Abstract
Background
A population-level survey (PLS) is an essential and standard method used in public health research that supports the quantification of sociodemographic events, public health policy development, and intervention designs. Data collection mechanisms in PLS seem to be a significant determinant in avoiding mistakes. Using electronic devices such as smartphones and tablet computers improves the quality and cost-effectiveness of public health surveys. However, there is a lack of systematic evidence to show the potential impact of electronic data collection tools on data quality and cost reduction in interviewer-administered surveys compared with the standard paper-based data collection system.
Objective
This systematic review aims to evaluate the impact of the interviewer-administered electronic data collection methods on data quality and cost reduction in PLS compared with traditional methods.
Methods
We conducted a systematic search of MEDLINE, CINAHL, PsycINFO, the Web of Science, EconLit, Cochrane CENTRAL, and CDSR to identify relevant studies from 2008 to 2018. We included randomized and nonrandomized studies that examined data quality and cost reduction outcomes, as well as usability, user experience, and usage parameters. In total, 2 independent authors screened the title and abstract, and extracted data from selected papers. A third author mediated any disagreements. The review authors used EndNote for deduplication and Rayyan for screening.
Results
Our search produced 3817 papers. After deduplication, we screened 2533 papers, and 14 fulfilled the inclusion criteria. None of the studies were randomized controlled trials; most had a quasi-experimental design, for example, comparative experimental evaluation studies nested on other ongoing cross-sectional surveys. A total of 4 comparative evaluations, 2 pre-post intervention comparative evaluations, 2 retrospective comparative evaluations, and 4 one-arm noncomparative studies were included. Meta-analysis was not possible because of the heterogeneity in study designs, types, study settings, and level of outcome measurements. Individual paper synthesis showed that electronic data collection systems provided good quality data and delivered faster compared with paper-based data collection systems. Only 2 studies linked cost and data quality outcomes to describe the cost-effectiveness of electronic data collection systems. Field data collectors reported that an electronic data collection system was a feasible, acceptable, and preferable tool for their work. Onsite data error prevention, fast data submission, and easy-to-handle devices were the comparative advantages offered by electronic data collection systems. Challenges during implementation included technical difficulties, accidental data loss, device theft, security concerns, power surges, and internet connection problems.
Conclusions
Although evidence exists of the comparative advantages of electronic data collection compared with paper-based methods, the included studies were not methodologically rigorous enough to combine. More rigorous studies are needed to compare paper and electronic data collection systems in public health surveys considering data quality, work efficiency, and cost reduction.
International Registered Report Identifier (IRRID)
RR2-10.2196/10678
Keywords: electronic data collection, demographic and health survey, tablet computer, smartphone, mobile phone
Introduction
Until well-established civil and vital statistics systems are in place in low- and middle-income countries (LMIC), monitoring sociodemographic events using data on vital societal statistics will remain dependent on alternative data sources. Public health surveys—such as censuses, demographic and health surveys (DHS), and health and demographic surveillance—serve as a data lifeline for these countries [1,2]. Mortality and morbidity indicators, service utilization, and population-level program impact evaluations are usually calculated from household-level data. Further analysis of these population-level epidemiologic indicators is helpful in identifying the determinants of mortality and morbidity. Data collection and management is the first step in the process of evidence generation from household surveys, in which data quality errors could be introduced or prevented. Avoiding errors at this stage is the first-line choice to avoid inherited errors in further data management processes [1,3,4].
The current classical data collection and management processes in LMIC are heavily dependent on paper-based manual methods [4,5]. Paper-based data collection requires extensive human and material resources, especially for large-scale surveys. It also incurs high printing and data entry costs and requires extra data quality assurance steps during and after data collection. Moreover, it takes a long time for an error-free data set to be ready for analysis [6,7]. The intrinsic mode of paper-based data collection affects the data quality, timeliness, and cost of survey implementation, among other factors [8-10].
The rapid development of the global telecommunications infrastructure provides an opportunity for mobile and wireless technologies (mobile health [mHealth]) to support health services and research. Harnessing this technology’s potential, particularly in LMIC where the disease burden is highest, is becoming a popular strategy led by relevant activities in World Health Organization member countries [11]. There are diverse mHealth solutions broadly categorized as a tool to support communication between health service institutions and individuals. These include health call centers; reminders to attend appointments; providing access to information and education for health care professionals, for example, access to electronic health care databases and clinical decision support systems; and supporting health monitoring and surveillance (eg, data collection and reporting in health surveys, surveillance, and patient monitoring) [12].
The implementation of tablet- or smartphone-based data collection tools is becoming increasingly popular in public health surveys to mitigate challenges encountered in paper-based data collection [13,14]. Compared with face-to-face interviews, a self-administering mode of electronic data collection tools could potentially increase the response rate among stigmatized groups. These tools have been tested in the contexts of drug abuse [15] and sexual health or HIV [16-18] in public health. The findings conclude that respondents prefer electronic data collection tools as a solution for reporting sensitive information.
Considering data collection in clinical trials, electronic clinical report forms (eCRF) show a potential advantage over paper-based clinical case report forms (CRF) [19-22]. Studies have identified the relative advantages of electronic data capturing tools in terms of data quality, timeliness, and implementation cost.
A handful of experience reports are available on the use of electronic data collection methods in health and demographic surveillance systems (HDSS) in the International Network for the Demographic Evaluation of Populations and Their Health (INDEPTH) network. The HDSS site in Malawi used an OpenHDS data system as a means of GPS data collection [23]. One surveillance site in Kenya also reported the adoption of technological innovation using OpenHDS to manage a large-scale malaria survey in western Kenya. The findings asserted that electronic data collection (EDC) enabled the collection of demographic and malaria data quickly and effectively. Moreover, the possibility of real-time data quality controls using the system led to an efficient workflow and subsequent cost savings [24]. The Kombewa HDSS in Kenya also collected data electronically using PDAs and computer notebooks [25,26]. Since 2010, the Magu HDSS site in Tanzania has used EDC to enable enumerators to record household information directly in the PDA [27]. The Dabat HDSS site in northwest Ethiopia also reported the use of PDAs as a means of data collection [6,28]. Most HDSS and DHS still use a paper-based data collection system, and those sites with EDC implementation experience have rarely published their experience or the comparative impact of EDC and paper-based data collections. Despite the individual implementation experiences that suggest that EDC tools can improve data quality and work efficiency and reduce overall survey costs, systematic reviews of the available evidence are limited. The focus of the available systematic reviews is primarily on the mixed potential of mHealth, not specifically on the impact of mobile devices on improving the data collection and management processes in surveys [13,29,30]. Therefore, the impacts of EDC tools in surveys need to be separately analyzed and reported. The available Cochrane systematic review on the impact of data quality parameters focuses on self-administered EDC tools and excludes interviewer-administered methods [14]. In the case of face-to-face interviews, the data collection process involves interaction between the questionnaire, respondent, and interviewer. The difference in the mode of questionnaire administration can have serious effects on data quality [9]. Moreover, conducting face-to-face surveys has more organizational costs involved than self-administered surveys.
Therefore, a systematic review that considers interviewer-administered data collection may complement this evidence. We found no systematic review that analyzed the data quality and cost-effectiveness of electronic and paper-based interview-administered public health surveys. The objective of this systematic review is to synthesize the evidence on the effect of using EDC systems on data quality and cost reduction in public health surveys, with a focus on studies reporting comparative impacts of paper-based data collection and EDC.
Methods
We registered a detailed protocol with PROSPERO, an international database of prospectively registered systematic reviews, with the registration number CRD42018092259. PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines were used to report our systematic review [31,32]. The protocol of this study has been published [33].
Inclusion Criteria
We assessed studies that investigated the effect of EDC methods on improving the data quality and cost-effectiveness in public health surveys or surveillance, compared with traditional paper-based data collection methods. We included all mobile apps with technologies that directly support the data collection process by enabling data collectors and interviewers to collect and send data as well as enabling supervisors and data managers to monitor the data collection process. The study participants included in our review are defined as data collection tool users who use a method of data collection.
Studies with the following characteristics were included:
The study compared either data quality or cost-effectiveness or both as primary outcomes and reported these in the paper.
The intervention consisted of mobile information and communication technology devices along with mobile apps, which include PDAs, cellphones, smartphones, and tablet computers—devices used specifically for data collection and reporting processes during surveys.
The control and intervention groups were compared in face-to-face interview-administered surveys conducted at the household level.
Demographic surveillance sites were based on clinical settings and not mandated for standard clinical trials (eg, CRF vs eCRF).
The paper was published between January 2008 and December 2018.
Search Information Source and Search Strategies
Studies were identified through systematic searching in the following electronic databases: MEDLINE via Ovid, CINAHL via EBSCO, PsycINFO via Ovid, EconLit via EBSCO, the Social Science Citation Index, the Science Citation Index via the Web of Science and CENTRAL, and the Cochrane Library (Table 1). In addition, the reference lists of all the included citations were screened. We also searched clinical trial registries for unpublished and in-progress studies.
Table 1.
Subject term translations for individual databases
| MEDLINE and Cochrane | PsycINFO | CINAHL |
| Mobile applications | Not available | Mobile applications |
| Computers, handheld | Mobile devices, computer peripheral devices | Computers, handheld |
| Electronic health records | Not available | Electronic health records |
| Cell phone | Cellular phones | Cell phone |
| Surveys and questionnaires | Surveys; questionnaires | Data collection methods |
| Interviews as topic | Interviews | Included in data collection methods |
| Costs and cost analysis | Costs and cost analysis | Costs and cost analysis |
| Data accuracy | Not available | Not available |
The search strategy reported in the protocol was refined and updated in collaboration with a research librarian. This strategy considered 3 categories: the technology or intervention used (eg, mobile device, mobile phone, mHealth, or EDC), area of application (eg, data collection, demographic and health survey, or large-scale survey), and the outcome of interest (eg, data quality, missing data, and cost-effectiveness). We linked synonyms and controlled vocabulary with Boolean operators OR and the categories with the operator AND. Textbox 1 presents the search strategy in MEDLINE via Ovid. Appropriate modifications to control for vocabulary and syntax were made to the search strategy for each database (Textbox 1). Additional search strategies for PsycINFO, CINAHL, Web of Science, and Cochrane databases are presented in the supplementary file (Multimedia Appendix 1). All searches were conducted in January 2019.
Search strategy in MEDLINE via Ovid.
MEDLINE(R) and Epub Ahead of Print, via Ovid
1. (((tablet or handheld or hand held or electronic) adj2 (device* or computer*)) or ((electronic or digital) adj2 (form? or data capture* or survey* or case report form? or data collection?)) or Open Data Kit or ODK or EDC or eCRF or eHealth or mHealth or digital health or Android or tablet? or PDA? or personal digital assistant? or app? or (mobile adj2 (technolog* or application? or app?)) or ((mobile or cell* or smart) adj2 phone*) or smartphone* or cellphone*).ti,ab.
2. exp “mobile applications”/
3. exp ”computers, handheld”/
4. exp “electronic health records”/
5. exp “cell phone”/
6. or/2-5
7. 1 or 6
8. (field work or fieldwork or HDSS or CAPI or computer assisted personal interviewing or questionnaire* or survey* or interview* or (population adj2 surveillance) or DHS or EDC or (data adj2 (gather* or captur*)) or (health and demographic surveillance system?)).ti,ab.
9. exp “surveys and questionnaires”/
10. exp “interviews as topic”/
11. or/9-10
12. 8 or 11
13. ((cost? adj2 (analy?s or comparison* or saving? or measure? or effectiv* or reduction? or reduce? or reduction or reducing or decrease? or decreasing)) or (cost benefit adj2 (analy?s or comparison* or measure?)) or (cost utility adj2 (analy?s or comparison* or measure?)) or economic evaluation? or quality control? or (data adj2 (quality or accuracy or accurate* or error? or error rate? or incomplete* or complete* or inaccurate* or inaccuracy or valid*))).ti,ab.
14. exp “costs and cost analysis”/
15. exp “data accuracy”/
16. or/14-15
17. 13 or 16
18. 7 and 12 and 17
19. Limit 18 to yr=“2008 -Current”
Study Selection
We imported all citations from all databases to EndNote for deduplication management and further screening. Although we planned to use the Covidence web-based screening tool to manage the title and abstract screening process, we finally chose the Rayyan QCRI (Qatar Computing Research Institute) screening tool because it is freely available and provides sufficient screening functionalities. Two authors (AZ, MPH in Health Informatics, and TN, MSc in Informatics) independently screened the titles, abstracts, and full text, based on the inclusion criteria. Disagreements and uncertainty on the screening results were first resolved through discussion among the reviewers, followed by consultation with the third (FF, Postdoc in Medical Informatics) and fifth authors (RR, Professor in Medical Informatics). We used a pretested and standardized (through calibration exercise) Microsoft Excel sheet for data extraction based on the inclusion criteria and the objectives of the review.
Data Management and Extraction Process
Two reviewers extracted the following information from the papers:
Bibliographic information (authors, titles, journals, and year of publication).
Characteristics of the intervention (eg, hardware, software, and networking).
Study methods (design, setting, participants, and sample size).
Assessed outcomes (data quality, cost-effectiveness, and others).
Quantitative or qualitative summary of the main findings, including descriptive frequencies and statistical tests.
The full description of the data extraction items can be accessed in the published protocol [33].
Risk of Bias or Quality Assessment
Randomized controlled trials are suitable for evaluating whether drugs are effective; however, for interventions that involve health care delivery modes, it may not be appropriate or possible to conduct a randomized controlled trial. We aimed to assess the quality of the data in the included studies using parameters such as random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. However, the included studies were neither randomized controlled trials nor nonrandomized trials with clinical outcomes; they were mainly prospective comparative experimental studies, cross-sectional studies, or historical secondary data record comparisons. The remaining studies were a one-time feasibility study or experience reports from the implementation or use of an EDC tool in public health practice.
Data Synthesis
There was substantial heterogeneity among the studies concerning the intervention (mobile electronic data capturing tools such as PDA, smartphone, or tablet computer and the app they used), outcome types (error rate and missing or inaccurate data), and level of outcome measurements (sample level, household level, and variable level) of the mHealth interventions and study outcomes.
The studies were found to be noncombinable, and combining these studies would not have been methodologically sound. Consequently, we performed a narrative synthesis of the studies.
Results
Study Selection and Characteristics
The search performed in the included databases yielded 3817 results. After deduplication, 2533 results were exported to the Rayyan QCRI screening tool (Figure 1). Of these, 2500 papers were discarded after title and abstract screening, as these papers clearly did not meet our criteria. Of the 33 full-text papers included, only 14 met the amended inclusion criteria. The original protocol was aimed at including comparative studies that addressed paper-based and electronic tools in the same study, conducted household-level data collection in a community field setting, and reported primary outcomes (data quality or cost-effectiveness) of both data collection tools in the same paper. Only 7 studies (that are heterogeneous) fulfilled these criteria. Due to the limited evidence, we extended our inclusion criteria to cover studies that use the tools in demographic surveys or surveillance systems in a clinical or hospital setting. We also included one-sided study design papers that only reported primary outcomes (cost or data quality) and EDC methods, without formal comparison with paper-based data collection methods. This widening of the scope provided an additional 7 papers (3 comparative and 4 noncomparative EDC papers) to bring the total to 14: 10 comparative and 4 noncomparative single-arm studies were included for final full-text extraction (Figure 1).
Figure 1.
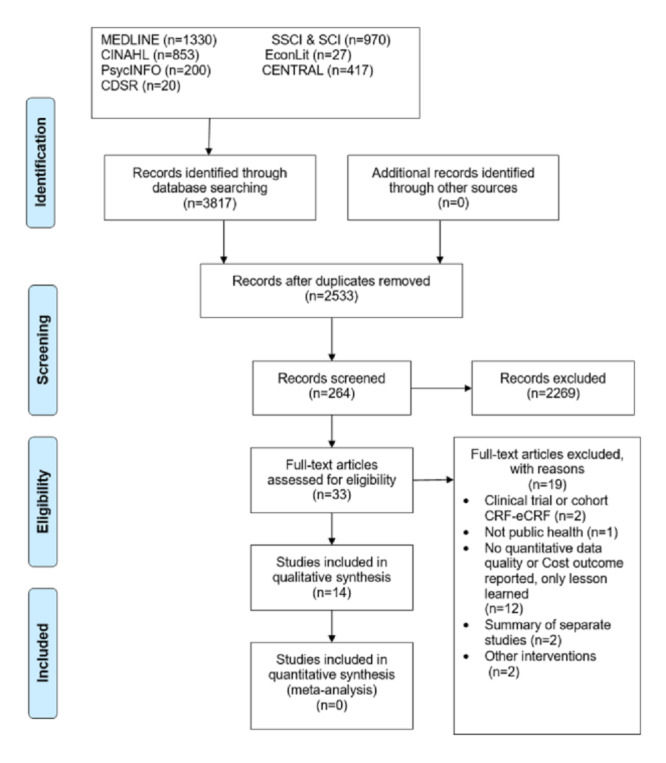
Screening process based on the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) flowchart template.
Study Characteristics
The final systematic synthesis analysis included 14 studies conducted in 12 LMIC. Of these 14, 10 [7,34-42] comparatively assessed the outcomes using paper-based data collection and EDC in the same analysis. Furthermore, 4 papers [43-46] reported either data quality or cost-related outcomes or both in a study conducted using an EDC tool (Table 2).
Table 2.
Study and content characteristics of the included papers
| Category | Studies, n | References | |
| Country (n=14) | |||
|
|
Kenya | 2 | [36,45] |
|
|
Ethiopia | 1 | [7] |
|
|
China | 2 | [42,43] |
|
|
Malawi | 1 | [39] |
|
|
India | 1 | [37] |
|
|
Philippines/Bangladesh | 1 | [34] |
|
|
Sudan | 1 | [35] |
|
|
Burkina Faso | 2 | [40,44] |
|
|
Tanzania | 1 | [38] |
|
|
South Africa | 1 | [41] |
|
|
Nigeria | 1 | [46] |
| Study setting (n=10) | |||
|
|
Household community setting | 7 | [7,34,35,37-40] |
|
|
Clinical/hospital setting | 3 | [36,41,42] |
| Comparison of paper-based and electronic data collection (n=10) | |||
|
|
Both from the same study | 8 | [34,35,37-42] |
|
|
Compared from 2 studies conducted at different times | 2 | [7,36] |
| Purpose of the study design (n=7) | |||
|
|
Primarily designed to evaluate paper-based and electronic tools | 5 | [35,37,39,41,42] |
|
|
Secondary byproduct of another primary survey | 2 | [7,36] |
| Types of outcomes (n=10) | |||
|
|
Only data quality outcomes | 1 | [35] |
|
|
Only cost outcomes | 2 | [34,40] |
|
|
Both cost and data quality outcomes | 7 | [7,36-39,41,42] |
| Level of data quality outcome assessment (n=8) | |||
|
|
Household level | 2 | [7,38] |
|
|
Questionnaire level | 4 | [7,35,41,42] |
|
|
Variable level | 3 | [36,37,39] |
| Type of data quality outcome comparison (n=8) | |||
|
|
Missing | 4 | [7,36,38,39] |
|
|
Inaccurate | 4 | [7,36,38,39] |
|
|
Mixed (identified as error) | 5 | [35,37,38,41,42] |
| Economic evaluation type (n=11) | |||
|
|
Complete input cost | 3 | [36,38,40] |
|
|
Partial (differential) cost | 8 | [7,37,39,41-43,45,46] |
| Usability/user preference evaluation (n=14) | |||
|
|
Reported after formal evaluation | 6 | [7,34,37-39,42] |
|
|
Reported with informal discussion | 1 | [36] |
|
|
No user evaluation information | 7 | [35,40,41,43-46] |
| Study, intervention, or evaluation year (n=10) | |||
|
|
2008-2012 | 5 | [36,38,41,42,44] |
|
|
2013-2018 | 5 | [34,35,37,43,46] |
| Publication year (n=14) | |||
|
|
2008-2012 | 2 | [42,46] |
|
|
2013-2018 | 12 | [7,34-41,43,45,46] |
Furthermore, 5 studies [35,37,39,41,42] were primarily intended to evaluate and compare data quality and cost-related outcomes from a prospective study design using paper-based and electronic tools. The remaining 5 papers [7,34,36,38,40] reported the outcomes from previous technology utilization experiences. The reported outcomes were not primarily intended to evaluate the tools; rather, data quality and cost-related outcomes were extracted from surveys at different times.
Regarding the settings, 7 studies [7,34,35,37-40] in the comparative group included data from a household-level survey, and 3 studies [36,41,42] conducted surveys in clinical settings or research centers.
Types of Outcomes
Data Quality Outcomes
Data quality outcomes, as defined in the methods section, comprise the frequency of errors (incomplete, missing, or inaccurate items) on 3 levels, based on the reported outcomes at the household, questionnaire, and variable levels. At the household level, the incidence of 1 or more errors among the total number of households included in the surveys and data analysis were reported in 2 papers [7,38]. Similarly, the frequency of 1 or more errors per complete questionnaire, regarded as a questionnaire-level error, was reported in 4 studies [7,35,41,42]. At a variable level, a count of the errors in a complete set of questions or variables in questionnaires measured as a variable error were mentioned in 3 papers (Figure 2) [36,37,39].
Figure 2.
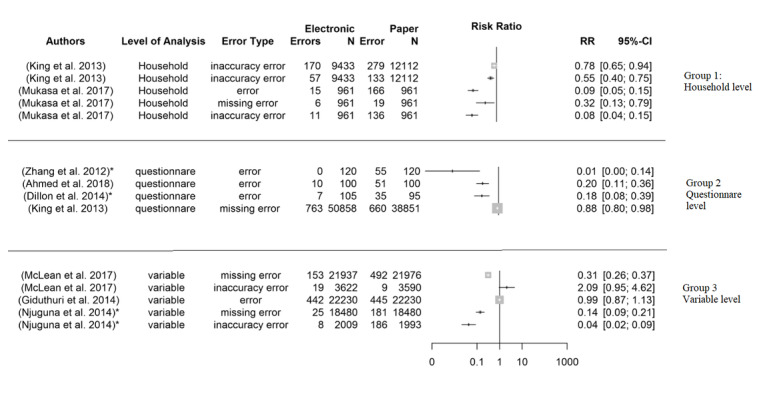
Forest plot comparison of heterogeneity characteristics of the data quality outcomes.
The cost of implementing electronic and paper-based data collection processes was estimated by most studies (12 out of 14). The majority of these estimated the partial or differential costs unique to that study and its EDC [7,37,39,41-43,45,46]. Only 3 papers listed the full implementation cost [36,38,40] for both the study and the EDC. Except 2 studies that compared costs per correctly entered data observation or error-free databases [39,41], none attempted to link the data quality outcome measures to the cost inputs.
Effect on Data Quality: Missing Data and Inaccuracy Errors
Errors are reported in terms of missing and inaccurate data in 4 studies [7,36,38,39], while a combination of both errors as a single error indicator is reported in 4 others [35,37,38,42] (Table 3).
Table 3.
Extracted data quality outcomes.
| Study and country | Type of comparison, study design, and settinga | Study population and sample unit | Intervention: device or app | Methods of paper-based and electronic tool administration | Outcome measurement characteristicsb | Result | Biases (selection, information, or confounding) |
| Ahmed et al [35], Sudan | B: Exploratory pilot study nested and experimented in cross-sectional household surveys |
|
EDC: Smartphone ODKe app | Daily sample randomization for EDC or PPDC. 1 respondent simultaneously interviewed by 2 interviewers with different tools (PPDC and EDC) |
|
|
Selection and information |
| King et al [7], Ethiopia | A: Pre- and postdesign in full EDC implementation evaluation in cross-sectional household surveys | Sample units:
|
EDC: For example, tablet computer or self-developed Android app | Paper and electronic surveys at different places and times, 1 data collector at a time |
|
|
Selection, information, and confounding |
| McLean et al [39], Malawi | A: Prospective evaluation nested and experimented in cross-sectional household surveys at HDSSg sites |
|
EDC: Tablet computer and smartphone, ODK app | Independent parallel EDC or PPDC, 1 data collector at a time |
|
|
Selection and information |
| Giduthuri et al [37], India | A: Prospective experimental comparative study designed for households selected randomly |
|
EDC: Tablet computer/ODK app | A respondent simultaneously interviewed by 2 interviewers with different tools (PPDC and EDC) |
|
|
Selection, information, and confounding |
| Mukasa et al [38], Tanzania | A: Retrospective record review of household survey data |
|
EDC or PDA BlackBerry customized HRSi | Repeated survey, PPDC first, followed by EDC, 1 data collector at a time |
|
|
Selection, information, and confounding |
| Zhang et al [42], China | B: Prospective comparison study conducted in a clinic |
|
EDC: smartphones, leased software | A respondent simultaneously interviewed by 2 interviewers with same tools (PPDC or EDC). Random assignation of respondents to one of the tools |
|
|
Selection, information, and confounding |
| Njuguna et al [36], Kenya | A: Pre- and postimplementation evaluation in hospital-based surveillance data collection |
|
EDC: smartphones, FASTj app | Paper and electronic surveys at the same place but different times, 1 data collector at a time |
|
|
Selection, information, and confounding |
| Dillon et al [14], South Africa | B: Prospective data collection in case control study in a hospital setting |
|
EDC: tablet computer, self-developed app | One data collector interviewed all respondents either with paper or with electronic tools in a random order |
|
|
Selection, information, and confounding |
aA: full implementation; B: pilot testing.
bA: type of outcome; B: stage of error assessment for paper questionnaire; C: error measurement level; D: questionnaire name/s similarities.
cPPDC: pen and paper data collection.
dEDC: electronic data collection.
eODK: Open Data Kit.
fBOLD: Burden of Obstructive Lung Disease.
gHDSS: health and demographic surveillance systems.
hRR: risk ratio.
iHRS: Household Registration System.
jFAST: Field Adapted Survey Toolkit.
An exploratory pilot study nested and experimented in a cross-sectional household survey in Sudan compared data quality errors, questions with no answers, or incorrect use of the skip pattern in 100 convenience samples. A pair of data collectors simultaneously interviewed each respondent—1 with pen and paper and 1 with electronic tools—and recorded the data separately. In the paper-based data collection, 51 of the 100 questions had one or more errors, compared with 10 errors in the electronically submitted forms [35].
A study in India by Giduthuri et al [37] also compared error rates between paper-based and electronically collected data from a comparative prospective experimental study. The data collectors were randomly assigned to use either pen and paper or EDC tools while interviewing each respondent simultaneously. Audio-recorded data during the survey were used as a reference to compare discrepancies and device-attributable errors. According to the reference, paper errors indicate when a paper entry was incorrect and when a tablet entry was missing because the paper-based tool interviewer (lead) did not follow the skip logic. Furthermore, tablet entries were incorrect or missing and paper entries were missing because of the electronic tool interviewer’s (lead) skip logic was considered an error of the electronic data collection tool. The mean number of paper-attributable errors was 4.68 (445/22,230, 2.01%), while the mean number of tablet-attributable errors was 4.65 (442/22,230, 1.99%); thus, no significant differences were observed [37].
A study in China compared smartphone and paper-based data collection in an infant feeding practice survey conducted in rural clinics. Purposive sampling techniques were used to select 120 mothers, 60 per survey tool group. Two data collectors with the same tool (paper or electronic) interviewed 60 mothers in random order, yielding 120 records for each tool. For the paper-based questionnaire, 55 of 120 questionnaires had 1 or more errors or missing data. The most frequent error was a missing confirmation of the default option, which was observed 156 times in 49 questionnaires. No missing error was reported for the EDC tool group [42]. The mean duration of an interview was 10.22 (SD 2.17) min for the smartphone method and 10.83 (SD 2.94) min for the pen and paper method. Moreover, database completion took 16 hours (including data entry, checking, and data cleaning) for pen and paper data collection (PPDC), while it took half an hour for EDC [42].
A prospective evaluation experiment conducted a nested, ongoing, cross-sectional household survey at HDSS sites in Malawi. In 3 weeks, 426 interviews with PPDC and 558 interviews with EDC were conducted. Data collectors independently interviewed different households in a 1 data collector in 1 home mode. Missing data were defined as not asked (blank; discounting not applicable blank questions) or as blank and entered as unknown combined. Internal validity was defined as a field with an impossible or inconsistent value and time for submission. In paper questionnaires, missing data were reported in 492 (2.2%) of 21,976 fields, compared with 153 (0.7%) of 21,937 fields in electronic forms (risk ratio [RR] 3.2, 95% CI 2.7-3.8). Internal inconsistencies were found in 19 (0.5%) of 3590 fields collected by PPDC compared to 9 (0.2%) of 3622 fields for EDC (RR 0.5, 95% CI 0.2-1.1) [39]. Moreover, the mean data availability duration in databases was 3.4 days (95% CI 3.0-3.7) in PPDC compared with 2.1 days (95% CI 2.0-2.3) for EDC. The mean number of interviews per day was similar for both groups at 10.7 (95% CI 8.7-12.6) for PPDC and 11.8 (95% CI 8.1-15.5) for EDC [39].
A tablet computer-based data collection system was implemented in a large-scale study of trachoma impact assessment surveys in Ethiopia [7]. Data quality outcomes were compared with a similar paper-based survey conducted 7 months earlier in a different part of the country. The sampling units were households (PPDC: 9433 vs EDC: 12,112), and the study enumerated 38,851 individuals in the PPDC survey and 50,858 in the EDC survey. Individuals enumerated with at least 1 blank field in a single respondent response were defined as missing data (1.7% for PPDC vs 1.5% for EDC; P=.01). Missing data at the household level for GPS with blank entries was also reported (EDC: 1.1% vs PPDC: 0.6%; P<.01). Inaccuracy errors were defined only in a percentage of households with an incorrect unique identifying number (PPDC: 2.3% vs EDC: 1.8%). Data entry and analysis were done in less than 1 day for EDC, while it took 14 days for data entry and an additional 5 days for double data entry discrepancy checks for PPDC [7].
Apart from the above studies, interview time or mean data availability duration in the databases were also reported in some papers. A study in Kenya reported faster data upload to a central database in EDC and a median duration for data upload of 7 days (range 1-13 days) after data collection for EDC and 21 days (range 4-56 days) for PPDC (P<.01) [36]. A combined report from Indonesia and the Philippines showed that the median time between data collection and data entry for PPDC surveys was approximately 3 months, compared with 2 days for EDC [34]. Time analysis from a large-scale survey in Tanzania reported that the median duration of an enumeration session per household was 9.4 min (90% central range 6.4-12.2) for paper surveys and 8.3 min (6.1-12.0) for electronic surveys (P=.01) [38].
Effect on Cost-effectiveness
Most of the studies reported cost analysis for the expenses incurred to conduct surveys using paper-based and electronic tools. However, the included studies varied significantly in the types and level of cost analysis reported in their groups. Most of the recommendations from the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [47] were not included. In Table 4, we provide basic information on the study and country, analytic method and model, participants per intervention, time horizon, discount rate, currency, included cost inputs, cost ranges, outcomes, consequence, and conclusion information.
Table 4.
Extracted cost information (based on Consolidated Health Economic Evaluation Reporting Standards evaluation template)
| Study and country | Analytic method or model | Interventions studied or population per group (1=PPDCa; 2=EDCb) | Time horizon, discount rate, currency (base year) | Included cost inputs and assumptions (1=PPDC; 2=EDC) | Data quality outcome link with input cost | Cost range of intervention (1=PPDC; 2=EDC) | Conclusions and remark |
| King et al [7], Ethiopia | Input cost analysis |
|
|
|
Not linked |
|
|
| McLean et al [39], Malawi | Input cost analysis Differential cost |
|
|
|
Not linked |
|
|
| Giduthuri et al [37], India | Differential input cost analysis |
|
|
|
Not linked | For 96 interviews, the cost is
|
|
| Mukasa et al [38], Tanzania | Cost and cost-effectiveness |
|
|
|
Error rate | For 1000 households, error-free data set:
|
|
| Zhang et al [42], China | Input cost analysis |
|
|
|
Not linked | Sample size: 60 each
|
|
| Njuguna et al [36], Kenya | Input cost analysis |
|
|
|
Not linked | First year:
Second year:
|
|
| Dillon et al [41], South Africa | Input and economic analysis |
|
|
|
Error rate | Salary cost per month:
|
|
| Flexman et al [34], Bangladesh and Philippines | Input data analysis |
|
|
|
Not linked |
|
|
| Lietz et al [40], Burkina Faso | Comparative input cost analysis |
|
|
|
Not assessed | Cost per household visit
|
|
aPPDC: pen and paper data collection.
bEDC: electronic data collection.
cNR: not reported
dHDSS: health and demographic surveillance systems.
eHMS: Household Morbidity Survey.
fCDA: Comprehensive Disease Assessment.
Studies from Ethiopia [7], Malawi [39], India [37], and Bangladesh and Philippines [34] reported a differential input cost unique to paper-based or electronic tools. For paper-based data collection, these were printing and data entry costs, and for EDC systems, the cost of electronic devices’ hardware, software, and accessories. The general assumption was that all other costs, such as personnel costs, were the same for both tools. Another cost assumption was that of the cost of small-scale, short-duration surveys. Such kinds of small costs were extrapolated to large-scale surveys with no clear information about the model or the cost assumptions followed to reach the large-scale costs. The studies concluded that EDC was expensive for small-scale surveys, as the initial investment in hardware and software outweighs the paper-based printing and data entry costs. However, large-scale surveys showed a significant decrease in cost for EDC surveys; for example, the paper-based survey cost was up to 65% higher per annum than the unique costs for the EDC system [39]. None of these studies linked the input cost with data quality errors.
Detailed cost information and the link between cost and data quality were reported in a retrospective data analysis in Tanzania. However, the base year for the cost was 2008, and deflator values of 209.5 for 2008 and 233.6 for 2016 were used to report the costs. For 1000 households, the cost per error-free data set was US $11,610 for PPDC and US $9380 for EDC. For error-free data sets, surveys using electronic tools—compared with paper-based tools—were 28% less expensive in recurrent costs and 19% less expensive in total costs [38].
A study in South Africa also reported cost inputs linked to data quality outcomes [41]. The formula presented by Walther et al [19] was used with minimum staffing: for PPDC, 1 field worker, 1 data entry clerk, and 1 data supervisor, and for EDC, 1 field worker and 1 data manager. In addition, 46 questions in a questionnaire, 5 interviews per day, 22 working days per month, 110 interviews per month, and 5060 questions per month were planned. The EDC salary cost per correctly entered question was 0.5 times that of PPDC. Overall, the cost per question was £0.18 for EDC and £0.20 for PPDC. The equipment cost for PPDC was £420, compared with £1036 for EDC. EDC saved £101.20 per month, and the EDC cost recoup time was reported as 6 months.
Lingani et al [40] in Burkina Faso and Njuguna et al [36] in Kenya reported a detailed financial cost comparison for the establishment of PPDC and EDC at HDSS and a hospital-based surveillance system, respectively. The Kenyan report indicated that during establishment, the cost of EDC was 9.4% higher than that of PPDC. However, after 2 years, EDC costs decreased by 7%, compared with PPDC (see Table 4 for detailed cost information).
Technology Characteristics, User Preference, and Acceptance
The mobile devices used for data collection included PDAs, smartphones, tablet computers, and notebooks (Table 5). The included studies (6/12, 50%) also reported the use of open source Android apps called open data kit apps to customize the software according to their needs. Microsoft Windows and BlackBerry operating systems were installed on mobile devices.
Table 5.
Extracted intervention or technology characteristics
| Study and country | Analytic method or model | Interventions studied or population per group (1=PPDCa; 2=EDCb) | Time horizon, discount rate, currency (base year) | Included cost inputs and assumptions (1=PPDC; 2=EDC) | Data quality outcome link with input cost |
| Njuguna et al [36], Ethiopia |
|
Direct to server with mobile network |
|
|
Data saved in the smartphone’s memory and later uploaded on to the server from convenient places |
| Ahmed et al [35], Sudan |
|
Mobile internet network |
|
|
Used ODK offline and submitted the data after restarting the smartphone |
| King et al [7], Ethiopia |
|
SDc card with password-protected downloading to the supervisor’s laptop |
|
|
Data stored first on the supervisor laptop and then uploaded to the local server to maintain the sovereignty and security of the data set |
| Flexman et al [34], Bangladesh and Philippines |
|
Direct to server with mobile network |
|
|
Purchase memory cards for the tablets to back up data locally |
| Giduthuri et al [37], India |
|
Encrypted and uploaded over a Wi-Fi connection to a central server after returning to the office | Not described |
|
Not described |
| Musaka et al [38], Tanzania |
|
Micro SD card |
|
|
Not described |
| McLean et al [39], Malawi |
|
Secured wireless network (not relayed on phone network) |
|
|
A dedicated, secured device charging area |
| Dillon et al [41], South Africa |
|
Data transfer through USB connections, avoiding the need for a constant internet connection |
|
|
Data transfer through USB |
| Jing et al [43], China |
|
Stored safely on the smartphone and uploaded to a computer secured by a password. Compiled data sent to the country coordinator |
|
|
Building a server in China that can be easily accessed would facilitate improved data security and immediate assignment of cause of death on smartphones at the time of interview |
| Byass et al [44], Burkina Faso |
|
Data copied for PDAs’ memory cards |
|
|
Saving data from the PDAs’ internal volatile memory to nonvolatile memory cards. Protective plastic cover for the safety of PDA was important |
| Maduga et al [46], Nigeria |
|
Trained to upload completed forms onto a secure server, with back-end access provided to only the research team lead |
|
|
The extra 2 phones served as backup in the event of malfunction or challenges with the global system for mobile communication mobile and data networks. Multiple SIM cards were provided in an attempt to mitigate the problem |
aFAST: Field Adapted Survey Toolkit.
bODK: Open Data Kit.
cSD: Secure Digital.
dHRS: Household Registration System.
eOS: operating system.
Data transfer from mobile devices to the central server was conducted using one of the following methods:
Direct transfer from the data collection site to the server using a mobile data network [34-36] and secure virtual private network [45].
Transfer using USB cable [41].
Data transfer to a server located in a foreign country using a mobile network was not considered appropriate in some studies due to data ownership or security concerns [7,43].
Technical Challenges in Electronic Data Collection
Limited or poor internet connectivity, occasional server communication interruptions, language challenges, the need for highly trained data collectors, device stack or freezing, device loss and breakdowns, and limited battery or power sources are among the technological challenges faced in the implementation of EDC systems (Table 5). The solutions include offline storage and transfer as soon as the data collectors obtain reliable internet connection (store and forward methods), transferring data using USB cables or secure digital cards, purchasing backup mobile devices or batteries, and using paper questionnaires at times of device malfunction.
Preference, Acceptability, and Feelings
A total of 6 papers [7,34,37-39,42] reported that user preferences, acceptance, and opinions were assessed using formal evaluation methods, such as individual or focus groups or qualitative interviews. Detailed comparative advantages and disadvantages of paper and electronics tools are reported in thematic-based tables in [7,38,39].
The use of smartphones to collect data was faster, easier to follow, and more convenient, as the data collectors did not have to carry cumbersome paper questionnaires and less space was needed to store their data collection tools. Additional functionalities—such as automatic retrieval of respondents and other members of the household or GPS functionalities—are also reported as an advantage.
The risk of data loss with paper-based surveys was perceived as being less than that with EDC, as paper questionnaires are tangible and enable immediate review, identification, and correction of mistakes. Paper surveys were also perceived to be easier for manipulating, adding, or changing data—for instance, including a household member absent during the survey who was later encountered by the survey team. The automated skip function was advantageous and time saving. The enumerators did not have to read the questions on every visit to the same household.
Enumerators described that the devices felt exciting, interesting, and prestigious, and they were skilled professionals in the eyes of the community. Some fieldworkers felt that EDC interviews took longer, and occasionally, devices froze during an interview.
Some studies that were excluded from our review also offer important insights about preference, acceptability, and local experiences [48-51].
Discussion
This systematic review synthesized the available comparative evidence on paper-based and electronic data collection tools and the potential effect of using these tools on data quality, implementation cost, and user preferences in interview-administered public health surveys. The systematic review included studies from 2008 to 2018 that were identified through multiple online electronic database searches. We identified more than 3500 papers and screened more than 2500 titles and abstracts to include 14 full-text papers based on our inclusion criteria. We extracted and synthesized available evidence regarding data quality, cost-effectiveness, timeliness, and user preferences. No paper has reported a study design with a classical randomized control approach. Randomization was reported to indicate either respondent allocation to paper-based or electronic tools or data collectors’ exposure to one of the data collection tools. Meta-analysis was not possible due to the heterogeneous nature of study designs, measurements and outcome types, and study settings. Instead, a narrative synthesis based on predefined data quality, cost and related outcomes’ acceptability, and preferences was conducted.
We employed a rigorous systematic review process to formulate the research questions, prepare individual database-tailored search strategies, execute searches in multiple databases, and independently screen and extract evidence from thousands of papers.
However, the results were inadequate for meta-analysis. The final included studies were heterogeneous and could not be combined to generate better estimates. Low-quantity and low-quality phenomena are becoming evident in most recent systematic reviews assessing mHealth or eHealth outcomes [13,29,30].
This scarcity might result from the following reasons: lack of primary studies with a rigorous study design, insufficient search strategy or review process, or unnecessary narrowing of a study focus. The most commonly reported reason is a lack of sufficient, well-planned, rigorous studies. The lack of evidence might be due to reluctance to evaluate the system after implementation and due to publication bias because of unsuccessful or disappointing findings [52]. Apart from a few studies [35,37,39,41,42], those included in our systematic review were not primarily designed to evaluate the impact of EDC compared with PPDC. Instead, the studies were a byproduct of survey experience from a comparative analysis using their secondary data. Information from such implementation practices can provide insights or lessons for readers, but the comparative outcomes might be influenced by unplanned and uncontrolled confounding variables [7,36]. Elimination of observed and unobserved factors that might otherwise plausibly explain the difference in outcomes in the study design can increase confidence in the assertion that the estimated impact constitutes the real impact of the tools. The studies included in this review suffered from multiple biases during sample size estimation, selection (purposive vs random selection), and data quality outcome measurement level (before or after data entry). We recommend that future research focus specifically on the mode of data collection measurement and on quantifying the impacts with sound research designs.
Generating a full economic evaluation of the evidence facilitates a comparison between interventions in terms of their costs and intended outcomes and can be used to inform decisionmakers or funders of the available choices among alternatives upon cost justification [53,54]. In our systematic review, we attempted to extract the available cost information using the CHEERS checklist [47]; however, most of the expected items in the checklist were not reported. A majority of the studies lacked a detailed description of unit costs, data sources, and cost calculations. Moreover, most used a time horizon of 1 year and failed to assess long-term costs and data quality effects. The rationale for the choice of the time horizon was also not explicitly stated.
Despite these limitations, the available cost data could provide clues regarding the existing cost parameters for paper- and electronic data collection systems. Two studies managed to link the cost of implementing EDC and PPDC tools to data quality. Such cost-effectiveness analyses should be encouraged in future studies. There is no clear answer or guideline to shape the type and level of rigorous studies in health information technology evaluation [55,56]. Details of evidence-based health informatics history, current practices, and future recommendations are discussed elsewhere [52]. Further debate and consensus among academics and researchers in biomedical informatics should continue to determine how and when health information technology evaluation is rigorous and produces good quality data [57].
The studies identified in this review were conducted in various countries and in the context of different health care systems. Generalizing and applying results from different contexts is difficult because of variations in clinical practice, costs, and their analysis. However, what was consistent across all studies was a lack of reporting on the feasibility of adopting these technologies based on economic and organizational factors.
It was surprising to see limited publications from global survey implementation organizations for the DHS and INDEPTH network groups, as those projects have many years of multinational implementation experience [4,58]; however, apart from experience reports, comparative evaluation studies in those areas are rare. Further evaluation research in these projects might produce evidence of data quality, cost, timeliness, and the success and failure factors for multinational projects.
There are positive perceptions regarding the acceptability, usability, and preference of EDC over PPDC among data collectors. This positivity is because technology enables data collectors to focus on their work, get immediate feedback regarding their mistakes, correct their errors while in the field, and leave few data quality issues to revisit. It is not known whether this excitement is a short-term effect immediately after the technology introduction or a stable long-term view based on longer exposure. A short period of technophilic or technophobic attitudes might lead to inaccurate overall impression of the tool, as accurate impression can only develop with longer exposure to the technology [59]. The generalizability and applicability of the results, given the different types of devices with different technical specifications and the rapid pace at which technology advances, need critical evaluation. The generalizability of the findings of this systematic review is also challenged by the studies themselves, considering the variations in the characteristics of data collectors, level of outcome measurements, settings of the survey, and the psychometric properties of the survey questionnaires.
Conclusions
This systematic review showed that, despite consistent claims of a positive impact of technology on data quality and cost-effectiveness, the available evidence is small in quantity and low in quality. Purposefully designed comparative studies assessing the impact of data quality and cost-effectiveness are needed for implementation in organizations and by decision makers.
Despite the heterogeneity and low quality of the included studies, their qualitative synthesis showed the superiority of EDC systems over paper-based systems for data quality, process efficiencies, and cost.
Comparative evaluation studies sourced from international survey-implementing organizations where their routine data collection mode is EDC can provide a better platform for impact evaluation research in large-scale surveys.
Acknowledgments
The authors would like to thank the Deutscher Akademischer Austauschdienst for funding the research stay of the principal investigator.
Abbreviations
- CHEERS
Consolidated Health Economic Evaluation Reporting Standards
- CRF
clinical case report form
- DHS
demographic and health survey
- eCRF
electronic clinical report form
- HDSS
health and demographic surveillance system
- INDEPTH
International Network for the Demographic Evaluation of Populations and Their Health
- mHealth
mobile health
- RR
risk ratio
Appendix
Additional search strategies.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Bocquier P, Sankoh O, Byass P. Are health and demographic surveillance system estimates sufficiently generalisable? Glob Health Action. 2017;10(1):1356621. doi: 10.1080/16549716.2017.1356621. http://europepmc.org/abstract/MED/28820344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timaeus I, Harpham T, Price M, Gilson L. Health surveys in developing countries: the objectives and design of an international programme. Soc Sci Med. 1988;27(4):359–68. doi: 10.1016/0277-9536(88)90270-5. [DOI] [PubMed] [Google Scholar]
- 3.Gerritsen A, Bocquier P, White M, Mbacké C, Alam N, Beguy D, Odhiambo F, Sacoor C, Phuc HD, Punpuing S, Collinson MA. Health and demographic surveillance systems: contributing to an understanding of the dynamics in migration and health. Glob Health Action. 2013 Jul 11;6(1):21496. doi: 10.3402/gha.v6i0.21496. http://europepmc.org/abstract/MED/23849188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Survey Organization Manual for Demographic and Health Surveys. ICF International. 2012. [2018-12-05]. https://dhsprogram.com/pubs/pdf/DHSM10/DHS6_Survey_Org_Manual_7Dec2012_DHSM10.pdf.
- 5.Ethiopia Demographic and Health Survey. Central Statistical Agency [Ethiopia] and ICF International. 2012. [2018-10-02]. https://dhsprogram.com/countries/Country-Main.cfm?ctry_id=65.
- 6.Okwaraji YB, Cousens S, Berhane Y, Mulholland K, Edmond K. Effect of geographical access to health facilities on child mortality in rural Ethiopia: a community based cross sectional study. PLoS One. 2012;7(3):e33564. doi: 10.1371/journal.pone.0033564. https://dx.plos.org/10.1371/journal.pone.0033564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King JD, Buolamwini J, Cromwell EA, Panfel A, Teferi T, Zerihun M, Melak B, Watson J, Tadesse Z, Vienneau D, Ngondi J, Utzinger J, Odermatt P, Emerson PM. A novel electronic data collection system for large-scale surveys of neglected tropical diseases. PLoS One. 2013;8(9):e74570. doi: 10.1371/journal.pone.0074570. https://dx.plos.org/10.1371/journal.pone.0074570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toninelli D, Pinter R, Pablo DP. Mobile Research Methods: Opportunities and Challenges of Mobile Research Methodologies. London, UK: Ubiquity Press; 2015. [Google Scholar]
- 9.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf) 2005 Sep;27(3):281–91. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]
- 10.Groves R. Survey Errors and Survey Costs. New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 11.World Health Organization . Global Diffusion of Ehealth: Making Universal Health Coverage Achievable. Report of the Third Global Survey on Ehealth. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 12.Labrique AB, Vasudevan L, Kochi E, Fabricant R, Mehl G. mHealth innovations as health system strengthening tools: 12 common applications and a visual framework. Glob Health Sci Pract. 2013 Aug 6;1(2):160–71. doi: 10.9745/ghsp-d-13-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun R, Catalani C, Wimbush J, Israelski D. Community health workers and mobile technology: a systematic review of the literature. PLoS One. 2013;8(6):e65772. doi: 10.1371/journal.pone.0065772. https://dx.plos.org/10.1371/journal.pone.0065772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcano Belisario JS, Jamsek J, Huckvale K, O'Donoghue J, Morrison C, Car J. Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst Rev. 2015 Jul 27;(7):MR000042. doi: 10.1002/14651858.MR000042.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004 Jul;99(7):885–96. doi: 10.1111/j.1360-0443.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 16.Adebajo S, Obianwu O, Eluwa G, Vu L, Oginni A, Tun W, Sheehy M, Ahonsi B, Bashorun A, Idogho O, Karlyn A. Comparison of audio computer assisted self-interview and face-to-face interview methods in eliciting HIV-related risks among men who have sex with men and men who inject drugs in Nigeria. PLoS One. 2014;9(1):e81981. doi: 10.1371/journal.pone.0081981. https://dx.plos.org/10.1371/journal.pone.0081981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauclair R, Meng F, Deprez N, Temmerman M, Welte A, Hens N, Delva W. Evaluating audio computer assisted self-interviews in urban South African communities: evidence for good suitability and reduced social desirability bias of a cross-sectional survey on sexual behaviour. BMC Med Res Methodol. 2013 Jan 31;13:11. doi: 10.1186/1471-2288-13-11. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richens J, Copas A, Sadiq ST, Kingori P, McCarthy O, Jones V, Hay P, Miles K, Gilson R, Imrie J, Pakianathan M. A randomised controlled trial of computer-assisted interviewing in sexual health clinics. Sex Transm Infect. 2010 Aug;86(4):310–4. doi: 10.1136/sti.2010.043422. [DOI] [PubMed] [Google Scholar]
- 19.Walther B, Hossin S, Townend J, Abernethy N, Parker D, Jeffries D. Comparison of electronic data capture (EDC) with the standard data capture method for clinical trial data. PLoS One. 2011;6(9):e25348. doi: 10.1371/journal.pone.0025348. https://dx.plos.org/10.1371/journal.pone.0025348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Jeannic A, Quelen C, Alberti C, Durand-Zaleski I, CompaRec Investigators Comparison of two data collection processes in clinical studies: electronic and paper case report forms. BMC Med Res Methodol. 2014 Jan 17;14:7. doi: 10.1186/1471-2288-14-7. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlović I, Kern T, Miklavcic D. Comparison of paper-based and electronic data collection process in clinical trials: costs simulation study. Contemp Clin Trials. 2009 Jul;30(4):300–16. doi: 10.1016/j.cct.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Rorie DA, Flynn RW, Grieve K, Doney A, Mackenzie I, MacDonald TM, Rogers A. Electronic case report forms and electronic data capture within clinical trials and pharmacoepidemiology. Br J Clin Pharmacol. 2017 Sep;83(9):1880–95. doi: 10.1111/bcp.13285. doi: 10.1111/bcp.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.di Pasquale A, McCann RS, Maire N. Assessing the population coverage of a health demographic surveillance system using satellite imagery and crowd-sourcing. PLoS One. 2017;12(8):e0183661. doi: 10.1371/journal.pone.0183661. https://dx.plos.org/10.1371/journal.pone.0183661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homan T, Di Pasquale A, Kiche I, Onoka K, Hiscox A, Mweresa C, Mukabana WR, Takken W, Maire N. Innovative tools and OpenHDS for health and demographic surveillance on Rusinga Island, Kenya. BMC Res Notes. 2015 Sep 1;8:397. doi: 10.1186/s13104-015-1373-8. https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-015-1373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko S, K'opiyo J, Kiche I, Wanyua S, Goto K, Tanaka J, Changoma M, Ndemwa M, Komazawa O, Karama M, Moji K, Shimada M. Health and demographic surveillance system in the western and coastal areas of Kenya: an infrastructure for epidemiologic studies in Africa. J Epidemiol. 2012;22(3):276–85. doi: 10.2188/jea.je20110078. http://joi.jlc.jst.go.jp/JST.JSTAGE/jea/JE20110078?from=PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sifuna P, Oyugi M, Ogutu B, Andagalu B, Otieno A, Owira V, Otsyula N, Oyieko J, Cowden J, Otieno L, Otieno W. Int J Epidemiol. 2014 Aug;43(4):1097–104. doi: 10.1093/ije/dyu139. http://europepmc.org/abstract/MED/25009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishamawe C, Isingo R, Mtenga B, Zaba B, Todd J, Clark B, Changalucha J, Urassa M. Int J Epidemiol. 2015 Dec;44(6):1851–61. doi: 10.1093/ije/dyv188. http://europepmc.org/abstract/MED/26403815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okwaraji YB, Webb EL, Edmond KM. Barriers in physical access to maternal health services in rural Ethiopia. BMC Health Serv Res. 2015 Nov 4;15:493. doi: 10.1186/s12913-015-1161-0. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-015-1161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS One. 2017;12(2):e0170581. doi: 10.1371/journal.pone.0170581. https://dx.plos.org/10.1371/journal.pone.0170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal S, Perry HB, Long L, Labrique AB. Evidence on feasibility and effective use of mHealth strategies by frontline health workers in developing countries: systematic review. Trop Med Int Health. 2015 Aug;20(8):1003–14. doi: 10.1111/tmi.12525. doi: 10.1111/tmi.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. https://dx.plos.org/10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009 Jul 21;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. https://dx.plos.org/10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeleke AA, Naziyok T, Fritz F, Röhrig R. Data quality and cost-effectiveness analyses of electronic and paper-based interviewer-administered public health surveys: protocol for a systematic review. JMIR Res Protoc. 2019 Jan 30;8(1):e10678. doi: 10.2196/10678. https://www.researchprotocols.org/2019/1/e10678/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaxman AD, Stewart A, Joseph JC, Alam N, Alam SS, Chowdhury H, Mooney MD, Rampatige R, Remolador H, Sanvictores D, Serina PT, Streatfield PK, Tallo V, Murray CJL, Hernandez B, Lopez AD, Riley ID. Collecting verbal autopsies: improving and streamlining data collection processes using electronic tablets. Popul Health Metr. 2018 Feb 1;16(1):3. doi: 10.1186/s12963-018-0161-9. https://pophealthmetrics.biomedcentral.com/articles/10.1186/s12963-018-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed R, Robinson R, Elsony A, Thomson R, Squire SB, Malmborg R, Burney P, Mortimer K. A comparison of smartphone and paper data-collection tools in the Burden of Obstructive Lung Disease (BOLD) study in Gezira state, Sudan. PLoS One. 2018;13(3):e0193917. doi: 10.1371/journal.pone.0193917. https://dx.plos.org/10.1371/journal.pone.0193917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Njuguna HN, Caselton DL, Arunga GO, Emukule GO, Kinyanjui DK, Kalani RM, Kinkade C, Muthoka PM, Katz MA, Mott JA. A comparison of smartphones to paper-based questionnaires for routine influenza sentinel surveillance, Kenya, 2011-2012. BMC Med Inform Decis Mak. 2014 Dec 24;14:107. doi: 10.1186/s12911-014-0107-5. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-014-0107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giduthuri JG, Maire N, Joseph S, Kudale A, Schaetti C, Sundaram N, Schindler C, Weiss MG. Developing and validating a tablet version of an illness explanatory model interview for a public health survey in Pune, India. PLoS One. 2014;9(9):e107374. doi: 10.1371/journal.pone.0107374. https://dx.plos.org/10.1371/journal.pone.0107374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukasa O, Mushi HP, Maire N, Ross A, de Savigny D. Do surveys with paper and electronic devices differ in quality and cost? Experience from the Rufiji Health and demographic surveillance system in Tanzania. Glob Health Action. 2017;10(1):1387984. doi: 10.1080/16549716.2017.1387984. http://europepmc.org/abstract/MED/29157182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean E, Dube A, Saul J, Branson K, Luhanga M, Mwiba O, Kalobekamo F, Geis S, Crampin AC. Implementing electronic data capture at a well-established health and demographic surveillance site in rural northern Malawi. Glob Health Action. 2017;10(1):1367162. doi: 10.1080/16549716.2017.1367162. http://europepmc.org/abstract/MED/28922071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lietz H, Lingani M, Sié A, Sauerborn R, Souares A, Tozan Y. Measuring population health: costs of alternative survey approaches in the Nouna Health and Demographic Surveillance System in rural Burkina Faso. Glob Health Action. 2015;8:28330. doi: 10.3402/gha.v8.28330. http://europepmc.org/abstract/MED/26257048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dillon DG, Pirie F, Rice S, Pomilla C, Sandhu MS, Motala AA, Young EH, African Partnership for Chronic Disease Research (APCDR) Open-source electronic data capture system offered increased accuracy and cost-effectiveness compared with paper methods in Africa. J Clin Epidemiol. 2014 Dec;67(12):1358–63. doi: 10.1016/j.jclinepi.2014.06.012. https://linkinghub.elsevier.com/retrieve/pii/S0895-4356(14)00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Wu Q, van Velthoven MH, Chen L, Car J, Rudan I, Zhang Y, Li Y, Scherpbier RW. Smartphone versus pen-and-paper data collection of infant feeding practices in rural China. J Med Internet Res. 2012 Sep 18;14(5):e119. doi: 10.2196/jmir.2183. https://www.jmir.org/2012/5/e119/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Joshi R, Sun J, Rosenthal SR, Tong M, Li C, Rampatige R, Mooney M, Lopez A, Yan LL. A feasibility study on using smartphones to conduct short-version verbal autopsies in rural China. Popul Health Metr. 2016;14:31. doi: 10.1186/s12963-016-0100-6. https://pophealthmetrics.biomedcentral.com/articles/10.1186/s12963-016-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byass P, Hounton S, Ouédraogo M, Somé H, Diallo I, Fottrell E, Emmelin A, Meda N. Direct data capture using hand-held computers in rural Burkina Faso: experiences, benefits and lessons learnt. Trop Med Int Health. 2008 Jul;13(Suppl 1):25–30. doi: 10.1111/j.1365-3156.2008.02084.x. doi: 10.1111/j.1365-3156.2008.02084.x. [DOI] [PubMed] [Google Scholar]
- 45.Ojwangʼ JK, Lee VC, Waruru A, Ssempijja V, Ngʼangʼa JG, Wakhutu BE, Kandege NO, Koske DK, Kamiru SM, Omondi KO, Kakinyi M, Kim AA, Oluoch T. Using information and communications technology in a national population-based survey. J Acquired Immun Def Syndrome. 2014;66:S123–9. doi: 10.1097/qai.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maduka O, Akpan G, Maleghemi S. Using android and open data kit technology in data management for research in resource-limited settings in the niger delta region of nigeria: cross-sectional household survey. JMIR Mhealth Uhealth. 2017 Nov 30;5(11):e171. doi: 10.2196/mhealth.7827. https://mhealth.jmir.org/2017/11/e171/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, CHEERS Task Force Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Br Med J. 2013 Mar 25;346:f1049. doi: 10.1136/bmj.f1049. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=23529982. [DOI] [PubMed] [Google Scholar]
- 48.Seebregts CJ, Zwarenstein M, Mathews C, Fairall L, Flisher AJ, Seebregts C, Mukoma W, Klepp K. Handheld computers for survey and trial data collection in resource-poor settings: development and evaluation of PDACT, a Palm Pilot interviewing system. Int J Med Inform. 2009 Nov;78(11):721–31. doi: 10.1016/j.ijmedinf.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Health Sci Pract. 2013 Jul 11;1(2):277–84. doi: 10.9745/ghsp-d-12-00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knipe DW, Pearson M, Borgstrøm R, Pieris R, Weerasinghe M, Priyadarshana C, Eddleston M, Gunnell D, Metcalfe C, Konradsen F. Challenges and opportunities of a paperless baseline survey in Sri Lanka. BMC Res Notes. 2014 Jul 15;7(1):-. doi: 10.1186/1756-0500-7-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sime H, Deribe K, Assefa A, Newport MJ, Enquselassie F, Gebretsadik A, Kebede A, Hailu A, Shafi O, Aseffa A, Reithinger R, Brooker SJ, Pullan RL, Cano J, Meribo K, Pavluck A, Bockarie MJ, Rebollo MP, Davey G. Integrated mapping of lymphatic filariasis and podoconiosis: lessons learnt from Ethiopia. Parasit Vectors. 2014 Aug 27;7:397. doi: 10.1186/1756-3305-7-397. https://parasitesandvectors.biomedcentral.com/articles/10.1186/1756-3305-7-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rigby M, Ammenwerth E. The need for evidence in health informatics. Stud Health Technol Inform. 2016;222:3–13. [PubMed] [Google Scholar]
- 53.Schweitzer J, Synowiec C. The economics of eHealth and mHealth. J Health Commun. 2012 May 02;17 Suppl 1(sup1):73–81. doi: 10.1080/10810730.2011.649158. [DOI] [PubMed] [Google Scholar]
- 54.LeFevre AE, Shillcutt SD, Broomhead S, Labrique AB, Jones T. Defining a staged-based process for economic and financial evaluations of mHealth programs. Cost Eff Resour Alloc. 2017;15:5. doi: 10.1186/s12962-017-0067-6. https://resource-allocation.biomedcentral.com/articles/10.1186/s12962-017-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pham Q, Wiljer D, Cafazzo JA. Beyond the randomized controlled trial: a review of alternatives in mhealth clinical trial methods. JMIR Mhealth Uhealth. 2016 Sep 9;4(3):e107. doi: 10.2196/mhealth.5720. https://mhealth.jmir.org/2016/3/e107/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Poucke S, Thomeer M, Heath J, Vukicevic M. Are randomized controlled trials the (g)old standard? From clinical intelligence to prescriptive analytics. J Med Internet Res. 2016 Jul 6;18(7):e185. doi: 10.2196/jmir.5549. https://www.jmir.org/2016/7/e185/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenhalgh T, Russell J. Why do evaluations of eHealth programs fail? An alternative set of guiding principles. PLoS Med. 2010 Nov 2;7(11):e1000360. doi: 10.1371/journal.pmed.1000360. https://dx.plos.org/10.1371/journal.pmed.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ethiopia Demographic Health Survey 2016. Central Statistical Agency (CSA) [Ethiopia] and ICF. 2016. [2018-08-12]. https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf.
- 59.Edith L. The Effect of Computer-assisted Interviewing on Data Quality: a Review of the Evidence. Utrecht University Repository. 2008. [2020-12-30]. https://dspace.library.uu.nl/handle/1874/44502.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional search strategies.


