Abstract
Cardiovascular diseases (CVDs) have gained increasing attention because of their high prevalence and mortality worldwide. Epidemiological studies revealed that intake of fruits, vegetables, nuts, and cereals could reduce the risk of CVDs, and their antioxidants are considered as the main contributors. Moreover, experimental studies showed that some antioxidant natural products and their bioactive compounds exerted beneficial effects on the cardiovascular system, such as polyphenols, polysaccharides, anthocyanins, epigallocatechin gallate, quercetin, rutin, and puerarin. The mechanisms of action mainly included reducing blood pressure, improving lipid profile, ameliorating oxidative stress, mitigating inflammation, and regulating gut microbiota. Furthermore, clinical trials confirmed the cardiovascular-protective effect of some antioxidant natural products, such as soursop, beetroot, garlic, almond, and green tea. In this review, we summarized the effects of some antioxidant natural products and their bioactive compounds on CVDs based on the epidemiological, experimental, and clinical studies, with special attention paid to the relevant mechanisms and clinical trials.
1. Introduction
Cardiovascular diseases (CVDs), such as coronary heart disease (CHD), hypertensive heart disease, heart failure, and stroke, are the leading cause of death worldwide [1]. CVDs could be caused by hypertension, dyslipidemia, atherosclerosis, oxidative stress, inflammation, and enteric dysbacteriosis [2–4]. Several synthetic drugs have been used to treat CVDs, but they showed some adverse effects, such as gastrointestinal reaction, hyperkalemia, and arrhythmias [5, 6]. On the other hand, accumulating evidence has shown that some antioxidant natural products could be a safe and effective alternative for the prevention and treatment of CVDs [7–11].
Natural products are rich in dietary fibers, polyphenols, vitamins, minerals, and other beneficial components, and possess many bioactivities, such as antioxidant, anti-inflammatory, anticancer, antidiabetic, antiobesity, hepatoprotective, immunoregulatory, antibacterial, and cardiovascular-protective effects [12–20]. Epidemiological studies found that people consuming more fruits, vegetables, teas, cereals, and nuts had a lower risk of CVDs, and the antioxidants in these natural products were considered as the main contributors [21–23]. Additionally, experimental researches showed that some antioxidant natural products and their active compounds could prevent and treat CVDs through different mechanisms of action [24–32]. Furthermore, clinical trials provided more reliable human evidence on some antioxidant natural products for the prevention and treatment of CVDs [33, 34]. The purpose of this review is to summarize the effect of some antioxidant natural products and their bioactive compounds on CVDs from the results of epidemiological, experimental and clinical studies in the last five years, and special attention was paid to the mechanisms of action and clinical trials.
2. Epidemiological Studies
Increasing epidemiological studies have suggested that the intake of some antioxidant natural products significantly attenuated the risk factors of CVDs (Table 1).
Table 1.
The effects of antioxidant natural products on CVDs from epidemiological studies.
| Plants | Components | Study type | Subjects | Results | Ref. |
|---|---|---|---|---|---|
| Fruit | NA | Cohort study | 512,891 Chinese | Lowering SBP and blood glucose, reducing the risks of cardiovascular death (HR: 0.60; 95% CI, 0.54-0.67), incident major coronary event (HR: 0.66; 95% CI, 0.58-0.75), ischemic stroke (HR: 0.75; 95% CI, 0.72-0.79), and hemorrhagic stroke (HR: 0.64; 95% CI, 0.56-0.74) | [38] |
| Fruit | NA | Cross-sectional study | 1,590 adults | Low fruit consumption was associated with increased BP in 50–59-year-old group (PR: 1.62; 95% CI, 1.09-2.41) | [43] |
| Fruit | Anthocyanin, flavanone | Cohort study | 43.880 healthy men | Higher anthocyanin intake was inversely associated with nonfatal myocardial infarction (HR: 0.87; 95% CI, 0.75-1.00). Higher flavanone intake was inversely associated with ischemic stroke (HR: 0.78; 95% CI, 0.62-0.97). | [44] |
| Fruit | Flavone | Cross-sectional study | 7,963 women aged ≥30 years | Inversely associated with SBP, TG, and TG/HDL-C. | [45] |
| Fruit | NA | Cohort study | 70,047 Chinese adults with CVD or hypertension | Inversely associated with CVD mortality (HR: 0.79; 95% CI, 0.73-0.86) | [46] |
| Fruit | NA | Cross-sectional study | 9,040 subjects aged ≥25 years | Inversely associated with CVD (OR: 0.86; 95% CI, 0.74-0.98) | [47] |
| Vegetable | NA | Cross-sectional study | 18,757 adolescents | Consuming ≥3 servings of vegetables lowered the risk of hypertension (OR: 0.74; 95% CI, 0.58-0.94) | [35] |
| Vegetable | Nitrate | Cohort study | 2,229 Australian aged ≥39 years | Inversely associated with CVD mortality (comparison of <69.5 mg/day intake of vegetable, 69.5-99.6 mg/day (HR: 0.53; 95% CI, 0.35-0.82), 99.7-137.8 mg/day (HR: 0.51; 95% CI, 0.32-0.80), and >137.8 mg/day (HR: 0.63; 95% CI, 0.41-0.95)) | [48] |
| Allium vegetable | NA | Cohort study | Adult men and women | Associated with a 64% reduced risk of CVD outcomes (HR: 0.36; 95% CI, 0.18-0.71) | [39] |
| Nut | NA | Cohort study | 16,217 participants with diabetes mellitus | Inversely associated with the total CVD incidence (HR: 0.83; 95% CI, 0.71-0.98), CHD incidence (HR: 0.80; 95% CI, 0.67-0.96), and CVD mortality (HR: 0.66; 95% CI, 0.52-0.84) | [49] |
| Nut | NA | 3 large cohort studies | 76,364 women 92,946 women 41,526 men |
Inversely associated with the total CVD (HR: 0.86; 95% CI, 0.79-0.93) and CHD (HR: 0.80; 95% CI, 0.72-0.89) | [50] |
| Nut | NA | 3 large cohort studies | 34,103 men 77,815 women 80,737 women |
Inversely associated with CVD (RR: 0.92; 95% CI, 0.86-0.98), CHD (RR: 0.94; 95% CI, 0.89-0.99), and stroke (RR: 0.89; 95% CI, 0.83-0.95) | [51] |
| Nut | NA | Cohort study | 61,364 Swedish adults | Inversely associated with risk of heart failure and atrial fibrillation 1-3 times/month: heart failure (HR: 0.87; 95% CI, 0.80-0.94) and atrial fibrillation (HR: 0.97; 95% CI, 0.93-1.02) 1-2 times/week: heart failure (HR: 0.80; 95% CI, 0.67-0.97) and atrial fibrillation (HR: 0.88; 95% CI, 0.79-0.99) 3 times/week: heart failure (HR: 0.98; 95% CI, 0.76-1.27) and atrial fibrillation (HR: 0.82; 95% CI, 0.68-0.99) |
[42] |
| Legume | NA | Cohort study | 6,504 Iranian middle-aged and older people | Inversely related to the risk of CVD events in old-aged Iranians (HR: 0.66; 95% CI, 0.45-0.98) but not in middle-aged Iranians | [52] |
| Curry (turmeric) | Curcumin | Cross-sectional study | Individuals aged 19-64 years | Lowering blood glucose and TG levels | [37] |
| Fruit, vegetable | NA | Cross-sectional study | 1,596 adolescents and young people in Tanzania and Uganda | Lowering the risk of hypertension (OR: 0.7; 95% CI, 0.50-0.98) | [53] |
| Fruit, vegetable | NA | Cross-sectional study | 229 patients with primary hypertension | Lowering BP, heart rate, and BMI | [54] |
| Fruit, vegetable | NA | Cohort study | 2,354 Ugandan newborns | Lowering BP | [21] |
| Fruit, vegetable | NA | Cohort study | 8,997 aging subjects | Fruit intake was inversely associated with BP but vegetable intake was not | [55] |
| Fruit, whole grain | Fiber | Cohort study | 17,007 young Mediterranean participants | Fruit (HR: 0.51; 95% CI, 0.27-0.95) or whole grain (HR: 0.43; 95% CI, 0.20-0.93) intake was inversely associated with CVD events. | [40] |
| Fruit, vegetable, cereal | Fiber | Cross-sectional study | 18,433 American adults | Total fiber (OR: 0.62; 95% CI, 0.52-0.75), cereal fiber (OR: 0.80; 95% CI, 0.69-0.98), and vegetable fiber (OR: 0.82; 95% CI, 0.69-0.98) were inversely associated with the risk of hypertension, but fruit fiber was not | [36] |
| Fruit, vegetable, legume, grain, nut | Fiber | Cohort study | 2,295 health professionals | Legume fiber (HR: 0.31; 95% CI, 0.15-0.65), fruit fiber (HR: 0.44; 95% CI, 0.22-0.89), and vegetable fiber (HR: 0.34; 95% CI, 0.16-0.72) were inversely associated with the CVD risks, but grain and nut fiber were not | [41] |
Note. NA: not available; CVD: cardiovascular disease; CHD: coronary heart disease; HR: hazard ratio; PR: prevalence ratio; OR: odds ratio; RR: relative ratio; CI: confidence interval; BW: body weight; BP: blood pressure; SBP: systolic blood pressure; TG: triglyceride; BMI: body mass index; HDL-C: high-density lipid protein cholesterol.
Several cross-sectional studies found that some dietary plants were beneficial for the prevention and management of CVDs. For instance, a cross-sectional study of 18,757 Chinese adolescents aged 13-17 years revealed that daily intake of at least 3 servings of vegetables (1 serving of vegetable was the size of an adult's fist) lowered the risk of hypertension (odds ratio (OR) = 0.74; 95% confidence interval (CI): 0.42-0.95) compared with daily consumption of vegetable <1 serving [35]. Additionally, an analysis of 18,433 American adults found that compared with the lowest tertile consumption of cereals, vegetables, and fruit fibers as well as their total fiber, the OR (95% CI) of hypertension for the highest tertile were 0.80 (0.69-0.98), 0.82 (0.69-0.98), 0.86 (0.71-1.04), and 0.62 (0.52-0.75), respectively, indicating that cereals, vegetables, and total fibers were inversely related with hypertension, but fruit fiber was not [36]. Moreover, the data from the Korea National Health and Nutrition Examination Survey showed that overweight older males and younger females who consumed a moderate amount of curry (2-3 times a month or once a week), mainly composed of turmeric, had significantly lower levels of blood glucose and triglyceride (TG) than a group who had low curry consumption (almost never, or once a month) [37].
A negative correlation between the intake of several edible plants and the incidence as well as mortality of CVDs was also observed in some cohort studies. A follow-up study recruiting 521,891 Chinese adults aged 30-79 years reported that participants who consumed fresh fruit daily had lower systolic blood pressure (SBP) and blood glucose level compared to those who never or rarely ate fresh fruit. The HR (95% CI) for cardiovascular death, incident major coronary events, ischemic stroke, and hemorrhagic stroke were 0.60 (0.54-0.67), 0.66 (0.58-0.75), 0.75 (0.72-0.79), and 0.64 (0.52-0.74), respectively, elucidating the protective effect of fresh fruit on the cardiovascular system [38]. Moreover, another analysis of 3,052 adults indicated that the habitual consumption of allium vegetables, such as garlic and onion, was related to a 64% decreased risk of CVD outcomes (HR = 0.36; 95% CI: 0.18-0.71) [39]. Additionally, a cohort study of young Mediterranean populations found that compared to the lowest quintile of fruit intake or whole grain intake, the HR (95% CI) of the risk of CVD events for the highest quintile were 0.51 (0.27-0.95) and 0.43 (0.20-0.93), respectively, showing the benefits of fruit or whole grain to prevent CVDs [40]. Furthermore, a prospective study of 2,295 Iranian adults pointed out that compared to the lowest tertile of dietary fiber intakes from grains, legumes, nuts, fruits, and vegetables, the hazard ratios (HR) (95% CI) of CVD risks for the highest tertile were 0.90 (0.44-1.86), 0.31 (0.15-0.65), 0.49 (0.24-1.02), 0.44 (0.22-0.89), and 0.34 (0.16-0.72), respectively, suggesting that dietary fiber from legumes, fruits, and vegetables were negatively related to CVDs, while fiber from grains and nuts had no significant association with CVDs [41]. However, a cohort study found that nut intake significantly lowered the risk of CVDs [42]. The reason could be that it was not fiber but other bioactive compounds in nuts that play a vital role in the prevention of CVDs, or that there were disparities of population, study design, and confounding factors in different studies, which need to be further investigated in the future.
In short, the collected epidemiological investigations illuminated the protective effects of some antioxidant natural products and their bioactive components on CVDs, although there were inconsistent results. In addition, based on the beneficial role of some plants in CVDs, it is advisable to increase the intake of some plant-based foods, such as fresh fruits, vegetables, legumes, cereals, and nuts, to reduce the risk of CVDs.
3. Experimental Studies
Many experimental studies investigated the effects of some antioxidant natural products and their bioactive compounds on CVDs (Table 2), and the relevant mechanisms are discussed below (Figure 1).
Table 2.
The effects of antioxidant natural products on CVDs from experimental studies.
| Plants | Components | Study type | Subjects | Dose & Time | Effects and mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Winged bean seed | Peptide |
In vitro
In vivo |
ACE and SD rats | 1 mM peptides, 3 h; 150 and 300 mg/kg BW, 24 h | Inhibiting ACE activity Lowering BP |
[61] |
| Solanum macrocarpon | Polyphenols |
In vitro
In vivo |
SHRs | 100 and 500 mg/kg BW | Inhibiting ACE/renin activities Lowering DBP and heart rate |
[62] |
| Citrus paradisi and Ocimum sanctum | Epigallocatechin gallate and quercetin | In vivo | SD rats | 2 g dried ground material in 200 mL water, 4 months | Reducing BP (renin and angiotensinogen↓) and reducing renal TG accumulation and lipid/protein oxidation (Citrus paradisi) Reducing BP via other mechanisms (Ocimum sanctum) |
[63] |
| Pigeon pea | Protein |
In vitro
In vivo |
ACE and SHRs | 100 mg/kg BW, 24 h | Inhibiting ACE/renin activities and scavenging free radicals Lowering BP |
[24] |
| Ficus deltoidea var. Kunstleri | NA | In vivo | SHRs | 500, 800, 1000, and 1300 mg/kg BW, 4 weeks | Lowering BP (ACE, angiotensin, aldosterone↓, and eNOS↑) and improving antioxidant capacity | [97] |
| Pueraria lobata | Puerarin | In vivo | SHRs | 40 and 80 mg/kg, 9 weeks | Lowering BP (eNOS, NO, and cGMP↑) | [98] |
| White mulberry fruit | Polysaccharides |
In vitro
In vivo |
Mesenteric artery and endothelial cells; SD rats and SHRs | 0.5 mg/mL; 5 mg/kg, 5 min | Inducing endothelium-dependent relaxation in rat mesenteric arteries and NO production in endothelial cells Lowering mean arterial BP |
[67] |
| Grape seed | Polyphenols | In vivo | Hypertensive rats | 375 mg/kg | Lowering BP (eNOS and Sirtuin-1↑) | [68] |
| Morus alba | Rutin |
In vitro
In vivo |
Mesenteric arteries; wild-type and eNOS-deficient mice |
8 mg/mL; 100, 200, and 400 mg/kg | Inducing endothelial vasorelaxation via a NO-dependent pathway Decreasing BP in wild-type mice, not in eNOS-deficient mice |
[66] |
| Phyllanthus niruri | NA |
In vitro
In vivo |
Endothelium-intact/denuded aorta rings; SHRs | 0.125-4 mg/mL; 1000 mg/kg BW, 2 weeks | Inducing vasorelaxation on endothelium-intact aorta rings Decreasing BP |
[99] |
| Scutellaria baicalensis Georgi | Baicalin |
In vitro
In vivo |
Thoracic aortas; SHRs | 0.1 mg/mL; 10, 50, 100, and 200 mg/kg BW, 0, 30, 60, 90, and 120 min | Relaxing SHR aortas in an endothelium-independent manner Reducing BP |
[100] |
| Heliotropium strigosum | Polyphenols | In vivo | Diabetic rabbits | 21 days | Improving lipid profile (TC, TG, and LDL-C↓) and lowering blood glucose | [25] |
| Mung bean sprouts | NA | In vivo | SD rats | 1 mL/200 g BW, 8 weeks | Lowering BP and improving lipid profile (LDL-C↓) | [72] |
| Red dragon fruit | NA | In vivo | SD rats | 4 weeks | Improving lipid profile (TC, TG, and LDL-C↓) and lowering blood glucose | [73] |
| Red dragon fruit peel | NA | In vivo | Hyperlipidemia male mice | 50, 100, 150, and 200 mg/kg BW, 30 days | Improving lipid profile (TC, TG↓, and HDL-C↑) | [74] |
| Citrus maxima | NA | In vivo | Wistar rats | 300 and 600 mg/kg BW, 14 days | Improving lipid profile (TC, TG↓, and HDL-C↑), lowering blood glucose, and increasing BW | [75] |
| Bitter melon | β-Sitosterol | In vivo | Hyperglycemia rats | 71.1 mg, 4 weeks | Improving lipid profile (TC, TG, LDL-C, fecal cholesterol secretion, cholesterol absorption↓, and HDL-C↑) and lowering blood glucose | [76] |
| Dried chokeberry | Anthocyanins | In vivo | SHRs | 50 mg/kg and 4 weeks | Ameliorating oxidative stress (TBARS↓ and FRAP↑) and lowering SBP and pulse pressure | [26] |
| Sweet cherry | Polyphenols | In vivo | Wistar rats | 5% and 10% (w/w) in food (fruits); 1% and 3% (w/w) in food (leaves), 12 weeks | Decreasing BW gain, ameliorating oxidative stress (SOD, GPx, CAT↑, and TBARS↓), and improving lipid profile (LDL-C+VLDL-C↓) | [82] |
| Wild rice | NA | In vivo | Hyperlipidemic rats | NA and 8 weeks | Ameliorating oxidative stress (TAC, SOD↑, and MAD↓), improving lipid profile (TG and TC↓), and mitigating inflammation (CRP and TNF-α↓) | [80] |
| Sambucus nigra L. | Polyphenols | In vivo | Wistar rats | 0.046 g/kg BW, 8 weeks | Ameliorating oxidative stress (TAC↑), lowering BP, and improving lipid profile (HDL-C↑) | [81] |
| Nepeta deflersiana | NA | In vivo | Wistar rats | 50 and 100 mg/kg BW, 25 days | Attenuating myocardial injuries, mitigating inflammation (TNF-α, IL-6, and IL-10↓), and improving oxidative stress (CAT, SOD, NO↑, and MDA↓) | [29] |
| Spinach | Nitrate | In vivo | Swiss-Kunming mice | 15, 30, and 60 mg/kg of nitrate, 28 days | Mitigating inflammation (CRP, TNF-α, and IL-6↓) and improving vascular endothelial function (NO↑ and endothelin-1↓), lipid profile (TC, TG, LDL-C↓, and HDL-C↑), and insulin resistance | [88] |
| Zygophyllum album roots | NA | In vivo | Wistar rats | 400 mg/kg BW, 60 days | Attenuating myocardial injuries, improving oxidative stress (MDA, PC↓, CAT, SOD, and GPx↑), and mitigating inflammation (TNF-α, IL-1β, IL-6, and nuclear factor-kappa B↓) | [84] |
| Spinacia oleracea | Lutein | In vivo | Wistar rats | 100, 200, and 300 mg/kg BW | Ameliorating myocardial necrosis via mitigating inflammation (TNF-α, IL-1β and IL-6↓) | [89] |
| Antidesma bunius | NA | In vivo | SD rats | 0.38, 0.76, and 1.52 g/kg, 12 weeks | Improving oxidative stress (MDA↓) and mitigating inflammation (TNF-α, IL-6, VCAM-1, and MCP-1↓) | [90] |
| Rice bran | Protein | In vivo | SD rats | 250 and 500 mg/kg, 6 weeks | Lowering BP (ACE↓, NO, and eNOS↑) and reducing arterial stiffening, vascular remodeling, and oxidative stress (SOD and MDA↓) | [83] |
| Polygoni multiflori Radix | 2,3,5,4′-Tetrahydroxy-stilbene-2-O-beta-D-glucoside | In vivo | ApoE(-/-) mice | 1.125 mg/g, 8 weeks | Inhibiting atherosclerotic plaque formation, improving lipid profile (TG and ox-LDL↓), mitigating inflammation (TNF-α, IL-6, VCAM-1, and ICAM-1↓), and regulating gut microbiota composition (Firmicutes/Bacteroidetes, Akkermansia↑, Proteobacteria, Tenericutes, and Helicobacter pylori↓) | [28] |
| Wasabi | Allyl isothiocyanate | In vivo | Wistar rats | 5% (w/w) in food, 8 weeks | Regulating gut microbiota composition to prevent the development of hypertension (Allobaculum, Sutterella, Uncl. S247, Uncl. Coriobacteriaceae, and Bifidobacterium↑) | [93] |
| Lycium ruthenicum Murray | Anthocyanins | In vivo | C57BL/6 mice | 200 mg/kg, 12 weeks | Improving oxidative stress (TAC, SOD, GPx↑, and MDA↓) and inflammation (TNF-α, IL-6, and IL-1β↓), regulating gut microbiota (Barnesiella, Alistipes, Eisenbergiella, Coprobacter, and Odoribacter↑), and increasing SCFA in cecal and feces | [94] |
| Tea | Polyphenols | In vivo | ApoE(-/-) mice | 1.6, 0.8, and 0.4 g/L tea polyphenols in drinking water | Lowering TC and LDL-C, decreasing the plaque area/lumen area, and promoting the proliferation of the intestinal Bifidobacteria | [95] |
| Berry mixture | Polyphenols | In vivo | Dahl salt-sensitive rats | 2 g, 9 weeks | Mitigating changes in the microbiota composition caused by the high-salt diet (phylum Bacteroidetes↑, Firmicutes, and Proteobacteria↓) | [96] |
Note. NA: not available; SHRs: spontaneously hypertensive rats; SD rats: Sprague-Dawley rats; BW: body weight; w/w: weight in weight; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACE: angiotensin-1 converting enzyme; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; cGMP: cyclic guanosine monophosphate; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; VLDL-C: very-low-density lipoprotein cholesterol; ox-LDL: oxidized low-density lipoprotein; TAC: total antioxidant capacity; FRAP: ferric ion-reducing antioxidant power; MDA: malondialdehyde; PC: protein carbonyls; TBARS: thiobarbituric acid reactive substances; SOD: superoxide dismutase; GPx: glutathione peroxidase; GR: glutathione reductase; CAT: catalase; TNF-α: tumor necrosis factor α; CRP: C reactive protein; IL-1β: interleukin-1β; IL-6: interleukin-6; IL-10: interleukin-10: VCAM-1: vascular cell adhesion molecule 1; ICAM-1: intercellular adhesion molecule 1; MCP-1: monocyte chemotactic protein 1; SCFA: short-chain fatty acids.
Figure 1.
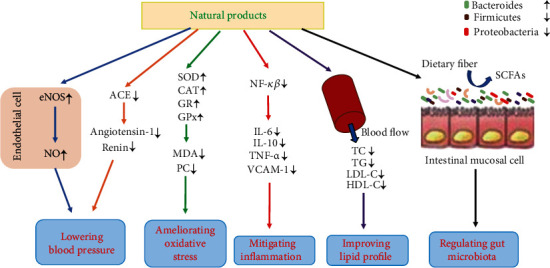
The mechanisms of natural products against cardiovascular diseases. Natural products could stimulate the activity of endothelial nitric oxide synthase (eNOS) and promote the release of nitric oxide (NO) to lower blood pressure; they can also inhibit the activity of angiotensin-1 converting enzyme (ACE) and decrease angiotensin-1 and renin to lower blood pressure. Natural products could promote the activities of antioxidant enzymes, like superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx), and decrease the concentration of peroxidative products, like malondialdehyde (MDA) and protein carbonyls (PC), to ameliorate oxidative stress. Through the nuclear factor-kappa B (NF-κβ) signaling pathway, natural products could decrease levels of inflammatory markers, like tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), and vascular cell adhesion molecule-1 (VCAM-1), to mitigate inflammation. Natural products could decrease the levels of total cholesterol (TC), triglyceride (TG), and low-density lipid protein cholesterol (LDL-C) and increase the level of high-density lipid protein cholesterol (HDL-C) to improve lipid profile. Natural products could increase the abundance of beneficial bacteria, like Bacteroides, and decrease the abundance of harmful bacteria, like Firmicutes and Proteobacteria, to regulate gut microbiota.
3.1. Reducing Blood Pressure
It's widely known that hypertension is an important risk factor for CVDs [56]. An analysis pointed out that every 10 mm Hg reduction in SBP markedly decreased the risk of major cardiovascular disease events in patients with a history of CVDs [57]. Some natural products are effective in the prevention and treatment of CVDs via reducing blood pressure. The hypotensive effect of these natural products was mainly related to the regulation of the renin-angiotensin system (RAS) and the release of nitric oxide (NO).
3.1.1. Regulating the Renin-Angiotensin System
Blood pressure regulation is a sophisticated process involving various organs and systems, among which RAS plays an important role in elevating blood pressure [58]. Regulating the activity of RAS, such as inhibiting the synthesis of angiotensin-1 converting enzyme (ACE) as well as the secretion of renin/angiotensin, is helpful to ameliorate blood pressure [59, 60]. Many experimental studies revealed that some natural products performed the blood pressure lowering efficacy mainly though the regulation of RAS. For example, a study showed a potent in vitro ACE inhibitory property of winged bean seed hydrolysate, as well as the in vivo hypotensive effect of the hydrolysate in a dose-dependent manner in Sprague-Dawley (SD) rats, indicating that the hydrolysate lowered blood pressure via suppressing the activity of ACE [61]. Another study found that Solanum macrocarpon leaf extract suppressed the in vitro activities of renin and ACE. The oral administration of the extract decreased SBP, diastolic blood pressure (DBP), and heart rate in spontaneously hypertensive rats. Rutin, caffeic acid, and myricetin were the major polyphenols in the extract [62]. Furthermore, a study pointed out that Ocimum sanctum and Citrus paradisi infusions possessed a hypotensive property. The infusion of Ocimum sanctum downregulated the gene expression of renin and angiotensinogen and reduced renal triglyceride accumulation and lipid/protein oxidation in SD rats, while the hypotensive effect of Citrus paradisi could be associated with other mechanisms [63].
3.1.2. Increasing the Release of NO
Accumulating evidence has proven that the generation of NO in endothelial cells is mainly activated by endothelial nitric oxide synthase (eNOS). NO could induce the relaxation of blood vessels, leading to the reduction of blood pressure [64, 65]. Hence, promoting the production of NO is an effective way to decrease blood pressure, which will protect the function of the cardiovascular system. There are findings suggesting that some natural products showed an antihypertensive effect via accelerating the release of NO, holding tremendous promise to prevent the development of hypertension and CVDs. For example, Morus alba induced endothelial vasorelaxation in mesenteric arteries via a NO-dependent pathway, and decreased blood pressure in wild-type mice. However, it failed to exert a hemodynamic effect in eNOS-deficient mice, which further testified to the antihypertensive action of Morus alba through a NO-dependent pathway [66]. Moreover, a study found that white mulberry fruit polysaccharides could provoke endothelium-dependent relaxation in rat mesenteric arteries and NO production in endothelial cells, and its intravenous injection induced the reduction of blood pressure in both normotensive rats and spontaneously hypertensive rats, while this effect was markedly attenuated in normotensive rats pretreated with the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME). These results suggested that the hypotensive effect of white mulberry fruit was mediated by the NO pathway [67]. Additionally, grape seed polyphenol extract promoted the production of NO and reduced the blood pressure in hypertensive rats via upregulating the expression of eNOS and Sirtuin-1 [68].
3.2. Improving Lipid Profile
Hyperlipidemia results from the metabolic abnormalities of lipids, leading to higher levels of lipids in plasma than normal ones, which can be generally characterized as higher levels of total cholesterol (TC), triglyceride (TG), and low-density lipid protein cholesterol (LDL-C) and a lower level of high-density lipid protein cholesterol (HDL-C) [69]. Increasing evidence suggested that hyperlipidemia was closely associated with atherosclerosis, playing an important role in the development of CVDs [70, 71]. Several experimental studies revealed the hypolipidemic effect of natural products. For example, an in vivo study found that after the treatment of mung bean sprouts, the SBP and LDL-C levels of SD rats in the high-fat diet group significantly lowered to the normal level [72]. Additionally, supplementing obese rats with red dragon fruit flour for 4 weeks markedly reduced the blood glucose, TC, TG, and LDL-C levels, while HDL-C had no significant difference [73]. Also, another study found that after oral administration of red dragon fruit peel powder for 30 days, TC, TG, and LDL-C levels of hyperlipidemic male mice declined in a dose-dependent manner, accompanied by an increase in HDL-C levels [74]. The two studies above showed that both the pulp and peel of red dragon fruit possessed promising blood lipid-lowering efficacy. Furthermore, after administration with Citrus maxima juice for 14 days, male Wistar rats showed a significant decrease of TC and TG, along with an increase of HDL-C [75]. Furthermore, feeding with fresh bitter melon fruit juice for 4 weeks markedly dropped down the levels of blood glucose, TG, TC, and LDL-C in hyperglycemia rats compared with the initial levels, but the HDL-C level was dramatically elevated. Meanwhile, bitter melon effectively improved the fecal cholesterol secretion and suppressed cholesterol absorption, posing a potent ability to improve lipid profile [76].
3.3. Ameliorating Oxidative Stress
Oxidative stress, a major cause of the CVDs, is the result of the reduction of antioxidant capacity and the production of excessive reactive oxygen species (ROS) [77–79]. Some natural products could improve oxidative stress via promoting the activities of antioxidant enzymes, like superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx) and decreasing the concentration of peroxidative products, like malondialdehyde (MDA) and protein carbonyls, hence promising to prevent and treat CVDs. A study showed that the intake of North American or Chinese wild rice effectively inhibited the formation of oxidative stress in hyperlipidemic rats via improving total antioxidant capacity, increasing SOD activity, and reducing MDA concentration. In addition, two wild rice varieties were also effective in suppressing hyperlipidemia and inflammation in rats [80]. Moreover, the polyphenol extract of Sambucus nigra L. ameliorated oxidative stress by enhancing total antioxidant capacity, and reduced both SBP and DBP in Wistar rats. Its combination with a renin inhibitor (Aliskiren) generated a superior antioxidant effect compared to administering the two separately, and it could also reduce the side effects of the antihypertensive agent [81]. Furthermore, the effects of dried chokeberry fruit extract on haemodynamic parameters, lipid profile, and oxidative stress were evaluated in spontaneously hypertensive rats, and anthocyanins, phenolic acids, and flavonoids in the extract were determined by the HPLC/DAD method. The extract rich in anthocyanins significantly reduced systolic and pulse pressures via increased diuresis. The thiobarbituric acid reactive substances (TBARS) in plasma and erythrocytes were significantly decreased in the treated group. The consumption of the extract also reduced lipid peroxidation through improving the ferric ion-reducing antioxidant power (FRAP) of plasma, but the activity of SOD in the treated group was significantly lower compared to the control group [26]. Additionally, the supplement of sweet cherry fruit and leaves to the high-fat–high-cholesterol diet in Wistar rats decreased body gain, improved liver function, and reduced inflammation and oxidative stress (by provoking the activities of SOD, GPx, GR, and CAT, and reducing the level of TBARS). The fruit and leaves reduced lipid accumulation in the liver and improved the lipid profile in serum. These effects could be from the regulation of the expression of fatty acid synthesis and oxidation-related genes [82]. In a previous study, the effects of rice bran protein hydrolysate on arterial stiffening, vascular remodeling, and oxidative stress were evaluated in rats fed a high-carbohydrate and high-fat diet. The hydrolysate supplementation significantly alleviated hyperglycemia, insulin resistance, dyslipidemia, hypertension, increased aortic pulse wave velocity, aortic wall hypertrophy, and vascular remodeling. The hydrolysate reduced the levels of ACE and tumor necrosis factor-alpha in plasma. The hydrolysate also alleviated oxidative stress by decreasing plasma MDA, reducing superoxide production, and suppressing p47 (phox) NADPH oxidase expression in the vascular tissues. The hydrolysate increased plasma nitrate/nitrite level and upregulated eNOS expression in the aortas of model group rats, indicating that the hydrolysate increased NO production [83]. In another study, Zygophyllum album root extract was analyzed using HPLC-DAD-ESI-QTOF-MS/MS, and twenty-six molecules were identified, including phenolic compounds and saponins. The extract significantly improved the heart injury markers, lipid peroxidation, protein oxidation, antioxidant capacity (SOD, CAT, and GPx), and DNA structure. The extract reduced the expressions of NF-kappa B, decreased plasmatic proinflammatory cytokine concentration, and suppressed the myocardial collagen deposition [84]. An in vivo study showed that apple polyphenol extract possessed a positive effect on vascular oxidative stress and endothelium function [85].
3.4. Mitigating Inflammation
Inflammatory response is a prominent pathological change in the development of CVDs, which can be characterized by increased levels of inflammatory markers, like tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), C reactive protein (CRP), monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion molecule-1 (VCAM-1) [86, 87]. It has been reported that some natural products were able to downregulate the expression of these cytokines and mitigate inflammation, which was a way of lowering the risk of CVDs. For example, an in vivo study pointed out that Nepeta deflersiana ethanolic extract effectively attenuated the myocardial injuries in Wistar rats by improving oxidative stress, inhibiting apoptosis, and mitigating inflammation. Nepeta deflersiana exerted an anti-inflammatory effect via the downregulation of the gene expression of TNF-α, IL-6, and IL-10 [29]. Another study found that the oral administration of Zygophyllum album root extract ameliorated the myocardial injuries in Wistar rats though the improvement of oxidative stress and the alleviation of inflammation. Zygophyllum album root extract decreased the plasma concentration of proinflammatory cytokines, like TNF-α, IL-1β, and IL-6 [84]. Additionally, spinach nitrate significantly lowered the elevated levels of serum CRP, TNF-α, and IL-6 induced by a high-fat and high-fructose diet in male mice, showing a strong anti-inflammatory capacity [88]. Also, a study demonstrated that Spinacia oleracea leaf methanolic extract dose-dependently attenuated isoproterenol-induced myocardial necrosis in male Wistar rats via mitigating the levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [89]. Moreover, Antidesma bunius extract significantly ameliorated the expressions of genes involved with proinflammatory cytokines, such as TNF-α, IL-6, VCAM-1, and MCP-1, showing great anti-inflammatory capacity [90].
3.5. Regulating Gut Microbiota
Recent interest has focused on the impact of gut microbiota on chronic diseases, especially CVDs. Increasing evidence has shown that gut microbiota was closely associated with the function of the cardiovascular system via contributing to the fermentation of dietary fiber in the colon, the production of short-chain fatty acids (SCFA), and the intestinal absorption of phytochemicals [91, 92]. Hence, it is of great significance to maintain the balance of intestinal flora to protect against CVDs. Some studies revealed that several natural products could regulate the homeostasis of gut microbiota. For example, a study demonstrated that wasabi powder prevented the development of hypertension in Wistar rats via changing the composition of gut microbiota, increasing the abundance of Allobaculum, Sutterella, Uncl. S247, Uncl. Coriobacteriaceae, and Bifidobacterium [93]. Moreover, treatment with anthocyanins extracted from Lycium ruthenicum Murray could not only improve oxidative stress and inflammation in C57BL/6 mice but also promote the proliferation of Barnesiella, Alistipes, Eisenbergiella, Coprobacter, and Odoribacter and increase the production of SCFA in cecal and feces [94]. Additionally, a study found that except for the improvement of lipid profile and inflammation, Polygoni multiflori Radix extract significantly inhibited atherosclerosis plaque formation in ApoE(-/-) mice via regulating gut microbiota composition [28]. Also, a study pointed out that tea polyphenols dose-dependently increased the abundance of intestinal Bifidobacteria in high-fat diet-fed ApoE(-/-) mice, and this increase negatively correlated with plaque area/lumen area ratios, suggesting that tea polyphenols could reduce atherosclerosis plaque induced by high-fat diet via increasing intestinal Bifidobacteria [95]. Furthermore, the intake of berry mixture, including blueberries, blackberries, raspberries, Portuguese crowberry, and strawberry tree fruit, increased the abundance of phylum Bacteroidetes, decreased the abundance of Firmicutes, and reduced the elevated abundance of Proteobacteria induced by a high-salt diet in Dahl salt-sensitive rats [96].
In brief, based on the in vitro and in vivo experimental studies, we summarized the potential mechanisms of some natural products protecting against CVDs, including reducing blood pressure, improving the lipid profile, ameliorating oxidative stress, mitigating inflammation, and regulating gut microbiota.
4. Clinical Trials
The benefits of reducing the risk of CVD events through the consumption of antioxidant natural products and their active ingredients have been studied in multiple clinical trials. Here, we summarize the protective effects of some natural products on CVDs (Table 3).
Table 3.
The effects of antioxidant natural products on CVDs from clinical studies.
| Plant types | Components | Study type | Subjects | Dose and time | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Fruits | ||||||
| Guava | NA | RCT | 45 healthy students | 400 g/day, 6 weeks | Lowering BP, TC, TG, and LDL-C | [101] |
| Soursop | NA | RCT | 143 hypertensive subjects | 2 × 100 g/day, 3 months | Lowering BP | [102] |
| Orange juice | Hesperidin and naringin | Controlled nonrandomized clinical study | 10 healthy women | 300 mL/day, 2 months | Improving LDL-C, blood glucose, insulin sensitivity, and gut microbiota metabolism | [103] |
| Haskap berry | Anthocyanin | Cross-over study | 20 adults aged 62-81 years | 400 mg anthocyanins | Lowering BP and improving episodic memory | [104] |
| Cherry juice | Anthocyanin | Pilot cross-over study | 6 young and 7 old adults | 300 mL or 100 mL, 3 times | Lowering BP and heart rate | [126] |
| Pomegranate extract | Polyphenols | RCT | 55 subjects without any symptomatic disease | Containing 210 mg punicalagins, 328 mg other pomegranate polyphenols, and 0-37 mg anthocyanins, 8 weeks | Lowering SBP | [127] |
| Plum juice | Anthocyanins | Pilot cross-over dose-timing study | 12 older (65+ years) and 12 younger (18-45 years) adults | 300 mL or 100 mL, 3 times | Reducing BP and cardiovascular responses | [128] |
| Noni and chokeberry juices | NA | RCT | 88 young adults | Noni juice 30 mL; chokeberry juice 200 mL | Lowering SBP, DBP, heart rate, and blood glucose (noni juice) Slightly lowering DBP (chokeberry juice) |
[129] |
| Vegetables | ||||||
| Tomato extract | NA | RCT | 65 patients with hypertension and a high risk of CVD | 213 mg/day, 4 weeks | Lowering DBP and mean arterial pressure | [105] |
| Beetroot juice | Nitrate | Open-label cross-over study | 17 patients with chronic kidney disease | Containing 300 mg nitrate, 4 hours | Lowering peripheral BP and mean arterial pressure | [130] |
| Beetroot juice | Nitrate | Double-blind cross-over study | 20 subjects with treated yet uncontrolled hypertension | Containing 12.9 mmol nitrate, 7 days | Increasing plasma nitrite and reducing BP | [131] |
| Beetroot juice | Nitrate | Feasibility trial | 40 hypertensive pregnant women | 70 mL/day, 8 days | Lowering DBP | [106] |
| Sateria palmifolia | NA | Quasiexperiment | 10 pregnant women | NA | Lowering BP | [132] |
| Eggplant powder | NA | RCT | 100 stressed participants with normal-high BP or stage 1 hypertension | 1.2 g/day, 12 weeks | Improving BP and psychological state | [107] |
| Beetroot, rocket salad and spinach | Nitrate | Semirandomized cross-over study | 11 men and 7 women | Each group containing 800 mg nitrate | Lowering BP | [133] |
| Spices | ||||||
| Satureja hortensis L. | NA | RCT | 47 patients with metabolic syndrome | 450 mg/day, 10 weeks | Lowering TC, TG, and LDL-C and increasing HDL-C | [109] |
| Cardamom | NA | RCT | 80 overweight and obese prediabetic women | 3 g, 2 months | Lowering TC and LDL-C | [108] |
| Garlic and cumin | NA | RCT | 75 patients with T2DM | Garlic powder: 300 mg/three times a day; cumin extract: 100 mg/twice a day, 2 months | Lowering BP | [110] |
| Cinnamon, cardamom, saffron, and ginger | NA | RCT | 204 patients with T2DM | 3 g cinnamon, 3 g cardamom, 1 g saffron, and 3 g ginger in a glass of black tea, respectively, 8 weeks | All showed potent effects on controlling BP and improving endothelial function | [33] |
| Nuts | ||||||
| Almond | NA | RCT | 86 overweight or obese adults | 15% energy from almond, 12 weeks | Lowering truncal and total body fat as well as DBP | [114] |
| Walnuts | NA | RCT | 211 participants | 30 g/day, 3 months | Lowering BP | [134] |
| Walnuts | NA | RCT | 100 overweight and obese participants | 15% energy from walnut, 6 months | Reducing BW, BMI, waist circumference, SBP, TC, and LDL-C | [115] |
| Walnuts | α-Linolenic acid | RCT | 42 adults at cardiovascular risk | 57–99 g/day, 6 weeks | Regulating gut microbiota | [135] |
| Cashew nut | NA | RCT | 300 Asian Indians with T2DM | 30 g/day, 12 weeks | Decreasing SBP and increasing HDL-C | [116] |
| Cashew nut | NA | RCT | 42 adults | 42 g/day, 4 weeks | No effect on risk factors of CVD | [136] |
| Mixed nuts | NA | RCT | 48 overweight and obese adults | 250 kcal, 4 and 8 weeks | Improving BW and glucose regulation | [117] |
| Teas | ||||||
| Green tea | Catechin | RCT | 1,075 postmenopausal women | Containing 1,315 mg catechins, 6 and 12 months | Lowering TC, LDL-C, and non-HDL-C | [118] |
| Kosen-cha | Catechin | Open-label pilot study | 6 obese subjects | 5 g/L, 12 weeks | Lowering BW, BMI, waist circumferences, and serum TG levels and improving insulin resistance, vascular function, and cardiac hypertrophy | [120] |
| Goishi tea | Polyphenols | RCT | 77 subjects | Containing 122 mg of polyphenols, 12 weeks | Increasing HDL-C and lowering TG | [137] |
| Black tea | Phytosterol | RCT | Subjects with mild hypercholesterolemia | Phytosterol-enriched functional black tea, 4 weeks | Lowering TC, LDL-C, and apolipoprotein B and improving oxidative stress | [119] |
| Others | ||||||
| Hibiscus sabdariffa | NA | Cross-over study | 25 men with 1% to 10% CVD risk | 250 mL, 0, 2, and 4 h | Reducing endothelial dysfunction and CVD risk | [138] |
| Hibiscus sabdariffa | NA | RCT | 46 patients with stage 1 hypertension | 2 cup/morning, 1 month | Reducing BP | [123] |
| Chamomile | NA | RCT | 50 diabetic patients | 200 mL/day, 4 weeks | Lowering TC, LDL-C, and creatinine | [139] |
| Soy flour | NA | 3 × 3 completely randomized repeated study | 75 postmenopausal women with prediabetes and prehypertension | 25 and 45 g/day, 12 weeks | Lowering fasting plasma glucose, fasting insulin, insulin resistance, and DBP | [124] |
| Navy beans and rice bran | NA | Pilot RCT | 38 children with abnormal cholesterol | 17.5 g/day cooked navy bean powder; 15 g/day heat-stabilized rice bran; 9 g/day navy beans and 8 g/day rice bran, 4 weeks | Modulating the plasma metabolome and reducing CVD risk | [140] |
| Oat noodles | NA | RCT | 84 healthy and mild hypercholesterolemic subjects | 100 g/day (replacing 1 or 2 meals of staple food), 10 weeks | Reducing TC/HDL-C and LDL-C/HDL-C ratios and blood pressure | [125] |
| Green coffee bean | NA | RCT | Patients with the metabolic syndrome | 400 mg twice/day, 8 weeks | Reducing SBP, insulin resistance, and abdominal obesity and inhibiting appetite | [141] |
Note. NA: not available; RCT: randomized controlled trial; CVD: cardiovascular disease; T2DM: type 2 diabetes mellitus; BW: body weight; BMI: body mass index; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol.
4.1. The Effects of Fruits on CVDs
Several clinical trials revealed the inverse relationship between the consumption of fruits and risk of CVDs. A randomized controlled trial (RCT) found that guava pulp significantly improved lipid profile by decreasing the levels of TC, TG, and LDL-C and increasing the level of HDL-C [101]. Another RCT pointed out that intake of 100 g soursop fruit twice per day for 3 months markedly decreased the levels of SBP, DBP, and serum uric acid in prehypertensive participants compared to the control group [102]. Additionally, a controlled nonrandomized clinical study showed that consuming 300 mL of orange juice for 2 months improved LDL-C, blood glucose, insulin sensitivity, and gut microbiota metabolism in healthy women [103]. Furthermore, a cross-over study found that after the intervention of 200 or 400 mg anthocyanin from haskap berry, the blood pressure levels in participants aged 62-81 years were significantly reduced [104]. Hence, consuming some fruits, like guava, soursop, and orange, is an effective way to prevent and manage CVDs.
4.2. The Effects of Vegetables on CVDs
Some vegetables also showed a protective effect on CVDs. An RCT found that the intake of 213 mg tomato extract for 4 weeks lowered DBP and mean arterial pressure in patients with hypertension and high risk of CVDs [105]. Moreover, several studies revealed the potent hypotensive efficacy of beetroot which was associated with its high content of nitrate. For example, a study demonstrated that consuming 70 mL beetroot juice significantly lowered the level of DBP in hypertensive pregnant women [106]. Additionally, daily consumption of 1.2 g eggplant powder markedly improved blood pressure and the psychological state of stressed participants with normal-high blood pressure or stage 1 hypertension [107]. Therefore, it is advisable to increase the intake of tomato, beetroot, and eggplant to protect the health of the cardiovascular system.
4.3. The Effects of Spices on CVDs
Similarly, some spices effectively reduced the risk of CVDs. An RCT showed that after intervention with 3 g cardamom for 2 months, the levels of TC and LDL-C in overweight and obese prediabetic women were remarkably lowered, while SBP, DBP, glycemic indices, and serum lipid values in the cardamom group did not significantly differ from the placebo group [108]. Another study compared the cardiovascular-protective effect of cardamom, cinnamon, saffron, and ginger, demonstrating that all of them showed potent abilities in controlling blood pressure and improving endothelial function [33]. Besides, daily ingestion of Satureja hortensis L. effectively improved the lipid profile in patients with metabolic syndrome by lowering TC, TG, and LDL-C and increasing HDL-C [109]. Moreover, garlic and cumin showed a strong hypotensive effect on patients with type 2 diabetes [110]. In brief, some spices, like cardamom, cinnamon, saffron, ginger, garlic, and cumin, hold great promise in preventing and treating CVDs.
4.4. The Effects of Nuts on CVDs
Nuts contain several antioxidant components and possess many bioactivities [111–113]. Moderate intake of nuts also attenuated the risk factors of CVDs. An RCT found that consumption of almond which provided 15% energy significantly lowered truncal and total body fat as well as DBP in overweight or obese adults [114]. Another 6-month-long RCT demonstrated that intake of walnut remarkably reduced body weight, body mass index, waist circumference, SBP, TC, and LDL-C [115]. Moreover, supplementing type 2 diabetes participants with cashew nut considerably reduced SBP and increased HDL-C, while significant differences were not observed in body weight, body mass index, glycemic, and other lipid variables [116]. Additionally, intake of mixed nuts, including almonds, cashews, hazelnuts, pecans, Brazil nuts, macadamia nuts, pistachios, walnuts, and peanuts, could attenuate CVD risk factors by improving body weight and glucose regulation, without exerting the negative effects on lipids compared with a common carbohydrate-rich snack [117].
4.5. The Effects of Teas on CVDs
As the second-most consumed beverage worldwide, the nutrition value of teas is extensively investigated. Several studies found that the consumption of teas could ameliorate the risk factors of CVDs. For example, supplementation with green tea extract, which contained 1,315 mg catechins, could significantly improve lipid profile in postmenopausal women by reducing the levels of TC, LDL-C, and non-HDL-C [118]. Furthermore, a study pointed out that the consumption of phytosterol-enriched functional black tea could lower TC, LDL-C, and apolipoprotein B in mild hypercholesterolemia subjects, accompanied with the amelioration of oxidative stress [119]. Moreover, an open-label pilot study found that after daily administration of 2 g/L kosen-cha, obese subjects showed a significant reduction of body weight, BMI, waist circumferences, and serum TG levels, as well as the improvement of insulin resistance, vascular function, and cardiac hypertrophy [120].
4.6. The Effects of Other Plants on CVDs
Other natural products also possessed the ability to protect the health of the cardiovascular system, such as cereals, legumes, and herbs [121, 122]. For example, drinking 2 cups of Hibiscus sabdariffa in the morning effectively reduced blood pressure in patients with stage 1 hypertension [123]. Besides, a 3 × 3 completely randomized repeated study showed that daily consumption of 25 g or 45 g soy flour markedly lowered the levels of fasting plasma glucose, fasting insulin, insulin resistance, and DBP in postmenopausal women with prediabetes and prehypertension [124]. Additionally, using oat noodles to replace 1 or 2 meals of staple food could reduce blood pressure, and improve lipid profile by lowering TC/HDL-C and LDL-C/HDL-C ratios [125]. Hence, supplementing the consumption of Hibiscus sabdariffa, soy, and oat helps to reduce the risk of CVDs.
In short, clinical trials involving different conditions of subjects illustrated that some antioxidant natural products could improve cardiovascular health and reduce the risk of CVDs, which might be related to decreasing blood pressure, regulating serum lipids, lowering blood glucose, and lowering body weight.
5. Conclusions
As a public health problem of global concern, CVDs have attracted considerable attention. Some antioxidant natural products have been proven capable of preventing CVDs. Multiple epidemiological investigations enrolling participants from different countries, different ages, and so on, suggested that the consumption of antioxidant natural products was beneficial to reduce the risk of CVD events. Moreover, results from experimental studies showed that some natural products exerted cardiovascular-protective effects via different mechanisms of action, such as reducing blood pressure, improving the lipid profile, ameliorating oxidative stress, mitigating inflammation, and regulating gut microbiota. Furthermore, clinical trials confirmed that some antioxidant natural products could prevent and treat CVDs. Supported by current evidence, some antioxidant natural products and their active compounds could be developed into functional foods or medicine for the prevention and treatment of CVDs. In the future, the effects of more antioxidant natural products on CVDs should be evaluated to find out more cardiovascular-protective natural products, and relative bioactive components should be isolated and identified. In addition, the mechanisms of action should be elucidated further. Furthermore, special attention should be paid to the safety of relative natural products and functional foods.
Acknowledgments
We thank Hang-Yu Li for the support given to this study. This study was supported by the National Key R&D Program of China (No. 2018YFC1604405), the China Central Public-Interest Scientific Institution Basal Research Fund (No. Y2020XK05), and the Key Project of Guangdong Provincial Science and Technology Program (No. 2014B020205002).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
D.-D.Z., R.-Y.G., and H.-B.L. conceptualized this study. D.-D.Z., M.L., A.S., Q.-Q.M., and B.-Y.L. wrote the original draft of this manuscript. R.-Y.G. and H.-B.L. wrote the manuscript and contributed in reviewing and editing. R.-Y.G. and H.-B.L. supervised the study. R.-Y.G. and H.-B.L. contributed in funding acquisition.
References
- 1.Wang H. D., Naghavi M., Allen C., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wg M., Benjamin E. J., Blaha M. J., et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:146–163. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liguori I., Russo G., Curcio F., et al. Oxidative stress, aging, and diseases. Clinical Interventions in Aging. 2018;Volume 13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang W. H. W., Kitai T., Hazen S. L. Gut microbiota in cardiovascular health and disease. Circulation Research. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziff O. J., Kotecha D. Digoxin: the good and the bad. Trends in Cardiovascular Medicine. 2016;26(7):585–595. doi: 10.1016/j.tcm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Giudicessi J. R., Ackerman M. J., Camilleri M. Cardiovascular safety of prokinetic agents: a focus on drug-induced arrhythmias. Neurogastroenterology and Motility. 2018;30(6, article e13302) doi: 10.1111/nmo.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller V., Mente A., Dehghan M., et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037–2049. doi: 10.1016/S0140-6736(17)32253-5. [DOI] [PubMed] [Google Scholar]
- 8.Rouhi-Boroujeni H., Heidarian E., Rouhi-Boroujeni H., Deris F., Rafieian-Kopaei M. Medicinal plants with multiple effects on cardiovascular diseases: a systematic review. Current Pharmaceutical Design. 2017;23:999–1015. doi: 10.2174/1381612822666161021160524. [DOI] [PubMed] [Google Scholar]
- 9.Tang G. Y., Meng X., Li Y., Zhao C. N., Liu Q., Li H. B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. 2017;9(8):p. 857. doi: 10.3390/nu9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X. Y., Meng X., Li S., Gan R. Y., Li Y., Li H. B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018;10(10):p. 1553. doi: 10.3390/nu10101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao Q. Q., Xu X. Y., Cao S. Y., et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe) Food. 2019;8(6):p. 185. doi: 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang A., Gan R. Y., Xu X. Y., Mao Q. Q., Zhang P. Z., Li H. B. Effects and mechanisms of edible and medicinal plants on obesity: an updated review. Critical Reviews in Food Science and Nutrition. 2020:1–17. doi: 10.1080/10408398.2020.1769548. In press. [DOI] [PubMed] [Google Scholar]
- 13.Cao S. Y., Li B. Y., Gan R. Y., et al. The in vivo antioxidant and hepatoprotective actions of selected Chinese teas. Food. 2020;9(3):p. 262. doi: 10.3390/foods9030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao J., Li S., Gan R. Y., Zhao C. N., Meng X., Li H. B. Targeting gut microbiota with dietary components on cancer: effects and potential mechanisms of action. Critical Reviews in Food Science and Nutrition. 2020;60(6):1025–1037. doi: 10.1080/10408398.2018.1555789. [DOI] [PubMed] [Google Scholar]
- 15.Xu X. Y., Zhao C. N., Cao S. Y., Tang G. Y., Gan R. Y., Li H. B. Effects and mechanisms of tea for the prevention and management of cancers: an updated review. Critical Reviews in Food Science and Nutrition. 2020;60(10):1693–1705. doi: 10.1080/10408398.2019.1588223. [DOI] [PubMed] [Google Scholar]
- 16.Xu X. Y., Zheng J., Meng J. M., et al. Effects of food processing on in vivo antioxidant and hepatoprotective properties of green tea extracts. Antioxidants. 2019;8(12):p. 572. doi: 10.3390/antiox8120572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao S. Y., Zhao C. N., Xu X. Y., et al. Dietary plants, gut microbiota, and obesity: effects and mechanisms. Trends in Food Science and Technology. 2019;92:194–204. doi: 10.1016/j.tifs.2019.08.004. [DOI] [Google Scholar]
- 18.Li B. Y., Xu X. Y., Gan R. Y., et al. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Food. 2019;8(10):p. 440. doi: 10.3390/foods8100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X., Li Y., Li S., Gan R. Y., Li H. B. Natural products for prevention and treatment of chemical-induced liver injuries. Comprehensive Reviews in Food Science and Food Safety. 2018;17(2):472–495. doi: 10.1111/1541-4337.12335. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q., Meng X., Li Y., et al. Natural products for the prevention and management of Helicobacter pylori infection. Comprehensive Reviews in Food Science and Food Safety. 2018;17(4):937–952. doi: 10.1111/1541-4337.12355. [DOI] [PubMed] [Google Scholar]
- 21.Lule S. A., Namara B., Akurut H., et al. Blood pressure risk factors in early adolescents: results from a Ugandan birth cohort. Journal of Human Hypertension. 2019;33(9):679–692. doi: 10.1038/s41371-019-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitok E., Sabate J. Nuts and cardiovascular disease. Progress in Cardiovascular Diseases. 2018;61(1):33–37. doi: 10.1016/j.pcad.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C. N., Meng X., Li Y., et al. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. 2017;9(6):p. 598. doi: 10.3390/nu9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olagunju A. I., Omoba O. S., Enujiugha V. N., Alashi A. M., Aluko R. E. Antioxidant properties, ACE/renin inhibitory activities of pigeon pea hydrolysates and effects on systolic blood pressure of spontaneously hypertensive rats. Food Science & Nutrition. 2018;6(7):1879–1889. doi: 10.1002/fsn3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood A., Sarfraz R. A., Bhatti I. A., Hussain F. Alpha-amylase inhibitory activity and blood glucose and lipid-lowering potential of Heliotropium strigosum. Oxidation Communications. 2016;39:108–117. [Google Scholar]
- 26.Cujic N., Savikin K., Miloradovic Z., et al. Characterization of dried chokeberry fruit extract and its chronic effects on blood pressure and oxidative stress in spontaneously hypertensive rats. Journal of Functional Foods. 2018;44:330–339. doi: 10.1016/j.jff.2018.02.027. [DOI] [Google Scholar]
- 27.Park S. W., Shin K. C., Yoou S.-K., et al. Effects of an ethanolic extract of mulberry fruit on blood pressure and vascular remodeling in spontaneous hypertensive rats. Clinical and Experimental Hypertension. 2019;41:280–286. doi: 10.1080/10641963.2018.1469645. [DOI] [PubMed] [Google Scholar]
- 28.Li F., Zhang T., He Y., et al. Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE−/− mice. Journal of Ethnopharmacology. 2020;247:p. 112232. doi: 10.1016/j.jep.2019.112232. [DOI] [PubMed] [Google Scholar]
- 29.al‑Taweel A. M., Raish M., Perveen S., et al. _Nepeta deflersiana_ attenuates isoproterenol-induced myocardial injuries in rats: Possible involvement of oxidative stress, apoptosis, inflammation through nuclear factor (NF)- κβ downregulation. Phytomedicine. 2017;34:67–75. doi: 10.1016/j.phymed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Jayachandran M., Chung S. S. M., Xu B. A critical review on diet-induced microbiota changes and cardiovascular diseases. Critical Reviews in Food Science and Nutrition. 2019;60:2914–2925. doi: 10.1080/10408398.2019.1666792. [DOI] [PubMed] [Google Scholar]
- 31.Tang G. Y., Meng X., Gan R. Y., et al. Health functions and related molecular mechanisms of tea components: an update review. International Journal of Molecular Sciences. 2019;20(24):p. 6196. doi: 10.3390/ijms20246196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang A., Cao S. Y., Xu X. Y., et al. Bioactive compounds and biological functions of garlic (Allium sativum L.) Food. 2019;8(7):p. 246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azimi P., Ghiasvand R., Feizi A., et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Blood Pressure. 2016;25(3):133–140. doi: 10.3109/08037051.2015.1111020. [DOI] [PubMed] [Google Scholar]
- 34.Peluso I., Raguzzini A., Catasta G., et al. Effects of high consumption of vegetables on clinical, immunological, and antioxidant markers in subjects at risk of cardiovascular diseases. Oxidative Medicine and Cellular Longevity. 2018;2018:9. doi: 10.1155/2018/5417165.5417165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Dong B., Zou Z., et al. Association between vegetable consumption and blood pressure, stratified by BMI, among Chinese adolescents aged 13-17 years: a national cross-sectional study. Nutrients. 2018;10(4):p. 451. doi: 10.3390/nu10040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun B., Shi X., Wang T., Zhang D. Exploration of the association between dietary fiber intake and hypertension among US adults using 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: NHANES 2007-2014. Nutrients. 2018;10:p. 1091. doi: 10.3390/nu10081091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon Y. Association of curry consumption with blood lipids and glucose levels. Nutrition Research and Practice. 2016;10(2):212–220. doi: 10.4162/nrp.2016.10.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du H., Li L., Bennett D., et al. Fresh fruit consumption and major cardiovascular disease in China. The New England Journal of Medicine. 2016;374:1332–1343. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahadoran Z., Mirmiran P., Momenan A. A., Azizi F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: a longitudinal follow-up study. Journal of Hypertension. 2017;35(9):1909–1916. doi: 10.1097/HJH.0000000000001356. [DOI] [PubMed] [Google Scholar]
- 40.Buil-Cosiales P., Martinez-Gonzalez M. A., Ruiz-Canela M., Diez-Espino J., Garcia-Arellano A., Toledo E. Consumption of fruit or fiber-fruit decreases the risk of cardiovascular disease in a Mediterranean young cohort. Nutrients. 2017;9:p. 295. doi: 10.3390/nu9030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirmiran P., Bahadoran Z., Khalili Moghadam S., Zadeh Vakili A., Azizi F. A prospective study of different types of dietary fiber and risk of cardiovascular disease: Tehran lipid and glucose study. Nutrients. 2016;8(11):p. 686. doi: 10.3390/nu8110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson S. C., Drca N., Bjorck M., Back M., Wolk A. Nut consumption and incidence of seven cardiovascular diseases. Heart. 2018;104(19):1615–1620. doi: 10.1136/heartjnl-2017-312819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domingos T. B., Pereira A. F., Yokoo E. M., Salles-Costa R. Low fruit consumption and omission of daily meals as risk factors for increased blood pressure in adults. The British Journal of Nutrition. 2016;116(4):683–691. doi: 10.1017/S0007114516002397. [DOI] [PubMed] [Google Scholar]
- 44.Cassidy A., Bertoia M., Chiuve S., Flint A., Forman J., Rimm E. B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. The American Journal of Clinical Nutrition. 2016;104(3):587–594. doi: 10.3945/ajcn.116.133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh J. S., Kim H., Vijayakumar A., Kwon O., Kim Y., Chang N. Association of dietary flavonoid intake with prevalence of type 2 diabetes mellitus and cardiovascular disease risk factors in Korean women aged ≥30 years. Journal of Nutritional Science and Vitaminology. 2017;63(1):51–58. doi: 10.3177/jnsv.63.51. [DOI] [PubMed] [Google Scholar]
- 46.Tian X., Du H., Li L., et al. Fruit consumption and physical activity in relation to all-cause and cardiovascular mortality among 70,000 Chinese adults with pre-existing vascular disease. PLoS One. 2017;12, article e173054 doi: 10.1371/journal.pone.0173054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H. A., Lim D., Oh K., Kim E. J., Park H. Mediating effects of metabolic factors on the association between fruit or vegetable intake and cardiovascular disease: the Korean National Health and Nutrition Examination Survey. BMJ Open. 2018;8(2, article e019620) doi: 10.1136/bmjopen-2017-019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu A. H., Bondonno C. P., Russell J., et al. Relationship of dietary nitrate intake from vegetables with cardiovascular disease mortality: a prospective study in a cohort of older Australians. European Journal of Nutrition. 2019;58(7):2741–2753. doi: 10.1007/s00394-018-1823-x. [DOI] [PubMed] [Google Scholar]
- 49.Liu G., Guasch-Ferre M., Hu Y., et al. Nut consumption in relation to cardiovascular disease incidence and mortality among patients with diabetes mellitus. Circulation Research. 2019;124(6):920–929. doi: 10.1161/CIRCRESAHA.118.314316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guasch-Ferre M., Liu X., Malik V. S., et al. Nut consumption and risk of cardiovascular disease. Journal of the American College of Cardiology. 2017;70(20):2519–2532. doi: 10.1016/j.jacc.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X., Guasch-Ferre M., Drouin-Chartier J.-P., et al. Changes in nut consumption and subsequent cardiovascular disease risk among US men and women: 3 large prospective cohort studies. Journal of the American Heart Association. 2020;9, article e013877 doi: 10.1161/JAHA.119.013877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nouri F., Sarrafzadegan N., Mohammadifard N., Sadeghi M., Mansourian M. Intake of legumes and the risk of cardiovascular disease: frailty modeling of a prospective cohort study in the Iranian middle-aged and older population. European Journal of Clinical Nutrition. 2016;70(2):217–221. doi: 10.1038/ejcn.2015.153. [DOI] [PubMed] [Google Scholar]
- 53.Nsanya M. K., Kavishe B. B., Katende D., et al. Prevalence of high blood pressure and associated factors among adolescents and young people in Tanzania and Uganda. Journal of Clinical Hypertension. 2019;21:470–478. doi: 10.1111/jch.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimani S., Mirie W., Chege M., Okube O. T., Muniu S. Association of lifestyle modification and pharmacological adherence on blood pressure control among patients with hypertension at Kenyatta National Hospital, Kenya: a cross-sectional study. BMJ Open. 2019;9, article e023995 doi: 10.1136/bmjopen-2018-023995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefler D., Malyutina S., Nikitin Y., et al. Fruit, vegetable intake and blood pressure trajectories in older age. Journal of Human Hypertension. 2019;33(9):671–678. doi: 10.1038/s41371-019-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loader T. B., Taylor C. G., Zahradka P., Jones P. J. H. Chlorogenic acid from coffee beans: evaluating the evidence for a blood pressure-regulating health claim. Nutrition Reviews. 2017;75:114–133. doi: 10.1093/nutrit/nuw057. [DOI] [PubMed] [Google Scholar]
- 57.Ettehad D., Emdin C. A., Kiran A., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 58.Weir M. R., Lakkis J. I., Jaar B., et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF-KDOQI controversies report. American Journal of Kidney Diseases. 2018;72(6):873–884. doi: 10.1053/j.ajkd.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 59.De Mello W. C. Local renin angiotensin aldosterone systems and cardiovascular diseases. Medical Clinics of North America. 2017;101(1):117–127. doi: 10.1016/j.mcna.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Horiuchi M. The protective arm of renin angiotensin system: recent research progress and expectation for new therapeutic approach. Japanese Journal of Clinical Medicine. 2016;74:1583–1589. [PubMed] [Google Scholar]
- 61.Chay S. Y., Salleh A., Sulaiman N. F., et al. Blood-pressure lowering efficacy of winged bean seed hydrolysate in spontaneously hypertensive rats, peptide characterization and a toxicity study in Sprague-Dawley rats. Food & Function. 2018;9(3):1657–1671. doi: 10.1039/C7FO01769C. [DOI] [PubMed] [Google Scholar]
- 62.Oluwagunwa O. A., Alashi A. M., Aluko R. E. Solanum macrocarpon leaf extracts reduced blood pressure and heart rate after oral administration to spontaneously hypertensive rats. Current Topics in Nutraceutical Research. 2020;17:282–290. [Google Scholar]
- 63.Gamboa-Gomez C., Perez-Ramirez I. F., Gonzalez-Gallardo A., Gallegos-Corona M. A., Ibarra-Alvarado C., Reynoso-Camacho R. Effect of Citrus paradisi and Ocimum sanctum infusions on blood pressure regulation and its association with renal alterations in obese rats. Journal of Food Biochemistry. 2016;40(3):345–357. doi: 10.1111/jfbc.12216. [DOI] [Google Scholar]
- 64.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circulation Research. 2017;120(4):713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 65.Farah C., Michel L. Y. M., Balligand J. L. Nitric oxide signalling in cardiovascular health and disease. Nature Reviews Cardiology. 2018;15(5):292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 66.Carrizzo A., Ambrosio M., Damato A., et al. Morus albaextract modulates blood pressure homeostasis through eNOS signaling. Molecular Nutrition & Food Research. 2016;60(10):2304–2311. doi: 10.1002/mnfr.201600233. [DOI] [PubMed] [Google Scholar]
- 67.Wang C., Cheng W., Bai S., et al. White mulberry fruit polysaccharides enhance endothelial nitric oxide production to relax arteries in vitro and reduce blood pressure in vivo. Biomedicine & Pharmacotherapy. 2019;116:p. 109022. doi: 10.1016/j.biopha.2019.109022. [DOI] [PubMed] [Google Scholar]
- 68.Pons Z., Margalef M., Bravo F. I., Arola-Arnal A., Muguerza B. Grape seed flavanols decrease blood pressure via Sirt-1 and confer a vasoprotective pattern in rats. Journal of Functional Foods. 2016;24:164–172. doi: 10.1016/j.jff.2016.03.030. [DOI] [Google Scholar]
- 69.El-Tantawy W. H., Temraz A. Natural products for controlling hyperlipidemia: review. Archives of Physiology and Biochemistry. 2018;125:128–135. doi: 10.1080/13813455.2018.1441315. [DOI] [PubMed] [Google Scholar]
- 70.Goldstein S. A., D’Ottavio A., Spears T., et al. Causes of death and cardiovascular comorbidities in adults with congenital heart disease. Journal of the American Heart Association. 2020;9, article e016400 doi: 10.1161/JAHA.119.016400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta M., Blumenthal C., Chatterjee S., et al. Novel emerging therapies in atherosclerosis targeting lipid metabolism. Expert Opinion on Investigational Drugs. 2020;29:611–622. doi: 10.1080/13543784.2020.1764937. [DOI] [PubMed] [Google Scholar]
- 72.Hadi N. S., Lestari D., Farmawati A., Ghozali A., Lestari L. A. The relationship between systolic blood pressure and LDL cholesterol male Sprague Dawley rats given high fat diet and mung bean sprouts (Phaseolus radiatus L.). In: Setyobudi R. H., Burlakovs J., Anne O., Soni P., VincevicaGaile Z., editors. UGM Annual Scientific Conference Life Sciences 2016; October 2016; Yogyakarta, Indonesia. pp. 26–35. [Google Scholar]
- 73.Febriani W., Sulaeman A., Setiawan B. Red dragon fruit flour and exercise improve blood glucose and lipid profile in obese rats. Jurnal Gizi Dan Pangan. 2016;11:175–182. [Google Scholar]
- 74.Hernawati, Setiawan N. A., Shintawatin R., Priyandoko D. The role of red dragon fruit peel (Hylocereus polyrhizus) to improvement blood lipid levels of hyperlipidaemia male mice. In: Aisyah S., Samsudin A., AlJupri Kusumawaty D., et al., editors. 4th International Seminar of Mathematics, Science and Computer Science Education. Bandung, Indonesia: Indonesia Univ Educ, Fac Math & Sci Educ; 2018. p. p. 1013. [Google Scholar]
- 75.Ani P. N., Aginam P. C. Effect of Citrus maxima juice on fasting blood glucose, lipid profile, liver enzyme and body weight. Nutrition & Food Science. 2018;48(5):755–763. doi: 10.1108/NFS-01-2018-0002. [DOI] [Google Scholar]
- 76.Rohajatien U., Harijono H., Estiasih T., Sriwahyuni E. Bitter melon (Momordica charantia L.) fruit decreased blood glucose level and improved lipid profile of streptozotocin induced hyperglycemia rats. Current Research in Nutrition and Food Science Journal. 2018;6(2):359–370. doi: 10.12944/CRNFSJ.6.2.11. [DOI] [Google Scholar]
- 77.Pignatelli P., Menichelli D., Pastori D., Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiologia Polska. 2018;76(4):713–722. doi: 10.5603/KP.a2018.0071. [DOI] [PubMed] [Google Scholar]
- 78.Kattoor A. J., Pothineni N. V. K., Palagiri D., Mehta J. L. Oxidative stress in atherosclerosis. Current Atherosclerosis Reports. 2017;19(11):p. 42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 79.Yang X., Li Y., Li Y., et al. Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Frontiers in Physiology. 2017;8:p. 600. doi: 10.3389/fphys.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H., Zhai C. K. Effects of Chinese and North American wild rice on blood lipids, oxidative stress, and inflammation factors in hyperlipidemic rats. Cereal Chemistry. 2016;93(4):357–363. doi: 10.1094/CCHEM-06-15-0119-R. [DOI] [Google Scholar]
- 81.Ciocoiu M., Badescu M., Badulescu O., Badescu L. The beneficial effects on blood pressure, dyslipidemia and oxidative stress of Sambucus nigra extract associated with renin inhibitors. Pharmaceutical Biology. 2016;54(12):3063–3067. doi: 10.1080/13880209.2016.1207088. [DOI] [PubMed] [Google Scholar]
- 82.Dziadek K., Kopec A., Piatkowska E. Intake of fruit and leaves of sweet cherry beneficially affects lipid metabolism, oxidative stress and inflammation in Wistar rats fed with high fat-cholesterol diet. Journal of Functional Foods. 2019;57:31–39. doi: 10.1016/j.jff.2019.03.044. [DOI] [Google Scholar]
- 83.Senaphan K., Sangartit W., Pakdeechote P., et al. Rice bran protein hydrolysates reduce arterial stiffening, vascular remodeling and oxidative stress in rats fed a high-carbohydrate and high-fat diet. European Journal of Nutrition. 2018;57(1):219–230. doi: 10.1007/s00394-016-1311-0. [DOI] [PubMed] [Google Scholar]
- 84.Feriani A., Tir M., Gómez-Caravaca A. M., et al. HPLC-DAD-ESI-QTOF-MS/MS profiling of Zygophyllum album roots extract and assessment of its cardioprotective effect against deltamethrin-induced myocardial injuries in rat, by suppression of oxidative stress-related inflammation and apoptosis via NF- κβ signaling pathway. Journal of Ethnopharmacology. 2020;247, article 112266 doi: 10.1016/j.jep.2019.112266. [DOI] [PubMed] [Google Scholar]
- 85.Cicero A. F. G., Caliceti C., Fogacci F., et al. Effect of apple polyphenols on vascular oxidative stress and endothelium function: a translational study. Molecular Nutrition & Food Research. 2017;61(11, article 1700373) doi: 10.1002/mnfr.201700373. [DOI] [PubMed] [Google Scholar]
- 86.Koene R. J., Prizment A. E., Blaes A., Konety S. H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews. Cardiology. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li T., Lu X., Sun Y., Yang X. Effects of spinach nitrate on insulin resistance, endothelial dysfunction markers and inflammation in mice with high-fat and high-fructose consumption. Food & Nutrition Research. 2016;60(1):p. 32010. doi: 10.3402/fnr.v60.32010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vutharadhi S., Jolapuram U., Kodidhela L. D. Nutraceutical inherent of _Spinacia oleracea_ Linn. methanolic leaf extract ameliorates isoproterenol induced myocardial necrosis in male albino Wistar rats via mitigating inflammation. Biomedicine & Pharmacotherapy. 2017;85:239–247. doi: 10.1016/j.biopha.2016.10.103. [DOI] [PubMed] [Google Scholar]
- 90.Udomkasemsab A., Ngamlerst C., Adisakwattana P., Aroonnual A., Tungtrongchitr R., Prangthip P. Maoberry (Antidesma bunius) ameliorates oxidative stress and inflammation in cardiac tissues of rats fed a high-fat diet. BMC Complementary and Alternative Medicine. 2018;18(1):p. 344. doi: 10.1186/s12906-018-2400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hills R. D., Jr., Pontefract B. A., Mishcon H. R., Black C. A., Sutton S. C., Theberge C. R. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11:p. 1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? Journal of Atherosclerosis and Thrombosis. 2017;24(7):660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomaz F. S., Altemani F., Panchal S. K., Worrall S., Nitert M. D. The influence of wasabi on the gut microbiota of high-carbohydrate, high-fat diet-induced hypertensive Wistar rats. Journal of Human Hypertension. 2020:1–11. doi: 10.1038/s41371-020-0359-8. In press. [DOI] [PubMed] [Google Scholar]
- 94.Peng Y., Yan Y., Wan P., et al. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Research International. 2020;130, article 108952 doi: 10.1016/j.foodres.2019.108952. [DOI] [PubMed] [Google Scholar]
- 95.Liao Z. L., Zeng B. H., Wang W., et al. Impact of the consumption of tea polyphenols on early atherosclerotic lesion formation and intestinal Bifidobacteria in high-fat-fed ApoE−/− mice. Frontiers in Nutrition. 2016;3:p. 42. doi: 10.3389/fnut.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gomes A., Oudot C., Macià, et al. Berry-enriched diet in salt-sensitive hypertensive rats: metabolic fate of (poly)phenols and the role of gut microbiota. Nutrients. 2019;11(11):p. 2634. doi: 10.3390/nu11112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Azis N. A., Agarwal R., Ismail N. M., et al. Blood pressure lowering effect of Ficus deltoidea var kunstleri in spontaneously hypertensive rats: possible involvement of renin-angiotensin-aldosterone system, endothelial function and anti-oxidant system. Molecular Biology Reports. 2019;46(3):2841–2849. doi: 10.1007/s11033-019-04730-w. [DOI] [PubMed] [Google Scholar]
- 98.Shi W., Yuan R., Chen X., et al. Puerarin reduces blood pressure in spontaneously hypertensive rats by targeting eNOS. The American Journal of Chinese Medicine. 2019;47:19–38. doi: 10.1142/S0192415X19500022. [DOI] [PubMed] [Google Scholar]
- 99.Bello I., Usman N. S., Dewa A., et al. Blood pressure lowering effect and vascular activity of Phyllanthus niruri extract: the role of NO/cGMP signaling pathway and β-adrenoceptor mediated relaxation of isolated aortic rings. Journal of Ethnopharmacology. 2020;250:p. 112461. doi: 10.1016/j.jep.2019.112461. [DOI] [PubMed] [Google Scholar]
- 100.Ding L., Jia C., Zhang Y., et al. Baicalin relaxes vascular smooth muscle and lowers blood pressure in spontaneously hypertensive rats. Biomedicine & Pharmacotherapy. 2019;111:325–330. doi: 10.1016/j.biopha.2018.12.086. [DOI] [PubMed] [Google Scholar]
- 101.Kumari S., Rakavi R., Mangaraj M. Effect of guava in blood glucose and lipid profile in healthy human subjects: a randomized controlled study. Journal of Clinical and Diagnostic Research: JCDR. 2016;10:4–7. doi: 10.7860/JCDR/2016/21291.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alatas H., Sja’bani M., Mustofa M., et al. The effects of soursop supplementation on blood pressure, serum uric acid, and kidney function in a prehypertensive population in accordance with the 2017 ACC/AHA guideline. Journal of Human Hypertension. 2020;34(3):223–232. doi: 10.1038/s41371-019-0235-6. [DOI] [PubMed] [Google Scholar]
- 103.Lima A. C. D., Cecatti C., Fidélix M. P., et al. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: controlled clinical trials. Journal of Medicinal Food. 2019;22(2):202–210. doi: 10.1089/jmf.2018.0080. [DOI] [PubMed] [Google Scholar]
- 104.Bell L., Williams C. M. A pilot dose-response study of the acute effects of haskap berry extract (Lonicera caerulea L.) on cognition, mood, and blood pressure in older adults. European Journal of Nutrition. 2019;58(8):3325–3334. doi: 10.1007/s00394-018-1877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osinska A. N., Begier-Krasinska B., Rzymski P., Krasinska A., Tykarski A., Krasinski Z. The influence of adding tomato extract and acetylsalicylic acid to hypotensive therapy on the daily blood pressure profiles of patients with arterial hypertension and high cardiovascular risk. Kardiochirurgia i torakochirurgia polska= Polish Journal of Cardio-Thoracic Surgery. 2017;14:245–252. doi: 10.5114/kitp.2017.72229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ormesher L., Myers J. E., Chmiel C., et al. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: A randomised, double-blind, placebo- controlled feasibility trial. Nitric Oxide. 2018;80:37–44. doi: 10.1016/j.niox.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Nishimura M., Suzuki M., Takahashi R., et al. Daily ingestion of eggplant powder improves blood pressure and psychological state in stressed individuals: a randomized placebo-controlled study. Nutrients. 2019;11(11):p. 2797. doi: 10.3390/nu11112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fatemeh Y., Siassi F., Rahimi A., et al. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: a randomized controlled trial. Journal of Diabetes & Metabolic Disorders. 2017;16(1):p. 40. doi: 10.1186/s40200-017-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nikaein F., Babajafari S., Mazloomi S. M., Zibaeenezhad M., Zargaran A. The effects of Satureja hortensis L. dried leaves on serum sugar, lipid profiles, hs-CRP, and blood pressure in metabolic syndrome patients: a double-blind randomized clinical trial. Iranian Red Crescent Medical Journal. 2017;19, article e34931 [Google Scholar]
- 110.Mansouri A., Vahed A. S., Shandadi H., Dashtban F., Arbabisarjou A. The effect of garlic and cumin on blood pressure and glycosylated hemoglobin in patients with type 2 diabetes. Bali Medical Journal. 2018;7(1):156–160. doi: 10.15562/bmj.v7i1.849. [DOI] [Google Scholar]
- 111.Lorenzon dos Santos J., Schaan de Quadros A., Weschenfelder C., Bueno Garofallo S., Marcadenti A. Oxidative stress biomarkers, nut-related antioxidants, and cardiovascular disease. Nutrients. 2020;12(3):p. 682. doi: 10.3390/nu12030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alasalvar C., Bolling B. W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. The British Journal of Nutrition. 2015;113:68–78. doi: 10.1017/S0007114514003729. [DOI] [PubMed] [Google Scholar]
- 113.Stevens-Barron J. C., de la Rosa L. A., Wall-Medrano A., et al. Chemical composition and in vitro bioaccessibility of antioxidant phytochemicals from selected edible nuts. Nutrients. 2019;11(10):p. 2303. doi: 10.3390/nu11102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhillon J., Tan S.-Y., Mattes R. D. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. The Journal of Nutrition. 2016;146(12):2513–2519. doi: 10.3945/jn.116.238444. [DOI] [PubMed] [Google Scholar]
- 115.Rock C. L., Flatt S. W., Barkai H.-S., Pakiz B., Heath D. D. Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutrition Journal. 2017;16(1):p. 76. doi: 10.1186/s12937-017-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohan V., Gayathri R., Jaacks L. M., et al. Cashew nut consumption increases HDL cholesterol and reduces systolic blood pressure in Asian Indians with type 2 diabetes: a 12-week randomized controlled trial. The Journal of Nutrition. 2018;148(1):63–69. doi: 10.1093/jn/nxx001. [DOI] [PubMed] [Google Scholar]
- 117.Abbaspour N., Roberts T., Hooshmand S., Kern M., Hong M. Y. Mixed nut consumption may improve cardiovascular disease risk factors in overweight and obese adults. Nutrients. 2019;11:p. 1488. doi: 10.3390/nu11071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Samavat H., Newman A. R., Wang R., Yuan J.-M., Wu A. H., Kurzer M. S. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo-controlled clinical trial. The American Journal of Clinical Nutrition. 2016;104(6):1671–1682. doi: 10.3945/ajcn.116.137075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orem A., Alasalvar C., Vanizor Kural B., et al. Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. Journal of Functional Foods. 2017;31:311–319. doi: 10.1016/j.jff.2017.01.048. [DOI] [Google Scholar]
- 120.Katanasaka Y., Miyazaki Y., Sunagawa Y., et al. Kosen-cha, a polymerized catechin-rich green tea, as a potential functional beverage for the reduction of body weight and cardiovascular risk factors: a pilot study in obese patients. Biological & Pharmaceutical Bulletin. 2020;43(4):675–681. doi: 10.1248/bpb.b19-00921. [DOI] [PubMed] [Google Scholar]
- 121.Giglio R. V., Patti A. M., Cicero A. F. G., et al. Polyphenols: potential use in the prevention and treatment of cardiovascular diseases. Current Pharmaceutical Design. 2018;24(2):239–258. doi: 10.2174/1381612824666180130112652. [DOI] [PubMed] [Google Scholar]
- 122.Cicero A. F. G., Colletti A. Effects of carotenoids on health: are all the same? Results from clinical trials. Current Pharmaceutical Design. 2017;23(17):2422–2427. doi: 10.2174/1381612823666170207095459. [DOI] [PubMed] [Google Scholar]
- 123.Jalalyazdi M., Ramezani J., Izadi-Moud A., Madani-Sani F., Shahlaei S., Ghiasi S. S. Effect of Hibiscus sabdariffa on blood pressure in patients with stage 1 hypertension. Journal of Advanced Pharmaceutical Technology & Research. 2019;10(3):107–111. doi: 10.4103/japtr.JAPTR_402_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thirunavukkarasu D., Kirubamani N. H., Naidu M. B. The effect of soy flour intake on systemic blood pressure and glycemic control in post-menopausal women with pre-diabetes and prehypertension. Indian Journal of Pharmaceutical Education and Research. 2017;51:349–354. [Google Scholar]
- 125.Liao M.-Y., Shen Y.-C., Chiu H.-F., et al. Down-regulation of partial substitution for staple food by oat noodles on blood lipid levels: a randomized, double-blind, clinical trial. Journal of Food and Drug Analysis. 2019;27(1):93–100. doi: 10.1016/j.jfda.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kent K., Charlton K. E., Jenner A., Roodenrys S. Acute reduction in blood pressure following consumption of anthocyanin-rich cherry juice may be dose-interval dependant: a pilot cross-over study. International Journal of Food Sciences and Nutrition. 2015;67:47–52. doi: 10.3109/09637486.2015.1121472. [DOI] [PubMed] [Google Scholar]
- 127.Stockton A., Farhat G., McDougall G. J., Al-Dujaili E. A. S. Effect of pomegranate extract on blood pressure and anthropometry in adults: a double-blind placebo-controlled randomised clinical trial. Journal of Nutritional Science. 2017;6, article e39 doi: 10.1017/jns.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Igwe E. O., Charlton K. E., Roodenrys S., Kent K., Fanning K., Netzel M. E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: a pilot crossover dose-timing study. Nutrition Research. 2017;47:28–43. doi: 10.1016/j.nutres.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 129.Nowak D., Goslinski M., Wesolowska A., Berenda K., Poplawski C. Effects of acute consumption of noni and chokeberry juices vs. energy drinks on blood pressure, heart rate, and blood glucose in young adults. Evidence-Based Complementary and Alternative Medicine. 2019;2019:9. doi: 10.1155/2019/6076751.6076751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kemmner S., Lorenz G., Wobst J., et al. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: a pilot study. Nitric Oxide. 2017;64:7–15. doi: 10.1016/j.niox.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 131.Kerley C. P., Dolan E., James P. E., Cormican L. Dietary nitrate lowers ambulatory blood pressure in treated, uncontrolled hypertension: a 7-d, double-blind, randomised, placebo-controlled, cross-over trial. The British Journal of Nutrition. 2018;119(6):658–663. doi: 10.1017/S0007114518000144. [DOI] [PubMed] [Google Scholar]
- 132.Lainata E. N., Mapanawang A. L., Husen S. H. Effect of lilin vegetable (Setaria palmifolia) against blood pressure decrease in pregnant women in Gosoma village Tobelo sub-district North Halmahera. International Journal Of Health Medicine and Current Research. 2017;2:540–544. [Google Scholar]
- 133.Jonvik K. L., Nyakayiru J., Pinckaers P. J. M., Senden J. M. G., van Loon L. J. C., Verdijk L. B. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. The Journal of Nutrition. 2016;146(5):986–993. doi: 10.3945/jn.116.229807. [DOI] [PubMed] [Google Scholar]
- 134.Ndanuko R. N., Tapsell L. C., Charlton K. E., Neale E. P., Batterham M. J. Effect of individualised dietary advice for weight loss supplemented with walnuts on blood pressure: the HealthTrack study. European Journal of Clinical Nutrition. 2018;72(6):894–903. doi: 10.1038/s41430-018-0123-0. [DOI] [PubMed] [Google Scholar]
- 135.Tindall A. M., McLimans C. J., Petersen K. S., Kris-Etherton P. M., Lamendella R. Walnuts and vegetable oils containing oleic acid differentially affect the gut microbiota and associations with cardiovascular risk factors: follow-up of a randomized, controlled, feeding trial in adults at risk for cardiovascular disease. The Journal of Nutrition. 2020;150(4):806–817. doi: 10.1093/jn/nxz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baer D. J., Novotny J. A. Consumption of cashew nuts does not influence blood lipids or other markers of cardiovascular disease in humans: a randomized controlled trial. The American Journal of Clinical Nutrition. 2019;109(2):269–275. doi: 10.1093/ajcn/nqy242. [DOI] [PubMed] [Google Scholar]
- 137.Ishida N., Iizuka M., Kataoka K., et al. Improvement of blood lipid profiles by Goishi tea polyphenols in a randomised, double-blind, placebo-controlled clinical study. International Journal of Food Sciences and Nutrition. 2018;69(5):598–607. doi: 10.1080/09637486.2017.1386629. [DOI] [PubMed] [Google Scholar]
- 138.Abubakar S. M., Ukeyima M. T., Spencer J. P. E., Lovegrove J. A. Acute effects of Hibiscus sabdariffa calyces on postprandial blood pressure, vascular function, blood lipids, biomarkers of insulin resistance and inflammation in humans. Nutrients. 2019;11(2):p. 341. doi: 10.3390/nu11020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaseb F., Yazdanpanah Z., Biregani A. N., Yazdi N. B., Yazdanpanah Z. The effect of chamomile (Matricaria recutita L.) infusion on blood glucose, lipid profile and kidney function in Type 2 diabetic patients: a randomized clinical trial. Progress in Food & Nutrition Science. 2018;20:110–118. [Google Scholar]
- 140.Li K. J., Borresen E. C., Jenkins-Puccetti N., Luckasen G., Ryan E. P. Navy bean and rice bran intake alters the plasma metabolome of children at risk for cardiovascular disease. Frontiers in Nutrition. 2018;4:p. 71. doi: 10.3389/fnut.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Roshan H., Nikpayam O., Sedaghat M., Sohrab G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: a randomised clinical trial. The British Journal of Nutrition. 2018;119(3):250–258. doi: 10.1017/S0007114517003439. [DOI] [PubMed] [Google Scholar]


