Abstract
Background
The objective of this study was to better understand the factors associated with the heterogeneity of in-hospital COVID-19 morbidity and mortality across France, one of the countries most affected by COVID-19 in the early months of the pandemic.
Methods
This geo-epidemiological analysis was based on data publicly available on government and administration websites for the 96 administrative departments of metropolitan France between March 19 and May 11, 2020, including Public Health France, the Regional Health Agencies, the French national statistics institute, and the Ministry of Health. Using hierarchical ascendant classification on principal component analysis of multidimensional variables, and multivariate analyses with generalised additive models, we assessed the associations between several factors (spatiotemporal spread of the epidemic between Feb 7 and March 17, 2020, the national lockdown, demographic population structure, baseline intensive care capacities, baseline population health and health-care services, new chloroquine and hydroxychloroquine dispensations, economic indicators, degree of urbanisation, and climate profile) and in-hospital COVID-19 incidence, mortality, and case fatality rates. Incidence rate was defined as the cumulative number of in-hospital COVID-19 cases per 100 000 inhabitants, mortality rate as the cumulative number of in-hospital COVID-19 deaths per 100 000, and case fatality rate as the cumulative number of in-hospital COVID-19 deaths per cumulative number of in-hospital COVID-19 cases.
Findings
From March 19 to May 11, 2020, hospitals in metropolitan France notified a total of 100 988 COVID-19 cases, including 16 597 people who were admitted to intensive care and 17 062 deaths. There was an overall cumulative in-hospital incidence rate of 155·6 cases per 100 000 inhabitants (range 19·4–489·5), in-hospital mortality rate of 26·3 deaths per 100 000 (1·1–119·2), and in-hospital case fatality rate of 16·9% (4·8–26·2). We found clear spatial heterogeneity of in-hospital COVID-19 incidence and mortality rates, following the spread of the epidemic. After multivariate adjustment, the delay between the first COVID-19-associated death and the onset of the national lockdown was positively associated with in-hospital incidence (adjusted standardised incidence ratio 1·02, 95% CI 1·01–1·04), mortality (adjusted standardised mortality ratio 1·04, 1·02–1·06), and case fatality rates (adjusted standardised fatality ratio 1·01, 1·01–1·02). Mortality and case fatality rates were higher in departments with older populations (adjusted standardised ratio for populations with a high proportion older than aged >85 years 2·17 [95% CI 1·20–3·90] for mortality and 1·43 [1·08–1·88] for case fatality rate). Mortality rate was also associated with incidence rate (1·0004, 1·0002–1·001), but mortality and case fatality rates did not appear to be associated with baseline intensive care capacities. We found no association between climate and in-hospital COVID-19 incidence, or between economic indicators and in-hospital COVID-19 incidence or mortality rates.
Interpretation
This ecological study highlights the impact of the epidemic spread, national lockdown, and reactive adaptation of intensive care capacities on the spatial distribution of COVID-19 morbidity and mortality. It provides information for future geo-epidemiological analyses and has implications for preparedness and response policies to current and future epidemic waves in France and elsewhere.
Funding
None.
Introduction
The spread of the COVID-19 pandemic has exhibited important heterogeneity between countries and regions.1 Spatial differences between incidence and mortality rates have been associated with factors as various as the arrival time of SARS-CoV-2,2 population age structure,3 urban development and population density,4 economic level,4 health system,5 climatic and meteorological factors,6 and anti-contagion policies and practices.7, 8, 9
In France, the first COVID-19 case was confirmed on Jan 24, 2020. An exponential epidemic then spread rapidly, resulting in a national lockdown from March 17, 2020. The first wave peaked on March 31, and lockdown ended on May 11 (panel ). By June 10, 2020, France was one of the most affected countries, with 150 000 cumulative cases confirmed by WHO and nearly 30 000 associated deaths.
Panel. The first wave of the COVID-19 epidemic and mitigation measures in France.
SARS-CoV-2 seemed to be already spreading in France in late December, 2019,10 but the first three patients prospectively diagnosed with COVID-19 were reported on Jan 24, 2020, in Bordeaux and Paris, all returning from Wuhan (China).11, 12, 13 In February, 2020, four clusters were reported in other areas, including Haute-Savoie, Oise, Morbihan, and Haut-Rhin. These clusters were mainly due to contact with people travelling from Singapore, China, Italy, or Egypt, and no specific spatial trends were identified at this stage. In Mulhouse city (Haut-Rhin), one religious event that took place from Feb 17 to Feb 21, 2020, brought together 2000–2500 participants from all over France and several other countries (eg, Belgium, Switzerland, Germany, and Burkina Faso). Many attendees became infected and then spread the virus onwards when returning home. The Oise cluster, near Paris, appeared in late February around a military airbase employing around 2500 people. Several airbase staff had been involved in the repatriation of a French citizen from China on Jan 31, 2020.
Following these initial sporadic cases and limited clusters, the COVID-19 epidemic wave rapidly spread from northeastern France (Grand-Est) towards north-central regions (Île-de-France and Haut-de-France). Given the exponential number and spatial diffusion of new cases and clusters, the first official mitigation measures were gradually applied from March 5, 2020, such as a ban on gathering of more than 5000 people, then 1000 people (March 14) and 100 people (March 16). On March 15, the first round of municipal elections took place but unessential services, including restaurants and cafes, were closed, followed by schools and main religious services the next day. On March 17, as France had already recorded a total of 7730 cases and 175 deaths, and as incidence was doubling every 3 days, a national lockdown was ordered by the government,14 and eventually extended until May 11. The daily incidence peak was reached on March 31, with 7578 new confirmed cases. However, considering the low level of testing capacities in France at this time of the epidemic, a model suggests that as many as 300 000 daily new infections might have occurred right before the lockdown onset.15 Hampered by the lockdown, the epidemic reached the rest of the country more slowly. In these other departments, the first deaths generally occurred after lockdown implementation, except for specific departments such as Corse-du-Sud (Corsica Island) and Morbihan (Brittany region). In the southeastern region, the most densely populated department (Bouche-du-Rhône including Marseille, the second largest French city) was more affected than the surrounding ones, in terms of incidence and mortality rates.
Research in context.
Evidence before this study
The spread of the COVID-19 pandemic has shown important heterogeneity between countries and regions, and France was one of the most affected countries in the early months of the pandemic. To assess the factors associated with the spatial differences between incidence and mortality rates, we searched PubMed for all articles up to Sept 28, 2020, using the query (COVID-19 OR SARS-CoV-2) AND ((France [title] AND epidemiology) OR (France [title] AND lockdown) OR (lockdown [title] AND (effect [title] OR effectiveness [title] OR impact [title])) OR “ecological study” OR (socioeconomic [title] OR demography [title] OR demographic [title] OR climate [title] OR climatic [title])). Some articles describing the initial COVID-19 clusters in France presented results of mechanistic or agent-based models at a coarse spatial scale, but none of the 456 articles described the first pandemic wave in France and analysed associated factors at a fine spatial scale. Similarly, the effect of the national lockdown in France was studied by only a few mechanistic or agent-based models. Worldwide, the importance of lockdown policies to drastically decrease COVID-19 cases and deaths was mostly suggested by model predictions, or by a few inter-country epidemiological comparisons. The influence of population age structure on COVID-19-associated mortality was highlighted at the country level but not studied at finer scales. COVID-19 incidence and mortality appeared to be higher in countries with a high socioeconomic status, but within affected countries or territories, populations with low socioeconomic status generally reported lower cases and mortality, suggesting a frequent testing and reporting bias.
Added value of this study
This geo-epidemiological multivariate analysis confirmed that the marked spatial heterogeneity of in-hospital COVID-19 cases and deaths across metropolitan France was strongly associated with the initial spread of the first pandemic wave before its efficient freezing by the national lockdown. Case fatality rate was not associated with the initial number of intensive care beds, suggesting that hospitals could rapidly scale up their capacities and organise medical evacuations to less affected areas. We found no independent association between in-hospital COVID-19 incidence and the four climates of metropolitan France.
Implications of all the available evidence
Epidemiological analyses should include COVID-19 pandemic spread, demographic structure, mitigation measures, and health-care capacities. All countries should adapt their preparedness plans to these key factors to better respond to current or future pandemic waves.
Although the spatial distribution of the first epidemic wave was heterogeneous across the country,15 no study has yet analysed the underlying combination of determinants of this spatial heterogeneity. We therefore did a geo-epidemiological analysis using data that were publicly available on government and administration websites to study the ecological factors associated with in-hospital COVID-19 incidence, mortality, and case fatality rates across France during the national lockdown. Our objective was to better understand the factors potentially associated with the heterogeneity of in-hospital COVID-19 morbidity and mortality across the country.
Methods
Parameters and data collection
In this geo-epidemiological study, we assessed the ecological factors associated with in-hospital COVID-19 incidence, mortality, and case fatality rates across the 96 administrative departments of France during the national lockdown. Ecological factors included the spatiotemporal spread of the epidemic before the national lockdown, demographic structure of the population, baseline intensive care capacities, baseline population health and health-care services, new chloroquine and hydroxychloroquine dispensations, economic indicators, urbanisation, and climate.
Numbers of suspected COVID-19 outpatients were not systematically recorded by the French surveillance system coordinated by Santé publique France (Public Health France). Indeed, most infectious cases, symptomatic or not, were not detected during the first epidemic wave, and, due to a shortage of tests, RT-PCR tests were reserved for in-hospital cases. However, confirmed (by RT-PCR) or probable (according to clinical and CT-scan signs) in-hospital COVID-19 cases and deaths were systematically compiled by Santé publique France from March 19, 2020, onwards. We downloaded these in-hospital data, aggregated at the departmental level, from the open data portal of the French Government (appendix pp 1–3), and we calculated cumulative values from March 19 to May 11, 2020, covering the first national lockdown. Incidence rate was defined as the cumulative number of in-hospital COVID-19 cases per 100 000 inhabitants, mortality rate as the cumulative number of in-hospital COVID-19 deaths per 100 000 inhabitants, and case fatality rate as the cumulative number of in-hospital COVID-19 deaths per cumulative number of in-hospital COVID-19 cases.
Before March 19, 2020, COVID-19-associated deaths at the departmental level were routinely declared by the Regional Health Agencies (Agences régionales de santé). These values were compiled as of Feb 7, 2020, in a separate publicly available database (appendix pp 1–2), which we used to calculate the relative lag between the first COVID-19 death and the lockdown on March 17 for each department, to account for the temporal progression of the epidemic wave across the country and the effect of the national lockdown. Because male sex and older age are associated with increased severity of disease,16, 17 we extracted the population age and sex structure estimated in 2020 for each department from the French national statistics institute (Institute national de la statistique et des études économiques; appendix pp 1–2). Considering that overwhelmed intensive care capacities might affect quality of care for patients with COVID-19, we also obtained the baseline number of intensive care unit (ICU) beds per department in 2018 from the website of the Ministry of Health (Ministère de solidarité et de la santé; appendix p 1).
During the early phase of the epidemic, some treatments were suggested to have antiviral properties (with a low level of evidence). Chloroquine and hydroxychloroquine were among such treatments, and were suggested to play a part in the spatial heterogeneity of COVID-19 incidence and mortality rates in France and some other countries.18, 19 We therefore extracted the number of new chloroquine and hydroxychloroquine dispensations at the departmental level between Jan 1 and April 19, 2020, from an official report of the French national drug agency (Agence nationale de la sécurité du médicament et des produits de santé) and the French national health insurance fund (Caisse nationale de l'Aussurance Maladie), which showed a surge in new dispensations of these two medications in pharmacies in the first week of March (appendix pp 1–2).
Given that structural health determinants and comorbidities have been associated with the severity of COVID-19, we also considered additional indicators associated with department-specific baseline population health and health-care services (appendix pp 1–2). We therefore included the baseline number of total deaths per department between March 19 and May 11, 2018, and between March 19 and May 11, 2019, indicators related to the availability of health-care resources, indicators of subsidised medical or dependency insurance, the proportion of hospital stays in several types of ward (eg, endocrinology, cardiology, pneumology, and medicine wards), and several economic indicators at the departmental level (appendix pp 1–3). To investigate the role of population density and connectivity in COVID-19 heterogeneity, we obtained indicators that describe urbanisation from the national institute of statistics and economics, which are described in the appendix (pp 1–3). Because France is climatically diverse, we characterised departments according to eight climate profiles, which are described in the appendix (pp 1–3). Finally, to consider spatial autocorrelation, we extracted the centroid coordinates of each department from a publicly available shapefile (appendix pp 1–3).
Statistical analysis
We calculated in-hospital COVID-19 incidence, mortality, and case fatality rates for each of the 96 administrative departments of metropolitan France. We mapped these indicators and deviations from nationwide rates. We also estimated spatial autocorrelation using Moran's I statistic for areal data (Queen criterion of contiguity).20
To analyse the factors associated with epidemiological indicators and avoid the curse of dimensionality and multicollinearities, we first summarised multidimensional factors using a non-supervised classification technique: hierarchical ascendant classification on principal component analysis.21 To classify departments into similar age–pyramid profiles, we did a principal component analysis on the relative composition of the 5-year age and sex population groups, and we then included the coordinates of each variable in the first 25 principal components in a hierarchical ascendant classification. Similarly, we used hierarchical ascendant classification based on principal component analysis coordinates to classify department-specific population health and health-care services, economy, climate, and urbanisation, on the basis of corresponding indicators (appendix pp 1–3). Sensitivity analyses were done through simulation studies (appendix pp 4–6).
We mapped the resulting classifications at the department level, as well as the lag between the first COVID-19-associated death and lockdown, ICU bed capacities, and the incidence of chloroquine and hydroxychloroquine dispensations.
To assess the relationships between these factors and in-hospital COVID-19 incidence, mortality, and case fatality rates, we used generalised additive models with a negative binomial regression to consider over-dispersion. A Gaussian kriging smoother (with a power exponential covariance function), based on the geographical coordinates of each department centroid (s(lon,lat)), was used to take spatial autocorrelation into account, as previously described.22 Log(population) was used as an offset to estimate standardised incidence ratios (equation 1) and standardised mortality ratios (equation 2), and log(cases) was used as an offset to estimate standardised fatality ratios (equation 3):
| (1) |
| (2) |
| (3) |
where s(lon,lat) was the Gaussian kriging smoother function using the longitude and latitude coordinates, and NegBin was the negative binomial distribution. For each outcome, the analysed cofactors are listed in the appendix (pp 1–2). Briefly, to model the in-hospital incidence rate, we considered the relative lag between the first COVID-19 death and the lockdown on March 17 (ie, the progression of the epidemic wave) and all risk factors, except for ICU capacities. To model the in-hospital mortality rate, we considered in-hospital COVID-19 cases, factors possibly associated with case severity (ie, population age structure and dispensation of chloroquine and hydroxychloroquine), and factors associated with case management capacities and preparedness (ie, progression of the epidemic wave, ICU bed capacity, population health, and health-care services), but not factors directly associated with incidence (ie, the economy, urbanisation, and climate). We considered similar factors to model the in-hospital case fatality rate, except that cases were used as an offset to estimate standardised fatality ratios.
We first separately analysed each factor with univariate models. Multivariate generalised additive models then used directed acyclic graphs23 to include variables that appeared to be important from a public health perspective, such as population age structure or population health and health-care services (appendix pp 7–8). In the multivariate mortality model, we considered the interaction between in-hospital cases and the relative lag between the first COVID-19-associated death and lockdown. Finally, we mapped Pearson residuals of multivariate models to highlight outliers (appendix p 9) and the deviation from the nationwide rates (appendix p 10). Spatial adjustments were verified by Moran I statistics on Pearson residuals. Sensitivity analyses of the three models were done by substituting the three outcomes (incidence, mortality rate, and case fatality rate) by emergency allergies and in-hospital allergies among patients at emergency departments, and in-hospital allergies among emergency allergies (appendix pp 11–13).
All analyses and maps were made using the software program R version 4.0.0 and the tidyverse, mgcv, FactoMineR, rgdal, spdep, and dagitty packages.
Role of the funding source
There was no funding source for this study.
Results
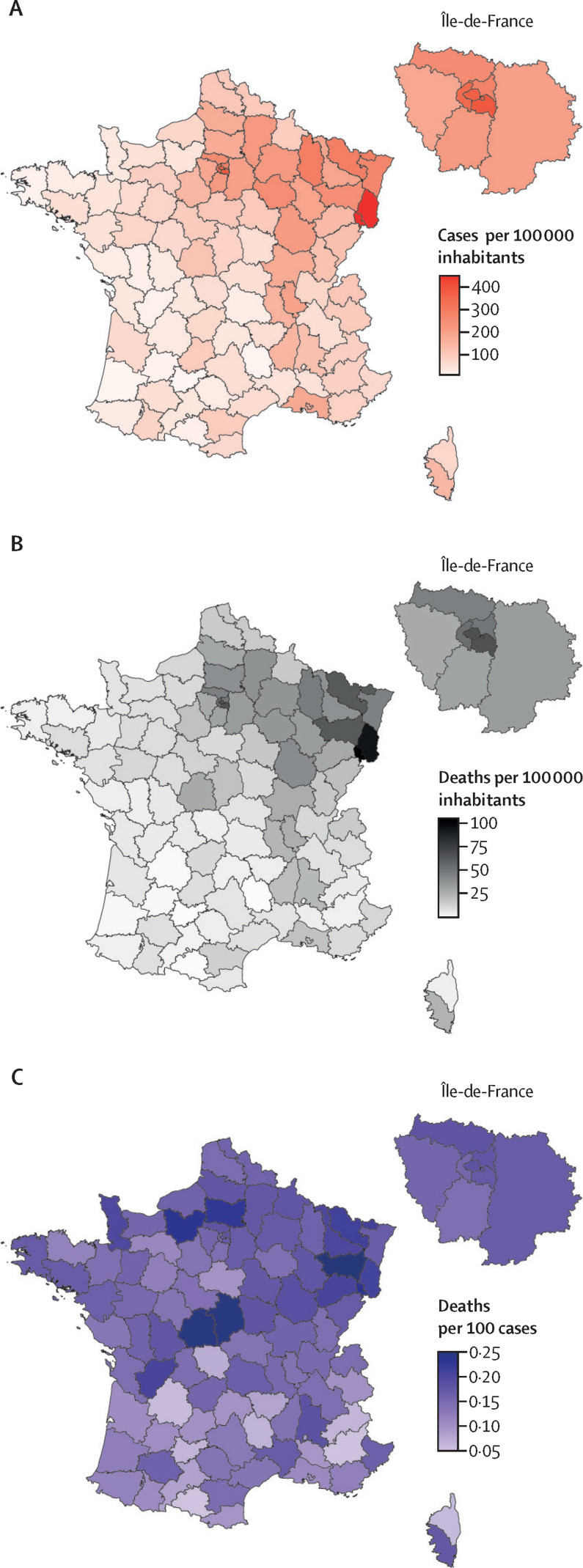
From March 19 to May 11, 2020, hospitals in metropolitan France notified a total of 100 988 COVID-19 cases, including 16 597 people who were admitted to the ICU and 17 062 deaths. These values corresponded to an overall cumulative in-hospital incidence rate of 155·6 cases per 100 000 inhabitants (range 19·4–489·5) across the 96 administrative departments (figure 1 ). The overall cumulative in-hospital mortality rate was 26·3 deaths per 100 000 inhabitants (range 1·1–119·2). Both indicators showed a marked heterogeneity following a north-to-south gradient (figure 1), with high spatial autocorrelations (both Moran's statistics 0·68; p<0·0001) related to the diffusion of the epidemic. The highest incidence and mortality rates were observed in Territoire-de-Belfort (489·5 cases and 119·2 deaths per 100 000 inhabitants) and Haut-Rhin (485·7 cases and 101·4 deaths per 100 000 inhabitants). In the department of Paris, the incidence was 397·6 cases per 100 000 inhabitants and the mortality rate was 74·9 deaths per 100 000, and in Bouches-du-Rhône (including Marseille, the second largest French city) the incidence was 200·5 cases per 100 000 and the mortality rate was 23·0 deaths per 100 000, which was higher than in surrounding departments. The overall cumulative in-hospital case fatality rate in metropolitan France was 16·9%. Case fatality rate also showed distinct heterogeneity (from 4·8% in Ariège to 26·2% in Indre; figure 1), but with a lower spatial autocorrelation (Moran's statistic 0·32; p<0·0001).
Figure 1.
Spatial heterogeneity of COVID-19 in France, showing cumulative in-hospital incidence (A), in-hospital mortality rate (B), and in-hospital case fatality rate (C)
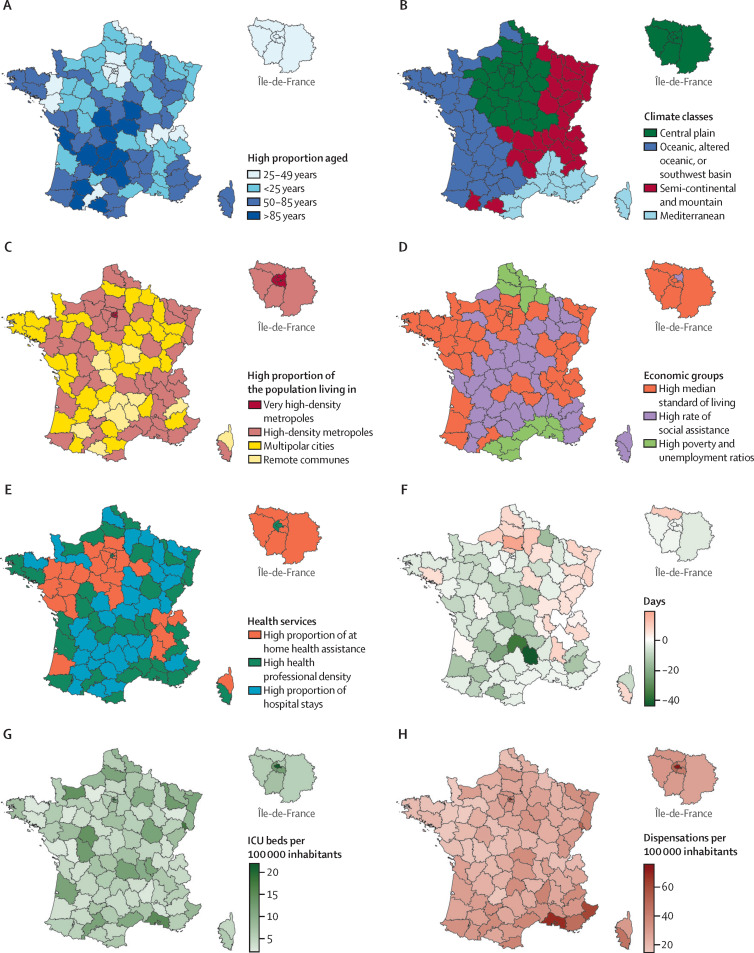
Across the 96 departments, the first COVID-19-associated death occurred at a median of 3 days after the lockdown (ie, a median relative lag of −3 days [IQR −7 to 1]; figure 2F ), with a maximum of 19 days in Oise, and a minimum of −44 days in Lozère. This lag shows the temporal progression of the epidemic wave across the country from northeastern and north-central departments. Hierarchical ascendant classification based on principal component analysis coordinates from age pyramids classified departments into four classes: high proportion aged 25–49 years (class 1), high proportion aged <25 years (class 2), high proportion aged 50–85 years (class 3), and high proportion aged >85 years (class 4; figure 2A). The overall density of ICU beds in 2018 in France was 8·1 per 100 000 inhabitants, ranging from 2·0 per 100 000 in Eure to 21·9 per 100 000 in Paris (figure 2G). Overall, the rate of new chloroquine and hydroxychloroquine dispensations in French pharmacies was 32·0 per 100 000 inhabitants from Jan 1 to April 19, 2020, ranging from 14·4 per 100 000 in Mayenne to 68·7 per 100 000 in Bouches-du-Rhône and 75·5 in Paris (figure 2H). Hierarchical ascendant classification based on principal component analysis classified departments into three classes of population health and health-care services: high proportion of the population receiving home health assistance (class 1), high health professional density (class 2), and high proportion of hospital stays (class 3; figure 2E). Classification for economic indicators also identified three classes: high median standard of living (class 1), high rate of social assistance (class 2), and high poverty and unemployment ratios (class 3; figure 2D). Classification for the urbanisation indicators fitted departments into four classes: very high proportion of the population living in metropolitan cities and high road density (class 1), high proportion of the population living in metropolitan cities and lower road density (class 2), high proportion of the population living in multipolar cities (class 3), and high proportion of the population living in remote communes (class 4; figure 2C). Finally, classification on climate indicators identified four department classes: central plains with modified oceanic climate (class 1), oceanic, altered oceanic, or southwest basin climate (class 2), semi-continental, submontane, or mountain climate (class 3), and mediterranean climate (class 4; fig 2B). The characteristics of the classes for these multidimensional variables are described in the appendix (pp 14–18).
Figure 2.
Maps of covariates, showing population age structure (A), climate classes (B), urbanisation (C), economic profile (D), population health and health-care services (E), the lag between the first COVID-19-associated death and lockdown (F), baseline intensive care capacity (G), and chloroquine and hydroxychloroquine dispensations in pharmacies (H)
Departments were classified into four climate classes (appendix pp 1–3, 14). ICU=intensive care unit.
In the univariate analysis, the incidence rate at the department level from March 19 to May 11, 2020, appeared to be associated with the lag between the first death and lockdown, population age structure, new chloroquine and hydroxychloroquine dispensations, and urbanisation (table 1 ). In the final multivariate model, higher cumulative COVID-19 incidence rates were associated with early arrival of the epidemic wave (for each day between the first death and lockdown, adjusted standardised incidence ratio 1·02, 95% CI 1·01–1·04; table 1). Compared with the very high-density departments of Paris and those surrounding it (class 1 of urbanisation), departments with a high proportion of the population living in metropolitan cities but with lower road density (class 2) showed a lower COVID-19 incidence rate (adjusted standardised incidence ratio 0·61, 0·37–1·02; table 1) in the multivariate analysis. In this model, population age structure, chloroquine and hydroxychloroquine dispensations, population health and health-care services, and climate did not appear to be associated with in-hospital COVID-19 incidence rates (table 1). This multivariate model explained 86·8% of the deviance in in-hospital COVID-19 incidence. Model Pearson residuals are mapped in the appendix (p 9) and showed no spatial correlation (Moran's statistic −0·12; p=0·95). None of the most affected departments showed important model residuals, and there were particularly minimal residuals in Paris and Bouches-du-Rhône (appendix p 9).
Table 1.
Factors associated with in-hospital COVID-19 incidence rate at the department level in metropolitan France
|
Univariate analyses |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| SIR (95% CI) | p value | aSIR (95% CI) | p value | ||
| Relative lag between first COVID-19-associated death and lockdown on March 17, 2020 | 1·02 (1·004–1·03) | 0·011 | 1·02 (1·01–1·04) | 0·0033 | |
| Population age structure estimated in 2020 per department | |||||
| Class 1: high proportion aged 25–49 years | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high proportion aged <25 years | 0·86 (0·64–1·15) | 0·29 | 0·87 (0·64–1·19) | 0·38 | |
| Class 3: high proportion aged 50–85 years | 0·72 (0·53–0·99) | 0·0407 | 0·68 (0·43–1·09) | 0·11 | |
| Class 4: high proportion aged >85 years | 0·96 (0·64–1·42) | 0·82 | 0·92 (0·51–1·66) | 0·77 | |
| Number of new chloroquine and hydroxychloroquine dispensations in pharmacies from Jan 1 to April 19, 2020 | 1·001 (1·0003–1·001) | 0·0008 | 1·00 (0·99–1·001) | 0·16 | |
| Baseline population health and health-care services | |||||
| Class 1: high proportion of the population receiving home health assistance | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high health professional density | 1·19 (0·94–1·51) | 0·15 | 1·07 (0·83–1·38) | 0·61 | |
| Class 3: high proportion of hospital stays | 1·07 (0·82–1·38) | 0·64 | 0·96 (0·71–1·31) | 0·81 | |
| Economic indicators* | |||||
| Class 1: high median standard of living | 1 (ref) | .. | .. | .. | |
| Class 2: high rate of social assistance | 1·07 (0·87–1·32) | 0·54 | .. | .. | |
| Class 3: high poverty and unemployment ratios | 0·97 (0·68–1·38) | 0·86 | .. | .. | |
| Urbanisation | |||||
| Class 1: very high proportion of population living in metropolitan cities and high road density | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high proportion of the population living in metropolitan cities and lower road density | 0·48 (0·30–0·78) | 0·0029 | 0·61 (0·37–1·02) | 0·059 | |
| Class 3: high proportion of population living in multipolar cities | 0·48 (0·29–0·79) | 0·0042 | 0·85 (0·46–1·55) | 0·59 | |
| Class 4: high proportion of population living in remote communes | 0·42 (0·24–0·74) | 0·0025 | 0·94 (0·47–1·88) | 0·86 | |
| Climate | |||||
| Class 1: central plains with modified oceanic climate | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: oceanic, altered oceanic, or southwest basin climate | 0·84 (0·56–1·26) | 0·41 | 0·81 (0·57–1·17) | 0·26 | |
| Class 3: semi-continental, submontane, or mountain climate | 0·93 (0·63–1·36) | 0·69 | 1·08 (0·76–1·54) | 0·67 | |
| Class 4: Mediterranean climate | 1·61 (0·86–3·00) | 0·14 | 1·72 (0·96–3·05) | 0·066 | |
Analyses were made using generalised additive models with a negative binomial regression, a Gaussian kriging smoother based on geographical coordinates, and log(population) as an offset. The multivariate model included confounders according to the directed acyclic graph. aSIRs were adjusted on the different cofactors and spatial structure. SIR=standardised incidence ratio. aSIR=adjusted standardised incidence ratio. Ref=reference.
Considering the directed acyclic graph analysis, economic groups were not included in the multivariate analysis.
In the univariate analysis, the mortality rate at the departmental level appeared to be positively associated with the lag between the first death and lockdown, the cumulative number of in-hospital COVID-19 cases from March 19 to May 11, 2020, ICU capacity, and new chloroquine and hydroxychloroquine dispensations (table 2 ). In the final multivariate model, higher COVID-19 mortality rates were associated with early arrival of the epidemic wave (for each day between the first death and lockdown, adjusted standardised mortality ratio 1·04, 95% CI 1·02–1·06) and with in-hospital COVID-19 case number (for each case adjusted standardised mortality ratio 1·0004, 1·0002–1·001), even after taking into account the interaction between both factors (table 2). Compared with departments with a high proportion of inhabitants aged 25–49 years (class 1 of population age structure), departments with a high proportion of inhabitants aged >85 years (class 4) had a much higher COVID-19 mortality rate (adjusted standardised mortality ratio 2·17, 1·20–3·90; table 2). In the multivariate model, chloroquine and hydroxychloroquine dispensations, ICU capacity, and population health and health-care services did not appear to be associated with COVID-19 in-hospital mortality rates (table 2). This multivariate model explained 82·1% of the deviance in in-hospital COVID-19 mortality. Model Pearson residuals are mapped in the appendix (p 9) and showed no spatial correlation (Moran's statistic −0·11; p=0·93). Residuals in Bouches-du-Rhône appeared higher than in surrounding departments, meaning that the observed mortality there was higher than the predicted mortality (appendix p 9).
Table 2.
Factors associated with in-hospital COVID-19 mortality rate at the department level in metropolitan France
|
Univariate analyses |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| SMR (95% CI) | p value | aSMR (95% CI) | p value | ||
| Number of in-hospital cases accumulated from March 18 to May 11, 2020 | 1·0002 (1·0001–1·0003) | <0·0001 | 1·0004 (1·0002–1·001) | <0·0001 | |
| Relative lag between first COVID-19-associated death and lockdown on March 17, 2020 | 1·03 (1·01–1·04) | 0·0009 | 1·04 (1·02–1·06) | 0·0001 | |
| Population age structure estimated in 2020 per department | |||||
| Class 1: high proportion aged 25–49 years | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high proportion aged <25 years | 0·85 (0·60–1·20) | 0·36 | 1·30 (0·92–1·83) | 0·14 | |
| Class 3: high proportion aged 50–85 years | 0·74 (0·51–1·06) | 0·10 | 1·41 (0·89–2·22) | 0·14 | |
| Class 4: high proportion aged >85 years | 0·96 (0·60–1·54) | 0·87 | 2·17 (1·20–3·90) | 0·010 | |
| Number of intensive care beds in 2018 | 1·002 (1·0004–1·003) | 0·015 | 1·00 (0·99–1·003) | 0·57 | |
| Number of new chloroquine and hydroxychloroquine dispensations in pharmacies from Jan 1 to April 19, 2020 | 1·001 (1·0002–1·001) | 0·0054 | 1·00 (0·99–1·001) | 0·28 | |
| Baseline population health and health-care services | |||||
| Class 1: high proportion of the population receiving home health assistance | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high health professional density | 1·19 (0·94–1·51) | 0·15 | 0·94 (0·69–1·29) | 0·70 | |
| Class 3: high proportion of hospital stays | 1·07 (0·82–1·38) | 0·64 | 0·87 (0·61–1·23) | 0·42 | |
Analyses were made using generalised additive models with a negative binomial regression, a Gaussian kriging smoother based on geographical coordinates, and log(population) as an offset. The multivariate model included confounders according to the directed acyclic graph. aSMRs were adjusted on the different cofactors, spatial structure, and the interaction between COVID-19 cases and temporal progression of the epidemic wave (p=0·062). SMR=standardised mortality ratio. aSMR=adjusted standardised mortality ratio. Ref=reference.
In the univariate analysis, the case fatality rate at the departmental level appeared to be associated with only the lag between the first death and lockdown (table 3 ). In the final multivariate model, higher case fatality rates were associated with early arrival of the epidemic wave (for each day between the first death and lockdown, adjusted standardised fatality ratio 1·01, 95% CI 1·005–1·02; table 3). Compared with departments with a high proportion of inhabitants aged 25–49 years (class 1), departments with a high proportion of inhabitants aged 50–85 years (class 3) and with a high proportion aged >85 years (class 4) had a higher case fatality rate (for class 4, adjusted standardised fatality ratio 1·43, 1·08–1·88; table 3) in the multivariate analysis. Compared with departments with a high proportion of the population receiving home health assistance (class 1 of population health and health-care services), departments with a high proportion of hospital stays (class 3) had a lower case fatality rate (adjusted standardised fatality ratio 0·83, 0·69–0·99; table 3). This multivariate model explained 44·6% of the deviance in in-hospital COVID-19 case fatality rates. Model Pearson residuals are mapped in the appendix (p 9) and showed no spatial correlation (Moran's statistic −0·12; p=0·94). Residuals appeared to be higher in less affected departments. In Bouches-du-Rhône, the model slightly overestimated the case fatality rate (appendix p 9).
Table 3.
Factors associated with in-hospital COVID-19 case fatality rate at the department level in metropolitan France
|
Univariate analyses |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| SFR (95% CI) | p value | aSFR (95% CI) | p value | ||
| Relative lag between first COVID-19-associated death and lockdown on March 17, 2020 | 1·01 (1·00–1·02) | 0·011 | 1·01 (1·005–1·02) | 0·0008 | |
| Population age structure estimated in 2020 per department | |||||
| Class 1: high proportion aged 25–49 years | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high proportion aged <25 years | 1·01 (0·91–1·13) | 0·84 | 1·05 (0·92–1·19) | 0·51 | |
| Class 3: high proportion aged 50–85 years | 1·08 (0·96–1·22) | 0·19 | 1·25 (1·03–1·52) | 0·025 | |
| Class 4: high proportion aged >85 years | 1·11 (0·93–1·33) | 0·25 | 1·43 (1·08–1·88) | 0·011 | |
| Number of intensive care beds in 2018 | 1·00 (0·99–1·0002) | 0·29 | 1·00 (0·99–1·002) | 0·77 | |
| Number of new chloroquine and hydroxychloroquine dispensations in pharmacies from Jan 1 to April 19, 2020 | 1·00 (0·99–1·0001) | 0·40 | 1·00 (0·99–1·001) | 0·89 | |
| Baseline population health and health-care services | |||||
| Class 1: high proportion of the population receiving home health assistance | 1 (ref) | .. | 1 (ref) | .. | |
| Class 2: high health professional density | 1·01 (0·91–1·12) | 0·86 | 0·95 (0·83–1·09) | 0·46 | |
| Class 3: high proportion of hospital stays | 1·00 (0·89–1·12) | 0·99 | 0·83 (0·69–0·99) | 0·066 | |
Analyses were made using generalised additive models with a negative binomial regression, a Gaussian kriging smoother based on geographical coordinates, and log(population) as an offset. The multivariate model included confounders according to the directed acyclic graph. aSFRs were adjusted for the different cofactors and spatial structure. SFR=standardised fatality ratio. aSFR=adjusted standardised fatality ratio. Ref=reference.
Discussion
This geo-epidemiological study highlights the clear spatial heterogeneity of in-hospital COVID-19 incidence and mortality rates across the departments of metropolitan France, following the spread of the epidemic before a national lockdown was declared on March 17, 2020. After classifying the multidimensional variables and completing multivariate analyses of the spatial structure of the epidemic, the delay that elapsed between the first COVID-19-associated death and the onset of the lockdown appeared to be positively associated with in-hospital incidence, mortality, and case fatality rates. In other words, morbidity and mortality were lower in departments where the general lockdown caught the epidemic at an earlier stage of its expansion. This finding suggests that the lockdown was an effective way to control the diffusion of this wave of the epidemic across the country. This effect of the lockdown strategy is in line with other publications that are based on modelling or observational studies. Among them, Salje and colleagues15 and Di Domenico and colleagues24 estimated that the lockdown reduced the reproductive number by 77% in France and 81% in Île-de-France. Using databases across 149 countries, Islam and colleagues25 also estimated that earlier implementation of lockdown was associated with a larger reduction in the incidence of COVID-19.
Mortality rate was also strongly associated with the incidence rate of in-hospital COVID-19 cases, even after adjusting for the interaction between incidence and the relative lag to lockdown. However, the number of ICU beds available in 2018 was not associated with mortality or case fatality rate. In addition, we found no association between baseline population health and health-care services and incidence and mortality rates. This finding suggests that hospitals managed to scale up their ICU capacity (≥100% increase in 21 departments; appendix p 19) or organise medical evacuations to less affected departments when necessary.
As was expected,3 in-hospital mortality and case fatality rates were higher in departments with older populations. Age has indeed been identified as the main risk factor of COVID-19 disease severity and death in many cohort studies.16, 17 We did not include the prevalence of comorbidities such as diabetes or obesity in our study because of a scarcity of available data at the departmental level. However, we considered several indicators associated with overall population health, such as basal mortality rate and the usual proportion of hospital stays in endocrinology, cardiology, pneumology, and medicine wards.
We included numbers of chloroquine and hydroxychloroquine dispensations in our models because previous statements suggested that this might be responsible for the lower COVID-19 burden observed in some regions.18, 19 However, our findings do not support this hypothesis because chloroquine and hydroxychloroquine dispensations were not associated with in-hospital incidence, mortality, or case fatality rates at the departmental level. We could not include data for in-hospital dispensations, which are not publicly available in France, and the prescription of chloroquine and hydroxychloroquine for COVID-19 was limited by a French regulation on May 26, 2020, after the end of our study period. Although univariate analyses showed important associations between in-hospital incidence and mortality rates and new chloroquine and hydroxychloroquine dispensations, these associations were positive (ie, higher chloroquine and hydroxychloroquine dispensations were associated with higher incidence or mortality rates). This association suggests a common confounding by indication,26 which was corrected by the multivariate analysis. Furthermore, our multivariate analysis explained almost all in-hospital COVID-19 incidence in the Paris and Bouches-du-Rhône departments, the two departments where chloroquine and hydroxychloroquine dispensations were the highest. The observed mortality in Bouches-du-Rhône was even higher than predicted by our model. Nevertheless, our ecological study was not designed to assess the effectiveness of chloroquine and hydroxychloroquine against COVID-19 at the individual level; these drugs have been shown not to be effective.27
Our multivariate model, which was adjusted for spatial heterogeneity and the delay between the first COVID-19 death and the start of the lockdown, did not identify any substantial association between in-hospital incidence and the four different climate zones, each of which hosts one of the four largest French metropolitan areas. Previous reports have suggested that temperature and humidity could affect the transmission of COVID-19. However, these studies analysed meteorological rather than climatic factors, or did not consider the temporal spread of the pandemic.6
Our study has several limitations. We only analysed COVID-19 in-hospital data. Testing was too low in France during the first wave of the epidemic to provide an accurate estimation of outpatient incidence. Furthermore, data on COVID-19 incidence and mortality in retirement homes have not yet been made available at the department level. Criteria for admission to hospital might not have been homogeneous across the departments, and more people with milder symptoms of COVID-19 might have been admitted to hospital in less affected departments or depending on local care policies.28 The age of patients admitted to hospital for COVID-19 has not been made available at the departmental level to account for these differences. This factor could partly account for why our multivariate analysis explained only 44·6% of the case fatality rate. Additional factors might also have been associated with case fatality rate, suggesting a need for further analysis at a more accurate scale. For instance, it was not possible to analyse ethnicity, because this characteristic is not recorded in French databases for legal reasons.
Finally, this was an ecological study rather than an individual population study, which introduces classical ecological fallacy. Even if the sensitivity analyses support our results, we cannot infer any direct individual risk,29 nor interpret the results in terms of causality. Moreover, the multidimensional reduction of economic indicators using hierarchical ascendant classification on principal component analysis, which allows numerous factors to be assessed and considers collinearities and the curse of dimensionality, might also have flattened the differences between departments and hidden possible associations.
In conclusion, our findings outline the effect of the COVID-19 pandemic wave in a country that could absorb the shock, thanks to a strong hospital system and a national lockdown. However, the findings indirectly underscore the weakness of its preventive and public health system, which could be useful for informing countries' preparedness for the current or future pandemic waves.
Data sharing
All the data are publicly available and can be downloaded using the URLs in the appendix (pp 1–3). The data analysed in the study can be provided as a spreadsheet upon request to the corresponding author.
Contributors
JG and SR developed the study idea and the analysis plan. JG, JL, LH, EM, FK-S, JD, LC, RP, and SR contributed to the literature review. JG, JL, MKB, and SR did the data investigation and management. All data were publicly available and all authors had full access to the data and analyses, and were responsible for the final decision to submit for publication. JG, JL, and SR were responsible for verifying the data used in the study. EL, LL, and AP participated in the data management and analysis. JG did the statistical analysis. JG and SR wrote the first draft. All authors contributed to the interpretation of the findings, reviewed the analysis, wrote the manuscript, and approved the final manuscript. JG had the final responsibility for the decision to submit for publication.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Khafaie MA, Rahim F. Cross-country comparison of case fatality rates of COVID-19/SARS-COV-2. Osong Public Health Res Perspect. 2020;11:74–80. doi: 10.24171/j.phrp.2020.11.2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middelburg RA, Rosendaal FR. COVID-19: how to make between-country comparisons. Int J Infect Dis. 2020;96:477–481. doi: 10.1016/j.ijid.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowd JB, Andriano L, Brazel DM. Demographic science aids in understanding the spread and fatality rates of COVID-19. PNAS. 2020;117:9696–9698. doi: 10.1073/pnas.2004911117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su D, Chen Y, He K. Influence of socio-ecological factors on COVID-19 risk: a cross-sectional study based on 178 countries/regions worldwide. medRxiv. 2020 doi: 10.1101/2020.04.23.20077545. published online Apr 29. (preprint). [DOI] [Google Scholar]
- 5.da Silveira Moreira R. COVID-19: intensive care units, mechanical ventilators, and latent mortality profiles associated with case-fatality in Brazil. Cad Saude Publica. 2020;36 doi: 10.1590/0102-311x00080020. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly KM, Auzenbergs M, Jafari Y, Liu Y, Flasche S, Lowe R. Effective transmission across the globe: the role of climate in COVID-19 mitigation strategies. Lancet Planet Health. 2020;4:e172. doi: 10.1016/S2542-5196(20)30106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiang S, Allen D, Annan-Phan S. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature. 2020;584:262–267. doi: 10.1038/s41586-020-2404-8. [DOI] [PubMed] [Google Scholar]
- 8.Jüni P, Rothenbühler M, Bobos P. Impact of climate and public health interventions on the COVID-19 pandemic: a prospective cohort study. CMAJ. 2020;192:e566–e573. doi: 10.1503/cmaj.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aleta A, Moreno Y. Evaluation of the potential incidence of COVID-19 and effectiveness of containment measures in Spain: a data-driven approach. BMC Med. 2020;18:157. doi: 10.1186/s12916-020-01619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deslandes A, Berti V, Tandjaoui-Lambotte Y. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard Stoecklin S, Rolland P, Silue Y. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiteri G, Fielding J, Diercke M. First cases of coronavirus disease 2019 (COVID-19) in the WHO European region, 24 January to 21 February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bal A, Destras G, Gaymard A. Molecular characterization of SARS-CoV-2 in the first COVID-19 cluster in France reveals an amino acid deletion in nsp2 (Asp268del) Clin Microbiol Infect. 2020;26:960–962. doi: 10.1016/j.cmi.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Légifrance Décret no 2020-260 du 16 mars 2020 portant réglementation des déplacements dans le cadre de la lutte contre la propagation du virus covid-19. March 16, 2020. https://www.legifrance.gouv.fr/eli/decret/2020/3/16/PRMX2007858D/jo/texte
- 15.Salje H, Kiem CT, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 17.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izoulet M. Countries which primarily use antimalarial drugs as COVID-19 treatment see slower dynamic of daily deaths. SSRN. 2020 doi: 10.2139/ssrn.3575899. published online April 21. (preprint). [DOI] [Google Scholar]
- 19.Raoult D. Comparaison des courbes épidémiques selon villes et pays. May 19, 2020. https://www.mediterranee-infection.com/comparaison-des-courbes-epidemiques-selon-villes-et-pays/
- 20.Tiefelsdorf M. The saddlepoint approximation of Moran's I's and local Moran's Ii's reference distributions and their numerical evaluation. Geogr Anal. 2002;34:187–206. [Google Scholar]
- 21.Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25:1–18. [Google Scholar]
- 22.Wood S. Generalized additive models: an introduction with R. 2006. https://www.crcpress.com/Generalized-Additive-Models-An-Introduction-with-R/Wood/p/book/9781584884743
- 23.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 24.Di Domenico L, Pullano G, Sabbatini CE, Boëlle P-Y, Colizza V. Impact of lockdown on COVID-19 epidemic in Île-de-France and possible exit strategies. BMC Medicine. 2020;18:240. doi: 10.1186/s12916-020-01698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam N, Sharp SJ, Chowell G. Physical distancing interventions and incidence of coronavirus disease 2019: natural experiment in 149 countries. BMJ. 2020;370 doi: 10.1136/bmj.m2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316:1818–1819. doi: 10.1001/jama.2016.16435. [DOI] [PubMed] [Google Scholar]
- 27.Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients. Clin Microbiol Infect. 2020;1:19–27. doi: 10.1016/j.cmi.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagier J-C, Million M, Gautret P. Outcomes of 3 737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson S, Montfort C. Ecological correlation studies. In: Elliott P, Wakefield J, Best N, Briggs D, editors. Spatial epidemiology methods and applications. Oxford University Press; Oxford: 2000. pp. 205–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are publicly available and can be downloaded using the URLs in the appendix (pp 1–3). The data analysed in the study can be provided as a spreadsheet upon request to the corresponding author.