Abstract
The three-orphan nuclear receptor 4A genes are induced by diverse stressors and stimuli, and there is increasing evidence that NR4A1 (Nur77), NR4A2 (Nurr1) and NR4A3 (Nor1) play an important role in maintaining cellular homeostasis and in pathophysiology. In blood-derived tumors (leukemias and lymphomas) NR4A expression is low and NR4A1−/−/NR4A3−/− double knockout mice rapidly develop acute myelocytic leukemia suggesting that these receptors exhibit tumor suppressor activity. Treatment of leukemia and most lymphoma cells with drugs that induce expression of NR4A1and NR4A3 enhances apoptosis and this represents a potential clinical application for treating this disease. In contrast, most solid tumor-derived cell lines express high levels of NR4A1 and NR4A2 and both receptors exhibit pro-oncogenic activities in solid tumors whereas NR4A3 exhibits tumor-specific activities. Initial studies with retinoids and apoptosis-inducing agents demonstrated that their cytotoxic activity is NR4A1-dependent and involved drug-induced nuclear export of NR4A1 and formation of a mitochondrial pro-apoptotic NR4A1- bcl-2 complex. Drug-induced nuclear export of NR4A1 has been reported for many agents/biologics and involves interactions with multiple mitochondrial and extramitochondrial factors to induce apoptosis. Synthetic ligands for NR4A1, NR4A2 and NR4A3 have been identified and among these compounds bis-indole derived (CDIM) NR4A1 ligands primarily act on nuclear NR4A1 to inhibit NR4A1-regulated pro-oncogenic pathways/genes and similar results have been observed for CDIMs that bind NR4A2. Based on results of laboratory animal studies development of NR4A inducers (blood-derived cancers) and NR4A1/NR4A2 antagonists (solid tumors) may be promising for cancer therapy and also for enhancing immune surveillance.
Keywords: cancer, nuclear receptor, nuclear translocation, cell signaling, apoptosis
INTRODUCTION
Background:
The 48 human nuclear receptors (NRs) play integral roles in maintaining cellular homeostasis and in pathophysiology and NR subfamily 4 (NR4A) consists of three orphan NRs for which there are no known physiological ligands (1,2). NR4A1 (Nur77), NR4A2 (Nurr1) and NR4A3 (Nor1) exhibit domain structures similar to other NRs; this includes N- and C- terminal domains containing activation function 1 (AF-1) and AF-2 [also ligand binding domain (LBD)] respectively and they flank a DNA-binding domain (DBD) and a hinge region (Fig. 1). The sequence homology of NR4A1, NR4A2 and NR4A3 is similar in the ligand binding AF-2, hinge and DBD but differ significantly in the N-terminal AF-1 domain (3–5) and there is evidence that this domain dictates some of the different functions of these orphan NRs (6–8). NR4A1, NR4A2 and NR4A3 are early immediate genes that exhibit overlapping and unique functions, and they characteristically are induced by diverse physical, physiological and pharmacological stimuli (rev. in (9,10)). In many solid tumor-derived cancer cells which exhibit enhanced metabolic rates, NR4As are overexpressed compared to corresponding non-transformed cells whereas most blood-derived tumors are characterized by low expression of NR4A (11,12) and these differences will be discussed below.
Figure 1.

Domain structure of NRs and similarities between NR4A1, NR4A2 and NR4A3.
NR4A interactions with cis-elements.
NRs are ligand activated transcription factors that bind their endogenous ligands (e.g.: hormones) or synthetic ligands and the ligand-bound receptor interacts with cis element in target gene promoters (1,2). This interaction can lead to recruitment of nuclear cofactors and results in modulation of gene expression. In addition, some receptors can act as ligand activated nuclear cofactors (11). Structurally-diverse ligands induce different conformational changes in the bound receptor and these selective receptor modulators can induce tissue- and gene-specific receptor agonist or antagonist activity. The NR4A subfamily are orphan receptors with no known endogenous ligands and they act through both nuclear and extranuclear pathways and can be influenced not only by synthetic receptor ligands but also by other agents that do not bind the receptor (12). X-ray crystallographic and functional studies of NR4A suggest that the ligand binding pocket contains bulky hydrophobic amino acid side chains that may preclude interactions with an endogenous ligand (5,8,13,14). There is extensive evidence that NR4A alone or in combination activate ligand-independent gene expression through direct or indirect interactions with cognate cis-elements (4,15,16), NR4As activate gene expression through binding as monomers, homodimers and heterodimers (with RXR) through interactions with an octanucleotide NGF1-β response element (NBRE), a Nur-responsive element (NuRE) and a DR5 motif (with RXR) respectively (Fig. 2) (17–19). NR4A1, NR4A2 and NR4A3 can also form heterodimers and only NR4A1 and NR4A2 but not NR4A3 heterodimerize with RXR (20,21). Recent high throughout studies showed that NR4A2 bound NuRE motifs which consists of two everted palindromic octanucleotides (ERO) with no spacer between the NBREs and two inverted repeats separated by 5 nucleotides (IR5) (22). Crystal structures of NR4A2-DBD interactions with ERO and IR5 have been identified as NR4A2 binding sites in multiple human genes and are structurally different from the “classical” NuRE identified in the pro-opiomelanocortin (POMC) gene (23). Analysis of NR4A3 interactions and chromatin immunoprecipitation (ChIP) -seq and identification of other NuREs that bind NR4A2 and NR4A3 have not been reported (23) and future studies using this approach will provide some basis for understanding functional and mechanistic differences between NR4A1, NR4A2 and NR4A3. These results are consistent with direct interactions of most NRs with their cognate consensus and non-consensus response elements. There is also evidence that NR4A1 interacts with DNA-bound specificity protein (Sp) transcription factors and act as a cofactor of Sp1 or Sp4. ChIP analysis showed that NR4A1 interacted with Sp1 or Sp4 bound to GC-rich promoter sequences in the survivin, β1-, β3- and β4-integrins, PAX-FOX01 and α5- and α6-integrin genes (24–27) and acts as a cofactor for Sp-dependent gene expression. NR4A1/Sp regulated gene expression is decreased by knockdown of Sp1/4 and also NR4A1 and there is evidence from RNAseq studies in Rhabdomyosarcoma (RMS) cells that many key pro-oncogenic factors are regulated by an NR4A1/Sp complex. This pathway for gene regulation is not unique to NR4A1 and has previously been observed for steroid hormone receptors and several RXR-binding receptors (28). Thus, activation of gene expression by NR4A involves interactions with different partner proteins and different cis-elements and this has been observed for other NRs. A unique feature of NR4A (particularly NR4A1) in cancer is that many apoptosis-inducing agents induce nuclear export of NR4A1 and this unusual pathway will be discussed below.
Figure 2.

Interactions of NR4A1 with cis-elements, DNA-bound RXR and DNA-bound-Sp.
NR4A knockout mice and cancer.
Individual knockout of individual NR4As in mice did not initially indicate a role for these receptors in carcinogenesis. Both NR4A1 and NR4A3 play comparable roles in induced apoptosis of β cells and negative selection of T-lymphocytes, however, NR4A1−/− mice were viable with no obvious phenotype (29–31). Studies with NR4A2−/− mice show the importance of the receptor for induction of the dopaminergic phenotype and these mice exhibit significant neuronal dysfunction and early mortality (32,33). NR4A3−/− mice were generated in two laboratories and their phenotypes were different. One study observed embryo lethality due to a failure to complete gastrulation (34) and the other report indicate that loss of NR4A3 resulted in inner ear defects (35). The role of NR4A1 in tumorigenesis was investigated in mouse models by comparing development of mouse cancer cell implants or xenografts in the presence or absence of NR4A1. For example, in a syngeneic mouse model using B16 melanoma cells the loss of NR4A1 enhanced tumor invasion and metastasis due to increased secretion of TNFα but decreased CSF-1R expression and tumor-infiltrating migratory activity (36). In contrast, NR4A1 enhanced tumor growth in mice bearing B16F1 melanoma cells and in these cells NR4A1 enhanced angiogenesis through regulation of VEGF expression (37). Expression of NR4A1 in mouse MV3 melanoma cells enhanced circulating tumor cell survival and metastasis (38). Similar results were observed in in vivo and in vitro studies using LLC and CMT93 colon cancer cells and the loss of NR4A1 in mice resulted in decreased tumor growth and metastasis (39). Thus, with the exception of APCmin/+ mice where NR4A1 loss resulted in enhanced intestinal tumors (40), most studies suggest that NR4A1 exhibits pro-oncogenic activity in solid tumors.
ROLE OF NR4A IN BLOOD-DERIVED TUMORS
Decreased NR4A and development of leukemias.
Mullican and coworkers first reported the remarkable phenotypic effects observed in combined NR4A1−/−/NR4A3−/− double knockout mice; the mice were smaller in size than their wild-type counterparts and all died within 3–4 weeks after birth from symptoms consistent with acute myeloid leukemia (AML) (41). This was accompanied by expansion of myeloid progenitors and hemopoietic stem cells, decreased expression of c-Jun, Jun-B and pro-apoptotic proteins such as Fas-L and TRAIL. Leukemic blasts from AML patients also exhibited low to non-detectable levels of NR4A1 and NR4A3, and similar results were observed in various leukemia-derived cell lines (41) which complemented the in vivo results and are consistent with tumor suppressor-like activity of NR4A1 and NR4A3 in combination. These observations were supported by an elegant study on the cancer outcomes of mice with “reduced NR4A gene dosage” (42), and these included NR4A1+/+/NR4A3+/+ (wild-type), single knockouts (NR4A1+/+/NR4A3−/−, NR4A1−/−/NR4A3+/+) and knockout/heterozygotes (NR4A1+/−/NR4A3−/−, NR4A1−/−/NR4A3+/−). Analysis of peripheral blood and other histological markers showed that wild-type and single knockout mice were normal whereas the knockout/heterozygote mice exhibited features consistent with mixed myelodysplastic/myeloproliferative neoplasms (MDS/MPN). Like the double knockout mice, NR4A1−/−/NR4A3+/− mice also exhibited AML. The MDS/MPN mice exhibited some changes in gene expression observed for NR4A1−/−/NR4A3−/− mice including decreased expression of JunB, egr1 and polo-like kinase 2 (Pik2).
Role of NR4A in lymphomas.
The expression and role of NR4A has also been investigated in lymphomas and it was reported that decreased expression of nuclear NR4A1 and NR4A3 was observed in patients with follicular lymphoma (FL) and diffuse large β-cell lymphoma (DLBCL) compared to their cells of origin. Decreased NR4A1 levels was associated with aggressive forms of FL and DLBCL and poor overall patient survival whereas NR4A2 was only detected in some samples and levels were similar in tumor and non-tumor tissue (43). NR4A3 is overexpressed in diffuse large B-cell lymphoma patients that responded favorably to chemotherapy whereas overexpression was not observed in patients that did not respond to therapy (44). Decreased NR4A1/NR4A3 expression was associated with decreased expression of apoptotic genes such as TRAIL, Puma and Bim, and in SuDHL4 lymphoma cells transfected with an NR4A1 expression plasmid there was a dramatic increase in apoptosis and this was accompanied by induction of pro-apoptotic genes TRAIL, Bim and Puma. An extensive analysis of DLBCL patients and subtypes showed that in germinal center B cell-like subtype, increased patient survival was associated with high cytoplasmic NR4A1 and genomic analysis indicate that this was associated with the ERK1/2 pathway (45). In another study (46) it was reported that in aggressive lymphomas decreased expression of NR4A3 was also associated with poor patient survival. Moreover, overexpression of NR4A3 in lymphoma cell lines induced apoptosis suggesting that in both leukemias and lymphomas the tumor suppressor-like activities of NR4A1 and NR4A3 are associated with their regulation of pro-apoptotic genes. Expression of NR4A3 is correlated with increased overall and event-frees survival of pediatric Pre-B-ALL patients but not the overall survival of AML patients (47). In contrast, analysis of NR4A1 expression in mantle cell lymphoma (MCL) showed that receptor was primarily located in the nucleus and levels were higher than in normal B cells from lymph nodes or tonsils (48). Moreover, there was a strong correlation between NR4A1 and Bruton tyrosine kinase (BTK) expression which is a key pro-oncogenic contributor to MCL. Knockdown of NR4A1 in MCL cells did not affect cell viability but enhanced drug (ibrutinib)-induced cell killing and genomic analysis after NR4A1 knockdown was consistent with the pro-oncogenic activity of NR4A1 in MCL cells and thus differed significantly from results obtained in leukemia and other types of lymphoma cells.
Drug/ligand-induced responses.
With the exception of MCL, there is evidence that the loss of NR4A1 and NR4A3 contributes to the development and expansion of blood derived leukemias and lymphomas, and the mechanism of silencing and drug-induced activation of the receptors has been investigated (49,50). Some studies report that histone deacetylase (HDAC) inhibitors induce expression of NR4A1 and NR4A3 in leukemia cells resulting in activation of proapoptotic pathways/genes and this is accompanied by enhanced histone acetylation (51). This effect of gene dosage was also observed in in vivo studies (42). Genome wide mapping of NR4A1 binding sites in human AML cells showed that NR4A1 targets 685 genes and regulates transcription in cooperation with distal ETS enhancers such as ERG and FLI-1 which promote recruitment of p300 (acetyltransferase) resulting in enhanced H3K27 acetylation (52). Analysis of the epigenetic status of NR4A1 and NR4A3 promoters showed that both promoters contained high levels of H3K4me3, however, comparative studies suggested that this was not an inhibitory mark and this was consistent with association of pol II occupancy on both promoters (53). A chemical screening assay identified dihydroergotamine (DHE), a drug which enhances NR4A expression and inhibits AML cell growth. Mechanistic studies show that DHE acts through a mechanism which reverses the promoter-paused pol II resulting in recruitment of the super elongation complex and increased elongation and enhanced gene expression (Fig. 3) (53). A recent study showed that DHE also targeted super enhancers of pro-oncogenic factors in leukemia and this includes MYC and results in decreased H3K27 acetylation thus providing a novel pathway for modulation of MYC expression in leukemia (54). Fenritinimide also induced expression of NR4A1 in AML cells and this was accompanied by nuclear export of NR4A1, interaction with bcl-2 resulting in induction of apoptosis (55). This pathway is similar to that observed in solid tumor derived cancer cells and will be further discussed in a subsequent section of the review. Thapsigargin induces NR4A3 levels in lymphoma cells and this drug-induced response mirrors the effect of NR4A3 overexpression resulting in inhibition of cell growth and induction of apoptosis (46). Cytosporone B (Csn-B) has been characterized as an NR4A1 ligand (56) and Csn-B induced apoptosis in lymphoma and immortalized B cells thus demonstrating potential drug efficacy of a compound that binds NR4A1 (41).
Figure 3.
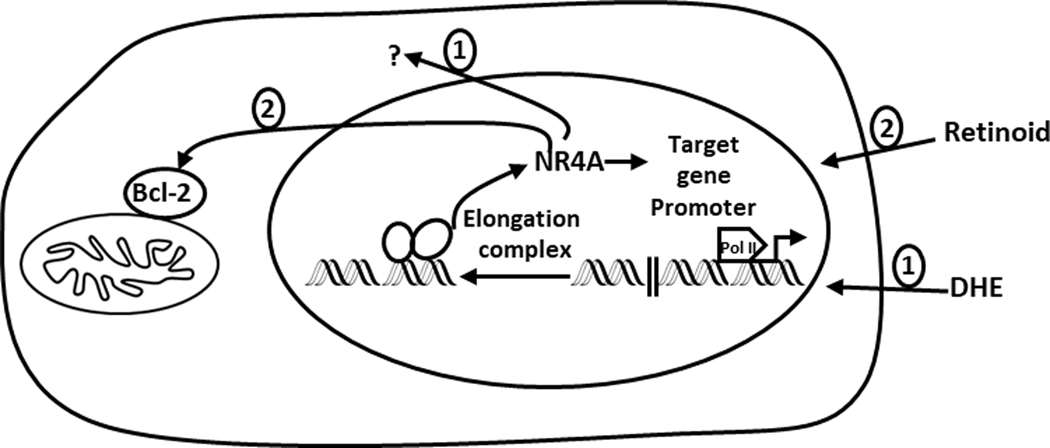
Basal and induced NR4A1 in blood-derived tumors-low expression of NR4A is observed in most blood-derived tumors due to promoter paused RNA Pol II and DHE induces NR4A tumor-expression through recruitment of the super elongation complex (49). There is also evidence for cytoplasmic NR4A in some lymphomas (42) and the retinoid Fenretinimide also induced nuclear export of NR4A1 which formed a complex with bcl-2 (51).
In contrast, treatment of MCL cells with 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH, CDIM8), a bis-indole derived NR4A1 ligand that acts as an antagonist in solid tumor-derived cells (57) also inhibited growth and synergistically enhanced ibrutinib-induced cytotoxicity. Moreover, in MCL cells Csn did not affect cell viability further confirming that in MCL cells NR4A1 exhibits pro-oncogenic activity (48). In summary there is evidence that NR4A1 and NR4A3 exhibit tumor suppressive-like activities in leukemias and some lymphomas, and drug-induced expression of these receptors is a potential treatment strategy. This is an area of research with future potential for clinical applications using NR4A inducing agents. MCL is the exception to these observations since NR4A1 exhibits tumor promoter-like activity and the underlying mechanisms that dictate these differences are not well defined. The tumor suppressor-like activity in most blood-derived cancers precludes the use of receptor ligands due to low NR4A expression and this contrasts with most studies in solid tumors as outlined below.
ROLE OF NR4A IN SOLID TUMORS
The expression, prognostic value, function, compound/ligand effects and mechanisms of action of NR4A receptors have been extensively investigated in solid tumors. However, there has been significantly more research on NR4A1 compared to NR4A2 or NR4A3. With one exception most in vivo studies indicate that NR4A1 (58) is pro-oncogenic in solid tumors indicating significant differences between the tumor suppressor-like activity of NR4A1 in blood-derived cancers, and the oncogenic-like activity of NR4A1 and NR4A2 in solid tumors.
NR4A expression, prognostic value and functions.
In contrast to blood-derived cancers, there is extensive evidence that NR4A1 is overexpressed in patients with multiple tumor types including breast, lung, pancreatic, ovarian, colon, endometrial, cervical and gastric cancers, rhabdomyosarcomas and melanomas (24,26,59–68). Moreover, high expression of NR4A1 in lung, breast, ovarian and colon cancers predict poor patient survival or prognosis (60,64–66) although a few prognostic studies are conflicting (69–74). The most convincing and consistent evidence demonstrating the pro-oncogenic activities of NR4A1 are results of gene silencing studies in solid tumor-derived cell lines which show that NR4A1 regulates one or more of cell proliferation, survival, migration/invasion and in some cells epithelial-mesenchymal – transition (Fig. 4). These effects have been observed in breast, colon, pancreatic, kidney, lung, rhabdomyosarcoma, melanoma, endometrial cancer cells (57,59–61,63,65,67,69,75–79). Although the pathways/genes associated with NR4A1 are complex and cell context specific, Figure 4 illustrates the role of NR4A1 in most solid tumor-derived cancer cell lines. This includes an important function for NR4A1 in TGFβ-induced invasion of breast and lung cancer cells (65,80,81). Studies in our laboratory showed that two NR4A1-regulated pro-reductant genes, namely thioredoxin domain-containing 5 (TXNDC5) and isocitrate dehydrogenase 1 (IDH1) were important for maintaining relatively high mTOR signaling and for decreasing intracellular reactive oxygen species (ROS) and ER stress (24,25,57). Knockdown of NR4A1 by RNA interference decreases TXNDC5 and IDH1 expression resulting in the induction of ROS and this is accompanied by activation of ER stress. Induction of ROS also inhibits mTOR signaling through induction of sestrin 2 (ROS-dependent) which activates AMPK resulting in mTOR inhibition (Fig. 4). This is observed in pancreatic, breast, lung, kidney and RMS cancer cells (25,57,60,77–79) and also in endometriotic cells where mTOR signaling is also inhibited by NR4A1 knockdown (82). A tumor specific effect of NR4A1 is observed in alveolar RMS (ARMS) where the unique PAX3-FOX01 fusion oncogene important for ARMS cell growth is also an NR4A1-regulated gene (26). As indicated in Figure 4, NR4A1 acts as a nuclear transcription factor or nuclear coactivator to modulate target gene expression. Most studies show that NR4A2 is also pro-oncogenic in solid tumor derived cell lines and like NR4A1 plays a role in cancer cell proliferation, survival and migration/invasion (76,83–94). For example, overexpression of NR4A2 in colon cancer cells enhanced chemoresistance and expression of NR4A2 was enhanced in colon cancer patients and was a negative prognostic factor (91). A recent study also reported that NR4A2 expression was a negative prognostic factor for glioblastoma patients and knockdown of the receptor by RNAi resulted in decreased growth, survival and invasion (94). Acinic cell carcinoma (ACC) is a salivary gland tumor which exhibits specific rearrangements [+(4;9)(q13;q31)] which result in enhanced expression of NR4A3 (95,96). Immunostaining of these carcinomas (97–101) shows that NR4A3 is highly expressed in most of these tumors (63/64) whereas NR4A2 (1/64) but not NR4A1 were also overexpressed compared to other salivary gland tumors (100,101). NR4A3 exhibits pro-oncogenic activity in acinic cell carcinomas and this is associated with increased cell proliferation and activation of NR4A-regulated genes and cooperative effects with MYB oncogene (95,96). NR4A3 knockdown did not affect the phenotype in glioblastoma cells (94) whereas other studies show that NR4A3 exhibits tumor suppressor like activity (102–105). Thus, in solid tumors NR4A1 and NR4A2 exhibit pro-oncogenic, and NR4A3 exhibits tumor suppressor-like activities and this contrasts with most blood-derived cancers where NR4A1/NR4A3 (combination) and possible NR4A2 are tumor suppressors.
Figure 4.
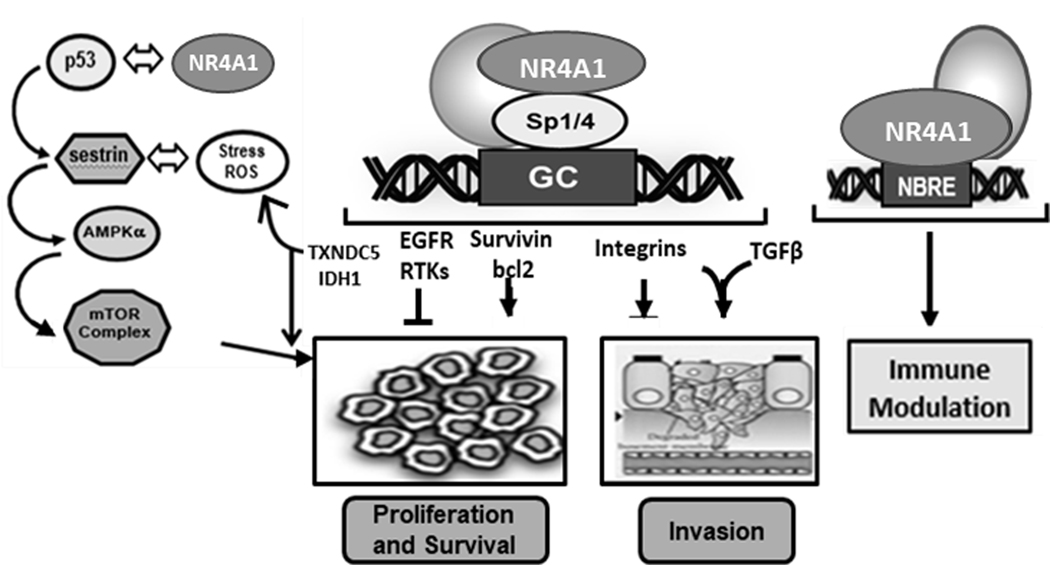
NR4A-regulated pathway/gene expression in solid tumors. These results were derived primarily from knockdown studies (of NR4A1) and were observed in multiple cancer cell lines (55–59, 61, 63, 65, 71–75).
Although NR4As are structurally similar and by definition are trans-acting factors that modulate gene expression (e.g.: Fig. 4), their paradoxical cell context dependent activities and differential effects of drugs/ligands are due in part to their intracellular interactions with other factors. Kurakula and co-workers (106) summarized the interactome for NR4A1, NR4A2 and NR4A3, and identified 64, 25 and 13 interacting factors respectively with only limited studies on interactions with NR4A2 and NR4A3. The NR4A interaction surfaces include all receptor domains; the 3 receptors interact with many different factors but only with a few proteins in common and this contributes to their different cell/tissue context-dependent activities. In addition, another key distinguishing feature of NR4As is that after activation by agents, ligands or stimuli their effects are due to both nuclear and/or extranuclear NR4A. Among the first reports on the role of NR4A1 in cancer were studies showing that the effects of structurally diverse apoptosis-inducing agents were due to the nuclear export of NR4A1 (107), whereas this has not been a distinguishing feature of NR4A2 or NR4A3. The following sections of the review will focus on agent/stimuli induced extranuclear and nuclear activation/inactivation of NR4A with most of the published studies on NR4A1.
Extranuclear NR4A action:
There is extensive evidence from knockdown and overexpression studies that NR4A1 regulates cancer cell proliferation, survival and migration/invasion (Fig. 4) and studies with retinoids and other apoptosis-inducing agents identified an important proapoptotic role for NR4A1 (107). Initial reports showed that the retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2 naphthalene carboxylic acid (AHPN, CD437) induced both retinoid receptor dependent or independent apoptosis in different cancer cell lines (107–113). Moreover, there was also evidence that AHPN-mediated induction of apoptosis in cancer cells was dependent on NR4A1 but did not require the DNA binding domain of the receptor. This response was blocked by leptomycin B, a nuclear export inhibitor and it was shown that AHPN-induced apoptosis was due to nuclear export of NR4A1 and mitochondrial targeting of the receptor (111). Similar effects were observed in cancer cells treated with many other structurally-diverse compounds including phobol esters, etoposide, cadmium, cholic acid derivatives, etoposide, HDAC inhibitors, dibutyltin derivatives, coumarin analogs, bile acids, oxidized analogs of bis-indole derived compounds, acetylshikonin analogs, and n-butylenephthalide and related compounds (111–124). Many of these agents not only induced nuclear export and apoptosis in cancer cells but also increased overall levels of NR4A1. Mechanistic studies with AHPN revealed that extranuclear NR4A1 interacted with mitochondrial bcl-2 resulting in formation of a pro-apoptotic complex that induced mitochondrial disruption, cytochrome c release and activation of the intrinsic apoptosis pathway (Fig. 5) (111,113). Subsequent studies report that NR4A1 binding to bcl-2 requires the loop region between the BH4 and BH3 domains of bcl-2 (113) and a site adjacent to the BH3 peptide binding crevice was recently shown to be involved in NR4A1- bcl-2 binding (125) Paclitaxel and a short NR4A1 peptide that interact with bcl-2 mimic the proapoptotic effects of NR4A1 (126,127) suggesting that design of small molecules that target the NR4A1 interacting sites of bcl-2 represent a novel class of apoptosis inducing agents (126,127). The extranuclear pro-apoptotic functions of NR4A2 have not been reported; whereas, transfection of NR4A3 into breast cancer cells induced apoptosis and interactions with bcl-2 were also observed (102). Thus, identification of ligand-dependent nuclear export of NR4A3 may also have some clinical potential for killing cancer cells (Fig. 5). There is also evidence that nuclear export of NR4A1 plays an integral role in many other pathways in cancer cells. Several agents including butyrate, sulindac and 5-fluorouracil also induce nuclear export of NR4A1 which is accompanied by induction of bax, cytochrome c release and apoptosis in colon cancer cells, however, in these studies NR4A1 did not target the mitochondria (128) (Fig. 5). It was assumed that other cytosolic factors were involved in the induction of apoptosis. Insulin-like growth factor binding protein 3 (IGFBP3) interacts directly with NR4A1 resulting in nuclear export and mitochondrial targeting of the receptor and this is associated with activation of JNK and inhibition of Akt (129,130).
Figure 5:
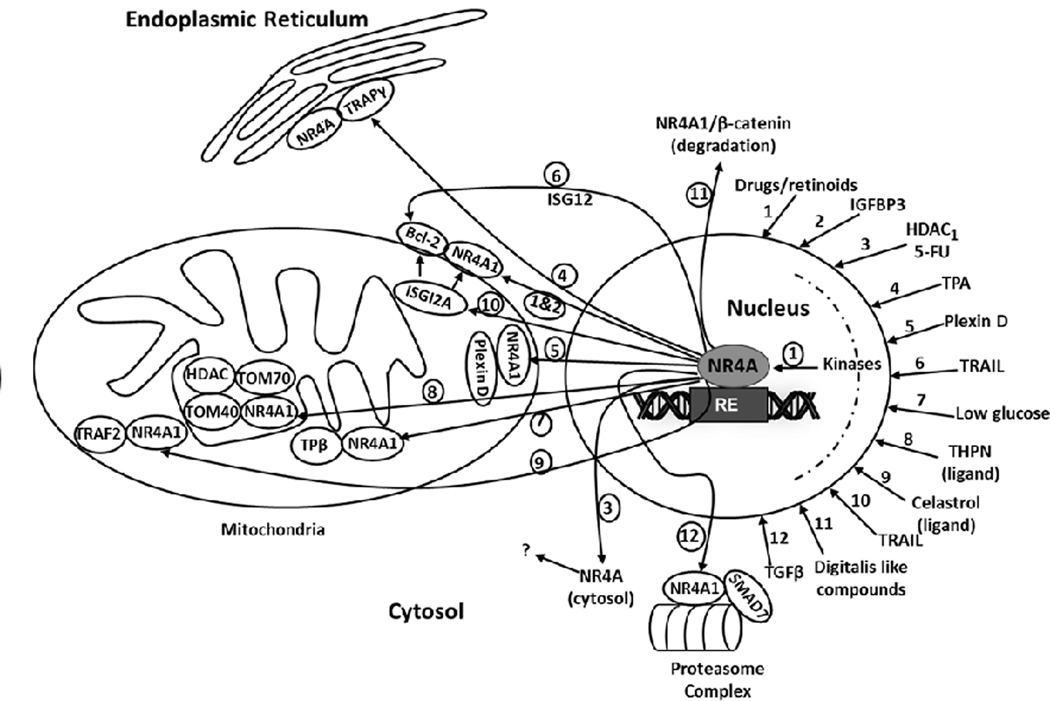
Drug/Ligand/TGFβ-induced nuclear export of NR4A1 and mitochondrial/cytosolic interactions; various inputs are indicated (1–12) and resulting outputs are also illustrated as 1–12 (circled). NR4A1 ligands such as Celastrol and THPN also bind the receptor. The drug/agent induced outputs illustrate the multiple interaction of extranuclear NR4A with mitochondrial targets as well as the endoplasmic reticulum and a proteasome complex.
In melanoma cells fatty acid oxidation (FAO) is important for generating ATP and cellular oxidants and this is observed in cells maintained under low glucose conditions. Glucose deprivation also resulted in nuclear export of NR4A1 to mitochondria and formation of a complex with TPβ a subunit of a mitochondrial functional protein (131). TPβ is important for fatty acid oxidation and NR4A1 protected TPβ from oxidation and thereby enhanced fatty acid oxidation and survival of melanoma cells maintained in low glucose medium (38). The novel NR4A1 binding compound 1-(3,4,5-trihydroxyphenyl)nonan-1-one (THPN) induces NR4A1 nuclear export to the mitochondria in melanoma cells, where it interacts with Tom40 and Tom70 resulting in disruption of the VDAC1 transition pore complex and activation of autophagic cell death pathways (132). Two recent studies identified 2-imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA) (131) and celastrol (133) a naturally occurring triterpenoid as NR4A1 ligands and the former compound induced nuclear export to mitochondria, possibly inducing apoptosis via interaction with bcl-2. Celastrol is a potent anticancer agent and treatment of liver cancer cells with this compound induces nuclear-to-mitochondrial translocation of NR4A1 which interacts with TRAF2; NR4A1 is ubiquitinated and interacts with p62/SQSTM1 resulting induction of autophagy (134). Plexin D1 is a receptor that interacts with its ligand Sema 3E to promote breast cancer survival and the Sema domain of Plexin D1 (SD1) acts as ligand binding trap and activates cell death pathways (135). Unliganded Plexin D1 interacts with cytosolic NR4A1 to induce apoptosis, however, the precise mechanism of this response and the role of bcl-2 was not defined. Phorbol esters induces ER stress in liver cancer cells and this is associated with nuclear export of NR4A1 and formation of an NR4A1-translocation-associated protein subunit γ (TRAPγ) complex (135). TRAIL-induced apoptosis in liver cancer cells involves NR4A1 nuclear export, formation of an NR4A1- bcl-2 mitochondrial complex which was dependent on interaction of NR4A1 with interferon stimulated gene 12a (ISG12a) for both nuclear export and enhanced bcl-2 interactions (136). Two digitalis-like compounds induced NR4A1 expression in colon cancer cells and this was accompanied by nuclear export of NR4A1 to the cytosol, where it interacts with β-catenin resulting in degradation of β-catenin (137). These examples of agent-induced nuclear export of NR4A1 (Fig. 5) illustrate the diverse pathways for nuclear export of NR4A1 and cell killing which are due to both mitochondrial disruption and non-mitochondrial effects. In contrast, TGFβ-induced invasion and metastasis of breast and lung cancer cells is an example of a pro-oncogenic pathway that is also dependent on NR4A1 and its nuclear export (65,80,81). This process involves TGFβ-induced nuclear export of NR4A1 which forms a cytosolic complex containing NR4A1, axin2, Arkadia and RNF12 which are necessary for proteasome-dependent degradation of the inhibitory SMAD-7. The loss or decrease of SMAD7-dependent inhibition of TGFβ signaling enhances TGFβ-induced cancer cell invasion. Most of the pathways associated with nuclear export of NR4A1 involve activation/inactivation of kinases which can be modulated by various kinase inhibitors; however, the role of individual kinases is both agent- and cell context dependent. For example, TGFβ-induced nuclear export of NR4A1 in breast cancers cells is p38-MAPK14 – dependent and blocked by p38 inhibitors. Whereas this same response is activated by c-Jun N-terminal kinase (JNK) in lung cancer cells and blocked by JNK inhibitors. TGFβ-dependent phosphorylation of NR4A1-Ser351 was observed after activation of different kinase pathways that induce nuclear export of NR4A1 in both breast and lung cancer cells (80,81). Phosphorylation of nuclear NR4A1 is important for nuclear export of the receptor and a recent study suggests a possible role for NR4A1 sumoylation in this process (138). These results demonstrate the remarkable and highly variable effects of agent/biologic-induced nuclear export of NR4A1 which interacts with multiple factors to induce apoptosis but also facilitates TGFβ-induced invasion of cancer cells (Fig. 5).
Nuclear functions of NR4As and their ligands.
RNA interference and overexpression experiments demonstrate that in the absence of ligands, NR4A1 regulates pro-oncogenic pathways and genes associated with cancer cell proliferation, survival and migration/invasion (Fig. 4) and in limited studies similar results have been observed for NR4A2. In contrast, NR4A3 exhibits oncogenic and tumor suppressor-like activity and has not been extensively studied in cancer. The identification of NR4As with important functional activities in cancer and other diseases has spurred development of receptor ligands that target NR4A as either agonists or antagonists. Wu and coworkers first identified cytosporone β (Csn-B) as an NR4A1 ligand (Fig. 6), however, Csn-B also induced apoptosis in cancer cells via nuclear export of the receptor to the mitochondria and this was also observed for THPN (55,56,132). Several Csn-B analogs also bound NR4A1 with KD values in the low μM range and not only induced mitochondrial localization of NR4A1 but also acted within the nucleus to repress expression of brain and reproductive organ-expressed protein (BRE) in gastric cancer cells (56). BRE is an NR4A1-regulated antiapoptotic/survival gene indicating that in gastric cancer cells Csn-B and its analogs are acting as an antagonist for this gene. In contrast, treatment of gastric cancer cells with Csn-B and related compounds induced activity in cells transfected with an NR4A1-responsive construct. Thus, the Csn-B compounds acts as selective NR4A1 modulators and their agonist or antagonist activities are gene- and function- dependent, and this is typical of selective receptor modulators (SRMs) for many other nuclear receptors (139).
Figure 6:

Structures of ligands that bind NR4As. The KD values for receptor binding by cytosporone B (1.68 μM) (56), celastrol (292 nM) (133) and chloroquine (0.27 μM) (151) have been reported. KD values for prostaglandin A2 and CDIM12 have not been reported and the KD value for CDIM8 is 0.56 μM (unpublished results).
Studies in the Safe laboratory have focused on bis-indole derived compounds (CDIMs) which bind NR4A1 and 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl)methane (CDIM8; DIM-C-pPhOH) and the p-carbomethoxyphenyl derivatives have been used as representative NR4A1 ligands (77–82). These compounds both induce and repress NR4A1-regulated genes but in terms of their functional responses they antagonize the nuclear NR4A1-regulated pro-oncogenic pathways/genes illustrated in Figure 4. Treatment of many solid tumor-derived cell lines with NR4A1 antagonists or knockdown of NR4A1 inhibits the pathways and gene illustrated in Figure 4. However, genomic or proteomic analysis demonstrate significant cell context-dependent differences in gene expression in pancreatic, kidney and breast cancer, and rhabdomyosarcoma cells (24,26,79,140). CDIM/NR4A1 antagonists also inhibit TGFβ-induced nuclear export of NR4A1 and cell invasion in lung and breast cancer cells suggesting that binding of the CDIM ligand inhibits some elements of nuclear export process (81,82). 1,1-Bis(3’-indolyl)-1-(4-chlorophenyl)methane (CDIM12) is an NR4A2 ligand and molecular modeling studies indicate that CDIM12 does not directly interact with the ligand binding. Modeling studies suggest that CDIM12 binds the coactivator region of NR4A2 and this needs to be further investigated. (141). Although transactivation studies in pancreatic cancer cells show that CDIM12 activates multiple genes and NR4A2-responsive constructs (142,143), CDIM12 exhibits functional antagonist activities and inhibits cancer cell growth and survival (94,144). Prostaglandin E2 (PGE2) is a cyclooxygenase-2 (COX-2) derived gene product that is pro-oncogenic in colon cancer and induces NR4A2 in both in vivo and in vitro models. The resulting changes in gene expression include enhanced osteopontin expression and fatty acid oxidation (145–147). PGE2-mediated activation of NR4A2 enhanced NR4A2/RXR-mediated expression of prolactin in stromal cells and the tumor-stromal prolactin signaling initiates prostate cancer and this is blocked by COX-2 inhibitors (148). Prostaglandin A2 (PGA2) has been identified as an NR4A3 ligand (149) (Fig. 6) and induces apoptosis and inhibits growth of breast and cervical cancer cells (150). In some cells it is possible that PGA2 enhances NR4A3-dependent tumor suppressor activity and acts as an agonist (151).
The high expression of NR4A receptors in solid tumors makes them potential targets for receptor ligands and this supported by studies on NR4A1 where Csn-B and related compounds bind and induce nuclear export and apoptosis (Fig. 5 and 6) whereas CDIMs primarily inactivate nuclear NR4A1-regulated pro-oncogenic pathway/genes (Fig. 4 and 6). Ligands for NR4A2 and NR4A3 have not been extensively investigated (Fig. 6) but may also be effective as anticancer agents. Applications of these selective NR4A modulators do not have to be confined to cancer since some of these compound (e.g.: CDIMs) show promise for treating endometriosis (82); Parkinson’s disease (141,152,153) and for enhancing learning and memory (154,155).
NR4A IN CANCER IMMUNE SURVEILLANCE
NR4A receptors are immediate early genes induced by T cell receptor signaling and play an important role in T cell development and immune responses (156). NR4A is expressed in Treg cells and in CD4+/CD8+ T cells and levels of NR4A dictate, in part, responsiveness to immunotherapy targeting tumor and immune cell checkpoints, and in CAR-T cell therapy (157–159). Analysis of tumor infiltrating lymphocytes (TIL) shows that in exhausted T cells NR4A is overexpressed and this is accompanied by enhanced expression of PD-L1 and Tim3, decreased expression of cytokines and low levels of cell killing (159). Loss of NR4A receptors partially reversed exhaustion resulting in tumor regression and increased survival (160). Hibino and coworkers (161) showed comparable results in mice lacking NR4A1 and NR4A2; moreover, after treatment or wild-type mice with campothecin or the cyclooxygenase-2 (COX-2) inhibitor SC-236 to decrease NR4A1 levels there was also a decrease in tumor volumes and an increase in CD8+/CD4+ ratios (161). Thus, loss of NR4A1 by genetic or pharmacological means enhanced immune surveillance. This was also observed using 1,1-bis(3’-indolyl)-1-(3-chloro-4-hydroxy-5-methoxyphenyl)methane (CDIM8–3-Cl-5-OCH3) which inhibited mammary tumor growth in both xenograft and syngeneic mouse models (140,162). Moreover, treatment with the CDIM/NR4A1 antagonist enhanced CD8+/CD4+ ratios in TILs and this was primarily due to decreased levels of CD4+. Thus, NR4A1 antagonists represent a novel class of drugs that enhance immune surveillance in a syngeneic mouse model using mouse 4T1 breast cancer cells and mechanistic studies also showed that PD-L1 is an NR4A1/Sp1 regulated gene that is downregulated by CDIM/NR4A1 antagonists (162).
SUMMARY
NR4A are structurally related NRs that activate gene expression through targeting common cis elements. NR4A1, NR4A2 and NR4A3 are activated by diverse stressors, however, their roles in cancer are diverse and paradoxical. In leukemias and most lymphomas NR4A1 and NR4A3 are tumor suppressor genes whereas the role of NR4A2 is not well defined. The mechanisms of NR4A-mediated genes and responses involve nuclear NR4A and require further investigation. In solid tumors both NR4A1 and NR4A2 are tumor promoter-like genes whereas NR4A3 exhibits tumor suppressor and promoter activities. NR4A1 has been intensively studied in solid tumor-derived human cancer cell lines and exhibits activity as a nuclear transcription factor and a nuclear cofactor. In addition, drug-induced nuclear export of NR4A1 results in the induction of apoptosis and this nuclear export pathway has primarily been observed for NR4A1 and not for NR4A2 or NR4A3. The extranuclear pro-apoptotic activity of NR4A1 in cancer cells and mouse models not only involves a unique interaction with bcl-2 but also interactions with variety of other partners in the mitochondria and cytosol. In contrast, TGFβ also induces nuclear export of NR4A1 where it exhibits tumor promoter-like activity and plays a role in degradation of inhibitory SMAD-7 in breast and lung cancer cells. The development of NR4A ligands is ongoing and it is clear that their activity as agonists or antagonists can be used to target blood derived and solid tumors, and these compounds can also be effective as enhancers of immune surveillance.
Acknowledgements:
Funding from the Kleberg Foundation (SS), Texas A&M AgriLife Research (SS), the Sid Kyle Chair Endowment (SS) and NIH (P30-ES029067) (SS) is gratefully acknowledged.
Footnotes
Conflicts of Interest: The CDIM technology has been licensed by Systems Oncology, Scottsdale AZ for development of clinical applications.
REFERENCES
- 1.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, and Mangelsdorf DJ (2006) Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y. (2007) Orphan nuclear receptors in drug discovery. Drug Discov Today 12, 440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy EP, Dobson AD, Keller C, and Conneely OM (1996) Differential regulation of transcription by the NURR1/NUR77 subfamily of nuclear transcription factors. Gene Expr 5, 169–179 [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen RF, Granas K, Johnsen H, Rolseth V, and Sterri S. (1995) Three related brain nuclear receptors, NGFI-B, Nurr1, and NOR-1, as transcriptional activators. J Mol Neurosci 6, 249–255 [DOI] [PubMed] [Google Scholar]
- 5.Saucedo-Cardenas O, Kardon R, Ediger TR, Lydon JP, and Conneely OM (1997) Cloning and structural organization of the gene encoding the murine nuclear receptor transcription factor, NURR1. Gene 187, 135–139 [DOI] [PubMed] [Google Scholar]
- 6.Maira M, Martens C, Batsche E, Gauthier Y, and Drouin J. (2003) Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol 23, 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wansa KD, Harris JM, and Muscat GE (2002) The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem 277, 33001–33011 [DOI] [PubMed] [Google Scholar]
- 8.Wansa KD, Harris JM, Yan G, Ordentlich P, and Muscat GE (2003) The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem 278, 24776–24790 [DOI] [PubMed] [Google Scholar]
- 9.Maxwell MA, and Muscat GE (2006) The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 4, e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearen MA, and Muscat GE (2010) Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24, 1891–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SO, Li X, Khan S, and Safe S. (2011) Targeting NR4A1 (TR3) in cancer cells and tumors. Expert Opin Ther Targets 15, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XK (2007) Targeting Nur77 translocation. Expert Opin Ther Targets 11, 69–79 [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, and Perlmann T. (2003) Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423, 555–560 [DOI] [PubMed] [Google Scholar]
- 14.Flaig R, Greschik H, Peluso-Iltis C, and Moras D. (2005) Structural basis for the cell-specific activities of the NGFI-B and the Nurr1 ligand-binding domain. J Biol Chem 280, 19250–19258 [DOI] [PubMed] [Google Scholar]
- 15.Paulsen RE, Weaver CA, Fahrner TJ, and Milbrandt J. (1992) Domains regulating transcriptional activity of the inducible orphan receptor NGFI-B. J Biol Chem 267, 16491–16496 [PubMed] [Google Scholar]
- 16.Davis IJ, Hazel TG, Chen RH, Blenis J, and Lau LF (1993) Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol 7, 953–964 [DOI] [PubMed] [Google Scholar]
- 17.Wilson TE, Fahrner TJ, Johnston M, and Milbrandt J. (1991) Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252, 1296–1300 [DOI] [PubMed] [Google Scholar]
- 18.Maira M, Martens C, Philips A, and Drouin J. (1999) Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol 19, 7549–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, and Drouin J. (1997) Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 17, 5946–5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlmann T, and Jansson L. (1995) A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev 9, 769–782 [DOI] [PubMed] [Google Scholar]
- 21.Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, and Perlmann T. (1996) Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol 10, 1656–1666 [DOI] [PubMed] [Google Scholar]
- 22.Jiang L, Dai S, Li J, Liang X, Qu L, Chen X, Guo M, Chen Z, Chen L, Wei H, and Chen Y. (2019) Structural basis of binding of homodimers of the nuclear receptor NR4A2 to selective Nur-responsive DNA elements. J Biol Chem 294, 19795–19803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Wei H, Yan N, Dai S, Li J, Qu L, Chen X, Guo M, Chen Z, and Chen Y. (2020) Structural basis of NR4A1 bound to the human pituitary proopiomelanocortin gene promoter. Biochem Biophys Res Commun 523, 1–5 [DOI] [PubMed] [Google Scholar]
- 24.Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, Wang H, and Safe S. (2010) Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res 70, 6824–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, and Safe S. (2016) Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget 7, 31257–31269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacey A, Rodrigues-Hoffman A, and Safe S. (2017) PAX3-FOXO1A Expression in Rhabdomyosarcoma Is Driven by the Targetable Nuclear Receptor NR4A1. Cancer Res 77, 732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedrick E, Li X, and Safe S. (2017) Penfluridol Represses Integrin Expression in Breast Cancer through Induction of Reactive Oxygen Species and Downregulation of Sp Transcription Factors. Mol Cancer Ther 16, 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safe S, and Kim K. (2008) Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. Journal of molecular endocrinology 41, 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng LE, Chan FK, Cado D, and Winoto A. (1997) Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J 16, 1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woronicz JD, Calnan B, Ngo V, and Winoto A. (1994) Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature 367, 277–281 [DOI] [PubMed] [Google Scholar]
- 31.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, and Milbrandt J. (1995) Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science 269, 532–535 [DOI] [PubMed] [Google Scholar]
- 32.Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, and Perlmann T. (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276, 248–250 [DOI] [PubMed] [Google Scholar]
- 33.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, and Conneely OM (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A 95, 4013–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeYoung RA, Baker JC, Cado D, and Winoto A. (2003) The orphan steroid receptor Nur77 family member Nor-1 is essential for early mouse embryogenesis. J Biol Chem 278, 47104–47109 [DOI] [PubMed] [Google Scholar]
- 35.Ponnio T, Burton Q, Pereira FA, Wu DK, and Conneely OM (2002) The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol Cell Biol 22, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XM, Wang JR, Shen T, Gao SS, He XS, Li JN, Yang TY, Zhang S, Gan WJ, Li JM, and Wu H. (2017) Nur77 deficiency in mice accelerates tumor invasion and metastasis by facilitating TNFalpha secretion and lowering CSF-1R expression. PLoS One 12, e0171347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, and Dvorak HF (2006) Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med 203, 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li XX, Wang ZJ, Zheng Y, Guan YF, Yang PB, Chen X, Peng C, He JP, Ai YL, Wu SF, Chien KY, Wu Q, and Chen HZ (2018) Nuclear Receptor Nur77 Facilitates Melanoma Cell Survival under Metabolic Stress by Protecting Fatty Acid Oxidation. Mol Cell 69, 480–492 e487 [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Li Y, Hou S, Bourbon PM, Qin L, Zhao K, Ye T, Zhao D, and Zeng H. (2020) Orphan nuclear receptor TR3/Nur77 biologics inhibit tumor growth by targeting angiogenesis and tumor cells. Microvasc Res 128, 103934. [DOI] [PubMed] [Google Scholar]
- 40.Yao LM, He JP, Chen HZ, Wang Y, Wang WJ, Wu R, Yu CD, and Wu Q. (2012) Orphan receptor TR3 participates in cisplatin-induced apoptosis via Chk2 phosphorylation to repress intestinal tumorigenesis. Carcinogenesis 33, 301–311 [DOI] [PubMed] [Google Scholar]
- 41.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, and Conneely OM (2007) Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med 13, 730–735 [DOI] [PubMed] [Google Scholar]
- 42.Ramirez-Herrick AM, Mullican SE, Sheehan AM, and Conneely OM (2011) Reduced NR4A gene dosage leads to mixed myelodysplastic/myeloproliferative neoplasms in mice. Blood 117, 2681–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deutsch AJ, Rinner B, Wenzl K, Pichler M, Troppan K, Steinbauer E, Schwarzenbacher D, Reitter S, Feichtinger J, Tierling S, Prokesch A, Scheideler M, Krogsdam A, Thallinger GG, Schaider H, Beham-Schmid C, and Neumeister P. (2014) NR4A1-mediated apoptosis suppresses lymphomagenesis and is associated with a favorable cancer-specific survival in patients with aggressive B-cell lymphomas. Blood 123, 2367–2377 [DOI] [PubMed] [Google Scholar]
- 44.Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, Ray TS, Koval MA, Last KW, Norton A, Lister TA, Mesirov J, Neuberg DS, Lander ES, Aster JC, and Golub TR (2002) Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 8, 68–74 [DOI] [PubMed] [Google Scholar]
- 45.Fechter K, Feichtinger J, Prochazka K, Unterluggauer JJ, Pansy K, Steinbauer E, Pichler M, Haybaeck J, Prokesch A, Greinix HT, Beham-Schmid C, Neumeister P, Thallinger GG, and Deutsch AJA (2018) Cytoplasmic location of NR4A1 in aggressive lymphomas is associated with a favourable cancer specific survival. Sci Rep 8, 14528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deutsch AJA, Rinner B, Pichler M, Prochazka K, Pansy K, Bischof M, Fechter K, Hatzl S, Feichtinger J, Wenzl K, Frisch MT, Stiegelbauer V, Prokesch A, Krogsdam A, Sill H, Thallinger GG, Greinix HT, Wang C, Beham-Schmid C, and Neumeister P. (2017) NR4A3 Suppresses Lymphomagenesis through Induction of Proapoptotic Genes. Cancer Res 77, 2375–2386 [DOI] [PubMed] [Google Scholar]
- 47.Lu Z, Xie J, Wu G, Shen J, Collins R, Chen W, Kang X, Luo M, Zou Y, Huang LJ, Amatruda JF, Slone T, Winick N, Scherer PE, and Zhang CC (2017) Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat Med 23, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Wang F, Lu L, Zhu F, Huang S, Nomie K, Zhang L, Yang DT, Huang W, Kahl BS, Safe S, Wang M, and Rui L. (2017) NR4A1 inhibition synergizes with ibrutinib in killing mantle cell lymphoma cells. Blood Cancer J 7, 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenzl K, Troppan K, Neumeister P, and Deutsch AJ (2015) The nuclear orphan receptor NR4A1 and NR4A3 as tumor suppressors in hematologic neoplasms. Curr Drug Targets 16, 38–46 [DOI] [PubMed] [Google Scholar]
- 50.Liu HB, Voso MT, Gumiero D, Duong J, McKendrick JJ, and Dear AE (2009) The anti-leukemic effect of a novel histone deacetylase inhibitor MCT-1 and 5-aza-cytidine involves augmentation of Nur77 and inhibition of MMP-9 expression. Int J Oncol 34, 573–579 [PubMed] [Google Scholar]
- 51.Zhou L, Ruvolo VR, McQueen T, Chen W, Samudio IJ, Conneely O, Konopleva M, and Andreeff M. (2013) HDAC inhibition by SNDX-275 (Entinostat) restores expression of silenced leukemia-associated transcription factors Nur77 and Nor1 and of key pro-apoptotic proteins in AML. Leukemia 27, 1358–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duren RP, Boudreaux SP, and Conneely OM (2016) Genome Wide Mapping of NR4A Binding Reveals Cooperativity with ETS Factors to Promote Epigenetic Activation of Distal Enhancers in Acute Myeloid Leukemia Cells. PLoS One 11, e0150450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudreaux SP, Duren RP, Call SG, Nguyen L, Freire PR, Narayanan P, Redell MS, and Conneely OM (2019) Drug targeting of NR4A nuclear receptors for treatment of acute myeloid leukemia. Leukemia 33, 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Call SG, Duren RP, Panigrahi AK, Nguyen L, Freire PR, Grimm SL, Coarfa C, and Conneely OM (2020) Targeting Oncogenic Super Enhancers in MYC-Dependent AML Using a Small Molecule Activator of NR4A Nuclear Receptors. Sci Rep 10, 2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong J, Kuang X, Lu T, Liu X, Cheng B, Wang W, Wei D, Li X, Zhang Z, Fang Q, Wu D, and Wang J. (2019) Fenretinide-induced Apoptosis of Acute Myeloid Leukemia Cells via NR4A1 Translocation into Mitochondria and Bcl-2 Transformation. J Cancer 10, 6767–6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, Li G, Xu Q, Zhang M, Weimer BC, Chen D, Cheng Z, Zhang L, Li Q, Li S, Zheng Z, Song S, Huang Y, Ye Z, Su W, Lin SC, Shen Y, and Wu Q. (2008) Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol 4, 548–556 [DOI] [PubMed] [Google Scholar]
- 57.Lee SO, Li X, Hedrick E, Jin UH, Tjalkens RB, Backos DS, Li L, Zhang Y, Wu Q, and Safe S. (2014) Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol Endocrinol 28, 1729–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu B, Yang JR, Jia Y, Zhang P, Shen L, Li XL, Li J, and Wang B. (2017) Overexpression of NR4A1 is associated with tumor recurrence and poor survival in non-small-cell lung carcinoma. Oncotarget 8, 113977–113986 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Muscat GE, Eriksson NA, Byth K, Loi S, Graham D, Jindal S, Davis MJ, Clyne C, Funder JW, Simpson ER, Ragan MA, Kuczek E, Fuller PJ, Tilley WD, Leedman PJ, and Clarke CL (2013) Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol 27, 350–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SO, Andey T, Jin UH, Kim K, Singh M, and Safe S. (2012) The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene 31, 3265–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, Khan S, Ramaiah SK, and Safe S. (2007) Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res 67, 674–683 [DOI] [PubMed] [Google Scholar]
- 62.Lee SO, Jin UH, Kang JH, Kim SB, Guthrie AS, Sreevalsan S, Lee JS, and Safe S. (2014) The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Molecular cancer research : MCR 12, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AG, Lim W, Pearen M, Muscat GE, and Sturm RA (2011) Regulation of NR4A nuclear receptor expression by oncogenic BRAF in melanoma cells. Pigment Cell Melanoma Res 24, 551–563 [DOI] [PubMed] [Google Scholar]
- 64.Wang JR, Gan WJ, Li XM, Zhao YY, Li Y, Lu XX, Li JM, and Wu H. (2014) Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis 35, 2474–2484 [DOI] [PubMed] [Google Scholar]
- 65.Zhou F, Drabsch Y, Dekker TJ, de Vinuesa AG, Li Y, Hawinkels LJ, Sheppard KA, Goumans MJ, Luwor RB, de Vries CJ, Mesker WE, Tollenaar RA, Devilee P, Lu CX, Zhu H, Zhang L, and Dijke PT (2014) Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat Commun 5, 3388. [DOI] [PubMed] [Google Scholar]
- 66.Delgado E, Boisen MM, Laskey R, Chen R, Song C, Sallit J, Yochum ZA, Andersen CL, Sikora MJ, Wagner J, Safe S, Elishaev E, Lee A, Edwards RP, Haluska P, Tseng G, Schurdak M, and Oesterreich S. (2016) High expression of orphan nuclear receptor NR4A1 in a subset of ovarian tumors with worse outcome. Gynecol Oncol 141, 348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhan YY, He JP, Chen HZ, Wang WJ, and Cai JC (2013) Orphan receptor TR3 is essential for the maintenance of stem-like properties in gastric cancer cells. Cancer Lett 329, 37–44 [DOI] [PubMed] [Google Scholar]
- 68.Sun L, Zhou R, Dong J, Liu S, Jiao Y, Wang L, Hu S, He P, Liu X, Zhao X, Jiang G, and Zhao Y. (2019) Lnc-NA inhibits proliferation and metastasis in endometrioid endometrial carcinoma through regulation of NR4A1. J Cell Mol Med 23, 4699–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu H, Lin Y, Li W, Sun Z, Gao W, Zhang H, Xie L, Jiang F, Qin B, Yan T, Chen L, Zhao Y, Cao X, Wu Y, Lin B, Zhou H, Wong AS, Zhang XK, and Zeng JZ (2011) Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells. FASEB J 25, 192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu H, Bi J, Peng Y, Huo L, Yu X, Yang Z, Zhou Y, Qin L, Xu Y, Liao L, Xie Y, Conneely OM, Jonkers J, and Xu J. (2017) Nuclear receptor NR4A1 is a tumor suppressor down-regulated in triple-negative breast cancer. Oncotarget 8, 54364–54377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wohlkoenig C, Leithner K, Olschewski A, Olschewski H, and Hrzenjak A. (2017) TR3 is involved in hypoxia-induced apoptosis resistance in lung cancer cells downstream of HIF-1alpha. Lung Cancer 111, 15–22 [DOI] [PubMed] [Google Scholar]
- 72.Wilson SR, Joshi AD, and Elferink CJ (2013) The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J Pharmacol Exp Ther 345, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bras A, Albar JP, Leonardo E, de Buitrago GG, and Martinez AC (2000) Ceramide-induced cell death is independent of the Fas/Fas ligand pathway and is prevented by Nur77 overexpression in A20 B cells. Cell Death Differ 7, 262–271 [DOI] [PubMed] [Google Scholar]
- 74.Li QX, Ke N, Sundaram R, and Wong-Staal F. (2006) NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol 21, 533–540 [DOI] [PubMed] [Google Scholar]
- 75.Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, and Zhang XK (2003) Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol 23, 8651–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ke N, Claassen G, Yu DH, Albers A, Fan W, Tan P, Grifman M, Hu X, Defife K, Nguy V, Meyhack B, Brachat A, Wong-Staal F, and Li QX (2004) Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res 64, 8208–8212 [DOI] [PubMed] [Google Scholar]
- 77.Hedrick E, Lee SO, Doddapaneni R, Singh M, and Safe S. (2015) Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocr Relat Cancer 22, 831–840 [DOI] [PubMed] [Google Scholar]
- 78.Mohankumar K, Li X, Sridharan S, Karki K, and Safe S. (2019) Nuclear receptor 4A1 (NR4A1) antagonists induce ROS-dependent inhibition of mTOR signaling in endometrial cancer. Gynecol Oncol 154, 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hedrick E, Lee SO, Kim G, Abdelrahim M, Jin UH, Safe S, and Abudayyeh A. (2015) Nuclear Receptor 4A1 (NR4A1) as a Drug Target for Renal Cell Adenocarcinoma. PLoS One 10, e0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedrick E, and Safe S. (2017) Transforming Growth Factor beta/NR4A1-Inducible Breast Cancer Cell Migration and Epithelial-to-Mesenchymal Transition Is p38alpha (Mitogen-Activated Protein Kinase 14) Dependent. Mol Cell Biol 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hedrick E, Mohankumar K, and Safe S. (2018) TGFbeta-Induced Lung Cancer Cell Migration Is NR4A1-Dependent. Molecular cancer research : MCR 16, 1991–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohankumar K, Li X, Sung N, Cho YJ, Han SJ, and Safe S. (2020) Bis-Indole-Derived Nuclear Receptor 4A1 (NR4A1, Nur77) Ligands as Inhibitors of Endometriosis. Endocrinology 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun L, Liu M, Sun GC, Yang X, Qian Q, Feng S, Mackey LV, and Coy DH (2016) Notch Signaling Activation in Cervical Cancer Cells Induces Cell Growth Arrest with the Involvement of the Nuclear Receptor NR4A2. J Cancer 7, 1388–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Komiya T, Coxon A, Park Y, Chen WD, Zajac-Kaye M, Meltzer P, Karpova T, and Kaye FJ (2010) Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene 29, 1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, and Tai HH (2009) Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis 30, 1606–1613 [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Yang J, Zou Y, Huang GL, and He ZW (2013) Orphan nuclear receptor nurr1 as a potential novel marker for progression in human prostate cancer. Asian Pac J Cancer Prev 14, 2023–2028 [DOI] [PubMed] [Google Scholar]
- 87.Llopis S, Singleton B, Duplessis T, Carrier L, Rowan B, and Williams C. (2013) Dichotomous roles for the orphan nuclear receptor NURR1 in breast cancer. BMC Cancer 13, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han Y, Cai H, Ma L, Ding Y, Tan X, Chang W, Guan W, Liu Y, Shen Q, Yu Y, Zhang H, and Cao G. (2013) Expression of orphan nuclear receptor NR4A2 in gastric cancer cells confers chemoresistance and predicts an unfavorable postoperative survival of gastric cancer patients with chemotherapy. Cancer 119, 3436–3445 [DOI] [PubMed] [Google Scholar]
- 89.Zhu B, Sun L, Luo W, Li M, Coy DH, Yu L, and Yu W. (2017) Activated Notch signaling augments cell growth in hepatocellular carcinoma via up-regulating the nuclear receptor NR4A2. Oncotarget 8, 23289–23302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inamoto T, Papineni S, Chintharlapalli S, Cho SD, Safe S, and Kamat AM (2008) 1,1-Bis(3’-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol Cancer Ther 7, 3825–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han Y, Cai H, Ma L, Ding Y, Tan X, Liu Y, Su T, Yu Y, Chang W, Zhang H, Fu C, and Cao G. (2013) Nuclear orphan receptor NR4A2 confers chemoresistance and predicts unfavorable prognosis of colorectal carcinoma patients who received postoperative chemotherapy. Eur J Cancer 49, 3420–3430 [DOI] [PubMed] [Google Scholar]
- 92.Han YF, and Cao GW (2012) Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol 18, 6865–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beard JA, Tenga A, and Chen T. (2015) The interplay of NR4A receptors and the oncogene-tumor suppressor networks in cancer. Cell Signal 27, 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karki K, Li X, Jin UH, Mohankumar K, Zarei M, Michelhaugh SK, Mittal S, Tjalkens R, and Safe S. (2020) Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas. J Neurooncol 146, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haller F, Bieg M, Will R, Korner C, Weichenhan D, Bott A, Ishaque N, Lutsik P, Moskalev EA, Mueller SK, Bahr M, Woerner A, Kaiser B, Scherl C, Haderlein M, Kleinheinz K, Fietkau R, Iro H, Eils R, Hartmann A, Plass C, Wiemann S, and Agaimy A. (2019) Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun 10, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee DY, Brayer KJ, Mitani Y, Burns EA, Rao PH, Bell D, Williams MD, Ferrarotto R, Pytynia KB, El-Naggar AK, and Ness SA (2020) Oncogenic Orphan Nuclear Receptor NR4A3 Interacts and Cooperates with MYB in Acinic Cell Carcinoma. Cancers (Basel) 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skaugen JM, Seethala RR, Chiosea SI, and Landau MS (2020) Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma. Cancer Cytopathol [DOI] [PubMed] [Google Scholar]
- 98.Nguyen L, Chopra S, Laskar DB, Rao J, Lieu D, Chung F, Kim ED, de Peralta-Venturina M, Bose S, and Balzer B. (2020) NOR-1 distinguishes acinic cell carcinoma from its mimics on fine-needle aspiration biopsy specimens. Hum Pathol 102, 1–6 [DOI] [PubMed] [Google Scholar]
- 99.Wong KS, Marino-Enriquez A, Hornick JL, and Jo VY (2020) NR4A3 Immunohistochemistry Reliably Discriminates Acinic Cell Carcinoma from Mimics. Head Neck Pathol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haller F, Skalova A, Ihrler S, Markl B, Bieg M, Moskalev EA, Erber R, Blank S, Winkelmann C, Hebele S, Baneckova M, Wiemann S, Muller S, Zenk J, Eils R, Iro H, Hartmann A, and Agaimy A. (2019) Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am J Surg Pathol 43, 1264–1272 [DOI] [PubMed] [Google Scholar]
- 101.Haller F, Moskalev EA, Kuck S, Bieg M, Winkelmann C, Muller SK, Ihrler S, Markl B, Eils R, Wiemann S, Iro H, Hartmann A, and Agaimy A. (2020) Nuclear NR4A2 (Nurr1) Immunostaining is a Novel Marker for Acinic Cell Carcinoma of the Salivary Glands Lacking the Classic NR4A3 (NOR-1) Upregulation. Am J Surg Pathol 44, 1290–1292 [DOI] [PubMed] [Google Scholar]
- 102.Fedorova O, Petukhov A, Daks A, Shuvalov O, Leonova T, Vasileva E, Aksenov N, Melino G, and Barlev NA (2019) Orphan receptor NR4A3 is a novel target of p53 that contributes to apoptosis. Oncogene 38, 2108–2122 [DOI] [PubMed] [Google Scholar]
- 103.Nie X, Zhang B, Li X, Xiang J, Xiao B, Ma J, Zhou M, Zhu S, Lu H, Gui R, Shen S, and Li G. (2003) Cloning, expression, and mutation analysis of NOR1, a novel human gene down-regulated in HNE1 nasopharyngeal carcinoma cell line. J Cancer Res Clin Oncol 129, 410–414 [DOI] [PubMed] [Google Scholar]
- 104.Ohkubo T, Ohkura N, Maruyama K, Sasaki K, Nagasaki K, Hanzawa H, Tsukada T, and Yamaguchi K. (2000) Early induction of the orphan nuclear receptor NOR-1 during cell death of the human breast cancer cell line MCF-7. Mol Cell Endocrinol 162, 151–156 [DOI] [PubMed] [Google Scholar]
- 105.Li W, Li X, Wang W, Li X, Tan Y, Yi M, Yang J, McCarthy JB, Xiong W, Wu M, Ma J, Su B, Zhang Z, Liao Q, Xiang B, and Li G. (2011) NOR1 is an HSF1- and NRF1-regulated putative tumor suppressor inactivated by promoter hypermethylation in nasopharyngeal carcinoma. Carcinogenesis 32, 1305–1314 [DOI] [PubMed] [Google Scholar]
- 106.Kurakula K, Koenis DS, van Tiel CM, and de Vries CJ (2014) NR4A nuclear receptors are orphans but not lonesome. Biochim Biophys Acta 1843, 2543–2555 [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, Chen GQ, Shroot B, Mercola D, and Zhang XK (1998) Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol Cell Biol 18, 4719–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holmes WF, Soprano DR, and Soprano KJ (2004) Synthetic retinoids as inducers of apoptosis in ovarian carcinoma cell lines. J Cell Physiol 199, 317–329 [DOI] [PubMed] [Google Scholar]
- 109.Holmes WF, Soprano DR, and Soprano KJ (2003) Early events in the induction of apoptosis in ovarian carcinoma cells by CD437: activation of the p38 MAP kinase signal pathway. Oncogene 22, 6377–6386 [DOI] [PubMed] [Google Scholar]
- 110.Pfahl M, and Piedrafita FJ (2003) Retinoid targets for apoptosis induction. Oncogene 22, 9058–9062 [DOI] [PubMed] [Google Scholar]
- 111.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, and Zhang X. (2000) Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289, 1159–1164 [DOI] [PubMed] [Google Scholar]
- 112.Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, Han YH, Dawson MI, and Zhang XK (2004) Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol 24, 9705–9725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, and Zhang XK (2004) Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116, 527–540 [DOI] [PubMed] [Google Scholar]
- 114.Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, and Su WJ (2002) Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol 8, 446–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chinnaiyan P, Varambally S, Tomlins SA, Ray S, Huang S, Chinnaiyan AM, and Harari PM (2006) Enhancing the antitumor activity of ErbB blockade with histone deacetylase (HDAC) inhibition. Int J Cancer 118, 1041–1050 [DOI] [PubMed] [Google Scholar]
- 116.Jeong JH, Park JS, Moon B, Kim MC, Kim JK, Lee S, Suh H, Kim ND, Kim JM, Park YC, and Yoo YH (2003) Orphan nuclear receptor Nur77 translocates to mitochondria in the early phase of apoptosis induced by synthetic chenodeoxycholic acid derivatives in human stomach cancer cell line SNU-1. Ann N Y Acad Sci 1010, 171–177 [DOI] [PubMed] [Google Scholar]
- 117.Gennari A, Bleumink R, Viviani B, Galli CL, Marinovich M, Pieters R, and Corsini E. (2002) Identification by DNA macroarray of nur77 as a gene induced by di-n-butyltin dichloride: its role in organotin-induced apoptosis. Toxicol Appl Pharmacol 181, 27–31 [DOI] [PubMed] [Google Scholar]
- 118.Shin HJ, Lee BH, Yeo MG, Oh SH, Park JD, Park KK, Chung JH, Moon CK, and Lee MO (2004) Induction of orphan nuclear receptor Nur77 gene expression and its role in cadmium-induced apoptosis in lung. Carcinogenesis 25, 1467–1475 [DOI] [PubMed] [Google Scholar]
- 119.Zhou Y, Zhao W, Xie G, Huang M, Hu M, Jiang X, Zeng D, Liu J, Zhou H, Chen H, Wang GH, and Zhang XK (2014) Induction of Nur77-dependent apoptotic pathway by a coumarin derivative through activation of JNK and p38 MAPK. Carcinogenesis 35, 2660–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen YL, Jian MH, Lin CC, Kang JC, Chen SP, Lin PC, Hung PJ, Chen JR, Chang WL, Lin SZ, and Harn HJ (2008) The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma cell therapy. Mol Pharmacol 74, 1046–1058 [DOI] [PubMed] [Google Scholar]
- 121.Hu Y, Chau T, Liu HX, Liao D, Keane R, Nie Y, Yang H, and Wan YJ (2015) Bile acids regulate nuclear receptor (Nur77) expression and intracellular location to control proliferation and apoptosis. Molecular cancer research : MCR 13, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanchez M, Xia Z, Rico-Bautista E, Cao X, Cuddy M, Castro DJ, Correa RG, Chen L, Yu J, Bobkov A, Ruvolo V, Andreeff M, Oshima RG, Matsuzawa SI, Reed JC, Zhang XK, Hansel D, Wolf DA, and Dawson MI (2018) Oxidized analogs of Di(1H-indol-3-yl)methyl-4-substituted benzenes are NR4A1-dependent UPR inducers with potent and safe anti-cancer activity. Oncotarget 9, 25057–25074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J, Zhou W, Li SS, Sun Z, Lin B, Lang YY, He JY, Cao X, Yan T, Wang L, Lu J, Han YH, Cao Y, Zhang XK, and Zeng JZ (2008) Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res 68, 8871–8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang LF, Lin PC, Ho LI, Liu PY, Wu WC, Chiang IP, Chang HW, Lin SZ, Harn YC, Harn HJ, and Chiou TW (2011) Overexpression of the orphan receptor Nur77 and its translocation induced by PCH4 may inhibit malignant glioma cell growth and induce cell apoptosis. J Surg Oncol 103, 442–450 [DOI] [PubMed] [Google Scholar]
- 125.Godoi PH, Wilkie-Grantham RP, Hishiki A, Sano R, Matsuzawa Y, Yanagi H, Munte CE, Chen Y, Yao Y, Marassi FM, Kalbitzer HR, Matsuzawa S, and Reed JC (2016) Orphan Nuclear Receptor NR4A1 Binds a Novel Protein Interaction Site on Anti-apoptotic B Cell Lymphoma Gene 2 Family Proteins. J Biol Chem 291, 14072–14084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, Town J, Cao X, Lin F, Zhai D, Kitada S, Luciano F, O’Donnell E, Cao Y, He F, Lin J, Reed JC, Satterthwait AC, and Zhang XK (2008) A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell 14, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferlini C, Cicchillitti L, Raspaglio G, Bartollino S, Cimitan S, Bertucci C, Mozzetti S, Gallo D, Persico M, Fattorusso C, Campiani G, and Scambia G. (2009) Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res 69, 6906–6914 [DOI] [PubMed] [Google Scholar]
- 128.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, and Augenlicht LH (2003) TR3/Nur77 in colon cancer cell apoptosis. Cancer Res 63, 5401–5407 [PubMed] [Google Scholar]
- 129.Lee KW, Ma L, Yan X, Liu B, Zhang XK, and Cohen P. (2005) Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J Biol Chem 280, 16942–16948 [DOI] [PubMed] [Google Scholar]
- 130.Lee KW, Cobb LJ, Paharkova-Vatchkova V, Liu B, Milbrandt J, and Cohen P. (2007) Contribution of the orphan nuclear receptor Nur77 to the apoptotic action of IGFBP-3. Carcinogenesis 28, 1653–1658 [DOI] [PubMed] [Google Scholar]
- 131.Zhang L, Liu W, Wang Q, Li Q, Wang H, Wang J, Teng T, Chen M, Ji A, and Li Y. (2018) New Drug Candidate Targeting the 4A1 Orphan Nuclear Receptor for Medullary Thyroid Cancer Therapy. Molecules 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang WJ, Wang Y, Chen HZ, Xing YZ, Li FW, Zhang Q, Zhou B, Zhang HK, Zhang J, Bian XL, Li L, Liu Y, Zhao BX, Chen Y, Wu R, Li AZ, Yao LM, Chen P, Zhang Y, Tian XY, Beermann F, Wu M, Han J, Huang PQ, Lin T, and Wu Q. (2014) Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat Chem Biol 10, 133–140 [DOI] [PubMed] [Google Scholar]
- 133.Hu M, Luo Q, Alitongbieke G, Chong S, Xu C, Xie L, Chen X, Zhang D, Zhou Y, Wang Z, Ye X, Cai L, Zhang F, Chen H, Jiang F, Fang H, Yang S, Liu J, Diaz-Meco MT, Su Y, Zhou H, Moscat J, Lin X, and Zhang XK (2017) Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol Cell 66, 141–153 e146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A, Treilleux I, Lecine P, Borg JP, Mehlen P, Chauvet S, and Mann F. (2013) Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 24, 673–685 [DOI] [PubMed] [Google Scholar]
- 135.Chen HZ, Wen Q, Wang WJ, He JP, and Wu Q. (2013) The orphan nuclear receptor TR3/Nur77 regulates ER stress and induces apoptosis via interaction with TRAPgamma. Int J Biochem Cell Biol 45, 1600–1609 [DOI] [PubMed] [Google Scholar]
- 136.Liu N, Wu Z, Chen A, Chai D, Li L, Zhang L, and Zheng J. (2019) ISG12a and its interaction partner NR4A1 are involved in TRAIL-induced apoptosis in hepatoma cells. J Cell Mol Med 23, 3520–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun Z, Cao X, Jiang MM, Qiu Y, Zhou H, Chen L, Qin B, Wu H, Jiang F, Chen J, Liu J, Dai Y, Chen HF, Hu QY, Wu Z, Zeng JZ, Yao XS, and Zhang XK (2012) Inhibition of beta-catenin signaling by nongenomic action of orphan nuclear receptor Nur77. Oncogene 31, 2653–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zarraga-Granados G, Mucino-Hernandez G, Sanchez-Carbente MR, Villamizar-Galvez W, Penas-Rincon A, Arredondo C, Andres ME, Wood C, Covarrubias L, and Castro-Obregon S. (2020) The nuclear receptor NR4A1 is regulated by SUMO modification to induce autophagic cell death. PLoS One 15, e0222072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Katzenellenbogen JA, O’Malley BW, and Katzenellenbogen BS (1996) Tripartite steroid hormone receptor pharmacology - interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol 10, 119–131 [DOI] [PubMed] [Google Scholar]
- 140.Hedrick E, Li X, Cheng Y, Lacey A, Mohankumar K, Zarei M, and Safe S. (2019) Potent inhibition of breast cancer by bis-indole-derived nuclear receptor 4A1 (NR4A1) antagonists. Breast Cancer Res Treat 177, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hammond SL, Popichak KA, Li X, Hunt LG, Richman EH, Damale PU, Chong EKP, Backos DS, Safe S, and Tjalkens RB (2018) The Nurr1 Ligand,1,1-bis(3’-Indolyl)-1-(p-Chlorophenyl)Methane, Modulates Glial Reactivity and Is Neuroprotective in MPTP-Induced Parkinsonism. J Pharmacol Exp Ther 365, 636–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X, Lee SO, and Safe S. (2012) Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3’-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochem Pharmacol 83, 1445–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li X, Tjalkens RB, Shrestha R, and Safe S. (2019) Structure-dependent activation of gene expression by bis-indole and quinoline-derived activators of nuclear receptor 4A2. Chem Biol Drug Des 94, 1711–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, and Kamat AM (2006) Inhibition of bladder tumor growth by 1,1-bis(3’-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor gamma agonists. Cancer Res 66, 412–418 [DOI] [PubMed] [Google Scholar]
- 145.Holla VR, Mann JR, Shi Q, and DuBois RN (2006) Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem 281, 2676–2682 [DOI] [PubMed] [Google Scholar]
- 146.Zagani R, Hamzaoui N, Cacheux W, de Reynies A, Terris B, Chaussade S, Romagnolo B, Perret C, and Lamarque D. (2009) Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology 137, 1358–1366 e1351–1353 [DOI] [PubMed] [Google Scholar]
- 147.Holla VR, Wu H, Shi Q, Menter DG, and DuBois RN (2011) Nuclear orphan receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. J Biol Chem 286, 30003–30009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng Y, Comaills V, Burr R, Boulay G, Miyamoto DT, Wittner BS, Emmons E, Sil S, Koulopoulos MW, Broderick KT, Tai E, Rengarajan S, Kulkarni AS, Shioda T, Wu CL, Ramaswamy S, Ting DT, Toner M, Rivera MN, Maheswaran S, and Haber DA (2019) COX-2 mediates tumor-stromal prolactin signaling to initiate tumorigenesis. Proc Natl Acad Sci U S A 116, 5223–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kagaya S, Ohkura N, Tsukada T, Miyagawa M, Sugita Y, Tsujimoto G, Matsumoto K, Saito H, and Hashida R. (2005) Prostaglandin A2 acts as a transactivator for NOR1 (NR4A3) within the nuclear receptor superfamily. Biol Pharm Bull 28, 1603–1607 [DOI] [PubMed] [Google Scholar]
- 150.Joubert AM, Panzer A, Bianchi PC, and Lottering ML (2003) The effects of prostaglandin A2 on cell growth, cell cycle status and apoptosis induction in HeLa and MCF-7 cells. Cancer Lett 191, 203–209 [DOI] [PubMed] [Google Scholar]
- 151.Kim CH, Han BS, Moon J, Kim DJ, Shin J, Rajan S, Nguyen QT, Sohn M, Kim WG, Han M, Jeong I, Kim KS, Lee EH, Tu Y, Naffin-Olivos JL, Park CH, Ringe D, Yoon HS, Petsko GA, and Kim KS (2015) Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc Natl Acad Sci U S A 112, 8756–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Miranda BR, Popichak KA, Hammond SL, Jorgensen BA, Phillips AT, Safe S, and Tjalkens RB (2015) The Nurr1 Activator 1,1-Bis(3’-Indolyl)-1-(p-Chlorophenyl)Methane Blocks Inflammatory Gene Expression in BV-2 Microglial Cells by Inhibiting Nuclear Factor kappaB. Mol Pharmacol 87, 1021–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De Miranda BR, Popichak KA, Hammond SL, Miller JA, Safe S, and Tjalkens RB (2015) Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicol Sci 143, 360–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chatterjee S, Walsh EN, Yan AL, Giese KP, Safe S, and Abel T. (2020) Pharmacological activation of Nr4a rescues age-associated memory decline. Neurobiol Aging 85, 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bridi MS, Hawk JD, Chatterjee S, Safe S, and Abel T. (2017) Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner. Neuropsychopharmacology 42, 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He YW (2002) Orphan nuclear receptors in T lymphocyte development. J Leukoc Biol 72, 440–446 [PubMed] [Google Scholar]
- 157.Ando M, Ito M, Srirat T, Kondo T, and Yoshimura A. (2020) Memory T cell, exhaustion, and tumor immunity. Immunol Med 43, 1–9 [DOI] [PubMed] [Google Scholar]
- 158.Sekiya T, Kashiwagi I, Inoue N, Morita R, Hori S, Waldmann H, Rudensky AY, Ichinose H, Metzger D, Chambon P, and Yoshimura A. (2011) The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat Commun 2, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen J, Lopez-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, and Rao A. (2019) NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, Hao J, Alekseev A, Khong H, Chen T, Huang R, Wu J, Zhao Q, Wu Q, Xu S, Wang X, Jin W, Yu S, Wang Y, Wei L, Wang A, Zhong B, Ni L, Liu X, Nurieva R, Ye L, Tian Q, Bian XW, and Dong C. (2019) Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 567, 525–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hibino S, Chikuma S, Kondo T, Ito M, Nakatsukasa H, Omata-Mise S, and Yoshimura A. (2018) Inhibition of Nr4a Receptors Enhances Antitumor Immunity by Breaking Treg-Mediated Immune Tolerance. Cancer Res 78, 3027–3040 [DOI] [PubMed] [Google Scholar]
- 162.Karki K, Wright GA, Mohankumar K, Jin UH, Zhang XH, and Safe S. (2020) A Bis-Indole-Derived NR4A1 Antagonist Induces PD-L1 Degradation and Enhances Antitumor Immunity. Cancer Res 80, 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]


