Abstract
Introduction
Kidney and simultaneous pancreas-kidney (SPK) transplant recipients can have prolonged postoperative hospitalization due to edema. Thrombo-embolic-deterrent (TED) stockings with intermittent pneumatic compression devices (TED+IPC) have been used to improve venous return during the perioperative period. The objective of this trial was to evaluate the effects of TED+IPC vs. muscle pump activator (MPA) devices on factors that could reduce postoperative complications and duration of hospitalization.
Methods
In this single-center, prospective, randomized, controlled trial, 221 kidney and SPK transplant recipients were randomized to either wearing TED+IPC or MPA for six days postoperatively. Groups were compared with respect to postoperative urine output, lower limb edema, weight, days in hospital, mobility, serum creatinine, delayed graft function, need for dialysis, and lower extremity blood flow.
Results
Patients in the MPA group had significantly higher urine output and less increase in mid-calf leg circumference and weight gain compared to the TED+IPC group (p=0.003, p=0.001, and p=0.003, respectively). The MPA group also experienced shorter hospitalization (p=0.038), higher femoral vein velocity (p=0.001), and took more steps (p=0.009). Incidence of delayed graft function (p=0.72) and number of dialysis runs (p=0.39) was not different between study groups. Subgroup analysis of primary endpoints in donation after cardiac death recipients and SPK recipients did not yield any significance between the study arms.
Conclusions
Postoperative use of the MPA device increases urine output, decreases leg edema, minimizes weight gain, and decreases duration of hospitalization after kidney transplantation. A larger and longer-term trial is needed to evaluate the impact on graft function.
Introduction
Kidney and simultaneous pancreas-kidney (SPK) transplantations can significantly reduce mortality and improve quality of life in patients with end-stage renal disease (ESRD) and select patients with diabetes and ESRD, respectively.1–5 Postoperatively, recipients may require a prolonged period of hospitalization secondary to a number of factors, including delayed graft function (DGF), delayed mobilization, and edema.6–8 Edema may also contribute to other postoperative complications, including impaired wound healing and ileus.9,10 Fluid management after renal transplantation can be challenging. The need to provide adequate pre-load to perfuse the newly transplanted allograft can conflict with efforts to avoid volume overload. The latter condition can be slow to correct, especially if the allograft is experiencing slow graft function or DGF. Management of lower-limb circulation and postoperative lower-limb edema is of special interest in kidney and SPK transplantation, given the site of implantation for the allograft vessels. The benefits of improving postoperative lower-limb circulation and correcting lower-limb edema has been demonstrated in trauma orthopedic surgery11 and lower-limb revascularization surgery,12 where improvements in mobility and surgical outcome have been demonstrated.
Thrombo-embolic-deterrent (TED) stockings and intermittent pneumatic compression (IPC) devices are the established standard of care for management of postoperative lower-limb edema and venous stasis.13 The devices can be uncomfortable to wear, and the large external pneumatic pump inhibits patient mobility. Muscle pump activator (MPA) devices have been found to be an effective alternative to IPC devices for management of postoperative lower-limb edema.14 The geko™ (Firstkind Ltd, U.K.) device is a portable, wireless MPA device, worn as a band over the legs bilaterally, just inferior to the fibular heads (Fig. 1). The resulting simultaneous contraction of tibialis, peroneus longus, and lateral gastrocnemius muscles compress the deep veins to increase blood flow in these vessels. In addition to greater improvements in both macrocirculation15 and microcirculation14 of the lower-limb in comparison to standard IPC pumps, the device is smaller, portable, and more comfortable to wear.16 Despite various studies demonstrating equal or greater efficacy in hemodynamic parameters, there is very limited high-quality evidence for the effects of MPA on clinically relevant measures.17 This randomized, controlled trial aims to elucidate the impact of MPA on lower-limb edema, renal function, and length of hospitalization in relation to standard TED+IPC after kidney and SPK transplantation. We hypothesized that the geko device used in the postoperative setting following kidney and SPK transplants would lead to improved lower-limb edema resulting in a decreased length of stay in hospital.
Fig. 1.

The The geko™ (Firstkind Ltd, U.K.) device worn as a band over the legs bilaterally, just inferior to the fibular heads.
Methods
Study design
This study was designed as an open-label, single-center, prospective, randomized, controlled trial. The study was conducted in accordance with the CONSORT 2010 guidelines and followed the principles of the Declaration of Helsinki. Institutional review approval was provided by the Western University Research Ethics Board (Protocol number 103618, ClinTrials.gov NCT01860820).
Participants
Patients undergoing kidney and SPK transplantation at London Health Sciences Centre were eligible for the study if they were ≥18 years old. Exclusion criteria included: age <18 years old, history of deep vein thrombosis (DVT), history of leg amputation, body mass index (BMI) >36, use of an implantable cardiac defibrillator, presence of deep brain stimulators, other contraindications to use of electrical stimulation devices, lack of ability to understand the risks and benefits of the study, and those who could not tolerate the MPA device stimulation.
Induction immunosuppressive therapy consisted of antithymocyte globulin (5–8 mg/kg IV) or basiliximab (20 mg IV on postoperative day [POD] 0 and 4) depending on the recipient’s immunological risk. Maintenance immunosuppressive regimen consisted of prednisone, tacrolimus, and mycophenolic acid, and were initiated while in hospital for all recipients. Serum drug levels were monitored closely, and dosages were adjusted according to accepted practices. Recipients of a renal transplant have their urinary catheters removed on POD 5 as a program standard. In cases when patients were discharged home prior to POD 5, their catheters were removed on the day of discharge.
Randomization and interventions
Patients deemed eligible for the study provided informed consent and underwent randomization prior to their transplant surgery. Randomization was performed by a study coordinator, independent of the patient’s clinical care, using an online computer generator sequence. Participants were placed into either the control (TED+IPC) or interventions (MPA, geko) arm based on randomization. The surgical team was blinded to patient’s group allocation at the time of surgery.
As per institutional standard, TED+IPC was used for intraoperative DVT prophylaxis for both groups. All participants were also placed on dalteparin 5000 IU subcutaneous injection daily for DVT prophylaxis starting on POD 1. Patients assigned to the MPA group were then switched over the geko device on POD 1. The device was properly fitted and adjusted for stimulation of the common peroneal nerve according to manufacturer’s instructions for use. Patients randomized to the control arm continued to wear the TED+IPC device postoperatively. On POD 6, or at the time of discharge if duration of hospitalization was less than six days, both groups had their devices removed. Postoperative care and discharge planning was performed by an independent nephrology team blinded to the randomization and uninvolved with the trial.
Outcomes
Co-primary endpoints were lower-limb edema and total urine output from POD 1–6. Lower-limb edema is reflected by the difference in calf circumference between POD 1 and 6, or day of discharge, whichever came first. Calf circumference is measured using a tape measure at 15 cm below the patella’s midpoint. Serum creatinine was used to directly report renal function. Based on a pilot study performed at our center suggesting a difference in lower-limb edema between the study groups, we sought to study a more clinically relevant metric as part of our primary outcome measure. We hypothesized that reduction in lower-limb circumference would translate into increased urine output, which is relevant to the early post-transplant period. Difference in patient weight was measured in kilograms between POD 1 and 6, or day of discharge, whichever came first.
Secondary endpoints included length of stay, occurrence of DGF (defined as the need for dialysis within one week of transplant), number of dialysis sessions postoperatively, renal blood flow, and mobility. Incidence of DGF was assessed dichotomously and further delineated by number of dialysis runs needed during the postoperative hospitalization period. Peak femoral vein velocity was measured in centimeter per second on Doppler ultrasound of the renal allograft on POD 6. During the postoperative period, participants in both groups wore pedometers and monitored their physical activity from POD 2–5. Postoperative incidence of DVT was also monitored.
Statistical analysis
Based on pilot study data, we expected the mean calf circumference in the TED+SCD group to increase by 3.5 cm with a standard deviation (SD) of 1.5. Given there is no defined minimal clinically important difference for calf circumference, we used the statistical convention of ½ SD to estimate this value. Therefore, we determined that in the MPA group, an increase in calf circumference to 2 cm would provide a moderate effect size of 0.5. With a desired power of 90%, we determined that sample size required for each group would be 86. We repeated this power calculation rationale for the co-primary endpoint of urine output from POD 1–6 using a baseline urine output in the TED+SCD group of 12.5 L with a SD of 8.4 L based on pilot study data. We arrived at the same required sample size of 86. We then accounted for approximately 20% attrition, arriving at 103 patients per group. Statistical tests were done with per-protocol analysis. GraphPad Prism was used to conduct a statistic analysis using a Students’ t-test for independent data groups, with p<0.05 representing the point of statistical significance. The normalcy of distribution of demographics and patient characteristics with outlier detection were assessed by Shapiro-Wilk’s test. Data are reported as mean ± SD unless otherwise specified.
Results
Between September 2015 and September 2017, 259 kidney and SPK transplantation were completed at London Health Sciences Centre. A total of 221 recipients were determined to be eligible for the study, provided informed consent, and were subsequently randomized (kidney transplant=204, SPK transplant=17; donation after brain death [DBD]=109, living donor=52, donation after cardiac death [DCD]=60). After randomization, two patients originally assigned to the intervention arm could not tolerate the stimulation from the MPA device and one participant in the intervention arm did not experience any muscle contraction despite optimization of device placement. These participants subsequently crossed over to the control arm within the first day of using the device, continued in the study until completion, and were analyzed according to treatment received. One hundred and eleven were eventually analyzed in the control arm and 110 in the intervention arm in the per protocol analysis. All participants adhered to wearing their device for the whole study period and there were no patients lost to followup, as relevant data collection was performed during hospitalization. Baseline characteristics and comorbidities were similar between groups (Tables 1, 2).
Table 1.
Baseline patient characteristics
| Recipient intervention | |||
|---|---|---|---|
|
| |||
| TED+IPC | MPA | p | |
| Number of participants | 111 | 110 | – |
| Age, years | 51.5±13.4 | 52.9±13.2 | 0.43 |
| Male:female | 64:47 | 69:41 | 0.44 |
| Weight | 77.47±16.9 | 78.71±17.6 | 0.59 |
| BMI (kg/m2) | 27.39±5.2 | 27.02±4.7 | 0.58 |
| Type of dialysis | 0.79 | ||
| HD | 69 | 67 | |
| PD | 30 | 28 | |
| Pre-emptive | 12 | 15 | |
| Type of donor | 0.27 | ||
| LD | 30 | 22 | |
| DBD | 55 | 54 | |
| DCD | 26 | 34 | |
| Cause of ESRD | 0.98 | ||
| DN | 37 | 26 | |
| HTNN | 12 | 9 | |
| DN + HTNN | 0 | 8 | |
| IgA nephropathy | 8 | 10 | |
| Alport syndrome | 2 | 2 | |
| Anatomic | 7 | 5 | |
| FSGS | 4 | 5 | |
| PCKD | 10 | 15 | |
| Other autoimmune | 10 | 8 | |
| Drug-induced | 3 | 5 | |
| GN/NS | 6 | 7 | |
| Congenital/genetic | 5 | 4 | |
| Distributive/systemic shock | 4 | 1 | |
| Unknown/other | 3 | 5 | |
Anatomic includes urinary tract obstruction and reflux. BMI: body mas index; DBD: donor after brain death; DCD: donor after cardiac death; DN: diabetic nephropathy; ESRD: end-stage renal disease; FSGS: focal segmental glomerulosclerosis; GN/NS: glomerulonephritis/nephrotic syndrome; HD: hemodialysis; HTNN: hypertensive nephrosclerosis; LD: living donor; PCKD: polycystic kidney disease; PD: peritoneal dialysis.
Table 2.
Recipient comorbidities
| Recipient intervention | ||
|---|---|---|
|
| ||
| Comorbidity | TED+IPC | MPA |
| Hypertension | 87 | 89 |
| Dyslipidemia | 36 | 54 |
| Diabetes | 29 | 28 |
| Hyperparathyroidism | 21 | 27 |
| Gout | 18 | 21 |
| GERD | 12 | 15 |
| Previous transplant | 12 | 4 |
| Neoplasm or pre-neoplastic process | 12 | 8 |
| Ischemic hearth disease/CHF | 8 | 1 |
| Hypothyroid | 7 | 11 |
| Obstructive sleep apnea | 7 | 7 |
| Infections | 6 | 5 |
| Gastrointestinal | 6 | 6 |
| Coronary artery disease | 5 | 12 |
| Osteoarthritis | 5 | 3 |
| Vascular disease | 5 | 3 |
| Autoimmune disease | 5 | 10 |
| Asthma/COPD | 5 | 3 |
| Genitourinary | 5 | 8 |
| CVA/TIA | 4 | 10 |
| Congenital/genetic | 3 | 4 |
| Gynecologic | 3 | 1 |
| Neurologic | 2 | 8 |
| Psychiatric | 2 | 11 |
| Thromboembolic disease | 2 | 3 |
| Osteoporosis | 1 | 1 |
| Other | 1 | 5 |
| Atrial fibrillation | 0 | 3 |
| Hematological | 0 | 6 |
| Cataract/glaucoma | 0 | 3 |
CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CVA: cerebral vascular accident; GERD: gastroesophageal reflux disease; IPC: intermittent pneumatic compression; MPA: muscle pump activator; TED: thrombo-embolic-deterrent; TIA: transient ischemic attack.
Primary endpoints
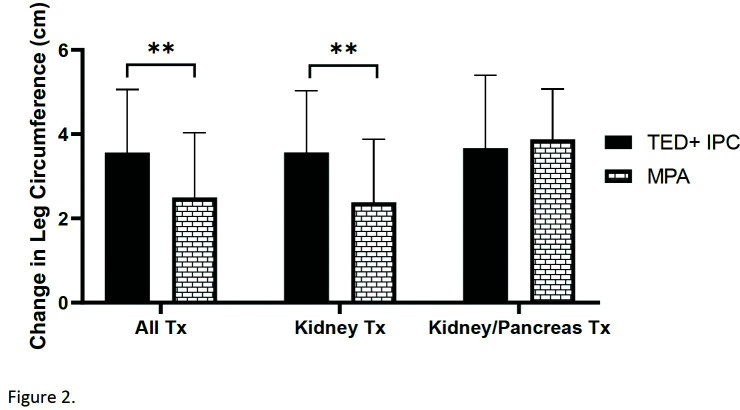
All patients experienced an increase in lower-limb circumference with postoperative fluid administration between the identified time points. The MPA group had an increase in calf circumference of 2.5±1.5 cm. This was significantly lower than the TED+IPC group, which had a mean increase in calf circumference of 3.6±1.5 cm (p=0.001) (Fig. 2). Similarly, the change in patient weight between POD 1 and 6 was also significantly less in the MPA group compared to the TED+IPC group (4.06±2.3 kg vs 5.18±2.8 kg, p=0.003) (Fig. 3).
Fig. 2.
Mean change in calf circumference between postoperative day 1 and 6. The MPA group had a significantly lower increase in calf circumference compared to the TED+IPC group (2.5±1.5 cm vs. 3.6±1.5 cm, p=0.001). IPC: intermittent pneumatic compression; MPA: muscle pump activator; TED: thrombo-embolic-deterrent.
Fig. 3.
Mean change in patient weight between postoperative day 1 and 6. The MPA group had a significantly lower increase in calf circumference compared to the TED+IPC group (4.06±2.3 kg vs. 5.18±2.8 kg, p=0.003). IPC: intermittent pneumatic compression; MPA: muscle pump activator; TED: thrombo-embolic-deterrent.
Patients who wore the MPA device made on average 15.99±8.8 L of urine between POD 1 and 6, in contrast to an average of 12.60±8.4 L made by TED+IPC group (p=0.003) (Table 3). Further analysis categorizing recipients by donor type also found a significant difference between the MPA and TED+IPC group in living and DBD donor kidneys (23.99±5.42 L vs. 18.90±7.64 L, p=0.009; 19.52±5.36 L vs. 12.85±7.13 L, p<0.001, respectively) (Table 4). However, serum creatinine on POD 6 was not significantly different between the two groups (162±171 μmol/L vs. 170±146 μmol/L, p=0.45). Although recipients who received DCD donor kidneys and used the MPA device made more urine than their TED+IPC counterparts, there was no statistical difference seen (5.60±4.01 L vs. 4.31±4.33 L, p=0.27).
Table 3.
Urine output between postoperative day 1–6
| Urine output (mL) | |||
|---|---|---|---|
|
| |||
| TED+IPC | MPA | p | |
| All Tx | 12595 | 15986 | 0.004 |
| Kidney Tx | 12457 | 16325 | 0.015 |
| Kidney/pancreas Tx | 4933 | 9662 | 0.98 |
The MPA group had significantly higher urine output in kidney transplant recipients but not SPK recipients. IPC: intermittent pneumatic compression; MPA: muscle pump activator; SPK: simultaneous pancreas-kidney; TED: thrombo-embolic-deterrent; Tx: transplant.
Table 4.
Primary endpoints analysis by donor type
| TED+IPC | MPA | p | |
|---|---|---|---|
| Living donor | |||
| Urine output (mL) | 18902 | 23989 | 0.009 |
| Weight (kg) | 4.90 | 2.82 | 0.005 |
| Calf size (cm) | 3.23 | 2.27 | 0.010 |
| DBD | |||
| Urine output (mL) | 12 832 | 19 292 | <0.001 |
| Weight (kg) | 4.87 | 3.91 | 0.05 |
| Calf size (cm) | 3.65 | 2.26 | <0.001 |
| DCD | |||
| Urine output (mL) | 4815.50 | 5496.38 | 0.27 |
| Weight (kg) | 6.04 | 4.97 | 0.23 |
| Calf size (cm) | 3.81 | 3.00 | 0.08 |
Urine output, weight and calf size were significantly different between the TED+IPC and the MPA recipients for those who received living donor or DBD donor kidneys. There was no difference in these measures seen between study groups for recipients who received DCD donor kidneys. DBD: donor after brain death; DCD: donor after cardiac death; IPC: intermittent pneumatic compression; MPA: muscle pump activator; TED: thrombo-embolic-deterrent.
In the SPK subpopulation, the differences in our co-primary endpoints between groups were not observed.
Secondary endpoints
The duration of hospitalization post-transplantation varied from 4–30 days, with the average length of stay being 8.3 days after kidney-only transplant and 14.6 days after SPK transplant (Fig. 4). Overall, the MPA group had a significantly shorter hospital stay compared to the TED+IPC group (8.15±3.5 days vs. 9.36±5.0 days, p=0.038).
Fig. 4.
Mean length of hospitalization. Participants in the MPA group had a significantly shorter hospital stay compared to the TED+IPC group (9.36±5.0 days vs. 8.15±3.5 days, p=0.038). No difference in length of hospitalization between the study groups was seen in SPK patients. IPC: intermittent pneumatic compression; MPA: muscle pump activator; TED: thrombo-embolic-deterrent.
With the MPA or TED+IPC devices in place, Doppler ultrasound on POD 6 showed higher femoral vein velocity in the MPA group (18.90±4.4 cm/s) compared to the TED+IPC group (14.41±5.1 cm/s, p=0.001). Despite improved venous blood flow, use of the MPA device was not associated with decreased rates of DGF (24% vs. 22%, p=0.72) or number of dialysis runs (56 vs. 73, p=0.39). A total of 80 participants wore pedometers to monitor their level of mobility on POD 2–5 (IPC=45, MPA=35). Those who wore the MPA device walked an average of 1231±189 steps, indicating a greater level of mobility compared to the control arm (1099±249 steps, p=0.009) (Fig. 5). There were two recipients in the MPA group and one recipient in the IPC group who developed lower limb DVTs. No statistical analysis was performed, as the study was not powered to assess differences in DVT incidence rates.
Fig. 5.
Average number of steps taken on postoperative day 1 to 6. The MPA group taken a significantly higher number of steps compared to the TED+IPC group (1231±189 steps vs 1099±249 steps, p=0.009). IPC: intermittent pneumatic compression; MPA: muscle pump activator; TED: thrombo-embolic-deterrent.
Discussion
With increasing healthcare costs and significant portions of the overall cost accruing from inpatient care, many surgical specialities have made efforts towards shortening the duration of postoperative hospitalization.18,19 Since being established in the early 2000s, Enhanced Recovery after Surgery (ERAS) has permeated through many surgical fields as an evidence-based, multimodal, multidisciplinary approach for care of the surgical patient.20,21 One of the tenants of ERAS is minimizing fluid shifts22 such that the sequelae of fluid overload can be avoided.23,24 This can be particularly difficult in transplant recipients, given the pre-existing electrolyte imbalance25 and generous fluid administration sometimes required to keep adequate intravascular volume to perfuse the allograft. Evidence has shown that external compression of the lower limbs to be effective in preventing the redistribution of fluids from the intravascular to extravascular compartments, thereby decreasing fluid demands and maintaining hemodynamic stability.26
In our study, we compared two devices that, through different mechanisms, compress the venous system of the lower limbs.13,27 Similar to previous findings,16,28 participants who used the MPA device were found to have significantly less lower-limb edema in the perioperative period. This has been explained by previous studies showing that stimulation of the intrinsic muscle pumps is more effective than external compression for increasing venous return14 and preventing extravascular redistribution of fluids around time of surgery.13 It stands to reason that with improved venous return from the lower limbs, there is better perfusion to the newly transplanted kidney to facilitate more urine production and, as a result, less fluid retention. This is reflected in our findings of higher urine output and less weight gain in participants who used the MPA deviceSubgroup analysis of recipients in the MPA group who received DCD donor kidneys, on average, made 1 L more urine than their TED+IPC counterparts. This was not statistically significant, although the sample size was small and the incidence of DGF in the DCD cohort was high (data not shown). As a result, urine output in the first week post-transplantation, in both groups, was low. In the early postoperative course, urine output has been found to be an independent predictive factor on graft survival and should, therefore, be optimized.29 Our results do suggest that the MPA device may have some clinical impact on fluid shifts, as the calf size measurements was trending towards statistical significance in the DCD cohort. Whether these non-significant improvements in fluid mobilization from the use of the MPA device impacts long-term graft function is unknown but warrants further investigations with longer followup.
We were able to demonstrate that the use of an MPA device was associated with a shorter length of hospitalization compared to the TED+IPC devices after kidney transplantation. Many factors are considered when determining a patient’s readiness to be discharged, including clinical parameters, such as ambulation, tolerance of oral intake, bowel motility, and pain control. In the subset of participants who wore pedometers, individuals assigned to use the MPA device were also found to have improved postoperative mobility. This could be related to the significant reduction in lower-extremity edema, which may increase the ease and comfort of ambulation. The portable nature of the MPA device also promotes ambulation, as it allows for greater freedom and independence to mobilize without having to manage the pneumatic pump device used with IPCs.30 Although evidence on effect of ambulation on gut mobilization is scarce,31–33 early mobilization is known to prevent perioperative pulmonary and thromboembolic complications34,35 and, thus, prevent additional days of hospitalization. We have also shown in a pilot study that significantly fewer patients described “some level of discomfort” on a patient satisfaction survey compared to TED+IPC (13% vs. 57%, p=0.003).36 This knowledge further supports the use of the MPA device.
Finally, we did not find any significance in our study parameters during secondary analysis of our SPK transplant cohort. This may be due to the small sample size of this subgroup. The lack of benefit may also be attributable to the more extended and complex nature of the surgery. The duration of the operation is longer for SPK recipients, given the additional vascular and bowel anastomosis. This can cause a greater stress response, with release of more cytokines leading to fluid redistribution, similar to what is seen in other major abdominal surgeries.37 SPK recipients would generally be without oral fluid intake postoperatively for longer, with potential for increased fluid losses through a nasogastric tube. This leads to more variable fluid prescription, making it more difficult to assess the effect of the MPA device on urine output, lower-limb edema, and patient weight.
To our knowledge, this is the first randomized, controlled trial looking at the effect of an MPA device on decreasing length of postoperative hospitalization. A significant strength of our study was accrual of an adequate sample size determined a priori, without attrition. Clinically relevant and objective measures were assessed, giving our results external validity.
This study is limited by its single-center design with short-term followup of the participants. The open-label design also created some limitations, as the patient and care teams were not blinded to the modality of lower-limb device due to the vastly discrepant size and appearance of the two devices. This represents a potential source of bias for postoperative decisions with regards to ambulation and discharge. Patients are typically mobilized with nursing staff help on POD 2 as per institutional care pathway. Physiotherapy is enlisted when patients have difficulties ambulating due to preoperative mobility limitations, or for newly identified mobility concerns. Since the care pathway implores mobilization on POD 2, and TED+IPC represents the standard of care, the care teams are not expected to significantly alter the enthusiasm to ambulate patients with TED+IPC relative to those with the MPA devices. Another limitation of the study design is a lack of documentation of perioperative fluid administration. However, fluid administration in the early postoperative period was protocolized so all patients were exposed to the same decisions for fluid prescription. The overall change in patient weight is a direct reflection of total fluid given in relation to diuresis. Thus, even though fluid administration to the MPA group may have been higher, there was less weight gain overall, and less low-limb edema, which would be consistent with less fluid shift from the intravascular to extravascular components. Finally, although the incidence of DVT was low overall, there were two incidences of DVT in the MPA group compared to one incidence in the TED+IPC group. Without adequate power to study this outcome, it is possible that the MPA device is less effective at preventing DVT formation compared to TED+IPC, although previously published reports have not found evidence to support this.17
Conclusions
Postoperative use of an MPA device decreases duration of hospitalization after kidney transplantation compared to when TED+IPC is used. This may be attributable to improved maintenance of intravascular volume leading to improved renal blood flow to the transplant allograft and, thus, increased urine output and decreased fluid retention. Further studies looking at long-term outcomes and with focus on the DCD kidney transplant population are needed.
Footnotes
Competing interests:The authors report no competing personal or financial interests related to this work
Funding: This study is support by an unrestricted investigator-initiated grant from Firstkind Ltd.
This paper has been peer-reviewed.
References
- 1.Ojo AO, Meier-Kriesche H-U, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation. 2001;71:82–9. doi: 10.1097/00007890-200101150-00014. [DOI] [PubMed] [Google Scholar]
- 2.Tyden G, Tollemar J, Bolinder J. Combined pancreas and kidney transplantation improves survival in patients with end-stage diabetic nephropathy. Clin Transplant. 2000;14:505–8. doi: 10.1034/j.1399-0012.2000.140510.x. [DOI] [PubMed] [Google Scholar]
- 3.Rabbat CG, Thorpe KE, Russell JD, et al. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–22. doi: 10.1681/ASN.V115917. http://www.ncbi.nlm.nih.gov/pubmed/10770970. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 5.Oniscu GC, Brown H, Forsythe JLR. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–65. doi: 10.1681/ASN.2004121092. [DOI] [PubMed] [Google Scholar]
- 6.Ounissi M, Cherif M, Abdallah TB, et al. Risk factors and consequences of delayed graft function. Saudi J Kidney Dis Transpl. 2013;24:243–6. doi: 10.4103/1319-2442.109564. [DOI] [PubMed] [Google Scholar]
- 7.Ho CK, Sun M, Au TW, et al. Pneumatic pump reduces leg wound complications in cardiac patients. Asian Cardiovasc Thorac Ann. 2006;14:452–7. doi: 10.1177/021849230601400602. [DOI] [PubMed] [Google Scholar]
- 8.Van Beijsterveld CA, Bongers BC, Den Dulk M, et al. The association between preoperative physical functioning and short-term postoperative outcomes: A cohort study of patients undergoing elective hepatic resection. HPB. 2019;21:1362–70. doi: 10.1016/j.hpb.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Boeckxstaens GE, De Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–11. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell G, Hucker T, Venn R, et al. Pathophysiology and clinical implications of perioperative fluid excess (multiple letters) Br J Anaesth. 2003;90:395–6. doi: 10.1093/bja/aeg526. [DOI] [PubMed] [Google Scholar]
- 11.Winge R, Bayer L, Gottlieb H, et al. Compression therapy after ankle fracture surgery: A systematic review. Eur J Trauma Emerg Surg. 2017;43:451–9. doi: 10.1007/s00068-017-0801-y. [DOI] [PubMed] [Google Scholar]
- 12.Slaa A, Dolmans D, Ho G. Treatment strategies and clinical aspects of lower limb edema following peripheral bypass surgery. Phlebology. 2014;29:18–25. doi: 10.1177/0268355514527689. [DOI] [PubMed] [Google Scholar]
- 13.Tessari M, Tisato V, Rimondi E, et al. Effects of intermittent pneumatic compression treatment on clinical outcomes and biochemical markers in patients at low mobility with lower limb edema. J Vasc Surg. 2018;6:500–10. doi: 10.1016/j.jvsv.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Bahadori S, Immins T, Wainwright TW. The effect of calf neuromuscular electrical stimulation and intermittent pneumatic compression on thigh microcirculation. Microvasc Res. 2017;111:37–41. doi: 10.1016/j.mvr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Williams KJ, Moore HM, Davies AH. Hemodynamic changes with the use of neuromuscular electrical stimulation compared to intermittent pneumatic compression. Phlebology. 2015;30:365–72. doi: 10.1177/0268355514531255. [DOI] [PubMed] [Google Scholar]
- 16.Aquil S, Alharbi B, Navaratnam R, et al. Use of a muscle pump activator leads to improved lower limb edema, lower limb blood flow, and urine output compared with standard TED stockings and compression devices following kidney transplant: A randomized controlled trial. Transplant Proc. 2019;7 doi: 10.1016/j.transproceed.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Hajibandeh S, Hajibandeh S, Ga A, et al. Neuromuscular electrical stimulation for the prevention of venous thromboembolism (Review) Cochrane Database Syst Rev. 2017;11:CD011764. doi: 10.1002/14651858.CD011764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OECD Library. Health spending (indicator) 2018. [Accessed July 28, 2020]. Available at: [DOI]
- 19.Fund TC. Inpatient hospital spending per capita. 2010. [Accessed July 28, 2020]. Available at: https://www.commonwealthfund.org/chart/2010/inpatient-hospital-spending-capita-2006.
- 20.Ljungqvist O, Scott M, Fearon K. Enhanced recovery after surgery a review. Clin Rev Educ. 2017;152:292–8. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 21.Kruszyna T, Niekowa B, Krasnicka M, et al. Enhanced recovery after kidney transplantation surgery. Transplant Proc. 2016;48:1461–5. doi: 10.1016/j.transproceed.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg. 2019;43:659–95. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 23.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: A randomised controlled trial. Lancet. 2002;359:1812–8. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 24.Bragg D, El-Sharkawy AM, Psaltis E, et al. Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr. 2015;34:367–76. doi: 10.1016/j.clnu.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Yee J, Parasuraman R, Narins RG. Selective review of key perioperative renal-electrolyte disturbances in chronic renal failure patients. Chest. 1999;115:125S–9S. doi: 10.1378/chest.115.suppl_2.149s. [DOI] [PubMed] [Google Scholar]
- 26.Kiefer N, Theis J, Putensen-Himmer G, et al. Peristaltic pneumatic compression of the legs reduces fluid demand and improves hemodynamic stability. Anesthesiology. 2018;114:536–44. doi: 10.1097/ALN.0b013e31820c3973. [DOI] [PubMed] [Google Scholar]
- 27.Tucker AT, Maass A, Bain DS, et al. Augmentation of venous, arterial, and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19:31–7. doi: 10.1055/s-0031-1278361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravikumar R, Williams KJ, Babber A, et al. Randomized controlled trial: Potential benefit of a footplate neuromuscular electrical stimulation device in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 2017;53:114–21. doi: 10.1016/j.ejvs.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Lai Q, Pretagostini R, Poli L, et al. Early urine output predicts graft survival after kidney transplantation. Transplant Proc. 2010;42:1090–2. doi: 10.1016/j.transproceed.2010.03.088. [DOI] [PubMed] [Google Scholar]
- 30.Aquil S, Sharma H, Alharbi B, et al. The impact of a muscle pump activator on incisional wound healing compared to standard stockings and compression devices in kidney and kidney-pancreas transplant recipients: A randomized controlled trial. Can Urol Assoc J. 2019;13:E341–9. doi: 10.5489/cuaj.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gero D, Gié O, Hübner M, et al. Postoperative ileus: in search of an international consensus on definition, diagnosis, and treatment. Langenbeck’s Arch Surg. 2017;402:149–58. doi: 10.1007/s00423-016-1485-1. [DOI] [PubMed] [Google Scholar]
- 32.Sahin E, Terzioglu F. The effect of gum chewing, early oral hydration, and early mobilization on intestinal motility after Cesarian birth. Worldviews Evidence-Based Nurs. 2015;12:380–8. doi: 10.1111/wvn.12125. [DOI] [PubMed] [Google Scholar]
- 33.Raue W, Haase O, Junghans T, et al. “Fast-track” multimodal rehabilitation program improves outcome after laparoscopic sigmoidectomy: A controlled prospective evaluation. Surg Endosc Other Interv Tech. 2004;18:1463–8. doi: 10.1007/s00464-003-9238-y. [DOI] [PubMed] [Google Scholar]
- 34.Partsch H, Blättler W. Compression and walking vs. bed rest in the treatment of proximal deep venous thrombosis with low molecular weight heparin. J Vasc Surg. 2000;32:861–9. doi: 10.1067/mva.2000.110352. [DOI] [PubMed] [Google Scholar]
- 35.Talec P, Gaujoux S, Samama CM. Early ambulation and prevention of postoperative thrombo-embolic risk. J Visc Surg. 2016;153:S11–4. doi: 10.1016/j.jviscsurg.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Alharbi B. The effect of the GekoTM device on post-kidney and pancreatic transplantation leg edema. Electron Thesis Diss Repos. 2016 Jan;:4371. [Google Scholar]
- 37.Klaschik S, Gehlen J, Neumann C, et al. Network of mediators for vascular inflammation and leakage is dysbalanced during cytoreductive surgery for late-stage ovarian cancer. Mediators Inflamm. 2019;2019:1–9. doi: 10.1155/2019/5263717. [DOI] [PMC free article] [PubMed] [Google Scholar]