Abstract
Objectives:
To assess prevalence of Personal Protective Equipment (PPE)-related symptoms and adverse reactions during Coronavirus Disease 2019 pandemics.
Methods:
We conducted an observational study among people exposed to various degree of infectious risk. Data were collected with a self-administered online questionnaire.
Results:
The entire cohort complained about a wide range of adverse reactions: respiratory symptoms affected 80.3% of respondents, 68.5% referred pressure-related skin lesions, fewer manifested a dermatosis of different grade or ocular symptoms. Most of the affected individuals belonged to healthcare staff and manifestations were predicted by wearing time (more than 6 h/d). Moreover, symptoms were higher in the healthcare staff wearing N95/FFP2 respirator mask.
Conclusions:
Given the crucial role of PPE to contain the pandemic infection, more attention has to be paid to exposed categories, establishing preventive measure of side effects to ensure total safety.
Keywords: adverse reactions, Covid19, healthcare workers, mask, personal protective equipment, respiratory symptoms, skin injuries
Since December 2019, Coronavirus disease, widely known as COVID-19, has broken out and rapidly spread from Wuhan, China.1 On February 20, 2020, the first patient was diagnosed with COVID-19 in Italy and developed respiratory failure leading to intensive care unit (ICU) admission in Lombardy, the most relevant international business, fashion and design connecting hub of northern Italy. Given the raising number and large diffusion of infection outside China, on March 11, 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a pandemic.2 As of November 12, 2020, 1,066,401 positive Italian cases of coronavirus have been recorded, including 387,758 people who fully recovered and 43,589 people dead, making Italy the tenth country in the world by number of total cases, after the United States of America, India, Brazil, France, Russia, Spain, United Kingdom, Argentina, Colombia, and Spain, and the sixth worldwide by number of deaths.3
At the beginning of this pandemic the emergent infection burden shocked not only the Health System but the entire society, from children to elderly people, involving daily life of all working fields. In fact, on March 7 Italian government imposed national quarantine and released numerous strict ordinances to regulate travels, activities, and personal relations, followed by similar restrictions dictated by Governments in each continent. Unfortunately, after a first decrease of infection spreading during summertime on Italian national territory, the new increase in positive cases’ numbers registered from September 2020, is suggesting a new pandemic's wave and further upcoming restrictions on daily life.
Due to airborne diffusion, social distancing and use of personal protective equipment (PPE) have been first highly suggested among Italian general population, becoming mandatory on October 7, both out- and in-doors, to prevent virus spreading.4 On the other hand, healthcare workers based in high-risk environments such as ICUs, isolation wards, emergency rooms, operating theatres, and general medical wards have been obliged to wear PPE and encouraged to have a strict hygiene of hands, staff, and working places.
While these latter measures have been intended to prevent risk of infection, they have caused some symptoms and adverse reactions, related to the extended period of use, as already reported in the previous SARS epidemic in 2002.5
Furthermore, the sudden outbreak of COVID-19 has led to widespread fear and general confusion on type and way of use of different PPE, with consequent misuse of the already limited protective equipment. In fact, according to the basic economic law of demand and supply, Italian population witnessed a dramatic increase of prices of surgical masks, respirator masks, hygiene products, etc due to the sharp demand's increase. However, this expensive equipment ended up being acquired by general population (with low-risk exposure) while unavailable for healthcare high risk categories. Unfortunately, PPE misuse has been very common, as documented by Italian newspapers and newscasts and by world scientific literature (eg, people wearing masks without covering the nose, reusing masks or gloves, wearing googles or face shield since experiencing fear of contagion even if not recommended…).6,7 Moreover, healthcare workers had to deal with PPE shortage, for example, being obliged to use FFP2 masks for more than 6 to 8 hours (in discord with productors’ instructions) or, even worse, reuse the same mask more than once.8 This scenario has led World Health Organization to diffuse recommendations for optimizing the availability of PPE.9
This study goes to focus on facial PPE use and aim to determine the prevalence of eventual related symptoms and adverse reactions in general population, and particularly in healthcare workers, during COVID-19 pandemic outbreak in Lombardy, Italy's most affected region.
MATERIAL AND METHODS
The study was performed among a sample of general population living in Lombardy, a region in the North of Italy. Population of study was randomly reached by email/phone numbers collected from outpatients’ database and healthcare workers. Questionnaires were sent to a total of 600 individuals since, with an expected a participation rate of 50%, this would lead to 300 participants that in turn would be sufficient to provide a 80% power to detect a odds ratio (OR) of 2, at a two-sided significance level of α = 0.05, between the two groups assuming a 30% of unexposed would present the symptoms.
The period of study coincided with the first wave of this pandemic in the Italian territory. At that time (from March to May 2020) Italy has been living imposition of quarantine for most of the population, with the exclusion of healthcare workers and people working in the basic goods’ production and distribution field (eg, pharmacy, foodstuffs, essential products for personal and environmental hygiene…). However, most of the Italians have spent almost all day at home (eg, smart-workers, students, and teachers involved in distance learning…) limiting out-doors activities to emergent occasions or supplying of first necessity goods.
In that period facial coverings have been highly suggested among general population, during quarantine restricted time spent out of the house, while mandatory in the healthcare system.
Population was interviewed on general data (sex, age), current position, and occupation. We determined the subgroups according to professional exposure to other people: health-care workers with high risk infection exposure (including doctors, nurses, and healthcare assistants); workers with high public exposure and people working by themselves or with low public contact.10
Anamnesis on medical history, current therapies, and allergies was collected. The entire cohort was asked about facial PPE wore including types of devices, wearing time per day, and per week. We collected data of self-perceived PPE-related respiratory, ocular and cutaneous facial symptoms (types, anatomical locations, severity, and onset), whether actions were taken by the PPE users to address the symptoms reported (Yes/No) and what preventive measures adopted (multiple choice). We disseminated the online self-administered questionnaire from April 20 to May 4, 2020, date when the imposed Italian National quarantine, carried out to contain the growing of infection, was converted in a less restricted phase II. Participants voluntarily used their cellphone to answer and submit the questionnaire response online within the 2 weeks. Questions were formulated with single or multiple-choice responses and Likert scale to assess severity of symptoms, allowing quick and accurate answers. We provided space for comments in selected cases. Inclusion criteria were age more than or equal to 18 years, regardless of sex and ethnicity, voluntary participation.
Distribution of continuous data was tested with the Shapiro-Wilk test. Normally distributed variables were expressed as mean ± standard deviation (SD). Categorical variables were reported as absolute values and corresponding percentages. Continuous variables were then compared using an independent-sample Student t test; categorical variables were compared with Chi-square test or Fisher exact test, when appropriate.
Linear logistic regression analysis was used to determine significant predictors of symptoms development. Variables with a univariate statistical significance of less than 0.05 were selected for inclusion into the multivariable model. Multivariate analysis, using stepwise forward selection, was finally performed to analyze the association of baseline characteristics with study endpoints, expressed as OR with 95% confidence interval (CI) and P values. All statistical tests were two-sided, and P values <0.05 were considered statistically significant. The Statistical analyses were performed using SPSS software version 25.0.0 (SPSS Inc., Chicago, IL). Graphs were performed using Prism version 8.0 (GraphPad).
Informed consent for data analysis and published images was provided by participants.
RESULTS
Among the 600 surveys that were distributed, 391 people responded (response rate more than 65.1%) and, among these, 381 gave their consent to data treatment and analysis. Over the entire cohort 126 were men (33.1%) while 255 were women (66.9%). Mean age was 35.0 ± 11.7 years. Respondents were distributed according to professional employment as following: 185 healthcare workers, 31 people with high public contact job, 165 with low public exposure (including students, smart workers, unemployed people, pensioners). Data about smoking habits, comorbidities, allergies and chronic therapy were collected; the three subgroups resulted homogeneous according to these characteristics as reported in Table S1.
All participants were interviewed about type of facial PPE used in daily life, according to their personal and job-related necessity (data shown in Table S2). Most of the population usually wore more than one type of PPE. Surgical mask was the most widespread device accounting on 72.4% of the entire cohort with an expected significant prevalence in healthcare workers (83.2% of the latter subgroup vs 58.1% in high exposure workers and 63.0% in low contact cohort, P < 0.001). Conversely, cotton/artisanal masks were prevalently used by low public exposure people (32.1%, P < 0.001). These data are in line with Italian government indication of use of “facial coverings” (not otherwise specified) among general population, in order to prevent virus spreading. Among respirator masks, the most commonly used was foldable without respiratory valve (36.2% of the respondents). High percentage of healthcare personnel wore masks combined to caps, goggles, and/or face shields (respectively 47.6%, 36.8%, 25.4%) while were nearly absent in people working outside the hospital (P < 0.001), as expected. As far as the wearing time, most of the entire cohort (37.5%) wore PPE from 3 to 6 days per week and 35.4% had a continuative use for more than 6 h/d. Obviously healthcare staff significantly represented the subclass of longer adopters of PPE (P < 0.001).
Onset of PPE-related symptoms was investigated among all three subgroups. More than 80% of respondents reported nasal symptoms such as obstruction or dyspnea, dry nose or crusting, sneezing or runny nose, and nasal itching. Two-hundreds and two people over the entire cohort complained about cutaneous symptoms (facial itching, skin rash or dermatitis, increased pore size or acne) as shown in Fig. 1, while pressure related symptoms accounted for 68.5% (nasal, facial or auricular pain, redness or decubitus lesions at zygoma, forehead, nose bridge, nasal dysmorphism) shown in Fig. 2. Ocular symptoms affected 133 people, while head itching occurred in 27.8% overall. Fifty-three respondents reported worsening of previously experienced allergic symptoms. Interestingly, more than 35% of respondents reported worsening of mood tone while nearly 10% complained about PPE related panic attacks. Most of the aforementioned symptoms got resolved in less than 1 hour. However, still 60.9% of the entire cohort claimed that reported symptoms did not prejudice their use or wearing time of PPE. Of note, nearly all symptoms showed a statistically significant difference in occurrence in health-care cohort over the other two subgroups (Table 1).
FIGURE 1.
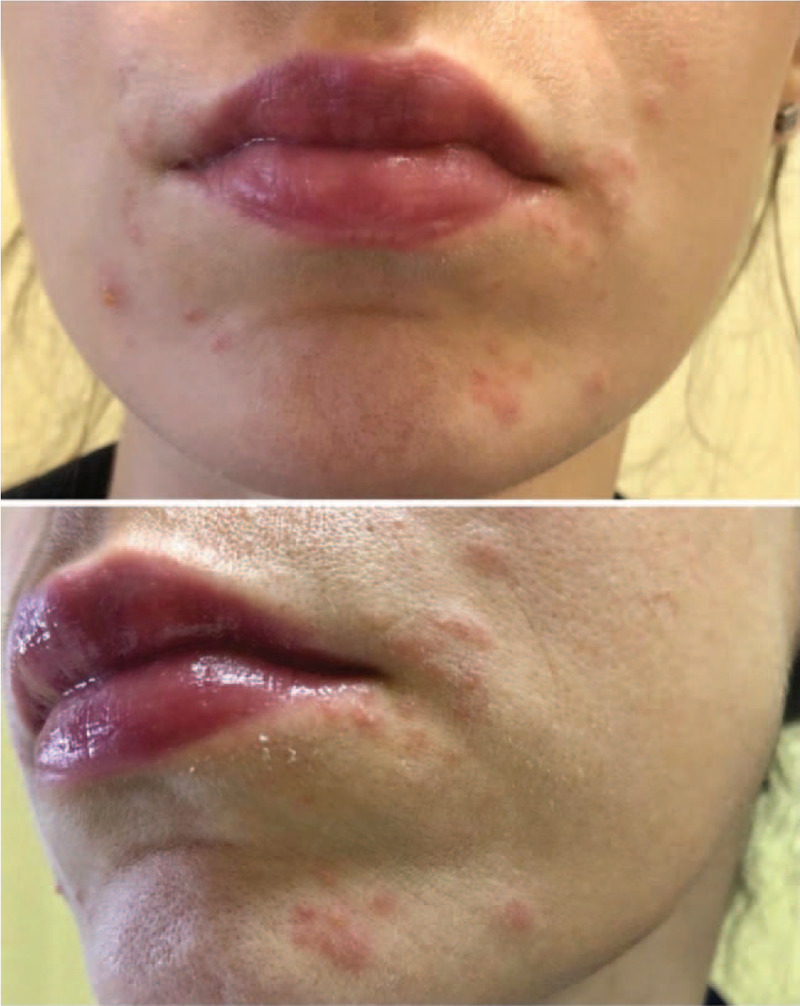
Facial dermatosis characterized by erythema and pustules corresponding to cheeks and chin skin usually covered by respirator mask.
FIGURE 2.
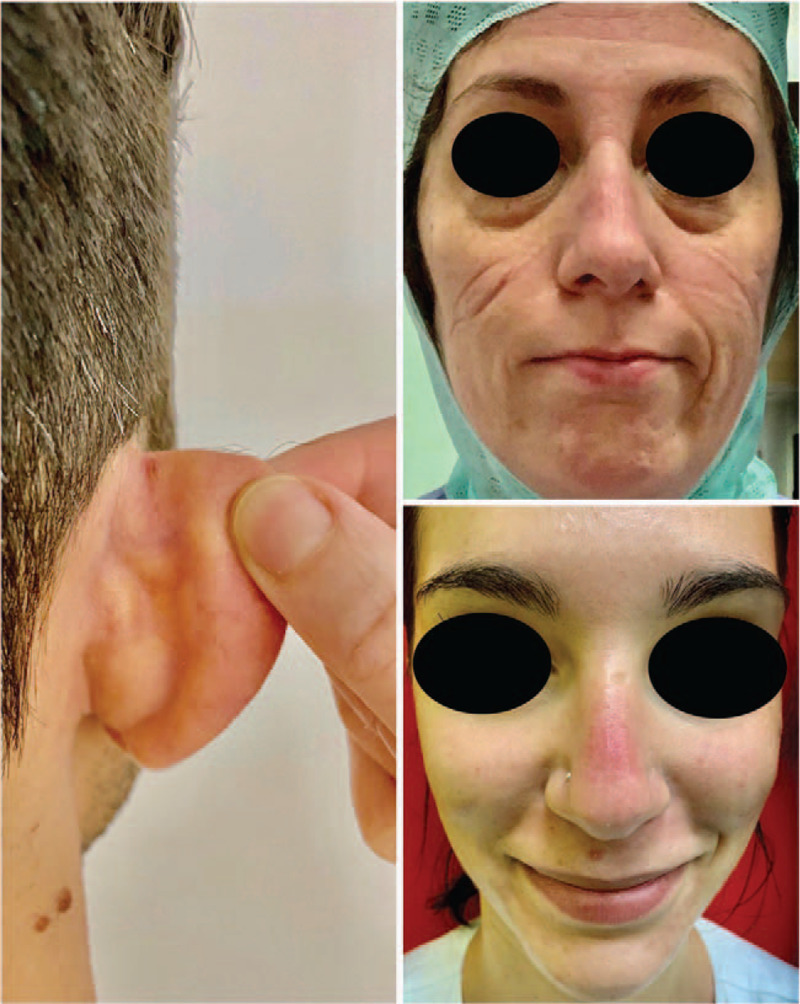
Device-related pressure lesions on auricular, zygoma, and nose bridge associated with use of facial personal protective equipment.
TABLE 1.
PPE-Related Adverse Reaction in Entire Study Cohort and the Three Subgroups Identified According to Professional Risk Exposure
| Entire Study Cohort (n = 381) | Healthcare Staff (n = 185) | Public Exposed Work (n = 31) | Low External Exposure (n = 165) | P Value | |
| Symptoms, n (%) | |||||
| Nasal symptoms (one or more of the following) | 306 (80.3) | 168 (90.8) | 28 (90.3) | 110 (66.7) | <0.001 |
| Nasal obstruction/dyspnea | 248 (65.1) | 136 (73.5) | 22 (71.0) | 90 (54.5) | 0.001 |
| Dry nose/Crusting | 161 (42.3) | 108 (58.4) | 12 (38.7) | 41 (24.8) | <0.001 |
| Sneezing/Runny nose | 152 (39.9) | 92 (49.7) | 11 (35.5) | 49 (29.7) | 0.001 |
| Itchy nose | 200 (52.5) | 118 (63.8) | 13 (41.9) | 69 (41.8) | <0.001 |
| Skin symptoms (one or more of the following) | 202 (53.0) | 121 (65.4) | 16 (51.6) | 65 (39.4) | <0.001 |
| Skin itching | 156 (40.9) | 86 (46.5) | 12 (38.7) | 58 (35.2) | 0.095 |
| Skin rash/Dermatitis | 77 (20.2) | 54 (29.2) | 6 (19.4) | 17 (10.3) | <0.001 |
| Acne/Increased pore size | 105 (27.6) | 79 (42.7) | 10 (32.3) | 16 (9.7) | <0.001 |
| Pressure-related symptoms (one or more of the following) | 261 (68.5) | 158 (85.4) | 21 (67.7) | 82 (49.7) | <0.001 |
| Nasal/facial pain | 155 (40.7) | 93 (50.3) | 15 (48.4) | 47 (28.5) | <0.001 |
| Redness zygoma/forehead | 158 (41.5) | 101 (54.6) | 11 (35.5) | 46 (27.9) | <0.001 |
| Decubitus lesions at zygoma/forehead | 54 (14.2) | 36 (19.5) | 5 (16.1) | 13 (7.9) | 0.008 |
| Redness nosebridge | 180 (47.2) | 114 (61.6) | 13 (41.9) | 53 (32.1) | <0.001 |
| Decubitus lesions at nosebridge | 50 (13.1) | 39 (21.1) | 4 (12.9) | 7 (4.2) | <0.001 |
| Nasal dysmorphism | 46 (12.1) | 26 (14.1) | 4 (12.9) | 16 (9.7) | 0.453 |
| Auricular pain | 166 (43.6) | 121 (65.4) | 10 (32.3) | 35 (21.2) | <0.001 |
| Ocular symptoms | 133 (34.9) | 83 (44.9) | 11 (35.5) | 39 (23.6) | <0.001 |
| Head itching | 106 (27.8) | 73 (39.5) | 6 (19.4) | 27 (16.4) | <0.001 |
| Worsening of allergy | 53 (13.9) | 36 (19.5) | 6 (19.4) | 11 (6.7) | 0.002 |
| Mood deflection | 135 (35.4) | 75 (40.5) | 10 (32.3) | 50 (30.3) | 0.126 |
| Panic attack | 37 (9.7) | 17 (9.2) | 3 (9.7) | 17 (10.3) | 0.940 |
Data are presented as n (%); bold is used to highlight significant P value (<0.05).
Focusing on the medical staff, all symptoms were significantly correlated with daily wearing time of PPE (Table S3–S4). Of note, wearing time more than 6 h/d has shown to highly predict occurrence of respiratory (OR 5.474, CI 95% 2.768 to 10.823, P < 0.001), cutaneous (OR 2.651, CI 95% 1.728 to 4.066, P < 0.001), ocular (OR 1.994, CI 95% 1.271 to 3.128, P = 0.003), and device-related pressure symptoms (OR 5.918, CI 95% 3.176 to 11.026, P < 0.001) in multivariate analysis (Fig. 3). No differences were shown on nasal and skin symptoms complain according to type of mask worn. However, as expected, N95/FFP2 mask have demonstrated to be a strong predictor of pressure related symptoms, with a four-folds increase of manifestation's risk (OR 3.887, CI 95% 2.301 to 6.566, P < 0.001) in multivariate analysis (Fig. 3). Accordingly, its own distinguishing feature of strict adherence to facial skin and skeleton explains the subsequent appearance of wide range of injuries from simple pain to redness or more severe device-related pressure ulcers (DRPUs), as reported in Table S5. Use of surgical cap had a statistically significant correlation with head itching, while demonstrated only weaker correlation with pressure related injuries, as done by goggles or face shield (Table S6-S7). Conversely, despite the prevalence of around 45% of ocular symptoms in health-care staff they are not significantly correlated with the use of goggles or face shield (Table S6).
FIGURE 3.
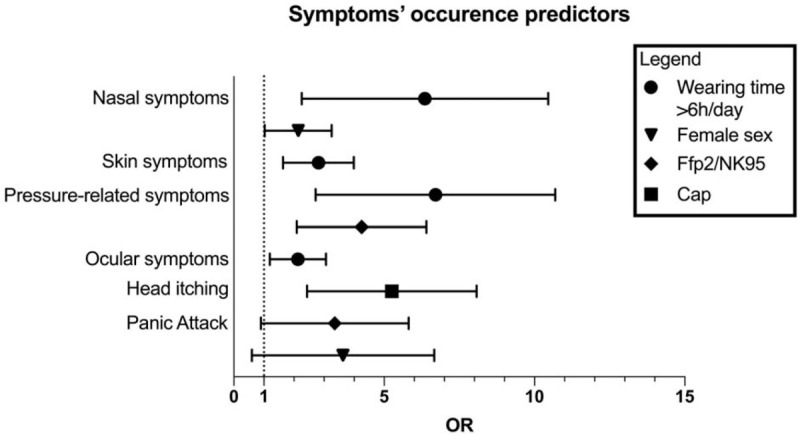
Multivariate analysis on symptoms predictors in the entire cohort.
We investigated the preventive measures adopted by respondents in light of the experienced symptoms (Table 2): 37.5% of the entire cohort declared frequent cleansing or facial peeling, while 28.4% adopted application of emollient ointments. Fifty people wore facial dressing to relieve pain or lesions on areas subjected to high pressure due to PPE. Of note, only 18 people felt concerned about manifestations related to PPE to justify the need of specialistic medical consultation (otolaryngologist, ophthalmologist, dermatologist, psychiatric, esthetical specialist).
TABLE 2.
Self-adopted Preventive Measures and Need of Medical Specialistic Consultation by General Population for PPE-Related Symptoms
| Entire Study Cohort (n = 381) | ||
| Self-adopted preventive measures, n (%) | ||
| Strips or facial dressing | 50 (13.1) | |
| Healing ointment | 27 (7.1) | |
| Emollient ointment | 108 (28.3) | |
| Frequent cleansing/facial peeling | 125 (37.5) | |
| Eye drops | 56 (14.7) | |
| Nasal rinse | 74 (19.4) | |
| Nasal ointment/spray | 39 (10.2) | |
| Painkillers | 13 (3.4) | |
| Need of medical specialistic consultation, n (%) (one or more of the following) | 18 (4.7) | |
| ENT visit | 9 (2.4) | |
| Ophthalmology visit | 3 (0.8) | |
| Dermatologic visit | 4 (1.0) | |
| Aesthetical visit | 4 (1.0) | |
| Psichological visit | 4 (1.0) | |
Data are presented as n (%).
DISCUSSION
COVID-19 outbreak has radically changed our daily life and routine, impacting on health and psychology of general population and workers directly involved in the global emergency. Pressure on Health System was intense and of paramount challenge, requiring reorganization and adaptation of departments and medical activities,11–13 establishment of criteria for hospitalization and intubation, management of communication with patients’ relatives.
Notably, since the beginning of the pandemic, health professionals have been on the frontline fighting the infection, within all settings of our healthcare system. According to Italian experience, healthcare sector has been necessarily the most involved working category, since directly engaged in assistance to COVID-19 affected patients. By November 12, 2020, 190 Italian doctors and 45 nurses died from Coronavirus disease.14 Being at risk of infection has become a constant fear to live with, along with experiencing emotional overload, often magnified by the shortage of suitable personal protective equipment, relentless work shifts, physical fatigue, reduction in human resources, and sometimes poor overall organization.15–18 Most of the time, healthcare staff worked 6 to 12 hours per day, unceasingly wearing PPE, including respirator masks, goggles, face shield, and protective gloves and gowns.
Interestingly, not all working related health afflictions are directly caused by the virus. Although crucial for personal protection against the emergent infection, use of PPE has brought a large cohort of physical and psychological symptoms, adding trouble to the daily struggle of healthcare workers.
Among all adverse reactions, the great majority of our cohort experienced nasal related symptoms, from the minor nasal dryness to the more severe subjective dyspnea. As examined in a previous study,19 this latter manifestation could be related to structural features of the face mask, highlighting a higher breathing resistance if respiratory valve is absent, in the folding mask compared with the cup type, and in nonwoven fabric masks than the cotton masks. However, these mechanical-demonstrated results do not find confirmation in our real-world population, maybe due to the psychological involvement in perceived symptom.
As far as cutaneous symptoms, we reported a wide range of manifestations from simple facial itching to more severe dermatosis. Nearly 30% of healthcare workers complained a kind of skin rash or dermatitis and these reactions are significantly associated with PPE wearing time. These data are in line with prevalence of self-reported contact dermatitis (31.5%) previously published.20–23
According to a multicenter Chinese study conducted during COVID-19 pandemic,24 nearly 42.8% of medical staff experienced at least one of the three major skin injuries: device-related pressure ulcers (DRPU), moist-associated skin damage (MASD), and skin tears. Higher prevalence was manifested in male healthcare population with an increased risk associated with sweating and wearing time. Most common anatomical sites were nose bridge, cheeks, ears, and forehead.24,25 Indeed, skin in these locations is predominantly submitted to both continuous static (strapping) and dynamic (sliding) frictional forces, caused by relatively stiff materials of face masks, googles, and shields that ident and damage the cutaneous and subcutaneous tissue. Moreover, the hot and humid microclimate created by the coverage of the facial skin with different types of PPE, along with the essential close fitting of respirator masks, are identified as causing factors of increased pore size and manifestation or exacerbation of acne.26
Of note, 68.5% of participants involved in our study suffered from pressure-related skin injuries. Among these, 40.7% complained about facial pain. It is well known that pain is expression of self-preservation, since it motivates the individual to withdraw from damaging situations, ensuring the integrity of the organism. In this perspective, pain is interpreted as first manifestation of a vicious cycle of DRPUs that, through a multistep mechanism made of tissue damage, inflammation, and ischaemia, eventually leads to tissue death.27 People wearing PPE, and particularly healthcare staff, should read pain as sign of initial tissue damage adopting measures to prevent more severe skin injuries, such as prompt removal of protective tools or application of facial dressing, as suggested by Gefen and Ousey.27 However, shortage of protective equipment, relentless work shifts combined with continuous exposure to infective risk on COVID-19 cases, has led to uninterrupted use of tight face masks and googles, provoking deep cutaneous lesions. Last but not least, these ulcers could be virus entry port, increasing risk of infection in medical staff.
CONCLUSIONS
COVID-19 pandemic has brought into sharp focus the paramount importance of personal protective equipment to prevent the widespread diffusion of this infectious disease. However, although essential to work safely, PPE have demonstrated to be potentially harmful due to their continuous and repetitive application. Implementation of PPE fitting and materials, establishment of preventive measure along with reduction of wearing time, avoiding overtime, are crucial to ensure safe and secure working condition to healthcare staff, leading to better management of emergency outbreak.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Antonio Sisinni for collaboration in statistical analysis in this study.
Footnotes
R.A.B. and M.F. contributed equally to this work.
Funding: none.
Declaration of Conflicting Interests: The authors declare that there is no conflicts of interest.
Clinical significance: COVID is an emerging infectious disease of pandemic proportions. PPE has demonstrated a double-edged sword role in transmission prevention with possible harmful side effects (dermatosis, pressure-related skin lesions, respiratory and psychological symptoms). Side effects’ preventive measures are crucial to ensure total safety in workers and general population.
Supplemental digital contents are available for this article.
REFERENCES
- 1.Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Milit Med Res 2020; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Director-General's opening remarks at the media briefing on COVID-19: 11 March 2020; 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-. Accessed March 30, 2020. [Google Scholar]
- 3. Johns Hopkins CSSE, Coronavirus COVID-19 Global Cases. Available at https://arcgis.com. Accessed November 12, 2020. [Google Scholar]
- 4. Ufficiale G. Decreto legge 7 ottobre 2020, n. 125; 2020. Available at: https://www.gazzettaufficiale.it/eli/id/2020/10/07/20G00144/sg (accessed November 13, 2020). [Google Scholar]
- 5.Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome – a descriptive study in Singapore. Contact Dermatitis 2006; 55:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobol Y, Siniver E, Yaniv G. Dishonesty and mandatory mask wearing in the COVID-19 pandemic. Econ Lett 2020; 197:109617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogoina D. Perspective piece COVID-19: The need for rational use of face masks in Nigeria. Am J Trop Med Hyg 2020; 103:33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otomaru H, Esi S, Quaye D, et al. Scenarios to Manage the Demand for N95 Respirators for Healthcare Workers During the COVID-19 Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO, World Health Organization (WHO). Rational Use of Personal Protective Equipment For Coronavirus Disease 2019 (COVID-19): Interim Guidance, 6 April 2020. 2020; 1–7. [Google Scholar]
- 10.Spinazzè A, Cattaneo A, Cavallo DM. COVID-19 outbreak in Italy: protecting worker health and the response of the Italian Industrial Hygienists Association. Ann Work Expo Health 2020; 64:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc 2020; 22:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topf MC, Shenson JA, Holsinger FC, et al. Framework for prioritizing head and neck surgery during the COVID-19 pandemic. Head Neck 2020; 42:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spina S, Marrazzo F, Migliari M, et al. The response of Milan's Emergency Medical System to the COVID-19 outbreak in Italy. Lancet 2020; 395:e49–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Italian Federation of Medicine and Dentistry [Federazione nazionale degli ordini dei medici chirurghi e degli odontoiatri (FNOMCeO)]. Available at: https://portale.fnomceo.it/elenco-dei-medici-caduti-nel-corso-dellepidemia-di-covid-19/. Accessed November 12, 2020. [Google Scholar]
- 15.Sim MR. The COVID-19 pandemic: major risks to healthcare and other workers on the front line. Occup Environ Med 2020; 77:281–282. [DOI] [PubMed] [Google Scholar]
- 16.World Heal Organ, World Health Organization. Coronavirus Disease (Covid-19) Outbreak: Rights, Roles and Responsibilities of Health Workers, Including Key Considerations for Occupational Safety. 2019; 1–3. [Google Scholar]
- 17.Mandrola J. CoViD-19 and PPE: Some of Us Will Die Because of the Shortage. Recenti Prog Med 2020; 2020:927206. [DOI] [PubMed] [Google Scholar]
- 18.Boškoski I, Gallo C, Wallace MB, et al. COVID-19 pandemic and personal protective equipment shortage: protective efficacy comparing masks and scientific methods for respirator reuse. Gastrointest Endosc 2020; 92:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao B, Wang Y, Ye X, et al. Impact of structural features on dynamic breathing resistance of healthcare face mask. Sci Total Environ 2019; 689:743–753. [DOI] [PubMed] [Google Scholar]
- 20.Mekonnen TH, Yenealem DG, Tolosa BM. Self-report occupational-related contact dermatitis: prevalence and risk factors among healthcare workers in Gondar town, Northwest Ethiopia, 2018—a cross-sectional study. Environ Health Prev Med 2019; 24:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizushima N. Autophagy: process and function. Genes Dev 2007; 21:2861–2873. [DOI] [PubMed] [Google Scholar]
- 22.Lin P, Zhu S, Huang Y, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol 2020; 183:190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus 19 disease (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatology Venereol 2020; 34:e353–e354. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Q, Liu Y, Wei W, et al. The prevalence, characteristics, and related factors of pressure injury in medical staff wearing personal protective equipment against COVID-19 in China: a multicentre cross-sectional survey. Int Wound J 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol 2020; 82:1215–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan KT, Greaves MW. N95 acne. Int J Dermatol 2004; 43:522–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gefen A, Ousey K. Update to device-related pressure ulcers: SECURE prevention. COVID-19, face masks and skin damage. J Wound Care 2020; 29:245–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


