Abstract
Background
Opinion polls on vaccination intentions suggest that COVID-19 vaccine hesitancy is increasing worldwide; however, the usefulness of opinion polls to prepare mass vaccination campaigns for specific new vaccines and to estimate acceptance in a country's population is limited. We therefore aimed to assess the effects of vaccine characteristics, information on herd immunity, and general practitioner (GP) recommendation on vaccine hesitancy in a representative working-age population in France.
Methods
In this survey experiment, adults aged 18–64 years residing in France, with no history of SARS-CoV-2 infection, were randomly selected from an online survey research panel in July, 2020, stratified by gender, age, education, household size, and region and area of residence to be representative of the French population. Participants completed an online questionnaire on their background and vaccination behaviour-related variables (including past vaccine compliance, risk factors for severe COVID-19, and COVID-19 perceptions and experience), and were then randomly assigned according to a full factorial design to one of three groups to receive differing information on herd immunity (>50% of adults aged 18–64 years must be immunised [either by vaccination or infection]; >50% of adults must be immunised [either by vaccination or infection]; or no information on herd immunity) and to one of two groups regarding GP recommendation of vaccination (GP recommends vaccination or expresses no opinion). Participants then completed a series of eight discrete choice tasks designed to assess vaccine acceptance or refusal based on hypothetical vaccine characteristics (efficacy [50%, 80%, 90%, or 100%], risk of serious side-effects [1 in 10 000 or 1 in 100 000], location of manufacture [EU, USA, or China], and place of administration [GP practice, local pharmacy, or mass vaccination centre]). Responses were analysed with a two-part model to disentangle outright vaccine refusal (irrespective of vaccine characteristics, defined as opting for no vaccination in all eight tasks) from vaccine hesitancy (acceptance depending on vaccine characteristics).
Findings
Survey responses were collected from 1942 working-age adults, of whom 560 (28·8%) opted for no vaccination in all eight tasks (outright vaccine refusal) and 1382 (71·2%) did not. In our model, outright vaccine refusal and vaccine hesitancy were both significantly associated with female gender, age (with an inverted U-shaped relationship), lower educational level, poor compliance with recommended vaccinations in the past, and no report of specified chronic conditions (ie, no hypertension [for vaccine hesitancy] or no chronic conditions other than hypertension [for outright vaccine refusal]). Outright vaccine refusal was also associated with a lower perceived severity of COVID-19, whereas vaccine hesitancy was lower when herd immunity benefits were communicated and in working versus non-working individuals, and those with experience of COVID-19 (had symptoms or knew someone with COVID-19). For a mass vaccination campaign involving mass vaccination centres and communication of herd immunity benefits, our model predicted outright vaccine refusal in 29·4% (95% CI 28·6–30·2) of the French working-age population. Predicted hesitancy was highest for vaccines manufactured in China with 50% efficacy and a 1 in 10 000 risk of serious side-effects (vaccine acceptance 27·4% [26·8–28·0]), and lowest for a vaccine manufactured in the EU with 90% efficacy and a 1 in 100 000 risk of serious side-effects (vaccine acceptance 61·3% [60·5–62·1]).
Interpretation
COVID-19 vaccine acceptance depends on the characteristics of new vaccines and the national vaccination strategy, among various other factors, in the working-age population in France.
Funding
French Public Health Agency (Santé Publique France).
Introduction
On March 11, 2020, WHO declared the SARS-CoV-2 outbreak in China to be a pandemic. In the spring of 2020, all European countries implemented physical distancing measures as an emergency response to contain COVID-19 and its associated death toll,1, 2 especially in older adults.3, 4, 5 As a second wave of SARS-CoV-2 infections hit Europe in the autumn of 2020, all countries increased the physical distancing measures that had been relaxed over the summer months.2 Such stop-and-go strategies are likely to remain in place until herd immunity is reached and SARS-CoV-2 can no longer circulate (ie, 60% or more of a country's population is immune to SARS-CoV-2 following infection or vaccination).4, 6 Because physical distancing measures aim to reduce SARS-CoV-2 transmission to the lowest levels,1, 4, 7 herd immunity can only be achieved by mass vaccination.6, 8
Research in context.
Evidence before this study
Following the PRISMA guidelines, a systematic search was done using OVID to identify all studies on COVID-19 vaccination intentions published in English from Jan 1 to Dec 31, 2020, on MEDLINE, Embase, and PsycINFO. One systematic review was identified using a combination of search terms (COVID-19 OR SARS-CoV-2) AND (vaccine OR immunization) AND (survey OR poll). Based on 126 surveys published before November, 2020, this review showed that COVID-19 vaccine hesitancy is increasing worldwide. However, the usefulness of these surveys to inform the preparation of mass vaccination campaigns is limited, as vaccination intentions depend on the framing of the question and are provided for an unspecified vaccine. Multiple new COVID-19 vaccines have been developed that differ on various characteristics, and it is not clear which, if any, of the available or possible vaccines people have in mind when completing the survey. Furthermore, vaccination intentions depend on the granularity of the proposed responses and outright vaccination refusal is seldom disentangled from vaccine hesitancy in the analysis, even though the antivaccine movement might be influential in the context of COVID-19.
Added value of this study
In this survey experiment, COVID-19 vaccination intentions were assessed from repeated choice tasks among vaccines with varying specified characteristics, while background information on vaccination was controlled for. Accordingly, we developed a behavioural model that allowed outright vaccination refusal (ie, serial refusal of vaccines regardless of vaccine characteristics) to be disentangled from vaccine hesitancy (ie, vaccine acceptance that depends on vaccine characteristics). This survey experiment shows that COVID-19 vaccine acceptance depends on the new vaccines' characteristics and the national vaccination strategy, among many other factors. As of early July, 2020, the behavioural model predicted that 29·4% (95% CI 28·6–30·2) of the French population of working age would refuse COVID-19 vaccination outright in mass vaccination centres despite the communication of herd immunity benefits. Furthermore, COVID-19 vaccine acceptance in this population ranged from 27·4% (26·8–28·0; for new vaccines manufactured in China, with 50% efficacy and a 1 in 10 000 risk of serious side-effects) to 61·3% (60·5–62·1; for new vaccines manufactured in the EU, with 90% efficacy and a 1 in 100 000 risk of serious side-effects).
Implications of all the available evidence
COVID-19 vaccine hesitancy seems to be increasing as mass vaccination campaigns draw closer in the working-age population. Anti-COVID-19 vaccination behaviour should be closely monitored as it could disrupt COVID-19 vaccination campaigns as happened in 2009 with vaccinations against influenza A(H1N1)pdm09. If the objective of a national vaccination strategy becomes herd immunity in the adult population rather than self-protection in older adults and patients with underlying chronic conditions, our results suggest that it would be more successful in France with COVID-19 vaccines made in the EU and a communication strategy emphasising the collective benefits of herd immunity in the working-age population.
As of Jan 6, 2021, two COVID-19 vaccines with greater than 90% efficacy to reduce symptomatic infection risk9, 10 have been approved in the EU, and 16 candidate vaccines are in phase 3 trials.11 However, COVID-19 vaccine hesitancy might represent a major hurdle to achieving herd immunity.6, 8 In a systematic review of 126 surveys on COVID-19 vaccination intentions, including 23 academic studies and 103 opinion polls published before November, 2020, Lin and colleagues showed that COVID-19 vaccine hesitancy is increasing worldwide.12 Intent to receive COVID-19 vaccination varies substantially across countries,12 with France recording the lowest rate among European countries,13 and is generally lower in the working-age population than in older people. Most people who intend not to be vaccinated report being worried about the safety of new COVID-19 vaccines.12
Although opinion polls highlight the issue of COVID-19 vaccine hesitancy, they are of limited use to prepare mass vaccination campaigns. Opinion polls ask about vaccination intentions, but responses depend on the framing of the question and are provided for unspecified vaccines.12 New vaccines differ on various characteristics.11 Higher vaccine efficacy or safety is expected to be associated with decreased hesitancy.12 The country of manufacture of the vaccine11 can be associated with increased hesitancy because people might perceive candidate vaccines to have been developed hastily in the USA,12, 14, 15 or might distrust vaccines developed in China or Russia.12, 16, 17 Mass vaccination centres might be the only place where vaccines can be administered because of logistical or cold storage constraints, but might also increase hesitancy.18 In addition, estimating vaccination intentions depends on the granularity of responses,12 but outright vaccination refusal is seldom disentangled from vaccine hesitancy in analyses.19 The antivaccine movement could be influential in the context of COVID-1914, 15, 16, 20 and disrupt mass vaccination campaigns, as in 2009 for vaccinations against influenza A(H1N1)pdm09.18, 21
This study investigates COVID-19 vaccine acceptance and its determinants in a representative working-age population (aged 18–64 years) in France. Understanding the determinants of COVID-19 vaccine acceptance in this group is important because it accounts for the majority of the French population, and herd immunity can only be reached if the mass vaccination campaign is successful in this group. It is not certain that vaccination uptake will be sufficient in this group given their lower risk of a severe form of COVID-19 and their low rate of compliance with seasonal influenza vaccination programmes compared with older adults (aged ≥65 years).22
In contrast to opinion polls, this study used a large-scale survey experiment in which vaccination intentions were assessed from repeated choice tasks among vaccines with varying characteristics, while controlling for background information on vaccination. This method allows precise estimation of outright vaccination refusal and vaccine acceptance for a range of realistic scenarios in a mass vaccination campaign. Furthermore, differences in behaviour across age categories were assessed with regard to possible age-based priorities for vaccination in the working-age population.6, 23
Methods
Study sample
We did a cross-sectional survey among a representative sample of 2000 adults aged 18–64 years in France. The survey was fielded around 3 weeks after the first lockdown was lifted on June 2, 2020.2 Participants were selected from an online survey research panel, which was developed and is maintained by the opinion survey research firm BVA (Paris, France) and consists of more than 700 000 French adults. Pre-existing information on the participants was used by BVA to determine eligibility and draw a stratified random sample, with oversampling of participants with low response rates. Sampling was stratified to match French official census statistics for gender, age, education, household size, area of residence (urban vs rural), and geographical region of residence (appendix p 2).
Participants completed a self-reported online questionnaire that elicited background information (including the stratification variables listed above) as well as information on vaccination behaviour: working status, whether they were a health-care worker, vaccination behaviour in the past, risk factors for a severe form of COVID-19 (current pregnancy, smoking status, body-mass index, hypertension, or a chronic condition other than hypertension), COVID-19 experience (had COVID-19 symptoms, had a test for SARS-CoV-2 infection, or knew someone who had COVID-19), and risk perceptions about COVID-19 severity (appendix p 3). Chronic conditions included in the survey were diabetes, asthma, other chronic lung disease, chronic arterial disease, chronic heart disease, chronic kidney disease, and cancer. In accordance with eligibility criteria for phase 3 vaccine trials,9, 10 participants who reported previous SARS-CoV-2 infection were excluded from the survey experiment (appendix p 4).
The data management of BVA is approved by the French National Commission for Data Protection in accordance with the General Data Protection Regulation of the EU (approval renewed on May 25, 2018). The survey experiment followed all requirements under French regulations.
Survey experiment
The survey experiment consisted of two sections at the end of the online questionnaire: background information on COVID-19 vaccination, and the elicitation of vaccine acceptance based on vaccine characteristics.
In the first section, all participants were provided with a full page presenting general information about COVID-19 vaccination (appendix p 6). In addition, each participant was randomly allocated by use of a random number generator to two different information blocks according to a full factorial design, with stratification by gender and educational level (some high school vs high school or university graduate). In block 1, participants were randomly assigned (1:1:1) to receive information about the collective benefits of herd immunity with a varying herd immunity target: more than 50% of adults aged 18–64 years old must be immunised (either by vaccination or infection); more than 50% of adults must be immunised (either by vaccination or infection); or no information on herd immunity.24 In block 2, participants were randomly assigned (1:1) to receive differing general practitioner (GP) advice about COVID-19 vaccination: recommendation or no opinion.18
In the second section, COVID-19 vaccine acceptance was elicited with use of a discrete choice experiment, in which participants completed a series of choice tasks based on vaccine characteristics.25 The hypothetical vaccines differed on four characteristics: vaccine efficacy to reduce the infection risk (50%, 80%, 90%, or 100%);26 vaccine safety in terms of the risk of serious side-effects (1 in 10 000 or 1 in 100 000 vaccinated people); vaccine manufacturer (headquarters in the EU, USA, or China); and where vaccinations are given (GP practice, local pharmacy, or mass vaccination centre). Based on these vaccine characteristics and levels, there were 2556 possible choice tasks between any two hypothetical vaccines. A D-efficient experimental design was used to reduce these choice tasks down to eight tasks with 16 hypothetical vaccines (appendix p 9). An example of a choice task is provided in table 1 . Participants completed a series of eight tasks (appendix p 10), choosing in each task between having one of two hypothetical vaccines (ie, only one vaccine, not both, could be selected) or no vaccination.
Table 1.
Choice task example in the discrete choice experiment
| Vaccination scenario A | Vaccination scenario B | No vaccination | |
|---|---|---|---|
| Risk of being infected with SARS-CoV-2 | The vaccine reduces your risk of having COVID-19 by 90% and reduces the risk of people around you becoming infected | The vaccine reduces your risk of having COVID-19 by 80% and reduces the risk of people around you becoming infected | Your risk of having COVID-19 depends on the number of cases in your area and the protective measures you take on a daily basis |
| Risk of rare but serious side-effects from the vaccine | 1 in 100 000 vaccinated people | 1 in 10 000 vaccinated people | No risk |
| Location of vaccine manufacturer | Headquarters in the EU | Headquarters in the USA | Not applicable |
| Place of vaccine administration | At your local pharmacy | At a mass vaccination centre | Not applicable |
| Choice* | I would be vaccinated in scenario A | I would be vaccinated in scenario B | I would not be vaccinated |
Participants were asked to choose between receiving one of two vaccines or no vaccination.
Outcome
The study outcome was COVID-19 vaccine acceptance in the working-age population in France. For the purpose of this study, we considered the population to contain two groups: those who would never choose to be vaccinated, regardless of vaccine characteristics (outright vaccination refusal); and those who are hesitant and whose choice to be vaccinated depends on vaccine characteristics. In our survey experiment, outright vaccination refusal was identified by serial non-vaccination in all eight choice tasks.
Statistical analysis
Full details on sample size calculation are provided in the appendix (p 2). We calculated the minimum sample size based on the assumption that COVID-19 vaccine acceptance is 50%. To estimate the probability of vaccine acceptance within 4% of the true value with a CI of 95%, based on each respondent completing eight choices, the minimum sample size was 300. Assuming 10% exclusions due to previous infection and to allow for random allocation in the full factorial design (three blocks on herd immunity × two blocks on GP advice on vaccination), the survey sample size was set to 2000 participants.27
We applied a single-hurdle repeated discrete choice model to account for a partition of the population between outright vaccine refusal and vaccine hesitancy in our data.28 The behavioural model is a two-part model that specifies one decision process underlying outright vaccination refusal and another decision process underlying vaccine hesitancy. The probability of outright vaccination refusal and associated factors was analysed with use of a logit regression model in the whole sample. The probability of vaccine acceptance based on vaccine characteristics and other factors was analysed with a conditional logit regression model for repeated discrete choices in the subsample of hesitant participants. Altogether, the two-part model provides an estimation of COVID-19 vaccine acceptance in the whole sample—ie, (1 – poutright vaccination refusal) × pvaccine acceptance.
The same set of independent factors was used to explain both decision processes and included the following: seven stratification variables used in the sampling procedure (gender, age group, educational level, household size [number of adults and number of children], area of residence, and region of residence), 12 variables related to vaccination behaviour (working status, health-care worker status, compliance with recommended vaccinations in the past, risk factors for severe COVID-19 [five variables, as detailed above], experience of COVID-19 [three variables], and perceived severity of COVID-19 if infected),18, 29 and the randomised information blocks. Compliance with recommended vaccinations in the past referred to vaccination for tetanus or before travelling abroad. Past compliance with recommended vaccinations was highly correlated with past immunisation behaviours against seasonal influenza or influenza A(H1N1)pdm09, which were discarded to avoid problems of multicollinearity (appendix p 3).
Probabilities and 95% CIs of outright vaccination refusal, vaccine acceptance in hesitant participants, and COVID-19 vaccine acceptance in the whole sample were estimated at the sample mean for 12 realistic vaccination scenarios based on the following: vaccine efficacy (50%26 or 90%9, 10), the risk of serious side-effects that could be assessable at different vaccination timepoints (detection threshold of 1 in 10 000 in vaccine phase 3 trials9, 10 or 1 in 100 000 shortly after the launch of worldwide vaccination campaigns), the location of the vaccine manufacturer (China, the USA, or the EU),11 and vaccination in mass vaccination centres with a herd immunity target of more than 50% of adults aged 18–64 years old but no opinion of GPs on vaccination.18 Probabilities and 95% CIs were similarly estimated after stratification by age group.
All analyses were based on two-sided p values, with p<0·05 considered to indicate statistical significance. The single-hurdle repeated discrete choice model was estimated with use of maximum likelihood techniques with R statistical software (version 3.6.3; appendix p 11).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of 2000 participants who were invited to and completed the online survey between June 22 and July 3, 2020, 58 (2·9%) reported previous SARS-CoV-2 infection and were excluded from the experiment. The remaining study sample (n=1942) was representative of adults aged 18–64 years residing in France, according to gender, age group, education level, household size, area of residence, and region of residence (table 2 ). In block 1, 652 (33·6%) participants were randomly allocated to receive information stating that more than 50% of adults aged 18–64 years old must be immunised (either by vaccination or infection) to reach herd immunity, 649 (33·4%) received information that more than 50% of adults must be immunised (either by vaccination or infection) to reach herd immunity; and 641 (33·0%) received no information on herd immunity. In block 2, 972 (50·1%) participants were randomly assigned to receive information stating that their GP recommended vaccination and 970 (49·9%) were told that their GP had no opinion on vaccination. Participants were evenly distributed across all groups in each block.
Table 2.
Characteristics and outright refusal of COVID-19 vaccination among study participants
| All participants (n=1942) |
Outright refusal of COVID-19 vaccination |
||||
|---|---|---|---|---|---|
| Yes (n=560) | No (n=1382) | p value | |||
| Survey stratification variables | |||||
| Gender | .. | .. | .. | <0·0001 | |
| Female | 993 (51·1%) | 343 (34·5%) | 650 (65·5%) | .. | |
| Male | 949 (48·9%) | 217 (22·9%) | 732 (77·1%) | .. | |
| Age, years | .. | .. | .. | <0·0001 | |
| 18–24 | 257 (13·2%) | 51 (19·8%) | 206 (80·2%) | .. | |
| 25–34 | 391 (20·1%) | 143 (36·6%) | 248 (63·4%) | .. | |
| 35–44 | 427 (22·0%) | 131 (30·7%) | 296 (69·3%) | .. | |
| 45–54 | 448 (23·1%) | 132 (29·5%) | 316 (70·5%) | .. | |
| 55–64 | 419 (21·6%) | 103 (24·6%) | 316 (75·4%) | .. | |
| Educational level | .. | .. | .. | <0·0001 | |
| Some high school | 854 (44·0%) | 301 (35·2%) | 553 (64·8%) | .. | |
| High school graduate | 420 (21·6%) | 116 (27·6%) | 304 (72·4%) | .. | |
| University graduate | 668 (34·4%) | 143 (21·4%) | 525 (78·6%) | .. | |
| Number of adults in household | .. | .. | .. | 0·077 | |
| 1 | 453 (23·3%) | 144 (31·8%) | 309 (68·2%) | .. | |
| 2 | 1038 (53·5%) | 303 (29·2%) | 735 (70·8%) | .. | |
| ≥3 | 451 (23·2%) | 113 (25·1%) | 338 (74·9%) | .. | |
| Number of children in household | .. | .. | .. | 0·045 | |
| 0 | 1125 (57·9%) | 298 (26·5%) | 827 (73·5%) | ||
| 1 | 379 (19·5%) | 121 (31·9%) | 258 (68·1%) | ||
| 2 | 265 (13·6%) | 81 (30·6%) | 184 (69·4%) | ||
| ≥3 | 173 (8·9%) | 60 (34·7%) | 113 (65·3%) | ||
| Area of residence | .. | .. | .. | 0·21 | |
| Rural area | 433 (22·3%) | 138 (31·9%) | 295 (68·1%) | .. | |
| Urban area of <100 000 inhabitants | 580 (29·9%) | 169 (29·1%) | 411 (70·9%) | .. | |
| Urban area of ≥100 000 inhabitants | 929 (47·8%) | 253 (27·2%) | 676 (72·8%) | .. | |
| Region of residence | .. | .. | .. | 0·34 | |
| Île-de-France (including Paris) | 373 (19·2%) | 94 (25·2%) | 279 (74·8%) | .. | |
| Northwest | 441 (22·7%) | 127 (28·8%) | 314 (71·2%) | .. | |
| Northeast | 436 (22·5%) | 139 (31·9%) | 297 (68·1%) | .. | |
| Southwest | 214 (11·0%) | 60 (28·0%) | 154 (72·0%) | .. | |
| Southeast | 478 (24·6%) | 140 (29·3%) | 338 (70·7%) | .. | |
| Variables related to vaccination behaviour | |||||
| Working status | .. | .. | .. | 0·15 | |
| Worker in the private sector | 830 (42·7%) | 220 (26·5%) | 610 (73·5%) | .. | |
| Worker in the public sector | 411 (21·2%) | 125 (30·4%) | 286 (69·6%) | .. | |
| Not working | 701 (36·1%) | 215 (30·7%) | 486 (69·3%) | .. | |
| Health-care worker | .. | .. | .. | 0·39 | |
| Yes | 124 (6·4%) | 40 (32·3%) | 84 (67·7%) | .. | |
| Not reported | 1818 (93·6%) | 520 (28·6%) | 1298 (71·4%) | .. | |
| Compliance with recommended vaccinations in the past | .. | .. | .. | <0·0001 | |
| Always | 989 (50·9%) | 182 (18·4%) | 807 (81·6%) | .. | |
| Sometimes | 711 (36·6%) | 237 (33·3%) | 474 (66·7%) | .. | |
| Never | 242 (12·5%) | 141 (58·3%) | 101 (41·7%) | .. | |
| Current pregnancy (among women) | .. | .. | .. | 0·64 | |
| Yes | 23 (2·3%) | 9 (39·1%) | 14 (60·9%) | .. | |
| Not reported | 970 (97·7%) | 334 (34·4%) | 636 (65·6%) | .. | |
| Smoking status | .. | .. | .. | 0·73 | |
| Former or current smoker | 1129 (58·1%) | 329 (29·1%) | 800 (70·9%) | .. | |
| Never smoker | 813 (41·9%) | 231 (28·4%) | 582 (71·6%) | .. | |
| Body-mass index, kg/m2 | .. | .. | .. | 0·35 | |
| ≥30 (obese) | 346 (17·8%) | 92 (26·6%) | 254 (73·4%) | .. | |
| 25 to <30 (overweight) | 569 (29·3%) | 158 (27·8%) | 411 (72·2%) | .. | |
| <25 (normal weight) | 1027 (52·9%) | 310 (30·2%) | 717 (69·8%) | .. | |
| Hypertension | .. | .. | .. | 0·037 | |
| Yes | 165 (8·5%) | 36 (21·8%) | 129 (78·2%) | .. | |
| Not reported | 1777 (91·5%) | 524 (29·5%) | 1253 (70·5%) | .. | |
| Chronic condition other than hypertension* | .. | .. | .. | <0·0001 | |
| Yes | 236 (12·2%) | 39 (16·5%) | 197 (83·5%) | .. | |
| Not reported | 1706 (87·8%) | 521 (30·5%) | 1185 (69·5%) | .. | |
| Had COVID-19 symptoms with no medical confirmation | .. | .. | .. | 0·048 | |
| Yes | 362 (18·6%) | 89 (24·6%) | 273 (75·4%) | .. | |
| Not reported | 1580 (81·4%) | 471 (29·8%) | 1109 (70·2%) | .. | |
| Had a negative test for SARS-CoV-2 infection | .. | .. | .. | 0·65 | |
| Yes | 50 (2·6%) | 13 (26·0%) | 37 (74·0%) | .. | |
| Not reported | 1892 (97·4%) | 547 (28·9%) | 1345 (71·1%) | .. | |
| Knows someone who had COVID-19 | .. | .. | .. | 0·0039 | |
| Yes, with hospital admission | 295 (15·2%) | 68 (23·1%) | 227 (76·9%) | .. | |
| Yes, without hospital admission | 522 (26·9%) | 136 (26·1%) | 386 (73·9%) | .. | |
| Not reported | 1125 (57·9%) | 356 (31·6%) | 769 (68·4%) | .. | |
| Perceived severity of COVID-19 if infected | .. | .. | .. | <0·0001 | |
| Very severe | 235 (12·1%) | 42 (17·9%) | 193 (82·1%) | .. | |
| Somewhat severe | 688 (35·4%) | 163 (23·7%) | 525 (76·3%) | .. | |
| Not particularly severe | 631 (32·5%) | 183 (29·0%) | 448 (71·0%) | .. | |
| Not severe at all | 105 (5·4%) | 49 (46·7%) | 56 (53·3%) | .. | |
| Do not know | 283 (14·6%) | 123 (43·5%) | 160 (56·5%) | .. | |
| Background information on COVID-19 vaccination | |||||
| Herd immunity against SARS-CoV-2 | .. | .. | .. | 0·27 | |
| >50% of adults aged 18–64 years old must be immunised (either by vaccination or infection) to reach herd immunity | 652 (33·6%) | 193 (29·6%) | 459 (70·4%) | .. | |
| >50% of adults must be immunised (either by vaccination or infection) to reach herd immunity | 649 (33·4%) | 197 (30·4%) | 452 (69·6%) | .. | |
| No information | 641 (33·0%) | 170 (26·5%) | 471 (73·5%) | .. | |
| General practitioner's advice on vaccination | .. | .. | .. | 0·79 | |
| Vaccination recommended | 972 (50·1%) | 283 (29·1%) | 689 (70·9%) | .. | |
| No opinion | 970 (49·9%) | 277 (28·6%) | 693 (71·4%) | .. | |
Data are n (%); percentages represent the distribution of variable categories among all participants or the split between outright refusal and no outright refusal of vaccination for participants in each category. p values are from χ2 test.
Diabetes (86 [36%] of 236 participants), asthma (88 [37%]), chronic lung disease other than asthma (47 [20%]), chronic arterial disease (13 [6%]), chronic heart disease (26 [11%]), chronic kidney disease (six [3%]), or cancer (21 [9%]).
560 (28·8%) participants were identified as refusing COVID-19 vaccination outright (table 2). In univariate analyses, outright vaccination refusal showed strongest associations (p<0·0001) with female gender, age (with an inverted U-shaped relationship), lower educational level, poorer compliance with recommended vaccinations in the past, no reported chronic condition (excluding hypertension), and lower perceived severity of COVID-19 if infected (table 2). Outright vaccination refusal was not associated with information blocks on the collective benefits of herd immunity (p=0·27) or GP's recommendation on vaccination (p=0·79).
Similar associations were found in the multivariate analysis (table 3 ). In particular, participants who never complied with recommended vaccinations in the past were significantly more likely to refuse any COVID-19 vaccine compared with those who always complied (adjusted odds ratio [OR] 5·59 [95% CI 4·03–7·79], p<0·0001). By contrast, participants who perceived COVID-19 as very severe if infected were significantly less likely to refuse any COVID-19 vaccine compared with participants who perceived COVID-19 as not severe at all (0·31 [0·18–0·55], p=0·0001).
Table 3.
Behavioural model of COVID-19 vaccination intentions
|
Outright refusal of COVID-19 vaccination (n=560) |
COVID-19 vaccine hesitancy (n=1382) |
||||
|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||
| Vaccine characteristics | |||||
| Efficacy | |||||
| 50% | .. | .. | 1 (ref) | · | |
| 80% | .. | .. | 0·70 (0·65–0·77) | <0·0001 | |
| 90% | .. | .. | 0·40 (0·38–0·42) | <0·0001 | |
| 100% | .. | .. | 0·36 (0·35–0·37) | <0·0001 | |
| Risk of serious side-effects | |||||
| 1 in 10 000 | .. | .. | 1 (ref) | .. | |
| 1 in 100 000 | .. | .. | 0·61 (0·56–0·66) | <0·0001 | |
| Region of vaccine manufacturer | |||||
| EU | .. | .. | 1 (ref) | .. | |
| China | .. | .. | 2·87 (2·42–3·52) | <0·0001 | |
| USA | .. | .. | 1·86 (1·64–2·15) | <0·0001 | |
| Place of vaccination administration | |||||
| Mass vaccination centre | .. | .. | 1 (ref) | .. | |
| General practitioner's practice | .. | .. | 0·80 (0·76–0·84) | <0·0001 | |
| Local pharmacy | .. | .. | 0·81 (0·76–0·86) | <0·0001 | |
| Background information on COVID-19 vaccination | |||||
| Herd immunity against SARS-CoV-2 | |||||
| No information | 1 (ref) | .. | 1 (ref) | .. | |
| >50% of adults aged 18–64 years old must be immunised (either by vaccination or infection) to reach herd immunity | 1·14 (0·87–1·48) | 0·35 | 0·76 (0·70–0·85) | <0·0001 | |
| >50% of adults must be immunised (either by vaccination or infection) to reach herd immunity | 1·16 (0·89–1·51) | 0·28 | 0·82 (0·75–0·92) | 0·0024 | |
| General practitioner's advice on vaccination | |||||
| No opinion | 1 (ref) | .. | 1 (ref) | .. | |
| Vaccination recommended | 1·01 (0·81–1·25) | 0·95 | 1·02 (0·92–1·15) | 0·67 | |
| Survey stratification variables | |||||
| Gender | |||||
| Female | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 0·57 (0·46–0·72) | <0·0001 | 0·82 (0·75–0·90) | 0·0002 | |
| Age, years | |||||
| 18–24 | 1 (ref) | .. | 1 (ref) | .. | |
| 25–34 | 1·99 (1·30–3·09) | 0·0017 | 1·37 (1·09–1·84) | 0·0011 | |
| 35–44 | 1·85 (1·19–2·90) | 0·0066 | 1·17 (0·95–1·52) | 0·13 | |
| 45–54 | 1·92 (1·25–3·00) | 0·0035 | 0·91 (0·77–1·11) | 0·37 | |
| 55–64 | 1·67 (1·07–2·61) | 0·025 | 0·72 (0·63–0·84) | 0·0014 | |
| Educational level | |||||
| Some high school | 1 (ref) | .. | 1 (ref) | .. | |
| High school graduate | 0·75 (0·56–1·00) | 0·054 | 0·99 (0·87–1·15) | 0·88 | |
| University graduate | 0·55 (0·41–0·72) | <0·0001 | 0·86 (0·77–0·97) | 0·029 | |
| Number of adults in household | 0·95 (0·80–1·12) | 0·51 | 1·00 (0·92–1·08) | 0·94 | |
| Number of children in household | 1·08 (0·96–1·22) | 0·17 | 1·04 (0·98–1·10) | 0·20 | |
| Area of residence | |||||
| Rural area | 1 (ref) | .. | 1 (ref) | .. | |
| Urban area of <100 000 inhabitants | 0·85 (0·63–1·14) | 0·28 | 1·00 (0·87–1·18) | 0·96 | |
| Urban area of ≥100 000 inhabitants | 0·94 (0·70–1·26) | 0·66 | 1·10 (0·95–1·31) | 0·21 | |
| Region of residence | |||||
| Île-de-France (including Paris) | 1 (ref) | .. | 1 (ref) | .. | |
| Northwest | 1·13 (0·78–1·65) | 0·52 | 1·09 (0·91–1·36) | 0·34 | |
| Northeast | 1·26 (0·88–1·81) | 0·21 | 1·11 (0·93–1·38) | 0·26 | |
| Southwest | 1·03 (0·66–1·59) | 0·90 | 1·14 (0·92–1·49) | 0·22 | |
| Southeast | 1·15 (0·81–1·63) | 0·43 | 1·34 (1·10–1·72) | 0·0005 | |
| Variables related to vaccination behaviour | |||||
| Working status | |||||
| Not working | 1 (ref) | .. | 1 (ref) | .. | |
| Worker in the private sector | 0·91 (0·69–1·20) | 0·51 | 0·79 (0·71–0·88) | 0·0004 | |
| Worker in the public sector | 1·12 (0·81–1·55) | 0·48 | 0·82 (0·73–0·95) | 0·017 | |
| Health-care worker | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 1·25 (0·79–1·94) | 0·33 | 1·18 (0·93–1·62) | 0·14 | |
| Compliant with recommended vaccination in the past | |||||
| Always | 1 (ref) | .. | 1 (ref) | .. | |
| Sometimes | 2·17 (1·72–2·76) | <0·0001 | 1·76 (1·47–2·19) | <0·0001 | |
| Never | 5·59 (4·03–7·79) | <0·0001 | 2·08 (1·49–3·44) | <0·0001 | |
| Current pregnancy | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 1·10 (0·42–2·73) | 0·84 | 1·45 (0·89–3·93) | 0·096 | |
| Smoking status | |||||
| Never smoker | 1 (ref) | .. | 1 (ref) | .. | |
| Former or current smoker | 0·80 (0·64–1·01) | 0·057 | 0·94 (0·85–1·05) | 0·30 | |
| Body-mass index, kg/m2 | |||||
| <25 (normal weight) | 1 (ref) | .. | 1 (ref) | .. | |
| 25 to <30 (overweight) | 0·93 (0·72–1·19) | 0·56 | 1·02 (0·91–1·18) | 0·70 | |
| ≥30 (obese) | 0·80 (0·59–1·10) | 0·18 | 1·01 (0·87–1·19) | 0·94 | |
| Hypertension | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 1·14 (0·72–1·78) | 0·57 | 0·72 (0·62–0·87) | 0·0065 | |
| Chronic condition other than hypertension | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 0·60 (0·40–0·87) | 0·0088 | 1·05 (0·90–1·25) | 0·55 | |
| Had COVID-19 symptoms with no medical confirmation | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 0·80 (0·60–1·07) | 0·13 | 0·85 (0·76–0·96) | 0·017 | |
| Had a negative test for SARS-CoV-2 infection | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 0·90 (0·42–1·78) | 0·76 | 1·03 (0·77–1·57) | 0·85 | |
| Knows someone who had COVID-19 | |||||
| Not reported | 1 (ref) | .. | 1 (ref) | .. | |
| Yes, with hospital admission | 0·86 (0·66–1·12) | 0·28 | 0·89 (0·80–1·00) | 0·062 | |
| Yes, without hospital admission | 0·78 (0·56–1·08) | 0·14 | 0·73 (0·66–0·83) | 0·0001 | |
| Perceived severity of COVID-19 if infected | |||||
| Not severe at all | 1 (ref) | .. | 1 (ref) | .. | |
| Very severe | 0·31 (0·18–0·55) | 0·0001 | 1·10 (0·83–1·65) | 0·52 | |
| Somewhat severe | 0·38 (0·24–0·62) | 0·0001 | 0·91 (0·73–1·21) | 0·49 | |
| Not particularly severe | 0·50 (0·32–0·80) | 0·0037 | 1·11 (0·86–1·58) | 0·44 | |
| Do not know | 0·76 (0·46–1·25) | 0·28 | 1·07 (0·82–1·57) | 0·64 | |
ORs and 95% CIs were estimated simultaneously with use of a two-part model to disentangle outright refusal of COVID-19 vaccination in the whole sample and COVID-19 vaccine acceptance in the subsample of hesitant participants. Results of the second part of the model (ie, vaccine hesitancy) represent odds of refusal of COVID-19 vaccines (rather than acceptance of COVID-19 vaccines). OR=odds ratio.
Among participants who did not refuse vaccination outright (1382 [71·2%]), vaccine hesitancy showed dependence on all the assessed vaccine characteristics, including vaccine efficacy and the location of the vaccine manufacturer (table 3). In this regard, the reduction of vaccine hesitancy for 90% (vs 50%) vaccine efficacy (OR 0·40 [95% CI 0·38–0·42], p<0·0001) was entirely offset if the vaccine was made in China rather than the EU (2·87 [2·42–3·52], p<0·0001). Vaccine hesitancy was significantly reduced with a 1 in 100 000 risk of serious side-effects compared with a 1 in 10 000 risk (0·61 [0·56–0·66], p<0·0001), and with vaccination at a GP practice (0·80 [0·76–0·84], p<0·0001) or local pharmacy (0·81 [0·76–0·86], p<0·0001) compared with at a mass vaccination centre. In addition, vaccine hesitancy significantly decreased with communication about the collective benefits of herd immunity (vs no information), especially with a herd immunity target of more than 50% of adults aged 18–64 years (0·76 [0·70–0·85], p<0·0001; table 3). By contrast, GP's advice on vaccination was not significantly associated with vaccine hesitancy (p=0·67).
Similar to outright vaccination refusal, independent predictors of vaccine hesitancy included female gender, age (with an inverted U-shaped relationship), lower educational level, poor compliance with recommended vaccinations in the past, and no reported hypertension (as a chronic condition; table 3). In contrast to vaccination refusal, vaccine hesitancy was not associated with the perceived severity of COVID-19 if infected, but was significantly decreased with working status (vs not working) and experience of COVID-19 (having had symptoms or knowing someone with COVID-19), and was increased in the southeast region versus Île-de-France.
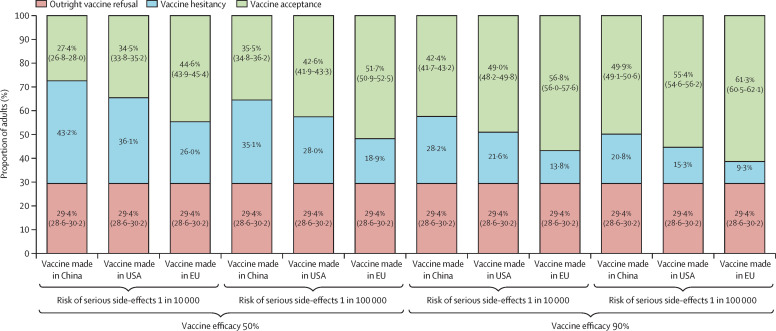
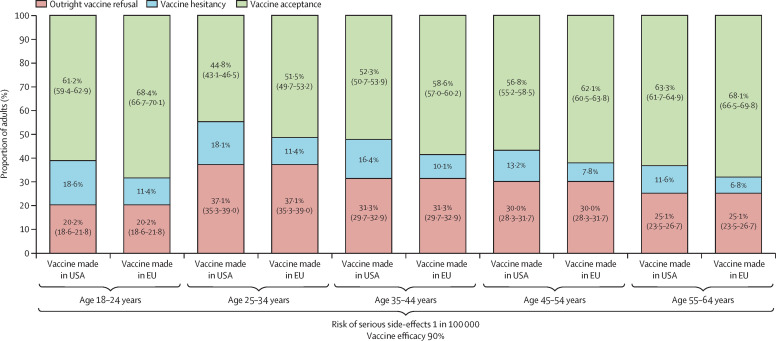
In the context of a mass vaccination campaign with vaccine administration in mass vaccination centres and communication about the collective benefits of herd immunity, the behavioural model predicted that outright refusal of COVID-19 vaccination would be present in 29·4% (95% CI 28·6–30·2) of the working-age population in France (appendix p 12). Furthermore, COVID-19 vaccine hesitancy would be maximised for a vaccine manufactured in China with 50% efficacy and a risk of serious side-effects detectable at a 1 in 10 000 threshold, resulting in 27·4% (26·8–28·0) vaccine acceptance (figure 1 ; appendix p 12). By contrast, COVID-19 vaccine hesitancy would be minimised for a vaccine manufactured in the EU with 90% efficacy and a risk of serious side-effects detectable at a lower threshold of 1 in 100 000, resulting in 61·3% (60·5–62·1) vaccine acceptance (figure 1; appendix p 12). As both outright vaccination refusal and vaccine hesitancy were associated with age with an inverted U-shaped relationship, COVID-19 vaccine acceptance would be maximised at both ends of the age spectrum in the working-age population (18–24 years 68·4% [66·7–70·1]; 55–64 years 68·1% [66·5–69·8]; figure 2 ).
Figure 1.
COVID-19 vaccine acceptance predicted in the French working-age population depending on vaccine efficacy, vaccine safety, and location of vaccine manufacturer
The probability of vaccine hesitancy in each scenario was calculated by deduction of the probabilities of outright refusal of vaccination and vaccine acceptance from 100%; appendix p 12).
Figure 2.
COVID-19 vaccine acceptance predicted in the French working-age population depending on age group, vaccine efficacy, vaccine safety, and location of vaccine manufacturer
The probability of vaccine hesitancy in each scenario was calculated by deduction of the probabilities of outright refusal of vaccination and vaccine acceptance from 100%; appendix p 12).
Discussion
This study shows that COVID-19 vaccination intentions in the French working-age population depend on the characteristics of new vaccines and the national vaccination strategy, among many other factors. In this survey experiment, COVID-19 vaccination intentions were assessed from repeated choice tasks among vaccines with varying characteristics. Accordingly, we developed a behavioural model that allowed us to disentangle outright vaccination refusal (serial non-vaccination regardless of vaccine characteristics) from vaccine hesitancy (vaccine acceptance based on vaccine characteristics).19 As of early July, 2020, 29% of the French working-age population would refuse any COVID-19 vaccine, 27% would accept COVID-19 vaccines provided in mass vaccination centres, even with less favourable characteristics (vaccine efficacy at 50% and risk of serious side-effects at 1 in 10 000) and a manufacturer based in China, and 43% would remain hesitant unless COVID-19 vaccines had better characteristics or were manufactured in the USA or EU. Both outright vaccination refusal and vaccine hesitancy showed independent associations with age with an inverted U-shaped relationship, suggesting that consideration should be given to increasing the priority level for vaccination of the youngest adults at high risk of SARS-CoV-2 transmission.
The study findings shed new light on anti-COVID-19 vaccination behaviour and its underlying mechanisms. Two results suggest that the outright vaccination refusal measured in this study is a good proxy of anti-COVID-19 vaccination behaviour. Outright vaccination refusal is associated with a high level of decision-making certainty: participants refused all 16 hypothetical vaccines with varying characteristics (appendix p 10). The double-hurdle model28 showed that a negligible proportion of those participants would accept vaccination even if presented with an ideal vaccine on all four characteristics (data not shown). Furthermore, we found no association between outright vaccination refusal and other experimental variables (collective benefits of herd immunity or recommendation of vaccination by the GP).
We found that anti-COVID-19 vaccination behaviour was strongly associated with certain characteristics of the participants: female gender, age with an inverted U-shaped relationship, lower educational level, poorer compliance with recommended vaccinations in the past, no report of a chronic condition, and lower perceived severity of COVID-19 if infected. These results corroborate other studies' findings on the determinants of COVID-19 vaccine hesitancy, in particular female gender12, 14, 15, 16, 30 and lower educational level.12, 15, 30 Other attitudinal characteristics suggest the exacerbation of underlying vaccine hesitancy mechanisms in anti-COVID-19 vaccination behaviour, namely distrust in vaccination and vaccination complacency.19
Outright vaccination refusal implies that people refused all four hypothetical vaccines that had 100% efficacy (appendix p 10) and all eight hypothetical vaccines that had the lowest risk of serious side-effects (1 in 100 000), which corresponds to a detection threshold that cannot be reached in vaccine phase 3 trials that have enrolled fewer than 100 000 people.9, 10 These findings suggest distrust in the effectiveness and safety of new COVID-19 vaccines. Outright vaccination refusal was also associated with lower compliance with immunisation in the past, reflecting the anchoring effect of negative attitudes towards vaccination in France and distrust in vaccine safety in particular.18, 21, 31 Other studies on COVID-19 vaccination intentions found that concern about vaccine safety was the main reason for refusing vaccination in France.14, 15 A more general distrust in health policy and services12, 14, 15, 16 was also suggested by three of our findings: the outright refusal of COVID-19 vaccination regardless of the country of the manufacturer, where the vaccination is given, and whether or not the GP recommends vaccination. Future studies should explicitly consider the role of trust in outright vaccination refusal and vaccine hesitancy.
In accordance with other studies on COVID-19 vaccination intentions,12, 14, 15 we found that vaccination complacency might fuel anti-COVID-19 vaccination behaviour, as outright vaccination refusal was strongly associated with a lower perceived severity of COVID-19 if infected and the absence of a reported chronic condition. Given concerns about vaccine safety in this group, the individual benefit–risk assessment of COVID-19 vaccination might be perceived as unfavourable. The risk of developing a severe form of COVID-19 is low in the working-age population and very low in adults younger than 50 years.3, 4, 5 Although the death toll from COVID-19 amounted to 50 000 deaths after the peak of the second wave of the epidemic in France, 553 (1·1%) of these deaths were recorded in people younger than 50 years of age (as of Nov 26, 2020),32 and were most likely attributable to underlying chronic conditions.5 Although the individual benefit–risk balance could be assessed more broadly, we found that outright vaccination refusal was insensitive to communication about both the individual and societal benefits of herd immunity as a way to return to a normal life.
COVID-19 vaccine hesitancy was sensitive to all four characteristics presented for the hypothetical vaccines. Hesitancy decreased with higher vaccine efficacy and lower risk of serious side-effects, and increased if vaccination was only accessible in mass vaccination centres rather than a GP practice or local pharmacy.18 Recommendation of vaccination by the GP would not be enough to compensate for COVID-19 vaccine hesitancy related to mass vaccination centres.18 The lack of significance of the GP's recommendation on vaccine acceptance might be because the related randomised statement (only one sentence) was presented before the discrete choice experiment (appendix p 6) and because four of eight choice tasks included a direct comparison of vaccination at GP practice versus mass vaccination centres (appendix p 10). Accordingly, vaccination at GP practice might have captured the expected effect of a GP's recommendation on vaccine acceptance. COVID-19 vaccine hesitancy increased if the vaccine manufacturer was not from the EU; the effect of 80% or even 90% (vs 50%) vaccine efficacy on hesitancy was entirely offset if the vaccine was made in the USA or China. Such distrust in foreign COVID-19 vaccines could be explained by the perception of rushed vaccine development, as made explicit by Operation Warp Speed in the USA,12, 14, 15 or a more profound distrust of Chinese products since the beginning of the SARS-CoV-2 pandemic.16, 17
Finally, this survey experiment provides causal evidence that communicating the collective benefits of herd immunity in terms of a herd immunity target is associated with significantly reduced COVID-19 vaccine hesitancy in the working-age population, confirming previous findings based on fictitious epidemics.24 This finding is important in the context of the COVID-19 pandemic because the working-age population accounts for the majority (58%) of the French population (French census on Jan 1, 2020) and herd immunity can only be reached if COVID-19 vaccine acceptance is substantial in this group.6, 8 In this study, COVID-19 vaccine acceptance was highest at both ends of the working-age spectrum, among the youngest adults at highest risk of SARS-CoV-2 infection7 and the oldest adults at highest risk of a severe form of COVID-19.4 These findings suggest that a strict COVID-19 vaccination prioritisation based on age from oldest to youngest adults23 could miss a major opportunity to achieve herd immunity more quickly by vaccinating the youngest adults who are very likely to accept vaccination. A strategy prioritising vaccination in the younger population, after those aged 50 years and older, could alleviate physical distancing measures in young adults who are at a very low risk of a severe form of COVID-19.6, 23
The main limitation of our study is the timing of the survey in early July, 2020. At this time, there was little information about COVID-19 candidate vaccines and vaccination locations, and people's vaccination intentions might have evolved over time with new information. Nevertheless, a full page of background information on COVID-19 vaccination was presented (appendix p 6), providing common background information to all respondents (who were also randomly allocated to the two information blocks). This design should control for much of the information that people have become aware of over time. The vaccine characteristics presented in the survey experiment represent four salient characteristics expected to be associated with COVID-19 vaccine hesitancy (efficacy, safety, country of the vaccine manufacturer, and place of vaccine administration). Other vaccine characteristics not included in this study are the risk of minor side-effects and duration of vaccine immunity; however, another study suggested that these factors would be less relevant to decision making than the factors we included in our study.17 In early July, 2020, the French population was recovering from the first wave of SARS-CoV-2 and some of the most stringent physical distancing measures in Europe.2 People's benefit–risk assessment of COVID-19 vaccination might have evolved over time in response to rising numbers of SARS-CoV-2 infections, reintroduced physical distancing measures since Oct 30, 2020,2 the approval of COVID-19 candidate vaccines with higher than 90% efficacy,9, 10 and vaccine safety concerns (eg, allergic reactions) that might not be captured by the presentation of absolute risks of serious side-effects. Additionally, the types of places that could be used for mass vaccination centres were not specified in the survey experiment. Respondents' interpretations of this feature might evolve as the location of mass vaccination centres is announced and further influence COVID-19 vaccine acceptance.
Another possible limitation is that we identified outright vaccination refusal as refusal to be vaccinated in all eight choice tasks, regardless of vaccine characteristics. This response pattern could also represent a fast way for respondents to complete the tasks; however, this behaviour is not commonly observed in discrete choice experiments.33 Furthermore, our results have content validity and are consistent with other studies of COVID-19 vaccination intentions that used different methods.12 A further limitation is that the death toll has been even more pronounced in those aged 50 years or older32 during the second wave compared with the first wave of SARS-CoV-2. As a result, the perceived severity of COVID-19 among the working-age population could either remain stable or decrease, which might reduce vaccine acceptance. Furthermore, as the start of a mass vaccination campaign draws nearer, the antivaccine movement could become more active, as suggested by an upward trend in COVID-19 vaccine hesitancy before November, 2020.12, 15 The dynamics of anti-COVID-19 vaccination behaviour should be closely monitored.
In summary, this survey experiment shows that COVID-19 vaccine acceptance in the working-age population depends on the characteristics of new vaccines and the national vaccination strategy, among many other factors. Acceptance of the first-in-race vaccines using an mRNA platform9, 10 is likely to be lower than expected in France because logistical and cold storage constraints might require mass vaccination centres, the vaccine is designated as an American vaccine9, 10 rather than German technology,9 and the national vaccination strategy prioritises the self-protection of older adults rather than herd immunity in the whole adult population.23 Whether or not the objective of a national vaccination strategy becomes herd immunity, our results suggest that it would be more successful in France with COVID-19 vaccines made in the EU and with a communication strategy emphasising the collective benefits of herd immunity in the working-age population.
Data sharing
Deidentified cross-sectional data from the analysis and the R program to estimate hurdle discrete choice models can be made available by the corresponding author after the authors' review of requests.
Acknowledgments
Acknowledgments
The Department of Methodology and Innovation in Prevention at the Teaching Hospital of Bordeaux is partially funded by the Regional Health Agency (Agence Régionale de Santé) of Nouvelle-Aquitaine. The Health Economics Research Unit at the University of Aberdeen is funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. We acknowledge Centre de Calcul Intensif d'Aix-Marseille for granting access to its high-performance computing resources.
Contributors
MS conceptualised the study, contributed to the overall design of the survey experiment, analysis, and interpretation of the data, wrote the first draft of the paper, and is the guarantor for the study. VW designed the discrete choice experiment and contributed to the analysis and interpretation of the data. PA and FA contributed to the discussion of public health implications and helped shape the overall interpretation. SL contributed to the overall design of the survey experiment, did the analysis including R programming of hurdle repeated discrete choice models, and contributed to interpretation of the data. All authors had access to all the data in the study and had final responsibility for the decision to submit for publication. The underlying survey data were verified by MS and SL.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Flaxman S, Mishra S, Gandy A. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control Weekly COVID-19 country overview. https://www.ecdc.europa.eu/en/covid-19/country-overviews
- 3.Verity R, Okell LC, Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salje H, Tran Kiem C, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson EJ, Walker AJ, Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan AB, Winskill P, Watson OJ. Imperial College London; London: 2020. Report 33: modelling the allocation and impact of a COVID-19 vaccine. [DOI] [Google Scholar]
- 7.Carrat F, de Lamballerie X, Rahib D. Seroprevalence of SARS-CoV-2 among adults in three regions of France following the lockdown and associated risk factors: a multicohort study. medRxiv. 2020 doi: 10.1101/2020.09.16.20195693. published online Sept 18. (preprint). [DOI] [Google Scholar]
- 8.Saad-Roy CM, Wagner CE, Baker RE. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020;370:811–818. doi: 10.1126/science.abd7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, El Sahly HM, Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020 doi: 10.1056/nejmoa2035389. published online Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 12.Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel) 2020;9:e16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarus JV, Ratzan SC, Palayew A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020 doi: 10.1038/s41591-020-1124-9. published online Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward JK, Alleaume C, Peretti-Watel P. The French public's attitudes to a future COVID-19 vaccine: the politicization of a public health issue. Soc Sci Med. 2020;265 doi: 10.1016/j.socscimed.2020.113414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacquin A-S, Altay S, de Araujo E, Chevallier C, Mercier H. Sharp rise in vaccine hesitancy in a large and representative sample of the French population: reasons for vaccine hesitancy. PsyArXiv. 2020 doi: 10.31234/osf.io/r8h6z. published online Nov 17. (preprint, version 2) [DOI] [Google Scholar]
- 16.Roozenbeek J, Schneider CR, Dryhurst S. Susceptibility to misinformation about COVID-19 around the world. R Soc Open Sci. 2020;7 doi: 10.1098/rsos.201199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreps S, Prasad S, Brownstein JS. Factors associated with US adults' likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzinger M, Flicoteaux R, Cortarenoda S, Obadia Y, Moatti JP. Low acceptability of A/H1N1 pandemic vaccination in French adult population: did public health policy fuel public dissonance? PLoS One. 2010;5 doi: 10.1371/journal.pone.0010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Johnson NF, Velásquez N, Restrepo NJ. The online competition between pro- and anti-vaccination views. Nature. 2020;582:230–233. doi: 10.1038/s41586-020-2281-1. [DOI] [PubMed] [Google Scholar]
- 21.Mereckiene J, Cotter S, Weber JT. Influenza A(H1N1)pdm09 vaccination policies and coverage in Europe. Euro Surveill. 2012;17 doi: 10.2807/ese.17.04.20064-en. [DOI] [PubMed] [Google Scholar]
- 22.Verger P, Fressard L, Cortaredona S. Trends in seasonal influenza vaccine coverage of target groups in France, 2006/07 to 2015/16: impact of recommendations and 2009 influenza A(H1N1) pandemic. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.48.1700801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haute Autorité de Santé Stratégie de vaccination contre le SARS-CoV-2—recommandations préliminaires sur la stratégie de priorisation des populations à vacciner. Nov 27, 2020. https://www.has-sante.fr/jcms/p_3221338/fr/strategie-de-vaccination-contre-le-sars-cov-2-recommandations-preliminaires-sur-la-strategie-de-priorisation-des-populations-a-vacciner
- 24.Betsch C, Böhm R, Korn L, Holtmann C. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:0056. [Google Scholar]
- 25.Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19:300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30773-8. published online Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8:373–384. doi: 10.1007/s40271-015-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Haefen RH, Massey DM, Adamowicz WL. Serial nonparticipation in repeated discrete choice models. Am J Agric Econ. 2005;87:1061–1076. [Google Scholar]
- 29.Bish A, Yardley L, Nicoll A, Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29:6472–6484. doi: 10.1016/j.vaccine.2011.06.107. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes A, Hoq M, Measey MA, Danchin M. Intention to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30724-6. published online Sept 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson HJ, de Figueiredo A, Xiahong Z. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi: 10.1016/j.ebiom.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santé publique France COVID-19 : point épidémiologique du 26 novembre 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-26-novembre-2020
- 33.Ladenburg J, Olsen SB. Augmenting short cheap talk scripts with a repeated opt-out reminder in choice experiment surveys. Resour Energy Econ. 2014;37:39–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified cross-sectional data from the analysis and the R program to estimate hurdle discrete choice models can be made available by the corresponding author after the authors' review of requests.