Abstract
Introduction
Secondary polycythemia is a known adverse effect of testosterone replacement therapy (TRT). Different testosterone formulations are available, with significantly different half-lives, which have varying influences on the development of secondary polycythemia. Herein, we compared the prevalence of secondary polycythemia in testosterone-deficient men treated with intranasal testosterone gel (Natesto®) vs. intramuscular testosterone cypionate (TC) therapy.
Methods
We performed a cross-sectional analysis of secondary polycythemia (hematocrit [Hct] ≥54%) in men who received TRT. We included a total of 60 men: 30 men who received Natesto (4.5% testosterone gel [tid, 5.5 mg/nostril, 11 mg/dose, 33 mg/day]), and 30 who received TC (between 0.5 and 1.0 mL or 100–200 mg intramuscularly weekly). A univariable and multiple regression analysis was performed considering last Hct measurement as the main outcome. The analyzed variables included were age, body mass index (BMI), smoking history, treatment group, and testosterone levels on followup.
Results
We identified polycythemia (Hct ≥54%) in 10% (3/30) of men who received TC. Additionally, in men treated with TC, 33.3% (10/30) had a Hct ≥50% during therapy. None of the men who received Natesto had a Hct ≥50% during therapy. On multivariable linear regression analysis, we demonstrated that the use of TC increased Hct by 3.24% (95% confidence interval [CI] 0.74–5.73%, p=0.012) compared to Natesto.
Conclusions
The prevalence of polycythemia in men treated with Natesto was markedly lower compared to the men who received TC therapy.
Introduction
The prevalence of middle-aged males diagnosed with testosterone deficiency is estimated to be 6.5 million by 2025, with an approximate 0.8% to 1.6% yearly decline in total testosterone after age 30.1 The most common treatment for males with testosterone deficiency is testosterone replacement therapy (TRT). In males 18–45 years old, testosterone prescription sales have quadrupled in the past decade.2 In addition to ameliorating testosterone deficiency symptoms, TRT can lead to increased muscle mass, bone mineral density, and improved insulin resistance.3,4 However, TRT has several common side effects, including increased in estradiol, acne, gynecomastia, and erythrocytosis.5–7 Commonly used testosterone (T) formulations include intramuscular (IM) injectables, transdermal gels, and subcutaneous pellets. While all TRT preparations are effective, the likelihood of the associated side effects are determined by method of administration, pharmacokinetics, and dosage.8 Initially described by Dobs et al, several studies examined the incidence of erythrocytosis associated with IM injections, in particular testosterone cypionate (TC) and enanthate, over transdermal formulations, have the highest incidence of erythrocytosis, almost approaching 40%.9
The erythrogenic effect of TRT has been recognized since the 1960s, however, the different mechanisms proposed to explain this observation remain poorly understood, including increased erythropoietin production by the kidneys,10 increased erythroid progenitor cells in bone marrow,11 and suppression of hepcidin leading to increased iron absorption and subsequent increase in systemic iron transport and erythropoiesis.12 Bachman et al showed that supraphysiological T levels suppressed hepcidin in a dose- and age-dependent manner by more than 50%, and resulted in an increased hematocrit (Hct), along with stable erythropoietin levels.12 Since the release of a new warning label in 2014 by the FDA, thrombotic and cardiovascular risks of testosterone therapy have come under increased scrutiny.13
We conducted a clinical trial with Natesto® — an intransal testosterone gel — and demonstrated that the majority of men were able to maintain their gross semen parameters during treatment.14 Natesto has a different pharmacokinetic profile and much shorter half-life compared to TC.15,16 It is believed that semen parameters were maintained during treatment with Natesto likely due to the short half-life and frequent troughs in serum T throughout the day and throughout the night, which allow for secretion of gonadotropin-releasing hormone (GnRH).14 Due to this this novel finding and unique pharmacokinetic profile, we hypothesized that the short half-life of Natesto (30 minutes) would contribute to lower prevalence of polycythemia as compared to the long half-life of TC (6.9 days).17 Our objective was to perform a cross-sectional analysis to evaluate the prevalence of secondary polycythemia for men on either Natesto or TC, and prospectively evaluate the changes in T and Hct after treatment.
Methods
Patient identification and data acquisition
We performed a cross-sectional analysis of Hct levels in men being treated for testosterone deficiency with either Natesto or TC. A total of 118 men were identified who received either Natesto or TC between May 2017 and November 2019. Furthermore, we analyzed the testosterone levels at the time of the highest Hct or last recorded Hct on followup after at least three months of therapy. After removing patients with missing data and/or lab work, 60 men remained: 30 who received Natesto and 30 who received TC. Men were diagnosed with testosterone deficiency according to American Urological Association (AUA) guidelines, which require both clinical symptoms of testosterone deficiency (i.e., decreased libido, energy, and erectile dysfunction), as assessed by history during their initial clinic encounter, in addition to biochemical evidence of low serum T (<300 ng/dL). All men had their labs drawn from the same lab between 7:00 am and 10:00 am. Men who were interested in preserving fertility were prescribed Natesto and men not interested in fertility were prescribed TC. Men receiving Natesto administered the drug three times daily based on manufacturer’s recommendations (Natesto 4.5% testosterone gel [t.i.d., 5.5 mg/nostril, 11 mg/dose, 33 mg/day]) (Haupt Pharma, Regensburg, Germany). Men on injectable TC formulations injected between 0.5 and 1.0 mL or 100–200 mg IM weekly.
We performed a cross-sectional analysis to evaluate the overall prevalence of secondary polycythemia during treatment with Natesto and TC. Since the definition of erythrocytosis in the literature ranges from 50–54%, we defined erythrocytosis as Hct ≥54% on followup based on the recommendations by the Endocrine Society Guidelines;18 this is the cutoff used in clinical practice to identify patients requiring cessation of treatment and/or phlebotomy. For a secondary endpoint, we assessed the number of men with a Hct above the other cutoffs defined by the Endocrine Society Guidelines (Hct >50%, Hct >52%) to help guide clinical decision-making. A post-hoc power analysis with n=60 (30 in each group) looking at incidence of Hct >50% and an alpha of 0.05 would result in a power of 95%. This study was submitted to and approved by the institutional review board of the University of Miami, Miller School of Medicine.
Statistical analysis
All statistical analysis was performed using Statistical Package for the Social Sciences (SPSS), version 24, with p<0.05 considered statistically significant. Categorical variables were presented as absolute values and frequencies and were analyzed with a Chi-squared or Fisher’s exact test as required. Continuous variables were reported as the mean ± standard deviation (SD) and the Student t-test was used to compare groups. A univariable and multivariable regression analysis was performed considering last Hct measurement as the main outcome. The analyzed variables included were age, body mass index (BMI), smoking history, treatment group, and testosterone levels on followup. To avoid multicollinearity, before running a multivariable regression analysis, we confirmed that none of the variables had a high degree of correlation (r>±0.7) and calculated the tolerance of each independent variable. Additionally, we calculated the Cook’s distance of each individual patient to determine the influence of data points, and performed the Durbin-Watson test to assess for autocorrelation in the residuals.
Results
The clinical and demographic characteristics of the analyzed men are shown in Table 1. The men treated with TC were older than men treated with Natesto (TC=54.9±11.8 years vs. Natesto=36.6±7.6 years, respectively). Men on TC had a longer mean treatment duration than men treated with Natesto (6.9±4.3 vs. 4.7±1.5 months, respectively). Smoking history was present in eight men (26.7%) treated with TC and six men (20%) treated with Natesto (p=0.542). There were no significant differences in baseline serum testosterone or Hct between the two groups (p>0.05) (Table 1).
Table 1.
Comparison between the clinical and demographic characteristics of men in the Natesto and TC group
| Overall | Natesto | TC | p | |
|---|---|---|---|---|
| Age | 45.3±13.2 | 36.6±7.6 | 54±11.8 | <0.001 |
| BMI | 29.7±5.8 | 30.5±6.8 | 28.6±4.6 | 0.281 |
| Smoking history | ||||
| No | 46 (76.7%) | 24 (80%) | 22 (73.3%) | |
| Yes | 14 (23.3%) | 6 (20%) | 8 (26.7%) | 0.542 |
| Baseline | ||||
| Testosterone | 244.5±101.4 | 228.7±57.7 | 260.4±130.6 | 0.231 |
| Hematocrit | 44.8±3.3 | 44.8±2.2 | 44.8±3.7 | 0.994 |
| Followup | ||||
| Testosterone | 811.7±388.8 | 640.3±302.4 | 983.1±394.1 | <0.001 |
| Hematocrit | 46.5±3.6 | 44.9±2.3 | 48.1±3.9 | <0.001 |
| Time followup (months) | 5.7±3.4 | 4.7±1.5 | 6.9±4.3 | 0.004 |
Mean ± standard deviation. BMI: body mass index; TC: testosterone cypionate.
Polycythemia (Hct ≥54%) prevalence in men on TC was 10% (3/30) vs. 0% (0/30) for men on Natesto. Additionally, for patients on TC, 33.3% (10/30) had a Hct ≥50% during therapy compared to Natesto, which had no patients with a Hct ≥50% during therapy (Fig. 1). On univariable linear regression, age and treatment group significantly contribute to Hct on followup. In the multivariable regression analysis model, there was independence of residuals (Durbin-Watson=1.999). There was no evidence of multicollinearity, as evaluated in the independent variables, with no results of tolerance less than 0.1, and there was no case with a Cook’s distance above 1. The multivariable regression model significantly predicted followup Hct (F[5,54]=2.822, p=0.025] and accounts for a variation in hematocrit of 13.4% (R2=0.207, adjusted R2=0.134). After adjusting for covariables, the treatment group significantly contributed to the model, showing that using TC increase Hct by 3.24% (95% confidence interval [CI] 0.74–5.73%, p=0.012) when compare to Natesto (Table 2).
Fig. 1.
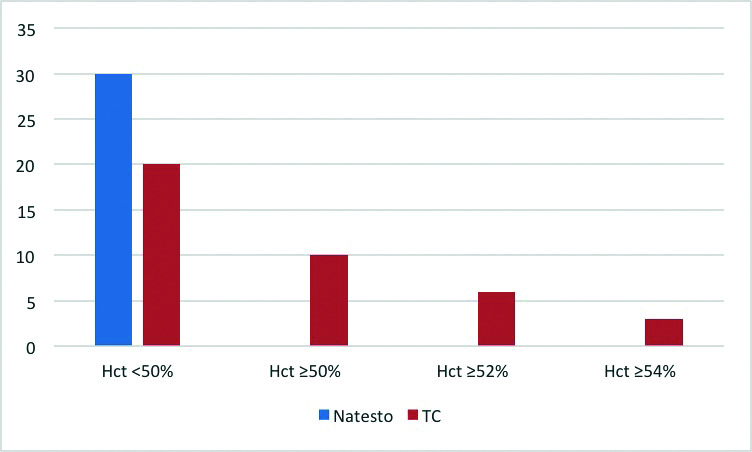
Followup hematocrit (Hct) levels during therapy with Natesto or testosterone cypionate (TC). Represents the number of patients reaching various Hct cutoffs during treatment with TC or Natesto.
Table 2.
Univariable and multivariable linear regression analysis used to predict followup hematocrit
| Variable | Univariable | Multiple linear regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| R2 | B | 95% CI | p | Adj. R2 | B | 95% CI | p | |||
|
| ||||||||||
| Lower | Upper | Lower | Upper | |||||||
| Age in years | 0.080 | 0.08 | 0.01 | 0.14 | 0.029 | 0.134 | −0.01 | 0.08 | −0.10 | 0.796 |
| Treatment group | ||||||||||
| Natesto | Ref. | Ref. | ||||||||
| TC | 0.200 | 3.15 | 1.50 | 4.81 | <0.001 | 3.24 | 0.74 | 5.73 | 0.012 | |
| BMI (kg/m2) | 0.0002 | −0.01 | −0.17 | 0.15 | 0.921 | 0.04 | −0.12 | 0.19 | 0.644 | |
| Smoking history | ||||||||||
| No | Ref. | Ref. | ||||||||
| Yes | 0.006 | 0.66 | −1.52 | 2.85 | 0.546 | 0.42 | −1.69 | 2.53 | 0.694 | |
| Followup testosterone (10 ng/dL increase) | 0.050 | 0.02 | −0.003 | 0.05 | 0.085 | 0.004 | −0.02 | 0.03 | 0.730 | |
B: unstandardized regression coefficient; BMI: body mass index; CI: confidence interval; R2: coefficient of determination; adj. R2: adjusted coefficient of determination; Ref.: reference value; TC: testosterone cypionate.
Discussion
To the best of our knowledge, this is the only study comparing the prevalence of secondary polycythemia in testosterone-deficient patients treated with either intranasal testosterone (Natesto) or IM TC therapy. There was a lower prevalence of secondary polycythemia (Hct ≥54%) in the cohort treated with Natesto (0%) compared to that on TC therapy (10%). In addition, using various cutoffs for Hct, significant differences were seen between treatment groups. In men treated with TC, 33.3% (10/30) had a Hct ≥50% and 20% (6/30) had a Hct ≥52% on followup, compared to men treated with Natesto, which had no one develop a Hct ≥50% during treatment. The prevalence of secondary polycythemia due to TC is comparable to previous studies that have evaluated this phenomenon.19
A study performed by Bachman et al showed that supraphysiological T levels suppressed hepcidin in a dose- and age-dependent manner by >50% and resulted with an increased Hct, along with stable erythropoietin levels. His study also supported the hypothesis that older men respond to testosterone with a relatively greater level of hepcidin suppression than younger men. This mechanism may be responsible for the difference in prevalence of polycythemia and change in Hct seen in TC patients, as the average age was 17.4 years older in our cohort. Since men interested in fertility preservation were treated with Natesto, this group was younger than the TC group, who were not interested in fertility preservation. Additionally, post-treatment levels of testosterone were significantly higher in the TC group than in the Natesto group (983.1±394.1 ng/dL vs. 640.3±302.4 ng/dL, respectively), and this could have contributed to the observed difference. However, in an attempt to further analyze the effect of different independent variables on followup Hct, we performed a multivariable analysis that was adjusted by variables including age, BMI, smoking history, and testosterone on followup. Our findings indicate that the most influential factor contributing to an increase in Hct on followup was the use of TC.
Previous studies have demonstrated that different T formulations may have varying effects on erythropoiesis, and some have proposed that the troughs in serum T between doses are most predictive of developing polycythemia.20,21 Importantly, the pharmacokinetic profile and three times daily dosing of Natesto allows T levels to return to baseline multiple times daily, whereas TC leads to steady state levels over days. This property of Natesto was highlighted in our published clinical trial, in which most men on Natesto maintained semen parameters during treatment. This was likely attributed to the maintenance of pulsatile secretion of GnRH that allowed daily release of gonadotropins.22 Regardless of the proposed mechanisms for the erythrogenic effect of T,10–12 Natesto’s short half-life and frequent troughs in T during treatment would have less of an influence on these mechanisms. Of note, although Natesto can provide symptom relief similar to that of TC, the three time daily administration that Natesto requires can be cumbersome for patients when compared to a single injection that lasts for weeks.22
The difference in the hematological effect from these different formulations can have substantial clinical implications. The Framingham study, a 34-year followup study that evaluated the risk between cardiovascular disease (CVD) mortality and Hct, found Hct to be an important risk factor for CVD mortality, including congestive heart disease, myocardial infarction, angina pectoris, and stroke.23 According to the Endocrine Society clinical practice guidelines, a Hct >50% should be a contraindication for initiation of T therapy, and a Hct >54% should warrant discontinuation of the medication.18 A previous systematic review and meta-analysis, which included 51 studies, found that an increase in Hct was the most frequent adverse effect experienced by men on T therapy (weighted mean difference 3.18%; 95% CI 1.35–5.01).24 Given the frequency of secondary polycythemia seen in men on TRT and the guidelines recommended by the Endocrine Society, having an option of TRT that minimizes the risk of polycythemia is optimal for clinical practice.
Our study had several limitations, including its retrospective nature, variability in dosages of TC taken, and although adequately powered, a relatively small sample size and a non-age-matched cohort. Strengths of the study are the novelty in the literature, adequate power, directly comparing the biochemical effects of TC and short-acting Natesto, and a multivariable regression analysis demonstrating mode of therapy as an independent predictor of polycythemia. Further prospective, randomized trials are needed in which men are matched by age, serum T, and Hct levels on baseline to confirm our findings.
Conclusions
The prevalence of polycythemia in men treated with Natesto were markedly lower compared to the men who received TC injections. Given that the most frequent adverse effect of TRT is the resulting polycythemia, Natesto could be a potential option if this is a concern, although larger, prospective studies with age-matched cohorts are needed to validate the finding in our cross-sectional study.
Footnotes
Competing interests: Dr. Ramasamy is a consultant for Acerus Pharmaceuticals and consultant/grant recipient for Endo Pharmaceuticals. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–7. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 2.Rao PK, Boulet SL, Mehta A, et al. Trends in testosterone replacement therapy use from 2003–2013 among reproductive-age men in the United States. J Urol. 2017;197:1121–6. doi: 10.1016/j.juro.2016.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycemic control, visceral adiposity, and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 4.Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–72. doi: 10.1093/gerona/56.5.M266. [DOI] [PubMed] [Google Scholar]
- 5.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 6.Coviello AD, Kaplan B, Lakshman KM, et al. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93:914–9. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: A systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.1016/S0025-6196(11)60964-6. [DOI] [PubMed] [Google Scholar]
- 8.Kovac JR, Rajanahally S, Smith RP, et al. Patient satisfaction with testosterone replacement therapies: The reasons behind the choices. J Sex Med. 2014;11:553–62. doi: 10.1111/jsm.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–78. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 10.Shahidi NT. Androgens and erythropoiesis. N Engl J Med. 1973;289:72–80. doi: 10.1056/NEJM197307122890205. [DOI] [PubMed] [Google Scholar]
- 11.Palacios A, Campfield LA, McClure RD, et al. Effect of testosterone enanthate on hematopoiesis in normal men. Fertil Steril. 1983;40:100–4. doi: 10.1016/S0015-0282(16)47185-2. [DOI] [PubMed] [Google Scholar]
- 12.Bachman E, Feng R, Travison T, et al. Testosterone suppresses hepcidin in men: A potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–7. doi: 10.1210/jc.2010-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigen R, O’Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 14.Masterson T, Molina M, Ibrahim E, et al. Natesto effects on reproductive hormones and semen parameters: Results from an ongoing single-center, investigator-initiated phase 4 clinical trial. Eur Urol Focus. 2018;4:333–5. doi: 10.1016/j.euf.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Rogol AD, Tkachenko N, Badorrek P, et al. Phase 1 pharmacokinetics and phase 3 efficacy of testosterone nasal gel in subjects with seasonal allergies. Can Urol Assoc J. 2018;12:E349–56. doi: 10.5489/cuaj.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogol AD, Tkachenko N, Bryson N. Natesto, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016;4:46–54. doi: 10.1111/andr.12137. [DOI] [PubMed] [Google Scholar]
- 17.MacIndoe JH, Perry PJ, Yates WR, et al. Testosterone suppression of the HPT axis. J Investig Med. 1997;45:441–7. [PubMed] [Google Scholar]
- 18.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar RR, Kaiser FE, Morley JE. Outcomes of long-term testosterone replacement in older hypogonadal males: A retrospective analysis. J Clin Endocrinol Metab. 1997;82:3793–6. doi: 10.1210/jcem.82.11.4387. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–53. doi: 10.1210/jc.85.8.2839. [DOI] [PubMed] [Google Scholar]
- 21.Ip FF, di Pierro I, Brown R, et al. Trough serum testosterone predicts the development of polycythemia in hypogonadal men treated for up to 21 years with subcutaneous testosterone pellets. Eur J Endocrinol. 2010;162:385–90. doi: 10.1530/EJE-09-0717. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy R, Masterson TA, Best JC, et al. Effect of Natesto on reproductive hormones, semen parameters and hypogonadal symptoms: A single-center, open-label, single-arm trial. J Urol. 2020. [DOI] [PubMed]
- 23.Gagnon DR, Zhang TJ, Brand FN, et al. Hematocrit and the risk of cardiovascular disease — the Framingham study: A 34-year followup. Am Heart J. 1994;127:674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]


