Abstract
Background:
Neurocysticercosis (NCC) is the most common parasitic infection of the brain and a leading cause of epilepsy in resource-limited settings. While NCC and Human Immunodeficiency Virus (HIV) co-infections have commonly been reported, there is little data on how they interact. As part of an observational study of HIV and cognition in Lusaka, Zambia, we identified a cluster of subjects with NCC. We hypothesized that neighborhood of residence may be an important factor driving clustering of NCC.
Methods:
34 subjects with HIV and 13 subjects without HIV (ages 8-17) were enrolled in a prospective cohort study. All subjects had Magnetic Resonance Imaging (MRI) of the brain performed and were evaluated for NCC. Standardized interviews were conducted to identify potential risk factors for NCC. Quantitative Geographic Information Systems (QGIS) was utilized to investigate the relationship between neighborhood of residence, HIV, and NCC.
Results:
Three of 34 subjects with HIV (8.82%) and one of 13 controls were found to have NCC. Geographic cluster analysis demonstrated that all subjects with NCC were clustered in two adjacent neighborhoods (Chawama and Kanyama) with lower rates of piped water (C-22.8%, K-26.7%) and flush toilets (C-14.0%, K-14.0%) than surrounding neighborhoods.
Discussion:
These results suggest that NCC cases among subjects with HIV may be partly attributable to residence in areas with low rates of piped water and limited access to flush toilets. Despite the small sample size, this study has identified the need for further research on NCC and HIV co-infection, and demonstrated the utility of geographic analysis in identifying potential associations.
Introduction:
Despite widespread introduction of combination antiretroviral therapy (cART), human immunodeficiency virus (HIV) remains a major cause of morbidity and mortality among children, with more than 1.8 million children infected worldwide [1]. HIV infection remains extremely prevalent in Zambia, with a national prevalence rate of 11.5% among adults ages 15-49 (UN AIDS), and more than 90,000 children living with HIV[1]. Numerous studies have found developmental delays, cognitive impairment, and abnormal neurological examinations in children with HIV [2], but there is little published research on the contribution of co-infections to neurologic outcomes in children with HIV. Despite its widespread prevalence and strong association with morbidity in resource-limited settings, neurocysticercosis (NCC) has received comparatively little attention compared to HIV [3]. Cysticercosis results from the ingestion of Taenia solium eggs by fecal-oral contamination, and causes NCC when parasites migrate to the brain and form cysts. Pigs are the intermediate host of taenia solium, and contact with pigs or undercooked pork is a major vector of transmission. NCC is the most common parasitic infection of the central nervous system with estimates of approximately 50 million people infected worldwide, although estimates are probably low due to subclinical disease and few reliable data on prevalence [3,4]. In addition, NCC is the most common cause of epilepsy in resource-limited settings and causes significant neuropsychiatric sequelae including cognitive impairment, memory loss and dementia [5–9]. NCC is endemic in Zambia, with some studies suggesting more than half of new cases of epilepsy in regions with the highest pig ownership are due to NCC [10,11]. Human cysticercosis involves a complex host-parasite interaction in which the immune response of the host and the level of the inflammatory reaction determine the symptoms and clinical manifestations of the disease [12] .
Because HIV and NCC are both highly prevalent diseases in many resource-limited settings, simultaneous diagnosis of both would be expected to occur in a substantial number of subjects even without a pathophysiologic interaction [13]. While NCC and HIV co-infections have intermittently been reported in case reports or small case series [13,14], there are little data on whether co-infection modifies the pathophysiology of either disorder. In addition, HIV may increase susceptibility to NCC by decreasing host immune responses that would limit systemic spread of cysticercosis [15,16]. Because so little is known about the influence of HIV infection on the frequency and the clinical progression of NCC, further research is necessary to investigate this potential association.
This research was conducted as a sub-study of the HIV-Associated Neurocognitive Disorders in Zambia (HANDZ) study. HANDZ is a prospective cohort study with a nested case-control component in Lusaka, Zambia evaluating cognitive outcomes among children and adolescents living with HIV (n=208) compared to HIV-exposed, uninfected (HEU) controls (n=208). Demographic data and Magnetic Resonance Imaging (MRI) were obtained from 47 subjects from this cohort of subjects. We identified 4 cases of NCC among the 47 imaged subjects (8.5%) in two adjacent constituencies, which is an unexpectedly high number within a small area. Lusaka, the capital city, is divided into “constituencies,” large neighborhoods defined geographically similar to zip code areas with single-member representation in the National Assembly of Zambia. These constituencies formed the basic unit of geographic analysis in this study. Subjects were mapped into their constituency of residency, and Geographic Information Systems (GIS) software was used to examine the relationship between HIV, NCC, and socioeconomic factors in Lusaka, Zambia.
Methods
Study Design & Setting:
This study was conducted as a sub-study of the HIV-Associated Neurocognitive Disorders in Zambia (HANDZ) study, a longitudinal study of cognition in children with and without HIV. In the primary HANDZ cohort, children and adolescents with HIV (n=208) were recruited from outpatient visits at the Pediatric Center of Excellence (PCOE) in Lusaka, Zambia, which is the major outpatient HIV care referral center in the country and serves children throughout the city of Lusaka as well as some children referred from other provinces. HIV exposed-uninfected (HEU) controls (n=208) were recruited from the community by a community health worker, with stratified sampling to ensure the control population was similar in age, sex, and neighborhood of residence.
Data Collection:
Each participant completed a standardized interview, chart review, and comprehensive neuropsychological testing and data were collected and managed using REDCap electronic data capture tools hosted at the University of Rochester Medical Center [17]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies. Subjects were seen at baseline and every 3 months of follow up for a planned total of 3 years. Subjects’ residence was identified on a map of Lusaka and approximate longitude and latitude was plotted. A socioeconomic status index ranging from 1 to 12 was constructed utilizing measures of income, wealth, parental education, and housing quality.
Magnetic resonance imaging (MRI) of the brain was conducted on the first 34 eligible subjects with HIV. The first 13 eligible control subjects were then enrolled utilizing stratified sampling to ensure a similar distribution of age and sex compared to imaged subjects with HIV. All subjects in the MRI sub-study with NCC identified on imaging were seen for a follow up visit to discuss MRI results, and had a structured interview and neurologic exam conducted by a neurologist to further assess any signs or symptoms of NCC. All subjects with NCC were referred for further clinical evaluation and treatment according to local guidelines.
MRI Protocol and Analysis:
Brain MRI sequences were performed using a 1.5T Siemens scanner located at Cancer Disease Hospital at University Teaching Hospitals in Lusaka, Zambia. No sedation or contrast agents were used. Sequences included: localizer scouts (3-plane); whole-brain sagittal three dimensional (SD) T1 weighted gradient echo sequence; T1, T2 and FLAIR sequences; DWI and ADC sequences; and Magnetic Resonance Angiography sequences. Each scan was anonymized and reviewed by a neuroradiologist with expertise in imaging in resource-limited settings who was blinded to HIV status and clinical history of the subject. MRI reads were annotated using the NeuroInterp database system [18]. NCC was identified based on MRI findings using standard diagnostic criteria, and categorized as noncystic, vesicular, colloidal vesicular and/or granular nodular. The location and number of NCC lesions was reported, as well as any associated findings. All subjects with NCC had their scans reviewed by a neurologist with expertise in NCC to ensure there was consensus on the diagnosis of NCC. Discordant reads were planned to be adjudicated by a second neuroradiologist; however, there were no discordant reads in this study.
Geographic Analysis:
Using demographic data and tools from Geographic Information Systems (GIS), geographical analysis was conducted on all HIV-infected and HEU subjects in the HANDZ study, regardless of participation in the MRI sub study. From the participant demographic forms, longitude and latitude coordinates of the subject’s addresses were approximated using Google Maps and OpenStreetMap. Maps were generated by importing the dataset from the HANDZ study with GPS coordinates and shapefiles of Zambian maps into QGIS 3.2.0 [19]. A specific shapefile map of the Lusaka constituencies was created and saved as the base layer. The dataset was merged with the GPS coordinates using the ‘Join’ tool in QGIS. With this analysis, the goal was to geographically place all study subjects (HIV-infected and HEU-matched controls) on a map of Lusaka, and visualize the location of those who were co-infected with NCC.
Confidentiality:
To protect the confidentiality of all subjects in the HANDZ study, the maps constructed are zoomed out to view the entire city without specific landmarks and the subject points are enlarged to prevent identification of individual residences.
Statistical Methods:
Standard statistical analyses were conducted using Microsoft Excel and Stata 14 (Version 14.2, College Station, Texas). Differences between subjects with NCC and subjects without NCC were evaluated using Fisher’s exact test for dichotomous variables, t-tests for normally distributed continuous variables, and Kruskall-Wallis rank test for non-normally distributed continuous variables. A manual backwards logistic regression model was used to evaluate risk factors. Clustering analysis was done using SaTScan software (Version 9.6; http://www.satscan.org) using a Bernoulli model. The Bernoulli data set was analyzed with purely spatial scan statistics. This creates a circular window centered on each possible grid point, creating an infinite number of distinct geographical circles with each circle evaluated as a possible candidate cluster. A p-value cutoff for significance was not utilized in the NCC cluster as the MRI substudy of HANDZ is a pilot study and was not powered to detect a specific effect size. A p-value cutoff of 0.05 was used for the cluster analysis for access to running water and pit latrine. Sample size was determined for the MRI substudy of HANDZ based on volumetric differences between groups; thus, this should be viewed as a convenience sample. Subjects without HIV could not be included in the SatScan analysis because they were recruited matched on neighborhood of residence. Therefore, neighborhood of residents was not random in subjects without HIV.
Ethics Statement:
This study was approved by the institutional review boards of the University of Rochester (protocol #00068985), the University of Zambia (reference #004-08-17), and the National Health Research Authority of Zambia. Verbal and written informed consent was sought from the parents of all subjects for participation in the study, and verbal and written assent was obtained from all subjects ages 12 and older.
Results:
Of 47 subjects within HANDZ who underwent MRI, 4 subjects (8.5% of total) were found to have NCC, with imaging suggesting the vesicular (active) phase of cysticercosis in all subjects. Demographic characteristics of subjects with and without NCC are described in Table 2, while a comparison of HIV+ vs. HIV− subjects is presented in Table 3. Subjects with NCC were unrelated to each other and had no direct contact with each other. All subjects were of black race. Prevalence of NCC was slightly higher among HIV infected subjects (8.8% vs 7.7%), though small sample size limits comparison between these groups. Subjects with NCC came from families with lower socioeconomic status index (6 vs. 6.5) and were less likely to live in households with running water (25% vs. 51%) and flush toilets (25% vs. 43%). However, none of these differences were statistically significant (See Table 2). All subjects with NCC denied any direct contact with livestock, pigs, or undercooked/uncooked meat. In a multivariable logistic regression model adjusting for age, sex, and socioeconomic status, factors associated with NCC included HIV status (OR 1.3), running water (adjusted OR 0.21) and presence of flush toilets in the home (adjusted OR 0.34). We evaluated whether risk factors for NCC were different in subjects with HIV and subjects without HIV. Access to running water and flush toilets were both significantly higher in subjects with HIV (see Table 3). All subjects with HIV were taking combination antiretroviral therapy, and the vast majority had undetectable viral loads, were classified at Clinical Stage 1 (i.e. asymptomatic HIV) according to the World Health Organization (WHO) HIV/AIDS Clinical Staging System [20], and had CD4 counts in the normal range. There were no differences in HIV-disease specific measures between subjects with and without NCC (see Table 4). Two of the subjects with NCC had single lesions while the other two subjects had multi-focal lesions. Two of the four subjects with neurocysticercosis (both HIV+) developed recurrent seizures during the follow up period of the study. Both subjects were treated with antiparasitics and anticonvulsants with resolution of seizures.
Table 2.
Demographics of Subjects with NCC and without NCC
| w/ NCC (n=4) | w/o NCC (n=43) | P value | |
|---|---|---|---|
| Mean age (+/− SD) | 11.00 +/− 3.83 | 12.49 +/− 2.35 | 0.26 |
| Percent Female (f/m) | 75% (3/1) | 49% (21/22) | 0.61 |
| HIV (+/−) | 75% (3/1) | 72% (31/12) | 1.00 |
| Median Socioeconomic Status Index | 6 (4.5-8) | 6.5 (5-9) | 0.09 |
| Running Water (yes/no) | 25% (1/3) | 51% (22/21) | 0.61 |
| Flush Toilet (yes/no) | 25% (1/3) | 43% (18/24) | 0.63 |
| Electricity (with/without) | 100% (4/0) | 91% (39/4) | 1.00 |
Table 3.
Demographics of Subjects with HIV and without HIV
| w/ HIV (n=34) | w/o HIV (n=13) | P value | |
|---|---|---|---|
| Mean Age | 12.4 +/− 2.55 | 12.2 +/− 2.42 | 0.83 |
| Percent Female (f/m) | 53% (18/16) | 46% (6/7) | 0.75 |
| Running Water (with/within) | 62% (21/13) | 15% (2/11) | 0.008 |
| Toilet (pit latrine/flush toilet) | 47% (16/17) (n=33) | 85% (11/2) | 0.044 |
| Electricity (with/without) | 94% (32/2) | 85% (11/2) | 0.30 |
Table 4.
HIV-Specific Characteristics of HIV+ Subjects with and without NCC
| w/ NCC (n=3) | w/o NCC (n=31) | P value | |
|---|---|---|---|
| Average Time on cART (years) | 5.67 +/− 3.79 | 7.45 +/− 2.23 | 0.22 |
| Most Recent CD4 Count | 778 +/− 312 | 676.64 +/− 266 | 0.54 |
| Most Recent WHO Stage (Stage 1/2/3/4) | 3/0/0/0 | 29/0/2/0 | 1.00 |
| Viral Load Undetectable (%) (undetectable/detectable) | 100% (3/0) | 81% (25/6) | 1.00 |
Geographic analysis demonstrated that the four subjects with NCC resided in two adjacent constituencies/neighborhoods (Chawama and Kanyama; see Figure 1). Other study subjects were distributed roughly equally over all constituencies of Lusaka that were represented in the study. Individual analysis of subjects with NCC did not reveal any other significant differences from the background study population. However, geographic analysis from HANDZ demographic data of Chawama and Kanyama demonstrated the lowest rates of running water among the constituencies in the Lusaka area (Chawama 22.8%, Kanyama 26.7%, compared to average rates of 54.9% in study subjects outside of those constituencies) and among the lowest rates of flush toilets (Chawama 14.0%, Kanyama 14.0%; compared to average rates of 47.1%; see Table 5 and Figure 2).
Figure 1 -.

MRI Subjects by Constituency in Lusaka
Alexandra Buda 3/26/19 EPSG:20934 Arc 1950/UTM Zone 34S
Stanford Earthworks, Google Maps, OpenStreetMaps, Central Statistical Office of Zambia
Table 5.
Access to Water, Electricity, Toilets, and Food Security by Constituency
| Constituency | Number of Subjects in MRI Study | Number of Subjects in HANDZ | Access to Running Water | Electricity | Flush Toilets | Food Insecurity |
|---|---|---|---|---|---|---|
| Chawama* | 9 | 57 | 22.8% | 87.7% | 14.0% | 66.7% |
| Chilanga | 2 | 23 | 39.1% | 56.5% | 30.4% | 43.5% |
| Chongwe | 1 | 6 | 50.0% | 50.0% | 33.3% | 50.0% |
| Kabwata | 5 | 38 | 68.4% | 86.8% | 68.4% | 44.7% |
| Kanyama* | 12 | 86 | 26.7% | 75.6% | 14.0% | 65.1% |
| Katuba | 1 | 9 | 33.3% | 44.4% | 33.3% | 55.6% |
| Lusaka Central | 1 | 11 | 36.4% | 63.6% | 27.3% | 54.5% |
| Mandevu | 8 | 120 | 27.5% | 72.5% | 9.2% | 70.8% |
| Matero | 5 | 21 | 66.7% | 85.7% | 66.7% | 42.9% |
| Munali | 3 | 29 | 72.4% | 89.7% | 55.2% | 51.7% |
| Total | 47 | 402 |
= Constituency with NCC
Figure 2 -.
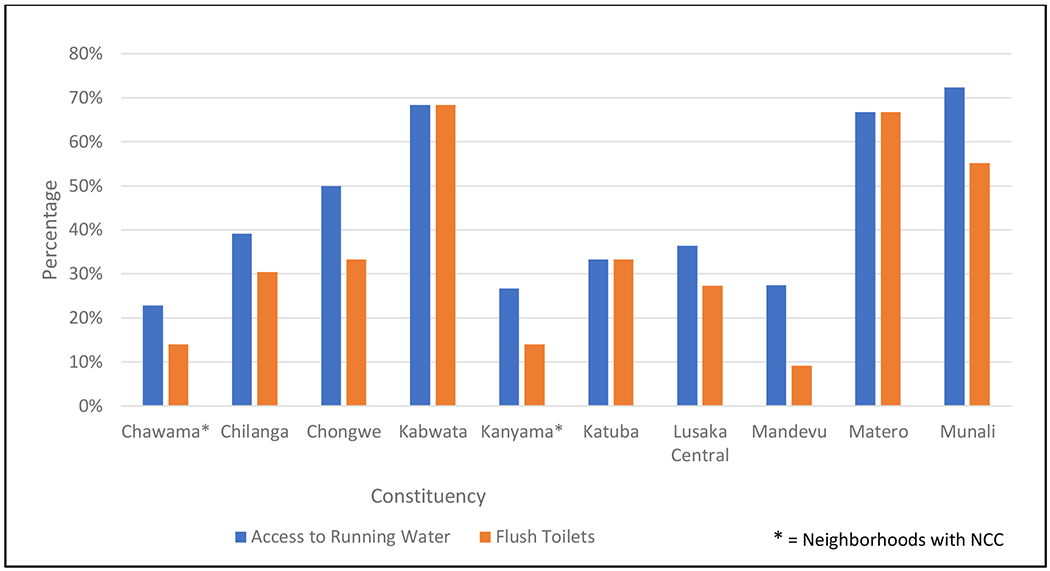
HANDZ Data: Access to Running Water and Flush Toilets
The SaTScan cluster analysis detected geographic clustering of NCC in these constituencies (see Figure 3). There was a 4-km radius cluster detected of 3 cases of NCC among the 34 subjects with HIV. Prevalence of NCC among HIV+ scanned subjects in this area was 25%, compared to the study population prevalence of 8.8%. (Observed/expected ratio of 2.83; log likelihood ratio 3.4).
Figure 3 –
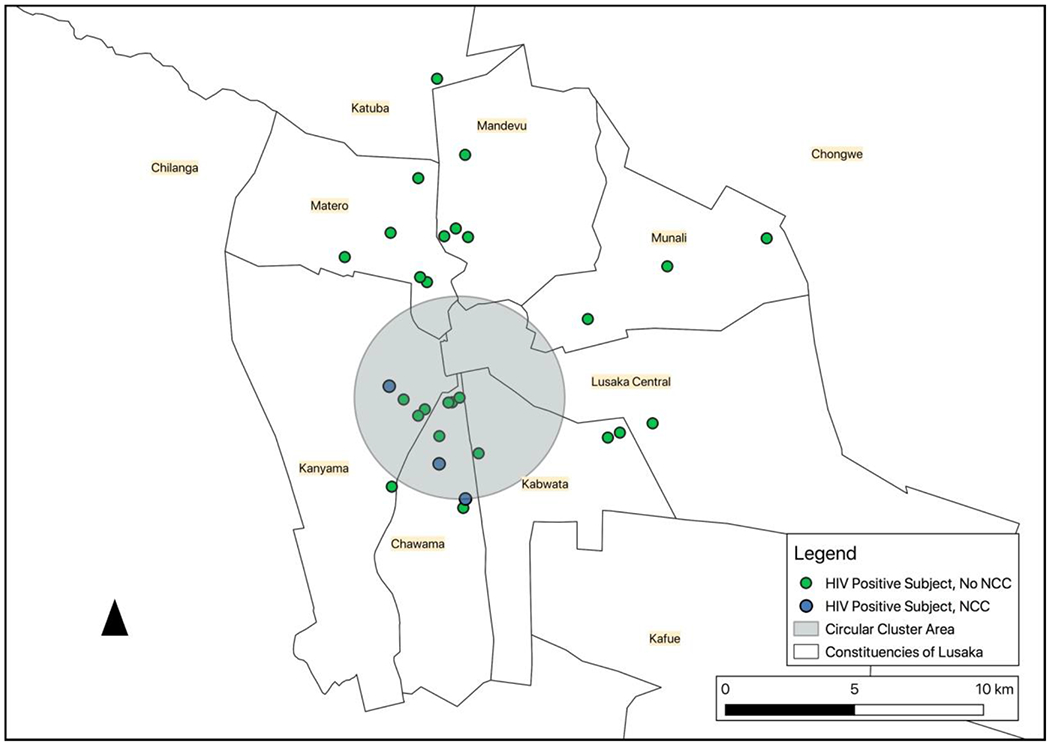
NCC Cluster in Lusaka
Alexandra Buda 3/26/19 EPSG:20934 Arc 1950/UTM Zone 34S
Stanford Earthworks, Google Maps, OpenStreetMaps, Central Statistical Office of Zambia
A second SaTScan cluster analysis detected geographic clustering of lack of water access and pit latrines among all of the subjects in HANDZ (n=395) (see Table 6 and Figure 4). There were two clusters (A+B) that showed high rates of pit latrines compared to the study population rate of 74.2%. Additionally, there were two clusters (C+D) that showed high rates of lack of running water compared to the study population rate of 62.3%.
Table 6:
Cluster Analysis Data
| Cluster | Radius | Rate | P-Value | Observed/Expected Ratio | Log Likelihood Ratio |
|---|---|---|---|---|---|
| Cluster A (Green) | 3.53 km | 94.7% (pit latrine) | <0.001 | 1.28 | 16.89 |
| Cluster B (Green) | 5.25 km | 90.7% (pit latrine) | <0.001 | 1.22 | 13.68 |
| Cluster C (blue) | 3.12 km | 82.4% (lack of water) | <0.001 | 1.32 | 12.78 |
| Cluster D (blue) | 4.31 km | 83.0% (lack of water) | <0.001 | 1.33 | 12.28 |
Figure 4 –
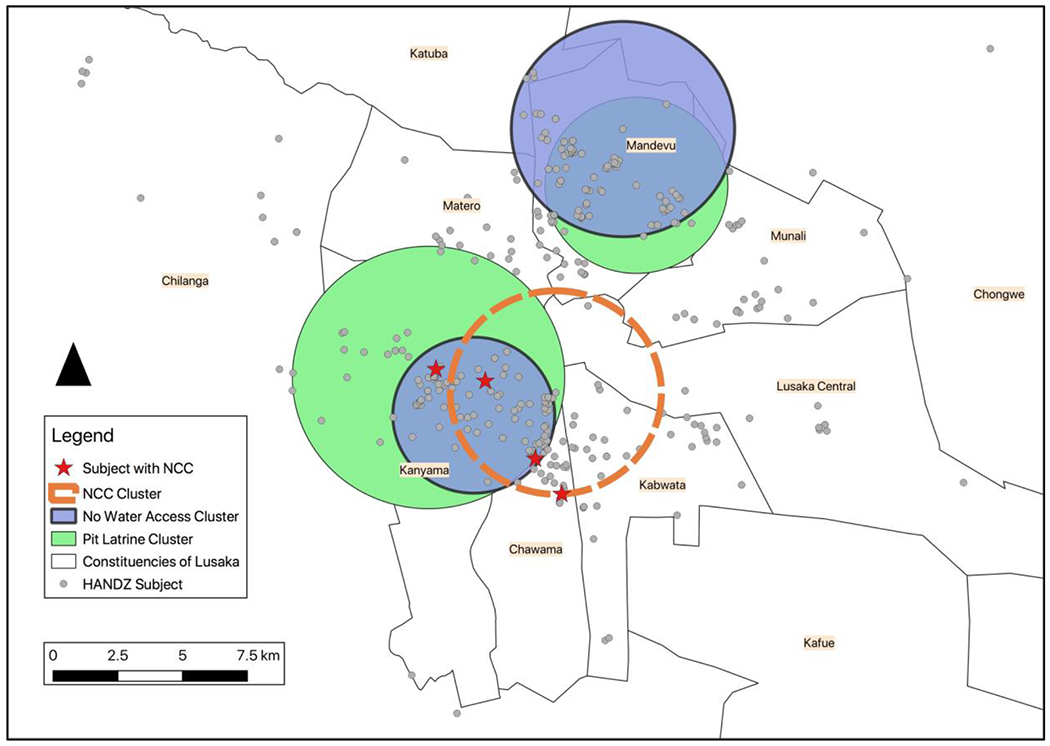
Lack of Water Access Cluster and Pit Latrine Clusters
Alexandra Buda 4/22/19 EPSG:20934 Arc 1950/UTM Zone 34S
Stanford Earthworks, Google Maps, OpenStreetMaps, Central Statistical Office of Zambia
Discussion
In this prospective study, we investigated possible clustering of cases of NCC among pediatric patients with and without HIV infection in Lusaka, Zambia. Absence of running water in the home and lack of flush toilets were identified as possible risk factors for NCC. Although small sample size prevents us from making any definitive conclusions, the association of NCC with lack of access to clean water and lack of adequate toilet facilities is consistent with what has previously been reported in the literature. Statistical testing indicated geographic clustering at a rate that would be unexpected by chance. All four subjects with NCC were from constituencies (Kanyama and Chawama) with low rates of piped water, limited access to flush toilets, and lower average socioeconomic status than surrounding areas. We did not identify any HIV-specific risk factors for NCC. However, our ability to detect immunosuppression-related effects on NCC was limited by the fact that subjects in the HANDZ study were almost all virally suppressed with relatively preserved immune function.
Although mapping of NCC cases by region or in relation to putative risk factors has previously been performed [21], recent advances in the technology of street level mapping open up the possibility of identifying clustering of infectious diseases at a very high level of spatial resolution. Our study suggests neighborhood of residence and socioeconomic factors may be risk factors in developing NCC. HIV has previously been reported in association with NCC in several case reports and small case series [13–15,22]. A single case control study to investigate the association between HIV and NCC failed to find an effect; however, this study was limited by small sample size and very low prevalence of NCC [14]. Further studies should investigate immune mechanisms that might predispose patients with HIV to develop NCC.
The prevalence of NCC (8.5%) was much higher in our study than expected. This was especially notable as subjects with known epilepsy were specifically excluded from participation, which should have resulted in lower rates of NCC in this population. To our knowledge, there have been no prior studies of NCC prevalence in the general population in Zambia. However, seroprevalence of cysticercosis in Zambia has ranged from 6.3%-12%, 5.8%-13% and 34%-39% based on copro-Ag-ELISA, serum Ag-ELISA, and sero-antibody detection, respectively [23]. The high rates of NCC in our study may have been driven in part by the HIV status of the majority of subjects. It is equally possible that these higher rates are driven by neighborhood effects, that is, subjects with HIV in Zambia may tend to live in poorer neighborhoods with increased risk of exposure to NCC.
Screening for neurocysticercosis should be considered among subjects presenting with new onset seizures in regions in which cysticercosis is prevalent. However, optimal screening strategies remain to be determined as neuroimaging is unavailable in many locations in which NCC is common. Where imaging is available, screening with CT or MRI is advised. Other screening strategies or potential prophylactic treatment for high risk patients need to be investigated in future studies.
Limitations, Bias, and Generalizability:
The small sample size limited our ability to perform statistical hypothesis testing. A larger appropriately powered future study is necessary to confirm the association between HIV and NCC and to investigate whether there are HIV-specific risk factors for NCC. A further limitation of this study is that we were only able to identify cysticercosis in subjects with lesions on MRI imaging. Cysticercosis cases without MRI lesions were not able to be evaluated in this study, as no serologic testing was performed. Another possible limitation is that we did not collect data on travel history to rural areas. While subjects denied any direct contact with pigs or undercooked meat, they may have traveled outside Lusaka which was not specifically asked. In addition, this study was performed at a single center in an urban area in Zambia, and generalizability to other areas with lower prevalence of cysticercosis and HIV may be limited. As we recruited subjects from a referral center, this could have biased our estimates of prevalence of NCC, as sicker patients may be more likely to attend a referral center. However, since all subjects were asymptomatic at the time of recruitment, and all were healthy outpatients recruited for this study during medication refill visits, this is unlikely to have contributed to bias in our study. Another possible limitation is that subjects with epilepsy were excluded from the parent HANDZ study. The HANDZ study is being used as a control population for the epilepsy research study in Zambia. Due to this prior research, subjects with epilepsy were excluded from the HANDZ study. This may have resulted in low rates of NCC due to subjects with epilepsy being excluded.
Conclusion
This study demonstrates the utility of geographic analysis in visualizing clustering of NCC cases, and can provide a blueprint for future studies. Further research on NCC and HIV must be done to explore association between socioeconomic characteristics and lifestyle factors that may make children more susceptible to co-infection. Geographic clustering of NCC in poorer neighborhoods with limited access to clean water and flush toilets as well as high rates of HIV may be one factor driving the association.
Supplementary Material
Table 1.
Inclusion/Exclusion Criteria for HANDZ Conducted in Lusaka, Zambia with Enrollment from 2017-2018
| Inclusion Criteria for pediatric subjects with HIV: | • Age 8-17 • Perinatally infected with HV with diagnosis confirmed by Western blot or DNA PCR • Participant currently taking cART with initiation of cART having taken place at least one year prior to enrollment |
| Inclusion Criteria for HIV-exposed Uninfected Controls: | • Age 8-17 • HIV negative status confirmed by immunoassay • Participant’s mother was HIV infected during or prior to pregnancy |
| Exclusion Criteria: | • Subjects with history of known CNS infection with organism other than HIV • Known history of epilepsy • Pregnancy • Subjects with chronic or acute medical or psychiatric conditions other than HIV that could potentially impact study participation or results including psychosis, malignancy, or chronic liver or renal failure • Subjects were excluded from the imaging sub-study if they had any medical or psychiatric condition which would inhibit their ability to have an unsedated MRI, including severe claustrophobia or indwelling metal objects. |
Acknowledgments
Other Information
This work was supported by the URMC Office of Medical Education International Research Grant and the HIV Associated Neurocognitive Disorders in Zambia (HANDZ) study. Support for HANDZ comes from the University of Rochester Center for AIDS Research (CFAR), an NIH funded program (P30 AI 045008), and the McGowan Foundation.
References
- 1.UNAIDS. Zambia Data Factsheet. 2017; http://www.unaids.org/en/regionscountries/countries/zambia. Accessed October 1, 2019.
- 2.Cohen S, Ter Stege JA, Geurtsen GJ, et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin Infect Dis. 2015;60(7):1111–1119. [DOI] [PubMed] [Google Scholar]
- 3.Fogang YF, Savadogo AA, Camara M, et al. Managing neurocysticercosis: challenges and solutions. Int J Gen Med. 2015;8:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia HH, Gonzalez AE, Evans CA, Gilman RH, Cysticercosis Working Group in P. Taenia solium cysticercosis. Lancet. 2003;362(9383):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciampi de Andrade D, Rodrigues CL, Abraham R, et al. Cognitive impairment and dementia in neurocysticercosis: a cross-sectional controlled study. Neurology. 2010;74(16):1288–1295. [DOI] [PubMed] [Google Scholar]
- 6.Forlenza OV, Filho AH, Nobrega JP, et al. Psychiatric manifestations of neurocysticercosis: a study of 38 patients from a neurology clinic in Brazil. J Neurol Neurosurg Psychiatry. 1997;62(6):612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez-Bermudez J, Higuera-Calleja J, Espinola-Nadurille M, Corona T. Neuropsychiatric disorders in patients with neurocysticercosis. Asia Pac Psychiatry. 2017;9(2). [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues CL, de Andrade DC, Livramento JA, et al. Spectrum of cognitive impairment in neurocysticercosis: differences according to disease phase. Neurology. 2012;78(12):861–866. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava S, Chadda RK, Bala K, Majumdar P. A study of neuropsychiatric manifestations in patients of neurocysticercosis. Indian J Psychiatry. 2013;55(3):264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwape KE, Blocher J, Wiefek J, et al. Prevalence of Neurocysticercosis in People with Epilepsy in the Eastern Province of Zambia. PLoS Negl Trop Dis. 2015;9(8):e0003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia HH, Rodriguez S, Friedland JS, Cysticercosis Working Group in P. Immunology of Taenia solium taeniasis and human cysticercosis. Parasite Immunol. 2014;36(8):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto Hernandez JL, Ostrosky Zeichner L, Tavera G, Gomez Avina A. Neurocysticercosis and HIV infection: report of two cases and review. Surg Neurol. 1996;45(1):57–61. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt V, Kositz C, Herbinger KH, et al. Association between Taenia solium infection and HIV/AIDS in northern Tanzania: a matched cross sectional-study. Infect Dis Poverty. 2016;5(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delobel P, Signate A, El Guedj M, et al. Unusual form of neurocysticercosis associated with HIV infection. Eur J Neurol. 2004; 11(1):55–58. [DOI] [PubMed] [Google Scholar]
- 16.Prasad S, MacGregor RR, Tebas P, Rodriguez LB, Bustos JA, White AC Jr. Management of potential neurocysticercosis in patients with HIV infection. Clin Infect Dis. 2006;42(4):e30–34. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potchen MJ, Kampondeni SD, Ibrahim K, et al. NeuroInterp: a method for facilitating neuroimaging research on cerebral malaria. Neurology. 2013;81(6):585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.QGIS Geographic Information System [computer program]. Version 3.2.0: Open Source Geospatial Foundation Project, http://qgis.osgeo.org/; 2018. [Google Scholar]
- 20.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach ANNEX 10, WHO clinical staging of HIV disease in adults, adolescents and children. 2nd ed Geneva: 2016: https://www.ncbi.nlm.nih.gov/books/NBK374293/. [PubMed] [Google Scholar]
- 21.Mwanjali G, Kihamia C, Kakoko DV, et al. Prevalence and risk factors associated with human Taenia solium infections in Mbozi District, Mbeya Region, Tanzania. PLoS Negl Trop Dis. 2013;7(3):e2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chianura L, Sberna M, Moioli C, Villa MR, Orcese C, Causarano R. Neurocysticercosis and human immunodeficiency virus infection: a case report. J Travel Med. 2006;13(6):376–380. [DOI] [PubMed] [Google Scholar]
- 23.Gabriel S, Mwape KE, Phiri IK, Devleesschauwer B, Dorny P. Taenia solium control in Zambia: The potholed road to success. Parasite Epidemiol Control. 2019;4:e00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


