Abstract
Neonatal extracorporeal membrane oxygenation (ECMO) is a life-saving procedure for critically ill neonates suffering from a potentially reversible disease, causing severe cardiac and/or respiratory failure and refractory to maximal conventional management. Since the 1970s, technology, management, and clinical applications of neonatal ECMO have changed. Pulmonary diseases still represent the principal neonatal diagnosis, with an overall 74% survival rate, and up to one-third of cases are due to congenital diaphragmatic hernia. The overall survival rate in cardiac ECMO is lower, with congenital heart defect representing the main indication. This review provides an overview of the available evidence in the field of neonatal ECMO. We will address the changing epidemiology, basic principles, technologic advances in circuitry, and monitoring, and deliver a current multidisciplinary management framework, focusing on ECMO applications, complications, and long-term morbidities. Lastly, areas for further research will be highlighted.
Conclusions: ECMO is a life support with a potential impact on long-term patients’ outcomes. In the next years, advances in knowledge, technology, and expertise may push neonatal ECMO boundaries towards more premature and increasingly complex infants, with the final aim to reduce the burden of ECMO-related complications and improve overall patients’ outcomes.
|
What is Known: • ECMO is a life-saving option in newborns with refractory respiratory and/or cardiac failure. • The multidisciplinary ECMO management is challenging and may expose neonates to complications with an impact on long-term outcomes. What is New: • Advances in technology and biomaterials will improve neonatal ECMO management and, eventually, the long-term outcome of these complex patients. • Experimental models of artificial placenta and womb technology are under investigation and may provide clinical translation and future research opportunities. |
Keywords: Neonate, Developmental Hemostasis, Anticoagulation, ECMO, Respiratory ECMO, Cardiac ECMO, ECMO management, Follow-up, ECMO complications
ECMO patients and trends
Neonatal extracorporeal membrane oxygenation (ECMO) begins in 1975 with Dr. Bartlett and colleagues at the University of Irvine, CA, USA, where they successfully placed on ECMO Esperanza, a 1-day-old newborn with severe persistent pulmonary hypertension of the newborn (PPHN), after failing conventional therapies [1]. Since then, neonatal ECMO has rapidly evolved due to the increased understanding of cardiopulmonary pathophysiology, advances in medical management, and ECMO technology [2–10].
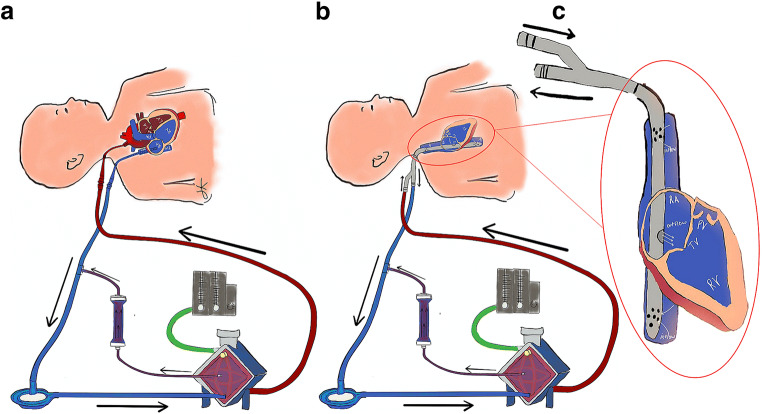
The principle of ECMO can be resumed as follows: the neonate’s deoxygenated blood is taken from the right heart; enters the ECMO circuit; passes through the membrane lung where oxygenation, temperature control, and carbon dioxide clearance are provided; and finally returns to either the arterial system (veno-arterial, VA ECMO, Fig. 1A) or the right heart (veno-venous, VV ECMO, Fig. 1B, C) [11].
Fig. 1.
Veno-Arterial (VA) ECMO with passive hemofiltration (A); Veno-Venous (VV) ECMO with dual lumen (DL) single bicaval cannula and passive hemofiltration (B); DL bicaval cannula (C)
From 1989 to 2020, the Extracorporeal Life Support Organization (ELSO) registry reported 43.707 neonates supported with ECMO worldwide [12].
The number of neonatal respiratory ECMO cases has decreased in the last decade, while cardiac indications remained stable [12]. ECMO survival is variable and depends on the underlying disease [13–19]. Nevertheless, pulmonary diseases still represent the primary diagnosis for neonatal ECMO (Table 1) [12]. In 2019, 280 newborns (160 respiratory, 93 cardiac, and 27 extracorporeal cardiopulmonary resuscitation (ECPR)) have been supported with ECMO in Europe.
Table 1.
Neonatal ECMO: diagnosis and survival rates between ELSO European and international report
| Etiology | ELSO European report* | ELSO international report° | Local reports | |||
|---|---|---|---|---|---|---|
| Incidence (%) | Survival (%) | Incidence (%) | Survival (%) | Survival (%) | References | |
| Pulmonary | 59.8 | 74 | 54.6 | 68 | 61.9–79.7 | 14–18 |
| CDH | 33.7 | 59 | 33.1 | 53 | 46–57.9 | 14–15, 17, 34–35 |
| MAS | 16 | 97 | 16.3 | 91 | 84.6–100 | 14–17, 34–35 |
| PPHN | 12 | 71 | 13.2 | 72 | 79.4–84.6 | 14–15, 35 |
| RDS | 0.7 | 100 | 0.7 | 85 | 92.5–92.9 | 14, 35 |
| Sepsis | 2.6 | 56 | 2.6 | 51 | 44–69 | 14, 16–17, 35, 38 |
| Pneumonia | 0.8 | 42 | 0.5 | 45 | 69^ | 16 |
| Other | 33.9 | 72 | 33.2 | 71 | 8.7–43 | 14, 16, 35 |
| Cardiac | 31.7 | 46 | 34.5 | 50 | 50–86 | 15, 18–19 |
| Congenital defect | 55.3 | 51 | 58.9 | 48 | 20–50 | 34, 69 |
| Cardiogenic shock | 4.9 | 36 | 4.9 | 50 | - | - |
| Cardiomyopathy | 0.4 | 0 | 0.8 | 40 | - | - |
| Myocarditis | 0.4 | 50 | 0.7 | 61 | 36 | 77 |
| Other | 38.9 | 55 | 34.9 | 54 | - | - |
| ECPR | 8.5 | 39 | 10.9 | 44 | 67 | 18 |
*Survival rates from 2015 to 2019, according to ELSO ECLS Registry Report, European Summary—July 2020 [13]
°Survival rates from 2015 to 2019, according to ELSO ECLS Registry Report, International Summary—July 2020 [12]
CDH, congenital diaphragmatic hernia; MAS, meconium aspiration syndrome; PPHN, persistent pulmonary hypertension of the newborn; RDS, respiratory distress syndrome; ECPR, extracorporeal cardiopulmonary resuscitation
^Combined survival of sepsis and/or pneumonia
ECMO criteria in neonates
ECMO may be offered to neonates with severe cardiac and/or respiratory failure, refractory to maximal conventional management, potentially reversible etiology, and high mortality risk [20–23]. ECMO criteria, as well as contraindications, are reported in Table 2 [22, 24].
Table 2.
Neonatal ECMO indications and contraindications (adapted from the ELSO Guidelines for Neonatal Respiratory Failure 2017 [22] and ELSO Guidelines for Pediatric Cardiac Failure 2018 [24])
| Neonatal respiratory ECMO | |
|
1. Oxygenation index (OI) > 40 for > 4h
| |
| 2. Failure to wean from 100% oxygen despite prolonged (> 48 h) maximal medical therapy or persistent episodes of decompensation | |
| 3. Severe hypoxic respiratory failure with acute decompensation (PaO2 < 40 mmHg) unresponsive to intervention | |
| 4. Severe pulmonary hypertension with evidence of right ventricular dysfunction and/or left ventricular dysfunction | |
| 5. Pressor-resistant hypotension | |
| Neonatal cardiac ECMO | |
| 1. Low cardiac output with evidence end-organ malperfusion despite maximal medical therapy | |
| 2. Refractory hypotension | |
| 3. Low cardiac output with increasing lactates levels (> 4 mmol/L) | |
| 4. Low cardiac output state with mixed venous oxygen saturation (or superior central venous oxygen saturation for single ventricles patients) < 50% | |
| Absolute contraindications | |
| 1. Lethal chromosomal disorder1 or another lethal anomaly | |
| 2. Irreversible brain damage | |
| 3. Uncontrolled bleeding | |
| 4. Grade III or greater intraventricular hemorrhage | |
| Relative contraindications | |
|
1. Irreversible organ damage (unless considered for organ transplant) 2. Weight < 2 kg 3. Postmenstrual age < 34 weeks 4. Mechanical ventilation > 10–14 days |
1Includes trisomy 13 and trisomy 18 (not trisomy 21)
Absolute and relative contraindications are the same for respiratory and cardiac ECMO. Gestational age (< 34 weeks) and low body weight (< 2000 g) have been associated with high rates of intracranial hemorrhage and mortality [20, 25–30]. Currently, the neonatal ECMO population is more critically ill than in the past, making patients’ management and cost-benefit assessment more challenging [9, 31]. However, there is wide center-to-center heterogeneity in the lower gestational age limits, based on the local level of expertise [28, 32].
Neonatal respiratory indications
Historically, meconium aspiration syndrome, PPHN, and neonatal respiratory distress syndrome were the most common ECMO indications in the neonatal period. Their incidence has recently declined due to perinatal care improvement [12, 14–17, 33–36]. The use of ECMO for pneumonia and neonatal sepsis has also declined. The universal maternal group B streptococcus (GBS) screening and intrapartum antibiotic prophylaxis induced an 80% reduction of GBS neonatal sepsis and, consequently, sepsis-related ECMO use [8, 37, 38]. Unfortunately, ECMO survival for septic shock did not improve [14, 16, 17, 36, 39]. This data could probably be explained by the increase of more aggressive and resistant pathogens, such as Escherichia coli [8, 40].
To date, one-third of ECMO neonatal diagnosis is represented by congenital diaphragmatic hernia (CDH). CDH ECMO has a mean survival rate of 50% that has remained unchanged over time [8, 12, 14, 15, 17, 22, 35, 36]. Although there are reports of a significant improvement in CDH ECMO survival rates in highly specialized centers over the past decade, the overall trend is due to the higher risk profile of the actual CDH population compared to the past [8, 41–47].
ECMO for congenital diaphragmatic hernia
The pathophysiology of CDH includes pulmonary hypoplasia and PPHN, which are responsible for life-threatening cardiorespiratory failure in the first weeks of life [22]. ECMO has the potential to rescue severe patients, avoiding iatrogenic lung injury, and providing time for improvement of pulmonary hypertension [45, 47–49].
Criteria predicting high mortality in CDH patients before ECMO have been recently published based on ELSO data [42, 50], while entry criteria suggested by ELSO are substantially the general ones for respiratory failure and pulmonary hypertension of any cause [22, 48, 51–54].
In the 5th Edition of the ECLS Red Book [55], CDH ECMO criteria have been integrated with the CDH EURO Consortium Consensus Guidelines Update 2015 (Table 3) [22, 48, 52, 56].
Table 3.
ECMO Criteria for CDH patients: a comparison between ELSO and CDH Euro Consortium Consensus indications
| ELSO* | CDH EURO Consortium Consensus° |
|---|---|
|
1. Inability to maintain preductal saturations > 85% or postductal saturations > 70%; 2. Respiratory acidosis with pH < 7.15 despite optimal ventilator management; 3. PIP > 28 cm H2O or MAP > 17 cm H2O is required to achieve saturation > 85%; 4. Refractory metabolic acidosis; 5. PaO2 < 40 for 4 h on FiO2 1.00; 6. Hypotension refractory to vasopressors; 7. OI > 40 for 4 h. |
1. Inability to maintain preductal saturations > 85% or postductal saturations > 70%; 2. Increased PaCO2 and respiratory acidosis with pH < 7.15 despite optimization of ventilator management; 3. PIP > 28 cm H2O or MAP > 17 cm H2O is required to achieve saturation > 85%; 4. Inadequate oxygen delivery with metabolic acidosis as measured by elevated lactate ≥ 5 mmol/L and pH < 7.15; 5. Systemic hypotension, resistant to fluid and inotropic therapy, resulting in urine output < 0.5 mL/kg/h for at least 12–24 h; 6. OI ≥ 40 present for at least 3 h. |
The optimal mode of ECMO in CDH is still debatable. A systematic review showed no differences in survival rates in the use of VV or VA mode. However, VA ECMO is more extensively used, not only for the advantage of providing both respiratory and cardiac support [57, 58] but also for the lack of adequate double-lumen cannulas for VV ECMO. Timing for optimal diaphragmatic repair in ECMO patients is controversial. Ideally, it is performed when the patient is stable [59, 60]. Some centers do prefer to delay surgery after ECMO weaning to limit the risk of hemorrhagic complications [60–62]. However, it has been suggested that early surgical repair while on ECMO may increase survival in the most severe forms of CDH [63–65]. Although the optimal timing has not been established, there is an increasing consensus that early repair within the first week of life would improve outcomes [49, 56].
CDH patients usually need long ECMO support, as either the severe vascular disease or pulmonary hypoplasia is less likely to improve over the weeks [8, 12, 42, 49, 66, 67]. Although time limits have not yet been established, a prolonged ECMO course is associated with increased mortality [49].
ECMO for tracheal and bronchial malformations
Congenital tracheobronchial malformations are rare disorders often associated with other intrathoracic anomalies (e.g., left pulmonary artery sling, bronchial extension, congenital heart disease) [68]. Currently, several operative approaches can be used for their treatment, including tracheal resection with end-to-end anastomosis, augmentation tracheoplasty with a pericardial patch, and slide tracheoplasty depending on the type, position, and length of the lesion. Peripheral ECMO (VA or VV) can be considered a valid alternative to cardiopulmonary bypass (CPB) to manage these complex lesions. ECMO requires a lower level of anticoagulation and lasts for several days; further, preoperative ECMO may facilitate the diagnostic workup (cardiac ultrasound, bronchoscopy, and computed tomography with three-dimensional reconstruction), often necessary due to the high prevalence of other intrathoracic anomalies. Intraoperative ECMO may allow surgeons to have better access to the operative field than CPB, which requires a mid sternotomy. Postoperative ECMO may allow lung resting with low-pressure mechanical ventilation favoring the healing of the sutures. The type of ECMO (VA vs. VV) varies among centers and surgical expertise. Despite its possible complications (bleeding, sepsis, neurologic injuries), ECMO may be considered the first extracorporeal approach in unstable neonates with an isolated tracheal lesion. Intraoperative conversion to CPB is used in case of concomitant cardiovascular anomalies [69].
ECMO for neonatal cardiac failure
The use of ECMO for neonatal cardiac failure has progressively increased in the last decade in Europe [13]. Currently, neonatal cardiac ECMO has reported a hospital survival of around 36–55%, which depends mainly on the indications [12, 13, 15, 18, 19]. Pre- and postoperative stabilization of congenital heart disease (CHD) are the most frequent ECMO indication, followed by cardiac arrest, cardiogenic shock, cardiomyopathies and myocarditis, and refractory arrhythmias [12, 13, 35, 70]. Preoperative stabilization (e.g., transposition of great arteries with pulmonary hypertension), failure to wean from cardiopulmonary bypass (stunned myocardium, pulmonary hypertension following cardiac surgery), or postoperative low cardiac output syndrome (Ross-Konno repair, truncus arteriosus repair, anomalous left coronary artery from the pulmonary artery repair, total anomalous pulmonary venous drainage repair) is the most frequent indication for ECMO in neonates with CHD [71, 72]. Risk factors associated with the use of ECMO in CHD are young age, low weight (< 3 kg), pre-ECMO clinical course (mechanical ventilation before surgery > 14 days, presence of fluid overload, cardiopulmonary resuscitation requirements), high complexity of the case according to the STAT classification, presence of shock or arrhythmias, and duration of cardiopulmonary bypass [73].
Cardiac failure associated with septic shock or cardiac arrest (ECPR) is also another ECMO indication in neonates [71]. Hospital survival in these groups is variable and ranges between 40 and 50% [18, 74, 75].
Causes of cardiac arrest and ECPR initiation may impact survival [55]. In the postcardiotomy population, patients with single ventricle physiology received ECMO for cardiac arrest (56%) and low cardiac output state (44%) [76]. ECPR due to hypoxemia or modified Blalock-Taussig shunt thrombosis reported a higher survival than ECPR for low cardiac output syndrome or right ventricular to pulmonary artery shunt thrombosis [71].
Neonatal myocarditis/cardiomyopathy is rare but often requires ECMO to maintain end-organ perfusion [71]. In these cases, ECMO may be used as a bridge to recovery (e.g., viral myocarditis), bridge to heart transplantation or ventricular assist device, or bridge to decision-making. Survival is poor for cardiomyopathies, often fatal, while it is relatively good for viral myocarditis [77, 78].
Arrhythmias (e.g., supraventricular tachycardia, ventricular tachycardia, bradycardia, or severe atrioventricular block) may occur in the perinatal period (primary arrhythmia), postoperatively, or in myocarditis/cardiomyopathy. ECMO support is generally used to maintain end-organ perfusion while optimizing pharmacological/surgical treatment (e.g., catheter ablation, pacemaker implantation) [79].
Timing for ECMO deployment in neonatal cardiac failure is still controversial; however, early initiation may reduce myocardium exposure and peripheral organs to hypoxia [80]. Cannulation for neonatal cardiac failure may be peripheral as well as central according to the circumstances (e.g., failure to wean from cardiopulmonary bypass, the need for high flow). Neonates unable to be weaned from cardiopulmonary bypass are generally supported with central cannulation, while neonates receiving ECPR can receive either a central or peripheral cannulation based on patient clinical history and surgical expertise. Current evidence describing the ECMO cannulation site’s frequency and its impact on outcomes is limited to retrospective single-center studies, mainly focused on ECPR. Further, conflicting results have been reported describing the neurologic complications of carotid versus central cannulation on mortality [81, 82].
ECMO duration is associated with outcome. Prolonged ECMO in children with cardiac disease carries a high mortality and reduces the chances of a successful heart transplant [83]. Survival drops from 45 % (overall survival of children with cardiac disease) to 23–25 % when ECMO duration is between 14 and 28 days and 13% for ECMO course longer than 28 days [71]. Notably, also neonatal respiratory ECMO duration is associated with poor outcome. Prolonged respiratory ECMO > 21 days has reported a survival of 23.5% [9].
Based on all these considerations, when evaluating ECMO as a bridge to a ventricular assist device, its duration should not be longer than 5–7 days [71, 84].
Optimizing end-organ perfusion and oxygen delivery awaiting myocardium recovery is the primary purpose of ECMO in neonatal cardiac failure, and its efficacy is generally reflected by the clearance of acidosis [70]. Thus, when lactate remains high, other causes of hypoperfusion should be appropriately evaluated. Among these, the persistence of residual lesions and the presence of a stunned and dilated myocardium may negatively impact on survival. Postoperative neonatal cardiac surgery patients unable to be weaned off ECMO should be evaluated for residual lesions, and when present, early correction should be advocated [70]. Unloading the left ventricle by creating an atrial shunt via a septostomy or placing another cannula in the left atrium is the most effective strategy to decompress and perfuse a failing left ventricle, especially when a low dose of inotropes is unable to improve ejection [85].
ECMO management
Neonatal ECMO should be provided in a dedicated intensive care unit by a multidisciplinary team, including neonatologists, surgeons, perfusionists, nurses, and psychologists. Psychological support for the family is crucial and should be planned in advance, sometimes also before delivering a special diagnosis (e.g., CDH). A high-quality communication between the ECMO team and the family may also help in ECMO withhold or withdrawal. ECMO management at the bedside starts with the circuit priming and with the choice of the cannulas.
Priming is generally constituted by a mixture of packed red cells and plasma [55]. Electrolytes are added to maintain physiologic levels. Cannulas are generally chosen according to the vessels’ size, often evaluated with ultrasound [86] (Table 4).
Table 4.
Characteristics of neonatal cannulas (based on Medtronic Bio-Medicus Pediatric Cannulas, technical sheet 2010; Maquet Avalon Elite Bi-Caval Dual Lumen Catheter, technical sheet 2015) and circuits (based on Permanent Life Support (PLS) System and Quadrox-iD Pediatric (Rotaflow Console with PLS Set))
| Cannula sizes and characteristics | |||||
| Cannula | Internal diameter (in.) | Size (Fr) | Tip lenght (cm) | Blood flow (L/min)* | Radiopacity |
| SL arterial | 1/4 | 6 | 10 | 0.35 | Metal spiral ends at 0.5 cm from the tip |
| 8 | 10 | 0.6 | |||
| 10 | 10 | 1.25 | |||
| 12 | 11 | 2 | |||
| SL venous | 1/4 | 8 | 10 | 0.4 | Metal spiral ends at 4 cm from the tip |
| 10 | 10 | 0.8 | |||
| 12 | 11 | 1.3 | |||
| 14 | 12 | 1.8 | |||
| SL arterial NextGen | 1/4 | 9 | 10 | 0.6 | Metal spiral ends at 0.2 cm from the tip |
| 11 | 10.5 | 1.2 | |||
| 13 | 11 | 2 | |||
| SL venous NextGen | 1/4 | 9 | 10 | 0.4 | Metal spiral ends at 0.2 cm from the tip |
| 11 | 10.5 | 0.8 | |||
| 13 | 11 | 1.3 | |||
| 15 | 11.5 | 1.8 | |||
| DL Bi-Caval | 1/4 | 13 | 11 | Arterial 0.5 | Metal spiral ends at the tip |
| Venous 0.65 | |||||
| 1/4 | 16 | 14 | Arterial 0.75 | ||
| Venous 1.0 | |||||
| Cannula selection | |||||
| Weight | VA circuit—SL arterial | VA circuit—SL venous | VV circuit—DL Bi-Caval | ||
| < 3 kg | 8 Fr | 8 Fr | - | ||
| 3–5 kg | 10–12 Fr | 10–14 Fr | 13 Fr | ||
| 4–8 kg | 10–12 Fr | 10–14 Fr | 16 Fr | ||
| Characteristics of 1/4 in. circuit | |||||
| Blood flow (L/min) | 0.2–2.8 | Oxygenator volume (mL) | 81 | ||
| Gas flow (L/min) | 0.1–5.6 | Centrifugal pump volume (mL) | 32 | ||
| Venous pressure (mmHg) | < − 80 | Hemoconcentrator volume (mL) | 17 | ||
SL, single lumen; DL, double lumen; VA, veno-arterial; VV, veno-venous; Fr, French
*Based on blood flow at 100 mmHg for arterial cannula and at − 40 mmHg for venous cannula
When the cannulas are placed at the neck (either surgical or percutaneously), the position is generally checked with chest X-ray or transthoracic echocardiography [21, 22, 87–89]. When ECMO is connected to the patient, the pump flow should be started slowly at 20 ml/kg/min and increased gradually over 5 to 10 min in order to achieve adequate oxygen delivery. For VA ECMO, pump flow is increased to achieve a pre-pump blood oxygen saturation (SpreO2) of 70–80%. On VV ECMO, instead, pump flow is increased to achieve an arterial saturation ≥ 80%.
Although most European centers use centrifugal pumps, differently to the USA, where the roller pump is common practice, this discrepancy in the management would not seem to impact on mortality even if O’Halloran et al. demonstrated contrasting data in favor of the use of roller versus centrifugal pump [90].
As the cardiopulmonary function is gradually replaced, blood gases must be strictly monitored and maintained within normal ranges [22]. SpreO2 is a proxy of tissue oxygenation status, as it reflects the ratio of oxygen delivery to oxygen consumption [22]. Ventilator settings should be tailored at “rest settings” to reduce the risk of VILI, although specific guidelines have not been defined [21, 22]. Serial lung mechanics measurements, such as the forced oscillation technique, may be used to titrate ventilatory support and chest physiotherapy, and support either lung recruitment maneuver and pharmacological treatment [91]. Extubation may be feasible during neonatal ECMO [92].
Fluid management during ECMO is crucial to limit fluid overload and edema [21]. Diuretics, slow continuous ultrafiltration, and continuous renal replacement therapy may be used to achieve dry weight [20, 22, 93]. Full nutritional support should be provided, either enterally or parenterally [22, 94]. Interaction between drugs and non-endothelial surfaces, sequestration into the circuit components, the increase of volume of distribution, and changes in the clearance of many drugs may alter pharmacokinetics and pharmacodynamics; thus, pharmacotherapy should be tailored accordingly [95, 96].
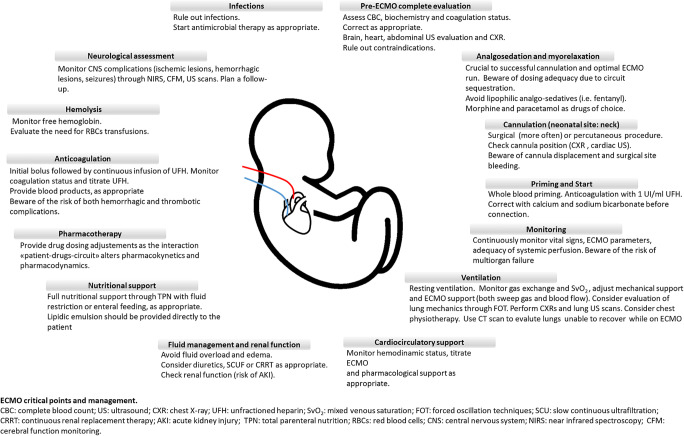
An overview of the critical points of ECMO management is provided in Fig. 2.
Fig. 2.
Overview of the critical points of ECMO management
Anticoagulation
When approaching this critical issue, maturational differences of the hematologic system across age, known as “developmental hemostasis,” should be considered [97–102]. Indeed, despite prolonged activated partial thromboplastin time (aPTT) at birth, the neonatal clotting system is balanced and gradually evolves towards a procoagulant phenotype over the first weeks of life [103, 104].
Further, the priming volume (often around three times the neonate’s circulating volume), the use of low ECMO flows, and the contact with foreign surfaces (polyvinyl chloride tubes and polymethylpenthene) may impact hemostasis [105–107]. Unfractionated heparin (UFH) is the anticoagulation of choice for neonatal ECMO despite its age-dependent variation in activity [22, 105, 108–112]. As critically ill neonates are also at increased risk of bleeding, namely intracranial hemorrhage, close hemostatic monitoring is required to detect mechanical complications and consumptive coagulopathy [22, 105–107, 112].
As each coagulation assay has advantages and limitations, the optimal test is lacking, and instead, a combination of them is usually performed to tailor UFH dosage and blood product replacement (Table 5) [105, 108–116]. Besides the use of plasmatic assays, such as aPTT and anti-Xa, many centers adopt whole blood-based tests such as activated clotting time, thromboelastography, or rotational thromboelastography [11, 22, 105, 109, 110, 117–123].
Table 5.
Assessment of coagulation status and management during neonatal ECMO
| Anticoagulation | |||||
|---|---|---|---|---|---|
| Administration of UFH: bolus of UFH 50 UI/kg at cannulation, followed by continuous infusion at 25 UI/kg/h | |||||
| Coagulation monitoring and management | |||||
| Parameter | Characteristics | Target | Intervention | Advantages | Disadvantages |
| AT |
- Sample: citrated plasma - Endpoint: available AT |
80–120% | Consider AT supplementation | - Possible optimization of UFH dose and effect |
- Lack of evidence of improved clinical outcome following AT supplementation - Possible increased risk of bleeding and thrombosis |
| ACT |
- Point of care test - Sample: whole blood - Endpoint: clot detection |
180–220 s | Titrate UFH infusion, especially at ECMO start |
- Small sample size (2–3 whole blood drops) - Low cost - Rapid and easy to perform - Suitable for transport |
- Least related to UFH doses and UFH changes - Poor correlation with aPTT at lower UFH (risk of underestimation of heparin effect) - Influenced by hemodilution, thrombocytopenia, platelet dysfunction, hypothermia, age, coagulation factors deficiencies - Analyzer and reagent dependent |
| aPTT |
- Clotting-based assay - Sample: citrated plasma - Endpoint: thrombus detection - Monitors intrinsic and common coagulation pathways (factors XII, XI, IX, X, V, II, fibrinogen) |
Ratio 1.5–2.5 times baseline |
Titrate UFH infusion Consider fresh-frozen plasma if aPTT is prolonged |
- Low cost, widely used, readily available - Suitable for transport - Can detect underlying factor deficiencies (congenital or acquired), vitamin K deficiency, DIC in presence of UFH by using heparinase |
- Lack of neonatal and pediatric ranges - Newborns have physiologically longer baseline levels compared to children and adults - Age-dependent effect of UFH on aPTT - Poor correlation with ACT and anti-Xa results in neonates - Mainly responsive to procoagulant drivers, does not reflect in vivo hemostasis - Influenced by UFH contamination of sample, hemodilution, coagulation factor deficiencies, and liver disease increased bilirubin, triglycerides, and plasma free Hb - Large blood sample size - Analyzer and reagent dependent - Risk of pre-analytic errors (i.e., suboptimal tube filling) |
| Anti-Xa |
- Functional assay - Sample: citrated plasma - Endpoint: bound Factor Xa |
0.3–0.7 IU/mL | Titrate UFH infusion |
- Direct measurement of heparin effect on Factor Xa - Can monitor the effect of LMWH and oral Anti-Xa drugs - Calibration of aPTT reference ranges |
- Anti-IIa effect not measured - Influenced by AT levels and assay type (exogenous AT, dextran sulfate additive), hyperbilirubinemia, triglycerides, and elevated plasma free Hb - High costs - Not available in all laboratories - Experienced staff needed |
| TEG |
- Point of care test - Sample: whole blood - Endpoint: clot formation, strength, and breakdown • R time: time to factor IIa generation and fibrin formation; • Angle and K: fibrin mesh formation; • MA: platelet function and platelet fibrin interaction; • LY30: clot lysis 30 min after MA |
R times in kaolin should be 2- to 3-fold longer than R times in heparinase (i.e., R times in kaolin 15–25 min) |
Titrate UFH infusion and blood products Long R times in heparinase: consider fresh-frozen plasma administration Low ratio R kaolin/R heparinase: consider increase heparin High ratio R kaolin/R heparinase: consider decrease heparin Low MA values: check platelet count and fibrinogen levels and correct |
- Small sample size - Rapid and easy to perform - Suitable for transport - Viscoelastic clotting tests with real-time global assessment of hemostasis (clot formation, strength, fibrinolysis) - Can monitor the role of fibrinogen and platelet - Can assess in vitro coagulation with UFH (kaolin) or without UFH (kaolin + heparinase), thus allowing to evaluate native hemostasis |
- Influenced by the reagents and plasma free Hb - Lack of neonatal ranges of TEG parameters for anticoagulation during ECMO |
| Platelets |
- Sample: EDTA blood - Endpoint: platelet count |
> 80,000–100,000 if high risk of bleeding > 45,000 if low risk of bleeding |
Consider administration of platelets (20 mL/kg) | - Low cost, widely used, readily available |
- Platelet count does not reflect platelet function - Platelets may stick to the ECMO circuit components, contributing to either circuit deterioration and bleeding risk in patients |
| Fibrinogen |
- Sample: citrated plasma - Endpoint: fibrinogen concentration |
> 100–150 mg/dL |
Consider administration of fibrinogen concentrate: - 50–70 mg/kg if fibrinogen < 50 mg/dL - 30 mg/kg if fibrinogen 50–100 mg/dL Consider fresh-frozen plasma |
- Low cost, widely used, readily available - Role in detecting hypercoagulability and DIC, including the concurrent evaluation of platelet count and D-dimers |
- Fibrinogen is usually depleted on ECMO and shows less sensitivity in detecting DIC |
| D-Dimers |
- Sample: citrated plasma - Endpoint: available fibrin split products |
< 300 μg/L |
If D-dimer levels increase: - Check the circuit for clots - Consider changing the oxygenator |
- Monitors fibrinolysis - Role in detecting hyperfibrinolysis and DIC together with fibrinogen status and platelet count trends |
- Low specificity |
| PT |
- Clotting-based assay - Sample: citrated plasma - Endpoint: thrombus detection - Monitors extrinsic coagulation pathway |
Ratio < 1.5 times baseline | Consider fresh-frozen plasma if PT is prolonged |
- Low cost, widely used, readily available - Suitable for transport - Can detect effects of vitamin K inhibitors and Anti-Xa agents |
- Does not reflect the UFH effect - Age, analyzer, and reagent dependent - Large blood sample size |
AT, antithrombin; UFH, unfractionated heparin; ACT, activated clotting time; aPTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; EDTA, ethylenediaminetetraacetic acid; LMWH, low molecular weight heparin; TEG, thromboelastography; PT, prothrombin time
Additional details and specific references are provided in the text
Although UFH has molecule- and age-dependent variation in activity, it is the anticoagulant of choice [22, 105, 108–112].
Normal antithrombin levels are essential for UFH efficacy; however, they are physiologically lower in neonates than in children and adults. Evidence for antithrombin supplementation has yet to be proven, and there are increasing concerns regarding thromboembolic events associated with AT supplementation, especially in critically ill newborns and children [124–128]. The presence of high levels of latent antithrombin, with potent pro-coagulative effects, in the commercially available products could explain this phenomenon [129].
Direct thrombin inhibitors are a promising alternative to UFH for anticoagulation in neonates, especially in heparin-induced thrombocytopenia, heparin resistance, or significant thrombosis. Dosing strategies and risk of adverse effects are still a matter of debate [22, 101, 105, 110, 130–134].
Overall, there is still a wide heterogeneity in coagulation management during neonatal ECMO across centers [109, 113, 135–141].
Weaning, decannulation, and withdrawal
As minimal support is reached to ensure tissue oxygenation, decannulation is considered. Trial-off support is performed to test the patient’s readiness for weaning before any attempt of decannulation [22].
During VV ECMO, native gas exchange is evaluated by progressively reducing sweep gas flow while adjusting ventilatory support. When the gas flow is stopped and gas exchange is adequate with minimal ventilator support, patients are ready to be weaned. During VA ECMO, the blood flow rate and gas flow is gradually reduced over time while adapting the ventilator setting and monitoring patients’ status. Alternatively, a bridge may be inserted into the circuit between the venous and the arterial side, to allow blood flow to re-circulate within the circuit, thus excluding the patients. Achieving low flow conditions in neonates is often impossible due to the required minimum flow for the ECMO devices (100–200 ml/min). Thus, other groups successfully promoted trial-off with a retrograde pump flow of the ECMO circuit [142]. If trial-off is tolerated, the patient is ready for decannulation [21, 22]. The carotid artery’s repair is rarely performed and is still a matter of debate [22, 143].
ECMO should also be discontinued if there is no hope of survival, there is no chance of organ replacement, or the patient has not shown any improvement during a reasonable amount of time, which must be defined on a case by case basis [21].
Complications
Critical issues related to hemostatic disturbances make hemorrhagic and thrombotic complications prevalent, with decreased survival rates [144, 145]. Circuit clotting is the most frequent mechanical complication, being the oxygenator the most common site. Coagulation abnormalities may lead to ischemic and hemorrhagic injuries [12, 22]. Since brain injuries are worrisome during neonatal ECMO, bedside imaging and neuromonitoring are mandatory during and after ECMO [22, 146–148].
Among the most severe neurological complications, seizures, ischemic infarction, and brain death are frequent and associated with poor prognosis [149, 150]. Cerebral function monitoring, continuous electroencephalography, and near-infrared spectroscopy may help to detect neurologic events, although pediatric literature is still limited [11, 151].
A degree of renal impairment, ranging from oliguria to renal failure, frequently occurs while on ECMO. Acute kidney injury and fluid overload are independent risk factors associated with increased mortality [152, 153]. Chronic kidney injury is less frequent since the long-term renal function is usually restored [154].
Cannulas, central lines, immobility, and general conditions are risk factors for infections associated with increased ECMO duration and higher mortality [20, 155]. Routine prophylaxis is not recommended unless a specific culture demonstrates an ongoing infection, while antibiotic coverage for cannulation should follow standard surgical prophylaxis [156–159].
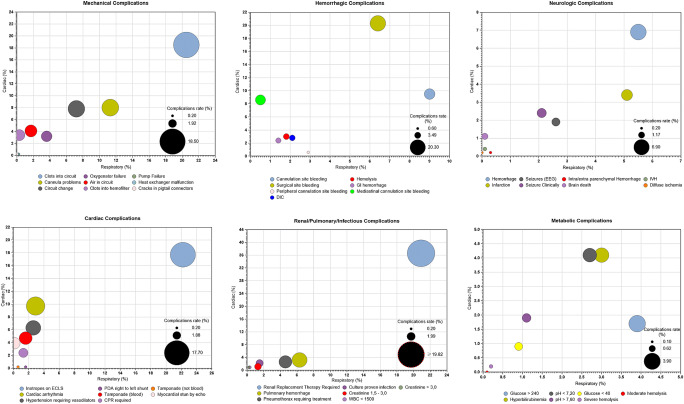
Arrhythmias and myocardial dysfunction are common in VA mode. Other complications, like cannula displacement and cardiac tamponade, are life-threatening and need to be promptly recognized and corrected (Fig. 3) [20].
Fig. 3.
Complications during neonatal respiratory and cardiac ECMO and incidence
Long-term morbidities and follow-up
Improved survival carries a higher risk of long-term morbidity among survivors depending on several factors, such as the primary underlying condition and adverse events arising during the ECMO course [12, 13, 49, 160–167].
Among respiratory ECMO patients, the risk of developmental disabilities is significantly increased [150, 168–172]. Bilateral sensorineural hearing loss, motor problems, learning difficulties, behavior disorders, and cognitive and neuropsychological impairments require specific assessment and multidisciplinary long-term follow-up [160, 161, 166, 167, 173–178].
Nevertheless, adult ECMO survivors show high self-esteem and are satisfied with their quality of life [179–181].
The need for oxygen supplementation, pulmonary hypertension, obstructive pattern with bronchospasm, asthma, decreased exercise tolerance, and chronic lung disease are among the most common conditions within the long-term respiratory morbidity [59, 161, 162, 173, 182–185].
The growth pattern is generally typical or slightly decreased in ECMO survivors, with good chances of catch-up growth during childhood and adolescence [162, 173, 186].
Most deaths occur while on bypass or early on in the hospital course, while late deaths occurring after ECMO discontinuation are uncommon and mainly affecting CDH patients (14.3%) [187].
There are considerable data regarding survival to hospital discharge of neonates receiving ECMO for cardiac failure; however, limited data exist on intermediate- and long-term outcomes. Among survivors, 40–60% reported new neurologic deficits and low health-related quality of life than healthy controls [188–190]. Neurologic complications may have variable features (language acquisition delay, motor development stages delay, behavioral problems) and represent a source of significant morbidity and long-term care requirements [190]. Due to the cohorts’ heterogeneity, which includes different age groups and different ECMO indications (ECPR, low cardiac output syndrome, septic shock, myocarditis), a robust conclusion cannot be achieved.
In the light of these considerations, standardized follow-up protocols among centers and data sharing are important to provide further knowledge and implement interventions to prevent and manage complications, improve quality assistance, and improve the overall quality of life.
Conclusions and future directions
Over the years, ECLS technology and expertise have considerably improved, pushing the lower limit of gestational age and suggesting that ECMO use may be potentially extended to more premature infants in the next years [28].
Experimental models of artificial placenta have been developed [191–193]. An ideal artificial placenta provides ECMO through the umbilical vessels, thus preserving major fetal blood vessels from cannulation. It uses low partial pressure of oxygen because of fetal hemoglobin features and increases hematocrit levels. No ventilation is required since the infant could “breathe” with fluid-filled lungs (perfluorocarbon liquid ventilation), reducing the risk of VILI to the developing lungs [194–197]. A trial using an artificial placenta in preterm lambs showed increased survival compared to mechanical-ventilated lambs [192]. An experimental extra-uterine system to physiologically support fetal lambs has shown encouraging results in terms of hemodynamic stability, oxygenation, and maintenance of fetal circulation [193].
Advances in technology, materials, and miniaturization of equipment would help to reduce ECMO-related complications. Biomimetic or biopassive tubing may mitigate the adverse effects of the contact between blood and non-endothelial surfaces, but their efficacy is partial [101]. The endothelization of the circuit would provide physiological regulation of coagulation and inflammatory response in ECMO patients. However, the in vitro process is technically challenging, takes time, and cannot be produced in urgent settings [198–201]; the in vivo process is currently not feasible, but ongoing research is promising. Lastly, paracorporeal lung assist devices might be explored in selected cases where traditional ECMO is not an option, although evidence for hybrid approaches is scarce [202].
Acknowledgments
The authors would like to thank all the “Neonatal ECMO Team Mangiagalli” of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the “Neonatal and Pediatric ECMO Team” of Children’s Hospital Bambino Gesù (Area Rossa), IRCCS, Rome, Italy; surgeons of the Department of Pediatric Surgery of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico; anesthesiologists of the Pediatric Anesthesiology and Intensive Care Unit; and nurses of the operating room of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico.
Abbreviations
- aPTT
Activated partial thromboplastin time
- CDH
Congenital diaphragmatic hernia
- CHD
Congenital heart disease
- CPB
Cardiopulmonary bypass
- ECMO
Extracorporeal membrane oxygenation
- ECPR
Extracorporeal cardiopulmonary resuscitation
- ELSO
Extracorporeal life support organization
- GBS
Group B streptococcus
- PPHN
Persistent pulmonary hypertension of the newborn
- SpreO2
Pre-pump blood oxygen saturation
- UFH
Unfractionated heparin
- VA ECMO
Veno-arterial extracorporeal membrane oxygenation;
- VILI
Ventilator-induced lung injury
- VV ECMO
Veno-venous extracorporeal membrane oxygenation
Author contributions
IA, GR, MDN, FMo, FMa, AA, and GC contributed conception and design of the manuscript; SK and GC developed the graphic part of the manuscript. IA, GR, MDN, and GC wrote the first draft of the manuscript. All authors contributed to manuscript critical revision and read and approved the submitted version.
Data availability
N/A
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
N/A
Consent to participate
N/A
Consent for publication
N/A
Code availability
N/A
Footnotes
Ilaria Amodeo and Matteo Di Nardo are co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ilaria Amodeo, Email: amodeoilaria@gmail.com.
Matteo Di Nardo, Email: matteo.dinardo@opbg.net.
Genny Raffaeli, Email: genny.raffaeli@unimi.it.
Shady Kamel, Email: kamelshady.o.f@gmail.com.
Francesco Macchini, Email: francesco.macchini@policlinico.mi.it.
Antonio Amodeo, Email: antonio.amodeo@opbg.net.
Fabio Mosca, Email: fabio.mosca@unimi.it.
Giacomo Cavallaro, Email: giacomo.cavallaro@policlinico.mi.it.
References
- 1.Bartlett RH. Esperanza: the first neonatal ECMO patient. ASAIO J. 2017;63(6):832–843. doi: 10.1097/MAT.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 2.Hintz SR, Suttner DM, Sheehan AM, Rhine WD, Van Meurs KP. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics. 2000;106(6):1339–1343. doi: 10.1542/peds.106.6.1339. [DOI] [PubMed] [Google Scholar]
- 3.Kennaugh JM, Kinsella JP, Abman SH, Hernandez JA, Moreland SG, Rosenberg AA. Impact of new treatments for neonatal pulmonary hypertension on extracorporeal membrane oxygenation use and outcome. J Perinatol. 1997;17(5):366–369. [PubMed] [Google Scholar]
- 4.Christou H, Van Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE, et al. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med. 2000;28(11):3722–3727. doi: 10.1097/00003246-200011000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Barrington KJ, Finer N, Pennaforte T, Altit G (2017) Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2017, Issue 1. Art. No.: CD000399. 10.1002/14651858.CD000399.pub3. Accessed 8 Jan 2021. [DOI] [PMC free article] [PubMed]
- 6.El Shahed AI, Dargaville PA, Ohlsson A, Soll R (2014) Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst Rev 2014, Issue 12. Art. No.: CD002054. 10.1002/14651858.CD002054.pub3. Accessed 8 Jan 2021. [DOI] [PMC free article] [PubMed]
- 7.Bartlett RH. Extracorporeal life support: history and new directions. Semin Perinatol. 2005;29(1):2–7. doi: 10.1053/j.semperi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood B, Newton D, Pallotto EK. Current trends in neonatal ECMO. Semin Perinatol. 2018;42(2):80–88. doi: 10.1053/j.semperi.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Sharma J, Sherman A, Rimal A, Haney B, Weiner J, Pallotto E. Neonatal respiratory extracorporeal membrane oxygenation and primary diagnosis: trends between two decades. J Perinatol. 2020;40(2):269–274. doi: 10.1038/s41372-019-0547-y. [DOI] [PubMed] [Google Scholar]
- 10.Konduri GG, Sokol GM, Van Meurs KP, Singer J, Ambalavanan N, Lee T, et al. Impact of early surfactant and inhaled nitric oxide therapies on outcomes in term/late preterm neonates with moderate hypoxic respiratory failure. J Perinatol. 2013;33(12):944–949. doi: 10.1038/jp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valencia E, Nasr VG. Updates in pediatric extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2020;34(5):1309–1323. doi: 10.1053/j.jvca.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Extracorporeal Life Support Organization (2020) ECLS Registry Report. International summary - July 2020. Available on January 8, 2021; from https://www.elso.org
- 13.Extracorporeal Life Support Organization (2020) ECLS Registry Report. European summary - July 2020. Available on January 8, 2021; from https://www.elso.org
- 14.Karimova A, Brown K, Ridout D, Beierlein W, Cassidy J, Smith J et al (2009) Neonatal extracorporeal membrane oxygenation: practice patterns and predictors of outcome in the UK. Arch Dis Child Fetal Neonatal Ed 94(2), F129-F132. [DOI] [PubMed]
- 15.Kuok CM, Tsao PN, Chen CY, Chou HC, Hsieh WS, Huang SC, Chen YS, Wu ET. Extracorporeal membrane oxygenation support in neonates: a single medical center experience in Taiwan. Pediatr Neonatol. 2017;58(4):355–361. doi: 10.1016/j.pedneo.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Schaible T, Hermle D, Loersch F, Demirakca S, Reinshagen K, Varnholt V. A 20-year experience on neonatal extracorporeal membrane oxygenation in a referral center. Intensive Care Med. 2010;36(7):1229–1234. doi: 10.1007/s00134-010-1886-5. [DOI] [PubMed] [Google Scholar]
- 17.Reiterer F, Resch E, Haim M, Maurer-Fellbaum U, Riccabona M, Zobel G, et al. Neonatal extracorporeal membrane oxygenation due to respiratory failure: a single center experience over 28 years. Front Pediatr. 2018;6(September):1–7. doi: 10.3389/fped.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi P, Glass K, Clark JB, Myers JL, Cilley RE, Ceneviva G, Wang S, Kunselman AR, Ündar A. Clinical outcomes of neonatal and pediatric extracorporeal life support: a seventeen-year, single institution experience. Artif Organs. 2019;43(11):1085–1091. doi: 10.1111/aor.13512. [DOI] [PubMed] [Google Scholar]
- 19.Hsu J, Chang CH, Chiang LT, Caffrey JL, Lin JW, Chen YS (2019) Survival analysis of extracorporeal membrane oxygenation in neonatal and pediatric patients – a nationwide cohort study. J Formos Med Assoc 118(9):1339–1346. 10.1016/j.jfma.2018.12.008 [DOI] [PubMed]
- 20.Fletcher K, Chapman R, Keene S (2018) An overview of medical ECMO for neonates. Semin Perinatol 42(2):68–79 [DOI] [PubMed]
- 21.Extracorporeal Life Support Organization (2017) ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. pp 1–26. Available on January 8, 2021; from https://www.elso.org
- 22.Wild KT, Rintoul N, Kattan J, Gray B, Keene S, Best D et al (2020) Extracorporeal Life Support Organization (ELSO): Guidelines for Neonatal Respiratory Failure. Asaio Journal. 66(5):463–70. [DOI] [PubMed]
- 23.Maclaren G, Conrad S, Peek G, Peek G, Maclaren G, Brodie D. ELSO Guidelines: Indications for pediatric respiratory extracorporeal life support. 2015. pp. 1–8. [Google Scholar]
- 24.Extracorporeal Life Support Organization. ECLS Guidelines 2018 - x Failure. 2018;1–43. Available on January 8, 2021; from http://www.elso.org
- 25.Hardart GE, Hardart MKM, Arnold JH. Intracranial hemorrhage in premature neonates treated with extracorporeal membrane oxygenation correlates with conceptional age. J Pediatr. 2004;145(2):184–189. doi: 10.1016/j.jpeds.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandrappa A, Rosenberg ES, Wagoner S, Jain L. Morbidity and mortality in late preterm infants with severe hypoxic respiratory failure on extra-corporeal membrane oxygenation. J Pediatr. 2011;159(2):192–198.e3. doi: 10.1016/j.jpeds.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMullan DM, Thiagarajan RR, Smith KM, Rycus PT, Brogan TV. Extracorporeal cardiopulmonary resuscitation outcomes in term and premature neonates. Pediatr Crit Care Med. 2014;15(1):9–16. doi: 10.1097/PCC.0b013e3182a553f3. [DOI] [PubMed] [Google Scholar]
- 28.Church JT, Kim AC, Erickson KM, Rana A, Drongowski R, Hirschl RB, Bartlett RH, Mychaliska GB. Pushing the boundaries of ECLS: outcomes in < 34 week EGA neonates. J Pediatr Surg. 2017;52(11):1810–1815. doi: 10.1016/j.jpedsurg.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 29.Revenis ME, Glass P, Short B. Lou. Mortality and morbidity rates among lower birth weight infants (2000 to 2500 grams) treated with extracorporeal membrane oxygenation. J Pediatr. 1992;121(3):452–458. doi: 10.1016/S0022-3476(05)81804-9. [DOI] [PubMed] [Google Scholar]
- 30.Cilley R, Zwischenberger J, Andrews A, Bowerman R, Roloff D, Bartlett R (1986) Intracranial hemorrhage during extracorporeal membrane oxygenation in neonates. Pediatrics 78(4):699–704. [PubMed]
- 31.Di Nardo M, Ore AD, Testa G, Annich G, Piervincenzi E, Zampini G, et al. Principlism and personalism. comparing two ethical models applied clinically in neonates undergoing extracorporeal membrane oxygenation support. Front Pediatr. 2019;7(JULY):1–9. doi: 10.3389/fped.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman RL, Peterec SM, Bizzarro MJ, Mercurio MR. Patient selection for neonatal extracorporeal membrane oxygenation: beyond severity of illness. J Perinatol. 2009;29(9):606–611. doi: 10.1038/jp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American College of Obstetricians and Gynecologists ACOG Committee Opinion No. 394: cesarean delivery on maternal request. Obstet Gynecol. 2007;110(6):1501. doi: 10.1097/01.AOG.0000291577.01569.4c. [DOI] [PubMed] [Google Scholar]
- 34.Yoder B. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002;99(5):731–739. doi: 10.1016/s0029-7844(02)01942-7. [DOI] [PubMed] [Google Scholar]
- 35.Yeo AS, Chong JH, Tan TH, Ng AS, Rajadurai VS, Chan YH (2014) Neonatal and Paediatric Extracorporeal Membrane Oxygenation (ECMO) in a Single Asian Tertiary Centre. Ann Acad Med Singap 43(7):355–61 [PubMed]
- 36.Brown KL, Sriram S, Ridout D, Cassidy J, Pandya H, Liddell M, Davis C, Goldman A, Field D, Karimova A. Extracorporeal membrane oxygenation and term neonatal respiratory failure deaths in the United Kingdom compared with the United States: 1999 to 2005. Pediatr Crit Care Med. 2010;11(1):60–65. doi: 10.1097/PCC.0b013e3181b0644e. [DOI] [PubMed] [Google Scholar]
- 37.Puopolo KM, Lynfield R, Cummings JJ. Management of infants at risk for group B streptococcal disease. Pediatrics. 2019;144(2):e20191881. doi: 10.1542/peds.2019-1881. [DOI] [PubMed] [Google Scholar]
- 38.Nair IS. Prevention of group B streptococcal early-onset disease in newborns: ACOG Committee Opinion, Number 797. Obstet Gynecol. 2020;135(2):e51–e72. doi: 10.1097/AOG.0000000000003668. [DOI] [PubMed] [Google Scholar]
- 39.Rambaud J, Guellec I, Léger PL, Renolleau S, Guilbert J (2015) Venoarterial extracorporeal membrane oxygenation support for neonatal and pediatric refractory septic shock. Indian J Crit Care Med 19(10):600–605 [DOI] [PMC free article] [PubMed]
- 40.Butt WW, Chiletti R (2020) ECMO for neonatal sepsis in 2019. Front. Pediatr 8:50. 10.3389/fped.2020.00050 [DOI] [PMC free article] [PubMed]
- 41.Ford J. Neonatal ECMO: current controversies and trends. Neonatal Netw. 2006;25(4):229–238. doi: 10.1891/0730-0832.25.4.229. [DOI] [PubMed] [Google Scholar]
- 42.Guner YS, Delaplain PT, Zhang L, Di Nardo M, Brogan TV, Chen Y, et al. Trends in mortality and risk characteristics of congenital diaphragmatic hernia treated with extracorporeal membrane oxygenation. ASAIO J. 2019;65(5):509–515. doi: 10.1097/MAT.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kays DW, Islam S, Perkins JM, Larson SD, Taylor JA, Talbert JL. Outcomes in the physiologically most severe congenital diaphragmatic hernia (CDH) patients: whom should we treat? J Pediatr Surg. 2015;50(6):893–897. doi: 10.1016/j.jpedsurg.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg. 2006;16(6):385–391. doi: 10.1055/s-2006-924751. [DOI] [PubMed] [Google Scholar]
- 45.Zalla JM, Stoddard GJ, Yoder BA. Improved mortality rate for congenital diaphragmatic hernia in the modern era of management: 15 year experience in a single institution. J Pediatr Surg. 2015;50(4):524–527. doi: 10.1016/j.jpedsurg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snoek KG, Greenough A, Van Rosmalen J, Capolupo I, Schaible T, Ali K, et al. Congenital diaphragmatic hernia: 10-year evaluation of survival, extracorporeal membrane oxygenation, and foetoscopic endotracheal occlusion in four high-volume centres. Neonatology. 2017;113(1):63–68. doi: 10.1159/000480451. [DOI] [PubMed] [Google Scholar]
- 47.Kays DW. ECMO in CDH: is there a role? Semin Pediatr Surg. 2017;26(3):166–170. doi: 10.1053/j.sempedsurg.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Brogan TV, Lequier L, Lorusso R, Peek G. Congenital diaphragmatic hernia and ECMO. In: Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal life support: the ELSO red book. 5. Michigan: Ann Arbor; 2017. [Google Scholar]
- 49.Grover TR, Rintoul NE, Hedrick HL. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia. Semin Perinatol. 2018;42(2):96–103. doi: 10.1053/j.semperi.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Guner YS, Nguyen DV, Zhang L, Chen Y, Harting MT, Rycus P, Barbaro R, di Nardo M, Brogan TV, Cleary JP, Yu PT. Development and validation of extracorporeal membrane oxygenation mortality-risk models for congenital diaphragmatic Hernia. ASAIO J. 2018;64(6):785–794. doi: 10.1097/MAT.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandrasekharan PK, Rawat M, Madappa R, Rothstein DH, Lakshminrusimha S. Congenital Diaphragmatic hernia – a review. Matern Heal Neonatol Perinatol. 2017;3(1):6. doi: 10.1186/s40748-017-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snoek KG, Reiss IKM, Greenough A, Capolupo I, Urlesberger B, Wessel L, Storme L, Deprest J, Schaible T, van Heijst A, Tibboel D, for the CDH EURO Consortium Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium Consensus - 2015 Update. Neonatology. 2016;110(1):66–74. doi: 10.1159/000444210. [DOI] [PubMed] [Google Scholar]
- 53.Russo FM, Eastwood MP, Keijzer R, Al-Maary J, Toelen J, Van Mieghem T, et al. Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49(6):704–713. doi: 10.1002/uog.16000. [DOI] [PubMed] [Google Scholar]
- 54.Oluyomi-Obi T, Kuret V, Puligandla P, Lodha A, Lee-Robertson H, Lee K, Somerset D, Johnson J, Ryan G. Antenatal predictors of outcome in prenatally diagnosed congenital diaphragmatic hernia (CDH) J Pediatr Surg. 2017;52(5):881–888. doi: 10.1016/j.jpedsurg.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Brogan TV, Lequier L, Lorusso R, Al E, et al. In: Red book. 5. Brogan TV, Lequier L, Lorusso R, et al., editors. Michigan: Ann Arbor; 2017. [Google Scholar]
- 56.Rafat N, Schaible T. Extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Front Pediatr. 2019;7(August):1–9. doi: 10.3389/fped.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puligandla PS, Grabowski J, Austin M, Hedrick H, Renaud E, Arnold M, Williams RF, Graziano K, Dasgupta R, McKee M, Lopez ME, Jancelewicz T, Goldin A, Downard CD, Islam S. Management of congenital diaphragmatic hernia: a systematic review from the APSA outcomes and evidence based practice committee. J Pediatr Surg. 2015;50(11):1958–1970. doi: 10.1016/j.jpedsurg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Guner YS, Khemani RG, Qureshi FG, Wee CP, Austin MT, Dorey F, Rycus PT, Ford HR, Friedlich P, Stein JE. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg. 2009;44(9):1691–1701. doi: 10.1016/j.jpedsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Grover TR, Murthy K, Brozanski B, Gien J, Rintoul N, Keene S, Najaf T, Chicoine L, Porta N, Zaniletti I, Pallotto EK, Children's Hospitals Neonatal Consortium Short-term outcomes and medical and surgical interventions in infants with congenital diaphragmatic hernia. Am J Perinatol. 2015;32(11):1038–1044. doi: 10.1055/s-0035-1548729. [DOI] [PubMed] [Google Scholar]
- 60.Golden J, Jones N, Zagory J, Castle S, Bliss D (2017) Outcomes of congenital diaphragmatic hernia repair on extracorporeal life support. Pediatr Surg Int 33(2):125–131 [DOI] [PubMed]
- 61.Bryner BS, West BT, Hirschl RB, Drongowski RA, Lally KP, Lally P, et al. Congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: does timing of repair matter? J Pediatr Surg. 2009;44(6):1165–1172. doi: 10.1016/j.jpedsurg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Partridge EA, Peranteau WH, Rintoul NE, Herkert LM, Flake AW, Adzick NS, Hedrick HL. Timing of repair of congenital diaphragmatic hernia in patients supported by extracorporeal membrane oxygenation (ECMO) J Pediatr Surg. 2015;50(2):260–262. doi: 10.1016/j.jpedsurg.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Prabhu S, Mattke AC, Anderson B, McBride C, Cooke L, Karl T, Alphonso N. Repair of congenital diaphragmatic hernia during extracorporeal life support: experience with six neonates. ANZ J Surg. 2016;86(9):711–716. doi: 10.1111/ans.13466. [DOI] [PubMed] [Google Scholar]
- 64.Dassinger MS, Copeland DR, Gossett J, Little DC, Jackson RJ, Smith SD. Early repair of congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg. 2010;45(4):693–697. doi: 10.1016/j.jpedsurg.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Fallon SC, Cass DL, Olutoye OO, Zamora IJ, Lazar DA, Larimer EL, Welty SE, Moise AA, Demny AB, Lee TC. Repair of congenital diaphragmatic hernias on extracorporeal membrane oxygenation (ECMO): does early repair improve patient survival? J Pediatr Surg. 2013;48(6):1172–1176. doi: 10.1016/j.jpedsurg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Prodhan P, Stroud M, El-Hassan N, Peeples S, Rycus P, Brogan TV, et al. Prolonged extracorporeal membrane oxygenator support among neonates with acute respiratory failure: a review of the Extracorporeal Life Support Organization registry. ASAIO J. 2014;60(1):63–69. doi: 10.1097/MAT.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 67.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A (2004) The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg 39(3):307–312. Discussion 307-12 [DOI] [PubMed]
- 68.Yeh Y-T, Liu C, Tsai H-L, Wu F-Y, Soong W-J, Lee Y-S, Tsao PC. A combination of tracheoplasty and tracheal stenting is an acceptable method of treating severe congenital tracheobronchial stenosis under extracorporeal membrane oxygenation. J Pediatr Surg. 2019;54(12):2492–2497. doi: 10.1016/j.jpedsurg.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 69.Antón-Pacheco JL, Cano I, Comas J, Galletti L, Polo L, García A, López M, Cabezalí D. Management of congenital tracheal stenosis in infancy. Eur J Cardio-thoracic Surg. 2006;29(6):991–996. doi: 10.1016/j.ejcts.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 70.Howard TS, Kalish BT, Wigmore D, Nathan M, Kulik TJ, Kaza AK, Williams K, Thiagarajan RR. Association of extracorporeal membrane oxygenation support adequacy and residual lesions with outcomes in neonates supported after cardiac surgery*. Pediatr Crit Care Med. 2016;17(11):1045–1054. doi: 10.1097/PCC.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 71.Roeleveld PP, Mendonca M. Neonatal cardiac ECMO in 2019 and beyond. Front Pediatr. 2019;7(August):1–13. doi: 10.3389/fped.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Nardo M, MacLaren G, Marano M, Cecchetti C, Bernaschi P, Amodeo A (2016) ECLS in pediatric cardiac patients. Front Pediatr 7:4 [DOI] [PMC free article] [PubMed]
- 73.Mascio CE, Austin EH, Jacobs JP, Jacobs ML, Wallace AS, He X et al (2014) Perioperative mechanical circulatory support in children: an analysis of the society of thoracic surgeons congenital heart surgery database. J Thorac Cardiovasc Surg 147(2):658–665. 10.1016/j.jtcvs.2013.09.075 [DOI] [PMC free article] [PubMed]
- 74.Shakoor A, Pedroso FE, Jacobs SE, Okochi S, Zenilman A, Cheung EW, Middlesworth W (2019) Extracorporeal cardiopulmonary resuscitation (ECPR) in infants and children: a single-center retrospective study. World J Pediatr Congenit Hear Surg 10(5):582–589 [DOI] [PubMed]
- 75.van Leeuwen Bichara GC, Furlanetto B, Gondim Teixeira L, Di Nardo M (2019) Is peripheral venovenous-arterial ECMO a feasible alternative to central cannulation for pediatric refractory septic shock? Intensive Care Med 45(11):1658–1660 [DOI] [PubMed]
- 76.Hoskote A, Bohn D, Gruenwald C, Edgell D, Cai S, Adatia I, van Arsdell G. Extracorporeal life support after staged palliation of a functional single ventricle: subsequent morbidity and survival. J Thorac Cardiovasc Surg. 2006;131(5):1114–1121. doi: 10.1016/j.jtcvs.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 77.Lin K-M, Li M-H, Hsieh K-S, Kuo H-C, Cheng M-C, Sheu J-J, Lin YJ. Impact of extracorporeal membrane oxygenation on acute fulminant myocarditis-related hemodynamic compromise arrhythmia in children. Pediatr Neonatol. 2016;57(6):480–487. doi: 10.1016/j.pedneo.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Cortina G, Best D, Deisenberg M, Chiletti R, Butt W. Extracorporeal membrane oxygenation for neonatal collapse caused by enterovirus myocarditis. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F370–F376. doi: 10.1136/archdischild-2016-312429. [DOI] [PubMed] [Google Scholar]
- 79.Dyamenahalli U, Tuzcu V, Fontenot E, Papagiannis J, Jaquiss R, Bhutta A, Morrow WR, Erickson CC, Imamura M, Prodhan P (2012) Extracorporeal membrane oxygenation support for intractable primary arrhythmias and complete congenital heart block in newborns and infants. Pediatr Crit Care Med 13(1):47–52 [DOI] [PubMed]
- 80.Ford MA, Gauvreau K, McMullan DM, Almodovar MC, Cooper DS, Rycus PT, et al. Factors associated with mortality in neonates requiring extracorporeal membrane oxygenation for cardiac indications. Pediatr Crit Care Med. 2016;17(9):860–870. doi: 10.1097/PCC.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 81.Teele SA, Salvin JW, Barrett CS, Rycus PT, Fynn-Thompson F, Laussen PC, Thiagarajan RR. The association of carotid artery cannulation and neurologic injury in pediatric patients supported with venoarterial extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2014;15(4):355–361. doi: 10.1097/PCC.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 82.Lasa JJ, Jain P, Raymond TT, Minard CG, Topjian A, Nadkarni V, Gaies M, Bembea M, Checchia PA, Shekerdemian LS, Thiagarajan R. Extracorporeal cardiopulmonary resuscitation in the pediatric cardiac population. Pediatr Crit Care Med. 2018;19(2):125–130. doi: 10.1097/PCC.0000000000001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merrill ED, Schoeneberg L, Sandesara P, Molitor-Kirsch E, O’Brien J, Dai H, et al. Outcomes after prolonged extracorporeal membrane oxygenation support in children with cardiac disease—Extracorporeal Life Support Organization registry study. J Thorac Cardiovasc Surg. 2014;148(2):582–588. doi: 10.1016/j.jtcvs.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 84.Villa CR, Lorts A, Riggs KW, Alten J, Morales DL. How small can you go? A 2.5-kg infant with pulmonary atresia and coronary atresia bridged to cardiac transplantation with a paracorporeal-continuous flow ventricular assist device. J Thorac Cardiovasc Surg. 2019;158(2):e67–e69. doi: 10.1016/j.jtcvs.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 85.Eastaugh LJ, Thiagarajan RR, Darst JR, McElhinney DB, Lock JE, Marshall AC (2015) Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease*. Pediatr Crit Care Med 16(1):59–65 [DOI] [PubMed]
- 86.Pantalos GM, Abel DE, Ravisankar A, Horrell TJ, Lind C, Funk A, Austin EH III, Mascio CE (2012) In vitro pumping performance evaluation of the ension pediatric cardiopulmonary assist system for venoarterial and venovenous ECMO. Cardiovasc Eng Technol 3(3):250–262
- 87.Macchini F, Di Cesare A, Morandi A, Ichino M, Raffaeli G, Conigliaro F et al (2019) Surgical expertise in neonatal extracorporeal membrane oxygenation (ECMO): a single center experience. Front Pediatr 27:7 [DOI] [PMC free article] [PubMed]
- 88.Mayer A, Raffaeli G, Schena F, Parente V, Sorrentino G, Macchini F, et al. Successful extracorporeal membrane oxygenation after incidental azygos vein cannulation in a neonate with right-sided congenital diaphragmatic hernia interruption of the inferior vena cava and azygos continuation. Front Pediatr. 2019;7(October):1–6. doi: 10.3389/fped.2019.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas TH, Price R, Ramaciotti C, Thompson M, Megison S, Lemler MS (2009) Echocardiography, not chest radiography, for evaluation of cannula placement during pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med 10(1):56–59 [DOI] [PubMed]
- 90.O’Halloran CP, Thiagarajan RR, Yarlagadda VV, Barbaro RP, Nasr VG, Rycus P et al (2019) Outcomes of infants supported with extracorporeal membrane oxygenation using centrifugal versus roller pumps: an analysis from the Extracorporeal Life Support Organization Registry. Pediatr Crit Care Med 20(12):1177–1184 [DOI] [PMC free article] [PubMed]
- 91.Raffaeli G, Veneroni C, Ghirardello S, Lavizzari A, Passera S, Mosca F et al (2018) Role of lung function monitoring by the forced oscillation technique for tailoring ventilation and weaning in neonatal ECMO: new insights from a case report. Front Pediatr 6:332. 10.3389/fped.2018.00332 [DOI] [PMC free article] [PubMed]
- 92.Costa J, Dirnberger DR, Froehlich CD, Beaty CD, Priest MA, Ogino MT. Awake Neonatal Extracorporeal Membrane Oxygenation. ASAIO J. 2020;66(5):e70–e73. doi: 10.1097/MAT.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 93.Chen H, Yu RG, Yin NN, Zhou JX. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 2014;18(1):1–11. doi: 10.1186/s13054-014-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stoppe C, Nesterova E, Elke G. Nutritional support in patients with extracorporeal life support and ventricular assist devices. Curr Opin Crit Care. 2018;24(4):269–276. doi: 10.1097/MCC.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 95.Raffaeli G, Pokorna P, Allegaert K, Mosca F, Cavallaro G, Wildschut ED et al (2019) Drug disposition and pharmacotherapy in neonatal ECMO: from fragmented data to integrated knowledge. Front. Pediatr 7:360. 10.3389/fped.2019.00360 [DOI] [PMC free article] [PubMed]
- 96.Raffaeli G, Cavallaro G, Allegaert K, Koch BCP, Mosca F, Tibboel D et al (2020) Sequestration of voriconazole and vancomycin into contemporary extracorporeal membrane oxygenation circuits: an in vitro study. Front. Pediatr 8:468. 10.3389/fped.2020.00468 [DOI] [PMC free article] [PubMed]
- 97.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L (1992) Maturation of the hemostatic system during childhood. Blood 80(8):1998–2005 [PubMed]
- 98.Favaloro EJ, Lippi G (2017) Translational aspects of developmental hemostasis: infants and children are not miniature adults and even adults may be different. Ann Transl Med 5(10):212 [DOI] [PMC free article] [PubMed]
- 99.Cheung PY, Sawicki G, Salas E, Etches PC, Schulz R, Radomski MW. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med. 2000;28(7):2584–2590. doi: 10.1097/00003246-200007000-00067. [DOI] [PubMed] [Google Scholar]
- 100.Monagle P, Newall F, Campbell J. Anticoagulation in neonates and children: pitfalls and dilemmas. Blood Rev. 2010;24(4–5):151–162. doi: 10.1016/j.blre.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 101.Sniderman J, Monagle P, Annich GM, MacLaren G (2020) Hematologic concerns in extracorporeal membrane oxygenation. Res Pract Thromb Haemost 4(4):455–468 [DOI] [PMC free article] [PubMed]
- 102.Cashen K, Meert K, Dalton H. Anticoagulation in neonatal ECMO: an enigma despite a lot of effort! Front Pediatr. 2019;7(September):1–11. doi: 10.3389/fped.2019.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raffaeli G, Tripodi A, Cavallaro G, Cortesi V, Scalambrino E, Pesenti N, Artoni A, Mosca F, Ghirardello S. Thromboelastographic profiles of healthy very low birthweight infants serially during their first month. Arch Dis Child - Fetal Neonatal Ed. 2020;105(4):412–418. doi: 10.1136/archdischild-2019-317860. [DOI] [PubMed] [Google Scholar]
- 104.Tripodi A, Raffaeli G, Scalambrino E, Padovan L, Clerici M, Chantarangkul V, Cavallaro G, Peyvandi F, Mosca F, Ghirardello S. Procoagulant imbalance in preterm neonates detected by thrombin generation procedures. Thromb Res. 2020;185:96–101. doi: 10.1016/j.thromres.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 105.Kamdar A, Rintoul N, Raffini L. Anticoagulation in neonatal ECMO. Semin Perinatol. 2018;42(2):122–128. doi: 10.1053/j.semperi.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Arnold P, Jackson S, Wallis J, Smith J, Bolton D, Haynes S. Coagulation factor activity during neonatal extracorporeal membrane oxygenation. Intensive Care Med. 2001;27(8):1395–1400. doi: 10.1007/s001340100991. [DOI] [PubMed] [Google Scholar]
- 107.Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, Davis AK. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015;29(2):90–101. doi: 10.1016/j.tmrv.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 108.Annich G, Adachi I. Anticoagulation for pediatric mechanical circulatory support. Pediatr Crit Care Med. 2013;14(5 Suppl 1):S37–S42. doi: 10.1097/PCC.0b013e318292dfa7. [DOI] [PubMed] [Google Scholar]
- 109.Saini A, Spinella PC. Management of anticoagulation and hemostasis for pediatric extracorporeal membrane oxygenation. Clin Lab Med. 2014;34(3):655–673. doi: 10.1016/j.cll.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 110.Barton R, Ignjatovic V, Monagle P. Anticoagulation during ECMO in neonatal and paediatric patients. Thromb Res. 2019;173(2017):172–177. doi: 10.1016/j.thromres.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 111.Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001;21(7):1094–1096. doi: 10.1161/hq0701.093686. [DOI] [PubMed] [Google Scholar]
- 112.Bridges B, Ranucci M, Lequier R. Anticoagulation and disorders of haemostasis. In: Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal life support: the ELSO red book. 5. Michigan: Ann Arbor; 2017. pp. 93–103. [Google Scholar]
- 113.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med. 2013;14(2):e77–e84. doi: 10.1097/PCC.0b013e31827127e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khaja WA, Bilen O, Lukner RB, Edwards R, Teruya J. Evaluation of heparin assay for coagulation management in newborns undergoing ECMO. Am J Clin Pathol. 2010;134(6):950–954. doi: 10.1309/AJCPGVD62LKKVDLH. [DOI] [PubMed] [Google Scholar]
- 115.Sulkowski JP, Preston TJ, Cooper JN, Duffy VL, Deans KJ, Chicoine LG, Minneci PC. Comparison of routine laboratory measures of heparin anticoagulation for neonates on extracorporeal membrane oxygenation. J Extra Corpor Technol. 2014;46(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen JB, Svensson B, Olsen R, Ezban M, Osterud B, Paulssen RH. Heparin induces synthesis and secretion of tissue factor pathway inhibitor from endothelial cells in vitro. Thromb Haemost. 2000;83(6):937–943. doi: 10.1055/s-0037-1613946. [DOI] [PubMed] [Google Scholar]
- 117.Padhya DP, Prutsky GJ, Nemergut ME, Schears GS, Flick RP, Farah W, Wang Z, Prokop LJ, Murad MH, Alsawas M. Routine laboratory measures of heparin anticoagulation for children on extracorporeal membrane oxygenation: systematic review and meta-analysis. Thromb Res. 2019;179(February):132–139. doi: 10.1016/j.thromres.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 118.Henderson N, Sullivan JE, Myers J, Wells T, Calhoun A, Berkenbosch J, Tzanetos DT. Use of thromboelastography to predict thrombotic complications in pediatric and neonatal extracorporeal membranous oxygenation. J Extra Corpor Technol. 2018;50(3):149–154. [PMC free article] [PubMed] [Google Scholar]
- 119.Newall F, Johnston L, Ignjatovic V, Monagle P. Unfractionated heparin therapy in infants and children. Pediatrics. 2009;123(3):e510–e518. doi: 10.1542/peds.2008-2052. [DOI] [PubMed] [Google Scholar]
- 120.Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, de Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95(2):362–372. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- 121.Kuhle S, Eulmesekian P, Kavanagh B, Massicotte P, Vegh P, Lau A, et al. Lack of correlation between heparin dose and standard clinical monitoring tests in treatment with unfractionated heparin in critically ill children. Haematologica [Internet] 2007;92(4):554–557. doi: 10.3324/haematol.10696. [DOI] [PubMed] [Google Scholar]
- 122.Maul TM, Wolff EL, Kuch BA, Rosendorff A, Morell VO, Wearden PD. Activated partial thromboplastin time is a better trending tool in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012;13(6):e363–e371. doi: 10.1097/PCC.0b013e31825b582e. [DOI] [PubMed] [Google Scholar]
- 123.Liveris A, Bello RA, Friedmann P, Duffy MA, Manwani D, Killinger JS, Rodriquez D, Weinstein S. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2014;15(2):e72–e79. doi: 10.1097/PCC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 124.Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016;42(4):505–520. doi: 10.1007/s00134-016-4225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wong TE, Nguyen T, Shah SS, Brogan TV, Witmer CM. Antithrombin concentrate use in pediatric extracorporeal membrane oxygenation: a multicenter cohort study∗. Pediatr Crit Care Med. 2016;17(12):1170–1178. doi: 10.1097/PCC.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 126.Stansfield BK, Wise L, Ham PB, Patel P, Parman M, Jin C, et al. Outcomes following routine antithrombin III replacement during neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 2017;52(4):609–613. doi: 10.1016/j.jpedsurg.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 127.Perry R, Stein J, Young G, Ramanathan R, Seri I, Klee L, Friedlich P. Antithrombin III administration in neonates with congenital diaphragmatic hernia during the first three days of extracorporeal membrane oxygenation. J Pediatr Surg. 2013;48(9):1837–1842. doi: 10.1016/j.jpedsurg.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 128.Niebler RA, Christensen M, Berens R, Wellner H, Mikhailov T, Tweddell JS. Antithrombin replacement during extracorporeal membrane oxygenation. Artif Organs. 2011;35(11):1024–1028. doi: 10.1111/j.1525-1594.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 129.Broman LM. When antithrombin substitution strikes back. Perfusion. 2020;35(1_suppl):34–37. doi: 10.1177/0267659120906770. [DOI] [PubMed] [Google Scholar]
- 130.Sanfilippo F, Asmussen S, Maybauer DM, Santonocito C, Fraser JF, Erdoes G, Maybauer MO. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. 2017;32(5):312–319. doi: 10.1177/0885066616656333. [DOI] [PubMed] [Google Scholar]
- 131.Scott LK, Grier LR, Conrad SA. Heparin-induced thrombocytopenia in a pediatric patient receiving extracorporeal membrane oxygenation managed with argatroban. Pediatr Crit Care Med. 2006;7(5):473–475. doi: 10.1097/01.PCC.0000231946.88688.07. [DOI] [PubMed] [Google Scholar]
- 132.Nagle EL, Dager WE, Duby JJ, Roberts AJ, Kenny LE, Murthy MS, et al. Bivalirudin in pediatric patients maintained on extracorporeal life support. Pediatr Crit Care Med. 2013;14(4):5–9. doi: 10.1097/PCC.0b013e31827200b6. [DOI] [PubMed] [Google Scholar]
- 133.Pieri M, Agracheva N, Bonaveglio E, Greco T, De Bonis M, Covello RD, et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: a case-control study. J Cardiothorac Vasc Anesth. 2013;27(1):30–34. doi: 10.1053/j.jvca.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 134.Ranucci M, Ballotta A, Kandil H, Isgrò G, Carlucci C, Baryshnikova E, Pistuddi V, the Surgical and Clinical Outcome Research Group Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care. 2011;15(6):R275. doi: 10.1186/cc10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paden ML, Rycus PT, Thiagarajan RR, ELSO Registry (2014) Update and outcomes in extracorporeal life support. Semin Perinatol 38(2):65–70 [DOI] [PubMed]
- 136.Cashen K, Dalton H, Reeder RW, Saini A, Zuppa AF, Shanley TP, Newth CJL, Pollack MM, Wessel D, Carcillo J, Harrison R, Dean JM, Meert KL, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) (2020) Platelet transfusion practice and related outcomes in pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med 21(2):178–185 [DOI] [PMC free article] [PubMed]
- 137.Verrijckt A, Proulx F, Morneau S, Vobecky S. Activated recombinant factor VII for refractory bleeding during extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2004;127(6):1812–1813. doi: 10.1016/j.jtcvs.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 138.Repessé X, Au SM, Bréchot N, Trouillet JL, Leprince P, Chastre J et al (2013) Recombinant factor VIIa for uncontrollable bleeding in patients with extracorporeal membrane oxygenation: report on 15 cases and literature review Crit Care 17(2), R55. [DOI] [PMC free article] [PubMed]
- 139.Swaminathan M, Shaw AD, Greenfield RA, Grichnik KP. Fatal thrombosis after factor VII administration during extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2008;22(2):259–260. doi: 10.1053/j.jvca.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 140.Downard CD, Betit P, Chang RW, Garza JJ, Arnold JH, Wilson JM. Impact of AMICAR on hemorrhagic complications of ECMO: a ten-year review. J Pediatr Surg. 2003;38(8):1212–1216. doi: 10.1016/S0022-3468(03)00270-7. [DOI] [PubMed] [Google Scholar]
- 141.Bui JD, Despotis GD, Trulock EP, Alexander Patterson G, Goodnough LT. Fatal thrombosis after administration of activated prothrombin complex concentrates in a patient supported by extracorporeal membrane oxygenation who had received activated recombinant factor VII. J Thorac Cardiovasc Surg. 2002;124(4):852–854. doi: 10.1067/mtc.2002.126038. [DOI] [PubMed] [Google Scholar]
- 142.Westrope C, Harvey C, Robinson S, Speggiorin S, Faulkner G, Peek GJ. Pump controlled retrograde trial off from VA-ECMO. ASAIO J. 2013;59(5):517–519. doi: 10.1097/MAT.0b013e31829f5e9f. [DOI] [PubMed] [Google Scholar]
- 143.Duggan EM, Maitre N, Zhai A, Krishnamoorthi H, Voskresensky I, Hardison D, Tice J, Pietsch JB, Lovvorn HN III (2015) Neonatal carotid repair at ECMO decannulation: patency rates and early neurologic outcomes. J Pediatr Surg 50(1):64–68 [DOI] [PMC free article] [PubMed]
- 144.Dalton HJ, Garcia-Filion P, Holubkov R, Moler FW, Shanley T, Heidemann S, Meert K, Berg RA, Berger J, Carcillo J, Newth C, Harrison R, Doctor A, Rycus P, Dean JM, Jenkins T, Nicholson C, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. 2015;16(2):167–174. doi: 10.1097/PCC.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, Moler FW, Shanley T, Pollack MM, Newth C, Berger J, Wessel D, Carcillo J, Bell M, Heidemann S, Meert KL, Harrison R, Doctor A, Tamburro RF, Dean JM, Jenkins T, Nicholson C, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017;196(6):762–771. doi: 10.1164/rccm.201609-1945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bulas D, Glass P. Neonatal ECMO: neuroimaging and neurodevelopmental outcome. Semin Perinatol. 2005;29(1):58–65. doi: 10.1053/j.semperi.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 147.Wien MA, Whitehead MT, Bulas D, Ridore M, Melbourne L, Oldenburg G, Short BL, Massaro AN. Patterns of brain injury in newborns treated with extracorporeal membrane oxygenation. Am J Neuroradiol. 2017;38(4):820–826. doi: 10.3174/ajnr.A5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schiller RM, van den Bosch GE, Muetzel RL, Smits M, Dudink J, Tibboel D, Ijsselstijn H, White T. Neonatal critical illness and development: white matter and hippocampus alterations in school-age neonatal extracorporeal membrane oxygenation survivors. Dev Med Child Neurol. 2017;59(3):304–310. doi: 10.1111/dmcn.13309. [DOI] [PubMed] [Google Scholar]
- 149.Polito A, Barrett CS, Wypij D, Rycus PT, Netto R, Cogo PE, Thiagarajan RR. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensive Care Med. 2013;39(9):1594–1601. doi: 10.1007/s00134-013-2985-x. [DOI] [PubMed] [Google Scholar]
- 150.Parish AP, Bunyapen C, Cohen MJ, Garrison T, Bhatia J. Seizures as a predictor of long-term neurodevelopmental outcome in survivors of neonatal extracorporeal membrane oxygenation (ECMO) J Child Neurol. 2004;19(12):930–934. doi: 10.1177/08830738040190120401. [DOI] [PubMed] [Google Scholar]
- 151.Tian F, Jenks C, Potter D, Miles D, Raman L. Regional cerebral abnormalities measured by frequency-domain near-infrared spectroscopy in pediatric patients during extracorporeal membrane oxygenation. ASAIO J. 2017;63(5):e52–e59. doi: 10.1097/MAT.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Askenazi DJ, Ambalavanan N, Hamilton K, Cutter G, Laney D, Kaslow R, Georgeson K, Barnhart DC, Dimmitt RA (2011) Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med 12(1):e1–e6 [DOI] [PubMed]
- 153.Fleming GM, Sahay R, Zappitelli M, King E, Askenazi DJ, Bridges BC, Paden ML, Selewski DT, Cooper DS. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes. Pediatr Crit Care Med. 2016;17(12):1157–1169. doi: 10.1097/PCC.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Paden ML, Warshaw BL, Heard ML, Fortenberry JD. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(2):153–158. doi: 10.1097/PCC.0b013e3181e2a596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12(3):277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 156.Extracorporeal Life Support Organization (2012) ELSO ID TASK FORCE Recommendation Summary. Available on January 8, 2021, from: https://www.elso.org/Portals/0/Files/ELSO-ID-Task-Force-Recommendations-Summary.pdf
- 157.O’Horo JC, Cawcutt KA, De Moraes AG, Sampathkumar P, Schears GJ. The evidence base for prophylactic antibiotics in patients receiving extracorporeal membrane oxygenation. ASAIO J. 2016;62(1):6–10. doi: 10.1097/MAT.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 158.Glater-Welt LB, Schneider JB, Zinger MM, Rosen L, Sweberg TM. Nosocomial bloodstream infections in patients receiving extracorporeal life support: variability in prevention practices: a survey of the Extracorporeal Life Support Organization members. J Intensive Care Med. 2016;31(10):654–659. doi: 10.1177/0885066615571540. [DOI] [PubMed] [Google Scholar]
- 159.Hsu M-S, Chiu K-M, Huang Y-T, Kao K-L, Chu S-H, Liao C-H. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hosp Infect. 2009;73(3):210–216. doi: 10.1016/j.jhin.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 160.Schiller RM, Madderom MJ, Reuser JJCM, Steiner K, Gischler SJ, Tibboel D et al (2016) Neuropsychological follow-up after neonatal ECMO. Pediatrics 138(5):e20161313; 10.1542/peds.2016-1313 [DOI] [PubMed]
- 161.McNally H, Bennett CC, Elbourne D, Field DJ (2006) United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics 117(5):e845–e854 [DOI] [PubMed]
- 162.Cortes RA, Keller RL, Townsend T, Harrison MR, Farmer DL, Lee H, Piecuch RE, Leonard CH, Hetherton M, Bisgaard R, Nobuhara KK (2005) Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg 40(1):36–46 [DOI] [PubMed]
- 163.Glass P, Bulas D, Wagner A, Rajasingham S, Civitello L, HPapero P et al (1997) Severity of brain injury following neonatal extracorporeal membrane oxygenation and outcome at age 5 years. Dev Med Child Neurol 39(7):441–448 [DOI] [PubMed]
- 164.The Collaborative UK (1998) ECMO Trial: follow-up to 1 year of age. Pediatrics 101(4):e1–e1 [DOI] [PubMed]
- 165.Robertson CMT, Finer NN, Sauve RS, Whitfield MF, Belgaumkar TK, Synnes AR, Grace MG (1995) Neurodevelopmental outcome after neonatal extracorporeal membrane oxygenation. Cmaj 152(12):1981–1988 [PMC free article] [PubMed]
- 166.Khambekar K, Nichani S, Luyt DK, Peek G, Firmin RK, Field DJ, et al. Developmental outcome in newborn infants treated for acute respiratory failure with extracorporeal membrane oxygenation: present experience. Arch Dis Child Fetal Neonatal Ed. 2006;91(1):21–25. doi: 10.1136/adc.2004.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Danzer E, Hoffman C, D’Agostino JA, Gerdes M, Bernbaum J, Antiel RM, et al. Neurodevelopmental outcomes at 5 years of age in congenital diaphragmatic hernia. J Pediatr Surg. 2017;52(3):437–443. doi: 10.1016/j.jpedsurg.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 168.Danzer E, Zarnow D, Gerdes M, D’Agostino JA, Siegle J, Bebbington MW, et al. Abnormal brain development and maturation on magnetic resonance imaging in survivors of severe congenital diaphragmatic hernia. J Pediatr Surg. 2012;47(3):453–461. doi: 10.1016/j.jpedsurg.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 169.Madderom MJ, Reuser JJCM, Utens EMWJ, Van Rosmalen J, Raets M, Govaert P, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: a nationwide multicenter study. Intensive Care Med. 2013;39(9):1584–1593. doi: 10.1007/s00134-013-2973-1. [DOI] [PubMed] [Google Scholar]
- 170.Graziani LJ, Gringlas M, Baumgart S. Cerebrovascular complications and neurodevelopmental sequelae of neonatal ECMO. Clin Perinatol. 1997;24(3):655–675. doi: 10.1016/S0095-5108(18)30163-5. [DOI] [PubMed] [Google Scholar]
- 171.Van Heijst AFJ, De Mol AC, IJsselstijn H. ECMO in neonates: neuroimaging findings and outcome. Semin Perinatol. 2014;38(2):104–113. doi: 10.1053/j.semperi.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 172.Mehta A. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med. 2013;2(4):40–47. doi: 10.5492/wjccm.v2.i4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.IJsselstijn H, Van Heijst AFJ. Long-term outcome of children treated with neonatal extracorporeal membrane oxygenation: increasing problems with increasing age. Semin Perinatol. 2014;38(2):114–121. doi: 10.1053/j.semperi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 174.Macrae DJ, Field DJ. Our study 20 years on: UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(5):841–843. doi: 10.1007/s00134-016-4229-3. [DOI] [PubMed] [Google Scholar]
- 175.Fligor BJ, Neault MW, Mullen CH, Feldman HA, Jones DT. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115(6):1519–1528. doi: 10.1542/peds.2004-0247. [DOI] [PubMed] [Google Scholar]
- 176.Murray M, Nield T, Larson-Tuttle C, Seri I, Friedlich PS. Sensorineural hearing loss at 9-13 years of age in children with a history of neonatal extracorporeal membrane oxygenation. Arch Dis Child Fetal Neonatal Ed. 2011;96(2):128–132. doi: 10.1136/adc.2010.186395. [DOI] [PubMed] [Google Scholar]
- 177.Van Den Hondel D, Madderom MJ, Goedegebure A, Gischler SJ, Mazer P, Tibboel D, et al. Sensorineural hearing loss and language development following neonatal extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2013;14(1):62–69. doi: 10.1097/PCC.0b013e31825b54ae. [DOI] [PubMed] [Google Scholar]
- 178.Schiller RM, Tibboel D. Neurocognitive outcome after treatment with(out) ECMO for neonatal critical respiratory or cardiac failure. Front Pediatr. 2019;7(November):1–9. doi: 10.3389/fped.2019.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Engle WA, West KW, Hocutt GA, Pallotto EK, Haney B, Keith RJ, Stewart DL, Knodel E, Suttner D, Chapman R, Thomas A, Schwerin B, Stork E, Crowley M, Piazza AJ, Heard ML, Gebregziabher N, Fadel W, Bartlett R. Adult outcomes after newborn respiratory failure treated with extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2017;18(1):73–79. doi: 10.1097/PCC.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 180.Madderom M.J. (2013) Long-term Follow-up of Children Treated with Neonatal Extracorporeal Membrane Oxygenation: neuropsychological outcome. Erasmus University Rotterdam. Available on 08 January 2021, from: http://hdl.handle.net/1765/40289
- 181.Toussaint LCC, Van Der Cammen-Van Zijp MHM, Janssen AJ, Tibboel D, Van Heijst AF, Ijsselstijn H (2016) Perceived motor competence differs from actual performance in 8-year-old neonatal ECMO survivors. Pediatrics 137(3):e20152724; 10.1542/peds.2015-2724 [DOI] [PubMed]
- 182.Hofhuis W, Hanekamp MN, Ijsselstijn H, Nieuwhof EM, Hop WCJ, Tibboel D, et al. Prospective longitudinal evaluation of lung function during the first year of life after extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(2):159–164. doi: 10.1097/PCC.0b013e3181e8946e. [DOI] [PubMed] [Google Scholar]
- 183.Hamutcu R, Nield TA, Garg M, Keens TG, Platzker ACG. Long-term pulmonary sequelae in children who were treated with extracorporeal membrane oxygenation for neonatal respiratory failure. Pediatrics. 2004;114(5):1292–1296. doi: 10.1542/peds.2003-1080-L. [DOI] [PubMed] [Google Scholar]
- 184.Jaillard SM, Pierrat V, Dubois A, Truffert P, Lequien P, Wurtz AJ, Storme L. Outcome at 2 years of infants with congenital diaphragmatic hernia: a population-based study. Ann Thorac Surg. 2003;75(1):250–256. doi: 10.1016/S0003-4975(02)04278-9. [DOI] [PubMed] [Google Scholar]
- 185.Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, Stolar CJH, et al. Outcomes of Congenital Diaphragmatic Hernia in the Modern Era of Management. J Pediatr. 2013;163(1):114–119.e1. doi: 10.1016/j.jpeds.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pierog A, Aspelund G, Farkouh-Karoleski C, Wu M, Kriger J, Wynn J, Krishnan U, Mencin A. Predictors of low weight and tube feedings in children with congenital diaphragmatic hernia at 1 year of age. J Pediatr Gastroenterol Nutr. 2014;59(4):527–530. doi: 10.1097/MPG.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 187.Iguchi A, Ridout DA, Galan S, Bodlani C, Squire K, O’Callaghan M, Brown KL. Long-term survival outcomes and causes of late death in neonates, infants, and children treated with extracorporeal life support. Pediatr Crit Care Med. 2013;14(6):580–586. doi: 10.1097/PCC.0b013e3182917a81. [DOI] [PubMed] [Google Scholar]
- 188.Peer SM, Emerson DA, Costello JP, Shu MK, Zurakowski D, Jonas RA, Berger JT, Nath DS (2014) Intermediate-term results of extracorporeal membrane oxygenation support following congenital heart surgery. World J Pediatr Congenit Hear Surg 5(2):236–240 [DOI] [PubMed]
- 189.Garcia Guerra G, Robertson CMT, Alton GY, Joffe AR, Moez EK, Dinu IA, Ross DB, Rebeyka IM, Lequier L, Western Canadian Complex Pediatric Therapies Follow-Up Group Health-related quality of life in pediatric cardiac extracorporeal life support survivors*. Pediatr Crit Care Med. 2014;15(8):720–727. doi: 10.1097/PCC.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 190.Fleck TPK, Dangel G, Bächle F, Benk C, Grohmann J, Kroll J, Siepe M, Höhn R, Kirschner J, Beyersdorf F, Stiller B. Long-Term follow-up on health-related quality of life after mechanical circulatory support in children. Pediatr Crit Care Med. 2017;18(2):176–182. doi: 10.1097/PCC.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 191.Schoberer M, Arens J, Lohr A, Seehase M, Jellema RK, Collins JJ, Kramer BW, Schmitz-Rode T, Steinseifer U, Orlikowsky T. Fifty years of work on the artificial placenta: milestones in the history of extracorporeal support of the premature newborn. Artif Organs. 2012;36(6):512–516. doi: 10.1111/j.1525-1594.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 192.Bryner BS, Mychaliska GB (2014) ECLS for preemies: the artificial placenta. Semin Perinatol 38(2):122–129 [DOI] [PubMed]
- 193.Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD et al (2017) An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun 8:15112 [DOI] [PMC free article] [PubMed]
- 194.Church JT, Perkins EM, Coughlin MA, McLeod JS, Boss K, Bentley JK et al (2018) Perfluorocarbons prevent lung injury and promote development during artificial placenta support in extremely premature lambs. Neonatology 113(4):313–321 [DOI] [PMC free article] [PubMed]
- 195.Mychaliska GB (2016) The artificial placenta: is clinical translation next? Pediatr Pulmonol 51(6):557–559 [DOI] [PMC free article] [PubMed]
- 196.Reoma JL, Rojas A, Kim AC, Khouri JS, Boothman E, Brown K, Grotberg J, Cook KE, Bartlett RH, Hirschl RB, Mychaliska GB (2009) Development of an artificial placenta I: pumpless arterio-venous extracorporeal life support in a neonatal sheep model. J Pediatr Surg 44(1):53–59 [DOI] [PMC free article] [PubMed]
- 197.Kading JC, Langley MW, Lautner G, Jeakle MMP, Toomasian JM, Fegan TL, Pfannes RA, Toor SC, Reiber MA, Kordell PR, Cornell MS, Bartlett RH, Rojas-Pena A, Mychaliska GB (2020) Tidal flow perfusion for the artificial placenta: a paradigm shift. ASAIO J 66(7):796–802 [DOI] [PMC free article] [PubMed]
- 198.Liu T, Liu S, Zhang K, Chen J, Huang N (2014) Endothelialization of implanted cardiovascular biomaterial surfaces: the development from in vitro to in vivo. J Biomed Mater Res - Part A 102(10):3754–3772 [DOI] [PubMed]
- 199.Lavery KS, Rhodes C, Mcgraw A, Eppihimer MJ (2017) Anti-thrombotic technologies for medical devices. Adv Drug Deliv Rev 112:2–11 [DOI] [PubMed]
- 200.Goh ET, Wong E, Farhatnia Y, Tan A, Seifalian AM (2015) Accelerating in situ endothelialisation of cardiovascular bypass grafts. Int J Mol Sci 16(1):597–627 [DOI] [PMC free article] [PubMed]
- 201.Pang JH, Farhatnia Y, Godarzi F, Tan A, Rajadas J, Cousins BG, Seifalian AM (2015) In situ endothelialization: bioengineering considerations to translation. Small 11(47):6248–6264 [DOI] [PubMed]
- 202.Maul TM, Nelson JS, Wearden PD (2018) Paracorporeal lung devices: thinking outside the box. Front Pediatr 6(September):1–13 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A