Supplemental Digital Content is available in the text.
Keywords: COVID-19, respiratory distress syndrome, troponin
Abstract
Background:
Knowledge gaps remain in the epidemiology and clinical implications of myocardial injury in coronavirus disease 2019 (COVID-19). We aimed to determine the prevalence and outcomes of myocardial injury in severe COVID-19 compared with acute respiratory distress syndrome (ARDS) unrelated to COVID-19.
Methods:
We included intubated patients with COVID-19 from 5 hospitals between March 15 and June 11, 2020, with troponin levels assessed. We compared them with patients from a cohort study of myocardial injury in ARDS and performed survival analysis with primary outcome of in-hospital death associated with myocardial injury. In addition, we performed linear regression to identify clinical factors associated with myocardial injury in COVID-19.
Results:
Of 243 intubated patients with COVID-19, 51% had troponin levels above the upper limit of normal. Chronic kidney disease, lactate, ferritin, and fibrinogen were associated with myocardial injury. Mortality was 22.7% among patients with COVID-19 with troponin under the upper limit of normal and 61.5% for those with troponin levels >10 times the upper limit of normal (P<0.001). The association of myocardial injury with mortality was not statistically significant after adjusting for age, sex, and multisystem organ dysfunction. Compared with patients with ARDS without COVID-19, patients with COVID-19 were older and had higher creatinine levels and less favorable vital signs. After adjustment, COVID-19–related ARDS was associated with lower odds of myocardial injury compared with non–COVID-19–related ARDS (odds ratio, 0.55 [95% CI, 0.36–0.84]; P=0.005).
Conclusions:
Myocardial injury in severe COVID-19 is a function of baseline comorbidities, advanced age, and multisystem organ dysfunction, similar to traditional ARDS. The adverse prognosis of myocardial injury in COVID-19 relates largely to multisystem organ involvement and critical illness.
Clinical Perspective.
What Is New?
Half of intubated patients with coronavirus disease 2019 (COVID-19) manifest myocardial injury, but mortality risk associated with myocardial injury is attenuated after adjustment for degree of critical illness.
Myocardial injury is less common in COVID-19 compared with conventional acute respiratory distress syndrome after adjusting for confounders of age, renal dysfunction, and degree of critical illness.
What Are the Clinical Implications?
Myocardial injury in COVID-19 is reflective of baseline risk and comorbidities and underlying multisystem organ dysfunction.
Most myocardial injury in COVID-19 is related to critical illness, but given isolated reports of frank myocarditis and other severe direct cardiac manifestations, it is important to identify these rare and distinct manifestations.
Myocardial injury manifested by elevations in cardiac troponin is common in patients with coronavirus disease 2019 (COVID-19).1–3 Myocardial injury has also been proposed as a prognostic factor.4,5 The pathogenesis of myocardial injury in COVID-19 is not established but is likely multifactorial, involving patient-, disease-, and treatment-specific factors.6 Important knowledge gaps remain in understanding the epidemiology and clinical implications of myocardial injury in COVID-19.
First, although crude mortality rates are higher in patients with COVID-19 with myocardial injury compared with those without, variable covariate adjustment has been performed in studies to date.2,4,7–12 The dictum that a single marker of myocardial injury is independently prognostic in severe COVID-19 should be investigated with comparative studies. Second, the determinants of myocardial injury in COVID-19 are not well-established. A conceptual model of COVID-19 myocardial injury includes systemic inflammation, hypoxemia, vasopressor requirement, and thrombophilia, which remain to be clarified.6 Third, it is not clear whether the incidence of myocardial injury in hospitalized patients with COVID-19 is truly higher than that observed in traditional acute respiratory distress syndrome (ARDS). ARDS is one of the most common causes of hypoxemic respiratory failure13 and manifests as acute hypoxemia within a week of a known pulmonary insult with bilateral pulmonary infiltrates not referable to alternate causes such as atelectasis or left heart failure.14 Up to 38% of patients with ARDS have been shown to have troponin levels above the 99th percentile cut point.15 Understanding whether the prevalence and pattern of myocardial injury in COVID-19 differ from ARDS unrelated to COVID-19 is important in defining the COVID-19 clinical phenotype, particularly given the ongoing debate as to whether COVID-19–related respiratory failure represents typical ARDS or not.16
We performed a retrospective cohort study of clinical factors and outcomes associated with myocardial injury in hospitalized patients critically ill with COVID-19 and a comparative study of myocardial injury within a COVID-19 cohort to myocardial injury within a cohort of patients with ARDS unrelated to COVID-19. We hypothesized that myocardial injury would be present in a significant number of patients with COVID-19 and that after adjusting for degree of critical illness, the association of myocardial injury with mortality in COVID-19 would be mitigated. We also hypothesized that the prevalence and prognostic value of troponin in COVID-19 would be similar to that of general ARDS after covariate adjustment.
Methods
Study Population
Data for patients with COVID-19 who required intubation were obtained from JH-CROWN (the Johns Hopkins Health System COVID-19 Precision Medicine Analytic Platform Registry). This registry aggregates electronic health data for all patients with COVID-19 across the 5 hospitals in the Johns Hopkins Healthcare System. We included all patients with confirmed COVID-19 who were intubated within our health system between March 15 and June 11, 2020, and who had troponin levels assessed within 24 hours of intubation. We focused our analysis only on intubated patients because of a priori scientific interest, because of high patient risk, and to facilitate a comparison of myocardial injury and outcomes with a previous cohort of intubated patients with ARDS.
For comparison with patients with ARDS without COVID-19, we used a subset of patients from a previous cohort study to assess myocardial injury in ARDS15,17 (MI-ARDS [Myocardial Injury in Acute Respiratory Distress Syndrome Study]). This cohort consists of patients with ARDS who were enrolled in clinical trials by the ARDS Network. The initial MI-ARDS consisted of patients with ARDS attributable to diverse causes including sepsis, trauma, transfusions, and pneumonia. To enable a comparison of diseases with similar pathophysiology, we chose to use only patients with ARDS who had ARDS attributable to pneumonia. We previously measured troponin using plasma taken within 24 hours of intubation in all patients.15,17 Clinical and demographic data and outcomes were collected as part of trial protocols.18,19 For this comparison, we used 506 patients with ARDS attributable to primary pneumonia. We chose to use primary pneumonia ARDS as the comparison group because of the inherent heterogeneity of the ARDS syndrome16,20 and because patients with COVID-19 manifest primary hypoxemic respiratory failure as an indication for intubation.21 The inclusion criteria and ARDS definition for patients in the MI-ARDS study included a ratio of partial pressure of oxygen (Pao2) to fraction of inspired oxygen (Fio2) <300, bilateral pulmonary infiltrates, and no clinical evidence of elevated left atrial pressure.
The study was approved by the Johns Hopkins University Institutional Review Board (approval number IRB00251735, committee IRB-3) as exempt from review because of the anonymized nature of the JH-CROWN registry. The data that support the findings of this study are available from the corresponding author on reasonable request.
Troponin Classification
All included patients in both cohorts had troponin levels assessed within 24 hours before or after intubation to ensure uniform temporality of the exposure variable. The 5 hospitals contributing data to the JH-CROWN registry use either clinical troponin T or troponin I assays. For patients with multiple troponin measurements within the 24 hours surrounding intubation, we used the highest value. To enable pooling of the cohort for analysis, we classified troponin as a categorical variable. Troponin was classified as less than upper limit of normal (ULN) for each assay, between 1 and 5 times ULN, between 5 and 10 times ULN, and >10 times ULN. The ULN for each assay is displayed in Table I in the Data Supplement. The distribution of patients within each clinical category was similar among the patients with troponin T and troponin I and similar when troponin was considered in quartiles. This categorization is analogous to the predetermined classification scheme in MI-ARDS. For troponin measurements in the ARDS population, a highly sensitive troponin I assay was used (Abbott ARCHITECT), which allows for detection of circulating troponin to a limit of detection of 2 ng/dL; the assays used in the patients with COVID-19 were clinical assays that were not highly sensitive (Table I in the Data Supplement). To enable comparison with the clinical troponin assays used in the patients with COVID-19, we categorized troponin I as <26 ng/L (corresponding to 99th percentile of a healthy reference population), between 26 and 130 ng/L, between 130 and 260 ng/L, and >260 ng/L. We also considered troponin as a binary variable in both cohorts as positive versus negative, with negative being below ULN for the patients with COVID-19 and <26 ng/L for the ARDS cohort. Troponin I and troponin T were considered as log-transformed continuous variables in separate linear regression models in the COVID-19 group to determine clinical characteristics associated with myocardial injury. For patients with troponin below ULN, we set values to halfway between 0 and ULN.
Study Outcomes and Covariates
The primary outcome for both groups was in-hospital mortality. Follow-up was complete for all patients. Covariates of interest included demographic and clinical information, laboratory values, inflammatory biomarkers, and ventilator parameters. The registry includes Elixhauser comorbidities22 generated using International Classification of Diseases–10 codes to identify previous medical history and chronic medical problems. Laboratory data were set to the worst value within the 24 hours before or after intubation. Ventilator data were set to the worst value within 24 hours after intubation. A secondary outcome of ventilator-free days was calculated as the number of days free of mechanical ventilation within the first 28 days after intubation, by convention.15
Statistical Analysis
Missing Data
Because data in the JH-CROWN registry were drawn from the electronic medical record, data were not complete for all covariates, as shown in Table II in the Data Supplement. Data were complete for the exposure of troponin assessment, the outcome of in-hospital death, demographics, and comorbidities. To address missing data, we performed multiple imputation to obtain unbiased estimates of the association between myocardial injury and outcome. Multiple imputation was performed using chained equations and 50 imputations.23 The variables that were complete were used as auxiliary variables. We used the “mi estimate” command in Stata, which combines the multiply imputed data sets using the Rubin formula.24 The results with and without multiple imputation were similar; therefore, we report the results using multiple imputed datasets. Interleukin-6 and fibrinogen had high levels of missingness and so were not imputed. Analyses incorporating those biomarkers were by complete-case analysis only. For descriptive analysis, comparisons were made using linear regression for continuous variables and logistic regression for categorical variables across the independent variable of interest (category of troponin, death, and COVID-19 status).
Survival Analysis
We categorized the exposure variable of troponin into clinical categories as described: below ULN, <5 times ULN, between 5 and 10 times ULN, and >10 times ULN. We also classified troponin as a dichotomous variable: positive or negative. In the COVID-19 population, we performed Cox proportional hazard models and Kaplan-Meier survival analysis to determine the association of myocardial injury with in-hospital mortality. We performed univariable analyses followed by progressive adjustment for age and sex and then age, sex, and multiorgan dysfunction (represented by creatinine, bilirubin, Pao2/FIo2 ratio, vasopressor use, and lactate levels). We chose these covariates on the basis of factors used to calculate the Sequential Organ Failure score.25 The Sequential Organ Failure score itself was not used because not all components were directly captured in the JH-CROWN database. The proportional hazard assumption was assessed by inspection of Schoenfeld residuals and time dependence of covariates. The assumption of proportionality was met. We also compared the association of myocardial injury with death in patients with COVID-19 and patients with ARDS without COVID-19. We assessed the interaction of COVID-19 status and myocardial injury with death with Cox proportional hazard models.
Determinants of Myocardial Injury
To identify determinants of troponin T and troponin I in COVID-19, we performed linear regression with log-transformed troponin levels as the dependent variable and clinical and demographic factors as the independent variable in univariable models. Factors with P<0.1 in univariable models were then assessed in adjusted models. The adjusted models included the factor of interest and covariates known to be associated with myocardial injury: age, sex, and creatinine level.
ARDS Comparison
To determine the degree of association of COVID-19 with myocardial injury as compared with traditional ARDS, we performed logistic regression with positive troponin as the dependent variable (corresponding to 26 ng/L for patients with ARDS without COVID-19 and troponin above the ULN for this COVID-19 cohort) and COVID-19 status compared with ARDS as the independent variable in univariable and multivariable models adjusting for age, sex, and multiorgan dysfunction. Analyses were performed using STATA 15. P value <0.05 was considered statistically significant.
Results
Factors Associated With Myocardial Injury in Patients With COVID-19
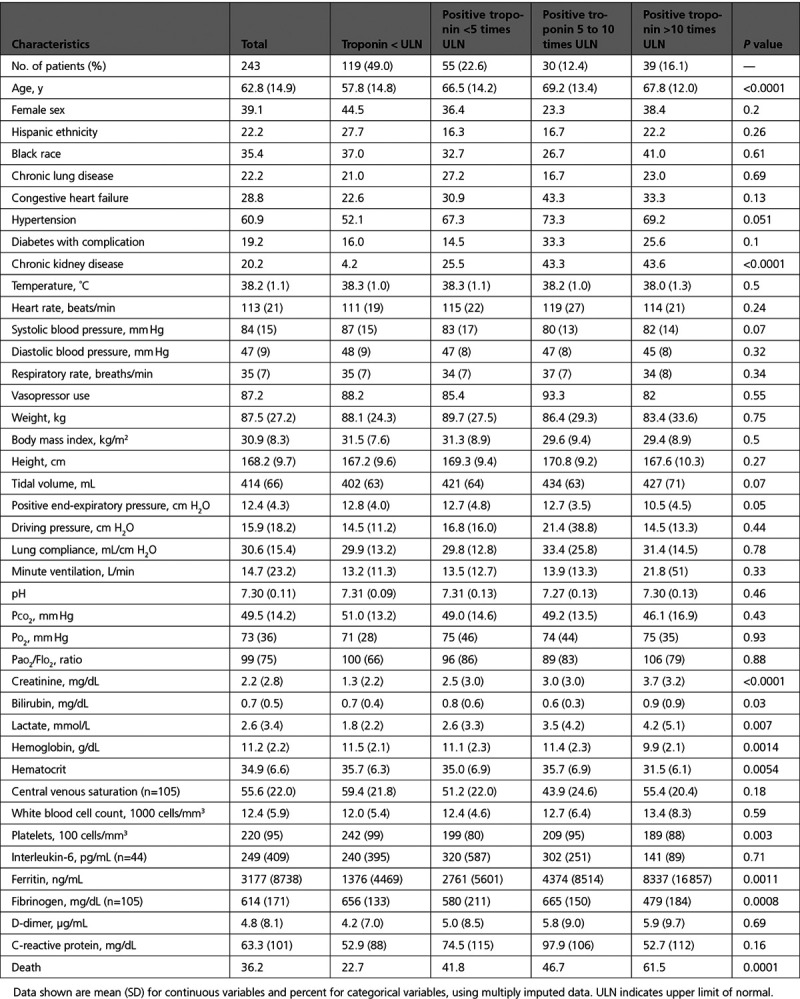
Of 328 intubated patients in our health system with COVID-19, 243 had troponin assessed within 24 hours of intubation and were included in the study. There were no major differences in age, sex, ethnicity, comorbidities, or death rates among patients who did versus did not have troponin checked (Table III in the Data Supplement). Of the study population, 54% had troponin I assessed and 46% troponin T. Patients assessed with troponin T were older with greater risk for death (Table IV in the Data Supplement). For included patients, mean age was 62.8 years, and a substantial minority had comorbidities of congestive heart failure, chronic lung disease, chronic kidney disease, or diabetes with complication (Table 1). More than 87% of patients received vasopressors and mean body mass index was 30.9 kg/m2. Of intubated patients with COVID-19, 51% had troponin levels above the ULN, including 16.1% with levels >10 times ULN. With higher troponin levels, patients had older age, higher proportion of chronic hypertension, and chronic kidney disease (Table 1). With higher troponin levels, patients had higher creatinine levels, higher lactate levels, and greater degree of anemia and thrombocytopenia as well as lower fibrinogen levels and higher ferritin levels (Table 1). Factors associated with troponin I and troponin T levels are displayed in Table V in the Data Supplement. After adjusting for age, sex, and creatinine, independent associations with troponin level included chronic kidney disease, higher lactate levels and white blood cell count, higher ferritin, and lower fibrinogen (Table V in the Data Supplement).
Table 1.
Characteristics of Intubated Patients With COVID-19, by Category of Troponin Level
Association of Myocardial Injury With Mortality in COVID-19
Overall mortality rate was 36.2%. Mortality was 22.7% among intubated patients with COVID-19 with troponin under the ULN and was greater with higher troponin levels, up to 61.5% among those with the highest troponin levels (P<0.001; Figure 1). The distribution of myocardial injury was also different comparing survivors with nonsurvivors: among survivors, 59% had troponin below the ULN and <10% troponin >10 times the ULN; among nonsurvivors, 31% had troponin below the ULN and 27% troponin >10 times ULN (P<0.001; Figure 1). Troponin I and Troponin T were also higher in nonsurvivors when considered as continuous variables (Table 2).
Figure 1.
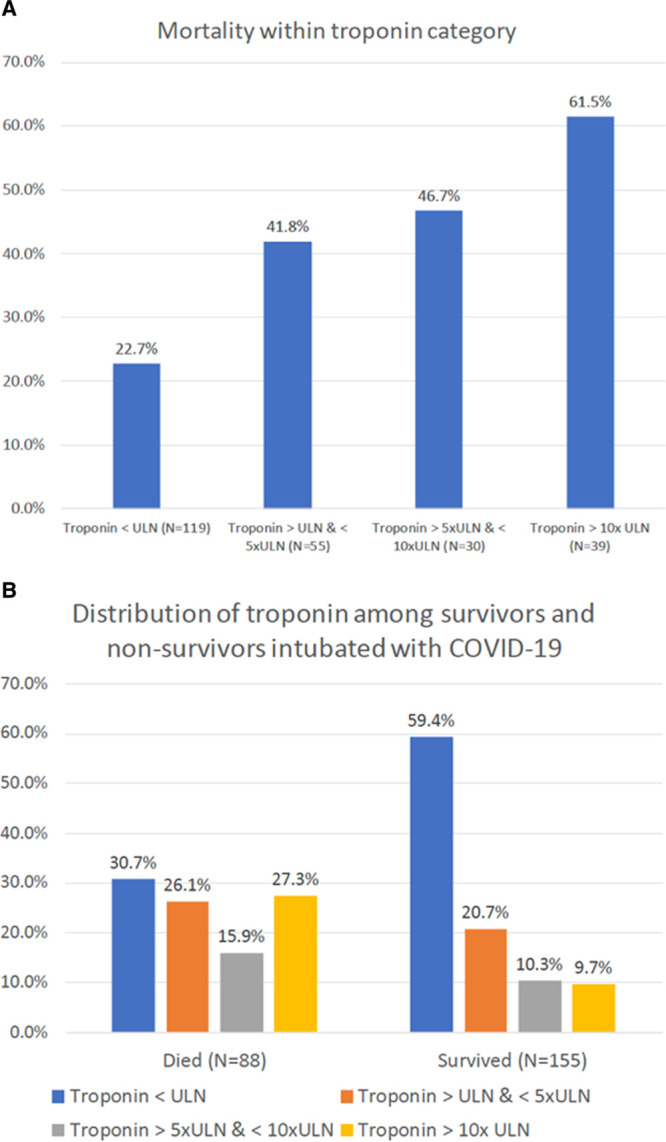
Mortality within each category of troponin level and distribution of troponin stratified by survival status.
A, Mortality within each category of troponin level (P<0.001 for difference in proportions and for trend). B, Distribution of troponin stratified by survival status (P<0.001 for difference in proportions). COVID-19 indicates coronavirus disease 2019; and ULN, upper limit of normal.
Table 2.
Characteristics of Intubated Patients With COVID-19, by Survival Status
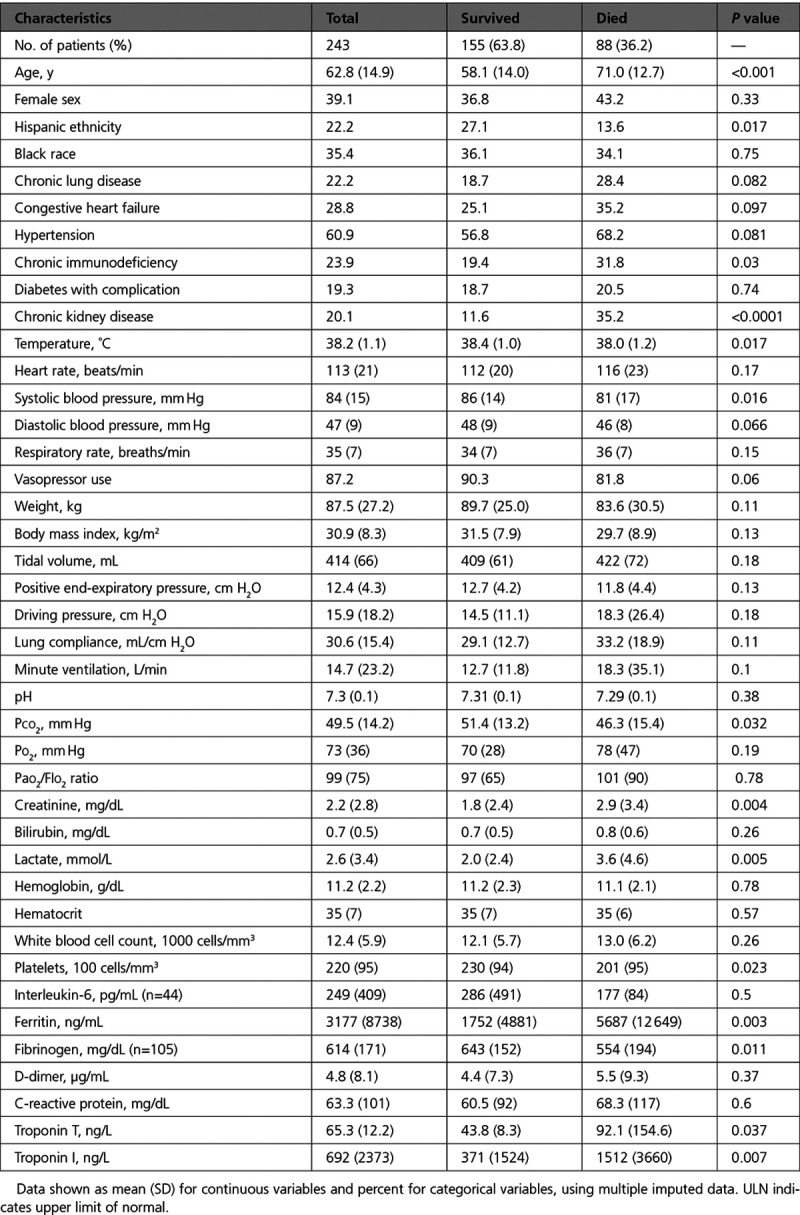
Other factors associated with mortality in descriptive analyses included older age, chronic kidney disease and higher creatinine, lactate levels, lower fibrinogen, and higher ferritin levels (Table 2). Positive troponin was associated with >2-fold increased hazard for mortality in unadjusted models (hazard ratio, 2.31 [95% CI 1.47–3.65]); the highest levels of troponin were associated with >3-fold risk of mortality compared with troponin below the ULN (hazard ratio, 3.17 [95% CI, 1.80–5.56]), as shown in Figures 2 and 3. The association of myocardial injury with mortality attenuated with progressive covariate adjustment and was no longer statistically significant after adjusting for age, sex, creatinine, bilirubin, Pao2/FIo2 ratio, vasopressor use, and lactate levels (Figure 3).
Figure 2.
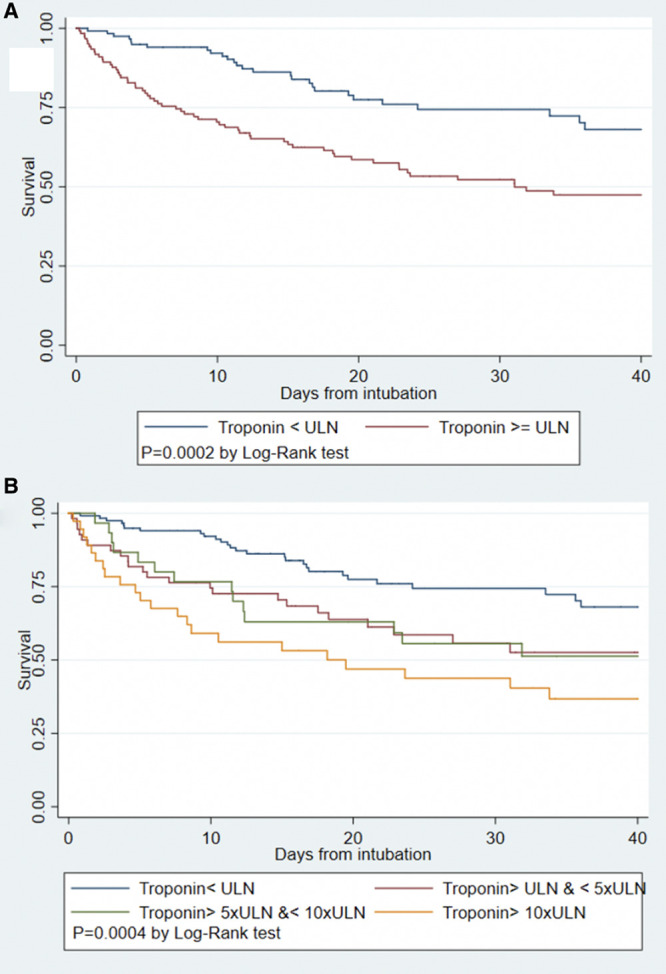
Kaplan-Meier survival curves for intubated patients with COVID-19.
Kaplan-Meier survival curves for intubated patients with COVID-19 by presence of any myocardial injury (A) and by category of troponin level (B). COVID-19 indicates coronavirus disease 2019; and ULN, upper limit of normal.
Figure 3.
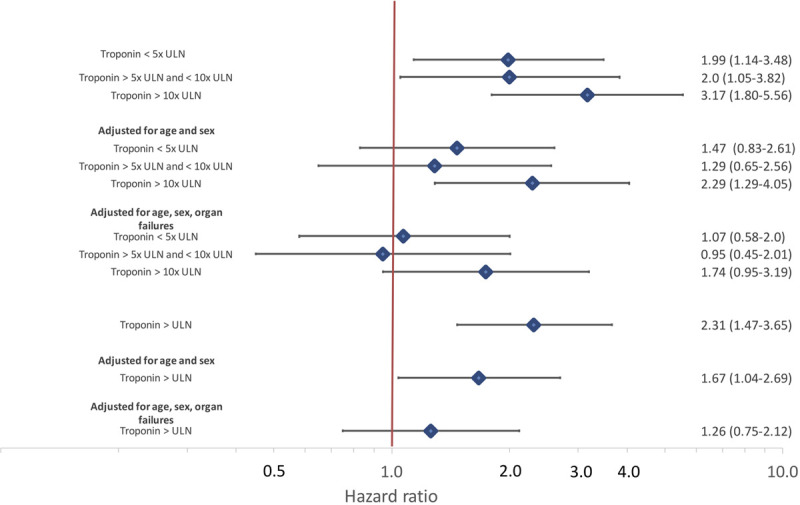
Univariable and adjusted hazard ratios for intubated patients with COVID-19 by presence of any myocardial injury and by category of troponin level.
Cox proportional hazard models adjusted first for age and sex and then for age, sex, creatinine, bilirubin, Pao2/FIo2 ratio, vasopressor use, and lactate levels. COVID-19 indicates coronavirus disease 2019; and ULN, upper limit of normal.
Comparison of Patients With COVID-19 Versus Patients With ARDS
Table 3 displays demographics and clinical characteristics of 243 intubated patients with COVID-19 and 506 patients with ARDS attributable to pneumonia from MI-ARDS. Patients with COVID-19 have a different clinical profile compared with patients with ARDS without COVID-19, including older age, fewer women, higher proportion of nonwhite race, higher body mass index and creatinine, less favorable vital signs, and worse oxygenation. The rate of any myocardial injury was similar between COVID-19 and ARDS: 51.0% in COVID-19 compared with 49.6% in ARDS (odds ratio for myocardial injury, 1.09 [95% CI, 0.78–1.44]; P=0.72). The distribution of troponin levels were also similar between COVID-19 and general ARDS (Table 3; P=0.37). After adjusting for sex, age, creatinine, bilirubin, Pao2/Fio2 ratio, and vasopressor use, COVID-19 was associated with lower odds of myocardial injury compared with ARDS (odds ratio, 0.55 [95% CI, 0.36–0.84]; P=0.005). Patients with COVID-19 had higher mortality than patients with ARDS without COVID-19: 36.2% vs 26.4% (P=0.007). In unadjusted analysis, mortality among patients with ARDS with and without myocardial injury and patients with COVID-19 without myocardial injury was similar; patients with COVID-19 with positive troponin had the highest mortality observed (Figure 4; P interaction=0.012). After adjusting for age, sex, creatinine, bilirubin, Pao2/FIo2 ratio, and vasopressor use, the interaction was no longer significant (P interaction=0.082).
Table 3.
Characteristics of Intubated Patients With COVID-19 Compared With Patients With ARDS Secondary to Pneumonia
Figure 4.
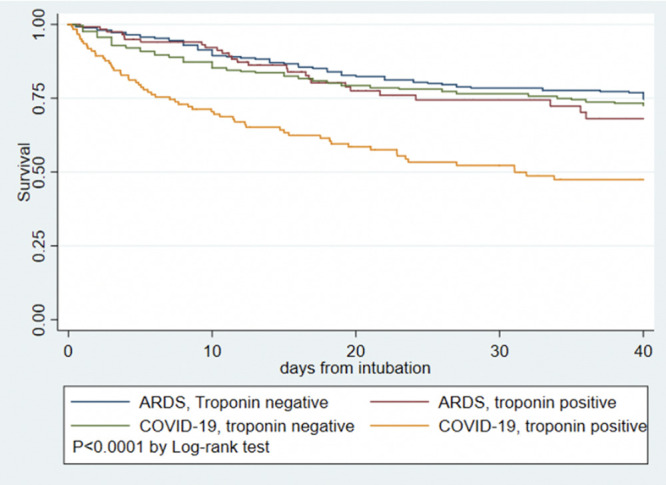
Kaplan-Meier survival curves for COVID-19 versus ARDS pneumonia and presence or absence of myocardial injury. ARDS indicates acute respiratory distress syndrome; and COVID-19, coronavirus disease 2019.
Discussion
Myocardial injury detected with cardiac biomarkers has garnered attention as a high-risk marker in COVID-19. Yet, the implications and pathogenesis of myocardial injury in COVID-19 remain unclear. In this study of myocardial injury in COVID-19 compared with traditional ARDS, we report several findings. First, half of intubated patients with COVID-19 manifest myocardial injury assessed by clinical troponin assays, which is associated with a graded increase in overall mortality. However, the magnitude of mortality risk is attenuated after adjustment for degree of critical illness, suggesting that myocardial injury is reflective of baseline risk and comorbidities and underlying multisystem organ dysfunction. Age, comorbidities, ferritin, and fibrinogen are associated with myocardial injury in COVID-19. Myocardial injury is actually less common in COVID-19 compared with conventional ARDS after adjusting for confounders of age, renal dysfunction, and degree of critical illness. Our findings place myocardial injury in COVID-19 in context of that observed in general ARDS, add to the evidence base for the usefulness of troponin as a prognostic biomarker, and suggest several avenues to further elucidate the pathogenesis of troponin elevation in COVID-19 and construct a conceptual model.
Epidemiology of Myocardial Injury in COVID-19
Of intubated patients with COVID-19, we report approximately half with myocardial injury, which is consistent with other reports of up to 44% rates of myocardial injury.2,3,8 Our study included only the most severely affected patients with COVID-19, who were intubated, explaining the high percentage of myocardial injury observed. High rates of myocardial injury are also observed in non–COVID-19–related critical illnesses including ARDS15,17 and sepsis,26 and we demonstrate that the unadjusted profile of myocardial injury is similar between patients with COVID-19 and a group of patients with ARDS secondary to pneumonia. However, the clinical profile of patients with COVID-19 compared with patients with conventional ARDS was higher risk, including more advanced age, more renal failure, and more severe lung disease. Given worse critical illness, more myocardial injury would be expected. After adjusting for severity of disease, therefore, the odds of myocardial injury in COVID-19 were lower than in conventional ARDS. The COVID-19 pandemic has resulted in a large number of critically ill patients presenting for care simultaneously, and it is debated which features of COVID-19 are unique to the virus versus facets of general critical illness.16,27 Our results suggest that myocardial injury in severe COVID-19 is not substantially different in magnitude from that in general ARDS and in fact may be of lesser magnitude adjusting for age and comorbidities, acknowledging that we are performing comparisons across disparate assays.
Contributors to Myocardial Injury in COVID-19
There are many potential mechanisms of elevated troponin in COVID-19, including thrombotic and plaque rupture events, supply–demand mismatch, and direct cardiac viral toxicity.28,29 We found that older age, acute and chronic renal dysfunction, and serum lactate levels were strongly associated with myocardial injury, consistent with studies in patients without COVID-19.15 The inflammatory and prothrombotic milieu of COVID-19 is hypothesized to also contribute to myocardial injury.30,31 Higher ferritin and lower fibrinogen were associated with troponin levels in our study. Higher ferritin levels may simply reflect more active systemic inflammation, although ferritin levels have also been associated with myocardial infarction in case–control studies.32 Ferritin has also been proposed to participate in the myocyte response to ischemia.33 The lower fibrinogen may reflect consumption, microvascular thrombosis, and endothelial dysfunction contributing to myocardial injury.34,35 Therefore, our work reinforces inflammation as a factor associated with myocardial injury and suggests that pathways involving coagulation and iron metabolism may be fruitful mechanistic areas of inquiry.
Prognostic Implications of Myocardial Injury in COVID-19
A growing evidence base supports that myocardial injury is associated with poor prognosis in COVID-19,8,29 and our findings support prior findings; in our critically ill patient population, myocardial injury was associated with >2-fold hazard for death. Yet the association of myocardial injury with mortality attenuated greatly after adjustment for age, sex, and multisystem organ dysfunction. We observed a similar pattern in the general ARDS population.15 These findings suggest that the association of myocardial injury with outcome in COVID-19 is a function of underlying critical illness and multisystem organ dysfunction, particularly concomitant renal dysfunction. The implication naturally follows that principles of critical care to optimize organ dysfunction would mitigate some of this risk and improve outcomes in patients with COVID-19 with myocardial injury. This premise is further supported by the fact that autopsy studies have not shown widespread direct myocarditis from COVID-19.36 MRI reports suggest abnormal myocardial signal in many patients with COVID-19,37–40 but patients with myocardial injury attributable to sepsis also manifest significant and common abnormalities on cardiac MRI.41 In our study, patients with COVID-19 with myocardial injury had worse prognosis than patients with pneumonia ARDS with myocardial injury; whether this incremental adverse prognosis is related to baseline comorbidities or to differing pathogenesis of myocardial injury such as thrombotic complications36 needs to be clarified. The fact that the interaction of myocardial injury with COVID-19 status lessened after covariate adjustment supports the fact that much of the difference relates to degree of critical illness.
COVID-19 Versus Traditional ARDS
It is debated whether COVID-19–related lung disease represents a form of traditional ARDS or has a distinct pathophysiology, with advocates of both viewpoints.42,43 We report crude rates of myocardial injury as similar to traditional ARDS, although after adjusting for clinical differences, the severely affected COVID-19 population had lower odds of myocardial injury. This could represent survivor bias, with individuals most severely affected experiencing cardiac arrest44–46 and death before surviving to assessment. Similar factors are associated with myocardial injury in both COVID-19 and traditional ARDS,15 including age, creatinine, and multisystem organ failure. This paradigm is supported by autopsy series suggesting virus involvement of pulmonary tissue with diffuse alveolar damage but rare microthrombi and endotheliitis; lymphocytic myocarditis was seen rarely but most patients had no evidence of direct cardiac involvement.36,47 Whether the hypercoagulable state and increased system inflammation observed in COVID-19 are unique features causing myocardial injury should be further investigated. Considering myocardial injury broadly, most cases seem related to critical illness, but there are isolated reports of frank myocarditis and other severe direct cardiac manifestations,1,48 and it is important to identify these rare and distinct manifestations.
Limitations
Limitations of our study include its observational nature; thus, hypotheses can be inferred but causal inference is not established. Our data are drawn from a single academic health system in a single state; in the context of a very heterogenous pandemic, reports from other health care systems are needed and anticipated. We chose a priori to focus only on intubated patients because of scientific interest and to enable our direct comparison to ARDS. Because our dataset is drawn from clinical care, there are missing data requiring imputation techniques, but results are similar in considering imputed and nonimputed data. In considering the comparison between ARDS and COVID-19, the MI-ARDS study was a cross-sectional study with troponin checked in all patients; in COVID-19, the decision to check troponin was made clinically. Thus, patients without troponin assessed were not included in the COVID-19 group, which could introduce bias. However, baseline characteristics and outcomes of patients with COVID-19 who did and did not have troponin checked were similar. An additional limitation relates to the fact that different assays were used within the hospitals admitting patients with COVID-19 and in the ARDS cohort, which provides challenges for direct comparisons. Outcomes of COVID-19 are variable across centers, which could reflect local epidemiology of the pandemic, patient risk profile, and local resources, among other factors. Echocardiography and other detailed cardiac imaging is not available in our data set, and formal cardiac imaging is often deferred in COVID-19 in lieu of informal point-of-care ultrasound. Thus, an assessment of ventricular function and wall motion abnormalities in patients with COVID-19 is not available in this study. This is an important area for future research.
Conclusions
Myocardial injury is common in severe COVID-19 as a function of baseline comorbidities, advanced age, and multisystem organ dysfunction. The adverse prognosis of myocardial injury in COVID-19 is a function of multisystem organ involvement, similar to generic ARDS. Markers of inflammation, iron metabolism, and thrombotic activity are associated with myocardial injury. Future studies of myocardial injury in COVID-19 should investigate any novel mechanisms and identify focused treatment of both primary cardiac involvement and the multisystem organ dysfunction.
Acknowledgments
The data used for this publication were part of JH-CROWN (the Johns Hopkins Health System COVID-19 Precision Medicine Analytic Platform Registry), which is based on the contribution of many patients and clinicians.
Sources of Funding
Dr Hays is supported by National Heart, Lung, and Blood Institute grant 1R01HL147660. Dr Lowenstein is supported by NIH grants R01HL134894 and R33HL141791, a grant from Novartis, and the Michel Mirowski MD Professorship in Cardiology. Dr Metkus is supported by the National Institutes of Health–funded Institutional Career Development Core at Johns Hopkins (project number 5KL2TR003099-02).
Disclosures
Dr Lowenstein receives research funding from Novartis for a clinical trial of treatment for patients with coronavirus disease 2019 (COVID-19; CRITICAL [Crizanlizumab for Treating COVID-19 Vasculopathy]; URL: https://www.clinicaltrials.gov; Unique identifier: NCT04435184). Abbott Laboratories provided reagents and financial support for MI-ARDS (the Myocardial Injury in Acute Respiratory Distress Syndrome Study; to principal investigator Dr Frederick Korley). MI-ARDS was designed and executed solely by the study investigators without industry involvement. Dr Post reports research support to an unrelated project from Abbott Laboratories for reagents. Dr Sokoll’s institution received funding and reagent support from Abbott Laboratories.
Supplemental Materials
Data Supplement Tables I–V
Supplementary Material
Footnotes
Sources of Funding, see page 563
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.050543.
This manuscript was sent to Allan S. Jaffe, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Contributor Information
Lori J. Sokoll, Email: lsokoll@jhmi.edu.
Andreas S. Barth, Email: abarth3@jhmi.edu.
Matthew J. Czarny, Email: mczarny@jhmi.edu.
Allison G. Hays, Email: ahays2@jhmi.edu.
Charles J. Lowenstein, Email: clowens1@jhmi.edu.
Erin D. Michos, Email: edonnell@jhmi.edu.
Eric P. Nolley, Email: enolley1@jhmi.edu.
Wendy S. Post, Email: wpost@jhmi.edu.
Jon R. Resar, Email: jresar@jhmi.edu.
David R. Thiemann, Email: dthiema1@jhmi.edu.
Jeffrey C. Trost, Email: jtrost2@jhmi.edu.
Rani K. Hasan, Email: rani.hasan@jhmi.edu.
References
- 1.Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis [published online April 19, 2020]. Am J Emerg Med. doi: 10.1016/j.ajem.2020.04.052. https://www.ajemjournal.com/article/S0735-6757(20)30280-1/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, Ren HW, Zuo Y, Li H, Wang J, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71:2089–2098. doi: 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendren NS, Drazner MH, Bozkurt B, Cooper LT., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, Guan X, Gary T, Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106:1142–1147. doi: 10.1136/heartjnl-2020-317062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, Wang K, Leng F, Wei S, Chen L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261–1267. doi: 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He XW, Lai JS, Cheng J, Wang MW, Liu YJ, Xiao ZC, Xu C, Li SS, Zeng HS. [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. ]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:456–460. doi: 10.3760/cma.j.cn112148-20200228-00137 [DOI] [PubMed] [Google Scholar]
- 12.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. ; Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 15.Metkus TS, Guallar E, Sokoll L, Morrow D, Tomaselli G, Brower R, Schulman S, Korley FK. Prevalence and prognostic association of circulating troponin in the acute respiratory distress syndrome. Crit Care Med. 2017;45:1709–1717. doi: 10.1097/CCM.0000000000002641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metkus TS, Guallar E, Sokoll L, Morrow DA, Tomaselli G, Brower R, Kim BS, Schulman S, Korley FK. Progressive myocardial injury is associated with mortality in the acute respiratory distress syndrome. J Crit Care. 2018;48:26–31. doi: 10.1016/j.jcrc.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193 [DOI] [PubMed] [Google Scholar]
- 19.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2675. doi: 10.1056/NEJMoa062200 [DOI] [PubMed] [Google Scholar]
- 20.Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:102 doi: 10.1186/s13054-020-2778-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57 doi: 10.1186/1471-2288-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 26.Masson S, Caironi P, Fanizza C, Carrer S, Caricato A, Fassini P, Vago T, Romero M, Tognoni G, Gattinoni L, et al. ; Albumin Italian Outcome Sepsis Study Investigators. Sequential N-terminal pro-B-type natriuretic peptide and high-sensitivity cardiac troponin measurements during albumin replacement in patients with severe sepsis or septic shock. Crit Care Med. 2016;44:707–716. doi: 10.1097/CCM.0000000000001473 [DOI] [PubMed] [Google Scholar]
- 27.Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17:787–789. doi: 10.1513/AnnalsATS.202004-325IP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval Y, Januzzi JL, Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723 doi: 10.1016/j.lfs.2020.117723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claeys D, Walting M, Julmy F, Wuillemin WA, Meyer BJ. Haemochromatosis mutations and ferritin in myocardial infarction: a case-control study. Eur J Clin Invest. 2002;32(Suppl 1):3–8. doi: 10.1046/j.1365-2362.2002.0320s1003.x [DOI] [PubMed] [Google Scholar]
- 33.Berenshtein E, Vaisman B, Goldberg-Langerman C, Kitrossky N, Konijn AM, Chevion M. Roles of ferritin and iron in ischemic preconditioning of the heart. Mol Cell Biochem. 2002;234–235:283–292. [PubMed] [Google Scholar]
- 34.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222 doi: 10.1186/cc4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Backer D, Donadello K, Favory R. Link between coagulation abnormalities and microcirculatory dysfunction in critically ill patients. Curr Opin Anaesthesiol. 2009;22:150–154. doi: 10.1097/ACO.0b013e328328d1a1 [DOI] [PubMed] [Google Scholar]
- 36.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho JS, Sia CH, Chan MY, Lin W, Wong RC. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung. 2020;49:681–685. doi: 10.1016/j.hrtlng.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui Y, Crouser ED, Raman SV. Nonischemic myocardial changes detected by cardiac magnetic resonance in critical care patients with sepsis. Am J Respir Crit Care Med. 2013;188:1037–1039. doi: 10.1164/rccm.201304-0744LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haudebourg AF, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A, Carteaux G. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:287–290. doi: 10.1164/rccm.202004-1226LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai PH, Lancet EA, Weiden MD, Webber MP, Zeig-Owens R, Hall CB, Prezant DJ. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mountantonakis SE, Saleh M, Coleman K, Kuvin J, Singh V, Jauhar R, Ong L, Qiu M, Epstein LM. Out-of-hospital cardiac arrest and acute coronary syndrome hospitalizations during the COVID-19 surge. J Am Coll Cardiol. 2020;76:1271–1273. doi: 10.1016/j.jacc.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paoli A, Brischigliaro L, Scquizzato T, Favaretto A, Spagna A. Out-of-hospital cardiac arrest during the COVID-19 pandemic in the province of Padua, Northeast Italy. Resuscitation. 2020;154:47–49. doi: 10.1016/j.resuscitation.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton-Cheh C, Zlotoff DA, Hung J, Rupasov A, Crowley JC, Funamoto M. Case 24-2020: a 44-year-old woman with chest pain, dyspnea, and shock. N Engl J Med. 2020;383:475–484. doi: 10.1056/NEJMcpc2004975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


