’s Disease patients (O’Kusky et al., 1999), who also have shown elevated TSPO expression (Lois et al., 2018). These observations support the use of TSPO as a marker of glial activation. Because in the CNS TSPO can be upregulated by microglia and/or astrocytes (Beckers et al., 2018; Lavisse et al., 2012), our study does not directly allow clarification of which glial cell subtype might contribute to the observed signal. For instance, several animal models have shown that initial upregulation of TSPO might be driven by microglia, whereas astrocytic TSPO upregulation might be maintained throughout the course of the disease (Chen et al., 2014; Chen and Guilarte, 2006; Kuhlmann and Guilarte, 2000; Liu et al., 2014; Martin et al., 2010). This phase-dependent activation of glial cells is supported by human post-mortem studies of multiple sclerosis, showing that in acute lesions, microglia and macrophages are the major cell contributors to TSPO expression, whereas in chronic lesions, astrocytes are the major contributors to TSPO expression (Cosenza-Nashat et al., 2009). However, because GWI is accompanied by sickness behavior (O’Callaghan and Miller, 2019), and given that microglia are largely the source of neuroinflammatory mediators that underlie sickness behaviors (Dantzer et al., 2008; Konsman et al., 2002; Maier, 2003; Watkins et al., 2007b), it seems likely that microglial activation would account for a significant proportion of the neuroinflammatory signal observed. In addition, several preclinical GWI studies implicate microglia and not astrocytes in neuroinflammation (Carreras et al., 2018; Locker et al., 2017). Furthermore, a recent study employing a dual-ligand approach suggests that in fibromyalgia, a chronic condition that shares clinical features with GWI, the TSPO signal might be indeed driven by microglia rather than astrocytes. In that study, we reported significant elevations in TSPO signal (which may reflect microglial or astrocytic contributions), but not in MAO-B signal [which is thought to reflect mostly astrocytic, but not microglial, contributions; (Albrecht et al., 2019)]. Similar approaches will need to be implemented to understand whether a similar interpretation can apply to GWI as well.
It is important to also stress that, in addition to being upregulated by glial cells within the CNS, TSPO is also highly expressed in activated macrophages and other peripheral immune cells (Lacor et al., 1996). For instance, a recent study has shown elevated TSPO expression in activated macrophages, fibroblast-like synoviocytes and CD4+ T lymphocytes in the synovial tissue of patients with rheumatoid arthritis (Narayan et al., 2018). In physiological conditions, the blood brain barrier (BBB) typically acts as a restriction to prevent easy recruitment into the CNS parenchyma of cells involved in the adaptive immunity response (with the exception of activated T cells) such as leukocytes (Ransohoff and Brown, 2012). However, several preclinical models of GWI document the presence of BBB disruptions [e.g., Abdel-Rahman et al., (2002)]. Indeed, a study in GW veterans detected CNS autoantibodies to glial fibrillary acidic protein, myelin basic protein, tau, tubulin and other neuro-glial proteins in the peripheral blood that would not be in circulation without at least prior BBB compromise at some point (Abou-Donia et al., 2017). Because the disruption in BBB permeability increases the likelihood of the CNS being infiltrated by activated macrophages or other cell types (Lopes Pinheiro et al., 2016), and given that TSPO is highly expressed in these cells, it is possible that the elevations in the brain levels of TSPO observed in this investigation might in part be due to this phenomenon. The recruitment of peripheral immune cells into the CNS was shown to be able to damage neuronal cells (Ransohoff and Brown, 2012), and thus might contribute to some of the symptoms of GWI such as cognitive/affective decrements and memory loss (Janulewicz et al., 2017; Jeffrey et al., 2019; Sullivan et al., 2018; Sullivan et al., 2003). However, future studies will need to directly measure BBB damage in veterans with GWI to assess the relevance of this mechanism to neuroinflammation.
In the present study, the brain TSPO PET signal did not correlate with circulating levels of proinflammatory cytokines, and when compared to HC, cytokine levels in GWI were not elevated. These results agree with several prior studies in patients with major depression (Richards et al., 2018; Setiawan et al., 2015), seasonal allergy (Tamm et al., 2018), schizophrenia (Coughlin et al., 2016) and in healthy participants imaged after administration of lipopolysaccharide (a potent immune activator) (Sandiego et al., 2015), which also reported no statistically significant correlations between brain TSPO signal and the majority of the peripheral markers of inflammation [although a prior study from our group did report a weak negative correlation between TSPO signal and IL-6 in chronic low back pain (Loggia et al., 2015)].
Why some veterans develop GWI while others do not is still yet to be answered. It is possible that the veterans with GWI have had other toxicant exposures or mTBIs that have ‘primed’ their glial cells for neuroinflammation at the exposure of further neurotoxicants and stressors (Blaylock and Maroon, 2011; Perry et al., 1985; Watkins et al., 2007a). Certainly, a larger sample size of veterans, ideally with detailed information about other brain insults including mTBIs, life stressors and the types of neurotoxicant exposures, would be required to begin answering some of these questions.
In conclusion, this study is the first to document an elevation of the neuroinflammatory glial marker, TSPO, in the brain of veterans with GWI. Further studies are required to validate and further refine these findings, and to determine whether glial modulation may be a viable therapy for GWI.
Supplementary Material
Supplementary figure 1: Correlation between [11C]PBR28 distribution volume ratios (DVR) and [11C]PBR28 standardized volume uptake (SUVR) within clusters from GWI vs HC group analysis.
Supplementary figure 2: Voxel-wise group difference in [11C]PBR28 standardized volume uptake (SUVR). Surface projection maps displaying areas with significantly elevated [11C]PBR28 SUVR in GWI (n=15) compared to HC (n=33), in voxel-wise analyses, data adjusted for sex, genotype and injected dose.
Supplementary figure 3: Voxel-wise group difference in [11C]PBR28 standardized volume uptake (SUVR). Volumetric maps displaying areas with significantly elevated [11C]PBR28 SUVR in GWI patients (n=15) compared to HC (n=33), in voxel-wise analyses, data adjusted for sex and genotype.
4. Acknowledgments
The study was supported by the following funding sources: DoD-W81XWH-14-1-0543 (MLL), International Association for the Study of Pain Early Career Award (MLL), R01-NS094306-01A1 (MLL), R01-NS095937-01A1 (MLL), R21-NS087472-01A1 (MLL), R01-AR064367 (VN, RRE), R01-AT007550 (VN), W81XWH-18-1-0549 (KS), Martinos Center Pilot Grant for Postdoctoral Fellows (DSA), Harvard Catalyst Advance Imaging Pilot Grant (JMH), P41RR14075, 5T32EB13180 (T32 supporting DSA), and P41EB015896. The authors thank Dr Norman Taylor and Dr Patricia Bachiller for assistance with data collection. The also thank Virginie Esain from Meso-Scale Discovery for her help with the cytokine analyses.
5. References
- Abou-Donia MB, Conboy LA, Kokkotou E, Jacobson E, Elmasry EM, Elkafrawy P, Neely M, Dale’Bass CR, Sullivan K, 2017. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicology and teratology 61, 36–46. [DOI] [PubMed] [Google Scholar]
- Abourbeh G, Theze B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dolle F, Tavitian B, Boisgard R, 2012. Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [(1)(8)F]DPA-714. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 5728–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Forsberg A, Sandstrom A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Hoglund CO, Catana C, Cervenka S, Akeju O, Lekander M, Cohen G, Halldin C, Taylor N, Kim M, Hooker JM, Edwards RR, Napadow V, Kosek E, Loggia ML, 2019. Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation. Brain Behav Immun 75, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Normandin MD, Shcherbinin S, Wooten DW, Schwarz AJ, Zurcher NR, Barth VN, Guehl NJ, Akeju O, Atassi N, Veronese M, Turkheimer F, Hooker JM, Loggia ML, 2018. Pseudoreference Regions for Glial Imaging with (11)C-PBR28: Investigation in 2 Clinical Cohorts. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 59, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassan Mohammed H, Mirshafiey A, Vahedi H, Hemmasi G, Moussavi Nasl Khameneh A, Parastouei K, Saboor-Yaraghi AA, 2017. Immunoregulation of Inflammatory and Inhibitory Cytokines by Vitamin D3 in Patients with Inflammatory Bowel Diseases. Scandinavian Journal of Immunology 85, 386–394. [DOI] [PubMed] [Google Scholar]
- Alshikho, Zürcher NR, Loggia ML, Cernasov P, Chonde DB, Garcia DI, Yasek JE, Akeju O, Catana C, Rosen BR, 2016. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology 87, 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshikho, Zurcher NR, Loggia ML, Cernasov P, Reynolds B, Pijanowski O, Chonde DB, Izquierdo Garcia, D., Mainero C, Catana C, Chan J, Babu S, Paganoni S, Hooker JM, Atassi N, 2018. Integrated magnetic resonance imaging and [(11) C]-PBR28 positron emission tomographic imaging in amyotrophic lateral sclerosis. Annals of neurology 83, 1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DM, Cotman CW, 1992. β-Amyloid stimulates glial cells in vitro to produce growth factors that accumulate in senile plaques in Alzheimer’s disease. Brain research 569, 141–145. [DOI] [PubMed] [Google Scholar]
- Arnett SV, Clark IA, 2012. Inflammatory fatigue and sickness behaviour — Lessons for the diagnosis and management of chronic fatigue syndrome. Journal of Affective Disorders 141, 130–142. [DOI] [PubMed] [Google Scholar]
- Banks CN, Lein PJ, 2012. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Archives of general psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Beckers L, Ory D, Geric I, Declercq L, Koole M, Kassiou M, Bormans G, Baes M, 2018. Increased Expression of Translocator Protein (TSPO) Marks Pro-inflammatory Microglia but Does Not Predict Neurodegeneration. Molecular imaging and biology 20, 94–102. [DOI] [PubMed] [Google Scholar]
- Binns J, Barlow C, Bloom F, J. Clauw D, Golomb B,C. Graves J, Hardie A, Knox M, Meggs J, W D. Nettleman M, 2008. Gulf War Illness and the Health of Gulf War Veterans. [Google Scholar]
- Blanchard M, Molina-Vicenty HD, Stein PK, Li X, Karlinsky J, Alpern R, Reda DJ, Toomey R, 2019. Medical Correlates of Chronic Multisymptom Illness in Gulf War Veterans. The American Journal of Medicine 132, 510–518. [DOI] [PubMed] [Google Scholar]
- Blaylock RL, Maroon J, 2011. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy—a unifying hypothesis. Surgical neurology international 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, Cropley V, Fujita M, Innis RB, Pike VW, 2008. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. Journal of medicinal chemistry 51, 17–30. [DOI] [PubMed] [Google Scholar]
- Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, Imaizumi M, Hong J, Pike VW, Innis RB, 2007. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 48, 2072–2079. [DOI] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, Sofroniew MV, 2016. Astrocyte roles in traumatic brain injury. Experimental Neurology 275, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB, 2007. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 4, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns CB, Panacek EA, Harken AH, Banerjee A, 2000. Bench to bedside: tumor necrosis factor-alpha: from inflammation to resuscitation. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 7, 930–941. [DOI] [PubMed] [Google Scholar]
- Calley CS, Kraut MA, Spence JS, Briggs RW, Haley RW, Hart J, 2010. The neuroanatomic correlates of semantic memory deficits in patients with Gulf War illnesses: a pilot study. Brain Imaging and Behavior 4, 248–255. [DOI] [PubMed] [Google Scholar]
- Carreras I, Aytan N, Mellott T, Choi J-K, Lehar M, Crabtree L, Leite-Morris K, Jenkins BG, Blusztajn JK, Dedeoglu A, 2018. Anxiety, neuroinflammation, cholinergic and GABAergic abnormalities are early markers of Gulf War illness in a mouse model of the disease. Brain research 1681, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW, 2010. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology 31, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan NR, Kapoor M, Prabha Singh L, Gupta RK, Chand Meena R, Tulsawani R, Nanda S, Bala Singh S, 2017. Heat stress-induced neuroinflammation and aberration in monoamine levels in hypothalamus are associated with temperature dysregulation. Neuroscience 358, 79–92. [DOI] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR, 2014. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain : a journal of neurology 137, 2193–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR, 2006. Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicological sciences : an official journal of the Society of Toxicology 91, 532–539. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Von Hehn CA, Woolf CJ, 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nature neuroscience 15, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ, 2014. Fibromyalgia: a clinical review. Jama 311, 1547–1555. [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff M, Connelly V, Campbell S, MacLean M, Barnes J, 2000. Conners’ continuous performance Test II (CPT II v. 5). Multi-Health Syst Inc 29, 175–196. [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC, 2009. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathology and applied neurobiology 35, 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG, 2016. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Translational psychiatry 6, e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC, 1983. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 17, 197–210. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, Mawle AC, Reeves WC, 1998. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. Jama 280, 981–988. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, James LM, Mahan MY, Joseph J, Georgopoulos A, Engdahl BE, 2016. Reduced Human Leukocyte Antigen (HLA) Protection in Gulf War Illness (GWI). EBioMedicine 3, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RK, Ursano RJ, Stuart JA, Engel CC, 2006. Stress and Stressors of the Early Phases of the Persian Gulf War. Philosophical Transactions: Biological Sciences 361, 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Gandhi P, Goyal A, Jiang L, Fang Y, Ouyang L, Ganji S, Buhner D, Ringe W, Spence J, Biggs M, Briggs R, Haley R, 2012. FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. Neurotoxicology 33, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas B, Makkai B, Kasa P, Gulya K, Bakota L, Varszegi S, Beliczai Z, Andersson J, Csiba L, Thiele A, Dyrks T, Suhara T, Suzuki K, Higuchi M, Halldin C, 2009. A comparative autoradiography study in post mortem whole hemisphere human brain slices taken from Alzheimer patients and age-matched controls using two radiolabelled DAA1106 analogues with high affinity to the peripheral benzodiazepine receptor (PBR) system. Neurochemistry international 54, 28–36. [DOI] [PubMed] [Google Scholar]
- Haley RW, Spence JS, Carmack PS, Gunst RF, Schucany WR, Petty F, Devous MD, Bonte FJ, Trivedi MH, 2009. Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Research: Neuroimaging 171, 207–220. [DOI] [PubMed] [Google Scholar]
- Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF, 2007. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology 28, 761–769. [DOI] [PubMed] [Google Scholar]
- Hudson AJ, Kiernan JA, Munoz DG, Pringle CE, Brown WF, Ebers GC, 1993. Clinicopathological features of primary lateral sclerosis are different from amyotrophic lateral sclerosis. Brain Research Bulletin 30, 359–364. [DOI] [PubMed] [Google Scholar]
- Hue B, Mony L, 1987. Actions of deltamethrin and tralomethrin on cholinergic synaptic transmission in the central nervous system of the cockroach (Periplaneta americana). Comparative biochemistry and physiology. C, Comparative pharmacology and toxicology 86, 349–352. [DOI] [PubMed] [Google Scholar]
- Imaizumi M, Kim HJ, Zoghbi SS, Briard E, Hong J, Musachio JL, Ruetzler C, Chuang DM, Pike VW, Innis RB, Fujita M, 2007. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neuroscience letters 411, 200–205. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Garcia D, Hansen AE, Forster S, Benoit D, Schachoff S, Furst S, Chen KT, Chonde DB, Catana C, 2014a. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 55, 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Garcia D, Sawiak SJ, Knesaurek K, Narula J, Fuster V, Machac J, Fayad ZA, 2014b. Comparison of MR-based attenuation correction and CT-based attenuation correction of whole-body PET/MR imaging. European journal of nuclear medicine and molecular imaging 41, 1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ML, Belichenko NP, Shuhendler AJ, Hoehne A, Andrews LE, Condon C, Nguyen TV, Reiser V, Jones P, Trigg W, Rao J, Gambhir SS, Longo FM, 2017. [(18)F]GE-180 PET Detects Reduced Microglia Activation After LM11A-31 Therapy in a Mouse Model of Alzheimer’s Disease. Theranostics 7, 1422–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz P, Krengel M, Quinn E, Heeren T, Toomey R, Killiany R, Zundel C, Ajama J, O’Callaghan J, Steele L, Klimas N, Sullivan K, 2018. The Multiple Hit Hypothesis for Gulf War Illness: Self-Reported Chemical/Biological Weapons Exposure and Mild Traumatic Brain Injury. Brain sciences 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz PA, Krengel MH, Maule A, White RF, Cirillo J, Sisson E, Heeren T, Sullivan K, 2017. Neuropsychological characteristics of Gulf War illness: A meta-analysis. PLOS ONE 12, e0177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz PA, Seth RK, Carlson JM, Ajama J, Quinn E, Heeren T, Klimas N, Lasley SM, Horner RD, Sullivan K, Chatterjee S, 2019. The Gut-Microbiome in Gulf War Veterans: A Preliminary Report. International Journal of Environmental Research and Public Health 16, 3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey MG, Krengel M, Kibler J, Zundel C, Klimas NG, Sullivan K, Craddock TJ, 2019. Neuropsychological Findings in Gulf War Illness: A Review. Frontiers in Psychology 10, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M, 2013. Glia and pain: is chronic pain a gliopathy? Pain 154 Suppl 1, S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GJ, Slater BCS, Leis LA, Rector TS, Bach RR, 2016. Blood Biomarkers of Chronic Inflammation in Gulf War Illness. PLOS ONE 11, e0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Akiyama H, Yamada T, McGeer PL, 1992. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol 140, 691–707. [PMC free article] [PubMed] [Google Scholar]
- Kolb A, Wehrl HF, Hofmann M, Judenhofer MS, Eriksson L, Ladebeck R, Lichy MP, Byars L, Michel C, Schlemmer HP, Schmand M, Claussen CD, Sossi V, Pichler BJ, 2012. Technical performance evaluation of a human brain PET/MRI system. European radiology 22, 1776–1788. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R, 2002. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in Neurosciences 25, 154–159. [DOI] [PubMed] [Google Scholar]
- Koo B-B, Michalovicz LT, Calderazzo S, Kelly KA, Sullivan K, Killiany RJ, O’Callaghan JP, 2018. Corticosterone potentiates DFP-induced neuroinflammation and affects high-order diffusion imaging in a rat model of Gulf War Illness. Brain, Behavior, and Immunity 67, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB, 2013. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain : a journal of neurology 136, 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW, 1996. Microglia: a sensor for pathological events in the CNS. Trends in neurosciences 19, 312–318. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR, 2000. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. Journal of neurochemistry 74, 1694–1704. [DOI] [PubMed] [Google Scholar]
- Lacor P, Benavides J, Ferzaz B, 1996. Enhanced expression of the peripheral benzodiazepine receptor (PBR) and its endogenous ligand octadecaneuropeptide (ODN) in the regenerating adult rat sciatic nerve. Neuroscience letters 220, 61–65. [DOI] [PubMed] [Google Scholar]
- Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, Ben Haim L, Lebon V, Remy P, Dolle F, Delzescaux T, Bonvento G, Hantraye P, Escartin C, 2012. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 10809–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li W, Dai L, Zhang T, Xia W, Liu H, Ma K, Xu J, Jin Y, 2014. Early repeated administration of progesterone improves the recovery of neuropathic pain and modulates spinal 18kDa-translocator protein (TSPO) expression. The Journal of steroid biochemistry and molecular biology 143, 130–140. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu H, Xu S, Tang Z, Xia W, Cheng Z, Li W, Jin Y, 2016. Spinal translocator protein alleviates chronic neuropathic pain behavior and modulates spinal astrocyte-neuronal function in rats with L5 spinal nerve ligation model. Pain 157, 103–116. [DOI] [PubMed] [Google Scholar]
- Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP, 2017. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. Journal of neurochemistry 142, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM, 2015. Evidence for brain glial activation in chronic pain patients. Brain : a journal of neurology 138, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Gonzalez I, Izquierdo-Garcia D, Zurcher NR, Wilkens P, Loggia ML, Hooker JM, Rosas HD, 2018. Neuroinflammation in Huntington’s Disease: New Insights with (11)C-PBR28 PET/MRI. ACS chemical neuroscience 9, 2563–2571. [DOI] [PubMed] [Google Scholar]
- Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B, de Vries HE, 2016. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochimica et biophysica acta 1862, 461–471. [DOI] [PubMed] [Google Scholar]
- Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, Shetty AK, 2019a. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain, Behavior, and Immunity 81, 430–443. [DOI] [PubMed] [Google Scholar]
- Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, Shetty AK, 2019b. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav Immun 81, 430–443. [DOI] [PubMed] [Google Scholar]
- Maier SF, 2003. Bi-directional immune–brain communication: implications for understanding stress, pain, and cognition. Brain, behavior, and immunity 17, 69–85. [DOI] [PubMed] [Google Scholar]
- Martin A, Boisgard R, Theze B, Van Camp N, Kuhnast B, Damont A, Kassiou M, Dolle F, Tavitian B, 2010. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30, 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule AL, Janulewicz PA, Sullivan KA, Krengel MH, Yee MK, McClean M, White RF, 2018. Meta-analysis of self-reported health symptoms in 1990–1991 Gulf War and Gulf War-era veterans. BMJ open 8, e016086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon PM, Nasrallah HA, Reeves RR, Ali JA, 2004. Hippocampal dysfunction in Gulf War Syndrome. A proton MR spectroscopy study. Brain research 1009, 189–194. [DOI] [PubMed] [Google Scholar]
- Narayan N, Owen DR, Mandhair H, Smyth E, Carlucci F, Saleem A, Gunn RN, Rabiner EA, Wells L, Dakin SG, Sabokbar A, Taylor PC, 2018. Translocator Protein as an Imaging Marker of Macrophage and Stromal Activation in Rheumatoid Arthritis Pannus. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 59, 1125–1132. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM, 2015. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. Journal of neurochemistry 133, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB, 2019. Neuroinflammation disorders exacerbated by environmental stressors. Metabolism: clinical and experimental 100s, 153951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kusky JR, Nasir J, Cicchetti F, Parent A, Hayden MR, 1999. Neuronal degeneration in the basal ganglia and loss of pallido-subthalamic synapses in mice with targeted disruption of the Huntington’s disease gene. Brain research 818, 468–479. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C, Weiner MW, Neylan TC, 2015. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 51, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo JO, Abdullah L, Evans J, Reed JM, Montague H, Mullan MJ, Crawford FC, 2014. Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 34, 109–127. [DOI] [PubMed] [Google Scholar]
- Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, Gunn RN, Rabiner EA, Wilkins MR, Reynolds R, Matthews PM, Parker CA, 2010. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30, 1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP, 2012. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 32, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganoni S, Alshikho MJ, Zurcher NR, Cernasov P, Babu S, Loggia ML, Chan J, Chonde DB, Garcia DI, Catana C, Mainero C, Rosen BR, Cudkowicz ME, Hooker JM, Atassi N, 2018. Imaging of glia activation in people with primary lateral sclerosis. NeuroImage. Clinical 17, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, 2002. TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol 174, 193–200. [DOI] [PubMed] [Google Scholar]
- Parbo P, Ismail R, Hansen KV, Amidi A, Marup FH, Gottrup H, Braendgaard H, Eriksson BO, Eskildsen SF, Lund TE, Tietze A, Edison P, Pavese N, Stokholm MG, Borghammer P, Hinz R, Aanerud J, Brooks DJ, 2017. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain : a journal of neurology 140, 2002–2011. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, 2014. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiological reviews 94, 1077–1098. [DOI] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U, Pekna M, 2014. The dual role of astrocyte activation and reactive gliosis. Neuroscience letters 565, 30–38. [DOI] [PubMed] [Google Scholar]
- Perry V, Hume DA, Gordon S, 1985. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15, 313–326. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT, 2007. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA, 2012. Innate immunity in the central nervous system. The Journal of Clinical Investigation 122, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, Adewuyi O, VanMeter JW, Baraniuk JN, 2013a. Exercise Challenge in Gulf War Illness Reveals Two Subgroups with Altered Brain Structure and Function. PLOS ONE 8, e63903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Timbol CR, Adewuyi O, Walitt B, VanMeter JW, Baraniuk JN, 2013b. Increased Brain White Matter Axial Diffusivity Associated with Fatigue, Pain and Hyperalgesia in Gulf War Illness. PLOS ONE 8, e58493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, Machado-Vieira R, Yuan P, Niciu MJ, Lyoo CH, Henter ID, Salvadore G, Drevets WC, Kolb H, Innis RB, Zarate CA Jr, 2018. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Research 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas S, Martin A, Arranz MJ, Pareto D, Purroy J, Verdaguer E, Llop J, Gomez V, Gispert JD, Millan O, Chamorro A, Planas AM, 2007. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 27, 1975–1986. [DOI] [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M, 2006. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiological reviews 86, 1309–1379. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW, 1995. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of neurology 38, 73–84. [DOI] [PubMed] [Google Scholar]
- Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL, 1989. A4 Protein in Alzheimer’s Disease: Primary and Secondary Cellular Events in Extracellular Amyloid Deposition. Journal of Neuropathology & Experimental Neurology 48, 674–691. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M, 2010. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature reviews. Drug discovery 9, 971–988. [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Connor KC, Huang Y, Carson RE, Hannestad J, Cosgrove KP, 2015. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proceedings of the National Academy of Sciences 112, 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH, 2015. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, 2000. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. American journal of epidemiology 152, 992–1002. [DOI] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR, 2012. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environmental health perspectives 120, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Bradford W, Stone C, Thompson TA, Heeren T, White RF, 2018. Neuropsychological functioning in military pesticide applicators from the Gulf War: Effects on information processing speed, attention and visual memory. Neurotoxicology and Teratology 65, 1–13. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Proctor SP, Devine S, Heeren T, White RF, 2003. Cognitive Functioning in Treatment-Seeking Gulf War Veterans: Pyridostigmine Bromide Use and PTSD. Journal of Psychopathology and Behavioral Assessment 25, 95–103. [Google Scholar]
- Tamm S, Cervenka S, Forsberg A, Estelius J, Grunewald J, Gyllfors P, Karshikoff B, Kosek E, Lampa J, Lensmar C, Strand V, Åkerstedt T, Halldin C, Ingvar M, Olgart Höglund C, Lekander M, 2018. Evidence of fatigue, disordered sleep and peripheral inflammation, but not increased brain TSPO expression, in seasonal allergy: A [11C]PBR28 PET study. Brain, Behavior, and Immunity 68, 146–157. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF, 2007a. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain, behavior, and immunity 21, 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF, 2007b. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain, behavior, and immunity 21, 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X-H, Wei X, Chen F-Y, Zang Y, Xin W-J, Pang R-P, Chen Y, Wang J, Li Y-Y, Shen K-F, 2013. The upregulation of translocator protein (18 kDa) promotes recovery from neuropathic pain in rats. Journal of Neuroscience 33, 1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex; a journal devoted to the study of the nervous system and behavior 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, 2011. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. The Journal of rheumatology 38, 1113–1122. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Michael Franklin C, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, John Reynolds W, Romano TJ, Jon Russell I, Sheon RP, 1990. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis & Rheumatism 33, 160–172. [DOI] [PubMed] [Google Scholar]
- Xanthos DN, Sandkühler J, 2013. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nature Reviews Neuroscience 15, 43. [DOI] [PubMed] [Google Scholar]
- Zelova H, Hosek J, 2013. TNF-alpha signalling and inflammation: interactions between old acquaintances. Inflammation research : official journal of the European Histamine Research Society … [et al. ] 62, 641–651. [DOI] [PubMed] [Google Scholar]
- Zhu B, Dong Y, Xu Z, Gompf HS, Ward SAP, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z, 2012. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiology of disease 48, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, Akeju O, Catana C, Rosen BR, Cudkowicz ME, Hooker JM, Atassi N, 2015. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. NeuroImage. Clinical 7, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Correlation between [11C]PBR28 distribution volume ratios (DVR) and [11C]PBR28 standardized volume uptake (SUVR) within clusters from GWI vs HC group analysis.
Supplementary figure 2: Voxel-wise group difference in [11C]PBR28 standardized volume uptake (SUVR). Surface projection maps displaying areas with significantly elevated [11C]PBR28 SUVR in GWI (n=15) compared to HC (n=33), in voxel-wise analyses, data adjusted for sex, genotype and injected dose.
Supplementary figure 3: Voxel-wise group difference in [11C]PBR28 standardized volume uptake (SUVR). Volumetric maps displaying areas with significantly elevated [11C]PBR28 SUVR in GWI patients (n=15) compared to HC (n=33), in voxel-wise analyses, data adjusted for sex and genotype.


