Abstract
Across species, lifespan is highly variable among individuals within a population. Even genetically identical Caenorhabditis elegans reared in homogeneous environments are as variable in lifespan as outbred human populations. We hypothesized that persistent inter-individual differences in expression of key regulatory genes drives this lifespan variability. As a test, we examined the relationship between future lifespan and the expression of 22 microRNA promoter::GFP constructs. Surprisingly, expression of nearly half of these reporters, well before death, could effectively predict lifespan. This indicates that prospectively long- vs. short-lived individuals have highly divergent patterns of transgene expression and transcriptional regulation. The gene-regulatory processes reported on by two of the most lifespan-predictive transgenes do not require DAF-16, the FOXO transcription factor that is a principal effector of insulin/insulin-like growth factor (IGF-1) signaling. Last, we demonstrate a hierarchy of redundancy in lifespan-predictive ability among three transgenes expressed in distinct tissues, suggesting that they collectively report on an organism-wide, cell non-autonomous process that acts to set each individual’s lifespan.
Research organism: C. elegans
Introduction
Across the tree of life, lifespan varies greatly, not only among species but also among individuals of the same species (Jones et al., 2014). Much effort has been devoted to discovering genetic and environmental factors associated with longevity in humans (Milman and Barzilai, 2016; Moskalev et al., 2014; Dato et al., 2017), and many lifespan-extending perturbations in model organisms like C. elegans have been discovered (Kenyon, 2010; Kenyon et al., 1993; Lucanic et al., 2013). However, little is known about how differences in lifespan arise among individuals within a population. Studies of identical twins demonstrate that lifespan is not particularly heritable, estimating that at most 30% of the variation in human lifespan can be attributed to genetics alone (Herskind et al., 1996; Ljungquist et al., 1998; Skytthe et al., 2003; McGue et al., 1993). Moreover, accounting for assortative mating reduces that figure to less than 10% (Ruby et al., 2018). Other factors like shared environment explain very little of the remaining variation between individuals (Herskind et al., 1996; Ljungquist et al., 1998; McGue et al., 1993). A reasonable fraction of the variance in human longevity is thus determined by non-genetic, non-environmental factors, likely of stochastic origin. Understanding what these processes are and how they arise provides insight into why some individuals live longer than others.
While these questions are difficult to study in humans, the self-fertile hermaphrodite Caenorhabditis elegans is an ideal model for investigating the role of stochastic events in the aging process. Even genetically identical C. elegans raised in standardized environments have widely different lifespans, displaying a degree of variability similar to that of genetically diverse human populations (Kirkwood et al., 2005; Vaupel et al., 1998; Zhang et al., 2016; Pincus et al., 2011). Specifically, the coefficient of variation (CV) of lifespan in inbred C. elegans is comparable to that of outbred human populations (CV estimates for C. elegans range from 0.19 to 0.23 vs. 0.17–0.21 in humans) (Zhang et al., 2016; Stroustrup et al., 2013; Gavrilova et al., 2012; Arias and Xu, 2017). The genetics of C. elegans aging are well understood (Kenyon, 2010) and its external environment is easily controlled. Furthermore, the short lifespan (≈2 weeks at 20°C), the ability to generate clonal, genetically identical populations, and the optically transparent body of C. elegans make the organism a tractable model to study how and why differences in lifespan arise.
It is often assumed that differences in lifespan among identical individuals are the result of differences in the random accumulation of damage over time. That is, by chance, some individuals encounter more frequent or injurious assaults from their external and/or internal environment than others, resulting in paths that become increasingly divergent with age. In this model, differences in exogenous or endogenous damage precede late-life biological differences between individuals (e.g. differences in gene expression), and ultimately differences in lifespan.
An alternate hypothesis, however, is that pre-existing biological differences lead some individuals to be more vs. less tolerant to such damage in the first place – which would then produce differences in ultimate lifespan. This might come about by a mechanism such as hormesis, in which individuals exposed to a biological stressor upregulate stress-response programs that persist past the original exposure and confer resistance to future stresses (Kumsta et al., 2017; Lithgow et al., 1995; Cypser and Johnson, 2002). Chance events early in life might cause some individuals but not others to stochastically enter into organismal states of heightened stress-vigilance, effectively committing them to longer future lifespans. Even small, stochastic fluctuations in the activity level of key regulatory genes could become stabilized and amplified by positive feedback loops, locking different individuals into distinct biological states. Indeed, feedback-stabilization of mutually exclusive gene-expression states is a hallmark of fate commitment decisions, from the lysis vs. lysogeny switch in bacteriophage lambda (Ptashne, 2011) to lineage commitment in hematopoiesis (Kato and Igarashi, 2019) and other cell types (Wang et al., 2009; Ferrell, 2012).
If long vs. short life is the result of stable differences in the gene-regulatory programs executed by different individuals, then long- and short-lived individuals should be distinguishable early in life by the expression of genes that are regulated by those programs. In other words, we propose that identifying genes whose expression early in life is predictive of future lifespan would provide strong evidence that differences in gene regulation and expression can lead previously identical individuals toward different lifespans.
Indeed, several genes and regulatory processes have been identified whose expression, long before death, predicts future lifespan in isogenic C. elegans (Pincus et al., 2011; Sánchez-Blanco and Kim, 2011; Rea et al., 2005; Bazopoulou et al., 2019). Expression of hsp-16.2 after exposure to heat shock correlates with the degree of hormetic lifespan extension afforded by that heat shock (Rea et al., 2005). This appears to be due to variable activation of thermosensory neurons after heat shock, leading (via variable IIS activity in intestinal cells) to heat-shock responses of different strengths (Burnaevskiy et al., 2019; Mendenhall et al., 2017). Likewise, expression of several genes associated with aging and/or IIS, including sod-3, a stress response gene often used as a reporter for the activity of the IIS-responsive transcription factor DAF-16, correlate with future lifespan when measured at middle age in unperturbed individuals (Sánchez-Blanco and Kim, 2011). Other more phenomenological predictors of lifespan, such as movement (Zhang et al., 2016; Hsu et al., 2009), size (Zhang et al., 2016; Pincus et al., 2011), redox state (Bazopoulou et al., 2019), and accumulation of autofluorescent material (Zhang et al., 2016; Pincus et al., 2011; Pincus et al., 2016) have also been described.
MicroRNAs (miRNAs), short non-coding RNAs that repress translation of many target transcripts, have been identified as both positive and negative markers of future longevity in C. elegans. Expression of mir-71 and mir-246, measured via fluorescence of promoter::GFP reporters, positively correlates with future lifespan in isogenic individuals, while expression of mir-239 negatively correlates (Pincus et al., 2011). Genetic manipulation of these miRNAs directly extends (mir-71, mir-246) or shortens (mir-239) lifespan through the insulin/insulin-like growth factor (IGF-1) signaling (IIS) and DNA damage response pathways, demonstrating that genetic predictors of lifespan may also act as functional determinants of longevity (de Lencastre et al., 2010). Other miRNAs have also been shown to both promote or antagonize longevity in C. elegans through canonical aging pathways (Boehm, 2005; Smith-Vikos et al., 2014; Yang et al., 2013).
While multiple predictive biomarkers of lifespan have been previously reported, these studies have been limited to small sets of candidate genes, generally hand-picked as reporters of either chronological age or IIS activity. Broadly, much of the work in this area, including our own, can be read to suggest that variability in IIS, which can be read out via (presumably rare) ‘biomarkers of longevity’, accounts for the bulk of variability in individual lifespan. In this work, we systematically revisit those conclusions by examining a large collection of promoter::GFP transgenes for their relationship with lifespan.
To this end, we employed a high-density individual culturing device developed by our lab (Zhang et al., 2016; Pittman et al., 2017) and automated microscopy and image processing to identify additional miRNA biomarkers of longevity. We chose to examine miRNAs because they regulate gene expression of many targets, they have been previously implicated in aging and lifespan, and, unlike transcription factors, their activity is well-represented by fluorescent promoter::GFP reporters. We screened 22 PmiRNA::GFP reporter strains and found 10 in which GFP levels robustly predict future lifespan among isogenic C. elegans. We used two of the most lifespan-predictive miRNA reporters, Pmir-47::GFP and Pmir-243::GFP, to investigate the specific pathways underlying stochastic variation in longevity. Our findings demonstrate that unlike most known biomarkers of longevity, these reporters are independent of the daf-16 branch of the IIS pathway in their ability to predict lifespan; however, the microRNAs mir-47 and mir-243 themselves are not functional determinants of lifespan. Analysis of dual reporter strains indicates that there is redundancy between predictive PmiRNA::GFP reporters, indicating that they act in a hierarchical manner to report on shared, cell non-autonomous lifespan determinants.
Overall, we find evidence that a large fraction of microRNA promoters (and, thus, perhaps all promoters) are engaged by transcriptional programs that reflect future lifespan. We find little evidence that these programs are specific to certain tissue types; indeed it appears that at least three GFPs redundantly report on a single, organism-wide, cell non-autonomous, daf-16-independent state that is associated with future lifespan. The fact that half of the promoter::GFP strains we tested did not correlate with lifespan suggests that this global state is transcriptional in nature: any lifespan-associated post-transcriptional mechanism would affect all GFP transcripts or proteins identically, regardless of the promoter sequence driving GFP expression. This stands in contrast to the case of interindividual variation in Phsp-16.2::GFP after heat shock, which is related, via IIS activity, to a global state of enhanced or decreased protein stability (Burnaevskiy et al., 2019). At a minimum, the transcriptional state we identified reflects early changes in organismal physiology leading to early vs. late death; at most, this state may in fact determine those physiological changes.
Results
Longitudinal observation of PmiRNA::GFP reporters
To identify genes whose expression early in life is predictive of future lifespan, we selected 22 integrated miRNA fluorescent reporters (PmiRNA::GFP) from a larger library of 73 transgenic strains (Martinez et al., 2008), using as the only selection criterion the ability to detect reporter fluorescence with short (<100 ms) exposure times at ×5 magnification on our imaging system. Most of the miRNAs corresponding to the selected reporters have no reported function, and in particular, no lifespan phenotype upon knockout (Table 1).
Table 1. miRNAs corresponding to PmiRNA::GFP reporters selected for this study and the predominant expression pattern and time window of expression, as observed at ×5 magnification.
Published regulatory functions and lifespan phenotypes for each miRNA are noted. Bold text indicates miRNAs that we found to be predictive of lifespan in this current study, as measured by correlation of PmiRNA::GFP expression with lifespan.
| miRNA | Expression pattern | Time window of expression | Regulatory function | Lifespan phenotype |
|---|---|---|---|---|
| let-7 | Ubiquitous | Embryo–death | Developmental timing (Reinhart et al., 2000) | – |
| lin-4 | Ubiquitous | L1–death | Developmental timing (Wightman et al., 1993) | lin-4(e912) are short-lived; lin-4 overexpression extends lifespan (Boehm, 2005) |
| mir-1 | Pharynx | Embryo–death | Synaptic function (Simon et al., 2008) | – |
| mir-228 | Neurons | Embryo–death | – | mir-228(n4382) are long-lived; mir-228 overexpression shortens lifespan (Smith-Vikos et al., 2014) |
| mir-240–786 | Uterus, gonad sheath | L3–death | Defecation (Kemp et al., 2012) | – |
| mir-241 | Hypodermis | L1–four dph | Developmental timing (Abbott, 2005) | – |
| mir-242 | Neurons | Embryo–death | – | – |
| mir-243 | Intestine | Embryo–death | – | – |
| mir-246 | Gonad sheath | L4–death | – | mir-246(n4636) are short-lived; mir-246 overexpression extends lifespan (de Lencastre et al., 2010) |
| mir-360 | Pharynx | Embryo–death | – | – |
| mir-47 | Hypodermis, vulva | Embryo–death | – | – |
| mir-51 | Intestine | Embryo–death | Developmental timing (Brenner et al., 2012), GABAergic synapses (Zhang et al., 2018) | – |
| mir-59 | Vulva | L4–death | – | – |
| mir-60 | Intestine | Embryo–death | Oxidative stress response (Kato et al., 2016) | mir-60(n4947) are long-lived under oxidative stress conditions (Kato et al., 2016) |
| mir-63 | Intestine | Embryo–death | – | – |
| mir-788 | Hypodermis | Embryo–four dph | – | – |
| mir-79 | Hypodermis | Embryo–five dph | Proteoglycan homeostasis (Pedersen et al., 2013) | – |
| mir-793 | Neurons | Embryo–death | – | – |
| mir-794 | Body wall muscle, intestine | Embryo–four dph | – | – |
| mir-84 | Pharynx, vulva | L1–death | Developmental timing (Abbott, 2005) | – |
| mir-85 | Gonadal sheath, uterus, spermatheca | L2–death | – | – |
| mir-90 | Body wall muscle, vulva | Embryo–death | – | – |
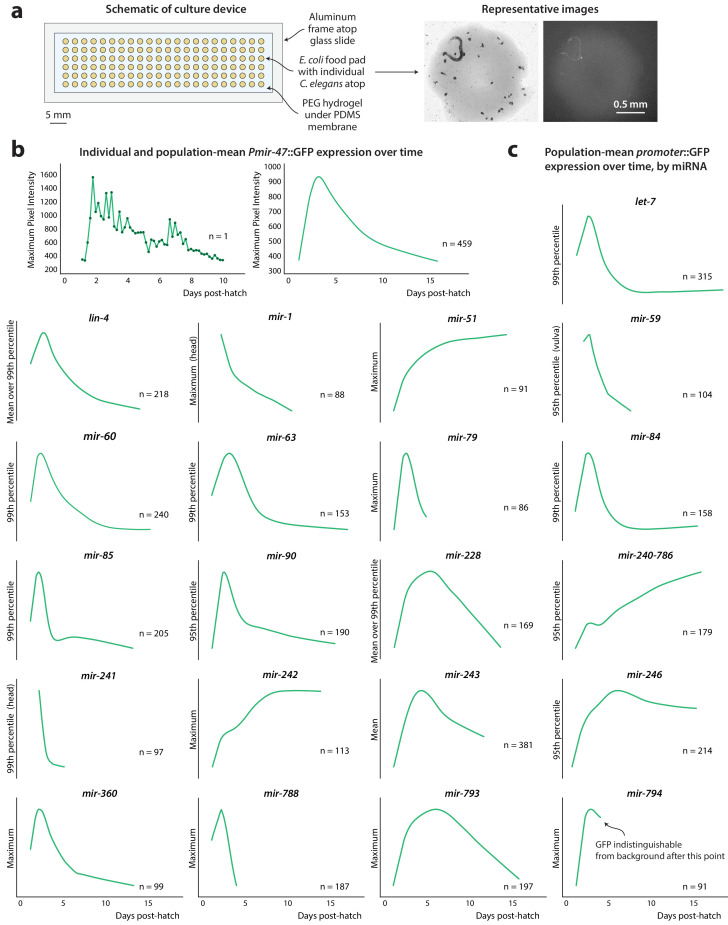
Each reporter was crossed into the temperature-sensitive sterile strain spe-9(hc88) and examined in a high-density single animal culture device previously developed by our lab (Figure 1a; Zhang et al., 2016; Pittman et al., 2017) maintained at 25°C. For each PmiRNA::GFP;spe-9(hc88) strain, we collected bright-field and fluorescence images of each individual every 4 hr from hatch until death. Using an in-house image analysis pipeline, the C. elegans in each bright-field image was automatically identified, defining a mask that separates ‘worm pixels’ from the image background. We converted images of GFP fluorescence, which contain many thousands of pixel intensities within the image region corresponding to a single animal, into a single summary statistic. This allowed us to quantify PmiRNA::GFP expression over time within a single individual (Figure 1b, left; see Materials and methods for details). We then averaged the fluorescence measurements across all individuals to capture the overall population trend in expression of each reporter over time (Figure 1b, right, and 1 c), which is consistent with individual expression trends (Figure 1—figure supplement 2). For some reporters, such as Pmir-788::GFP, expression analysis was limited to shortened time windows, as late in life, reporter fluorescence becomes indistinguishable from background autofluorescence (see Table 1).
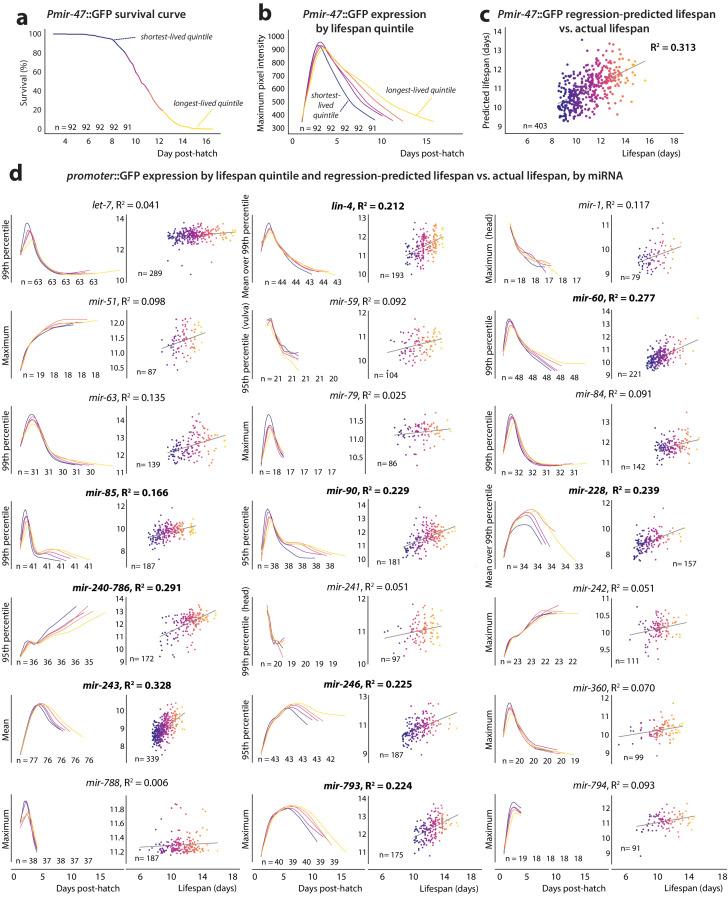
Figure 1. PmiRNA::GFP expression over time.
(a) Schematic of high-density single animal culture device and representative bright-field and fluorescent images acquired from a single Pmir-47::GFP; spe-9(hc88) individual. (b) Timecourse of Pmir-47::GFP expression for a single animal from hatch until death (left). Expression is measured as the maximum pixel intensity within the image region comprising that individual, from images acquired every 4 hr. LOWESS regression showing the average population timecourse of Pmir-47::GFP expression, collated from five biological replicates comprising more than 400 individuals (right). (c) Population timecourse of expression for all PmiRNA::GFP reporters. (Fluorescent values are in arbitrary units; in all cases the bottom of the y-axis is approximately the noise floor of the camera sensor).
Figure 1—figure supplement 1. Representative fluorescence images taken of individuals at 3 dph (top right panels) and 7 dph (bottom right panels) from each PmiRNA::GFP reporter strain.
Figure 1—figure supplement 2. Timecourse of expression for 10 randomly selected individuals from all PmiRNA::GFP reporters.
Figure 1—figure supplement 3. LOWESS trends for expression of predictive PmiRNA::GFP reporters over time (solid line) and standard deviation (dashed line).
We observed several categories of temporal dynamics among the reporters. Expression of some, such as Plet-7::GFP, Pmir-788::GFP, Pmir-79::GFP, Pmir-85::GFP, and Pmir-84::GFP, peak during young adulthood and sharply decline thereafter, in some cases becoming undetectable with age. Expression of others, like Pmir-793::GFP, Pmir-246::GFP, and Pmir-228::GFP, also decrease with age but exhibit a slower decline or plateau after young adulthood. Overall, the majority of PmiRNA::GFP reporters decreased with age, in agreement with previously published microarray (Ibáñez-Ventoso et al., 2006) and small-RNA sequencing studies (de Lencastre et al., 2010; Kato et al., 2011). Unusual for miRNAs, Pmir-51::GFP, Pmir-242::GFP, and Pmir-240–786::GFP show an increase in expression over time (Figure 1c). This increase was also observed with small RNA sequencing (de Lencastre et al., 2010; Kato et al., 2011), indicating that the PmiRNA::GFP reporters generally reflect age-related trends in endogenous transcription of miRNAs. In contrast to sequencing, however, this work provides a more detailed picture of expression trends over time.
PmiRNA::GFP reporters are predictive biomarkers of future lifespan
In order to visualize the relationship between PmiRNA::GFP expression and longevity (if any), we binned populations of the PmiRNA::GFP reporter strains into cohorts based on eventual lifespan (Figure 2a) and plotted average reporter expression for each lifespan-cohort over time (Figure 2b and d). For a subset of PmiRNA::GFP reporters, the average expression level and rate of change of expression appeared to differ substantially between cohorts in mid- to late- adulthood, indicating that reporter expression may be predictive of an individual’s future lifespan. To test this quantitatively, we performed a multivariate regression on each individual animal’s average level of GFP expression and the trend in GFP expression (positive or negative slope) against future lifespan (Figure 2c and d; Table 2). Because the temporal dynamics of the different PmiRNA::GFP reporters vary substantially, we used a sliding time window to determine the optimal span of expression data to include in the regression for each reporter strain. We examined GFP expression bounded between 3 days post-hatch (dph; approximately the first day of adulthood) and the time of 90% population survival. Note that that the time of 90% survival varied among strains, due to per-strain differences and, to a lesser extent, batch and seasonal effects (Table 2). We performed regressions using GFP levels at all possible windows within the overall bounds, by independently moving beginning and ending timepoints of the window at 12 hr intervals. Individuals that died before or within the chosen window were censored from analysis to avoid truncation effects (these short-lived individuals necessarily have fewer expression measurements, which can confounding analysis). The window that resulted in the maximum correlation between expression and lifespan was selected (Table 2). Similar results obtain using a simple, fixed time-window between 3 days post-hatch and the 90% survival timepoint, or repeating the variable-window analysis bounded between three dph and the 95% survival timepoint (tables S1 and S2 in Supplementary file 1).
Figure 2. PmiRNA::GFP expression vs.future lifespan.
(a) Survival curve for Pmir-47::GFP; spe-9(hc88) individuals pooled from five biological replicates. Animals are binned into color-coded quintiles based on eventual lifespan. The number of individuals in each quintile is indicated. (b) LOWESS regression of each quintile’s average Pmir-47::GFP expression (as measured by maximum intensity per individual at each timepoint) is plotted over time for the quintiles in panel a. (c) Joint regression of both the mean level of each individual’s Pmir-47::GFP expression between 5 and 8.5 days post-hatch, and the slope of that expression over that time, against future lifespan yields an R2 of 0.313. Each dot represents an individual animal and is color-coded based on lifespan. (d) Cohort-level expression timecourse and joint regression of slope and mean expression against future lifespan for all PmiRNA::GFP reporters. This mean and slope were measured across the optimal time window for each reporter’s correlation with future lifespan (specified in Table 2). Correlation coefficients > 0.15 are indicated by bolded text.
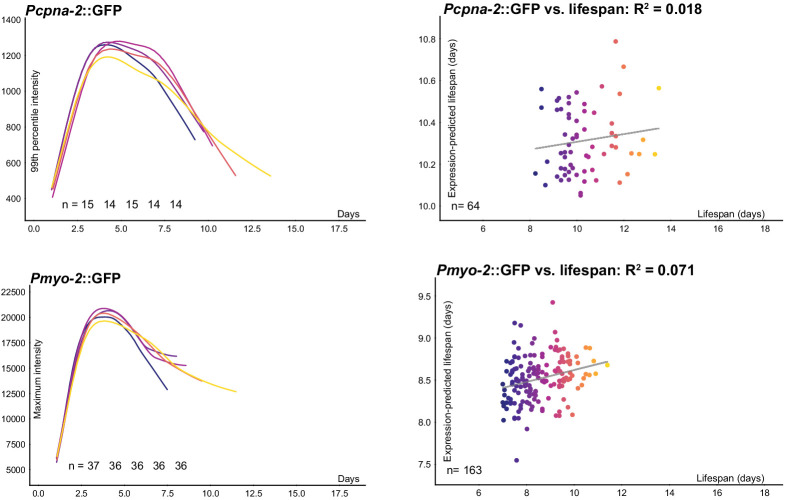
Figure 2—figure supplement 1. Expression of non-miRNA reporters Pcpna-2::GFP and Pmyo-2::GFP is plotted over time for each lifespan cohort.
Table 2. Correlation of PmiRNA::GFP reporters with lifespan.
A joint regression of slope and average expression against lifespan was performed using a sliding time window (minimum width of 12 hr) beginning at 3 days post-hatch and ending at the 90% survival timepoint, which we observed to be variable between strains. The optimal time window and highest correlation achieved is reported. R2 values exceeding 0.15 are indicated by bolded text. The 95% confidence interval (CI) for each joint correlation coefficient is also shown. The direction of correlation, derived from the individual regression on slope and mean expression (which we observed to always correlate in the same direction), is indicated by (+) or (-), respectively.
| miRNA | N | Measure of pixel intensity | Time window (dph) | Slope R2 | Mean R2 | Joint R2 | Joint 95% CI |
|---|---|---|---|---|---|---|---|
| let-7 | 289 | 99th percentile | 6.0–9.5 | 0.024 | 0.012 | 0.041 | [−0.002, 0.085] |
| lin-4 | 193 | Mean over 99th percentile | 4.5–9.0 | 0.211 | 0.029 | 0.212 (+) | [0.124, 0.306] |
| mir-1 | 79 | Maximum (head) | 6.0–7.5 | 0.102 | 0.025 | 0.117 | [0.008, 0.234] |
| mir-47 | 403 | Maximum | 5.0–8.5 | 0.158 | 0.158 | 0.313 (+) | [0.240, 0.388] |
| mir-51 | 87 | Maximum | 8.0–8.5 | 0.025 | 0.048 | 0.098 | [−0.010, 0.211] |
| mir-59 | 104 | 95th percentile (vulva) | 4.5–5.0 | 0.002 | 0.092 | 0.092 | [−0.020, 0.186] |
| mir-60 | 221 | 99th percentile | 7.5–8.5 | 0.035 | 0.238 | 0.277 (+) | [0.137, 0.402] |
| mir-63 | 139 | 99th percentile | 5.0–9.5 | 0.118 | 0.053 | 0.135 | [0.047, 0.230] |
| mir-79 | 86 | Maximum | 3.5–4.0 | 0.005 | 0.010 | 0.025 | [−0.033, 0.076] |
| mir-84 | 142 | 99th percentile | 9.0–9.5 | 0.000 | 0.091 | 0.091 | [−0.016, 0.187] |
| mir-85 | 187 | 99th percentile | 3.5–7.0 | 0.157 | 0.052 | 0.166 (+) | [0.075, 0.257] |
| mir-90 | 181 | 95th percentile | 8.0–8.5 | 0.014 | 0.215 | 0.229 (+) | [0.116, 0.340] |
| mir-228 | 157 | Mean over 99th percentile | 5.0–7.0 | 0.194 | 0.070 | 0.239 (+) | [0.129, 0.350] |
| mir-240–786 | 172 | 95th percentile | 7.5–8.5 | 0.043 | 0.268 | 0.291 (-) | [0.168, 0.419] |
| mir-241 | 97 | 99th percentile (head) | 3.0–3.5 | 0.013 | 0.037 | 0.051 | [−0.038, 0.133] |
| mir-242 | 111 | Maximum | 4.5–5.5 | 0.050 | 0.010 | 0.051 | [−0.041, 0.130] |
| mir-243 | 339 | Mean | 3.5–7.5 | 0.299 | 0.110 | 0.328 (+) | [0.254, 0.409] |
| mir-246 | 187 | 95th percentile | 6.5–8.5 | 0.124 | 0.124 | 0.225 (+) | [0.124, 0.332] |
| mir-360 | 99 | Maximum | 4.0–5.0 | 0.070 | 0.000 | 0.070 | [−0.032, 0.157] |
| mir-788 | 187 | Maximum | 3.0–3.5 | 0.000 | 0.005 | 0.006 | [−0.029, 0.037] |
| mir-793 | 175 | Maximum | 6.0–10.0 | 0.166 | 0.091 | 0.224 (+) | [0.146, 0.312] |
| mir-794 | 91 | Maximum | 3.0–3.5 | 0.009 | 0.082 | 0.093 | [−0.032, 0.209] |
Expression of 10 of the 22 tested PmiRNA::GFP reporters exhibited an ability to reproducibly predict lifespan with a joint correlation coefficient (R2) of at least 0.15: the reporters for miRNAs lin-4, mir-47, mir-60, mir-85, mir-90, mir-228, mir-240–786, mir-243, mir-246, and mir-793 (Table 2). The R2 value in this case represents the fraction of total inter-individual variation in lifespan that can be accounted for by the inter-individual variation in measured GFP levels of a single reporter.
The ability to predict lifespan does not appear to be a generic property of promoter::GFP constructs, as over half of the examined reporters did not correlate substantially with lifespan. Furthermore, two non-miRNA reporters we tested, Pmyo-2::GFP and Pcpna-2::GFP, were not substantially predictive of lifespan (Figure 2—figure supplement 1 and table S3 in Supplementary file 1). Moreover, the optimal time window for regression (i.e. the time window of expression data producing the maximum correlation coefficient value) varied between reporters, suggesting that the reporters may provide readouts of different phases of the aging process. Some reporters exhibited broad (at least 2 days) optimal time windows spanning young adulthood (Pmir-243::GFP, Pmir-85::GFP) or mid-to-late adulthood (Plin-4::GFP, Pmir-47::GFP, Pmir-228::GFP, Pmir-246::GFP, Pmir-793::GFP). Other reporters like Pmir-60::GFP, Pmir-90::GFP, and Pmir-240–786::GFP were optimally predictive in narrower time windows centered at late adulthood. For the latter set of reporters, the average expression value tended to be more predictive of future lifespan than the slope of an individual’s expression trendline. For the earlier predictors, the slope of the trendline tended to be as or more predictive of lifespan than the the average expression value (Table 2). Neither the maximum expression value an individual achieved nor the timepoint at which maximum expression was reached were substantially predictive of future lifespan for any of the PmiRNA::GFP reporters (table S4 in Supplementary file 1). A positive correlation between Pmir-246::GFP expression and lifespan has been previously published and lin-4 and mir-228 are known to play roles in longevity and the aging process; however, to our knowledge, an association between lifespan and expression of the other miRNAs in unperturbed C. elegans has not been reported (Table 1).
In each case, extended lifespan was correlated with retention of young-adult gene-expression levels and/or trends. Specifically, all reporters except for Pmir-240–786::GFP both decreased in expression throughout aging and correlated positively with future lifespan. Conversely, Pmir-240–786::GFP increases in expression with age (Figure 1) and was negatively correlated with future lifespan. In other words, for reporters where expression levels start high and decrease over time, long lifespan is predicted by high (‘youthful’) expression levels and a delay in their decline. By the same token, for Pmir-240–786::GFP, with low young-adult expression that increases with age, extended periods of ‘youthful’ low expression predict extended lifespan.
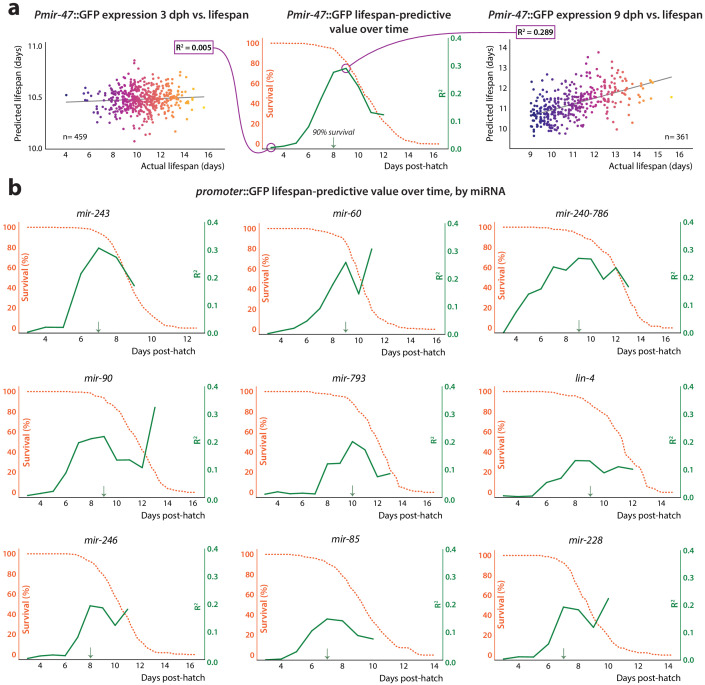
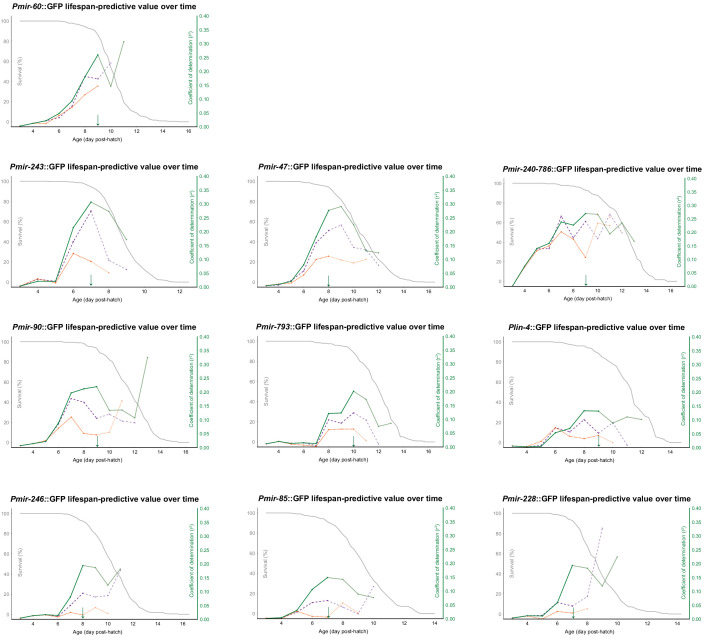
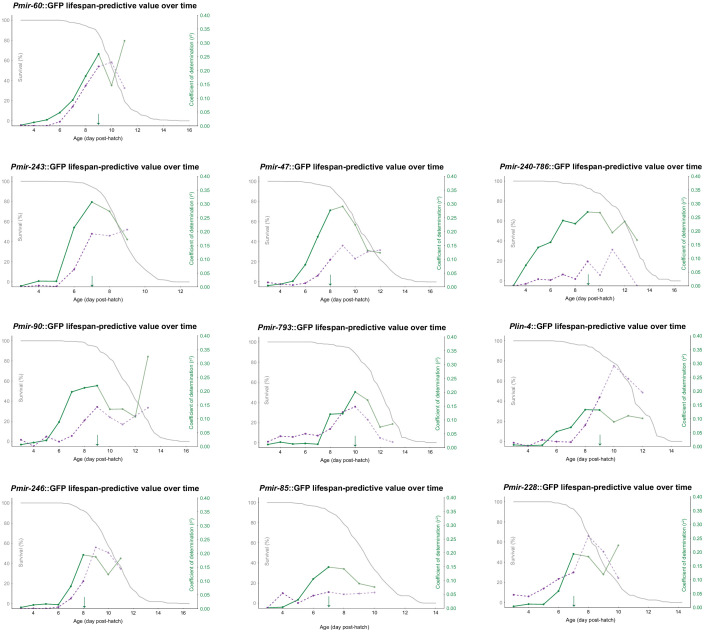
We next examined how the lifespan-predictive power of the PmiRNA::GFP reporters changes throughout adulthood. For every 24 hr period post-hatch, we jointly regressed each individual’s average expression and the slope of the expression trend over those 24 hr against that individual’s eventual lifespan (Figure 3a). We plotted the resulting correlation coefficient over time to illustrate how the predictive power of the reporter changes with aging (Figure 3a and b). Expression of predictive PmiRNA::GFP reporters generally begins to correlate with future lifespan when measured at mid to late adulthood, becoming more predictive over time. Comparing the correlation plot with the population survival curve revealed that reporter predictivity peaks simultaneously with the beginning of population die-off, continuing to significantly correlate with lifespan as more individuals in the population perish (Figure 3a and b). However, reporters do not simply correlate with imminent death, as for this analysis we excluded individuals that perished within the 24 hr time window. Moreover, excluding even those that perish within the subsequent 24 or 48 hr does not abolish predictive power for most reporters (Figure 3—figure supplement 1). None of the reporters we examined showed substantial correlation with future lifespan at timepoints less than 5 days post-hatch (roughly young adulthood). This result would be expected if reporter expression levels were identical across individuals early in life, and individuals’ expression levels only diverged later in adulthood. However, we found that the degree of inter-individual variability in reporter levels is largely consistent throughout life (Figure 1—figure supplements 2 and 3). This suggests that only at mid-adulthood do pre-existing inter-individual differences in PmiRNA::GFP expression become coupled to the aging process and future lifespan.
Figure 3. Lifespan-predictive ability vs.time.
(a) Construction of correlation plot for Pmir-47::GFP. Mean and slope of expression values for each individual during a sliding 24 hr time window, starting at 3 dph, were regressed against future lifespan. The regressions for 48–72 hr (window centered at 3 dph, left) and 192–216 hr (9 dph, right) are shown. At center, the correlation coefficient from each regression is plotted against the age at the middle of the regression window (solid line). The survival curve for the population is overlaid (dashed line). The 90% survival timepoint is indicated by the green arrow. (b) Correlation plots for remainder of predictive PmiRNA::GFP reporters.
Figure 3—figure supplement 1. Mean and slope of PmiRNA::GFP expression in a sliding 24 hr window was regressed against future lifespan, only for individuals surviving the subsequent 24 hr (green), 48 hr (purple), and 72 hr (orange).
Figure 3—figure supplement 2. Mean and slope of PmiRNA::GFP expression (green) and the 95th percentile of autofluorescence intensity (purple) for each individual during a 24 hr time window were regressed on future lifespan.
Interestingly, we found that autofluorescence intensity, a phenomenological measure that negatively correlates with lifespan (Pincus et al., 2016), is predictive of future longevity at approximately the same time in adulthood as are most of the PmiRNA::GFP reporters we examined (Figure 3—figure supplement 2). However, PmiRNA::GFP expression and autofluorescence provide largely non-redundant information with respect to future lifespan, based on the near-additivity of correlation coefficients in single vs. joint regression (table S5 in Supplementary file 1). This suggests that although both act as biomarkers of future lifespan, they likely report on distinct aspects of the aging process.
Variable expression of mir-47 and mir-243 are not determinants of individual variability in lifespan
Expression of the PmiRNA::GFP constructs can only correlate with future lifespan if there is some biological process which both (a) determines individual lifespan and (b) directly or indirectly regulates transcription of the GFP reporters. To learn more about the nature of this process, we investigated the genetic requirements for the correlation between lifespan and GFP levels in the two most lifespan-predictive reporters, Pmir-47::GFP and Pmir-243::GFP.
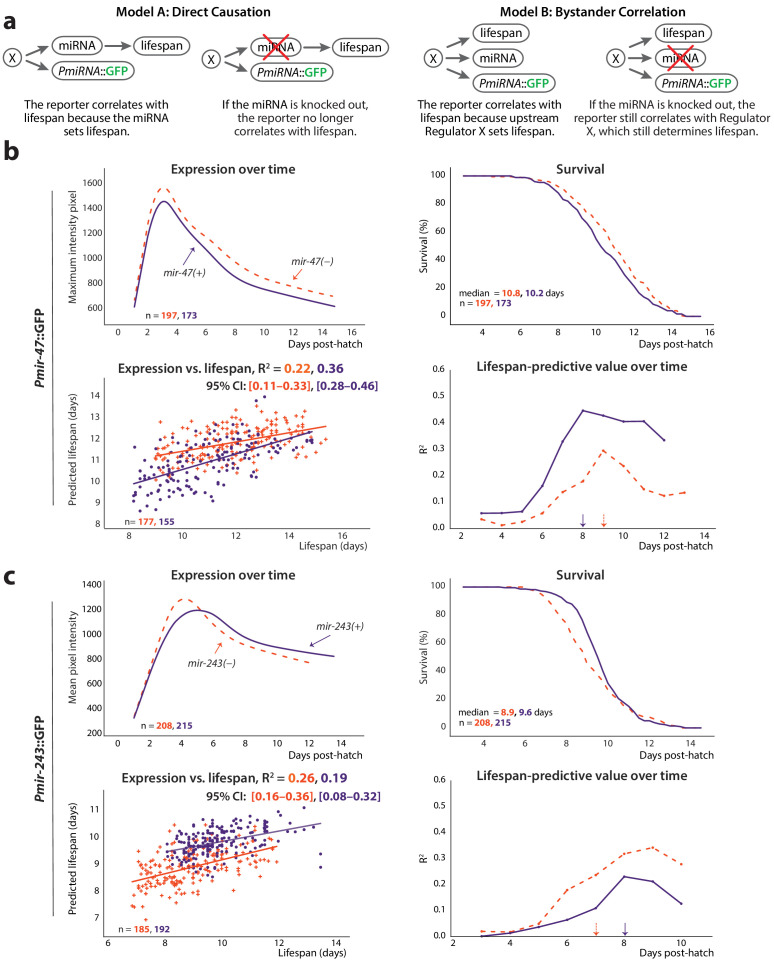
One straightforward possibility is that the PmiRNA::GFP constructs are honest reporters for their endogenous, cognate miRNAs, which determine individual lifespan by variability in their own expression (‘Direct Causation’ model; Figure 4a). Alternately, the endogenous miRNAs themselves may have nothing to do with the true lifespan-determining pathway; in this scenario, reporter expression is a downstream readout of pathways, processes and/or transcription factor activity that affect lifespan but do not require the activity of the endogenous miRNA (‘Bystander Correlation’ model; Figure 4a). Under the former model, activity of the cognate miRNA is a critical causal link to lifespan; absent that miRNA, variability in GFP would be decoupled from variability in lifespan. Under the latter model, loss of the miRNA would not influence the relationship between GFP expression and lifespan.
Figure 4. Non-involvement of endogenous miRNAs.
(a) Two models of the possible relationship between the endogenous miRNA and lifespan: ‘direct causation’ in which the miRNA is directly involved in determining individual lifespans, and ‘bystander correlation’ in which it is not. The predicted effect of knocking out the endogenous miRNA on the correlation between PmiRNA::GFP levels and future lifespan is shown for each scenario. (b) Comparison of Pmir-47::GFP expression over time (top left), survival (top right), regression on slope and mean of expression against lifespan (bottom right), and lifespan-predictive value (bottom left) between mir-47(+) (solid lines) and mir-47(gk167) (dashed lines) backgrounds. The time windows for regression were those that maximized correlation with future lifespan: 72–194 and 72–215 hr post-hatch for mir-47(+) and mir-47(gk167), respectively. (c) Comparison of Pmir-243::GFP expression over time (top left), survival (top right), regression on slope and mean of expression against lifespan (bottom left), and lifespan-predictive value (bottom right) between mir-243(+) (solid lines) and mir-243(n4759) (dashed lines) backgrounds. The time windows for regression were 72–192 and 72–164 hr post-hatch for mir-243(+) and mir-243(n4759), respectively. The 90% survival ages for each genotype are indicated by arrows. The 95% CI for each regression correlation coefficient is shown in brackets. In scatter plots, the (o) and (+) symbols correspond to the regressions in wild-type or miRNA-mutant genotypes, respectively. All figures pool data from two biological replicates.
To distinguish these two possibilities, we tested whether Pmir-47::GFP and Pmir-243::GFP were still predictive of lifespan in a genetic background lacking the cognate endogenous miRNAs (mir-47(gk167) and mir-243(n4759), respectively). As before, we performed regressions on average expression and the slope of the expression trendline in windows between three dph and the 90% survival timepoint; we also measured the lifespan-predictive value of the reporters over time in discrete 24 hr time periods. The correlation of Pmir-47::GFP expression with lifespan was somewhat reduced in the mir-47(gk167) mutant compared to mir-47(+) (R2 = 0.22 vs. 0.36) and exhibited a slightly different temporal window for lifespan prediction (7–9 dph vs. 6–8 dph) (Figure 4b). The correlation of Pmir-243::GFP expression with lifespan was actually increased in the mir-243(n4759) mutant compared to mir-243(+) (R2 = 0.257 vs. 0.193), and exhibited a different temporal window (6–7 dph vs. 7–8 dph) (Figure 4c). Overall lifespans were unaffected in mir-47(gk167) and mir-243(n4759) compared to strains wild-type at those loci (median lifespan = 10.8 vs. 10.2 days and 8.9 vs. 9.6 days, respectively). Because both reporters correlated positively with lifespan, a short-lived phenotype might be expected if either miR-243 or miR-47 played a functional role in determining lifespan. The lack of substantial lifespan difference further suggests that neither miRNA plays a direct functional role in determining lifespan.
Overall, the correlation of Pmir-47::GFP and Pmir-243::GFP with lifespan was not abolished in the absence of the endogenous miRNAs. This provides evidence for the ‘Bystander Correlation’ model of Figure 4a, implying that lifespan may be largely determined by transcriptional regulators of the PmiRNA::GFP transgenes (and presumably of the endogenous miRNAs as well), or regulatory pathways/processes even further upstream, rather than via the activity of the miRNAs themselves.
Lifespan-predictive abilities of Pmir-47::GFP and Pmir-243::GFP are independent of daf-16
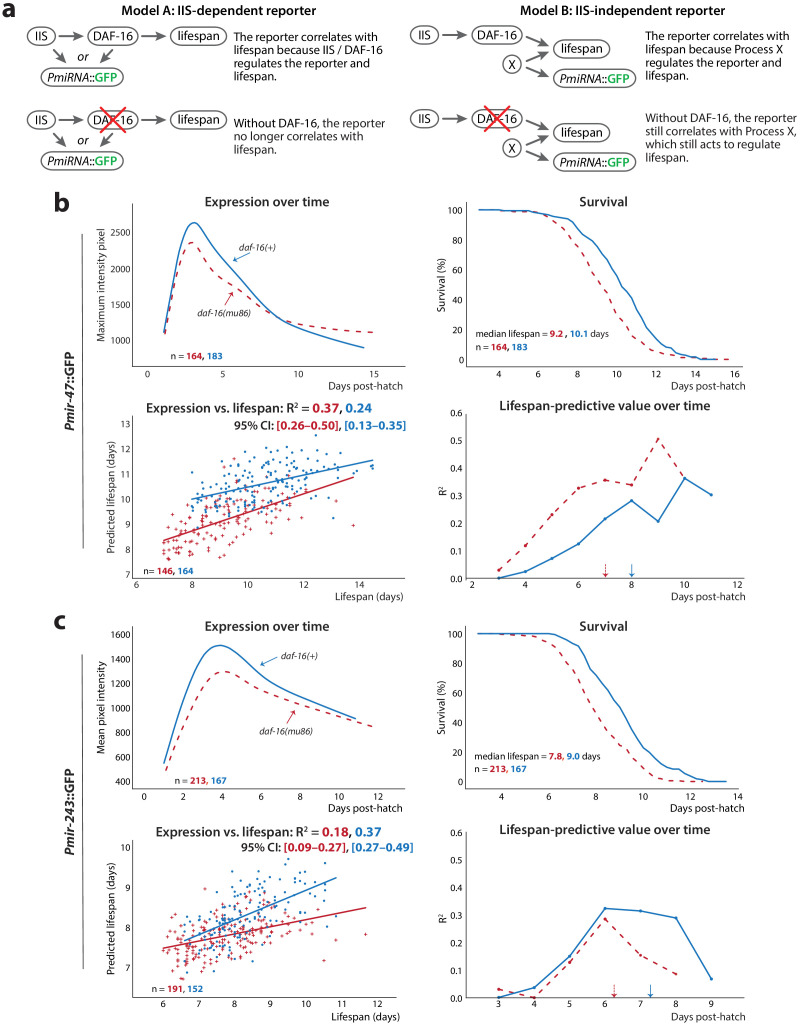
As the abundance and/or activity of miR-47 and miR-243 does not appear to directly affect lifespan, Pmir-47::GFP and Pmir-243::GFP must instead serve as markers of other pathways or processes that influence lifespan. We wondered if these might include insulin/insulin-like growth factor (IGF-1) signaling (IIS), a canonical aging pathway in C. elegans that is highly conserved across taxa (Kenyon, 2010). The primary effector of the IIS pathway in C. elegans is the FOXO transcription factor DAF-16, which modulates the expression of a number of downstream genes that influence aging and lifespan (Kenyon, 2010; Murphy et al., 2003). DAF-16 activity is a common genetic requirement for genes, pathways, and processes that have been reported to increase lifespan in C. elegans, including several microRNAs (Pincus et al., 2011; Boehm, 2005; Boulias and Horvitz, 2012). It is therefore plausible that inter-individual variability in insulin signaling or signal-responsiveness leads to variability in DAF-16 activity and thus to inter-individual differences in subsequent lifespan. Indeed, two previously characterized lifespan-predictive gene-expression reporters, Psod-3::GFP and Pmir-71::GFP, both require functional DAF-16 to correlate with future lifespan. This suggests those transgenes predict future lifespan by reporting on inter-individual variability in DAF-16 (and presumably IIS) activity. To test if Pmir-47::GFP and Pmir-243::GFP similarly report on DAF-16 activity, we examined whether GFP levels still correlated with lifespan in the absence of DAF-16 (Figure 5a). Specifically, we crossed each reporter into a daf-16(mu86);spe-9(hc88) background and assayed lifespan-predictive ability in side-by-side experiments with the reporter in the reference background (spe-9(hc88) only).
Figure 5. Non-involvement of IIS.
(a) For PmiRNA::GFPs to correlate with lifespan, each must report on some lifespan-determining process. The reported-on process may be IIS-dependent (left) or IIS-independent (right); these scenarios can be distinguished by the effects of knocking out the IIS effector daf-16 on the correlation between PmiRNA::GFP and future lifespan. (b) Comparison of Pmir-47::GFP expression over time (top left), survival (top right), regression on slope and mean of expression against lifespan (bottom right), and lifespan-predictive value (bottom left) between daf-16(+) (solid lines) and daf-16(mu86) (dashed lines) backgrounds. The optimal time windows for regression were 72–190 and 72–164 hr post-hatch for daf-16 wild-type and daf-16(mu86), respectively. (c) Comparison of Pmir-243::GFP expression over time (top left), survival (top right), regression on slope and mean of expression against lifespan (bottom left), and lifespan-predictive value (bottom right) between daf-16(+) (solid lines) and daf-16(mu86) (dashed lines) backgrounds. The optimal time windows for regression were 72–158 and 72–144 hr post-hatch for daf-16(+) and daf-16(mu86), respectively. The 90% survival ages for each genotype are indicated by arrows. The 95% CI for each regression correlation coefficient is shown in brackets. In scatter plots, the (o) and (+) symbols correspond to the regressions in daf-16(+) and daf-16(mu86), respectively. All figures pool data from two biological replicates.
Regression on average expression and the slope of the expression trendline between 3 dph and the 90% survival timepoint showed that the correlation of Pmir-47::GFP or Pmir-243::GFP expression with lifespan was not substantially suppressed in the absence of daf-16 (bottom left panels of Figure 5b and c, respectively). For Pmir-47::GFP, the R2 values vs. lifespan were 0.24 for daf-16(+) and 0.37 for daf-16(-); for Pmir-243::GFP, the R2 values were 0.37 for daf-16(+) and 0.18 for daf-16(-). Similarly, plotting the lifespan-predictive value of Pmir-47::GFP or Pmir-243::GFP over time shows that the predictive power of each reporter is not reduced in the daf-16(mu86) background (bottom right of Figure 5b and c, respectively). As expected, the predictive windows are slightly shifted between daf-16(mu86) and wild-type backgrounds due to the short-lived phenotype of daf-16(mu86) (top right of Figure 5b and c). Interestingly, peak levels of Pmir-243::GFP and Pmir-47::GFP were slightly but consistently reduced in the daf-16(mu86) background, suggesting that DAF-16 may play a minor role in the transcriptional regulation of these reporters.
Pmir-240–786::GFP, Pmir-793::GFP, and Pmir-47::GFP hierarchically report on a single lifespan-determining process
The lifespan-predictive PmiRNA::GFP reporters we identified cannot all provide independent information. Because the correlation coefficients with future lifespan (Table 2) sum to more than one, at least some subset of these reporters must necessarily be at least partially redundant with one another. This raises an obvious question: to what degree are the reporters independent? At one extreme, all 10 reporters might reflect the activity of a single lifespan-determining pathway. Alternately, there may be a small handful of such pathways that are reported on by different sets of the PmiRNA::GFPs.
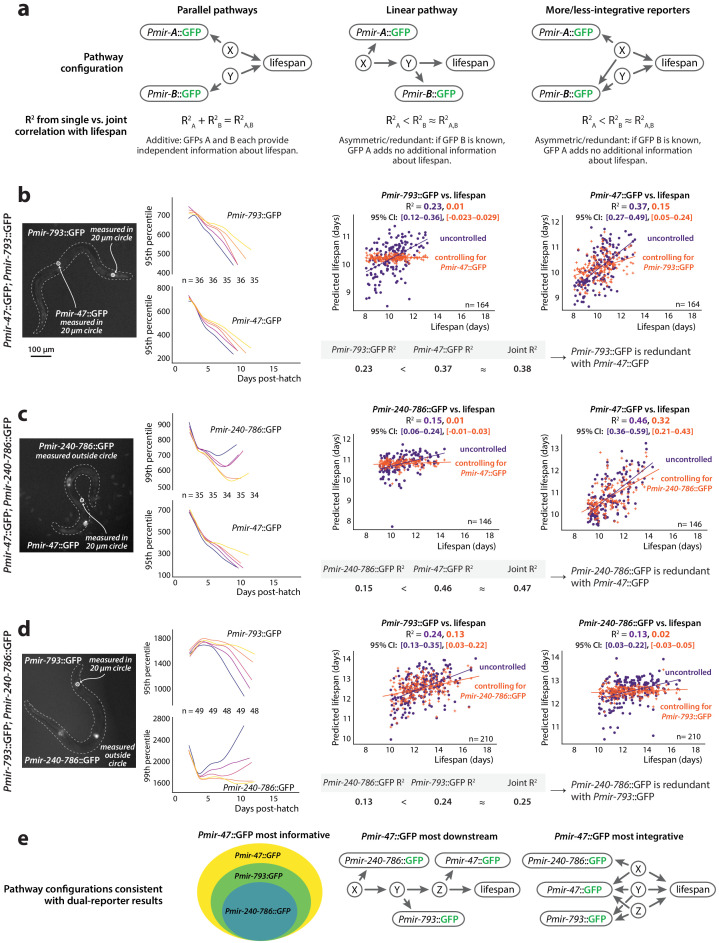
If two reporters provide information about distinct, independent biological processes, then when both reporters are measured in the same individual, the lifespan estimate using both reporters will be better than when using either individually. More specifically, if the information that each reporter provides about future lifespan is completely independent, then the R2 of a joint regression using both measurements to predict future lifespan will be the sum of the R2-values from the two single regressions. Alternatively, if two PmiRNA::GFP transgenes both report on the same process, then expression of one transgene will be correlated with the other, and, equivalently, the joint regression will not be substantially more predictive of lifespan than either single regression alone (Figure 6a).
Figure 6. Redundancy of reporters.
(a) Three models depicting possible additive or redundant relationships between lifespan-determining processes X and Y and two PmiRNA::GFP reporters A and B, with respect to their correlation vs. future lifespan. (b) Pmir-793::GFP and Pmir-47::GFP expression for different lifespan cohorts over time (left) and regression on slope and mean of each reporter from 3 to 8 dph controlled for expression of the other reporter (right). (c) Pmir-47::GFP and Pmir-240–786::GFP expression for different lifespan cohorts over time (left) and regression on slope and mean of each reporter from 3 to 9 dph controlled for expression of the other reporter (right). (d) Pmir-793::GFP and Pmir-240–786::GFP expression for different lifespan cohorts over time (left) and regression on slope and mean of each reporter from 3 to 10 dph controlled for expression of the other reporter (right). The tables in panels a–c compare correlation coefficients for single PmiRNA::GFP reporters to the joint correlation coefficient obtained by regressing on both reporters. In scatter plots, the (o) and (+) symbols correspond to the uncontrolled and controlled regressions, respectively. All figures represent data from two biological replicates. (e) Interpretations of the relationships among these three reporters and the reported-on lifespan-determining processes. Informational hierarchy (left): Pmir-47::GFP provides strictly more information about future lifespan than the other two reporters, and Pmir-793::GFP provides strictly more information than Pmir-240–786::GFP expression. This is consistent with (at least) two different mechanistic hierarchies. Signaling hierarchy (center): the informational hierarchy may suggest that Pmir-47::GFP provides lifespan-predictive information strictly more downstream than the other reporters. Integration hierarchy (right): alternately, Pmir-47::GFP could provide information that integrates among more independent lifespan determinants.
Figure 6—figure supplement 1. Pairwise correlations among GFP levels 3–8dph for each biomarker.
To begin to count the number of lifespan-determining processes reported on by the PmiRNA::GFPs we identified, we investigated the degree of redundancy among Pmir-47::GFP, Pmir-793::GFP and Pmir-240–786::GFP. We chose these reporters because they are among the strongest predictors of future lifespan, and can be easily spatially resolved because they are primarily expressed in distinct tissues. We thus constructed three dual-reporter strains comprising all pairs of these three transgenes, and for each strain measured GFP levels of both transgene independently. In specific, we manually annotated fluorescence images to delineate tissues in which each transgene was specifically expressed, and extracted our GFP measurements from those regions only, rather than the whole worm (see Materials and methods and Figure 6b–d).
To determine whether each PmiRNA::GFP in the dual-reporter strain provided independent information about longevity, we regressed each reporter individually against future lifespan and compared the resulting R2-values to that produced by multivariate regression of both reporters against lifespan. As a complementary analysis, we also calculated the semipartial correlation coefficient between each single reporter and future lifespan, statistically controlling for the expression of the other reporter. Specifically, the R2 from semipartial correlation between reporter A and lifespan, controlling for reporter B, represents the fraction of variation in lifespan that is uniquely associated with variability in reporter A but not reporter B. In the case that reporter A is fully redundant with reporter B, then reporter A will no longer correlate with future lifespan at all after controlling for the expression levels of reporter B (semipartial R2 = 0).
In the dual Pmir-47::GFP; Pmir-793::GFP strain, expression of each reporter is individually predictive of future lifespan, as we previously observed (Figure 6b, R2 = 0.37 and 0.23 for Pmir-47::GFP and Pmir-793::GFP respectively). We found that Pmir-793::GFP expression correlates strongly with Pmir-47::GFP expression (Figure 6—figure supplement 1), which suggests that they are co-regulated and thus potentially redundant with respect to future lifespan. Indeed, joint regression incorporating both reporters produces a correlation coefficient no greater than regressing against Pmir-47::GFP alone (Figure 6b, R2 = 0.38). This suggests that Pmir-793::GFP levels provide no information about future lifespan that is not also reflected in Pmir-47::GFP expression. As a confirmation, the semipartial correlation between Pmir-793::GFP and lifespan, controlling for Pmir-47::GFP, is (as expected) nearly zero (Figure 6b, left plot). In contrast, Pmir-47::GFP expression remains somewhat predictive of lifespan even after controlling for Pmir-793::GFP. Thus, Pmir-47::GFP and Pmir-793::GFP are not independent predictors of future lifespan and likely report on a single lifespan-determining pathway. Because Pmir-47::GFP expression contains strictly more information about lifespan than Pmir-793::GFP, we infer that Pmir-47::GFP likely reports on activity of that pathway at a somewhat more downstream position with respect to lifespan determination, or integrates additional information from a separate pathway (Figure 6a, center and right).
Similar results obtain for Pmir-47::GFP and Pmir-240–786::GFP. Expression of each reporter was individually predictive of lifespan (Figure 6c, R2 = 0.46 and 0.15 for Pmir-47::GFP and Pmir-240–786::GFP, respectively), but expression levels of the reporters are correlated (Figure 6—figure supplement 1) and regression on both reporters again results in a correlation coefficient nearly equal to regressing on Pmir-47::GFP alone (Figure 6c, R2 = 0.47). Semipartial correlation analysis was similar to the previous case (Figure 6c), indicating that Pmir-240–786::GFP is wholly redundant with Pmir-47::GFP but not vice-versa. We thus infer that, of the reporters we examined, Pmir-47::GFP expression either integrates information most broadly across lifespan-determining pathways, or reports on a step the farthest downstream in a single lifespan-determining pathway.
Unsurprisingly, Pmir-240–786::GFP and Pmir-793::GFP also were correlated and did not act as independent predictors of lifespan (Figure 6d; R2 = 0.13 and 0.24 for Pmir-240–786::GFP and Pmir-793::GFP, respectively, with a joint R2 = 0.25, which is close to the figure for Pmir-793::GFP alone and much less than 0.37, the sum of the two individual values). Confirmed by semipartial correlation analysis, this suggests that Pmir-240–786::GFP is almost wholly redundant with Pmir-793::GFP (save approximately 4% of lifespan variation which the former transgene can predict independent of Pmir-793::GFP). We infer that Pmir-793::GFP reports on information that is downstream of (or more broadly integrated than) Pmir-240–786::GFP, with respect to lifespan determination.
Overall, our data is consistent with a model wherein Pmir-240–786::GFP, Pmir-793::GFP, and Pmir-47::GFP expression provide hierarchical information about a lifespan-determining pathway or pathways. While there are multiple scenarios consistent with our results, all reflect the informational hierarchy depicted in Figure 6e (left), where Pmir-47::GFP provides strictly more information about lifespan than Pmir-793::GFP, which itself provides strictly more information than Pmir-240–786::GFP. Such a hierarchy is consistent with a scenario in which Pmir-47::GFP provides the most downstream report on a single lifespan-determination process (Figure 6e, center). It is also consistent with a scenario in which Pmir-47::GFP reports on three distinct lifespan-determination pathways, Pmir-793::GFP reports on two of those, and Pmir-240–786::GFP on a single pathway (Figure 6e, right). Last, it could also be that each transgene reports on the exact same step of a single pathway, but that the different transgenes also reflect different degrees of extraneous, unrelated transcriptional input. Each model has in common, however, the key fact that the bulk of the three transgenes’ correlation with lifespan is due to their mutual transcriptional relationship with a single lifespan-determining process.
It is particularly interesting in this context to recall that each of these reporters is expressed in distinct tissues; moreover, the expression trend of Pmir-240–786::GFP (increasing throughout life) is quite different from the other two reporters (which peak at mid-adulthood and decline). Nevertheless, the levels of these three reporters are all correlated (or anticorrelated) and provide largely redundant information about a single lifespan-determining process. Such redundancy and correlations among reporters expressed in distinct tissues could not exist without information flow between tissues, with the most likely candidate being a cell non-autonomous signaling process. We therefore conclude that Pmir-47::GFP, Pmir-793::GFP, and Pmir-240–786::GFP likely report on a global, cell non-autonomous (and DAF-16-independent) process of lifespan determination, which affects the entire animal’s physiology.
Discussion
In order to understand the relationship between inter-individual variation in transcriptional / gene-regulatory states and future lifespan, we used tools previously developed by our lab (Zhang et al., 2016; Pittman et al., 2017) to examine expression of 22 PmiRNA::GFP reporters (Martinez et al., 2008), across hundreds of individuals throughout life. This dataset provides a window into transgene-expression dynamics at a temporal and spatial resolution unprecedented for longitudinal experiments. Compared to previous work which followed a candidate-gene approach (Pincus et al., 2011; Sánchez-Blanco and Kim, 2011; Rea et al., 2005), this is the first systematic, unbiased examination of the relationship between promoter activity and future lifespan. Overall, we find that the ability to predict future lifespan is not a rare property, as 10 of the 22 transgenes tested were robustly and repeatedly correlated with individual longevity. We were not able to identify a clear pattern among lifespan-predictive vs. non-predictive miRNA promoters in terms of tissue of expression or temporal pattern in gene expression; instead, it appears that many transgenes, across many tissues, report on the activity of a smaller number of lifespan-determining processes. While previous studies have implicated inter-individual differences in IIS and more specifically, DAF-16 activity, in lifespan variability, we have identified at least one distinct, organism-wide transcriptional program that acts independently of daf-16 in reflecting or determining future lifespan.
When measured while at least 90% of the population remained alive, 10 PmiRNA::GFP reporters met our criteria as predictors of longevity (Figure 2, Table 2), explaining between 17% and 33% of the variability in lifespan among genetically identical individuals. Overall, we found that longevity is generally associated with the preservation of young-adult reporter expression states. Among microRNA reporters that predict lifespan, those with high young-adult levels that decrease with age are positively correlated with longevity, such that long life is associated with high and/or non-decreasing GFP expression. Conversely, the single lifespan-predictive reporter that has low young-adult levels and increases with age is negatively correlated with lifespan; long life is associated with maintenance of low / non-increasing GFP levels. The fact that both an individual’s average level of expression and the slope of expression over time can inform future lifespan suggests that both magnitude and maintenance of expression may be important for longevity. However, it is important to note that not all genes that change with time are predictive of future lifespan. For 12 of the 22 reporters examined, maintenance of ‘youthful’ expression patterns had no relationship with lifespan.
Nevertheless, we were surprised to find that nearly half of the PmiRNA::GFP reporters we tested did correlate with lifespan, especially as we did not take into account any prior association with aging or lifespan in selecting which reporters to examine. While lifespan-predictive reporters have previously been identified among hand-picked candidate genes (Pincus et al., 2011; Sánchez-Blanco and Kim, 2011), only unbiased screens such as the one we conducted can determine whether this is a relatively common or rare property. Overall, we conclude that it is not particularly uncommon or unusual for a transcriptional reporter to predict future lifespan, at least among those driven by microRNA promoters.
The ability to predict future lifespan is not a completely generic property of all GFP transgenes, however: in addition to the 12/22 the PmiRNA::GFP reporters that did not correlate with future lifespan, neither of the non-miRNA reporters we tested correlated strongly with lifespan (Figure 2—figure supplement 1); negative results for other transcriptional reporters have also been published (Sánchez-Blanco and Kim, 2011). These negative results also suggest that it is not simply the case that the ability to express any GFP transgene reflects a healthy, pro-longevity state. This conclusion is also bolstered by the observation that there exist promoter::GFP constructs whose expression negatively correlates with future lifespan (identified both in the present work and in previous publications Pincus et al., 2011). While recent work has shown that the capacity to fold and stabilize proteins may relate to the ability of Phsp-16.2::GFP to predict future lifespan after a heat shock (Burnaevskiy et al., 2019), our findings suggest that general protein expression capacity does not relate to future lifespan in un-heat-shocked animals.
More generally, similar logic suggests that none of the post-transcriptional steps that influence the measured GFP signal (e.g. the rates of mRNA degradation, translation, protein folding, protein degradation, and/or GFP photobleaching) explain the relationship between GFP levels and future lifespan among the strains we examined. This is because all of the PmiRNA::GFP reporters we examined produce identical mRNA transcripts; only the transcriptional control of these reporters differs. Thus, the salient difference between reporters that do vs. do not correlate with lifespan must necessarily be at the level of their transcriptional regulation.
Further study is required to identify the precise mechanism by which the transcription of these transgenes is regulated in a lifespan-predictive manner. One obvious possibility is transcription-factor binding in the promoter regions of the transgenes. However, position-specific effects are also plausible. Each multi-copy transgene is randomly integrated into the genome, and local regulatory sequences or chromatin accessibility can also influence the level of transgene expression. Regardless, our finding that many transgenes possess this property implies that is not an particularly rare property of promoter sequences, insertion site, or transgene structure.
Larger-scale studies will be necessary to determine whether a substantial fraction of promoters / integration sites across the genome act in a lifespan-responsive fashion, or whether this property is enriched at specific loci, or among promoters of regulatory genes like miRNAs or transcription factors.
No simple properties distinguish the PmiRNA::GFP reporters that predict future lifespan from those that do not. For every lifespan-predictive reporter, there are non-predictive reporters with nearly identical temporal trends in expression (Figure 1; Table 2), and non-predictive reporters with expression in the same tissues/organs (Table 1, Figure 1—figure supplement 1). This suggests that no single tissue is critical for individual lifespan, but instead that lifespan-determining processes act organism-wide, involving multiple tissues in a cell non-autonomous manner.
We next set out to determine the identity of the lifespan-determining cellular or genetic processes that these PmiRNA::GFP transgenes report on. The most straightforward hypothesis is that if expression of a PmiRNA::GFP reporter is positively correlated with lifespan, that is because the endogenous, reported-on miRNA acts to prolong lifespan. Several lines of evidence, however, suggest that this is not generally the case. First, the majority of predictive reporters we identified correspond to miRNAs with no published lifespan or aging phenotype (Table 1). lin-4, mir-228, and mir-246 have been previously associated with longevity; knockout and overexpression studies have reported lin-4 and mir-246 as positive regulators of lifespan and mir-228 as a negative regulator (de Lencastre et al., 2010; Boehm, 2005; Smith-Vikos et al., 2014). We found that as expected, reporters for lin-4 and mir-246 correlate positively with lifespan; surprisingly, Pmir-228::GFP also correlates positively with lifespan. It is important to note, however, that it should not be assumed that genes that change the population’s mean lifespan when removed or overexpressed are necessarily those that act to determine inter-individual variation in lifespans among a genetically unperturbed population. In particular, the presence of regulatory feedback and buffering processes can dramatically complicate this relationship.
Regardless, a second line of evidence also suggests that the relationship between several of the PmiRNA::GFP reporters and lifespan is not via the activity of the endogenous, reported-on miRNA. Specifically, the correlation of two of the predictive reporters, Pmir-243::GFP and Pmir-47::GFP, with lifespan is not abrogated in the absence of the endogenous miRNA (Figure 4). Were these microRNAs a key part of the lifespan-determining transcriptional program reported on by the GFP levels, the correlation between GFP levels and lifespan would have disappeared absent the microRNAs themselves. In addition, we also observed no substantial difference in lifespan between wild-type and mir-243 and mir-47 loss of function mutants.
These results indicate that neither mir-243 nor mir-47 play a direct functional role in determining lifespan in wild-type individuals. Our results are not surprising given most single miRNA (Miska et al., 2007) or even entire miRNA family (Alvarez-Saavedra and Horvitz, 2010) loss-of-function mutants are not required for normal development or viability; often phenotypes are only observed in sensitized backgrounds (Brenner et al., 2010) or when multiple members of a miRNA family are absent (Brenner et al., 2012). While mir-243 is not reported to be part of a family, mir-47 shares over 70% sequence identity with mir-46 (Ibáñez-Ventoso et al., 2008); thus, redundancy between family members could mask a functional role for mir-47 in aging.
Overall, this suggests that Pmir-243::GFP and Pmir-47::GFP (and potentially other of the lifespan-predictive reporters we identified) act as indirect, transcriptional reporters of some separate lifespan-determining process. Inspection of the promoter regions of these genes can provide hypotheses about the factors that may be both acting to determine lifespan and to control expression of these GFPs. While little is known in general about transcriptional control of miRNAs, much less the individual transcription factors involved (Turner and Slack, 2009), there is suggestive evidence. Yeast one-hybrid assays performed with C. elegans genes (Fuxman Bass et al., 2016) identify PQM-1, ELT-4, and HLH-30, transcription factors that are known to promote longevity (Bansal et al., 2014; Tepper et al., 2013; Lin et al., 2018), as likely to bind to the promoter sequence of mir-243. ZTF-8, a transcription factor that mediates the DNA damage response (Kim and Colaiácovo, 2014), may interact with the mir-47 promoter.
Regardless of the immediate transcriptional control of Pmir-243::GFP and Pmir-47::GFP, it is nevertheless the case that the expression of these reporters must in some way be linked to one or more lifespan-determining processes or pathways. As the insulin/IGF-1-like signaling pathway (IIS), specifically the branch controlled by DAF-16, is arguably the best-studied ‘longevity pathway’ in C. elegans, we asked whether these transgenes predict lifespan by correlating with the level of DAF-16 activity. Because the reporters still predict lifespan in an IIS-deficient daf-16 mutant, we conclude that this is not the case (Figure 5). This is surprising given that many other longevity-predictive reporters, such as Pmir-71::GFP and Psod-3::GFP, do require daf-16 to correlate with future lifespan (Pincus et al., 2011; Sánchez-Blanco and Kim, 2011). (Note that these latter results are as expected, because the endogenous miRNA mir-71, interacts genetically with IIS to functionally influence lifespan [de Lencastre et al., 2010], and because sod-3 is a direct transcriptional target of DAF-16 [Sánchez-Blanco and Kim, 2011]) Likewise the correlation between Phsp-16.2::GFP and lifespan after heat shock has also been proposed to be IIS-dependent (Burnaevskiy et al., 2019; Mendenhall et al., 2017). However, despite the importance of DAF-16 in determination of lifespan, both at a population and an individual level, Pmir-243::GFP and Pmir-47::GFP report on one or more processes or pathways that are genetically distinct from daf-16.
Beyond the precise identity of the lifespan-determining processes that are reported on by these PmiRNA::GFPs, we also attempted to estimate the overall number of such processes that might be at play. Because the correlation coefficients sum to greater than one (Table 2), some of the PmiRNA::GFPs must redundantly report on the same processes. We thus wondered whether we had identified 10 transgenes that each served as noisy measures of one single process (one extreme), or reporters for six to eight independent lifespan-determining processes. Fortunately, several of the highly lifespan-predictive PmiRNA::GFPs (including Pmir-47::GFP, which we had extensively analyzed as above) were expressed in distinct, non-overlapping tissues. This allowed us to simultaneously measure the lifespan-predictive signal from pairs of reporters in the same individuals. We found that Pmir-793::GFP and Pmir-240–786::GFP did not provide any information about future lifespan that was not already captured by Pmir-47::GFP expression. Furthermore, Pmir-240–786::GFP was completely redundant with Pmir-793::GFP. As such, all three GFPs must necessarily report at least in part on a single lifespan-determining process. This redundancy stands in stark contrast with the fact that these genes are expressed in very distinct tissues (Table 1; Figure 1—figure supplement 1) and with distinct temporal expression patterns (Figures 1 and 2). This suggests that Pmir-47::GFP, Pmir-793::GFP, and Pmir-240–786::GFP report on a cell non-autonomous process that affects and integrates among multiple tissues throughout aging.
High-resolution analysis of lifespan curves has suggested that a single state of ‘biological resilience’ emerges from the interactions of multiple, densely-interlinked lifespan-determining genes/processes (Stroustrup, 2016). Such a state of resilience might be expected to be cell- and tissue-autonomous; if so, it is possible that these PmiRNA::GFPs report on some aspects of overall resilience or biological reserve. Note, however, that Pmir-47::GFP expression, measured specifically from the vulva, does provide significant prediction of future lifespan that is not captured by Pmir-793::GFP or Pmir-240–786::GFP. Thus, in addition to shared cell and/or tissue non-autonomous processes, Pmir-47::GFP may also report on separate, tissue-specific processes. Indeed, vulval tissue integrity has been identified as an important determinant of health and survival C. elegans (Leiser et al., 2016).
We set out to explore the hypothesis that inter-individual differences in lifespan arise from fate-commitment-like mechanism, whereby certain individuals persistently maintain transcriptional states that assure future stress resistance and extended lifespan. Overall, our results clearly demonstrate that individuals with distinct future fates can in fact be distinguished on the basis of gene-regulatory states (as read out by a number of PmiRNA::GFP reporters). A developmental, or even young-adult, origin to these transcriptional states we identified appears unlikely, however: none of the reporters were particularly predictive of lifespan before approximately the third day of adulthood. Despite substantial inter-individual variation in GFP expression early in adulthood (Figure 1—figure supplements 2 and 3), the window in which that expression variability correlates with remaining lifespan begins roughly a day or so before the start of the population die-off and ends when approximately 25% of the population remains alive (Figure 3). This suggests that the lifespan-determining transcriptional processes that the PmiRNA::GFPs report on are set relatively late in life. This contrasts with the early-adult window in which major changes to the stress-response machinery take place (Labbadia and Morimoto, 2015), which we had originally suspected might be a key inflection point at which inter-individual differences in lifespan-determining stress-resistance pathways emerge.
The hypothesis that gene-regulatory programs determine individual lifespan should be compared against the null hypothesis that inter-individual variability in lifespan is solely due to stochastic differences in accumulation of damage throughout life. It is possible that the lifespan-predictive PmiRNA::GFPs we identified are simply very early reporters of the transcriptional consequences of lifespan-limiting stochastic damage (such as pathogenesis [Zhao, 2017; Podshivalova et al., 2017] or intestinal permeabilization [Gelino et al., 2016]). This would be consistent with ‘youthful’ gene-expression levels predicting long life: a change in gene expression would be indicative of an individual having received a potentially lethal insult. Alternately, and equally consistent with our data, it may be that loss of youthful, protective gene-expression states is a risk factor for suffering exogenous insults (e.g. for becoming bacterially infected), or for being unable to recover from such insults (e.g. for unsuccessfully countering a bacterial infection). In this scenario, differences in the timing of the departure from an optimal gene-expression program drives differences in individual lifespan. The fact that the reporters remain somewhat predictive of future lifespan even when excluding individuals that die within the subsequent 24–48 hr (Figure 3—figure supplement 1) provides some evidence for this latter view. However, it is also possible that the transgenes report on stochastic damage that leads only slowly to death. Further genetic and functional analysis of these lifespan-predictive reporters will be necessary to conclusively determine whether they lie upstream or downstream of exogenous, lifespan-limiting insults.
Overall, we find that it is quite common for microRNA-promoter::GFP transgene expression to predict an individual’s future lifespan, at mid-to-late adulthood. Moreover, at least three and potentially as many as all 10 of the 22 transgenes we examined (≈15–50%) redundantly report on a single, cell non-autonomous, IIS-independent, organismal state that relates to future lifespan. This state (whatever its specific molecular identity) is thus likely to be highly connected to many aspects of organismal biology.
Ultimately, this work raises a host of questions. What is the origin and identity of this state? Is it early responses to fatal damage? A state that controls the risk of suffering such damage? What specific transcription factors / chromatin accessibility changes define this state, and/or act on these transgenes in a lifespan-predictive fashion? We believe that answering these questions will shed light on the overall nature of the aging process, and identify gene regulators that not only identify prospectively short-lived individuals, but can be manipulated to switch those individuals toward long-lived fates.
Materials and methods
Strains
The following strains were obtained from the Caenorhabditis Genetics Center (CGC): VT1735 (Pmir-788::GFP), VT1541 (Pmir-360::GFP), VT1733 (Pmir-60::GFP), VL405 (Pmir-63::GFP), VL440 (Pmir-47::GFP), VT1153 (Plet-7::GFP), VT1072 (Plin-4::GFP), VL412 (Pmir-79::GFP), VT1379 (Pmir-59::GFP), VT1474 (Pmir-243::GFP), VT1189 (Pmir-241::GFP), VT1607 (Pmir-246::GFP), VT1485 (Pmir-228::GFP), VT1600 (Pmir-85::GFP), VT1481 (Pmir-51::GFP), VT2020 (Pmir-793::GFP), VT2021 (Pmir-794::GFP), VL370 (Pmir-240–786::GFP), VT1470 (Pmir-242::GFP), VT1160 (Pmir-84::GFP), VT1589 (Pmir-90::GFP), VT1665 (Pmir-1::GFP), PD4793 (Pmyo-2::GFP; Ppes-10::GFP; F22B7.9::GFP), B010652 (Pcpna-2::GFP) CF1038 (daf-16(mu86)), MT15454 (mir-243(n4759)), VC328 (mir-47(gk167)).
All miRNA reporter (PmiRNA::GFP) strains were selected from a larger library originally created by Martinez et al., 2008. Briefly, miRNA promoters were defined as 300 to 2000 base pair regions upstream of the mature miRNA or stem-loop sequence and cloned via Gateway cloning. The GFP and 3′ UTR for the constructs were cloned from the ‘Fire Lab Vector Kit’ construct pPD95.75, containing a GFP with artificial introns and an unc-54 3′ UTR. The transgenic strains were created via microparticle bombardment to generate low-copy random integrants, with UNC-119 rescue used as a selection marker.
All reporter strains were crossed into BA671 (spe-9(hc88)), a temperature-sensitive sterile mutant with a wild-type lifespan at 25.5 °C (Fabian and Johnson, 1994). Strains were maintained at 20°C and for all assays embryos were shifted to 25°C to prevent reproduction in the single-animal culture apparatus.
Single-animal culture
For longitudinal analysis, C. elegans were reared in high-density single-animal culture devices (‘worm corrals’), which we have described previously (Zhang et al., 2016; Pittman et al., 2017). In brief, 8-armed PEG-thiol (Jenkem Technology) and PEG-diacrylate (Sigma-Aldrich) are dissolved in a modified nematode growth medium (NGM) at concentrations of 133 mg/mL and 37 mg/mL, respectively, and filter-sterilized. The PEGs are mixed in a 1:1 ratio and cholesterol in ethanol is added to a final concentration of 4 µL/mL. The final media is poured into an aluminum frame adhered to a glass slide with PDMS (Dow Corning) and allowed to cure into a solid hydrogel at room temperature for approximately 2 hr. E. coli OP50-1, resuspended to 50% w/v from an overnight culture, is deposited into an array of 0.4 µL droplets onto the gel. A pretzel-stage embryo is placed into each droplet via eyelash pick. Approximately 1 mL liquid PDMS is poured onto the gel; the PDMS crosslinks to the gel and cures into a solid form within 48 hr. The culture devices were housed on a microscope stage (Leica) in a custom-built climate chamber held at 25°C and 90% relative humidity.
Image acquisition
Images of individual animals were automatically acquired every 4 hr at ×5 magnification using custom-built image acquisition and autofocus software. A bright-field, autofluorescence, and fluorescence image were taken of each animal at each timepoint. Fluorescence images were taken with a DAPI/FITC/TRITC filter (Semrock, DA/FI/TX-3X-A-000) and Lumencor Spectra X light source and were used to measure accumulation of autofluorescence material and transgene expression. For autofluorescence, an exposure time of 70 ms and 556/20 nm (center wavelength/bandwidth) excitation filter was used; fluorescence in these red emission wavelengths increases linearly with age and has been shown to correlate well with remaining lifespan (Pincus et al., 2016). An exposure time of 30–70 ms was manually chosen for each reporter strain (as strains vary considerably in brightness), with 40 ms most commonly used. Exposure times were consistent for all images within a given strain. A 480/17 nm excitation filter was used for all GFP imaging. Flat-field images of a fluorescent slide (Chroma) were collected before every image acquisition to control for spatial variation in illumination.
Image measurement
All images were corrected for sensor noise (dark-current) and spatial variation in illumination (flat-field). Images were automatically segmented using a convolutional neural network to determine pixels contained within the worm (Ghiasi and Fowlkes, 2016). Whole-animal summary statistics (e.g. 99th percentile intensity) were calculated from the fluorescence intensity distribution of these pixels. All measurements were performed in this fashion unless specifically indicated. Summary statistics are often generated by simply taking the average fluorescence intensity within the worm region. However, because the reporters we examined vary greatly in spatial and temporal expression pattern as well as absolute intensity, we found that no single summary statistic, such as the mean, was appropriate in all cases to robustly capture expression levels of each different reporter, or to distinguish GFP from background autofluorescence. For example, while taking the mean intensity across all ‘worm pixels’ can often reliably measure the fluorescence of a bright PmiRNA::GFP reporter expressed in a large tissue such as the intestine, the same mean may mostly capture intestinal autofluorescence for a reporter that is dim, or expressed only in a smaller tissue like the pharynx or vulva (Figure 1—figure supplement 1). Furthermore, expression of most reporters decreases with time while autofluorescence increases, further challenging robust, automated detection of reporter expression with measurements like mean intensity. Thus, we calculated a number of potential summary statistics, including 95th percentile intensity, the mean of pixels over the 99th percentile intensity, and maximum intensity (see Materials and methods), and for each reporter selected the one that most reliably captured bona-fide PmiRNA::GFP expression in both young and aged animals (Table 2). Our selection was based on manual comparisons of images of reporter expression with maps identifying the set of pixels measured by each summary statistic (i.e. if the pixels at the 95th-percentile intensity level were never co-localized with reporter-gene expression, the 95th percentile intensity was not considered an appropriate summary statistic). Figure 1—figure supplement 1 shows examples of such maps. Among plausible summary statistics, we selected those which produced the least measurement noise timepoint-to-timepoint.
For some strains with dim, spatially restricted fluorescence, measurements were performed on specific structures (e.g. head) rather than whole-worm images to avoid inadvertently measuring autofluorescence. We manually annotated the center of these structures from brightfield images and extracted summary measurements from fluorescence-image pixels within a circle of defined radius from those center-points. For Pmir-1::GFP, a circle with a radius of 5 pixels (6.5 µm) centered at the pharynx was used. For Pmir-241::GFP, a circle with a radius of 25 pixels (32.5 µm) centered at the pharynx was used. For Pmir-59::GFP, a circle with a radius of 25 pixels (32.5 µm) centered at the vulva was used to extract expression measurements.
For dual reporter strains, Pmir-47::GFP and Pmir-793::GFP expression was extracted from manually annotated circular regions centered at the vulva and pharynx. These are the predominant areas of detectable expression, especially in aged animals. Pmir-240–786::GFP expression was measured from areas of the worm excluding the manually annotated region for Pmir-47::GFP or Pmir-793::GFP. Varying the radius of the circular region did not substantially affect correlation with lifespan (data not shown).
Time of hatch, first egg lay, and death were annotated manually for all animals to determine lifespan. Individuals that hatched prior to the start of the experiment were annotated as hatching at t = 0; this timepoint is within at most 3 hr the actual hatch time (Zhang et al., 2016). No more than 5% of eggs in a given experiment fail to hatch in our system; often all hatch successfully. Of those that do hatch,<5% (typically 1–2%) are excluded from further imaging and analysis due to production of offspring (the majority of the excluded individuals) or larval arrest (a small minority). In a minority of cases, additional individuals were excluded due to localized failure of the ‘worm corral’ device: mold contamination and/or gel desiccation. The remaining individuals were indistinguishable from those in other biological replicates.
All fluorescence measures made for every animal used in all figures and tables (and associated life-stage annotations) are provided in easily parsed text files as Source data 1.
Data and statistical analysis
For visualizing population-level or cohort-level expression over time, each biological replicate was rescaled by median expression intensity and median lifespan before pooling. Expression data were fit to trendlines using LOWESS smoothing to facilitate visualization of expression levels over time. For regression and other correlative analyses, all expression values were z-transformed relative to each biological replicate and time rescaled by median lifespan of the replicate to facilitate data pooling and prevent replicate-dependent effects. Regression results from raw data from each biological replicate are consistent with the pooled analyses (table S6 in Supplementary file 1).
Single and multivariable regression of biomarkers versus lifespan was performed using ordinary least-squares regression, and the coefficient of determination (R2) calculated according to the standard formula. We aimed for a minimum sample size of 80 individuals, which leads to a statistical power of 95% to identify a true correlation of R2 >0.15 with p<0.05. We have found empirically that below R2 = 0.15, correlations with future lifespan are not generally robust across experimental replicates. Multivariable regressions for PmiRNA::GFP reporters were performed using both average expression and the slope of a least-squares linear fit line to an individual’s expression data points in a defined time window. Confidence intervals for correlation coefficients were determined by bootstrap analysis. Each pooled reporter sample was resampled with replacement 1000 times and regressions were performed on the resampled data. The 2.5th and 97.5th percentiles of the resampled correlation coefficients were used to construct the 95% confidence interval.
Individuals were excluded from analysis if they did not survive through the entire time window under consideration. Unless otherwise noted, time windows were chosen such that 10% or less of the total population was excluded.
Acknowledgements
We thank Tim Schedl, Drew Sinha, Will Pittman and Eric Kim for helpful discussions and thoughtful feedback on the manuscript. This work was supported by NIH grants T32 HG000045 and R01 AG057748, and a Beckman Young Investigator award from the Arnold and Mabel Beckman Foundation. Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Zachary Pincus, Email: zpincus@wustl.edu.
Jan Gruber, Yale-NUS College, Singapore.
Jessica K Tyler, Weill Cornell Medicine, United States.
Funding Information
This paper was supported by the following grants:
National Institute on Aging R01 AG057748 to Matthew C Mosley, Zachary Pincus, Isaac B Plutzer.
National Human Genome Research Institute T32 HG000045 to Holly E Kinser.
Arnold and Mabel Beckman Foundation Beckman Young Investigator to Holly E Kinser, Zachary Pincus.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing, Designed the experiments, conducted the experiments, analyzed the data and wrote the data analysis software.
Investigation, Writing - review and editing, Conducted the experiments.
Data curation, Investigation, Conducted the experiments.
Conceptualization, Software, Supervision, Funding acquisition, Methodology, Writing - original draft, Project administration, Writing - review and editing, Designed the experiments, wrote the data analysis software, wrote the image acquisition and image analysis software.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files. Source data files have been provided for all figures and tables.
References
- Abbott AL. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005;9:403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Saavedra E, Horvitz HR. Many families of C. elegans microRNAs are not essential for development or viability. Current Biology. 2010;20:367–373. doi: 10.1016/j.cub.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Xu J. National Vital Statistics Report. Centers for Disease Control and Preventio; 2017. https://www.cdc.gov/nchs/nvss/index.htm [Google Scholar]
- Bansal A, Kwon ES, Conte D, Liu H, Gilchrist MJ, MacNeil LT, Tissenbaum HA. Transcriptional regulation of Caenorhabditis elegans FOXO/DAF-16 modulates lifespan. Longevity & Healthspan. 2014;3:5. doi: 10.1186/2046-2395-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazopoulou D, Knoefler D, Zheng Y, Ulrich K, Oleson BJ, Xie L, Kim M, Kaufmann A, Lee YT, Dou Y, Chen Y, Quan S, Jakob U. Developmental ROS individualizes organismal stress resistance and lifespan. Nature. 2019;576:301–305. doi: 10.1038/s41586-019-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M. A developmental timing MicroRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Boulias K, Horvitz HR. The C. elegans microRNA mir-71 acts in neurons to promote germline-mediated longevity through regulation of DAF-16/FOXO. Cell Metabolism. 2012;15:439–450. doi: 10.1016/j.cmet.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner JL, Jasiewicz KL, Fahley AF, Kemp BJ, Abbott AL. Loss of individual microRNAs causes mutant phenotypes in sensitized genetic backgrounds in C. elegans. Current Biology. 2010;20:1321–1325. doi: 10.1016/j.cub.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner JL, Kemp BJ, Abbott AL. The mir-51 family of microRNAs functions in diverse regulatory pathways in Caenorhabditis elegans. PLOS ONE. 2012;7:e37185. doi: 10.1371/journal.pone.0037185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnaevskiy N, Sands B, Yun S, Tedesco PM, Johnson TE, Kaeberlein M, Brent R, Mendenhall A. Chaperone biomarkers of lifespan and penetrance track the dosages of many other proteins. Nature Communications. 2019;10:1–12. doi: 10.1038/s41467-019-13664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans Induce Stress Hormesis and Extended Longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.B109. [DOI] [PubMed] [Google Scholar]
- Dato S, Rose G, Crocco P, Monti D, Garagnani P, Franceschi C, Passarino G. The genetics of human longevity: an intricacy of genes, environment, culture and microbiome. Mechanisms of Ageing and Development. 2017;165:147–155. doi: 10.1016/j.mad.2017.03.011. [DOI] [PubMed] [Google Scholar]
- de Lencastre A, Pincus Z, Zhou K, Kato M, Lee SS, Slack FJ. MicroRNAs both promote and antagonize longevity in C. elegans. Current Biology. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian TJ, Johnson TE. Production of age-synchronous mass cultures of Caenorhabditis elegans. Journal of Gerontology. 1994;49:B145–B156. doi: 10.1093/geronj/49.4.B145. [DOI] [PubMed] [Google Scholar]
- Ferrell JE. Bistability, bifurcations, and Waddington's epigenetic landscape. Current Biology. 2012;22:R458–R466. doi: 10.1016/j.cub.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxman Bass JI, Pons C, Kozlowski L, Reece-Hoyes JS, Shrestha S, Holdorf AD, Mori A, Myers CL, Walhout AJ. A gene-centered C. elegans protein-DNA interaction network provides a framework for functional predictions. Molecular Systems Biology. 2016;12:884. doi: 10.15252/msb.20167131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova NS, Gavrilov LA, Severin FF, Skulachev VP. Testing predictions of the programmed and stochastic theories of aging: comparison of variation in age at death, menopause, and sexual maturation. Biochemistry. Biokhimiia. 2012;77:754–760. doi: 10.1134/S0006297912070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, Panowski S, Hansen M. Intestinal autophagy improves healthspan and longevity in C. elegans during Dietary Restriction. PLOS Genetics. 2016;12:e1006135. doi: 10.1371/journal.pgen.1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi G, Fowlkes CC. Laplacian pyramid reconstruction and refinement for semantic segmentation. In: Randy G, Yuzuru T, Wolfgang W, editors. Lecture Notes in Computer Science. Springer Verlag; 2016. pp. 519–534. [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 danish twin pairs born 1870-1900. Human Genetics. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Hsu A-L, Feng Z, Hsieh M-Y, Xu XZS. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans neurobiol. Aging. 2009;30:1498–1503. doi: 10.1016/j.neurobiolaging.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ibáñez-Ventoso C, Vora M, Driscoll M. Sequence relationships among C. elegans, D. melanogaster and human microRNAs highlight the extensive conservation of microRNAs in biology. PLOS ONE. 2008;3:e2818. doi: 10.1371/journal.pone.0002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlén J, García MB, Menges ES, Quintana-Ascencio PF, Caswell H, Baudisch A, Vaupel JW. Diversity of ageing across the tree of life. Nature. 2014;505:169–173. doi: 10.1038/nature12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Chen X, Inukai S, Zhao H, Slack FJ. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA. 2011;17:1804–1820. doi: 10.1261/rna.2714411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Kashem MA, Cheng C. An intestinal microRNA modulates the homeostatic adaptation to chronic oxidative stress in C. elegans. Aging. 2016;8:1979–2005. doi: 10.18632/aging.101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Igarashi K. To be red or white: lineage commitment and maintenance of the hematopoietic system by the "inner myeloid". Haematologica. 2019;104:1919–1927. doi: 10.3324/haematol.2019.216861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BJ, Allman E, Immerman L, Mohnen M, Peters MA, Nehrke K, Abbott AL. miR-786 regulation of a fatty-acid elongase contributes to rhythmic calcium-wave initiation in C. elegans. Current Biology. 2012;22:2213–2220. doi: 10.1016/j.cub.2012.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kim HM, Colaiácovo MP. ZTF-8 interacts with the 9-1-1 complex and is required for DNA damage response and double-strand break repair in the C. elegans germline. PLOS Genetics. 2014;10:e1004723. doi: 10.1371/journal.pgen.1004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, Omholt S, Westendorp RG. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mechanisms of Ageing and Development. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kumsta C, Chang JT, Schmalz J, Hansen M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nature Communications. 2017;8:14337. doi: 10.1038/ncomms14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. Repression of the heat shock response is a programmed event at the onset of reproduction. Molecular Cell. 2015;59:639–650. doi: 10.1016/j.molcel.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Jafari G, Primitivo M, Sutphin GL, Dong J, Leonard A, Fletcher M, Kaeberlein M. Age-associated vulval integrity is an important marker of nematode healthspan. Age. 2016;38:419–431. doi: 10.1007/s11357-016-9936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X-X, Sen I, Janssens GE, Zhou X, Fonslow BR, Edgar D, Stroustrup N, Swoboda P, Yates JR, Ruvkun G, Riedel CG. DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and longevity. Nature Communications. 2018;9:06624-0. doi: 10.1038/s41467-018-06624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. PNAS. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist B, Berg S, Lanke J, McClearn GE, Pedersen NL. The effect of genetic factors for longevity: a comparison of identical and fraternal twins in the swedish twin registry. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1998;53A:M441–M446. doi: 10.1093/gerona/53A.6.M441. [DOI] [PubMed] [Google Scholar]
- Lucanic M, Lithgow GJ, Alavez S. Pharmacological lifespan extension of invertebrates. Ageing Research Reviews. 2013;12:445–458. doi: 10.1016/j.arr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NJ, Ow MC, Reece-Hoyes JS, Barrasa MI, Ambros VR, Walhout AJ. Genome-scale spatiotemporal analysis of Caenorhabditis elegans microRNA promoter activity. Genome Research. 2008;18:2005–2015. doi: 10.1101/gr.083055.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Vaupel JW, Holm N, Harvald B. Longevity is moderately heritable in a sample of danish twins born 1870-1880. Journal of Gerontology. 1993;48:B237–B244. doi: 10.1093/geronj/48.6.B237. [DOI] [PubMed] [Google Scholar]
- Mendenhall A, Crane MM, Tedesco PM, Johnson TE, Brent R. Caenorhabditis elegans genes affecting interindividual variation in Life-span biomarker gene expression. The Journals of Gerontology: Series A. 2017;72:1305–1310. doi: 10.1093/gerona/glw349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman S, Barzilai N. Dissecting the mechanisms underlying unusually successful human health span and life span. Cold Spring Harbor Perspectives in Medicine. 2016;6:a025098. doi: 10.1101/cshperspect.a025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Abbott AL, Lau NC, Hellman AB, McGonagle SM, Bartel DP, Ambros VR, Horvitz HR. Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLOS Genetics. 2007;3:e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalev AA, Aliper AM, Smit-McBride Z, Buzdin A, Zhavoronkov A. Genetics and epigenetics of aging and longevity. Cell Cycle. 2014;13:1063–1077. doi: 10.4161/cc.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Pedersen ME, Snieckute G, Kagias K, Nehammer C, Multhaupt HA, Couchman JR, Pocock R. An epidermal microRNA regulates neuronal migration through control of the cellular glycosylation state. Science. 2013;341:1404–1408. doi: 10.1126/science.1242528. [DOI] [PubMed] [Google Scholar]
- Pincus Z, Smith-Vikos T, Slack FJ. MicroRNA predictors of longevity in Caenorhabditis elegans. PLOS Genetics. 2011;7:e1002306. doi: 10.1371/journal.pgen.1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus Z, Mazer TC, Slack FJ. Autofluorescence as a measure of senescence in C. elegans: look to red, not blue or green. Aging. 2016;8:889–898. doi: 10.18632/aging.100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman WE, Sinha DB, Zhang WB, Kinser HE, Pincus Z. A simple culture system for long-term imaging of individual C. elegans. Lab on a Chip. 2017;17:3909–3920. doi: 10.1039/C7LC00916J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podshivalova K, Kerr RA, Kenyon C. How a mutation that slows aging can also disproportionately extend End-of-Life decrepitude. Cell Reports. 2017;19:441–450. doi: 10.1016/j.celrep.2017.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Principles of a switch. Nature Chemical Biology. 2011;7:484–487. doi: 10.1038/nchembio.611. [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in Isogenic populations of Caenorhabditis elegans. Nature Genetics. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Wright KM, Rand KA, Kermany A, Noto K, Curtis D, Varner N, Garrigan D, Slinkov D, Dorfman I, Granka JM, Byrnes J, Myres N, Ball C. Estimates of the heritability of human longevity are substantially inflated due to assortative mating. Genetics. 2018;210:1109–1124. doi: 10.1534/genetics.118.301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blanco A, Kim SK. Variable pathogenicity determines individual lifespan in Caenorhabditis elegans. PLOS Genetics. 2011;7:e1002047. doi: 10.1371/journal.pgen.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, Kim JK. The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell. 2008;133:903–915. doi: 10.1016/j.cell.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skytthe A, Pedersen NL, Kaprio J, Stazi MA, Hjelmborg JV, Iachine I, Vaupel JW, Christensen K. Longevity studies in GenomEUtwin. Twin Research. 2003;6:448–454. doi: 10.1375/136905203770326457. [DOI] [PubMed] [Google Scholar]
- Smith-Vikos T, de Lencastre A, Inukai S, Shlomchik M, Holtrup B, Slack FJ. MicroRNAs mediate dietary-restriction-induced longevity through PHA-4/FOXA and SKN-1/Nrf transcription factors. Current Biology. 2014;24:2238–2246. doi: 10.1016/j.cub.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup N, Ulmschneider BE, Nash ZM, López-Moyado IF, Apfeld J, Fontana W. The Caenorhabditis elegans lifespan machine. Nature Methods. 2013;10:665–670. doi: 10.1038/nmeth.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup N. The temporal scaling of Caenorhabditis elegans. Ageing. 2016;530:103–107. doi: 10.1038/nature16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell. 2013;154:676–690. doi: 10.1016/j.cell.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MJ, Slack FJ. Transcriptional control of microRNA expression in C. elegans: promoting better understanding. RNA Biology. 2009;6:49–53. doi: 10.4161/rna.6.1.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Wang L, Walker BL, Iannaccone S, Bhatt D, Kennedy PJ, Tse WT. Bistable switches control memory and plasticity in cellular differentiation. PNAS. 2009;106:6638–6643. doi: 10.1073/pnas.0806137106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen D, He Y, Meléndez A, Feng Z, Hong Q, Bai X, Li Q, Cai G, Wang J, Chen X. MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age. 2013;35:11–22. doi: 10.1007/s11357-011-9324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WB, Sinha DB, Pittman WE, Hvatum E, Stroustrup N, Pincus Z. Extended twilight among isogenic C. elegans Causes a Disproportionate Scaling between Lifespan and Health. Cell Systems. 2016;3:333–345. doi: 10.1016/j.cels.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Fan Z, Qiao P, Zhao Y, Wang Y, Jiang D, Wang X, Zhu X, Zhang Y, Huang B, Lu J, Li X. miR-51 regulates GABAergic synapses by targeting rab GEF GLO-4 and lysosomal trafficking-related GLO/AP-3 pathway in Caenorhabditis elegans. Developmental Biology. 2018;436:66–74. doi: 10.1016/j.ydbio.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Two forms of death in ageing Caenorhabditis elegans. Nat Commun. 2017;8:1–8. doi: 10.1038/ncomms15458. [DOI] [PMC free article] [PubMed] [Google Scholar]