Abstract
Drug screens leading to successful targeted therapies in cancer have been mainly based on cell viability assays identifying inhibitors of dominantly acting oncogenes. In contrast, there has been little success in discovering targeted therapies that reverse the effects of inactivating mutations in tumor suppressor genes. BAP1 is one such tumor suppressor that is frequently inactivated in a variety of cancers, including uveal melanoma, renal cell carcinoma, and mesothelioma. Since BAP1 is an epigenetic transcriptional regulator of developmental genes, we designed a two-phase drug screen involving a cell-based rescue screen of transcriptional repression caused by BAP1 loss, followed by an in vivo screen of lead compounds for rescue of a BAP1-deficient phenotype with minimal toxicity in Xenopus embryos. The first screen identified 9 compounds, 8 of which were HDAC inhibitors. The second screen eliminated all except one compound due to inefficacy or toxicity. The resulting lead compound, quisinostat, has a distinctive activity spectrum, including high potency against HDAC4, which was recently shown to be a key target of BAP1. Quisinostat was further validated in a mouse model and found to prevent the growth of BAP1-mutant uveal melanomas. This innovative strategy demonstrates the potential for identifying therapeutic compounds that target tumor suppressor mutations in cancer.
Introduction
The concept behind targeted cancer therapy is to attack specific molecular vulnerabilities in cancer cells while sparing normal cells (1). To date, most such efforts have been focused on the enzymatic inhibition of mutant oncogenes such as KIT and BRAF (2). However, many cancer driver mutations occur in recessively acting tumor suppressor genes (3), which are notoriously recalcitrant to therapeutic intervention due to the difficulty in restoring lost function (4). One such tumor suppressor is BAP1, which is mutated in numerous cancer types, including uveal melanoma (UM), renal cell carcinoma, mesothelioma, and cholangiocarcinoma (5-9). BAP1 encodes a ubiquitin C-terminal hydrolase that deubiquitinates numerous substrates, including histone H2A, whereby it regulates transcription of genes involved in development, differentiation and other processes (8,10-12). Importantly, BAP1 mutations are often associated with increased metastatic risk and decreased survival (5,6,9), suggesting that targeting these mutations may inhibit metastasis. As such, a therapeutic strategy for targeting BAP1 mutations is urgently needed, but to date no such therapy has been identified.
Although synthetic lethality screens are a common strategy for identifying compounds that target tumor suppressors (13,14), drugs that induce cell death often have a narrow therapeutic window with dose limiting toxicity (15). These properties would further limit the use of such drugs in the adjuvant setting, where the goal is to treat patients at high risk for metastasis but who are still healthy. Consequently, we took a different approach to identify compounds that counteract the effects of BAP1 mutations (Fig. 1). As a primary high-throughput in vitro screening strategy, we used reversal of transcriptional repression caused by BAP1 loss, rather than cell death as the assay endpoint. Next, we screened the lead compounds using our recently described BAP1-deficient Xenopus embryo phenotype (11) as an in vivo tool for assessing rescue of the phenotype while simultaneously evaluating for toxicity. The final candidate identified by this approach was the HDAC inhibitor quisinostat, which has a distinctive activity spectrum that may explain its efficacy and low toxicity. Finally, we validated the ability of this compound to block the growth of tumors in vivo using a mouse UM model, thereby nominating quisinostat as a high value candidate for adjuvant therapy in patients with high risk BAP1-mutant UM.
Figure 1.
Overall screening strategy. Compound libraries are subjected to a high-throughput whole-cell transcription rescue screen to identify compounds that restore BAP1 target gene expression in cells knocked down for BAP1. Compounds that pass this screen are tested for their ability to rescue a BAP1-deficient phenotype and simultaneously screened for toxicity in a Xenopus embryo model. The lead compound is tested for its ability to halt tumor growth in a mouse model of uveal melanoma.
Materials and Methods
Cell lines
Prior to conducting experiments, all cell lines used in this study were tested for mycoplasma contamination by direct PCR assay on spent media using forward (GGGAGCAAACAGGATTAGATACCCT) and reverse (TGCACCATCTGTCACTCTGTTAACCTC) primers. PDX-derived BAP1-mutant UM cell lines MM28 and MP46, and BAP1-wildtype UM cell line MP41 were generously provided by Dr. Sergio Roman-Roman and maintained in 5% pO2 in DMEM/F12 with 10% heat-inactivated FBS, 1% penicillin/streptomycin, 2 mM Glutamax™, and 0.5% insulin-transferrin-selenium. Established BAP1-wildtype UM cell lines 92.1 and Mel202 were obtained from ATCC and maintained in 20% pO2 in RPM1 with 10% HI-FBS, 1% penicillin/streptomycin and 2 mM Glutamax™ (ThermoFisher Scientific). 92.1 and Mel202 cells were engineered to inducibly knockdown BAP1 expression by creating shRNA constructs targeting three regions in BAP1 (CGTCCGTGATTGATGATGATA, CCCTGTATATGGATTTATCTT, and CCACAACTACGATGAGTTCAT, collectively known as shBAP1). These were cloned into Tet-pLKO-puro vector (Addgene #21915) and packaged into lentiviral particles by transient transfection into H293T cells, as previously described (16). The lentiviral particles were then transduced into the cells and selected with puromycin (2 ug/mL) after 48 hrs. Transduced cells were then clonally selected, and individual clones were plated at <80% confluence and induced with doxycycline hyclate (1 μg/mL) for 48 hrs. Whole cell lysates were analyzed by western blot and probed for BAP1 (H300; Santa Cruz) and beta-actin (#4967S, Cell Signaling). The clones with optimal BAP1 knockdown (>85% depletion) were used for in the study. Uninduced cells were used as control.
RNA-Seq
RNA was isolated with Direct-zol RNA kit (Zymo), and melanin pigment was removed using OneStep PCR Inhibitor Removal Kit (Zymo), according to the manufacturers’ instructions. Libraries were prepared using the NEBNext® Ultra™ kit and sequenced on the Illumina NextSeq 500 (single-end 75 base pair sequencing). Sequencing quality was assessed using FastQC (v0.11.3). Reads were trimmed using trim galore, aligned to the human genome build hg38/GRCH38 using STAR (17) and counts were generated using RSEM (18). Differential expression was performed using DESeq2 (19). LogFC value <−0.6 and padj value <0.01 were used as cutoff criteria to identify commonly down-regulated genes in Class 2 BAP1-mutant versus Class 1 BAP1-wildtype tumors from TCGA, as well as in cell lines with conditional BAP1 knockdown (92.1 and Mel202).
Compounds
Library of 1280 Pharmacologically Active Compounds (LOPAC1280 library) was obtained from Sigma-Aldrich. A custom in-house epigenetic library (UM-CTI) was assembled and curated by the University of Miami Center for Therapeutic Innovations (20-23). The compounds were purchased from Selleckchem, Biovision, Tocris, The Structural Genomics Consortium (SGC), Epigenetix, SIGMA, and Cayman.
Cell-based transcription rescue screen
To calculate the Z-factor value for GLO1 expression, shBAP1-inducible 92.1 cells were plated in 384-well plates at 250 cells/well and induced with doxycycline (1 ug/mL) for 48 hrs. Since the chemical compound libraries were reconstituted in 100% DMSO, cells were treated with increasing concentration of the solvent (0, 0.1, 0.3 and 0.9 %) for an additional 72 hrs to assess its effect on the assay performance. Cells were then washed with PBS and treated with lysis buffer including DNase I, followed by Stop Solution then placed on ice to begin cDNA synthesis using the Taqman Reverse Transcription kit (ThermoFisher Cat. No. N8080234). Reverse transcriptase enzyme was mixed with Reverse Transcription Buffer and added to a PCR reaction plate. Cell lysates were added to the PCR plate and placed in the thermal cycler at 37°C for 60 minutes, 95°C for 5 minutes and 4°C indefinitely. Taqman gene expression Master Mix protocol was used for qPCR (ThermoFisher Cat. No. 4369016) and Taqman primers GLO1 (Assay ID Hs00198702_m1, ThermoFisher) and 18S (Cat. 319413E, ThermoFisher). The cDNA and Master Mix samples were placed at 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60 °C for 1 minute in the Applied Biosystems™ QuantStudio™ 6 Real-Time PCR system. The CT values obtained from the QuantStudio data analysis software were analyzed by calculating fold-change. Gene expressions were normalized to 18S mRNA expression. Z-factor value for DMSO gradient was calculated as previously described (24). For the cell-based screen, 92.1-Tet-shBAP1 cells were plated in 384-well plates at 250 cells/well and induced with doxycycline (1 μg/mL) for 48 hrs. The wells were then treated in duplicate for 72 hours with the LOPAC1280 library or the UM-CTI library at a final concentration of 10 uM or 1 uM, respectively. Z prime value was calculated for all plates. The RQ values were converted to percent of positive controls. A cutoff criterion was set at 70% above the mean of positive controls and the hit compounds were identified.
Frog embryo phenotype rescue and toxicity screen
The animal protocols used in this work were evaluated and approved by the Institutional Animal Care and Use Committee (IACUC) of the Universityof Miami (#18-031). All activities were performed in compliance with federal state, and institutional regulations. Xenopus laevis embryos were prepared as previously described (11). When embryos reached the 1-cell stage, they were injected with 15 ng of control morpholino CtrlMO (AGCTTTCGTTCATGTAACCTCCTCA) or bap1-directed morpholino Bap1MO (AGCCTTTATTCATGTTGCCTCCTCC) using a Nanoject II Auto-Nanoliter Injector (Drummond Scientific, Inc). After injections the embryos were maintained in 4% ficoll/MMR solution at 18 °C until they reached stage 6 and then transferred to 0.1 MMR buffer containing varying concentrations of each compound tested. Embryos were then incubated for additional 48 hrs at 21°C and scored for morphological abnormalities at early neurula stage and/or in the early tailbud stages. For each drug concentration, embryos were evaluated for phenotype expression using our previously established grading system (11). A total of n=300 embryos from 3 different females were evaluated.
Mouse xenograft validation
The animal protocols used in this work were evaluated and approved by the Institutional Animal Care and Use Committee (IACUC) of the Universityof Miami (#15-197). All activities were performed in compliance with federal state, and institutional regulations. Mice were randomized with respect to sex using the block randomization technique. For each mouse, 2 million MM28, MP46 or MP41 UM cells were resuspended in ice-cold Matrigel (Corning 354248) and injected subcutaneously into the intrascapular region above the fat pad in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ JAX® immunodeficient (NSG) mice. Drug or vehicle treatments were initiated 3 weeks after the graft date for MP46 and after 2 weeks for MP41 and MM28 xenografts, due to differences in growth rates. Quisinostat was resuspended in 100% DMSO (50mg/ml) and then reconstituted in 20% hydroxypropyl-β-cyclodextrin saline solution (final pH 8.7) at a final concentration of 0.17% (w/v) or 3.57mM quisinostat. Quisinostat or vehicle was administered via intraperitoneal injection on Mondays and Thursdays at a dose of 10mg/kg body weight. Xenografts were imaged every 2 weeks by ultrasound (Vevo3100; Fujifilm Visualsonics, Canada) to assess tumor growth over time.
Cell Viability Assay (MTT Assay)
UM cells were plated in sextuplicate wells (1.0 x 103 cells per well) and treated with increasing concentrations of MEKi (trametinib) and/or quisinostat for 72h. Cell viability was determined using MTT assay as previously described (Faiao-Flores et al., 2013) with the addition of 100ul of DMSO added to each well prior to the isopropanol solution.
Statistical analysis
Unless otherwise specified, statistical significance was assessed using Chi square test for ordinal variables and Mann-Whitney test for continuous variables using MedCalc version 19.1.
Results
Cell-based high-throughput gene expression rescue screen
Based on the hypothesis that BAP1 loss promotes tumor progression and metastasis by altering the transcription of key genes, we sought to identify a representative BAP1-regulated gene that is silenced by BAP1 loss in UM in order to screen for compounds that restore its transcription. First, we analyzed RNA-seq data from the 80 UM samples from The Cancer Genome Atlas (TCGA) to identify genes that are differentially expressed between Class 1 and Class 2 tumors (Fig. 2A and Supplementary Table S1), as previously defined (25). Then, we performed a similar differential expression analysis of RNA-seq data obtained from BAP1-wildtype 92.1 and Mel202 UM cells engineered to inducibly express shRNA directed against BAP1 (Fig. 2A, Supplementary Table S1 and Fig. S1A). Only 4 genes were differentially expressed in all 3 systems: GLO1, EDARADD, DAPL1, and BAP1 itself (Fig. 2B-C). The largest change in expression was observed for GLO1 (Fig. 2C), which was chosen for subsequent high-throughput screens.
Figure 2.
Primary transcription rescue screen. A, RNA-seq analysis showing overall numbers of differentially expressed genes (0.6<LogFC<−0.6, FDR<0.01) in Mel202 and 92.1 BAP1-wildtype uveal melanoma cell lines depleted of BAP1 using shRNA, and Class 1 BAP1-wildtype versus Class 2 BAP1-mutant uveal melanomas from TCGA. B, Modified Venn diagram showing overlap of genes down-regulated with BAP1 loss in these 3 systems, showing only 4 genes in common to all. C, Comparison of RNA-seq derived FPKM values of the 4 commonly down-regulated genes following shRNA-mediated knock down of BAP1 in the indicated cell lines, shows the greatest loss of expression for GLO1 in 92.1 cells, which were used for the high-throughput screen. D, Summary of results of high-throughput screen of LOPAC1280 library and UM-CTI epigenetic library in 92.1 uveal melanoma cells engineered with inducible shRNA against BAP1. BAP1-WT, uninduced; BAP1-KD, induced. E, Representative graphs of the lead compounds that passed the dose-response validation test. Quantification was performed by qPCR of GLO1 expression under primary assays conditions in triplicate.
We created a high-throughput platform to screen for compounds that rescue GLO1 expression in 92.1 UM cells. The optimized platform demonstrated a high-throughput compatibility Z-factor value of 0.518 (Supplementary Fig. S1B). An initial screen of the LOPAC1280 library of pharmacologically active compounds resulted in 11 hits, based on ≥70% rescue of GLO1 expression compared to DMSO control (Fig. 2D). We then screened a custom in-house epigenetic library of 180 compounds, which yielded 8 hits, all HDAC inhibitors (Fig. 2D). Dose-response assays were performed for all preliminary hits to eliminate false positives and to determine half maximal active concentration (IC50). IC50 values were then used to compare potency of each candidate compound to restore GLO1 expression. Only one of LOPAC1280 library compounds, ouabain, passed the dose-response validation test, for an overall hit rate for this library of 0.08% (Fig. 2E). Ouabain is a cardiac glycoside and Na/K-ATPase inhibitor that was not deemed a viable candidate due to potential cardiac side effects. Remarkably, all 8 hits from the in-house epigenetic library passed the dose-response validation test, for an overall hit rate of 4.4% for the epigenetics library (Fig. 2D and E, and Supplementary Table S2).
In vivo developmental rescue screen
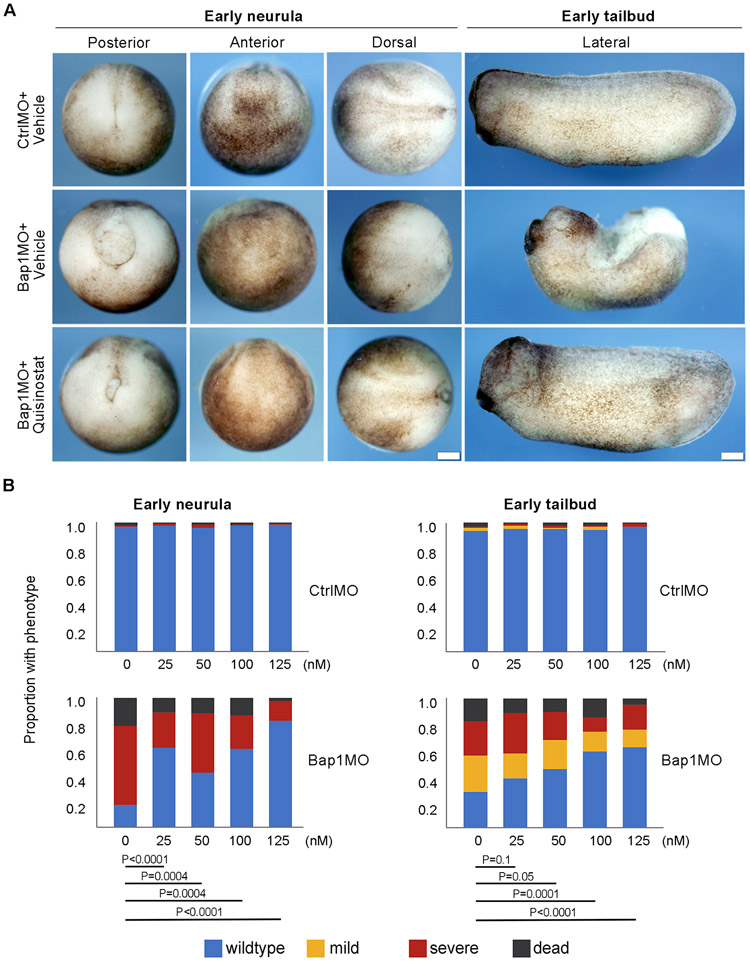
Next, using IC50 values generated in the primary assay we employed our recently described BAP1-deficient Xenopus embryo developmental model to screen the remaining lead compounds for both efficacy and toxicity (11). Trapoxin A was eliminated, as this was an irreversible HDAC inhibitor. Pracinostat and AR-42 (IC50 for GLO1 rescue 3.73 μM and 2.65 μM, respectively), demonstrated no toxicity but also no rescue (Supplementary Fig. S2). Therefore, these and other compounds with IC50>1 μM (trichostatin A, givinostat, and LAQ824) were eliminated. CUDC-907 (GLO1 rescue IC50=0.81 μM) caused severe toxicity in control and BAP1-deficient embryos. In contrast, quisinostat (GLO1 rescue IC50=0.46 μM) effectively rescued the BAP1-deficient phenotype in the nanomolar range with no detectable toxicity in control embryos even at micromolar concentrations (Fig. 3A-C). Since MEK inhibitors have shown some activity in UM(26), we tested the effects of combining quisinostat with the MEK inhibitor trametinib, but this resulted only in a modest additive effect (Supplementary Fig. S3).
Figure 3.
Phenotypic rescue and lack of toxicity using quisinostat in Xenopus embryos. A, Representative embryos with control inactive morpholino (CtrlMO) or bap1-targeting morpholino (Bap1MO) treated with DMSO vehicle or quisinostat (125 nM) at early neurula and early tailbud stages, showing rescue of wildtype phenotype by quisinostat. Control-treated BAP1-deficient embryos showed failure of blastopore closure and elongation defect, as previously described (11). Scale bar, 250uM. B, Quantification of dose-dependent quisinostat rescue in Bap1MO-injected BAP1 deficient embryos, and lack of toxicity in CtrlMO-injected control embryos. A total of n=300 embryos from 3 different females were evaluated. P values for Bap1MO-injected embryos are indicated. None of the P values for CtrlMO-injected embryos approached statistical significance.
In vivo validation of quisinostat in mouse model of uveal melanoma
To test the potential clinical value of quisinostat in the adjuvant setting for high-risk BAP1-mutant UM, we evaluated whether this compound could repress the growth of small UM xenografts using an established mouse intrascapular fat pad model (27). We tested two BAP1-mutant (MM28 and MP46) and one BAP1-wildtype (MP41) PDX-derived cell lines (27). Quisinostat markedly reduced tumor growth over a 2-month period in both BAP1-mutant lines (MM28 and MP46), whereas it did not affect the growth of the BAP1-wildtype line (MP41) (Fig. 4A-C).
Figure 4.
BAP1 loss sensitizes uveal melanoma cells to quisinostat in vivo. A, Time course of tumor growth in a mouse intrascapular fat pad xenograft model using BAP1-mutant MM28 and MP46 and BAP1-wildtype MP41 uveal melanoma cells treated with quisinostat versus DMSO vehicle control. B, Final weight at necropsy of tumors treated with quisinostat versus control. C, Representative examples of tumors treated with quisinostat versus vehicle control at necropsy. Q, quisinostat; V, vehicle. P values: *** < 0.0005, ** < 0.005, * < 0.05.
Discussion
Few drugs have been identified that target mutations in tumor suppressors, despite the pervasiveness of these aberrations in human cancer. In this study, we identify quisinostat as a potential targeted therapy in BAP1-mutant UM, and we demonstrate the potential value of a novel multi-step drug screening strategy for identifying such compounds. Capitalizing on the known role of BAP1 as an epigenetic regulator of gene expression, we used an initial high throughput drug screen in which transcriptional restoration of a reporter gene, rather than cell death, was used as the endpoint. This resulted in no viable candidates from a library of pharmacologically active compounds, but multiple hits from an epigenetic compound library, all of which were HDAC inhibitors, lending credibility to the specificity of this approach. The secondary screen involved our distinctive in vivo BAP1-deficient embryonic developmental model to assess for efficacy and toxicity. This screen eliminated several candidates due to toxicity and/or lack of efficacy, leaving quisinostat as the lead candidate. Finally, we validated that BAP1 loss sensitized UM tumors to quisinostat in vivo.
Quisinostat has a distinctive activity spectrum (28), including the highest potency of any compound in the Probes & Drugs portal (https://www.probes-drugs.org/home/) for HDAC4. Interestingly, most clinically available HDAC inhibitors have very low potency for HDAC4, which was recently identified as a key downstream target of BAP1 (11). We previously showed that earlier generation HDAC inhibitors such as valproic acid and vorinostat could induce differentiation and transcriptomic rewiring of UM cells in vitro, but only at higher concentrations (29). The low potency of these compounds, especially against HDAC4, may explain their lack of efficacy in BAP1 mutant UM (30) and their dose-limiting toxicity (31). Indeed, although quisinostat and CUDC-907 have comparable nanomolar affinity for HDAC1, HDAC2, HDAC11 and HDAC10 (Supplementary Table S2), CUDC-907 has low affinity for HDAC4 and failed to pass our secondary in vivo screen (Supplementary Fig. S2). Similarly, Pracinostat and AR-42 with low affinity for HDAC4 also failed the secondary in vivo screen (Supplementary Table S2 and Fig. S2). Moreover, the lower potency of quisinostat in a BAP1-wildtype UM cells (32) is consistent with preferential sensitivity of BAP1-mutant UM to this compound.
Our screening approach differs conceptually from synthetic lethal screens, which have suggested that BAP1 mutations may increase vulnerability to PARP inhibitors (33), which are in clinical trials in the metastatic setting (ClinicalTrials.gov Identifier: NCT03207347). In contrast, the epigenetic mechanism of action of an epigenetic compound such as quisinostat may be more appropriate in the adjuvant setting to prevent the progression of micrometastatic disease to overt metastasis in patients with high-risk UM and other BAP1-mutant cancers. Our study provides a proof of principle and framework for future screens of additional compound libraries to identify novel therapeutics targeting loss of BAP1 and other tumor suppressors.
Data and Materials Availability
All data are available in the main text or the supplementary material.
Supplementary Material
Implications.
Few drugs have been identified that target mutations in tumor suppressors. Using a novel 2-step screening approach, strategy, we identified quisinostat as a candidate for therapy in BAP1-mutant uveal melanoma. HDAC4 is implicated as a key target in uveal melanoma and perhaps other BAP1-mutant cancers.
Acknowledgments
This work was supported by DOD W81XWH-15-1-0578 (J.W.H.), Alcon Research Institute (J.W.H.), Research to Prevent Blindness, Inc. Senior Scientific Investigator Award (J.W.H.), a generous gift from Dr. Mark J. Daily (J.W.H), NCI P30CA240139 (Sylvester Comprehensive Cancer Center), NEI P30EY014801 (Bascom Palmer Eye Institute), and Research to Prevent Blindness Unrestricted Grant (Bascom Palmer Eye Institute). We acknowledge the assistance of the Molecular Therapeutics Shared Resource (MTSR), Biostatistics & Bioinformatics Shared Resource (BBSR), Oncogenomics Shared Resources (OGSR), and Cancer Modeling Shared Resource (CMSR) of the Sylvester Comprehensive Cancer Center of the University of Miami Miller School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Potential Conflicts of Interest
J.W. Harbour is the inventor of intellectual property related to prognostic testing in uveal melanoma. He is a paid consultant for Castle Biosciences, licensee of his intellectual property, and he receives royalties from the commercialization of his intellectual property. Other authors declare no competing financial interests.
REFERENCES
- 1.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017;17:286–301 [DOI] [PubMed] [Google Scholar]
- 2.Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A, Hubbard BP, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 2018;17:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018;173:371–85 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris LG, Chan TA. Therapeutic targeting of tumor suppressor genes. Cancer 2015;121:1357–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, Leng N, Pavia-Jimenez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nature genetics 2012;44:751–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nature genetics 2011;43:668–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer 2013;13:153–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nature genetics 2013;45:1470–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 2010;465:243–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuznetsov JN, Aguero TH, Owens DA, Kurtenbach S, Field MG, Durante MA, et al. BAP1 regulates epigenetic switch from pluripotency to differentiation in developmental lineages giving rise to BAP1-mutant cancers. Sci Adv 2019;5:eaax1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campagne A, Lee MK, Zielinski D, Michaud A, Le Corre S, Dingli F, et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat Commun 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature 2019;575:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerby-Arnon L, Pfetzer N, Waldman YY, McGarry L, James D, Shanks E, et al. Predicting cancer-specific vulnerability via data-driven detection of synthetic lethality. Cell 2014;158:1199–209 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Hu X, Han C, Wang L, Zhang X, He X, et al. Targeting tumor suppressor genes for cancer therapy. Bioessays 2015;37:1277–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiederschain D, Wee S, Chen L, Loo A, Yang G, Huang A, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle 2009;8:498–504 [DOI] [PubMed] [Google Scholar]
- 17.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England) 2013;29:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janczura KJ, Volmar CH, Sartor GC, Rao SJ, Ricciardi NR, Lambert G, et al. Inhibition of HDAC3 reverses Alzheimer's disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc Natl Acad Sci U S A 2018;115:E11148–e57 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Zeier Z, Esanov R, Belle KC, Volmar CH, Johnstone AL, Halley P, et al. Bromodomain inhibitors regulate the C9ORF72 locus in ALS. Exp Neurol 2015;271:241–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volmar CH, Salah-Uddin H, Janczura KJ, Halley P, Lambert G, Wodrich A, et al. M344 promotes nonamyloidogenic amyloid precursor protein processing while normalizing Alzheimer's disease genes and improving memory. Proc Natl Acad Sci U S A 2017;114:E9135–e44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustafi S, Camarena V, Volmar CH, Huff TC, Sant DW, Brothers SP, et al. Vitamin C Sensitizes Melanoma to BET Inhibitors. Cancer Res 2018;78:572–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 1999;4:67–73 [DOI] [PubMed] [Google Scholar]
- 25.Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun 2018;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goncalves Avezedo J, Emmons MF, Faiao-Flores F, Aplin AE, Harbour JW, Licht JD, et al. Decitabine limits escape from MEK inhibition in uveal melanoma. Pigment Cell Melanoma Res 2019 [DOI] [PMC free article] [PubMed]
- 27.Nemati F, Sastre-Garau X, Laurent C, Couturier J, Mariani P, Desjardins L, et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin Cancer Res 2010;16:2352–62 [DOI] [PubMed] [Google Scholar]
- 28.Arts J, King P, Marien A, Floren W, Belien A, Janssen L, et al. JNJ-26481585, a novel "second-generation" oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res 2009;15:6841–51 [DOI] [PubMed] [Google Scholar]
- 29.Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012;18:408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol 2018;10:1758834018757175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atmaca A, Al-Batran SE, Maurer A, Neumann A, Heinzel T, Hentsch B, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer 2007;97:177–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heijkants R, Willekens K, Schoonderwoerd M, Teunisse A, Nieveen M, Radaelli E, et al. Combined inhibition of CDK and HDAC as a promising therapeutic strategy for both cutaneous and uveal metastatic melanoma. Oncotarget 2018;9:6174–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan G, Sidhu GS, Williamson EA, Jaiswal AS, Najmunnisa N, Wilcoxen K, et al. Synthetic lethality in malignant pleural mesothelioma with PARP1 inhibition. Cancer Chemother Pharmacol 2017;80:861–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.