Abstract
Using high-throughput analysis methods, the present study sought to determine the impact of prenatal high fat dietary manipulations on isolation-induced ultrasonic vocalization production in both male and female Fmr1 mutants on PD9. Prior to breeding, male FVB/129 Fmr1 wildtype and female Fmr1 heterozygous breeding pairs were assigned to one of three diet conditions: standard lab chow, omega-3 fatty acid enriched chow, and a diet controlling for the fat increase. Prenatal exposure to omega-3 fatty acids improved reductions in the number of calls produced by Fmr1 heterozygotes females. Moreover, diminished spectral purity in the female Fmr1 homozygous mouse was rescued by exposure to both high fat diets, though these effects were not seen in the male Fmr1 knockout. Prenatal dietary fat manipulation also influenced several aspects of vocalization production, such as the number of calls produced and their fundamental frequency, aside from effects due to loss of Fmr1. Specifically, in males, regardless of genotype, prenatal exposure to high omega-3’s increased average fundamental frequency of calls. These data support the need for future preclinical and clinical work elucidating the full potential of prenatal high fat diets as a novel therapeutic alternative for Fragile X syndrome.
Keywords: Fragile X syndrome, Ultrasonic vocalizations, MATLAB, Autism, Gender, High fat
INTRODUCTION
Dysfunction in early communication is a facet of many different neurodevelopmental conditions and as such, represents an important diagnostic criterion. Previous studies have shown that children who will later be diagnosed with autism spectrum disorder (ASD) exhibit altered crying behaviors compared to neurotypical controls and can elicit a differential response from the listener, suggesting that this crying behavior could impact later childhood development [1–3]. In preclinical murine models, this prelingual behavior can be modeled by studying ultrasonic vocalizations (USVs), allowing for the study of different potential treatments. These USVs are whistle-like sounds that occur between 30 and 90 kHz [4], and in pups they can serve as a distress call to the dam, eliciting maternal retrieval [5, 4, 6]. Many different mouse models of ASD, including the Fmr1 knockout mouse model, display early postnatal alterations in this behavior when temporarily isolated from the dam [7–13].
The Fmr1 model is a commonly utilized mouse model that seeks to mirror Fragile X syndrome, a psychiatric condition caused by mutations in the FMR1 gene, which encodes for fragile x mental retardation protein (FMRP). This mouse model is also considered an appropriate model for studying intellectual disability and ASD, given that mutations in this gene are one of the most prevalent contributors to inherited intellectual disability [14] and the most common genetic contributor to ASD [15]. In preclinical studies, loss of the Fmr1 gene has been shown to result in a variety of behavioral alterations that mirror the human condition, including hyperactivity, altered anxiety, deficits in cognitive function and changes in sensorimotor gating behaviors [16–22]. In addition, a myriad of both clinical and preclinical studies have demonstrated significant alterations in communicative behavior, both in adulthood [23–26], as well as in early development [27, 28, 11, 29]. Recent preclinical work from our lab has demonstrated that on postnatal day (PD) 8 male knockouts exhibit longer calls, and call-type analysis indicated sex-specific differences in the types of calls produced between the wildtype and Fmr1 mutant littermates [29]. However, it is unclear how these deficits respond to different pharmacological treatments.
Mounting preclinical evidence has demonstrated that post-weaning treatment with omega-3 fatty acids can improve aspects of the Fmr1 phenotype, including deficits in cognitive function and accompanying changes in inflammatory signaling markers [30]. Previous data from our lab has expanded upon these initial findings, demonstrating that prenatal treatment with omega-3 fatty acids attenuated hyperactivity and fear learning deficits (Nolan et al, In Review). However, no previous study has examined how omega-3 fatty acids impact the robust vocalization phenotypes previously demonstrated in Fmr1 mutant mice. There is some evidence that the phenotypes for Fmr1 mutant mice are amenable to treatment, as treatment with minocycline reverses deficits in calling rate in the adult male Fmr1 knockout model [10]. Despite evidence that a high fortified diet in omega-3 fatty acids is therapeutic when given in adulthood for Fmr1 mutant mice, it is unknown whether prenatal omega-3 fatty acids could improve communication deficits present in pups. The current study aimed to address this gap by examining isolation-induced ultrasonic recordings in male and female WT and Fmr1 KO pups receiving prenatal administration of omega-3 fatty acids, compared to a high fat control and standard laboratory chow groups.
MATERIALS AND METHODS
Animals/Experimental Paradigm
All procedures were performed in accordance with Baylor University Institutional Care and Use Committee and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Male Fmr1+/+ and female Fmr1+/− FVB.129P2-Pde6b+Tyrc-ch Fmr1tm1Cgr/J (Jackson Labs Stock No: 004624) mice originally from Jackson Labs were housed at Baylor University and bred to produce wildtype (WT) and Fmr1 knockout (KO) offspring. Moreover, to produce female KOs for the USV paradigm, female heterozygous breeders were bred with male KOs. Thus, heterozygous females may come from either breeding paradigm. The colony was maintained on a 12-hour light/dark cycle (lights on at 7 am). Breeders were placed on one of the three experimental diets (Standard, Omega-3 and Control Fat) one week prior to pairing (Full paradigm shown in Figure 1A). The latter two diets had identical lipid content (50g/kg). The “Omega-3” condition received a custom diet from Teklad enriched with fish oil and dyed with food coloring to distinguish it from the control condition. To control for potential effects due to simply increasing fat content, the Control Fat Teklad custom diet contained olive and palm oils with a separate food coloring. Both parents and offspring were maintained on the assigned diet throughout pregnancy and during the postnatal period. For a list of ingredients, see Table 1. On postnatal day (PD) 7, pups were separated from parents and toe clippings were collected to be sent out for genotyping and preserved in 70% ethanol (Mouse Genotype, Escondido, CA, USA). Animals were housed in the Special Research Unit at the Baylor Science Building. All subjects and breeders had access to food and water ad libitum.
Figure 1. Paradigm overview and effects on offspring body weight.
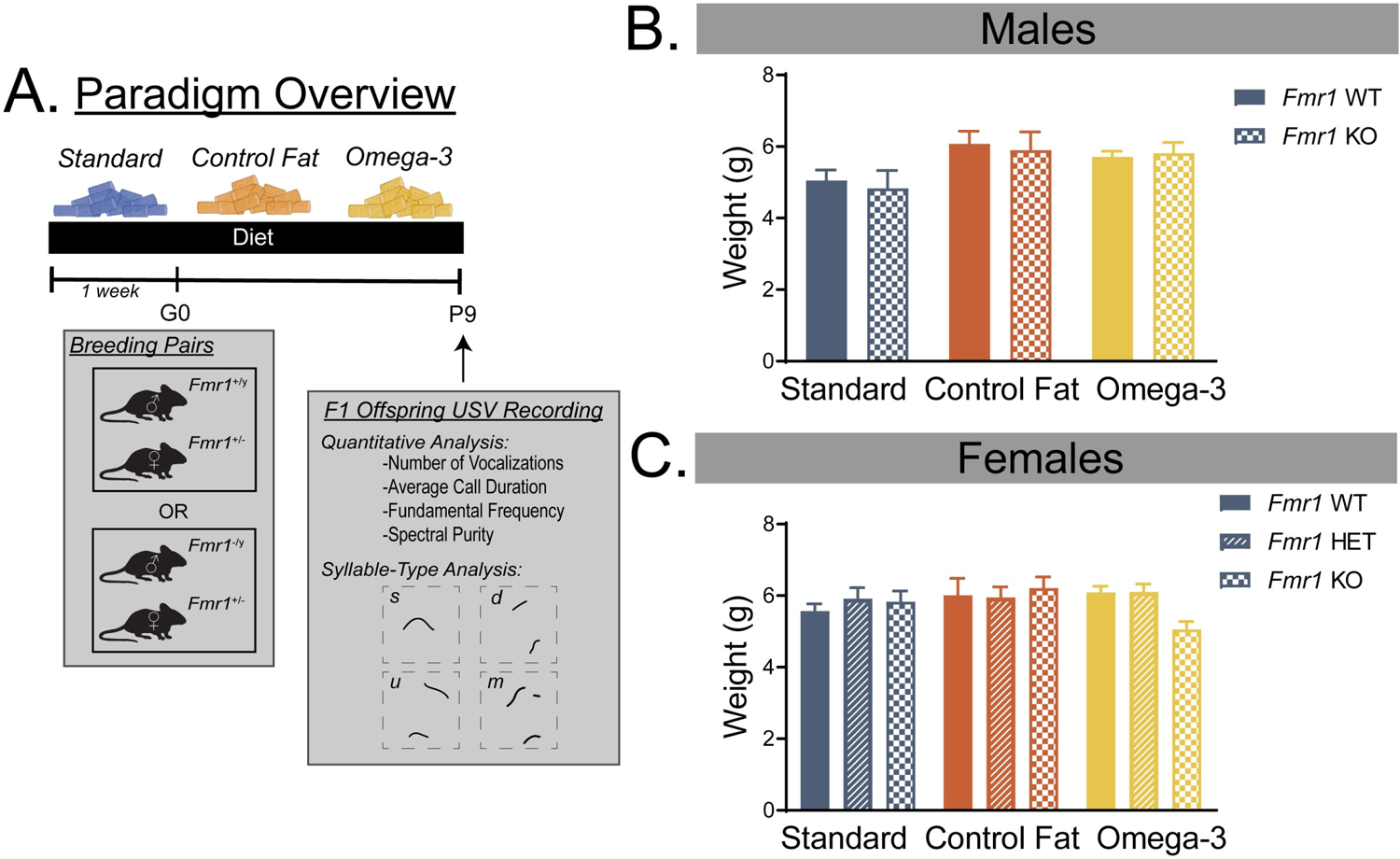
A. Overview of the paradigm used in the present study. B. Prenatal exposure to both the control fat and omega-3 diets increased body weight measurements on PD9 in males, regardless of genotype. C. Neither genotype or diet significantly influenced body weight in female pups. Data are expressed as mean ± SEM. A designation of “b” indicates that this group differed from the “a” comparison group at the level of p < 0.05.
Table 1. Diet Ingredients for Prenatal Paradigm.
Ingredients for the custom experimental diets in the prenatal supplementation paradigm (Custom Diet TD.160486 and TD.160487).
| Ingredients, g/kg | (Control Fat) | (Omega-3 Diet) |
|---|---|---|
| Casein | 200.0 | 200.0 |
| L-Cystine | 3.0 | 3.0 |
| Corn Starch | 341.60 | 341.60 |
| Maltodextrin | 132.0 | 132.0 |
| Sucrose | 100.0 | 100.0 |
| Canola Oil | 13.0 | 10.0 |
| Olive Oil | 65.0 | 20.0 |
| Palm Oil | 42.0 | 10.0 |
| Fish Oil | - | 80.0 |
| Cellulose | 50.0 | 50.0 |
| Mineral Mix AIN-93G | 35.0 | 35.0 |
| Vitamin Mix AIN-93 | 10.0 | 10.0 |
| Choline Bitartrate | 2.5 | 2.5 |
| Calcium Phosphate, dibasic | 3.1 | 3.1 |
| Calcium Carbonate | 1.0 | 1.0 |
| Magnesium Oxide | 0.154 | 0.154 |
| Cupric Carbonate | 0.0038 | 0.0038 |
| Ferric Citrate | 0.2352 | 0.2352 |
| Sodium Selenite (0.0455% in sucrose) | 1.25 | 1.25 |
| Vitamin K1, phylloquinone | 0.0003 | 0.0003 |
| Vitamin B12 (0.1% in mannitol) | 0.025 | 0.025 |
| TBHQ, antioxidant | 0.028 | 0.028 |
| Total | 1000.0 | 1000.0 |
Recording USVs
On PD9, isolation-induced ultrasonic vocalizations were recorded from pups. Briefly, male and female pups from the prenatal paradigm were brought down from the colony room in their home cage with the parents and weighed. They were then habituated to the testing room for 30 minutes prior to testing. At the time of testing, pups were separated from their parents into a clean housing pan with fresh bedding, warmed to ambient nesting temperature (~35° C) using a heating pad. During the testing window, pups were transferred into a 40 cm × 40 cm × 30 cm acrylic sound attenuating chamber for 2 minutes and vocalizations between 0 and 125 kilohertz (kHz) were recorded using a condenser microphone (CM16/CMPA, Avisoft Bioacoustics, Germany). The chamber was approximately room temperature (22° C). This microphone was connected to a recording interface (UltraSoundGate 116Hb, Avisoft Bioacoustics). Each pup was chosen randomly and recorded for 2 minutes, before being weighed, marked, and returned to the warmed littermate cage. An experimenter remained in the room to monitor the gain and other aspects of acquisition. Recordings were arranged such that pups were not away from the dam for longer than 30 minutes. After the last pup was tested, they were returned to the home cage with the parents. After excluding all non-vocalizers (Standard WT = 1, Standard KO = 1, Control Fat WT = 0, Control Fat KO = 4, Omega-3 WT = 1, Omega-3 KO = 1), the final sample size for the prenatal paradigm was as follows for males: Standard WT = 23, Standard KO = 12, Control Fat WT = 19, Control Fat KO = 14, Omega-3 WT = 14, Omega-3 KO = 13. After excluding non-vocalizers (Standard WT = 3, Standard HET = 1, Standard KO = 0, Control Fat WT = 0, Control Fat HET = 6, Control Fat KO = 3, Omega-3 WT = 4, Omega-3 HET = 2, Omega-3 KO = 0), the final sample size for the females was as follows: Standard WT = 32, Standard HET = 29, Standard KO = 10, Control Fat WT = 15, Control Fat HET = 20, Control Fat KO = 12, Omega-3 WT = 11, Omega-3 HET = 20, Omega-3 KO = 7. A nonsignificant chi-square indicated that the proportion of non-vocalizers was similar across the sexes, χ2 = 0.71, p = 0.41.
USV MATLAB Data Extraction
All WAV files were cleaned and processed using an automated analysis, freely available from: (http://jarvislab.net/research/mouse-vocal-communication/) using MathWork’s MATLAB software [31]. Sonograms were processed using the graphical user interface according to methods described previously [31, 32]. In the Sonogram Parameters section, we set Min Frequency to 15,000 Hz, Max Frequency to 125,000 Hz, the sampling frequency to 256 kHz, and the Threshold to 0.3. In the Whistle Options section, we set the Purity Threshold to 0.075, the Min Duration of the syllable to 3 ms, the Min Frequency sweep to 20,000 Hz, and the Filter Duration to 3 ms. For consistency, we also set the Min Note Duration to 3 ms, and the Min Note Count to 1. Following sonogram processing, density inter-syllable interval (ISI) was determined using the accompanying song-analysis guided analysis Excel file (available at the same website). From this analysis, the following variables could be assessed: number of calls, average call duration, fundamental frequency (frequency averaged from individual calls), and spectral purity (instantaneous maximum power at the peak frequency normalized by the instantaneous total power in the spectrum). For more information on how this method compares to previous cleaning methods used in our lab, see our previous works comparing MATLAB data extraction from our previously used hand scoring methods in the present model [29]. Further, we also classified the different syllables into their specific subtypes (representatives shown in Figure 1; for more information on syllable taxonomy, see [33]) and characterized how these distributions might be shifted by diet and genotype factors.
Statistical Analyses
All data were analyzed using GraphPad Software 7.0 (San Diego, CA) or IBM SPSS Statistics 23 (Aramonk, NY). Due to the differing numbers of genotype levels for males (WT, KO) and females (WT, HET, KO), the two sexes were analyzed separately. For males, a 2 × 3 (Genotype [WT,KO] x Diet [Standard, Control Fat, Omega-3]) analysis of variance (ANOVA) was run for each dependent variable. For females, a 3 × 3 (Genotype [WT, HET, KO] x Diet [Standard, Control Fat, Omega-3]) analysis of variance (ANOVA) was run for each dependent variable. Significant interactions of genotype and diet were followed up with the use of a unique identifier for all groups (i.e. “Standard WT”) and subsequent analysis. If there were multiple significant main effects, multiple comparisons were conducted using Fisher’s LSD comparisons (Standard WT vs Standard KO, Standard WT vs Control Fat KO, Standard WT vs Omega-3 KO). For the syllable-type analysis, a three-way chi-square was used for each sex: (genotype x diet x syllable-type). Significant chi-square statistics were followed up with Z-testing for column proportions. All comparisons were conducted within each diet group and referenced to their wildtype counterpart (i.e. Male Standard KOs were only compared to Male Standard WTs). For all inferential statistics, the level of significance remained at p < 0.05.
RESULTS
Exposure to both experimental diets increases body weight on PD9 in males but not females, regardless of genotype.
For males, there was no significant effect of genotype on body weight at PD9, Fgenotype(1, 94) = 0.10, p = 0.75. There was no significant interaction of genotype and diet, Fdiet x genotype(2, 94) = 0.10, p = 0.90. There was however, a significant main effect of diet, Fdiet (1, 94) = 4.56, p = 0.01 (Figure 1B). Post-hoc Fisher’s LSD testing revealed that, compared to the standard diet condition, males given both the control fat (p = 0.004) and the omega-3 (p = 0.04) dietary conditions weighed significantly more at PD9. This effect was similar across the two groups (Control Fat vs Omega-3, p = 0.52).
For females, there was no significant main effect of genotype, Fgenotype(1, 151) = 0.52, p = 0.60, or diet, Fdiet (1, 151) = 0.78, p = 0.46, on body weight (Figure 1C). Moreover, there was no significant interaction of the two factors, Fdiet x genotype(2, 151) = 1.16, p = 0.33.
Fmr1 knockout males show no changes in spectral and temporal aspects of vocalizations on PD9.
The analyses for males and females were conducted separately due to the addition of a heterozygous group for females. For males, an ANOVA was run and found no effect of genotype for: number of calls, Fgenotype(1, 89) = 0.19, p = 0.66 (Figure 2A), average call duration, Fgenotype(1, 89) = 3.60, p = 0.06 (Figure 2B), fundamental frequency, Fgenotype(1, 89) = 0.96, p = 0.33 (Figure 2C), or spectral purity, Fgenotype(1, 89) = 2.38, p = 0.13 (Figure 2D). No effect of diet was detected for any of the following variables: number of calls, Fdiet(2, 89) =1.54, p = 0.22, average duration, Fdiet(2, 89) = 1.52, p = 0.23, or spectral purity, Fdiet(2, 89) = 0.94, p = 0.40. However, fundamental frequency was significantly altered by diet, Fdiet(2, 89) = 4.44, p = 0.02. Post-hoc analyses with Fischer’s LSD indicated that the omega-3 diet (“b”) increased the fundamental frequency, compared to both standard (“a”) and control fat (“b”) conditions. No significant interactions of genotype and diet for any variable measured were detected: number of calls, Fdiet x genotype(2, 89) =0.28, p = 0.76, average duration, Fdiet x genotype(2, 89) = 0.52, p = 0.60, fundamental frequency, Fdiet x genotype(2, 89) = 0.78, p = 0.46, or spectral purity, Fdiet x genotype(2, 89) = 0.14, p = 0.87. Overall, the only significant finding in males demonstrated that fundamental frequency was significantly increased in pups receiving the omega-3 fatty acid diet.
Figure 2. Prenatal exposure to an omega-3 fatty acid enriched diet reverses aspects of vocalization production on PD9 in female Fmr1 mutants.
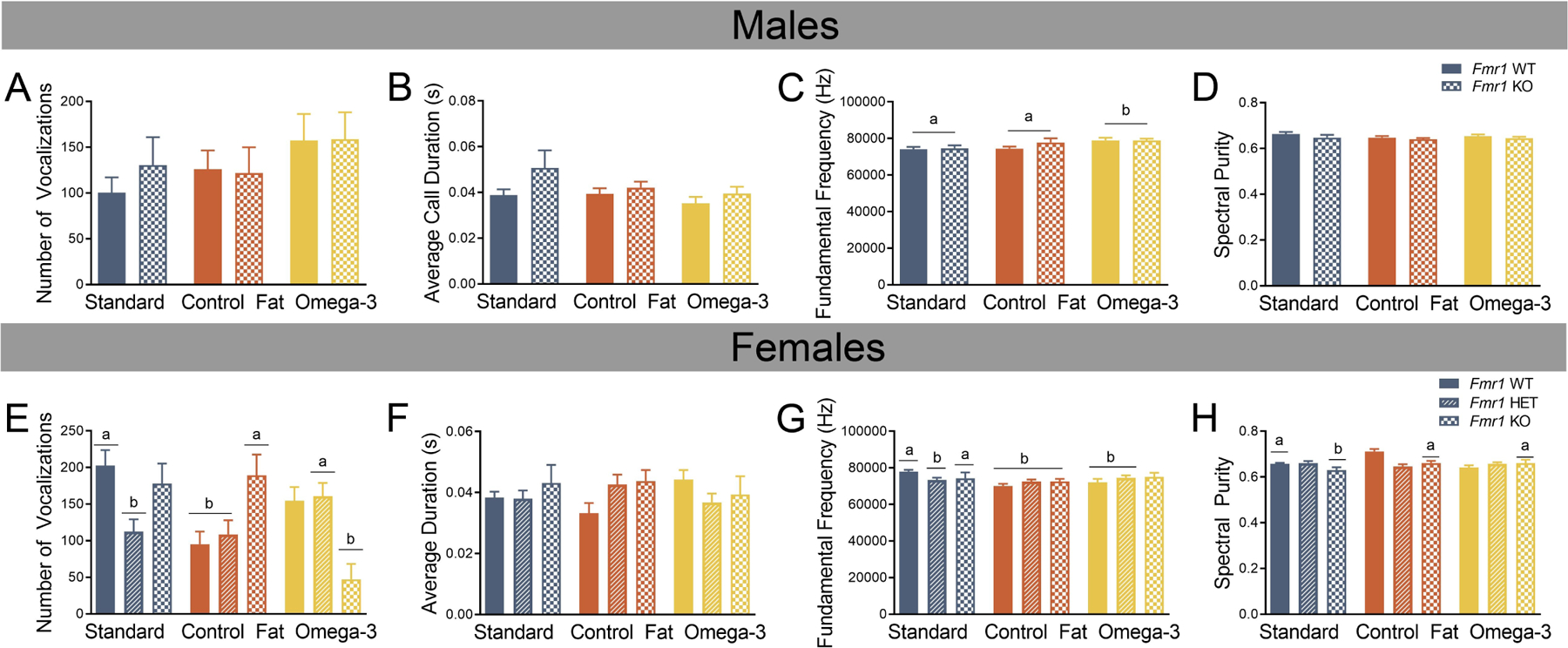
A. Number of vocalizations was unaffected by diet and genotype in males. B. Average duration of vocalizations was unaffected by diet and genotype in males. C. In males, fundamental frequency was significantly increased in subjects receiving the prenatal omega-3 diet, regardless of genotype. D. Spectral purity was unaffected by diet or genotype in males. E. Compared to the female Standard WT, both the female Standard Het and the female Control Fat Het showed reduced number of calls, and this was protected against by exposure to the omega-3 fatty acid diet. F. Average duration was unaffected by both genotype and diet in females. G. In females, fundamental frequency was reduced by heterozygous loss of Fmr1 and unaffected by the dietary condition. Moreover, the female WTs receiving the two experimental diets showed similar reductions in fundamental frequency. H. In the Standard Diet condition, homozygous loss of Fmr1 resulted in diminished spectral purity, however, exposure to the two experimental diet protected against this effect. Data are expressed as mean ± SEM. A designation of “b” indicates that this group differed from the “a” comparison group at the level of p < 0.05.
Prenatal exposure to an omega-3 fatty acid enriched diet reverses aspects of vocalization production on PD9 in female Fmr1 mutants.
For females, there was no main effect of genotype for the number of calls, Fgenotype(2, 147) = 0.91, p = 0.40 (Figure 2E). No main effect of diet was detected for number of calls, Fdiet(2, 147) = 2.77, p = 0.07. However, a significant interaction was detected, Fdiet x genotype(2, 147) = 6.52, p = 0.001, and was followed up with the creation of the unique “group” identifier for all 9 groups (e.g. “Standard WT” or “Standard Het”). Post-hoc Fischer’s LSD analyses indicated a few comparisons of interest (Figure 2E). First, in the standard diet condition, heterozygous deletion of Fmr1 in the two experimental dietary conditions resulted in a decrease in the number of calls produced [(a) Standard HET vs (b) Standard WT, p = 0.001]. While the control fat diet had no effect on this [(b) Control Fat HET vs (a) Standard WT, p = 0.001], and the decrease in calls in the HET mice was blocked by exposure to the omega-3 fatty diet, [(a) Standard WT vs (a) Omega-3 HET, p = 0.11]. Second, exposure to the control fat condition decreased the number of vocalizations produced in the wildtypes [(a) Standard WT vs (b) Control Fat WT, p = 0.001], but had no impact on the Fmr1 KO [(a) Standard WT vs (a) Control Fat KO, p = 0.67). Third, exposure to the omega-3 condition decreased the number of vocalizations in the Fmr1 KO [(a) Standard WT vs (b) Omega-3 KO, p = 0.001).
No main effect of genotype was detected for average duration, Fgenotype(2, 147) = 0.63, p = 0.53 (Figure 2F), nor was there a main effect of diet on average duration of calls produced, Fdiet(2, 147) = 0.004, p = 0.996. The interaction between genotype and diet was not significant for average duration, Fdiet x genotype(2, 147) = 1.73, p = 0.15 (Figure 2F).
No main effect of genotype was detected for fundamental frequency, Fgenotype(2, 147) = 0.09, p = 0.92 (Figure 2G). Diet did, however, affect fundamental frequency, Fdiet(2, 147) = 4.17, p = 0.02. Post-hoc analyses with Fischer’s LSD indicated that exposure to the control fat diet lowered the fundamental frequency, compared to the standard diet control, at the level of p < 0.05. This was not the case for the omega-3 diet condition (p = 0.10). Moreover, a significant interaction of genotype and diet, Fdiet x genotype(2, 147) = 2.91, p = 0.02, was followed up by Fisher’s LSD analyses (Figure 2G). First, in the standard diet condition, heterozygous loss of Fmr1 decreased fundamental frequency [(a) Standard WT vs (b) Standard Het, p = 0.003). This was only true in the heterozygous condition but not homozygous deletion [(a) Standard WT vs (b) Standard KO, p = 0.09]. In the control fat condition, all three genotypes displayed diminished fundamental frequency (“a” vs “b” p < 0.01) compared to the standard WT. It should be noted that the magnitude of this difference was not different between the HET and KOs in this dietary group (p > 0.05). Similarly, exposure to the omega-3 condition reduced fundamental frequency in the wildtype animals [(a) Standard WT vs (b) Omega-3 WT, p = 0.006]. The reduction in fundamental frequency shown by the standard diet HETs effect was similar for heterozygous deletion in the omega-3 condition, [(a) Standard WT vs (b) Omega-3 HET p = 0.05].
Results also indicate that loss of Fmr1 resulted in diminished spectral purity, Fgenotype(2, 147) = 3.33, p = 0.04 (Figure 2H). Similar to fundamental frequency, there was a main effect of diet on spectral purity, Fdiet(2, 147) = 4.17, p = 0.02. Post-hoc Fisher’s LSD analyses indicated no significant effects however, which may be due to the significant two-way interaction. The significant interaction, Fdiet x genotype(2, 147) = 7.01, p = 0.001, was followed up with post-hoc Fisher’s LSD analyses using a unique group identifier (i.e. Standard WT) (Figure 2H). First, results indicated that homozygous loss of Fmr1 resulted in reduced spectral purity in the standard diet condition [(a) Standard WT vs (b) Standard KO, p = 0.05). This effect was ameliorated by exposure to both the control fat [(a) Standard WT vs (a) Control Fat KO, p = 0.79) and omega-3 diets [(a) Standard WT vs (a) Omega-3 KO, p = 0.86].
Syllable-type analysis reveals subtle deficits in utilization profiles that is differentially affected by dietary manipulations.
Again, males and females were split for analysis and syllable-type analyses were conducted for each dietary group such that each treatment group was referenced back to its own control (Standard Male WT vs Standard Male KO) (for more information, see Statistical Analysis Methods). Results revealed that male KOs in the standard diet condition utilized a significantly different syllable-type repertoire, χ2 (3) = 91.48, p < 0.001. Z-tests for column proportions indicated that Fmr1 KOs produced proportionally fewer “s” syllable types than WTs and more “u” and “m” syllable types at the level of p < 0.05 (Figure 3A). A different pattern was shown in the control fat dietary condition, χ2 (3) = 20.93, p < 0.001 (Figure 3B). Z-tests for column proportions indicated that KOs in this group exhibited proportionally more “s” syllable types and less “m” syllable types. The omega-3 KO group showed a very similar distribution to the standard KO when compared to their dietary group WT animals, χ2 (3) = 23.48, p < 0.001 (Figure 3C). Z-tests revealed that, similar to the comparison between the standard WT and KOs, the omega-3 KO males produced significantly fewer “s” syllable types, as well as more “u” and “m” syllable types. Overall, these results suggest that the omega-3 diet did not shift the distribution of syllable-types, while the control fat significantly altered the KOs syllable-type production.
Figure 3. Prenatal omega-3 diet did not shift the distribution of syllable-types in males, while the control fat significantly altered the male KO syllable-type production.
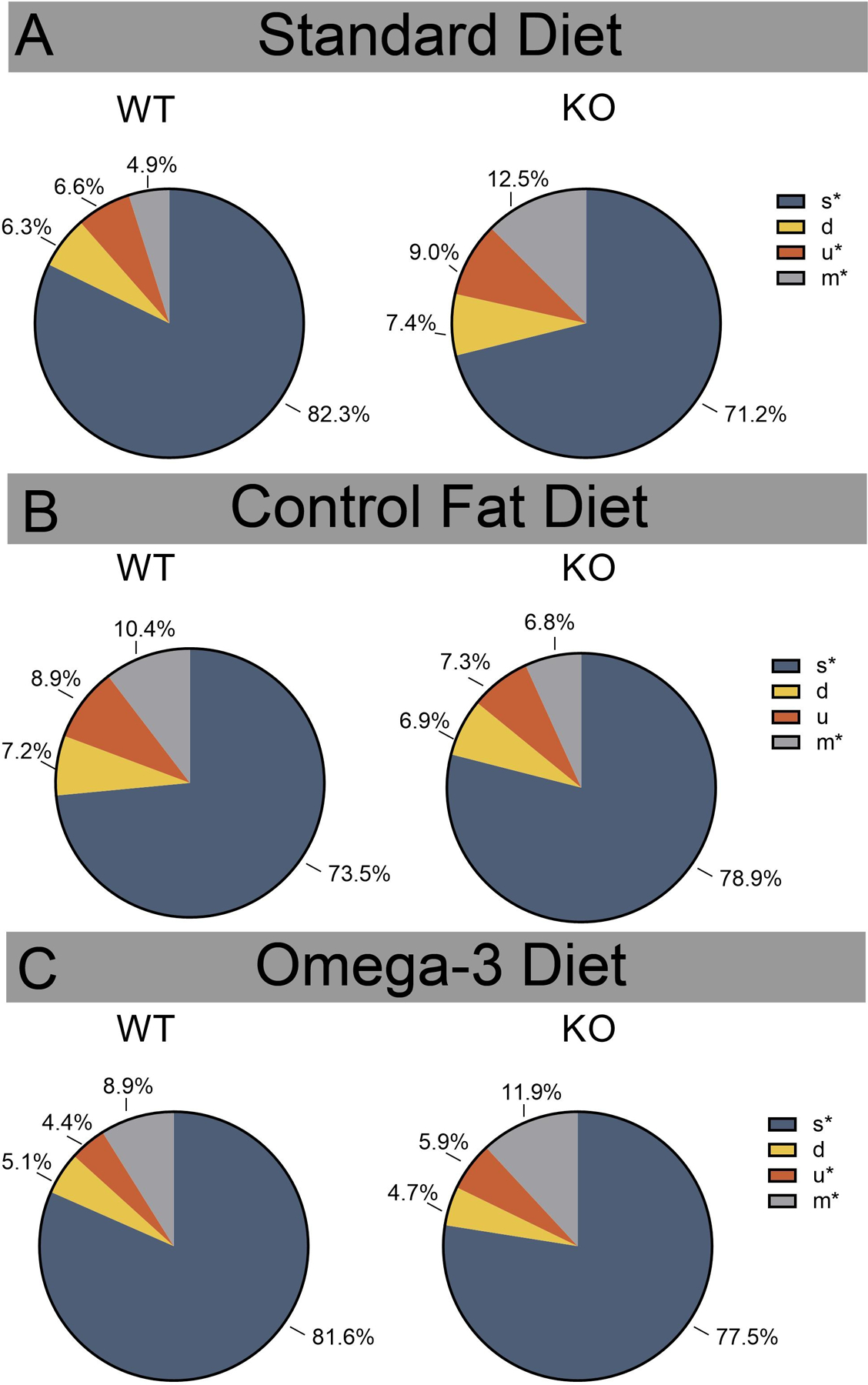
A. In the standard diet condition, Fmr1 KOs produced proportionally fewer “s” syllable types than WTs and more “u” and “m” syllable types. B. In the control fat condition, KOs exhibited proportionally more “s” syllable types and less “m” syllable types. C. The omega-3 KO group, similar to the standard KOs, produced significantly fewer “s” syllable types, as well as more “u” and “m” syllable types. Data are expressed as mean ± SEM. * = p < 0.05.
In females, mutation (collapsed across HETs and KOs) of Fmr1 resulted in a significant shift in the distribution of syllable production, χ2 (6) = 33.43, p < 0.001 (Figure 4A). Further testing showed that the standard HET produced proportionally more “s” syllable types and fewer “d” and “m” syllable types. Z-tests also revealed that compared the standard WT, the standard KO produced fewer “d” syllable types. A significant shift was also detected in the control fat diet group, χ2 (6) = 99.34, p < 0.001 (Figure 4B). Z-tests revealed that the control fat HET produced fewer “s” as well as more “d”, “u”, and “m” syllable types. The KOs in this dietary condition produced fewer “s” as well as more “d” and “m” syllable types, compared to the WT group. Finally, a significant shift was also detected for the omega-3 dietary group, χ2 (6) = 40.67 p < 0.001 (Figure 4C). Z-tests revealed HETs in this group produced significantly more “u” syllable types, while KOs produced fewer “s” and more “d” syllable-types compared to their respective WT groups. Similar to the results for males, the control fat diet further shifted the call-type profile in the HET and KO animals. The omega-3 partially rescued production in the HET group and further shifted the profile in the KO animal.
Figure 4. Prenatal omega-3 diet partially rescued syllable-type production differences in the HET, while both the omega-3 and control fat further shifted the female KO syllable-type production.
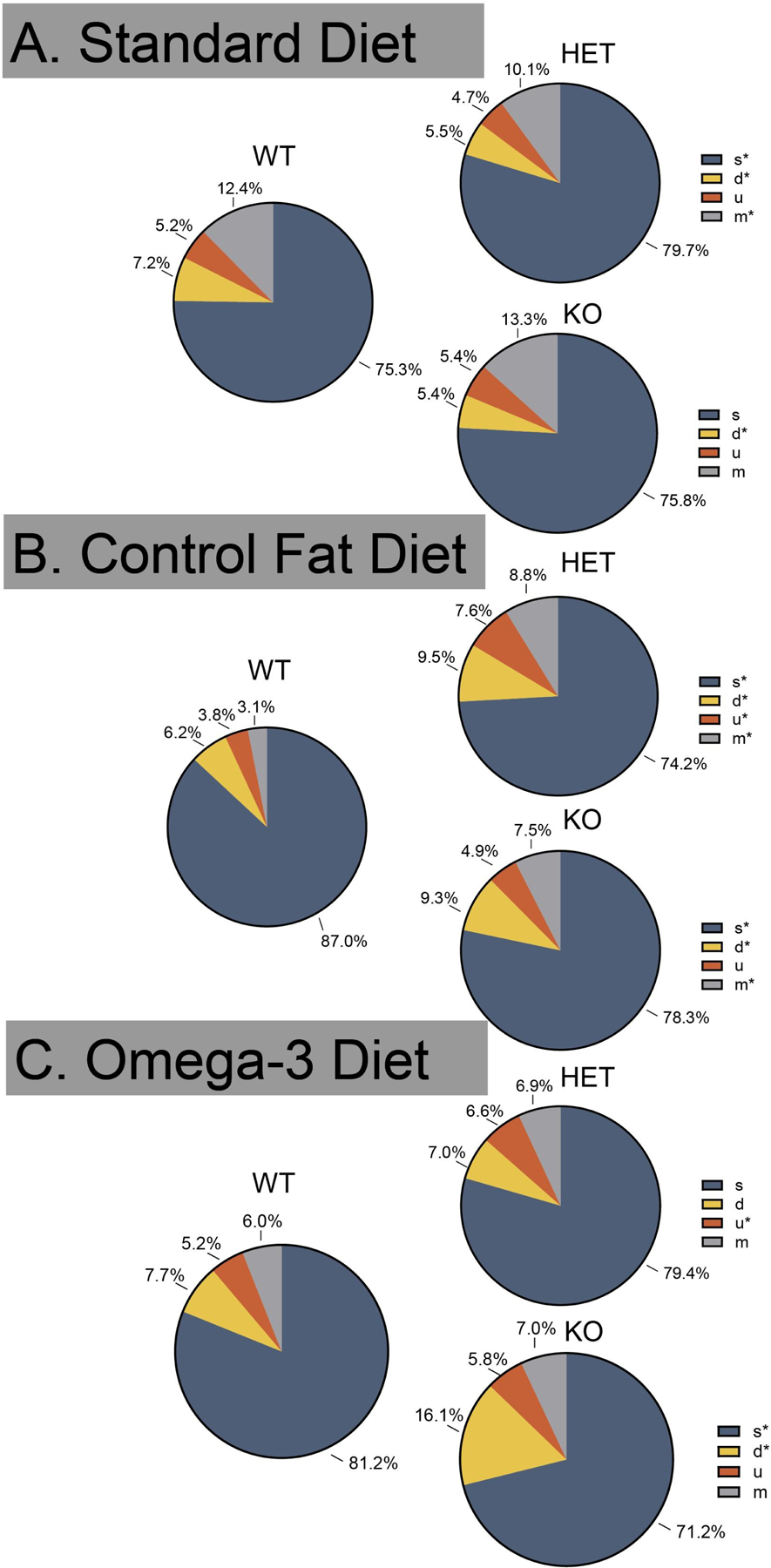
A. The standard HET produced proportionally more “s” syllable types and fewer “d” and “m” syllable types, while the standard KO produced fewer “d” syllable types. B. The control fat HET produced fewer “s” as well as more “d”, “u”, and “m” syllable types. The KOs in this dietary condition produced fewer “s” as well as more “d” and “m” syllable types. C. In the omega-3 group, HETs produced significantly more “u” syllable types, while KOs produced fewer “s” and more “d” syllable-types compared to their respective WT groups. Data are expressed as mean ± SEM. * = p < 0.05.
DISCUSSION
The present study presents evidence that prenatal administration of high fat diet is able to reverse some deficits in a sex-specific manner in the Fmr1 mutant model. Specifically, prenatal exposure to omega-3 fatty acids improved reductions in the number of calls produced by female Fmr1 heterozygotes. Diminished spectral purity in the female Fmr1 homozygous mouse was rescued by exposure to both high fat diets. Aside from the effects due to loss of Fmr1, prenatal dietary fat manipulation also influenced several aspects of vocalization production, such as the number of calls produced and their fundamental frequency. Specifically, in males, regardless of genotype, prenatal exposure to high omega-3 increased average fundamental frequency of calls. Together, the results for vocalization behavior in the current study provide support for the impact of these dietary manipulations on vocalization behavior and add to our understanding of the potential therapeutic value of omega-3 fatty acids, by comprehensively evaluating their effect on an early behavioral marker early in development.
Our results show that experimental manipulation of prenatal dietary fat influenced various aspects of vocalization behavior in the Fmr1 model on PD9, including the spectral purity. Spectral purity is calculated as “the instantaneous maximum power at the peak frequency normalized by the instantaneous total power in the spectrum, averaged across the entire syllable” [34]. Of biological relevance, congenitally deaf mice have a lower spectral purity than their hearing-intact counterparts, suggesting that auditory feedback is integral to the development of these vocalizations [35]. This postulation is potentially contentious, as the construct of vocal learning is typically considered unique to a few species of birds, humans, cetaceans, bats, elephants and pinnipeds [36, 37]. According to this definition, vocalizations produced in mice are instead considered an innate behavior. Indeed, it should be noted that number, structure and usage of vocalizations has been shown to not differ in congenitally deaf mouse pups [38]. Moreover, normative production of most aspects of vocalizations occurs without the presence of a normally developed cerebral cortex, suggesting this is a fundamental reflexive behavior [39]. The current study presents novel evidence that homozygous deletion of Fmr1 in female pups reduced spectral purity, which may reflect disruption of auditory brainstem networks shown previously in this model [40]. Moreover, we found that this diminished spectral purity could be rescued by exposure to either the high fat or high omega-3 diets, and this effect was specific to the loss of Fmr1. Prenatal DHA levels have been shown to significantly influence the development of the auditory brainstem in other rodent models [41, 42]. Thus, it is possible that rescue of spectral purity deficits due to increased fatty acid consumption during this early period reflect an effect on a lesser considered pathology in the Fmr1 model.
The current study sought to examine the possibility that the proposed treatment may differentially impact male and female Fmr1 mutants, as previous works have indicated the impact of Fmr1 loss on vocalization is also differential across the two sexes [11, 29]. Our results showed that in addition to the changes in spectral purity, both the number of vocalizations and fundamental frequency were vulnerable to manipulations of fat content in females, but not in males. Overall, these results suggest that communication behavior in females may be more sensitive to dietary manipulations during the prenatal and early neonatal period. However, further investigations are necessary, as these results do not conclusively point to one diet as optimal for vocalization development. Moreover, it’s possible that the results of the current study reflect a complex combination of sex-specific behavioral effects of loss of Fmr1 and the sex-specific nature of broad biological processes, like the trajectory of neurodevelopment or fatty acid metabolism [43]. In support of the latter, clinical findings have demonstrated that female children are born with higher levels of omega-3 fatty acids, suggesting a disparity in fetal metabolism [44]. Therefore, it is possible that the results of the present study reflect higher prenatal utilization of these dietary fats during development, explaining why the females exhibited a stronger response. However, previous studies have also supported the sex-specific trajectory of vocalization production early in development, though few studies have investigated these sexual behavioral dimorphisms early in development [45, 46, 21, 11, 47, 29]. It is possible that the sex-specificity in the present results reflect differences in the underlying model, as males showed few differences at this stage. Previous studies have corroborated differential effects of maternal high-fat diet on USV behaviors in male and female offspring, suggesting this is indeed a complex mechanism [48]. In sum, the exact combination of these factors at play in the present study is unclear, and future studies should elaborate on this question.
The present study indeed presents evidence that prenatal administration of high fat diet is able to reverse some deficits in a sex-specific manner in the Fmr1 mutant model. While these implications are strong, it should be noted that these results were from a single test day and USV is known to exhibit variation across the postnatal period [49, 11]. Therefore, the results may be owing to differences in the timing of the trajectory resulting from loss of Fmr1 rather than a general difference. Aside from the implications for the present model, the present study also reinforces important experimental considerations for the field of behavioral neuroscience. It has long since been understood that many factors can influence the trajectory of a behavioral experiment [reviewed in [50]]. Environmental factors, such as the timing of the test in relations to the light cycle, noise in the animal facility, or sex of the experimenter, can have significant influences on the behavioral output, depending on the task [51]. The variable of diet is of great importance considering the significant variation in available “standard” laboratory chows and often this dietary information is not included in the final published manuscript [52–54]. In addition to the factors listed above, such as sex of the experimenter and time of day, the present study finds that prenatal exposure to a “nonstandard” diet can also elicit changes in ultrasonic vocalization production during the early postnatal period, which has been demonstrated to, in turn, impact maternal care and subsequent development [55]. Broadly, these findings support the need to consider and expound on dietary variables when characterizing the results of a behavioral experiment.
Acknowledgements
The authors would like to thank the Lugo lab for their helpful review of the manuscript and the vivarium staff at Baylor University for their excellent management of facilities.
This work was supported by National Institutes of Health (NIH) (grant number: NS088776) to JNL.
Funding Sources
This work was supported by the National Institutes of Health (NIH) to JNL [Grant Number: NS088776] and by a Baylor University URSA grant to JNL. Neither funder had any role in the preparation of the manuscript or data.
Footnotes
The authors declare no conflicts of interest.
Statement of Ethics and a Disclosure Statement
The research in this manuscript was approved by the Baylor University Institutional Care and Use Committee and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. None of the authors have any conflicts of interest to report.
REFERENCES
- 1.Esposito G, Venuti P. Comparative Analysis of Crying in Children With Autism, Developmental Delays, and Typical Development. Focus Autism Other Dev Disabl. 2009. 2009/5/13.
- 2.Venuti P, Caria A, Esposito G, De Pisapia N, Bornstein MH, de Falco S. Differential brain responses to cries of infants with autistic disorder and typical development: an fMRI study. Res Dev Disabil. 2012. 2012;33(6):2255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito G, Hiroi N, Scattoni ML. Cry, Baby, Cry: Expression of Distress As a Biomarker and Modulator in Autism Spectrum Disorder. Int J Neuropsychopharmacol. 2017. 2017;20(6):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behavioural brain research. 2001. November 1;125(1–2):49–56. [DOI] [PubMed] [Google Scholar]
- 5.Smith JC. Responses of adult mice to models of infant calls. J Comp Physiol Psych. 1976;90(12):1105. [Google Scholar]
- 6.Branchi I, Santucci D, Alleva E. Analysis of ultrasonic vocalizations emitted by infant rodents. Current Protocols in Toxicology. 2006;30(1):13.12.1–13.12.14. [DOI] [PubMed] [Google Scholar]
- 7.Keller A, Saucier D, Sheerin A, Yager J. Febrile convulsions affect ultrasonic vocalizations in the rat pup. Epilepsy Behav. 2004. 2004/10;5(5):649–54. [DOI] [PubMed] [Google Scholar]
- 8.Cox ET, Hodge CW, Sheikh MJ, Abramowitz AC, Jones GF, Jamieson-Drake AW, et al. Delayed developmental changes in neonatal vocalizations correlates with variations in ventral medial hypothalamus and central amygdala development in the rodent infant: Effects of prenatal cocaine. Behav Brain Res. 2012. 2012;235(2):166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012. 2012/5;26(4):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 2012. 2012;1439:7–14. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds CD, Nolan SO, Jefferson T, Lugo JN. Sex-specific and genotype-specific differences in vocalization development in FMR1 knockout mice. Neuroreport. 2016. December 14;27(18):1331–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder MS, Lugo JN. NS-Pten knockout mice show sex- and age-specific differences in ultrasonic vocalizations. Brain Behav. 2017. 2017;7(11):e00857–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan SO, Hodges SL, Condon SM, Muhammed IDA, Tomac LA, Binder MS, et al. High seizure load during sensitive periods of development leads to broad shifts in ultrasonic vocalization behavior in neonatal male and female C57BL/6J mice. Epilepsy Behav. 2019. 2019/6/1;95:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996. 1996;64(1):196–97. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004. 2004;129A(3):225–34. [DOI] [PubMed] [Google Scholar]
- 16.Bakker. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994. 1994;78(1):23–33. [PubMed] [Google Scholar]
- 17.D’Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997. 1997;76(2):367–76. [DOI] [PubMed] [Google Scholar]
- 18.de Vrij FMS, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008. 2008;31(1):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009. 2009/2;46(2):94–102. [DOI] [PubMed] [Google Scholar]
- 20.Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010. 2010/8;9(6):562–74. [DOI] [PubMed] [Google Scholar]
- 21.Ding Q, Sethna F, Wang H. Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behavioural brain research. 2014. September 1;271:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolan SO, Reynolds CD, Smith GD, Holley AJ, Escobar B, Chandler MA, et al. Deletion of Fmr1 results in sex-specific changes in behavior. Brain Behav. 2017. 2017/10;7(10):e00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodapp EMDRM, Leckman JF. Behavior and development in fragile X syndrome. London: Sage; 1994. [Google Scholar]
- 24.Abbeduto L, Hagerman RJ. Language and communication in fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3(4):313–22. [Google Scholar]
- 25.Belagodu AP, Johnson AM, Galvez R. Characterization of ultrasonic vocalizations of fragile X mice. Behav Brain Res. 2016. 2016. [DOI] [PubMed]
- 26.Hodges SL, Nolan SO, Reynolds CD, Lugo JN. Spectral and temporal properties of calls reveal deficits in ultrasonic vocalizations of adult Fmr1 knockout mice. Behav Brain Res. 2017. 2017;332:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One. 2012. 2012;7(9):e44816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai JK, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA. Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res. 2014. 2014;259:119–30. [DOI] [PubMed] [Google Scholar]
- 29.Nolan SO, Hodges SL, Lugo JN. High-throughput analysis of vocalizations reveals sex-specific changes in Fmr1 mutant pups. Genes, Brain and Behavior. 2019. [DOI] [PubMed]
- 30.Pietropaolo S, Goubran MG, Joffre C, Aubert A, Lemaire-Mayo V, Crusio WE, et al. Dietary supplementation of omega-3 fatty acids rescues fragile X phenotypes in Fmr1-Ko mice. Psychoneuroendocrinology. 2014. November;49:119–29. [DOI] [PubMed] [Google Scholar]
- 31.Chabout J, Jones-Macopson J, Jarvis ED. Eliciting and analyzing male mouse ultrasonic vocalization (USV) songs. Journal of visualized experiments : JoVE. 2017. May 09(123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan SO, Hodges SL, Condon SM, Muhammed IDA, Tomac LA, Binder MS, et al. High seizure load during sensitive periods of development leads to broad shifts in ultrasonic vocalization behavior in neonatal male and female C57BL/6J mice. Epilepsy & Behavior. 2019. 2019/06/01/;95:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chabout J, Sarkar A, Dunson DB, Jarvis ED. Male mice song syntax depends on social contexts and influences female preferences. Frontiers in behavioral neuroscience. 2015;9:76–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: The mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS One. 2012;7(10):e46610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arriaga G, Jarvis ED. Mouse vocal communication system: Are ultrasounds learned or innate? Brain and Language. 2013. 2013/01/01/;124(1):96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janik VM, Slater PJ. The different roles of social learning in vocal communication. Animal behaviour. 2000. July;60(1):1–11. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004. 2004/6;1016:749–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammerschmidt K, Whelan G, Eichele G, Fischer J. Mice lacking the cerebral cortex develop normal song: insights into the foundations of vocal learning. Scientific reports. 2015. March 06;5:8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammerschmidt K, Whelan G, Eichele G, Fischer J. Mice lacking the cerebral cortex develop normal song: insights into the foundations of vocal learning. Scientific Reports. 2015. March 06;5:8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruby K, Falvey K, Kulesza RJ. Abnormal neuronal morphology and neurochemistry in the auditory brainstem of Fmr1 knockout rats. Neuroscience. 2015;303:285–98. [DOI] [PubMed] [Google Scholar]
- 41.Stockard JE, Saste MD, Benford VJ, Barness L, Auestad N, Carver JD. Effect of docosahexaenoic acid content of maternal diet on auditory brainstem conduction times in rat pups. Developmental Neuroscience. 2000;22(5–6):494–99. [DOI] [PubMed] [Google Scholar]
- 42.Haubner LY, Stockard JE, Saste MD, Benford VJ, Phelps CP, Chen LT, et al. Maternal dietary docosahexanoic acid content affects the rat pup auditory system. Brain Research Bulletin. 2002. 2002/05/01/;58(1):1–5. [DOI] [PubMed] [Google Scholar]
- 43.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. The American Journal Of Clinical Nutrition. 2011. December;94(6 Suppl):1914s–19s. [DOI] [PubMed] [Google Scholar]
- 44.Colquhoun I, Bunday S. A lack of essential fatty acids as a possible cause of hyperactivity in children. Medical Hypotheses. 1981. 1981/05/01/;7(5):673–79. [DOI] [PubMed] [Google Scholar]
- 45.Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135(3):999–1009. [DOI] [PubMed] [Google Scholar]
- 46.Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes, Brain and Behavior. 2010;9(6):562–74. [DOI] [PubMed] [Google Scholar]
- 47.Nolan SO, Reynolds CD, Smith GD, Holley AJ, Escobar B, Chandler MA, et al. Deletion of Fmr1 results in sex-specific changes in behavior. Brain and behavior. 2017. October;7(10):e00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abuaish S, Tse EK, McGowan PO. Perinatal high-fat diet impairs pup retrieval and induces sex-specific changes in ultrasonic vocalization characteristics of rat pups. Developmental psychobiology. 2020;62(4):436–45. [DOI] [PubMed] [Google Scholar]
- 49.Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PloS one. 2011;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Meer P, Raber J. Mouse behavioural analysis in systems biology. The Biochemical Journal. 2005;389(Pt 3):593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999. June 4;284(5420):1670–2. [DOI] [PubMed] [Google Scholar]
- 52.Wise A, Gilburt DJ. The variability of dietary fibre in laboratory animal diets and its relevance to the control of experimental conditions. Food and Cosmetics Toxicology. 1980. 1980/01/01/;18(6):643–48. [DOI] [PubMed] [Google Scholar]
- 53.Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008;7(4):277–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giles K, Guan C, Jagoe TR, Mazurak V. Diet composition as a source of variation in experimental animal models of cancer cachexia. J Cachexia Sarcopenia Muscle. 2016;7(2):110–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abuaish S, Spinieli RL, McGowan PO. Perinatal high fat diet induces early activation of endocrine stress responsivity and anxiety-like behavior in neonates. Psychoneuroendocrinology. 2018;98:11–21. [DOI] [PubMed] [Google Scholar]


