Abstract
POLA1 encodes the catalytic unit of DNA polymerase α, which together with the Primase complex, launches the DNA replication process. While complete deficiency of this essential gene is presumed to be lethal, at least two conditions due to partial POLA1 deficiency have been described. The first genetic syndrome to be mapped to POLA1 was X-linked reticulate pigmentary disorder (XLPDR, MIM #301220), a rare syndrome characterized by skin hyperpigmentation, sterile multiorgan inflammation, recurrent infections, and distinct facial features. XLPDR has been shown to be accompanied by profound activation of type I interferon signaling, but unlike other interferonopathies, is not associated with autoantibodies or classical autoimmunity. Rather, it is accompanied by marked Natural Killer (NK) cell dysfunction, which may explain the recurrent infections seen in this syndrome. To date, all XLPDR cases are caused by the same recurrent intronic mutation, which results in gene missplicing. Several hypomorphic mutations in POLA1, distinct from the XLPDR intronic mutation, have been recently reported and these mutations associate with a separate condition, van Esch-O’Driscoll syndrome (VEODS, MIM #301030). This condition results in growth retardation, microcephaly, hypogonadism, and in some cases, overlapping immunological features to those seen in XLPDR. This review summarizes our current understanding of the clinical manifestations of POLA1 gene mutations with an emphasis on its immunological consequences, as well as recent advances in understanding of its pathophysiologic basis and potential therapeutic options.
INTRODUCTION
Innate immunity relies on the ability of the host to detect unique molecular patterns displayed by potential pathogens. Viruses and their distinctive replication cycles generate a variety of aberrant nucleic acids recognized by innate immune sensors (1). Signaling events downstream of these sensors result in the activation of key kinases, TBK1/IKKε and IKK1/IKK2, which in turn activate transcription factors from the Interferon Regulatory transcription Factor (IRF) and Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) families. Ultimately, this cascade culminates in the expression of a variety of pro-inflammatory factors, including type I interferon (IFN-I), a group of closely related cytokines that promote antiviral innate immunity (1, 2). IFN-I acts both in an autocrine and paracrine manner through an interferon-α receptor complex (IFNAR1/2), which engages downstream signaling kinases TYK2 and JAK1, and the STAT1 and STAT2 transcription factors. Activated STAT1 and STAT2, acting together with IRFs, lead to further expression of a set of Interferon-Stimulated Genes (ISGs) to restrict virus infection (2). In addition, to avoid exaggerated responses, several negative regulators of IFN-I signaling, such as USP18 and ISG15 genes, are also induced (3).
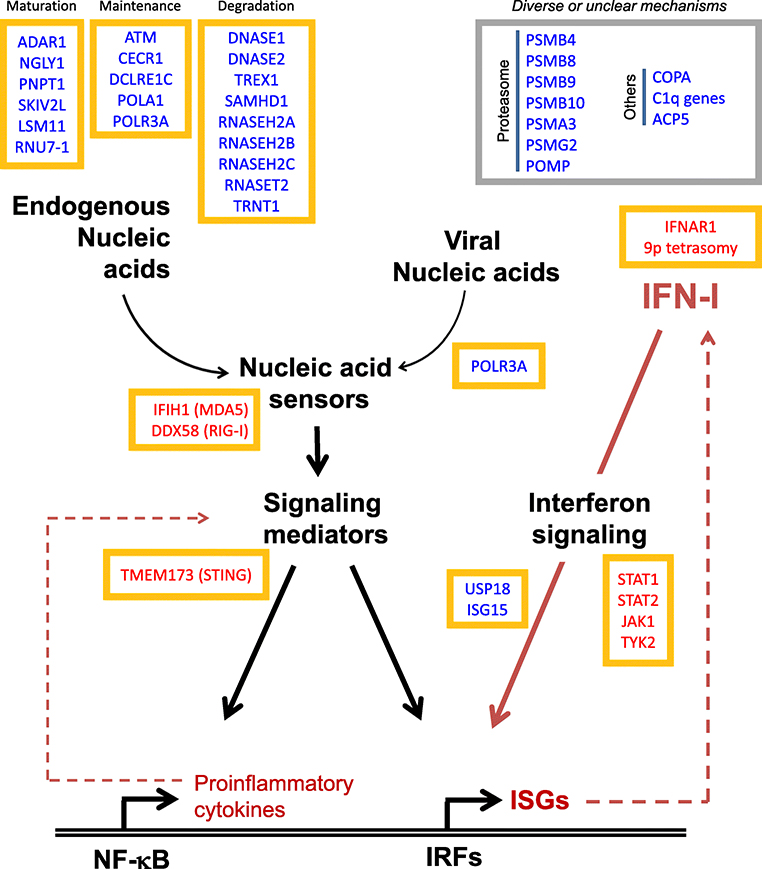
Mutations that disable or impair virus-sensing mechanisms can lead to immunodeficiency or autoinflammation in humans (2, 3). Conversely, mutations in genes involved in other aspects of nucleic acid metabolism can also lead to accumulation of immunologically active aberrant nucleic acid, which are similarly recognized by nucleic acid sensors resulting in sterile IFN-I production (Figure 1) (4, 5). The resulting diseases are characterized by myriad autoinflammatory manifestations and are referred to as type I interferonopathies (2, 3, 6). In some instances, interferonopathy is also associated with mutations impairing the protein-degradation system, specifically mutations in genes encoding proteasome subunits, in what has been termed proteasome-associated autoinflammatory syndromes (7).
Figure 1:
Schematic representation of the basic architecture of antiviral innate immune response. Many of the genes implicated in type I interferonopathies are depicted, with gain-of-function and loss-of-function mutations denoted with red and blue font color, respectively. Genes are grouped into functional clusters based on their role in interferon signaling.
The notion that Mendelian disorders characterized by autoinflammatory manifestations could be grouped under the category of “type I interferonopathies” was first proposed in 2011 (6). The prototypical disorder in this group, Aicardi-Goutières syndrome (AGS), is caused by mutations in a subset of genes involved in nucleic acid processing (ADAR1, LSM11, RNU7–1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, TREX1) or sensing (MDA5) (8–14). Irrespective of the underlying mutation, AGS is accompanied by profound IFN-I activation (in both circulating white cells and patient-derived cells in culture) and results in a clinical picture that resembles intrauterine viral infections with brain inflammation and calcifications as prominent manifestations. At this point, mutations in over 30 genes have been associated with immune dysregulation and increased activation of IFN-I signaling (Figure 1) (2, 3). A large number of these genes are involved in nucleic acid metabolism, others are innate nucleic acid sensors or their downstream signaling intermediaries, and others are classical components in interferon signaling downstream of its surface receptors (STATs, ISG15, USP18, IFNAR2) (2, 6, 8–13, 15–30). A compelling argument for IFN-I as a central event in AGS pathogenesis is the clinical improvement observed from blocking IFN-stimulated signaling with JAK inhibitors, as recently reported in several clinical trials (29–32).
Recent studies indicate that mutations in POLA1, encoding the catalytic subunit of DNA polymerase-α (33, 34), result in various clinical manifestations that include type-I interferon activation and immunodeficiency (2, 3, 24, 35, 36). The principal syndrome, X-linked reticulate pigmentary disorder (XLPDR, MIM #301220), is the result of a recurrent intronic mutation in POLA1 (35), while mutations elsewhere in this gene lead to a variety of developmental alterations, at times accompanied by immune dysregulation (37). In this review, we summarize our current knowledge of the clinical manifestations of POLA1 mutations, as well as the underlying pathophysiologic mechanisms that link this gene to human disease.
CLINICAL FEATURES
X-linked pigmentary reticulate disorder (XLPDR)
XLPDR was first described in 1981 by Partington in a large family in Ontario, Canada (38, 39). The disorder is characterized by facial and ectodermal developmental features, skin manifestations and immune dysregulation (35, 36, 38–43). Its cause is a recurrent unique intronic mutation in POLA1, which has been found in all patients with classical manifestations of XLPDR. To date, less than 50 cases have been reported, but it is likely that this is a gross underestimation resulting from limited awareness about this condition among clinicians.
The full spectrum of XLPDR occurs exclusively in males (Table), whereas female carriers only develop patchy skin involvement (35, 44, 45). Typically, the disorder is evident within the first 6 months of life. Many reported patients had low birth weights (35, 43, 44), although this growth delay does not persist postnatally, as most patients achieve normal overall height as adults. During early infancy, the disease is dominated by recurrent or persistent diarrhea, recurrent lung infections, feeding difficulty (at times requiring gastrostomy tube insertion) and poor growth. As such, most patients experience failure to thrive during infancy and undergo extensive diagnostic testing to try to identify an underlying cause (35, 38, 40). Sometimes, due to the history of recurrent pulmonary infections, patients are misdiagnosed as having cystic fibrosis (35, 44).
TABLE.
Clinical Manifestations of XLPDR
| Organ system | Manifestations | Pathologic findings | |
|---|---|---|---|
| Universal | Common | ||
| Skin and appendices | Males: Diffuse reticular hyperpigmentation Females: Blaschko’s line hyperpigmentation |
Hypohidrosis Poor thermoregulation Facial telangiectasias |
Melanophages in the dermis layer Amyloid deposition Basket-weave stratum corneum Hyperpigmentation of the basal layer |
| Facial and Ectodermal Developmental features | Low upswept hairline Coarse, unruly hair Broad upswept eyebrows |
Toe abnormalities Digital clubbing Oral/dental anomalies |
|
| Lungs | Recurrent bacterial infections Bronchiectasis |
Chronic lung disease (obstructive and restrictive features) | |
| Gastrointestinal | Recurrent diarrhea (at times bloody) in infancy Colitis Jejunal inflammation |
Crypt abscesses, architectural distortion of the mucosa Increased intraepithelial apoptosis and eosinophilic inflammation Mucosal ulceration, focal epithelial regenerations and areas of early granulation tissue. |
|
| Kidney | Urethral strictures Hematuria Renal abscess |
||
| Ophthalmology | Keratitis Corneal dyskeratosis Blindness |
Cornea: dense accumulations of eosinophilic lamellar material between the epithelium and Bowman’s membrane with focal nodular thickenings. | |
| Infectious phenotype | Recurrent bacterial pneumonia | Recurrent otitis media Recurrent sinusitis |
|
Cutaneous manifestations tend to develop after infancy and during in the first few years of life (38, 39, 44). Skin lesions begin to appear between 4 months and 5 years of age and are asymptomatic in nature. The lesions may start as hyperpigmented macules or patches and adopt a reticular pattern with time. Typically, they appear first in the neck, lateral surfaces of the face, axillae, dorsal surfaces of the hands, forearms, elbows, chest, and lower extremities including the knees. In addition, the lesions are less pronounced in the central part of the face and back, and spare mucous membranes (44). When biopsied, the skin demonstrates accumulation of melanophages, dermal histiocytes containing melanin granules through to represent phagocytic content. In many patients, the dermis also displays accumulation of amyloid material. Female carriers of the mutation do not develop systemic manifestations of XLPDR and only suffer from limited hyperpigmentation along Blaschko’s lines, regions of the skin with clonal X chromosome inactivation in keratinocytes. The hyperpigmented lesions are thought to represent regions of the skin where the normal allele has been silenced (45). As in males, these hyperpigmented areas appear during childhood and tend to fade away with age. Skewed X-chromosome inactivation has been reported in circulating leukocytes, possibly explaining the lack of systemic manifestations (46).
During early childhood most patients are also noted to produce minimal or no sweat (hypohidrosis) (35, 44). Impaired thermoregulation and hyperthermia can occur if sweating is severely impaired, and many patients avoid sun exposure or require cold baths to avoid hyperthermia. Moreover, attempts at sweat chloride testing to exclude cystic fibrosis often fail due to difficulties in sample collection, leading to diagnostic ambiguity that persists until other disease manifestations become evident and the correct diagnosis is reached (44). The lack of sweating is thought to represent impaired development of sweat glands, as seen in ectodermal dysplasia. Dental anomalies, which can be part of the spectrum of ectodermal dysplasia, are also seen in some patients (35, 40, 44). However, other manifestations of ectodermal dysplasia, such as loss of hair follicles or even patches of complete loss of epidermis, are not seen in XLPDR patients. This stands in contrast to loss-of-function mutations in the NF-κB pathway, such as mutations of IKBKG, which also lead to immunodeficiency and ectodermal dysplasia that can include more profound manifestations (47).
As XLPDR patients become older, the unique facies that characterizes this syndrome becomes quite evident. Patients have a low and upswept frontal hairline, along with coarse upswept eyebrows and unruly hair (40). Combined with hyperpigmentation and hypohidrosis, this physical appearance is nearly universal in XLPDR and is quite pathognomonic when the clinician is aware of this condition.
Recurrent pulmonary infections occur in more than 90% of XLPDR patients, leading to bronchiectasis and chronic pulmonary disease (35, 39–41). Identified organisms include Streptococcus pneumonia, Haemophilus influenza, Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, Mycobacterium avium and Candida spp. Severe infections resulting in mechanical ventilation, sepsis and even death can occur. In fact, the original report of the large Ontario family described by Partington included several young males who died of severe pneumonia in early life (38). Many patients report a history consistent with viral upper respiratory infections that are complicated by bacterial superinfection. However, viral pathogens in XLPDR patient population are poorly documented, most likely as a result of limited testing. The degree to which immune defects or chronic structural bronchial alterations contribute to the occurrence of more unusual pathogens, such as Pseudomonas or atypical Mycobacteria, is not known. Advanced chronic lung disease can occur, and we are aware of two patients who died from severe lung infections while waiting for lung transplantation.
Upper respiratory infections (sinusitis, otitis) are common but other invasive infections (meningitis, osteomyelitis, urinary infections, or skin infections) are not. Sepsis, when it occurs, is within the context of severe pulmonary infections. Severe, disseminated or uncommon viral infections, such as disseminated herpes virus infections, are not characteristic of this syndrome either (35).
Gastrointestinal involvement is common, with infantile bloody diarrhea frequently reported (32, 35, 38, 44). In most instances, this subsides spontaneously later in childhood. In a few instances when this has been endoscopically evaluated, patients have had severe colitis with histologic features consistent with ulcerative colitis but presenting in infancy and one case requiring colectomy has been reported (38, 39). A recent XLPDR case report involved a patient with a picture resembling pediatric-onset ulcerative colitis with eosinophil-rich inflammatory infiltrates in the colonic mucosa (32). Another case of persistent jejunal inflammation and protein-losing enteropathy has been reported in an adult with XLPDR (48).
The eyes are usually affected as well. Patients develop inflammation of the cornea, which can lead to corneal scarring and blindness (43). Interestingly, other inflammatory eye disorders have not been reported. Patients typically present with severe photophobia and visual impairment. As the eye involvement progresses, some have undergone corneal transplantation but recurrent inflammation affecting the transplanted cornea has been observed, resulting in blindness (40, 43). Patients may also develop urethral inflammation resulting in scarring and stricture formation. This can present with gross hematuria and voiding difficulty, and may need to be treated with recurrent dilations (35, 40).
Van Esch-O’Driscoll syndrome (VEODS)
A series of patients with an assortment mutations in coding and splice sites or POLA1, different from the XLPDR-causing mutation, develop a phenotype distinct from XLPDR (37). The common features of these patients are severe intrauterine growth delay, short stature, and various degrees of developmental delay, including microcephaly. Several patients also had evidence of hypogonadism. The skin and facial features of XLPDR are absent in these patients and only one of the patients was noted to have recurrent severe infections (pulmonary and others). Eventually, all new cases have been grouped into a new syndrome (VEODS, MIM #301030), within the larger group of X-linked intellectual disability (37).
GENETICS
X-linked pigmentary reticulate disorder
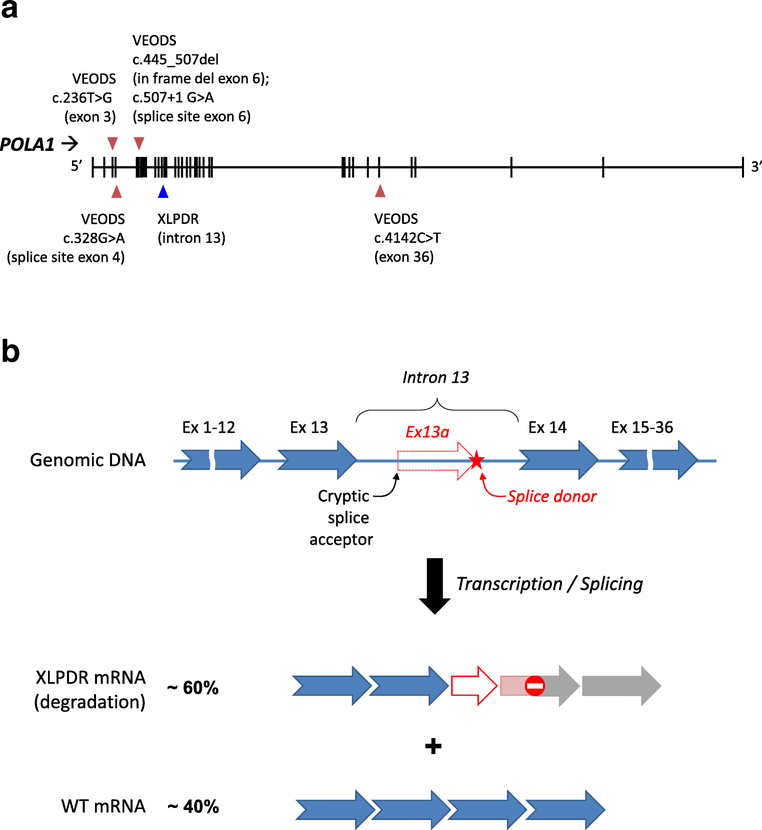
While prior linkage analyses identified a 4.9 Mb interval of chromosome X as the location of the causative mutation (42, 49), various exome sequencing approaches initially failed to identify a causal genetic lesion in the linked interval. Thus, to uncover the causative mutation, whole genome sequencing of four unrelated XLPDR was performed. All XLPDR probands shared the same unique intronic variant mapping to intron 13 of POLA1, (NM_016937.3:c.1375–354A>G) (35). The variant was absent from all known genomic databases and direct genotyping also failed to identify this variant among 1,133 genomes from an ethnically diverse cohort. By contrast, more than 20 unrelated XLPDR probands harbored the NM_016937.3:c.1375–354A>G variant, which segregated with XLPDR or carrier status among tested families (35).
The XLDPR-causing mutation creates a new splice donor site in intron 13, which is located 76 nucleotides downstream of a cryptic splice acceptor sequence in the same intron. This leads to partial missplicing of POLA1 pre-mRNA, introducing a novel exon 13a into a proportion of POLA1 transcripts (Figure 2A and B) (35). Normally spliced POLA1 transcripts are reduced by ~ 40–60% (as is the full-length protein), while the mis-spliced transcript containing exon 13a is expressed at very low levels, probably owing to inherent instability of this transcript. Although the aberrant exon 13a introduces a frameshift mutation, there is no evidence that a truncated protein is expressed in XLPDR-derived cells (35).
Figure 2:
A. Schematic representation of the POLA1 gene and the mutations implicated in VEODS and XLPDR syndromes. B. Model depicting the effect of the XLPDR mutation on POLA1 mRNA splicing. The mutation in intron 13 introduces a splice donor site (red star) resulting in the introduction of an aberrant exon (13a, red arrow), leading to frameshift and early termination.
XLPDR lacks allelic heterogeneity, meaning that the disorder is uniquely associated with the NM_016937.3:c.1375–354A>G intronic variant. Only two cases with XLPDR did not have this mutation, and in both instances the cases had atypical clinical manifestations (35). The reason for this lack of allelic heterogeneity is not completely clear. While this could suggest that the mutant allele behaves as a dominant or gain-of-function allele, cellular phenotypes are completely rescued by increased expression of POLA1 protein, suggesting that the disorder is indeed recessive. It has been speculated that the XLPDR mutation may result in its unique phenotype through differences in the degree of missplicing occurring at the tissue level, or alternatively, that the syndrome arises only within a narrow range of deficiency for this essential gene.
Van Esch-O’Driscoll syndrome (VEODS)
All other POLA1 hypomorphic mutations described to date (6 patients from 5 families with various non-XLPDR mutations) lead to a different clinical phenotype (Figure 2A) (37). A splice site mutation in at least one of the families results in decreased POLA1 protein expression that is greater than that seen in XLPDR. In some other affected families, the mutant protein is expressed at comparable levels but displays evidence of decreased activity. As such, this syndrome may reflect a state of more profound or more widespread POLA1 deficiency, and the growth retardation shared by all the affected patients may be a reflection of this. Furthermore, while severe germline mutations in POLA1 probably result in embryonic lethality, moderate mutations in other genes encoding components of the Pol-α/Primase complex may be expected to result in related phenotypes. Indeed, a recent report highlights that mutations in PRIM1, encoding the catalytic subunit of the Primase complex (50), results in phenotypic similarities. These patients have a complex phenotype that included intrauterine growth delay, short stature, microcephaly, as well as immune dysfunction (recurrent serious pulmonary infections, NK cell cytopenia, and other more severe immune defects).
PATHOPHYSIOLOGY
Type I interferonopathy
Unlike classical autoimmune diseases, XLPDR is not associated with autoantibodies or self-reactive T- or B-cells. To understand the underlying pathophysiology of POLA1 deficiency states, gene expression was profiled in dermal fibroblasts from XLPDR patients (35). The analysis revealed dramatic activation of interferon-stimulated genes (ISGs) in patient-derived cells, which was normalized by restoring normal expression of POLA1. A similar activation of ISG expression was also evident in blood samples from other XLPDR patients. Moreover, at least one of the patients with VEODS also displayed similar over-activation of type I interferon (37).
As the catalytic subunit of DNA Polymerase-α, the canonical activity of POLA1 is to mediate the extension of RNA primers generated by Primase at origins of DNA replication during the S phase (33, 51). This activity was shown to be also important for the generation of RNA:DNA hybrids in the cytosol, such that patient-derived fibroblasts or cells expressing a catalytically inactive form of POLA1 have reduced levels of cytosolic RNA:DNA (35). This material has a dampening effect on spontaneous activation of endogenous nucleic acid sensors, but the specific mechanisms by which endogenous cytosolic RNA:DNA hybrids regulate antiviral signaling remain unclear (35). It is however important to note that mutations in the RNASEH2 subunits, the most common underlying genetic cause for AGS syndrome, result in significant accumulation of genomic RNA:DNA hybrids and interferon activation (5). Despite the fact that POLA1 and RNASEH2 deficiencies mediate paradoxically opposing effects on cellular RNA:DNA content, it is not known that the hybrids in both conditions are comparable in any way.
Immunodeficiency
Unlike most other interferonopathies, XLPDR is associated with recurrent infections, particularly bacterial lung infections. In an effort to explain this disease manifestation, extensive immune phenotyping has been performed. These patients have normal IgG, IgM and IgA levels; several display low or high IgE levels of unclear significance. In the few instances tested, patients were found to mount a normal antibody response after vaccination. In addition, XLPDR is not associated with anti-nuclear or other autoantibodies. Phagocyte numbers (neutrophils and monocytes) as well as oxygen burst activity are also normal (35). In contrast, while most lymphocyte populations in circulation are normal, patients with XLPDR display reduced numbers of Natural Killer (NK) cells. Using a standard cutoff of less than 50 × 103 cells/mL to define severe NK cell lymphopenia (52), XLPDR patients were below this threshold 50% of the time, with mature NK cells (CD3–CD56dim subpopulation) being particularly affected (32, 36).
XLPDR patients display not only numerical NK deficiency, but also an impaired direct cytotoxicity toward cancer cells in vitro (32, 36). In fact, normal NK cells display reduced cytotoxicity upon POLA1 gene silencing, with reduced cytotoxic granule mobilization, but the mechanism that links this gene to the cytotoxic NK cell response remains unexplained (36). Despite their low number, the proliferative capacity of XLPDR-derived NK cells was normal in vitro; similarly, the rates of apoptotic and necrotic cells during NK cell proliferation did not differ between XLPDR and unaffected individuals. This is in line with prior observations that dermal fibroblasts and lymphocytes derived from XLPDR and VEODS patients also display normal grow and DNA replication rates (35–37). Interestingly, in cells derived from VEODS patients the distance between origins of replication was increased, and under conditions of replication stress these cells demonstrated 2-fold increase in stalled replication forks and lower replication pace (37).
NK cell development can be affected in many immunodeficiency syndromes, but few genetic causes of pure NK cell deficiency have been identified to date. These include autosomal dominant GATA2 mutations, deficiencies of MCM4, MCM10, GINS1, RTEL1, and IRF8, as well as the mutations in CD16 that leads to a functional NK cell deficiency (53, 54). MCM4, MCM10, and GINS1 genes encode component of the helicase complexes functionally linked to DNA polymerase-α during the DNA replication process (51), and fibroblasts from patients with XLPDR or VEODS have reduced MCM4 protein expression (36). Furthermore, MCM4 and GINS1 mutations have clinical overlap with XLPDR and VEODS, and are associated with growth retardation, recurrent infections (including pneumonias, bronchiectasis and respiratory failure), and NK cell deficiency with predominant depletion of mature CD3–CD56dim NK cells (54–57).
While severe NK cell lymphopenia as seen in patients with MCM4 or GINS1 mutations is classically associated with severe herpesvirus infections (53, 54), this has not been reported in XLPDR or VEODS. The most probable explanation is that the degree of NK lymphopenia is not as severe in POLA1 deficiency states compared to MCM4 of GINS1 mutations, where NK cells are nearly completely absent. However, a link between NK cell lymphopenia and lung infections has been noted in MCM4 patients (53, 55), and may also account for more severe lung infections in patients with common variable immunodeficiency (52). Whether the immune deficient state, particularly the NK cell deficiency seen in these syndromes, can promote tumor development, is not known. To date, no excess neoplasia has been reported in XLPDR or VEODS. However, given the very few cases in the world known to have these conditions, we must assume that our understanding of the full spectrum of the clinical phenotype is still incomplete.
Growth delay and endocrinopathies
This is exclusively seen in patients with VEODS, and resembles the phenotype of patients with MCM4 and GINS1 mutations. Although patient-derived cells replicate in culture at a normal rate, it is likely that poor growth is the result of alterations in DNA replication rates and cell proliferation during development. Finally, several of these patients have hypogonadism: the cause of this is at present unknown, but it is of interest that hypoadrenalism (another steroid hormone producing gland) is also seen in patients with MCM4 mutations (37, 55, 56).
PATIENT OUTCOMES AND AVAILABLE TREATMENTS
Most XLPDR patients stabilize with age and have an overall less complicated clinical course after adolescence. Gastrointestinal and urinary tract complications are progressively less active, and the pace of infections tends to decrease. However, those who have severe lung damage remain prone to recurrent pneumonia and may succumb to severe infections. Hypohidrosis is irreversible and remains a problem for life. XLPDR patients have normal fertility and the mutation has been transmitted to their female offspring. On the other hand, the clinical course of VEODS past childhood has yet to be described.
Management of other XLPDR symptoms is largely supportive. Conventional management of recurrent lung infections with antibiotics is essential; many patients receive inhaled prophylactic management akin to cystic fibrosis patients. Urethral strictures are treated with sequential dilations. Eye involvement is progressive, leading to blindness, and recurs after corneal transplantation.
Recently, a number of reports suggest encouraging results with the use of JAK inhibitors baricitinib and ruxolitinib in several distinct type I interferonopathies (29–31, 58–60). In fact, one XLPDR patient with refractory colitis was treated with tofacitinib with positive response of the colitis and no exacerbation of pulmonary infections (32). Other options that may be worth considering in the future are interferon receptor neutralizing antibodies, which are being actively pursued in the treatment of lupus where they show particular promise (61, 62).
A path for definitive treatment for XLPDR is at present unclear, but it is tempting to speculate whether the immunologic disturbance is predominantly driven by the hematopoietic compartment. The clinical course of the eye involvement is consistent with this possibility. If so, the disorder might be amenable to hematopoietic stem cell transplantation and could even be suitable for gene therapy and autologous stem cell transplant. Given the rarity of the disorder, and the present absence of an animal model, these questions remain unanswered.
CONCLUSIONS
Mutations in POLA1 produce at least two distinct clinical syndromes. XLPDR is a complex immunologic disorder, and displays a complete lack of allele heterogeneity, suggesting that the syndrome only develops under the specific effects of the intronic mutation. Whether this is due to a specific gene dose effect or tissue-specific missplicing remains unknown. Given the highly syndromic combination of features for XLPDR, we anticipate that greater awareness of this condition will lead to increased diagnoses of this condition. On the other hand, it appears that more damaging mutations of this gene result in VEODS, which lead to growth delay, intellectual disability and at times may be also accompanied by immune dysfunction. Interestingly, clinical and immunologic similarities connect these presentations to mutations in PRIM1, as well as in other factors present in the replication fork such as MCM4, MCM10, and GINS1. Besides supportive care, JAK inhibitors may emerge as important tools to treat the autoinflammatory manifestations of XLPDR. In the end, the paucity of reported cases to date and the lack of an animal models are current barriers to an improved understanding of these conditions.
Acknowledgments:
EB is supported by the “Pollock Family Center for Research in Inflammatory Bowel Disease” program from University of Texas Southwestern Medical Center.
Footnotes
DECLARATIONS
Conflicts of interest/Competing interests
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol 2015;15(2):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uggenti C, Lepelley A, Crow YJ. Self-Awareness: Nucleic Acid-Driven Inflammation and the Type I Interferonopathies. Annu Rev Immunol 2019;37:247–67. [DOI] [PubMed] [Google Scholar]
- 3.Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J Exp Med 2016;213(12):2527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim YW, Sanz LA, Xu X, Hartono SR, Chedin F. Genome-wide DNA hypomethylation and RNA:DNA hybrid accumulation in Aicardi-Goutieres syndrome. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Annals of the New York Academy of Sciences. 2011;1238:91–8. [DOI] [PubMed] [Google Scholar]
- 7.Brehm A, Kruger E. Dysfunction in protein clearance by the proteasome: impact on autoinflammatory diseases. Semin Immunopathol 2015;37(4):323–33. [DOI] [PubMed] [Google Scholar]
- 8.Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, et al. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet 2012;44(11):1243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, et al. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet 2009;41(7):829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokatayev V, Hasin N, Chon H, Cerritelli SM, Sakhuja K, Ward JM, et al. RNase H2 catalytic core Aicardi-Goutieres syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J Exp Med 2016;213(3):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kameli R, Amanat M, Rezaei Z, Hosseionpour S, Nikbakht S, Alizadeh H, et al. RNASET2-deficient leukoencephalopathy mimicking congenital CMV infection and Aicardi-Goutieres syndrome: a case report with a novel pathogenic variant. Orphanet J Rare Dis 2019;14(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice GI, Rodero MP, Crow YJ. Human disease phenotypes associated with mutations in TREX1. J Clin Immunol 2015;35(3):235–43. [DOI] [PubMed] [Google Scholar]
- 13.Rice GI, Del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 2014;46(5):503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uggenti C, Lepelley A, Depp M, Badrock AP, Rodero MP, El-Daher MT, et al. cGAS-mediated induction of type I interferon due to inborn errors of histone pre-mRNA processing. Nat Genet 2020. [DOI] [PubMed] [Google Scholar]
- 15.Belot A, Wassmer E, Twilt M, Lega JC, Zeef LA, Oojageer A, et al. Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr Rheumatol Online J. 2014;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Bel KL, Ragotte RJ, Saferali A, Lee S, Vercauteren SM, Mostafavi SA, et al. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol 2017;139(6):2016–20 e5. [DOI] [PubMed] [Google Scholar]
- 17.Duncan CJA, Thompson BJ, Chen R, Rice GI, Gothe F, Young DF, et al. Severe type I interferonopathy and unrestrained interferon signaling due to a homozygous germline mutation in STAT2. Sci Immunol 2019;4(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckard SC, Rice GI, Fabre A, Badens C, Gray EE, Hartley JL, et al. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat Immunol 2014;15(9):839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdos M, Jakobicz E, Soltesz B, Toth B, Bata-Csorgo Z, Marodi L. Recurrent, Severe Aphthous Stomatitis and Mucosal Ulcers as Primary Manifestations of a Novel STAT1 Gain-of-Function Mutation. Frontiers in immunology. 2020;11:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira CR, Crow YJ, Gahl WA, Gardner PJ, Goldbach-Mansky R, Hur S, et al. DDX58 and Classic Singleton-Merten Syndrome. J Clin Immunol 2019;39(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannelou A, Wang H, Zhou Q, Park YH, Abu-Asab MS, Ylaya K, et al. Aberrant tRNA processing causes an autoinflammatory syndrome responsive to TNF inhibitors. Ann Rheum Dis 2018;77(4):612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gul E, Sayar EH, Gungor B, Eroglu FK, Surucu N, Keles S, et al. Type I IFN-related NETosis in ataxia telangiectasia and Artemis deficiency. J Allergy Clin Immunol 2018;142(1):246–57. [DOI] [PubMed] [Google Scholar]
- 23.Meuwissen ME, Schot R, Buta S, Oudesluijs G, Tinschert S, Speer SD, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med 2016;213(7):1163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyts I, Casanova JL. A human inborn error connects the α’s. Nat Immunol 2016;17(5):472–4. [DOI] [PubMed] [Google Scholar]
- 25.Pestal K, Funk CC, Snyder JM, Price ND, Treuting PM, Stetson DB. Isoforms of RNA-Editing Enzyme ADAR1 Independently Control Nucleic Acid Sensor MDA5-Driven Autoimmunity and Multi-organ Development. Immunity. 2015;43(5):933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picard C, Thouvenin G, Kannengiesser C, Dubus JC, Jeremiah N, Rieux-Laucat F, et al. Severe Pulmonary Fibrosis as the First Manifestation of Interferonopathy (TMEM173 Mutation). Chest. 2016;150(3):e65–71. [DOI] [PubMed] [Google Scholar]
- 27.Rodero MP, Tesser A, Bartok E, Rice GI, Della Mina E, Depp M, et al. Type I interferon-mediated autoinflammation due to DNase II deficiency. Nature communications. 2017;8(1):2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sifuentes-Dominguez L, Starokadomskyy P, Welch J, Gurram B, Park JY, Koduru P, et al. Mosaic Tetrasomy 9p Associated With Inflammatory Bowel Disease. Journal of Crohn’s & colitis. 2019;13(11):1474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meesilpavikkai K, Dik WA, Schrijver B, van Helden-Meeuwsen CG, Versnel MA, van Hagen PM, et al. Efficacy of Baricitinib in the Treatment of Chilblains Associated With Aicardi-Goutieres Syndrome, a Type I Interferonopathy. Arthritis Rheumatol 2019;71(5):829–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLellan KE, Martin N, Davidson JE, Cordeiro N, Oates BD, Neven B, et al. JAK 1/2 Blockade in MDA5 Gain-of-Function. J Clin Immunol 2018;38(8):844–6. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez GAM, Reinhardt A, Ramsey S, Wittkowski H, Hashkes PJ, Berkun Y, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018;128(7):3041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legeret C, Meyer BJ, Rovina A, Deigendesch N, Berger CT, Daikeler T, et al. JAK inhibition in a patient with X-linked reticulate pigmentary disorder. Journal of Clinical Immunology. 2020;epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranovskiy AG, Tahirov TH. Elaborated Action of the Human Primosome. Genes (Basel). 2017;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suwa Y, Gu J, Baranovskiy AG, Babayeva ND, Pavlov YI, Tahirov TH. Crystal Structure of the Human Pol alpha B Subunit in Complex with the C-terminal Domain of the Catalytic Subunit. J Biol Chem 2015;290(23):14328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starokadomskyy P, Gemelli T, Rios JJ, Xing C, Wang RC, Li H, et al. DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat Immunol 2016;17(5):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starokadomskyy P, Wilton KM, Krzewski K, Lopez A, Sifuentes-Dominguez L, Overlee B, et al. NK cell defects in X-linked pigmentary reticulate disorder. JCI Insight. 2019;4(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Esch H, Colnaghi R, Freson K, Starokadomskyy P, Zankl A, Backx L, et al. Defective DNA Polymerase alpha-Primase Leads to X-Linked Intellectual Disability Associated with Severe Growth Retardation, Microcephaly, and Hypogonadism. Am J Hum Genet 2019;104(5):957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Partington MW, Marriott PJ, Prentice RS, Cavaglia A, Simpson NE. Familial cutaneous amyloidosis with systemic manifestations in males. Am J Med Genet 1981;10(1):65–75. [DOI] [PubMed] [Google Scholar]
- 39.Partington MW, Prentice RS. X-linked cutaneous amyloidosis: further clinical and pathological observations. Am J Med Genet 1989;32(1):115–9. [DOI] [PubMed] [Google Scholar]
- 40.Anderson RC, Zinn AR, Kim J, Carder KR. X-linked reticulate pigmentary disorder with systemic manifestations: report of a third family and literature review. Pediatr Dermatol 2005;22(2):122–6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Guarino M, Torrelo A, Fernandez-Lorente M, Fraile G, Garcia-Sagredo JM, Jaen P. X-linked reticulate pigmentary disorder: report of a new family. European journal of dermatology : EJD. 2008;18(1):102–3. [DOI] [PubMed] [Google Scholar]
- 42.Jaeckle Santos LJ, Xing C, Barnes RB, Ades LC, Megarbane A, Vidal C, et al. Refined mapping of X-linked reticulate pigmentary disorder and sequencing of candidate genes. Hum Genet 2008;123(5):469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pezzani L, Brena M, Callea M, Colombi M, Tadini G. X-linked reticulate pigmentary disorder with systemic manifestations: a new family and review of the literature. Am J Med Genet A. 2013;161(6):1414–20. [DOI] [PubMed] [Google Scholar]
- 44.Starokadomskyy P, Sifuentes-Dominguez L, Gemelli T, Zinn AR, Dossi MT, Mellado C, et al. Evolution of the skin manifestations of X-linked pigmentary reticulate disorder. The British journal of dermatology. 2017;177(5):e200–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim B, Seo S, M. K. X-Linked reticulate pigmentary disorder in a female patient. The International Journal of Dermatology. 2010;49:421–5. [DOI] [PubMed] [Google Scholar]
- 46.Mégarbané H, Boehm N, Chouery E, Bernard R, Salem N, Halaby E, et al. X-linked reticulate pigmentary layer. Report of a new patient and demonstration of a skewed X-inactivation. Genetic counseling (Geneva, Switzerland). 2005;16(1):85–9. [PubMed] [Google Scholar]
- 47.Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, et al. Genomic rearrangement in NEMO impairs NF-κB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405(6785):466–72. [DOI] [PubMed] [Google Scholar]
- 48.Fraile G, Norman F, Reguero ME, Defargues V, Redondo C. Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) in a man with a diagnosis of X-linked reticulate pigmentary disorder (PDR). Scand J Gastroenterol 2008;43(4):506–10. [DOI] [PubMed] [Google Scholar]
- 49.Gedeon AK, Mulley JC, Kozman H, Donnelly A, Partington MW. Localisation of the gene for X-linked reticulate pigmentary disorder with systemic manifestations (PDR), previously known as X-linked cutaneous amyloidosis. Am J Med Genet 1994;52(1):75–8. [DOI] [PubMed] [Google Scholar]
- 50.Parry DA, Tamayo-Orrego L, Carroll P, Marsh JA, Greene P, Murina O, et al. PRIM1 deficiency causes a distinctive primordial dwarfism syndrome. Genes & Development. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain R, Aggarwal AK, Rechkoblit O. Eukaryotic DNA polymerases. Curr Opin Struct Biol 2018;53:77–87. [DOI] [PubMed] [Google Scholar]
- 52.Ebbo M, Gerard L, Carpentier S, Vely F, Cypowyj S, Farnarier C, et al. Low Circulating Natural Killer Cell Counts are Associated With Severe Disease in Patients With Common Variable Immunodeficiency. EBioMedicine. 2016;6:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol 2013;132(3):515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mace EM, Paust S, Conte MI, Baxley RM, Schmit MM, Patil SL, et al. Human NK cell deficiency as a result of biallelic mutations in MCM10. J Clin Invest 2020;130(10):5272–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest 2012;122(3):814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest 2012;122(3):821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cottineau J, Kottemann MC, Lach FP, Kang YH, Vely F, Deenick EK, et al. Inherited GINS1 deficiency underlies growth retardation along with neutropenia and NK cell deficiency. J Clin Invest 2017;127(5):1991–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briand C, Fremond ML, Bessis D, Carbasse A, Rice GI, Bondet V, et al. Efficacy of JAK1/2 inhibition in the treatment of chilblain lupus due to TREX1 deficiency. Ann Rheum Dis 2019;78(3):431–3. [DOI] [PubMed] [Google Scholar]
- 59.Fremond ML, Rodero MP, Jeremiah N, Belot A, Jeziorski E, Duffy D, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol 2016;138(6):1752–5. [DOI] [PubMed] [Google Scholar]
- 60.Kothur K, Bandodkar S, Chu S, Wienholt L, Johnson A, Barclay P, et al. An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology. 2018;90(6):289–91. [DOI] [PubMed] [Google Scholar]
- 61.Oon S, Wilson NJ, Wicks I. Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clin Transl Immunology. 2016;5(5):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B, Higgs BW, Chang L, Vainshtein I, Liu Z, Streicher K, et al. Pharmacogenomics and translational simulations to bridge indications for an anti-interferon-alpha receptor antibody. Clin Pharmacol Ther 2013;93(6):483–92. [DOI] [PubMed] [Google Scholar]