Abstract
Objectives.
Defecation requires relaxation of the internal and external anal sphincters. High anal resting pressure is associated with painful constipation, defecatory disorders, and increased healthcare utilization in constipated patients; the mechanisms are unclear. Perhaps patients with a high anal resting pressure have a less distensible canal, which impedes defecation.
Methods.
In 50 of 64 participants (33 healthy and 17 constipated women), anal pressures and distensibility were measured respectively with manometry and balloon distention combined with magnetic resonance imaging; rectal balloon expulsion time (BET) was also studied.
Results.
The BET (P=0.006) was longer and the mean (SD) rectoanal pressure gradient (−58[40] vs −34[26] mmHg, P=0.03) was more negative in constipated than healthy women; anal resting pressure was not different. During anal distention, the balloon expanded rapidly at an opening pressure of 49 (18) mmHg, which was lower (P<0.0001) than resting pressure (90 [25] mmHg). The resting pressure was correlated with the opening pressure (r=0.57, P<0.0001) and inversely (r=−0.38, P=0.007) with maximum volume but not with anal distensibility (volume-pressure slope). In healthy women, the difference (opening-resting pressure) was correlated with anal relaxation during evacuation (r=0.35, P=0.04). Anal distensibility and sensory thresholds were not different between constipated and healthy women.
Conclusions.
Among healthy and constipated women, a greater anal resting pressure is correlated with greater opening pressure and lower maximum volume during distention, and, hence, provides a surrogate marker of anal distensibility. The difference (opening–resting pressure), which reflects anal relaxation during distention, is correlated with anal relaxation during evacuation. Anal resting pressure and distensibility were comparable in healthy and constipated women.
Keywords: constipation, pelvic floor dysfunction, EndoFlip, compliance
Introduction
Anal manometry is widely used to diagnose defecatory disorders (DD)1,2. During evacuation, a reduced rectal propulsive force, high anal pressure, inadequate anal relaxation, and a less positive or more negative rectoanal gradient are the most widely used manometry criteria for DD1–3. Some studies and a consensus document consider high anal resting pressure, also known as anal hypertension, as a feature of pelvic floor dysfunction, not only in DD, but also in patients with chronic anal fissure, levator ani syndrome or proctalgia fugax4–6. Among constipated patients, anal hypertension may define a phenotype, that is characterized by painful constipation, normal colon transit, and normal rectal sensation7 and is associated with increased healthcare utilization8.
Anal resting pressure is primarily maintained by the internal and to a lesser extent by the external anal sphincter9. Supporting a role for the internal anal sphincter, isosorbide dinitrate, the adrenergic α1-receptor antagonist alfuzosin, and internal anal sphincterotomy reduced anal resting pressure in patients with anal hypertension10–13. Pudendal nerve blockade reduced anal resting pressure and the electromyography amplitude of the external anal sphincter and puborectalis muscle measured at rest in healthy women and men, which demonstrates the contribution of the external anal sphincter and puborectalis to resting tone14,15. Conceptually, increased contraction of the internal and/or external anal sphincters may cause anal hypertension.
The mechanism(s) by which anal hypertension impairs rectal evacuation are unknown. In achalasia of the esophagus, esophageal retention is partly explained by a less distensible lower esophageal sphincter16. It is conceivable that anal hypertension is a surrogate marker of a less distensible anal sphincter that impedes the passage of stool through the anal canal. The EndoFLIP device has been used to investigate anal distensibility in healthy people and in patients with fecal incontinence (FI) but not in DD17. Patients with FI generally have decreased or normal but not high anal resting pressure18. Hence, the relationship between anal hypertension and anal distensibility has been not evaluated.
The anal canal is profusely lined with free and organized nerve endings19, which mediate the sensation of light touch and pressure and allow humans to discriminate between solid and liquid stool and gas20. Reduced anal sensation, which is manifest by a diminished ability to detect a temperature change, has been implicated in the pathophysiology of fecal incontinence21. The anal canal also contains Golgi-Mazzoni bodies and Pacinian corpuscles that detect tension and pressure. However, anal sensitivity to distention has not been evaluated19. Perhaps increased anal perception of distention may explain symptoms such as anal pain in patients with DD, especially those with anal hypertension.
The aims of this study were to compare anal pressures at rest and during evacuation, anal distensibility, and the perception of anal distention in healthy controls and patients with chronic constipation.
Methods
Design
The Mayo Clinic Institutional Review Board approved these studies. All participants provided informed consent. In 39 healthy and 25 constipated women, rectoanal pressures were measured with high-resolution anorectal manometry. Anal distensibility was assessed with anal balloon distention on two occasions, with and without magnetic resonance imaging (MRI).
Participants
All participants, who were aged 18 years or older, were interviewed and underwent a physical examination. Neither healthy controls nor patients had clinically-significant systemic diseases. They were not taking medications (eg, opioids) that affect gastrointestinal motility. In addition, controls did not have a functional bowel disorder by Rome 3 criteria, documented grade 3 or 4 obstetric anorectal laceration, or any previous anorectal surgery22. Patients had symptoms of chronic constipation for at least 1 year. The mean (SD) age and BMI were not significantly different between 39 healthy women (34 [13] y, 25 [3] kg/m2) and 25 constipated women (36 [15] y, 25 [6] kg/m2).
Procedures
Anorectal Manometry
After two sodium phosphate enemas (Fleet; C.B. Fleet), rectoanal pressures were measured in the left lateral position at rest, during squeeze, and simulated evacuation with high-resolution anorectal manometry (Manoscan™; currently Medtronic Inc). Pressures were measured for 20s at rest, during squeeze (voluntary contraction of the anal sphincter, 3 attempts), simulated evacuation with an empty rectal balloon, and a Valsalva maneuver23. All studies were analyzed with the commercially-available version of the software (Manoview AR v3.0; Medtronic Inc)23.
Rectal balloon expulsion time
Participants had up to 3 minutes to expel a 4-cm-long balloon filled with 50 ml water from the rectum in privacy while seated on a commode24,25. The BET was noted and the balloon was removed if participants could not expel the balloon within 3 minutes. Consistent with studies that used a similar balloon in healthy volunteers from our and other centers, a BET greater than 60s was considered to be prolonged (ie, abnormal)24,26.
Anal distensibility
Anal distensibility was evaluated with a barostat (Mayo Clinic, Rochester MN) with and separately without magnetic resonance imaging (MRI) (Figure 1). In previous studies, the anal pressure and diameter were approximately 100 mmHg and 1.3 cm during the rectal evacuation of barium27–29. Hence, the Mayo Clinic Institutional Review Board permitted anal distention with a modified barostat that generates a maximum pressure of 99 mmHg.
Figure 1.
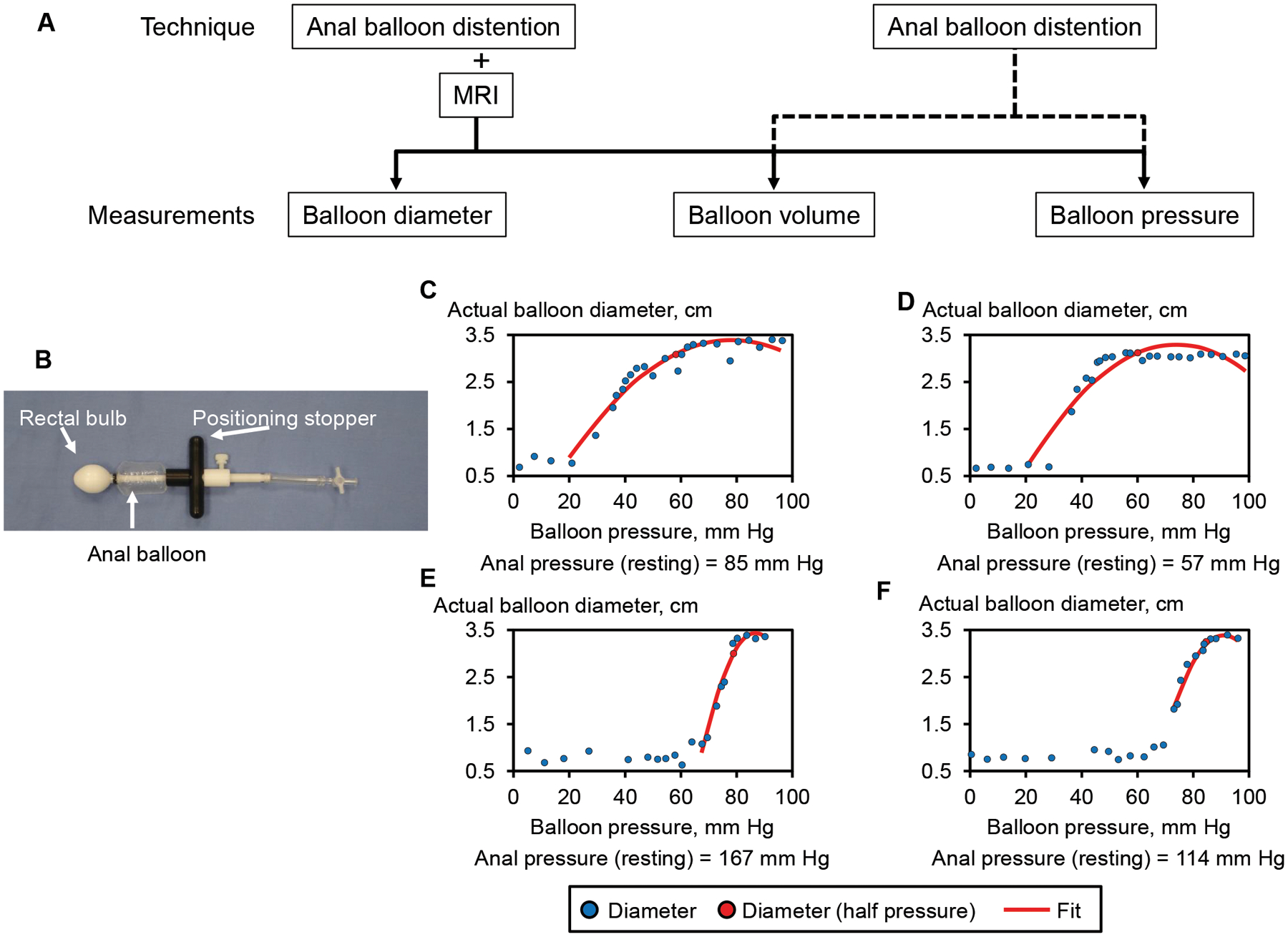
Techniques, measurements (Panel A), and the anal catheter (Panel B). Representative examples of balloon pressure-diameter curves and corresponding fitted curves are shown in healthy women (C and E) and constipated patients (D and F). In all four examples, the opening pressure during distention is lower than anal resting pressure. The opening pressure was lower in panels C and D (resting pressure was 85 and 57 mmHg) than panels E and F (resting pressure was 167 and 114 mmHg).
The barostat was connected to a balloon that was tied at both ends to a custom-manufactured device (Mui Scientific, Mississauga, Ontario, Canada) made of delrin (Figure 1). At its upper end, a cylindrical bulb secured this device in the rectum. At the outer end, a positioning stopper secured the device outside the anal orifice to prevent displacement of the balloon into the rectum. This length of the anal balloon was individualized to each participant based on the length of the high pressure zone measured with manometry. The manometry was performed just before anal distention. The balloon was 1.5, 2 cm, 2.5 cm, and 3 cm long respectively in 2, 3, 5, and 54 participants. The balloon was inflated in 5 ml steps from 0 ml to a maximum volume of 50 ml or to 99 mmHg, whichever came first, and deflated to 0 ml thereafter. Designed to achieve a quasi-static state in which there is a minimal flow of air between the barostat and the balloon, the pressure was maintained for 30 seconds at each step30.
There were two differences between the experiments with and without MRI. During the MRI studies, the barostat, which was outside the MRI scanner room, was connecting to the anal balloon by a 9.2 m long tube. During anal distention without MRI, this tube was 1.7 m long. Barostat pressure and volume were measured with both techniques. The balloon volume and maximum diameter were also measured on the MRI images.
During anal distention, there is compression of air and expansion of the polyethylene tubing connecting the barostat to the anal balloon. Hence, only a fraction of the air displaced from the barostat is delivered to the anal balloon. In vitro studies were used to estimate the volume actually delivered to the balloon during the in vivo studies. These in vitro studies were performed using the equipment for anal distention with and separately without MRI. During the in vitro studies, pressure-volume relationships were evaluated while distending the balloon in a rigid tube with a diameter of 1.0 cm. At each pressure during the in vivo distention, the balloon volume at the corresponding pressure during in vitro distention was determined. The sum of that volume and the volume of the central rod provided the estimated volume at each pressure. The estimated balloon diameter was derived from the estimated volume based on the assumption that the anal balloon is a cylinder. During anal distention with and without MRI, the estimated volume and diameter were measured. During anal distention with MRI, the actual volume and diameter were also measured on the images.
Anal pressure-volume relationships were analyzed as follows. First, the opening pressure beyond which the anal diameter began to increase was identified by inspecting the data. Then, all points between the opening and maximum pressures for the pressure-volume and pressure-diameter curves were fitted to a quadratic function. The summary parameters, which are listed here in the sequence in which they occur (ie, from low to high pressures), are as follows: opening pressure, half-maximum pressure (Prhalf), which is halfway between opening pressure and maximum pressure, the volume and diameter at Prhalf, maximum pressure, measured volume and diameter at maximum pressure. Except for the Prhalf, the volume, and diameter at this pressure, which were derived from the fitted curves, the remaining parameters, including the overall slope of the pressure-volume and pressure-diameter relationship, were calculated from the actual parameters.
MR imaging
During MRI distensions, thirteen axial SSFSE images were obtained along the long axis of the anal canal, from the rectum to the anal verge, at each step of distension. Other relevant SSFSE parameters included 5mm thickness, 192×192 acquired matrix, reconstructed at 256×256, 0.8594 pixel spacing, 220 mm FOV, flip angle 90° and TE/TR of 79/1800 ms. The echo time varied slightly due to gradient constraints relating to the rotated imaging plane. At each distending pressure, a region of interest was drawn by a single author (MS), around every slice with the balloon (AnalyzePro 1.0, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The balloon volume was calculated by integrating the cross-sectional areas at each distending pressure. The maximum balloon diameter was measured perpendicular to the long axis of the catheter.
Anal sensation
The pressure and volume thresholds for first sensation, desire to defecate, and urgency were recorded during anal distention; the threshold was the first sensation of each symptom. The pressures and volumes for sensations that were not perceived by participants were censored31. For example, when the first sensation was not perceived, the threshold for the desire to defecate, or urgency, or the maximum pressure during the curve, whichever came first, was used and labeled as “censored”, in contrast to sensations that were perceived (ie, occurred).
Statistical analysis
Wilcoxon’s rank sum test compared the anal resting pressure and distensibility parameters between healthy and constipated patients. During the MRI studies, actual and estimated distensibility parameters were compared with Wilcoxon’s signed rank test and Lin’s concordance correlation coefficient (CCC). Bland-Altman plots with 95% limits of agreement were generated to visualize the degree of agreement between actual and estimated distensibility parameters32. Sensory thresholds were compared using separate proportional hazard regression models for each threshold. These models estimated the “risk” (probability) for reporting a specific sensation threshold over increasing pressure steps analogous to a survival analysis of the risk for an event (e.g. death) over time. The associations between manometry and distension parameters were determined using Spearman’s rank correlation and linear regression models. Data are summarized as Mean (SD). All analyses used JMP software (version 9.4, SAS Cary, NC).
Results
Study Flow and Clinical Features
Complete datasets were available in 50 participants, i.e., 33 healthy women (age: 32 [11] y, BMI: 26 [4] kg/m2) and 17 constipated women (age: 33 [15] y, BMI: 24 [6] kg/m2). In the remaining 14 participants (ie, 6 healthy and 8 constipated women), anal distention was curtailed at a pressure lower than the anal resting pressure because the balloon was displaced outside the anal canal in 10 participants (4 healthy and 6 constipated; functional constipation [5 patients] or IBS-C [6 patients]) or because of patient discomfort (4 participants). Among these 17 patients, 7 (41%) had symptom criteria for constipation-predominant IBS and 10 (59%) had functional constipation. The symptoms included infrequent bowel movements (35%), hard stools (53%), excessive straining (82%), anal digitation (53%), anal blockage (76%) during defecation, and the feeling of incomplete evacuation after defecation (82%).
The balloon expulsion time was longer (P = 0.006) in constipated women (70 [75] s) than in controls (13 [10] s). It was shorter than 60s in all controls and longer than 60s, indeed 180s, in 5 of 17 constipated women (29%).
Rectoanal pressures
The anal pressures at rest and during evacuation were not significantly different between healthy and constipated women (Table 1). However, 10 of 17 constipated women (59%) but only 8 of 33 healthy women (24%, P < 0.03) had anal hypertension (ie, resting pressure greater than 111 or 93 mmHg respectively in women aged less than 50 years or 50 years and older). The anal canal was longer in constipated than healthy women (3.8 [0.7] cm vs 3.2 [1.1] cm, P = 0.02).
Table 1.
Anal manometry and anal balloon distension
| Parameter | Healthy women (n=33) | Constipated women (n=17) | P-value |
|---|---|---|---|
| Anorectal manometry | |||
| Anal pressure at rest, mm Hg | 90 (25) | 94 (26) | .59 |
| Anal canal length, cm | 3.2 (1.1) | 3.8 (.7) | .02* |
| Anal pressure during squeeze, mm Hg† | 235 (53) | 194 (53) | .01* |
| Anal pressure during evacuation, mm Hg | 71 (25) | 86 (28) | .06 |
| Anal pressure change (after – before) during evacuation, mm Hg | −19 (28) | −8 (19) | .1 |
| Rectoanal gradient during evacuation, mm Hg | −34 (26) | −58 (40) | .03* |
| Anal distension with MRI – actual parameters | |||
| Opening pressure, mm Hg | 49 (18) | 46 (19) | .68 |
| Balloon diameter at Prhalf, cm | 1.7 (.8) | 1.9 (.8) | .47 |
| Balloon volume at Prhalf, ml | 7.8 (4.9) | 7.7 (4.3) | .98 |
| Slope for balloon diameter, mm Hgĉm | .05 (.03) | .04 (.03) | .41 |
| Slope for balloon volume, mm Hĝml | .30 (.18) | .35 (,29) | .95 |
| Maximum balloon pressure, mm Hg | 84 (14) | 85 (15) | .66 |
| Maximum balloon diameter, cm | 2.2 (.7) | 2.5 (.6) | .24 |
| Maximum volume, ml | 11.8 (6.2) | 12.5 (4.8) | .77 |
| Anal distension with MRI – estimated parameters | |||
| Opening pressure, mm Hg | 49 (21) | 50 (25) | .86 |
| Balloon diameter at Prhalf, cm | 1.7 (.5) | 1.7 (.7) | .85 |
| Balloon volume at Prhalf, ml | 6.6 (3.5) | 7.8 (5.7) | .54 |
| Maximum balloon pressure, mm Hg | 88 (14) | 90 (13) | .92 |
| Maximum balloon diameter, cm | 2.1 (.7) | 2.4 (.8) | .28 |
| Maximum balloon volume, ml | 11.1 (5.8) | 14.5 (8.5) | .18 |
| Anal distension without MRI – estimated parameters | |||
| Opening pressure, mmHg | 46 (26) | 44 (26) | .96 |
| Balloon diameter at Prhalf, cm | 1.4 (.6) | 1.4 (.8) | .97 |
| Balloon volume at Prhalf, ml | 5.3 (4.7) | 6.2 (5.8) | .73 |
| Maximum balloon pressure, mm Hg | 86 (20) | 93 (14) | .18 |
| Maximum balloon diameter, cm | 2.3 (.8) | 2.2 (.8) | .99 |
| Maximum balloon volume, ml | 12.4 (7.8) | 13.1 (8.2) | .59 |
| Sensory thresholds | |||
| Pressure threshold for first sensation, mm Hg | 34 (26) | 41 (28) | .07 |
| Volume threshold for first sensation, ml | 1.3 (1.6) | 3.3 (3.8) | .02* |
| Pressure threshold for desire to defecate, mm Hg | 53 (29) | 48 (30) | .54 |
| Volume threshold for desire to defecate, ml | 3.8 (5.1) | 4.4 (4.6) | .75 |
| Pressure threshold for urgency, mm Hg | 69 (28) | 57 (28) | .34 |
| Volume threshold for urgency, ml | 6.3 (6.8) | 6.3 (6.1) | .72 |
These values are derived from the squeeze maneuver with the highest squeeze pressure
P<0.05
During evacuation, the anal pressure declined to a greater extent (P = 0.09) in healthy (−19 [28] mm Hg) than in constipated women (−8 [19] mm Hg). The rectoanal gradient was less negative (P = 0.03) in healthy than constipated women (−34 [26] mm Hg vs −58 [40] mm Hg).
Comparison of actual and estimated anal distensibility
During anal distention, the balloon volume noticeably increased at the opening pressure (Figures 1 and 2). The Mean (SD) R2 values for goodness of fit between actual data and fitted curves were 0.93 (0.16) for actual balloon diameter, 0.94 (0.08) for actual balloon volume, 0.93 (0.14) for estimated balloon diameter, and 0.95 (0.14) for estimated balloon volume. None of these actual and estimated parameters (ie, opening pressure, Prhalf, balloon volume and diameter at Prhalf,, maximum balloon volume and diameter, and the overall slope of the pressure-volume relationship) were significantly different between healthy and constipated women (Table 1).
Figure 2.
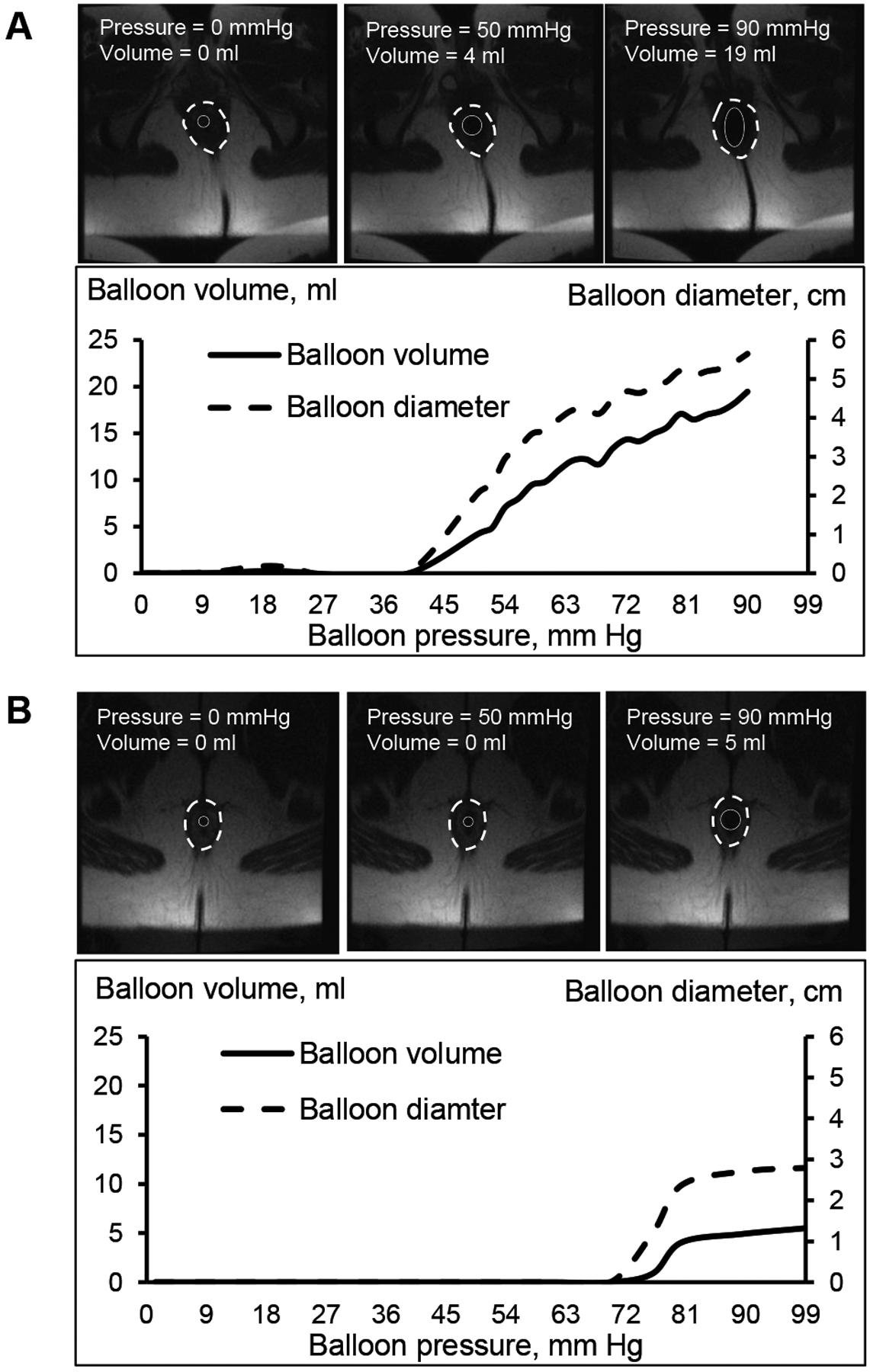
Representative images of anal distention. By comparison to Panel A (healthy woman), Panel B shows a constipated patient with a greater resting anal pressure (108 versus 88 mmHg), a greater opening pressure (72 mmHg versus 38 mmHg), and a lower maximum diameter (3 cm versus 6 cm). The solid and broken white circles respectively demarcate the anal balloon and anal sphincter.
For the comparisons between actual and estimated balloon volume and diameter, the concordance correlation coefficients were respectively 0.56 (95% CI, 0.35 – 0.72) and 0.54 (95% CI, 0.31 – 0.71). The actual and estimated maximum parameters were not significantly different in healthy women (volume: P = 0.71; diameter: P = 0.12) or constipated patients (volume: P = 0.40; diameter: P = 0.05). In the Bland-Altman analysis, the mean of the differences between the actual and estimated volume was different from zero (P = 0.01) (Figure 3A). In all participants, the estimated maximum volume overestimated the actual maximum volume by 0.18 ml (95% limit of agreement, −11.8ml to 11.5ml). The mean of the differences between the actual and estimated maximum balloon diameter was different from zero (P = 0.03) (Figure 3B). The estimated maximum diameter underestimated the actual maximum diameter by 0.10 cm, with a 95% limit of agreement between −1.19 cm and 1.39 cm.
Figure 3.
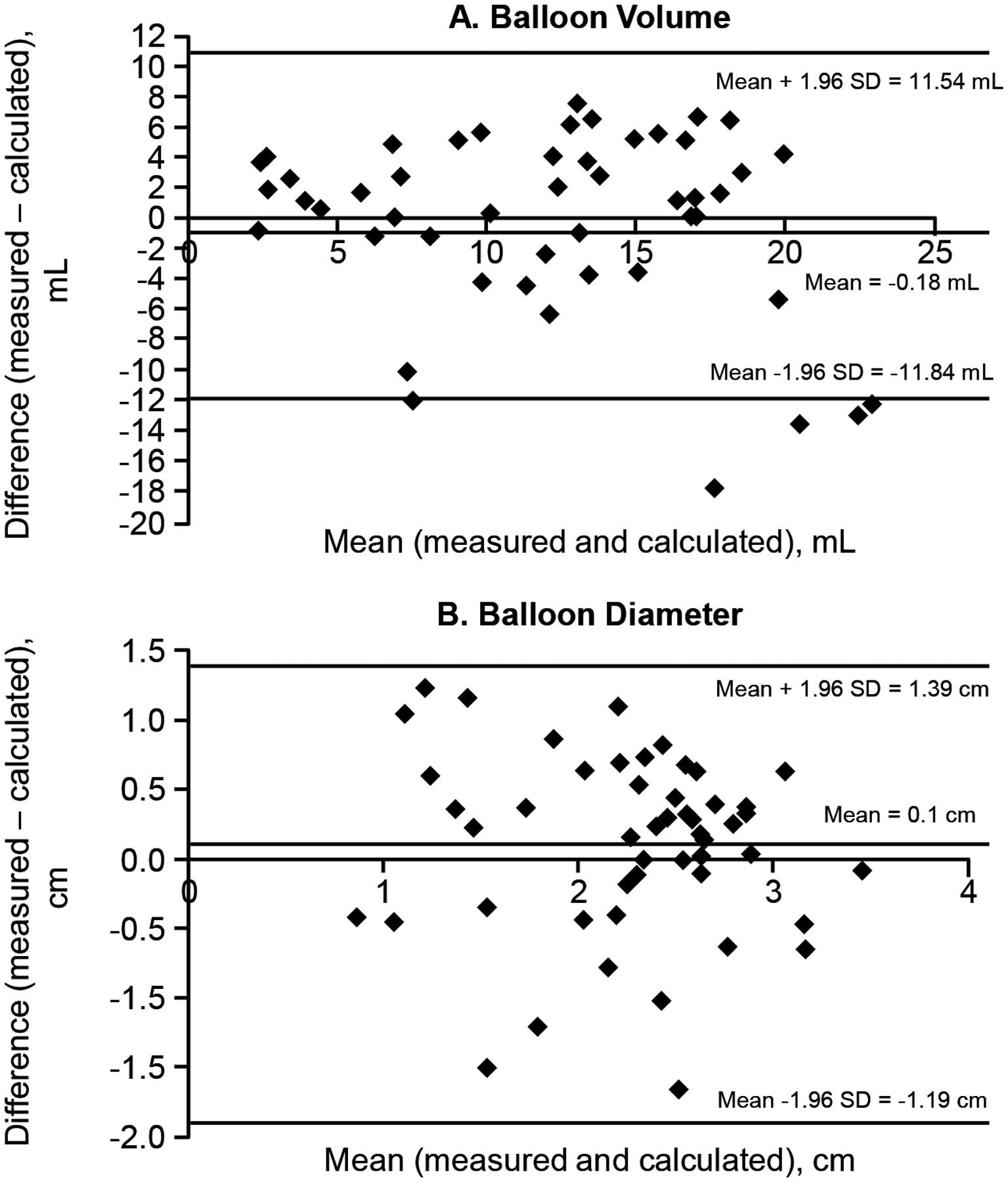
Bland Altman plots showing the agreement between actual and estimated balloon volume (Panel A) and balloon diameter (Panel B) during anal distention. The horizontal lines for each plot are the mean and mean±1.96*SD.
Assessment of anal distensibility without MRI
Similar to the experiments with MRI, in these experiments the estimated opening pressure, volume and diameter at Prhalf, pressure, volume, and diameter at maximum distention were not significantly different between healthy and constipated women (Table 1). The R2 values for closeness of fit between the actual data and the fitted curves were 0.85 (0.18) for balloon diameter and 0.83 (0.18) for balloon volume.
Except for the volume threshold for first sensation, which was greater in constipated patients than healthy women (P = .02), the pressure and volume sensory thresholds were not significantly different between healthy women and constipated patients (Table 1).
Relationship between anal pressures and distensibility
The opening pressure was correlated with the anal resting pressure in healthy (r = 0.55, P = 0.001) and constipated women (r = 0.66, P = 0.004) (Figure 4). In the linear regression model, resting anal pressure accounted for 23% of the observed variance in opening pressure in healthy women and 42% of this variance in constipated women.
Figure 4.
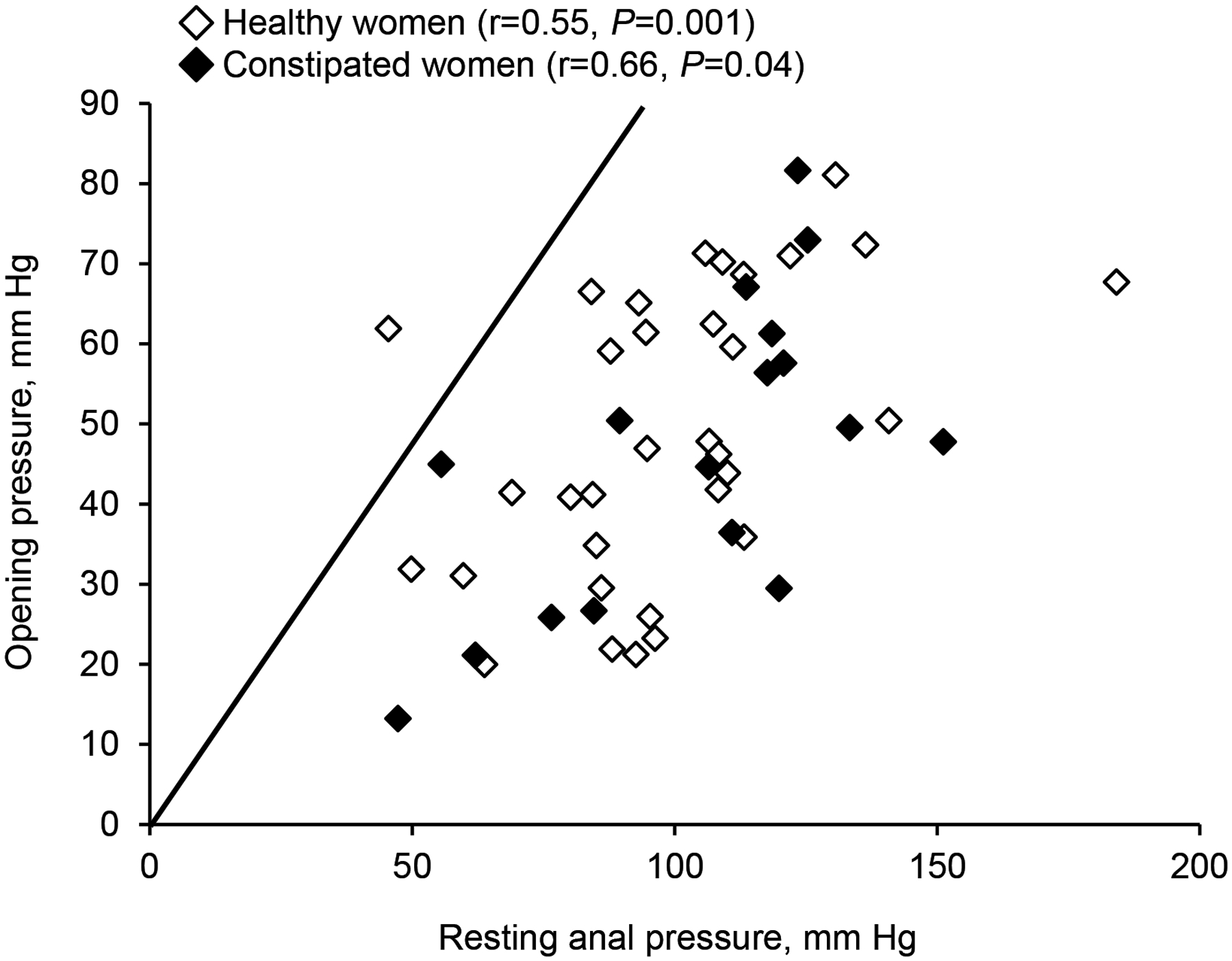
Relationship between resting anal pressure and anal opening pressure during anal distension with MRI. The opening pressure, which is the point at which the anal canal opens, is lower than the anal resting pressure.
In most participants, the opening pressure was lower than the anal resting pressure (Figure 4). Perhaps the difference between anal opening and resting pressure reflects the magnitude of anal relaxation during distention. This difference was correlated with the anal relaxation during evacuation in healthy (r = 0.35, P = 0.04), but not in constipated women (r = 0.13, P = 0.63). The difference between opening and resting pressure was used to categorize patients into three groups: less than the 25th percentile value (9 healthy and 4 constipated women), between 25th and 75th percentile values (14 healthy and 10 constipated women), and greater than 75th percentile value (10 healthy and 3 constipated women). Participants in whom anal relaxation during distention was lower than the 25th percentile values had greater relaxation (P = 0.05) during evacuation (−29 [32] mmHg) than participants in whom anal relaxation during distention exceeded the 75th percentile value (−6 [18] mmHg) or between the 25th and 75th percentile values (−13 [23] mmHg) (Figure 5).
Figure 5.
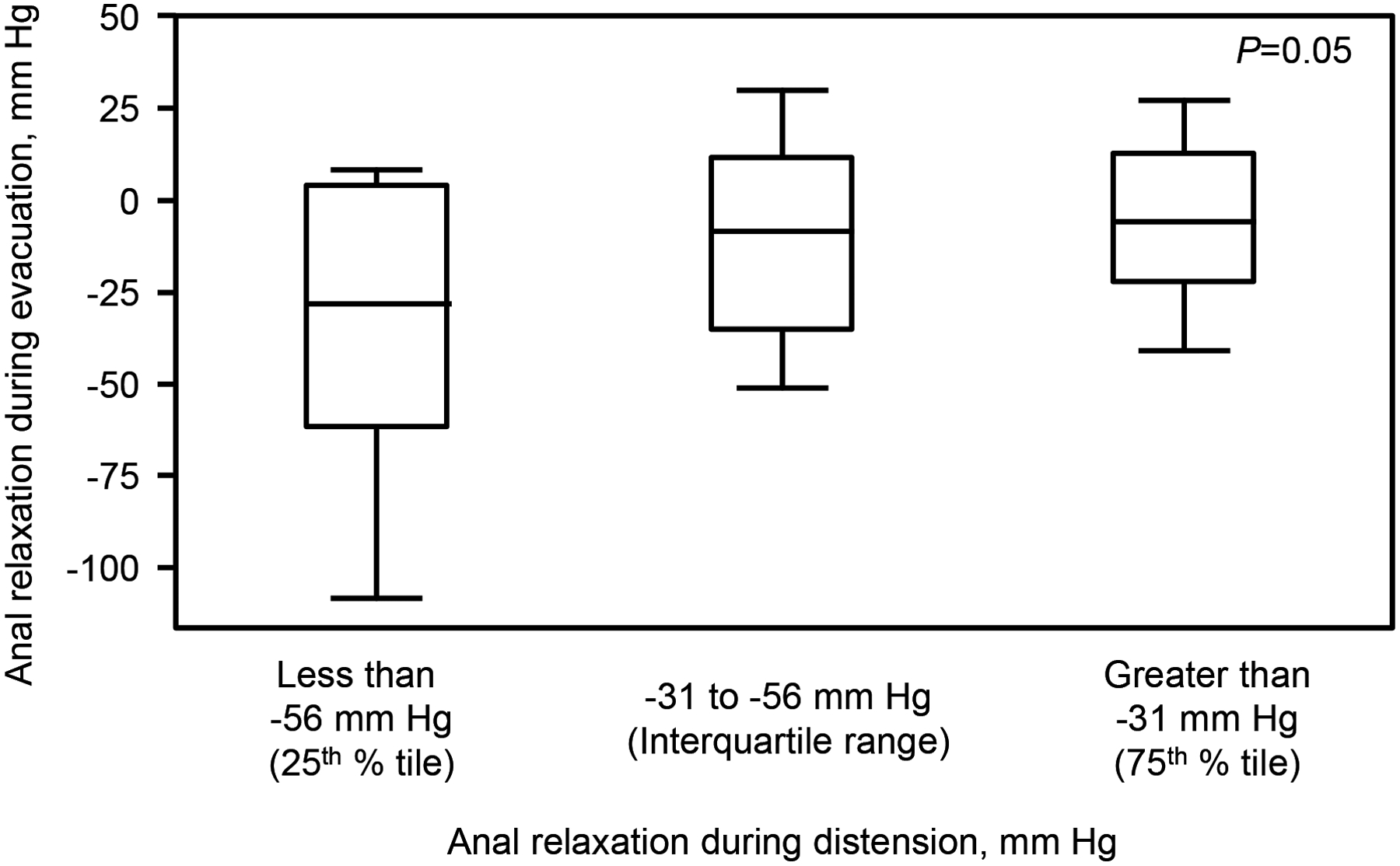
Relationship between anal relaxation during distension with MRI and evacuation in healthy and constipated women. Anal relaxation during distention was associated (P=0.05, ANOVA) with anal relaxation during evacuation. The box represents the mean (SD); whiskers represent minimum and maximum values.
The maximum balloon volume was inversely correlated with anal resting pressure in healthy women (r = −0.38, P = 0.03) and constipated patients (r = −0.50, P = 0.04). However, the anal resting pressure was not correlated with the change in balloon diameter/pressure in healthy (r = 0.32, P = 0.07) or constipated women (r = 0.27, P = 0.30) or the change in balloon volume/pressure in healthy (r = −0.02, P = 0.90) or constipated women (r = 0.14, P = 0.59).
Discussion
Using needle electromyography, Melzak and Porter observed that anal distention was associated with contraction followed by relaxation of the external anal sphincter33. They surmised that distention-induced relaxation enables “unresisted evacuation of the rectum once it has started.” This study used a new technique to assess anal distensibility, which was compared for the first time between healthy and constipated women. There are five cardinal observations. First, the opening pressure during anal distention is lower than the anal resting pressure in most people. Second, the anal canal was less distensible in participants with a greater anal resting pressure, which may impede the passage of stool. Third, the difference between opening pressure and anal resting pressure, which reflects distention-induced anal relaxation, was correlated with anal relaxation during evacuation in healthy women but not in constipated women. Fourth, compared to healthy women, constipated women had a lower rectoanal gradient during evacuation and a longer BET but similar anal resting pressure. Fifth, there were no significant differences in anal distensibility between healthy and constipated women, perhaps because only five women had DD and because 24% of healthy women had anal hypertension.
The EndoFLIP technique has been used to evaluate anal distensibility in patients with fecal incontinence but not constipation17,34. The configuration of anal pressure-volume relationships assessed with the EndoFLIP technique and with a barostat is similar. However, there are differences between these techniques. The EndoFLIP technique uses impedance to estimate the cross-sectional area; these estimates have not been independently validated with imaging35. In this study, balloon volume and diameter were directly measured with MRI. The estimated balloon volume and diameter were significantly correlated with the corresponding actual parameters, which validates these measurements. Second, the EndoFLIP balloon, which is over 6.4 cm long, is displaced, even at lower distending volumes, proximally into the rectum and distally outside the body, which may affect the accuracy of the measured anal distensibility. In this study, the length of the anal balloon used in this study was individualized in each participant. Studies in which the anal balloon was displaced were not considered for further analysis. These differences probably explain why several anal distensibility parameters are markedly different between this study and the EndoFLIP technique. For example, the opening or inflection pressure is 10 mmHg or lower for the EndoFLIP technique versus approximately 45 mmHg in this study35. Since 10 mmHg is much lower than and insufficient to overcome anal resting tone, this early rise in balloon volume perhaps reflects expansion into the rectum or outside the body. Among healthy women, the maximum balloon volume was 11.8 ± 6.2 ml in this study. By contrast, with the EndoFLIP technique, the anal canal opening balloon volume was approximately 20–30 ml in healthy participants36, likely because the balloon had partly expanded into the rectum and outside the body. Third, studies with the EndoFLIP technique estimated anal distensibility by dividing the cross sectional area at a given volume, typically 40ml, by the pressure17,36. In this study, anal distensibility was estimated by the change in volume divided by the change in pressure across a range of pressures, similar to the colon and rectum30,37.
The EndoFLIP assessment was not superior to rectoanal pressures measured with high resolution or high definition manometry for discriminating between healthy people and fecal incontinence17,34. In this study, anal distensibility was not superior to high resolution manometry for discriminating between healthy and constipated women, perhaps because only 5 constipated patients had a DD and also because the distribution of anal resting pressures was similar in healthy and constipated women.
The pressure and volume thresholds for first sensation desire to defecate, and urgency were not different between healthy controls and DD patients. Allowing for differences between techniques, the pressure thresholds are considerably higher during anal than rectal distention. For example, the median rectal and mean anal thresholds for the desire to defecate measured with a barostat were 16 mmHg in a different study and 53 mmHg in this study38. Since rectal distention with anal sampling prompts the call to defecate, it is teleological that sensory thresholds are lower in the rectum than the anal canal39. By contrast, in the anal canal, mucosal sensation, which includes the ability to detect longitudinal and rotatory motion, is exquisite and more relevant (ie, for sampling anal contents) than anal distention40.
This study had several strengths and corresponding limitations. In 14 participants, anal distensibility could not be accurately measured because the anal balloon was displaced into the rectum or outside the anus. The absence of significant differences in anal distensibility between healthy controls and constipated patients is probably not due to a type II error. A post-hoc sample size estimate based on the observed 3 mm Hg mean difference in inflection pressure between controls and patients suggests that 567 participants in each group are necessary to determine if this difference is statistically significant with a power of 80% and an alpha error of .05. Since only 5 of 17 constipated patients had an abnormal BET, a larger sample size is necessary to ascertain if constipated patients with an abnormal BET have reduced anal distensibility. Further studies are also necessary to compare anal distensibility measured with a barostat and with the EndoFLIP technique and to ascertain the contribution of the internal and/or external anal sphincters to increased resting pressure and reduced distensibility.
In summary, this study validated a technique to evaluate anal distensibility with barostat-driven anal balloon distention alone and in combination with MRI. The anal resting pressure was correlated with greater opening pressure and lower maximum volume during distention, and, hence provides a surrogate marker of anal distensibility. The constipated women had a lower (i.e., more negative) rectoanal gradient and longer balloon expulsion time. The opening pressure during anal distention was lower than the anal resting pressure, which suggests that the anal relaxed during distention. The magnitude of distention-induced anal relaxation was correlated with relaxation during anal relaxation during evacuation. Anal distensibility was not different between healthy and constipated women.
Acknowledgements and Disclosures
Funding. This study was supported by USPHS NIH Grant R01 DK78924.
Footnotes
Conflicts of interest. None.
References
- 1.Bharucha AE, Wald A. Chronic Constipation. Mayo Clin Proc. 2019;94(11):2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Lacy BE. Chronic Constipation: Mechanisms, Evaluation and Management. Gastroenterology. 2020;158(5):1232–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma M, Muthyala A, Feuerhak K, Narayanan SP, Bailey KR, Bharucha AE. Improving the Utility of High Resolution Manometry for the Diagnosis of Defecatory Disorders in Women with Chronic Constipation. Neurogastroenterol and Motility. 2020. July 01:e13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology. 2005;128:1199–1210. [DOI] [PubMed] [Google Scholar]
- 5.Ratuapli S, Bharucha AE, Noelting J, Harvey D, Zinsmeister AR. Phenotypic Identification and Classification of Functional Defecatory Disorders Using High Resolution Anorectal Manometry Gastroenterology. 2013;144:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrington EV, Heinrich H, Knowles CH, et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020;32(1):e13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanfranchi GA, Bazzocchi G, Brignola C, Campieri M, Labo G. Different patterns of intestinal transit time and anorectal motility in painful and painless chronic constipation. Gut. 1984;25(12):1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staller K, Barshop K, Kuo B, Ananthakrishnan AN. Resting anal pressure, not outlet obstruction or transit, predicts healthcare utilization in chronic constipation: a retrospective cohort analysis. Neurogastroenterol Motil. 2015;27(10):1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18(7):507–519. [DOI] [PubMed] [Google Scholar]
- 10.Schouten WR, Blankensteijn JD. Ultra slow wave pressure variations in the anal canal before and after lateral internal sphincterotomy. International Journal of Colorectal Disease. 1992;7(3):115–118. [DOI] [PubMed] [Google Scholar]
- 11.Eckardt VF, Schmitt T, Bernhard G. Anal ultra slow waves: a smooth muscle phenomenon associated with dyschezia. Digestive Diseases & Sciences. 1997;42(12):2439–2445. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty S, Feuerhak K, Muthyala A, Harmsen WS, Bailey KR, Bharucha AE. Effects of Alfuzosin, an Alpha-1 Alphadrenergic Antagonist on Anal Pressures and Bowel Habits, in Women With and Without Defecatory Disorders. Clinical Gastroenterology & Hepatology. 2018;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthyala A, Feuerhak KJ, Harmsen WS, Chakraborty S, Bailey KR, Bharucha AE. Effects of psychosensory stimulation on anal pressures: Effects of alfuzosin. Neurogastroenterol Motil. 2019;31(7):e13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaderrama NM, Liu J, Nager CW, et al. Evidence for the innervation of pelvic floor muscles by the pudendal nerve. Obstet Gynecol. 2005;106(4):774–781. [DOI] [PubMed] [Google Scholar]
- 15.Frenckner B, Euler CV. Influence of pudendal block on the function of the anal sphincters. Gut. 1975;16(6):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clinical Gastroenterology & Hepatology. 2017;15(3):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leroi AM, Melchior C, Charpentier C, et al. The diagnostic value of the functional lumen imaging probe versus high-resolution anorectal manometry in patients with fecal incontinence. Neurogastroenterol Motil. 2018;30(6):e13291. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duthie H, Gairns F. Sensory nerve-endings and sensation in the anal region of man. Br J Surg. 1960;47:584–594. [DOI] [PubMed] [Google Scholar]
- 20.Read M, Read N. Role of anorectal sensation in preserving continence. Gut. 1982;23:345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvioli B, Bharucha AE, Rath-Harvey D, Pemberton JH, Phillips SF. Rectal compliance, capacity and rectoanal sensation in fecal incontinence. Am J Gastroenterol. 2001;96(7):2158–2168. [DOI] [PubMed] [Google Scholar]
- 22.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. [DOI] [PubMed] [Google Scholar]
- 23.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people—Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterology and Motility : the official journal of the European Gastrointestinal Motility Society. 2019:e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratuapli S, Bharucha AE, Harvey D, Zinsmeister AR. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil. 2013;25(12):e813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prichard DO, Lee T, Parthasarathy G, Fletcher JG, Zinsmeister AR, Bharucha AE. High-resolution Anorectal Manometry for Identifying Defecatory Disorders and Rectal Structural Abnormalities in Women. Clinical Gastroenterology & Hepatology. 2017;15(3):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazor Y, Prott G, Jones M, Kellow J, Ejova A, Malcolm A. Anorectal physiology in health: A randomized trial to determine the optimum catheter for the balloon expulsion test. Neurogastroenterol Motil. 2019;31(4):e13552. [DOI] [PubMed] [Google Scholar]
- 27.Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW. Defecography in normal volunteers: results and implications. Gut. 1989;30(12):1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goes RN, Simons AJ, Masri L, Beart RW Jr. Gradient of pressure and time between proximal anal canal and high-pressure zone during internal anal sphincter relaxation. Its role in the fecal continence mechanism. Dis Colon Rectum. 1995;38(10):1043–1046. [DOI] [PubMed] [Google Scholar]
- 29.Broens P, Vanbeckevoort D, Bellon E, Penninckx F. Combined radiologic and manometric study of rectal filling sensation. Dis Colon Rectum. 2002;45(8):1016–1022. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha AE, Hubmayr RD, Ferber IJ, Zinsmeister AR. Viscoelastic properties of the human colon. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2001;281(2):G459–466. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M, Feuerhak K, Zinsmeister AR, Bharucha AE. A pharmacological challenge predicts reversible rectal sensorimotor dysfunctions in women with fecal incontinence. Neurogastroenterol Motil. 2018;30(10):e13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England). 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 33.Melzak J, Porter NH. Studies of the Reflex Activity of the External Sphincter Ani in Spinal Man. Paraplegia. 1964;1:277–296. [DOI] [PubMed] [Google Scholar]
- 34.Zifan A, Sun C, Gourcerol G, Leroi AM, Mittal RK. Endoflip vs high-definition manometry in the assessment of fecal incontinence: A data-driven unsupervised comparison. Neurogastroenterol Motil. 2018;30(12):e13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alqudah MM, Gregersen H, Drewes AM, McMahon BP. Evaluation of anal sphincter resistance and distensibility in healthy controls using EndoFLIP. Neurogastroenterol Motil. 2012;24(12):e591–599. [DOI] [PubMed] [Google Scholar]
- 36.Gourcerol G, Granier S, Bridoux V, Menard JF, Ducrotte P, Leroi AM. Do endoflip assessments of anal sphincter distensibility provide more information on patients with fecal incontinence than high-resolution anal manometry? Neurogastroenterol Motil. 2016;28(3):399–409. [DOI] [PubMed] [Google Scholar]
- 37.Law N-M, Bharucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit and sensation in humans. American Journal of Physiology:Gastrointestinal and Liver Physiology. 2001;281(5):G1228–G1237. [DOI] [PubMed] [Google Scholar]
- 38.Andrews C, Bharucha AE, Camilleri M, et al. Rectal sensorimotor dysfunction in women with fecal incontinence. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2007;292(1):G282–289. [DOI] [PubMed] [Google Scholar]
- 39.Fox M, Thumshirn M, Fruhauf H, Fried M, Schwizer W. Determinants of fecal continence in healthy, continent subjects: a comprehensive analysis by anal manometry, rectal barostat and a stool substitute retention test. Digestion. 2011;83(1–2):46–53. [DOI] [PubMed] [Google Scholar]
- 40.Rogers J Testing for and the role of anal and rectal sensation. Baillieres Clin Gastroenterol. 1992;6(1):179–191. [DOI] [PubMed] [Google Scholar]


