Abstract
Marine sedimentary ancient DNA (sedaDNA) is increasingly used to study past ocean ecosystems, however, studies have been severely limited by the very low amounts of DNA preserved in the subseafloor, and the lack of bioinformatic tools to authenticate sedaDNA in metagenomic data. We applied a hybridisation capture ‘baits’ technique to target marine eukaryote sedaDNA (specifically, phyto- and zooplankton, ‘Planktonbaits1’; and harmful algal bloom taxa, ‘HABbaits1’), which resulted in up to 4- and 9-fold increases, respectively, in the relative abundance of eukaryotes compared to shotgun sequencing. We further used the bioinformatic tool ‘HOPS’ to authenticate the sedaDNA component, establishing a new proxy to assess sedaDNA authenticity, “% eukaryote sedaDNA damage”, that is positively correlated with subseafloor depth. We used this proxy to report the first-ever DNA damage profiles from a marine phytoplankton species, the ubiquitous coccolithophore Emiliania huxleyi. Our approach opens new avenues for the detailed investigation of long-term change and evolution of marine eukaryotes over geological timescales.
Subject terms: Bioinformatics, Palaeoecology, Microbial biooceanography, Evolutionary ecology, Metagenomics, Eukaryote
Introduction
Over the past decade marine sedimentary ancient DNA (sedaDNA) has become increasingly used to study past ocean ecosystems and oceanographic conditions. The novelty of using sedaDNA lies in its enormous potential to detect genetic signals of taxa that do and don’t fossilise—meaning that, in theory, it is possible to go beyond standard environmental proxies and facilitate reconstruction of past marine ecosystems across the entire food web. For example, sedaDNA has revealed relationships between past marine community composition and paleo-tsunami episodes in Japan over the past 2000 years1, oxygen minimum zone expansions in the temperate Arabian Sea region over 43 thousand years (kyr)2, and Arctic sea-ice conditions spanning 100kyr3. While the logistical challenge of acquiring undisturbed sediment cores from the deep seafloor remains, the field of sedaDNA research is rapidly advancing due to new ship-board core sampling procedures that allow far greater contamination control, and improvements in sample processing, sequencing technologies and bioinformatic tools4.
Among the huge diversity of marine eukaryotes, phytoplankton are particularly useful targets to study past ocean conditions. Phytoplankton are free-floating, unicellular microalgae fulfilling two important functions: (1) they form the base of the marine food web supporting virtually all higher trophic organisms (e.g., 5), and (2) are highly useful environmental indicators due to their sensitivity to changing physical and chemical oceanographic conditions6. After phytoplankton die, they sink to the seafloor where small proportions of their DNA are able to become entombed and preserved in sediments under favorable conditions, over time forming long-term records of past ocean and climate conditions. Using the small subunit ribosomal RNA gene (18S rRNA, a common taxonomic marker gene), we recently determined the fraction of marine eukaryote sedaDNA preserved in Tasmanian coastal sediments to be a mere 1.37% of the total sedaDNA pool7. A slightly higher proportion of eukaryote sedaDNA (and also higher diversity) may be captured by combining multiple taxonomic markers, e.g., the small and large subunit ribosomal RNA gene8. However, rather than analysing only part of the total sedaDNA pool (such as eukaryote marker genes within a large metagenomic dataset), a more cost-effective approach is to increase marine eukaryote sedaDNA yield through optimised extraction and sample processing that maximise sequencing of sedaDNA from the intended target organisms.
Metagenomic approaches extract and analyse the ‘total’ DNA in a sample (‘shotgun’ style), irrespective of the source organism, facilitating recovery of DNA sequences from any organism in proportion to their original presence in that sample. As a result, metagenomic approaches are well suited to the study of microbial and environmental ancient DNA (e.g., 9–11) including sedaDNA. This approach does not prescribe the target DNA fragment size and, importantly also preserves fragment size variability and damage patterns characteristic of ancient DNA that are vital to assess the authenticity of potential ancient genetic signals. However, achieving the sequencing depth required to detected and quantify organisms present at low relative abundance (such as eukaryotes in marine sedaDNA) can be very expensive. Therefore, there is a need for more targeted approaches that allow the detailed investigation of specific organisms, model or non-model, for which reads may otherwise go undetected in shotgun data.
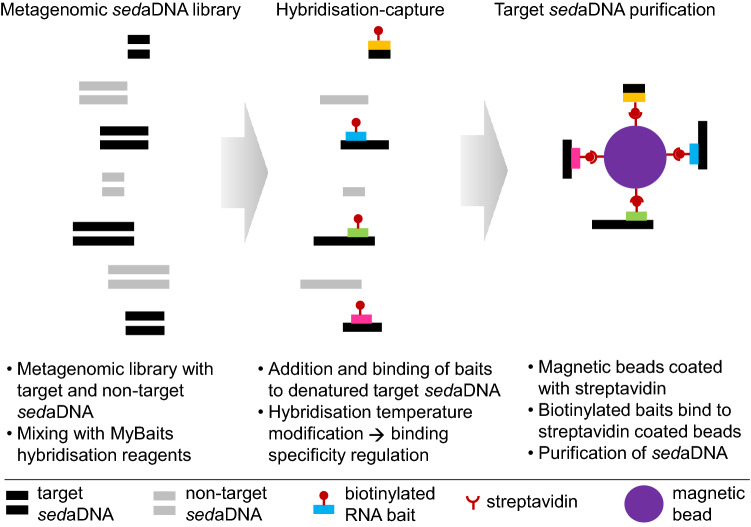
Hybridisation capture techniques are an increasingly popular method to focus the metagenomic analysis towards loci of interest, such as specific sequences to investigate particular groups of organisms12,13. Hybridisation capture uses short RNA probes (also called ‘baits’) designed to be complementary to DNA sequences of interest (e.g., taxonomic marker genes; Fig. 1). By binding to the target sequence, these genetic baits ‘capture’ DNA fragments from DNA extracts in a manner that preserves size variability, along with DNA damage patterns that can be used to examine whether sequences appear ancient. Additionally, careful bait design (i.e., selection of target sequences) and optimisation of the application protocol (e.g., hybridisation-temperature settings) allow differing levels of specificity in the capture process. While such ‘baits’ approaches have previously been used to investigate human, animal and even environmental DNA14–16, their application to marine sediments to capture sedaDNA from key primary producers and environmental indicator organisms (e.g., eukaryotic phytoplankton) remains untested.
Figure 1.
Schematic of hybridisation capture applied to marine sedimentary ancient DNA (sedaDNA). The three main steps are the preparation of a metagenomic sedaDNA library, hybridisation capture using RNA baits (in this study: Planktonbaits1 and HABbaits1) that are biotinylated, which enables binding of baits to streptavidin-coated magnetic beads (multiple baits per bead possible, schematic not to scale). For further technical details see Methods [and 12,17].
The assessment of sedaDNA authenticity has been hindered by a lack of established approaches to identify and analyse DNA damage patterns of rare ancient microorganisms in metagenomic samples (such as eukaryotes in marine sedaDNA). For example, software commonly used to detect DNA damage patterns, such as ‘mapDamage’, computes nucleotide misincorporation and fragmentation patterns by mapping next-generation sequencing reads against a reference genome18,19. This requires high-quality modern reference genomes, or species where ancient DNA is available in sufficient quantity (e.g., animals20 or humans21), but neither is generally possible with marine eukaryote sedaDNA. There is a lack of high-quality reference sequences for the thousands of marine organisms occurring in the global ocean, and the threshold of ~ 250 reads per species required to analyse and plot DNA damage patterns in mapDamage22 is often not reached in sedaDNA. Recently, Hübler et al.23 developed a new bioinformatic tool HOPS—‘Heuristic Operations for Pathogen Screening’—based on the mapDamage algorithm, to identify and authenticate bacterial pathogens in ancient metagenomic samples and extract this information for further downstream analysis. In combination with hybridisation capture to generate a larger number of ancient eukaryote sequences, HOPS has the potential to allow the assessment of sedaDNA authenticity based on DNA damage profiles from key marine eukaryotes, even if only very few sequences are available (≥ 50 reads per species23).
In this work, we develop and apply two hybridisation capture bait sets for the first such analysis of marine sediments, targeting (i) marine phyto- and zooplankton very broadly for general paleoplankton assessment (Planktonbaits1), and selected key phytoplankton groups (especially, diatoms, dinoflagellates and coccolithophores) that are either highly abundant or the cause of harmful algal blooms (HABs) in our study region off the East Australian coast (HABbaits1). Based on samples from two coastal sediment cores collected near Maria Island, Tasmania, we demonstrate: (1) the effectiveness of Planktonbaits1 and HABbaits1 to maximise sedaDNA originating from eukaryote targets relative to shotgun data; (2) the authenticity of both shotgun- and baits-derived sequencing data via HOPS; (3) examine relationships between the ‘ancient’ DNA fraction and subseafloor depth through the development of a new sedaDNA proxy (‘% eukaryote sedaDNA damage’); and (4) generate the first-ever DNA damage profile for a keystone marine phytoplankton, the coccolithophore Emiliania huxleyi.
Methods
Samples
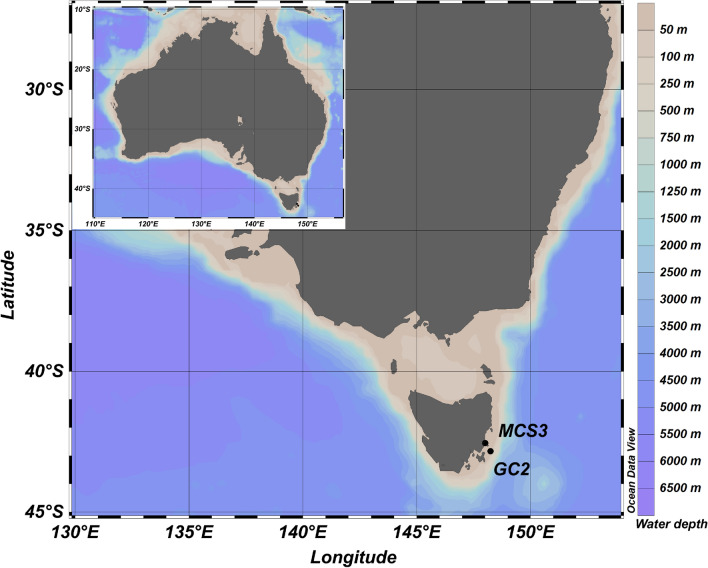
Cores were collected during the RV Investigator voyage IN2018_T02 (19 and 20 May 2018, respectively, Fig. 2) to Tasmania, from sites in the Mercury Passage and Maria Island (Fig. 2). We collected one KC Denmark Multi-Core (MCS3, inner core diameter 10 cm, 36 cm long, estimated to cover the last ~ 145 years based on 210Pb dating at the Australian Nuclear Science and Technology Organisation (ANSTO, Lucas Heights, Sydney) in the Mercury Passage (MP, 42.550 S, 148.014 E; 68 m water depth), and one gravity core (GC2; inner core diameter 10 cm, 3 m long) offshore from Maria Island (42.845 S, 148.240 E; 104 m) composed of 2 sections; GC2A (bottom) and GC2B (top) estimated to cover the last ~ 8950 years based on 210Pb and 14C dating, ANSTO). The untreated cores were immediately sealed with plastic caps and sealed with duct-tape, stored initially on-board at 10 °C, followed by transport to and storage at 4 °C at ANSTO. To minimise contamination during core splitting and subsampling (October, 2018, ANSTO), we wiped working benches, sampling and cutting tools with bleach and 80% EtOH, changed gloves immediately when contaminated with sediment, and wore appropriate PPE at all times (gloves, facemask, hairnet, disposable lab gown). We removed the outer ~ 1 cm of the working core-half (working from bottom to the top of the core), then collected plunge samples by pressing sterile 15 mL centrifuge tubes (Falcon) ~ 2 cm deep into the sediment core centre at 5 cm depth intervals. All sedaDNA samples were immediately frozen at − 20 °C and transported to the Australian Centre for Ancient DNA (ACAD), Adelaide. For this study, a total of 30 samples were selected from both cores, representing ~ 2 cm depth intervals within the upper 36 cm of MCS3 and GC2, and ~ 20 cm depth intervals in GC2 downcore from 36 cm below seafloor (cmbsf).
Figure 2.
Map of coring sites, inshore (MCS3) and offshore (GC2) of Maria Island, Tasmania, South-East Australian Coast. Map created in ODV (Schlitzer, R., Ocean Data View, https://odv.awi.de, 2018).
SedaDNA extractions
We prepared sedaDNA extracts and sequencing libraries at ACAD's ultra-clean ancient (GC2) and forensic (MCS3) facilities following ancient DNA decontamination standards24. All sample tubes were wiped with bleach on the outside prior to entering the laboratory for subsampling. Our extraction method followed the optimised (“combined”) approach outlined in detail previously7, with a minor modification in that we stored the final purified DNA in TLE buffer (50 μL Tris HCL (1 M), 10 μL EDTA (0.5 M), 5 mL nuclease-free water) instead of customary Elution Buffer (Qiagen) (see Supplementary Material Methods). To monitor laboratory contamination, we used extraction blank controls (EBCs) by processing 1–2 (depending on the extraction-batch size) empty bead-tubes through the extraction protocol. A total of 30 extracts were generated from sediment samples and 7 extracts from EBCs.
RNA-baits design
We designed two RNA hybridisation bait-sets, one targeting phyto- and zooplankton for a more detailed overview of plankton diversity (hereafter ‘Planktonbaits1’), and one targeting specific plankton organisms and their predators to enable detailed investigation of HABs, especially those caused by dinoflagellates, in coastal marine ecosystems (hereafter, ‘HABbaits1’). Planktonbaits1 was based on 18S-V9 and 16S-V4 sequences of major phyto- and zooplankton groups, whereas we designed HABbaits1 from a collection of LSU, SSU, D1-D2-LSU, COI, rbcL and ITS sequences for specific marine target organisms often associated with HABs in our study region (Table 1).
Table 1.
Planktonbaits1 and HABbaits1.
| Bait set | Target taxa | Targeted gene/gene region | Database from which sequences were acquired |
|---|---|---|---|
| Planktonbaits1 | Ciliophora, MALV, Dinophyceae, Archaeplastida, Euglenida, Telonemia, Haptophyta, Cryptophyta, Katablepharidophyta, Chlorarachnea, Phaeodarea, Foraminifera, Acantharea, Other_Radiolaria, RAD, Collodaria, MAST, Bicoeca, MOCH, Raphidophyceae, Pinguiophyceae, Phaeophyceae, Chrysophyceae-Synurophyceae, Pelagophyceae, Dictyochophyceae, Bolidophyceae-and-relatives, Bacillariophyta | 18S-V9 | W2_PR2_V925 |
| Trichodesmium erythraeum, Prochlorococcus marinus, Synechococcus sp. | 16S-V4 |
SILVA26 |
|
| HABbaits1 | Dinoflagellates | LSU: SILVA; SSU: PR227 or NCBI (https://www.ncbi.nlm.nih.gov/); D1D2: PHYTOPK28S-D1D228; ITS: BOLD29 (http://www.boldsystems.org/) or NCBI; rbcL: BOLD; COI: BOLD or NCBI | |
| Alexandrium tamarense Group 1 (A. catenella) | LSU, D1D2, ITS, COI | ||
| Alexandrium tamarense Group 2 (A. mediterraneum) | LSU, D1D2, ITS, COI | ||
| Alexandrium tamarense Group 3 (A. tamarense) | LSU, D1D2, ITS, COI | ||
| Alexandrium tamarense Group 4 (A. pacificum) | LSU, D1D2, ITS, COI | ||
| Alexandrium tamarense Group 5 (A. australiense) | LSU, D1D2, ITS, COI | ||
| Gymnodinium catenatum | LSU, D1D2, ITS, COI | ||
| Noctiluca scintillans | LSU, D1D2 | ||
| Tripos (Ceratium) furca | LSU, SSU, D1D2 | ||
| Tripos (Ceratium) fusus | LSU, SSU, D1D2, COI | ||
| Tripos sp. (genus) | SSU | ||
| Tripos muelleri | LSU, SSU | ||
| Diatoms | |||
| Pseudo-nitzschia sp. (genus) | LSU, D1D2, SSU, ITS | ||
| Pseudo-nitzschia cuspidata | LSU, D1D2, ITS, rbcL | ||
| Pseudo-nitzschia pungens | LSU, D1D2, ITS, rbcL | ||
| Haptophytes | |||
| Emiliania huxleyi | LSU, D1D2, rbcL, COI | ||
| Cnidarians | |||
| Aurelia spp. | LSU, D1D2, ITS, COI | ||
| Cyanea spp. | LSU, ITS, COI | ||
| Physalia | LSU, ITS, COI | ||
| Molluscs | |||
| Crassostrea gigas | LSU, D1D2, ITS, COI | ||
| Ostrea angasi | LSU, COI | ||
| Mytilus galloprovincialis | LSU, D1D2, ITS, COI | ||
| Modiolus spp. | LSU, D1D2, ITS, COI | ||
| Genes involved in toxin production | |||
| SxtA | |||
Target taxa of Plankton- and HABbaits1 genes/gene regions and source databases. For HABbaits1, all listed databases were searched for each gene (region) per target taxon, and, if available, the longest sequence was selected and included.
Planktonbaits1
To design Planktonbaits1 we downloaded the W2_V9_PR2 database25 (containing 18S-V9 rDNA and rRNA sequences of marine protists and their predators, downloaded on 30 July 2018), deduplicated using Geneious software (Geneious NZ), and filtered the remaining sequences to keep only those from major phyto- and zooplankton groups (Table 1). In collaboration with Arbor Biosciences, USA, we designed RNA baits based on these 15,035 target sequences by masking any repeating Ns (i.e., any consecutive Ns that were < 10 in a row were converted to Ts, with ultimately 0.1% masked), padding short targets to 84 nucleotides (nt) (i.e., any target less than 84 nt was padded with Ts up to 84 nt in length). This procedure provided 41,798 raw baits of 80 nt with 3 × tiling (creating an even coverage, i.e., one bait every ~ 27 nt). The raw baits were BLASTed against ArborBioscience’s in-house RefSeq database containing 5584 bacterial genome and plasmid sequences (downloaded from NCBI, May, 2018), and any baits leading to hits were removed (except for 785 loci from cyanobacterial taxa that we intended to keep, see below). This filtering step provided 36,836 baits, which were collapsed into 15,942 final baits (i.e., eliminating redundancy based on identity and overlap; using > 83% overlap, and > 95% identity). We added five 16S-V4 rRNA sequences (the prokaryotic equivalent of the small subunit ribosomal rRNA gene) of common marine cyanobacteria (one Trichodesmium erythraeum sequence, and two Prochlorococcus marinus and Synechococcus sp. sequences each), acquired from the SILVA database26; Table 1). To check and ensure target-taxon specificity, these five cyanobacterial sequences were mapped against a non-target sequence (Escherichia coli 16S RefSeq sequence NR_114042.1), then reverse-transcribed to DNA, and BLASTed to the same NCBI RefSeq database described above. BLAST hits of < 60 bp alignment length and < 80% identity were removed, and only those baits with < 50 BLAST hits were kept, resulting in 10 cyanobacterial baits. Consequently, Planktonbaits1 contained a total of 15,952 RNA baits targeting the 18S-V9 region of a broad diversity of phytoplankton and their predators and the 16SV4 region of three cyanobacteria.
HABbaits1
To design HABbaits1 we manually collated a total of 805 LSU, SSU, D1-D2-LSU, COI, rbcL and ITS sequences for specific marine target organisms often associated with harmful algal bloom events in our study region, primarily dinoflagellates but also certain diatoms, a coccolithophore, jelly- and shellfish and the saxitoxin A4 gene, involved in paralytic shellfish toxin production by the dinoflagellates Gymnodinium catenatum and some species of the genus Alexandrium (Table 1). As with Planktonbaits1, we worked in collaboration with Arbor Biosciences, USA, to design RNA baits based on the collated sequences (converting consecutive (< 10) Ns to Ts and RNA sequences to DNA, masking input sequences for simple repeats (0.4%)), attaining 23,064 raw 80 nt baits (using 3 × tiling, as for Planktonbaits1, see section “Planktonbaits1”). Each bait candidate was BLASTed against three target genomes (the oyster Crassostrea gigas, coccolithophore Emiliania huxleyi, mussel Mytilus galloprovincialis), and four non-target genomes (diatoms Fragilariopsis cylindrus, Phaeodactylum tricornutum, dinoflagellate Symbiodinium minutum, diatom Thalassiosira pseudonana, jellyfish Clytia hemisphaerica), and a hybridisation melting temperature (Tm)* was estimated for each hit assuming standard myBaits buffers and conditions (Tm is defined as the temperature at which 50% of molecules are hybridised). For each target bait candidate, one BLAST hit with the highest Tm was first discarded from the results (allowing for 1 hit in the genome), and only the top 500 hits (by bit score) were considered. Based on the distribution of remaining calculated Tm's, we filtered out non-specific baits using stringent (only specific baits pass) criteria (i.e., bait candidates pass if they satisfy one of these conditions: (a) no hits with Tm above 60 °C, (b) ≤ 2 hits 62.5–65 °C, (c) ≤ 10 hits 62.5–65 °C and at least 1 failing flanking bait, (d) ≤ 10 hits 62.5–65 °C, 2 hits 65–67.5 °C, and < 2 passing flanking baits, (e) ≤ 2 hits 62.5–65 °C, 1 hit 65–67.5 °C, 1 hit 70 °C or above, and < 2 passing flanking baits. Bait candidates were removed when a hit was determined after BLASTing them against the non-target genomes. This highly stringent filtering procedure for HABbaits1 was applied to ensure maximum target-specificity of our selected HAB species, and resulted in a total of 15,310 baits for this set.
Library preparations and hybridisation capture
We prepared sequencing libraries from all DNA extracts following previously established protocols11. Briefly, a 20 µL aliquot of DNA was repaired (15 min, 25 °C) in a 40 µL reaction using T4 DNA polymerase (New England Biolabs). After purifying the DNA (MinElute Reaction Cleanup Kit, Qiagen), a ligation step followed (T4 DNA ligase, Fermentas) in which truncated Illumina-adapter sequences containing two unique 5 base-pair (bp) barcodes were attached to the double-stranded DNA30 (60 min, 22 °C). DNA purification (MinElute Reaction Cleanup Kit, Qiagen) was performed, followed by a fill-in reaction with adapter sequences (Bst DNA polymerase, New England Biolabs; 30 min, 37 °C, with polymerase deactivation for 10 min, 80 °C). After barcode ligation, we prepared metagenomic shotgun libraries following a previously described protocol7, with slight modifications described in Supplementary Material Methods.
For sequencing library preparations for the hybridisation capture we followed the MyBaits Manual17 (Arbor Biosciences, USA). The latter recommends a minimum of 100 ng DNA in 7 µL as input for hybridisation capture reactions, however, based on pilot trials with three marine sediment samples (not shown), we determined that this minimum input can be reduced to ~ 50 ng if sedaDNA concentrations are very low, as was the case for our samples. To achieve at least ~ 50 ng input DNA in 7 µL, we re-amplified remaining sedaDNA of most of our shotgun libraries (cleaned post-IS7/IS8 PCR products) in a second IS7/IS8 PCR (one 75 µL reaction with 9 µL DNA input per sample, using 10 amplification cycles and the same reagent composition as for shotgun IS7/IS8 PCRs, see Supplementary Material Methods). We combined the barcoded EBCs (1 µL each, using a 1 in 10 dilution of EBC_A24029 due to its comparably high DNA concentration relative to the other EBCs) in one PCR reaction (7 µL EBC DNA template total) for the downstream enrichments. After re-amplification, the sedaDNA was cleaned using AxyPrep magnetic beads (1:1.8 library:beads) and quantified using Qubit DNA assays. Samples for which the initial IS7/IS8 PCR provided comparatively high DNA concentrations were not re-amplified prior to hybridisation capture. Using this procedure, we generated 62.53 ± 25.92 ng of DNA (23.24–171.75 ng; 0.07 ng for the EBC pool) for use as input material for the hybridisation capture with Planktonbaits1 and HABbaits1.
Hybridisation capture followed the MyBaits Manual17 with slight modifications. In the Hybridisation Mix (“HYBs”) we used 3 µL baits per reaction, and in the Blockers Mix (“LIBs”) we used the blockers Nimblegen SeqCapEZ (a plant repetitive elements blocker), Block O and Block A (Salmon Sperm DNA and P5/P7 block, respectively, both provided with the MyBaits kit), and we added 7 µL of DNA template. We combined LIBs and HYBs per sample in a Thermocycler (Thermoscientific) once the latter had been at hybridisation temperature for 5 min. For Planktonbaits1 we set the hybridisation temperature to 60 °C as per the manufacturer’s recommendation for short and damaged DNA molecules, and the hybridisation reaction to 40 h. For HABbaits1, we set the hybridisation temperature to 65 °C for the first 3 h to favour highly specific binding, followed by a decrease to 60 °C for the remaining 37 h of the hybridisation capture reaction. We prepared the beads for batches of 8 reactions in 1.7 mL tubes by washing the beads twice with binding buffer, then adding binding buffer and 48 µL yeast tRNA (= 480 µg per 240 mL beads) in a third washing step, followed by brief vortexing and incubation of the solution on a rotary mixer (30 min, room temperature), pelleting on a magnetic rack, and two more washes with binding buffer. We performed bead-hybrid binding for 20 min at 60 °C, with agitation by pipette-mixing, and briefly centrifuging to collect after 5 min. Subsequent washes and library resuspensions (in 40 µL Buffer EBT (EB (Qiagen) with 0.05% Tween20 (Sigma Aldrich)) were performed as per protocol for non-KAPA HiFi HotStart polymerase amplification (incubation at 95 °C, pelleting of beads and collection of sedaDNA containing buffer EBT).
GaII Indexing PCRs (using different indices for HABbaits1 and Planktonbaits1) were performed as for shotgun sequencing libraries (Supplementary Material Methods), but we used one 100 µL reaction and 16 amplification cycles per sample. Initially, we used 12 and 24 µL hybridisation capture sedaDNA template generated from MCS3 and GC2S1 samples as we assumed relatively high and low DNA concentrations, respectively. For samples GC2B 15–16.5 cm, GC2B 75–76.5 cm and GC2A 65–66.5 cm we used 12 µL DNA template due to previous experimental trials. Following amplification, very low DNA concentrations were determined for all HABbaits1 samples and Planktonbaits1 samples MCS3 2–3.5 cm, MCS3 4–5.5 cm, GC2B 85–86.5 cm and GC2A 75–76.5 cm. Therefore, we used the remaining hybridisation capture material (26 µL from MCS3 and 14 µL from GC2S1 samples) from these samples in a second GaII Indexing PCR (100 ul reaction, 16 cycles). We combined the initial and supplementary GaII PCR products per sample and concentrated to 15 µL (20 min, 45 °C) using a CentriVap concentrator (Labconco, USA). To clean the PCR products we used AxyPrep beads (1:1.1 PCR products:beads), eluted the beads in 30 µL nuclease-free H2O and assessed DNA quantity and quality through TapeStation. We prepared an equimolar (6 nM) sequencing pool from all samples, which we concentrated using CentiVap (45 min, 45 °C) to 110 µL, and cleaned using AxyPrep beads (1:1.1 sequencing pool:beads). Following DNA quantity and quality assessment using Qubit, TapeStation, and Fragment Analyzer, we performed one more AxyPrep clean-up (same ratio). We ran final DNA quantity and quality checks via Fragment Analyser and qPCR, and prepared a sequencing pool (mean fragment size 225 bp, 2.75 nM) for submission to Illumina sequencing (HiSeq XTen, 2 × 150 bp cycle). Sequencing was performed at the Australian Cancer Research Foundation Cancer Genomics Facility & Centre for Cancer Biology, Adelaide, Australia, and at the Garvan Institute of Medical Research, KCCG Sequencing Laboratory (Kinghorn Centre for Clinical Genomics), Darlinghurst, Australia.
Data analysis
Bioinformatics
Bioinformatic processing and filtering of the sequencing data, hereafter referred to as datasets ‘Shotgun’, ‘Planktonbaits1’ and ‘HABbaits1’, followed established protocols previously described7, with the exception that we used the NCBI Nucleotide database (ftp://ftp.ncbi.nlm.nih.gov/blast/db/FASTA/nt.gz, downloaded November 2019) as the reference database to align our sedaDNA sequences to (allowing us to run all three datasets against the same database; see Supplementary Material Methods). All species detected in EBCs (Supplementary Material Table 1) were subtracted from the sample data, and hereafter the term ‘samples’ refers to sediment-derived data post-EBC subtraction. For each dataset (Shotgun, Planktonbaits1 and HABbaits1), we used MEGAN6 Community Edition v6.18.10 to rank our assigned reads by domain and exported these read counts. We determined relative abundances per domain per sample, and the average and standard deviation per domain across all samples from MCS3 and GC2S1 (separately for each site due to relatively high variability in relative abundance between them, see results). To quantify the increase in the proportion of our target domain Eukaryota using Planktonbaits1 and HABbaits1 relative to Shotgun, we determined the ratio between the average relative abundance per domain between Planktonbaits1:Shotgun, and HABbaits1:Shotgun.
Ancient DNA authenticity assessment and damage analysis
To assess the authenticity of our Shotgun, Planktonbaits1 and HABbaits1 sedaDNA we ran the ‘MALTExtract’ and ‘Postprocessing’ tools of the HOPS v0.33-2 pipeline23. The latter included the use of the NCBI mapping and NCBI tree files (13 Nov 2019) provided with HOPS (https://github.com/rhuebler/HOPS/tree/external/Resources). Configurations deviating from the default HOPS settings included topMaltEx = 0.10, minPIdent = 95, meganSummary = 1, and destackingOff = 1. We processed each dataset using the ‘def_anc’ mode, which provided results for all filtered reads (‘default’) as well as all reads that had at least one damage lesion in their first 5 bases from either the 5′ or 3′ end (‘ancient’)23. Generally, HOPS determines DNA damage patterns separately for individual taxa, i.e., requires an input list of target taxa for which to compare the sedaDNA sequences identified in our samples to their modern references. We used two taxa screening lists with the aim to generate sedaDNA damage profiles for a representative regional eukaryotic plankton species: (a) the first taxa list simply specified the single word ‘Eukaryota’, which prompts HOPS to run through each eukaryote taxon identified, thereby allowing a general assessment of the amount of eukaryote sequences categorised as ‘default’ or ‘ancient’ in each of our samples and EBCs; and (b) our second taxa list contained the names of the specifically selected marine organisms included in HABbaits1, which are known to be common in our Tasmanian study region (Table 1).
Subsequently to running (a) we used the HOPS-generated ‘RunSummary’ output (containing read counts per taxon classified as either ancient or default) to determine eukaryote-derived percent damage in each dataset (Shotgun, Planktonbaits1 and HABbaits1). Separately for each dataset, we subtracted all taxa with no read counts in both the ancient or default output, and taxa for which read counts were determined in either the ancient or default output (or both) of EBCs (Supplementary Material Table 2). Next, we summed the number of eukaryote reads per sample for ancient and default outputs (total reads) and calculated the proportion between these ancient and default totals, with the proportion of ancient reads providing a ‘% eukaryote sedaDNA damage’ measure per sample. Subsequent to running (b) on all three datasets (Shotgun, Planktonbaits1 and HABbaits1), we used the MaltExtract Interactive Plotting Application (MEx-IPA, by J. Fellows Yates; https://github.com/jfy133/MEx-IPA) to visualise sedaDNA damage profiles (ancient reads only) of the target phytoplankton taxa (Table 1), however, sufficient ancient reads to generate these profiles for all samples in all three datasets were only consistently achieved for the coccolithophore Emiliania huxleyi.
Statistics
To determine relationships between % eukaryote sedaDNA damage and subseafloor depth and test the ‘% eukaryote sedaDNA damage’ measure’s validity as sedaDNA authenticity proxy, we performed two-tailed Pearson correlation analyses between the % eukaryote sedaDNA damage determined in Shotgun, Planktonbaits1 and HABbaits1 (n = 27 each, excluding 3 samples, see section “Proportions of Eukaryota in Shotgun, Planktonbaits1 and HABbaits1”) and subseafloor depth using the software PAST31.
Results
Proportions of Eukaryota in Shotgun, Planktonbaits1 and HABbaits1
After filtering, we retained between 4.6 (GC2A 15–16.5 cm) and 16.2 M (GC2B 5–6.5 cm) reads per sample for Shotgun, between 0.1 (MCS3 4–5.5 cm) and 4.6 M (GC2A 115–116.5 cm) reads per sample for Planktonbaits1 and between 0.2 (GC2A 45–46.5 cm) and 2.8 M (GC2A 115–116.5 cm) reads for HABbaits1. We retrieved no data (or nearly no data, thus not representative) for 3 out of 30 samples and these samples were excluded from downstream processing. The 3 samples were MCS3 0–1.5 cm with Shotgun, GC2B 115–116.5 cm with Planktonbaits1 and HABbaits1, and GC2A 85–86.5 cm with HABbaits1—likely due to low template DNA concentrations (see Supplementary Material). Our EBCs for Shotgun, Planktonbaits1 and HABbaits1 detected a total of 121, 69 and 28 eukaryote taxa (Supplementary Material Table 1). Of note was the identification of Cyprinus carpio (European/Common carp) sequences in all Shotgun, most Planktonbaits1 and three HABbaits1 EBCs. Being a freshwater species unlikely to occur offshore Tasmania, we considered cross-contamination from samples to EBCs irrespective of extraction batch unlikely. Most likely, this is a reagent-derived contaminant as we have detected this species in extraction blanks of unrelated datasets (not shown). Here, C. carpio was removed from downstream analyses and does not impact on our results, however, this finding emphasises the importance of sequencing controls and filtering sedaDNA data accordingly to remove contaminants.
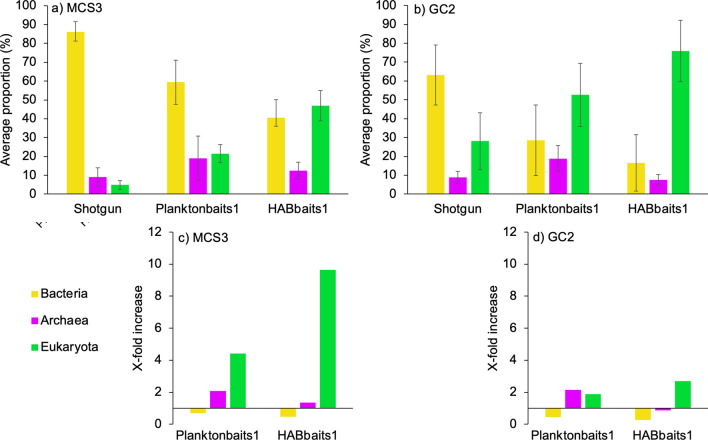
Based on alignments using the NCBI Nucleotide database, the majority of Shotgun reads were assigned to Bacteria (86 ± 5% and 63 ± 16% for MCS3 and GC2, respectively; Fig. 3a,b), and a relatively small portion to Eukaryota (5 ± 2% and 28 ± 15% for MCS3 and GC2, respectively, Fig. 3a,b). This small proportion of Eukaryota increased to 21 and 53% in MCS3 and GC2 using Planktonbaits1 (4.4 × and 1.9 × over Shotgun, respectively), and 47 and 76% in MCS3 and GC2 using HABbaits1 (9.6 × and 2.7 × over Shotgun respectively) (Fig. 3). Planktonbaits1 and HABbaits1 were efficient in the targeted enrichment of Eukaryota sedaDNA from marine sediments, with comparatively little ‘bycatch’ of Bacteria and Archaea (i.e., a decrease in the proportion of Bacteria and a < 2.1 × increase in Archaea relative to Shotgun; Fig. 3c,d). Planktonbaits1 included three cyanobacterial targets, therefore, some capture of bacterial sequences was expected; less than Shotgun but more than HABbaits1 (Fig. 3a,b).
Figure 3.
Proportions of reads assigned to Bacteria, Archaea and Eukaryota using Shotgun, Planktonbaits1 and HABbaits1. (a,b) Average proportion of reads and standard deviation across inshore MCS3 (n = 9) and offshore GC2 (n = 18) samples, respectively. (c,d) Increase in the proportion of Bacteria, Archaea and Eukaryota in Planktonbaits1 and HABbaits1 relative to Shotgun for MCS3 and GC2 samples, respectively, based on average proportions shown in (a,b).
Assessment of sedaDNA authenticity
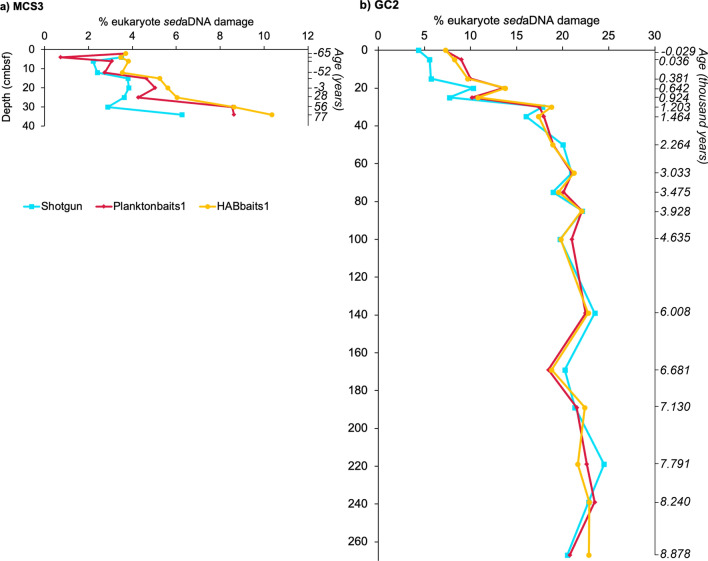
For both inshore MCS3 and offshore GC2, the ‘% eukaryote sedaDNA damage’ determined per sample increased with sub-seafloor depth for each of the three datasets Shotgun, Planktonbaits1 and HABbaits1 (Fig. 4). At the seafloor surface, we determined sedaDNA damage of ≤ 4% at MCS3, and between 4 and 10% at GC2, which, at both sites, slightly increased with depth until ~ 25 cmbsf (to about ~ 10% and 10–15% at MCS3 and GC2, respectively), before a steeper increase between ~ 25 and 35 cmbsf, and, in offshore GC2, remained relatively stable at ~ 20 and 25% below 35 cmbsf (> 1400 years of age). Correlation analyses showed that this increase of the % eukaryote sedaDNA damage with increasing subseafloor depth was highly significant for each dataset (Table 2). Additionally, the % eukaryote sedaDNA damage of the three different datasets (Shotgun, Planktonbaits1 and HABbaits1) were significantly positively correlated with each other, indicating that the original proportions of eukaryote sedaDNA damage patterns preserved in Shotgun were maintained in our hybridisation capture approach using both Planktonbaits1 and HABbaits1.
Figure 4.
Eukaryote sedaDNA damage with subseafloor depth. Shown is the increase in % eukaryote sedaDNA damage with depth (centimetres below seafloor, cmbsf) in both (a) MCS3, (b) GC2. See Table 2 for correlation between % eukaryote sedaDNA damage per dataset (Shotgun, Planktonbaits1, HABbaits1) and depth, and amongst the datasets. Age of sediment cores is recorded relative to the year 1950 (reference date for radiocarbon dating), i.e., MCS3 is ~ 145 years old (a) and GC2 ~ 8946 years old (b), and negative ages indicate years post-1950 (e.g., an age of -65 equals the year 2015).
Table 2.
Summary statistics of correlation analysis between % eukaryote sedaDNA damage and subseafloor depth.
| Depth (cmbsf) | Shotgun % damage | Planktonbaits1% damage | HABbaits1% damage | |
|---|---|---|---|---|
| MCS3 | ||||
| Depth (cmbsf) | 0.01361 | 0.00241 | 0.00026 | |
| Shotgun % damage | 0.77773 | 0.00032 | 0.00229 | |
| Planktonbaits1% damage | 0.86805 | 0.92695 | 0.00018 | |
| HABbaits1 A:D | 0.93138 | 0.87002 | 0.93812 | |
| GC2 | ||||
| Depth (cmbsf) | 0.00064 | 0.00072 | 0.00040 | |
| Shotgun % damage | 0.72638 | 9.84E-14 | 7.35E-13 | |
| Planktonbaits1% damage | 0.72182 | 0.98541 | 1.89E-14 | |
| HABbaits1% damage | 0.74356 | 0.98121 | 0.98815 | |
Pearson correlation coefficients r (in italics, lower matrix triangle) and corresponding two-tailed probability that r is uncorrelated (upper triangle of matrix; i.e., all values < 0.05 denote a significant correlation) between subseafloor depth (cmbsf) and Shotgun, Planktonbaits1 and HABbaits1% eukaryote sedaDNA damage (n = 27 each).
DNA damage profiles of the marine coccolithophore Emiliania huxleyi
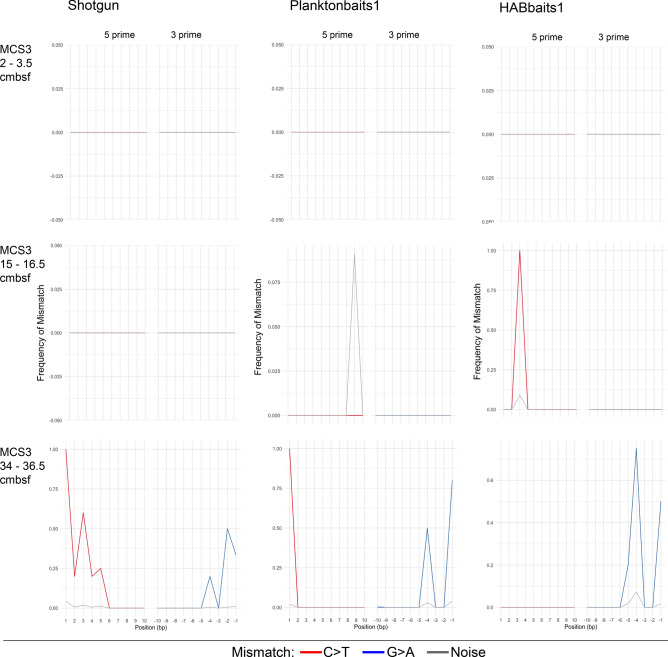
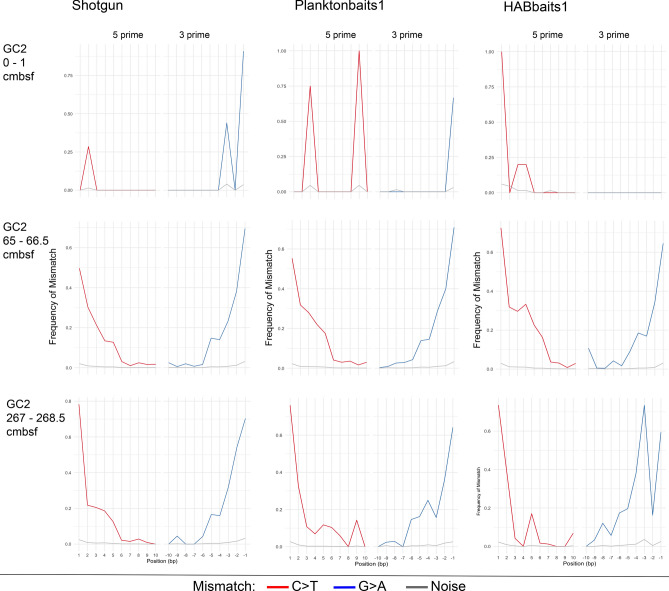
The sedaDNA damage analysis provided DNA damage profiles for most of the target taxa on our selected taxa list (taxa list ‘b’). However, the number of ancient sequences assigned to the ubiquitous coccolithophore Emiliania huxleyi was much higher, allowing the generation of more detailed sedaDNA damage profiles, consistently across all three datasets. Ancient E. huxleyi sequences ranged from a total of 0–10 reads in inshore MCS3 and 5–2651 in offshore GC2 for Shotgun, from 0 to 7 in MCS3 and 1 to 947 in GC2 for Planktonbaits1, and from 0 to 11 in MCS3 and 1 to 1183 in GC2 for HABbaits1. A lower representation of ‘ancient’ sequences in inshore MCS3 is consistent with our observation of lower % eukaryote sedaDNA damage in sediments above ~ 35 cmbsf (i.e., the complete length of MCS3) (see section “Assessment of sedaDNA authenticity”. Damage profiles for E. huxleyi sedaDNA are much more variable in inshore MCS3 (and in the upper ~ 25 cmbsf of GC2; Figs. 5, 6; Supplementary Material Fig. 1, 2) than the profiles of deeper, more stable offshore GC2 samples, likely resulting from a scarcity of ancient reads in the upper sediment layers and sedaDNA damage patterns not being as pronounced as in deeper GC2 samples.
Figure 5.
SedaDNA damage profiles of Emiliania huxleyi in MCS3. E. huxleyi sedaDNA damage profiles (frequency of mismatch against base pair position) per sample for Shotgun, Planktonbaits1 and HABbaits1 in MCS3. The red and blue lines denote C > T substitutions in 5′ direction and G > A substitutions in 3′ direction, respectively, for all ancient alignments. Grey lines denote estimated noise23. Shown are selected samples only (top, mid-depth and bottom sample), for damage profiles of all samples see Supplementary Material Fig. 1.
Figure 6.
SedaDNA damage profiles of Emiliania huxleyi in GC2. E. huxleyi sedaDNA damage profiles (frequency of mismatch against base pair position) per sample for Shotgun, Planktonbaits1 and HABbaits1 in GC2. The red and blue lines denote C > T substitutions in 5′ direction and G > A substitutions in 3′ direction, respectively, for all ancient alignments. Grey lines denote estimated noise23. Shown are selected samples only (top, mid-depth and bottom sample), for damage profiles of all samples see Supplementary Material Fig. 2.
The E. huxleyi sedaDNA damage profiles of Shotgun, Planktonbaits1 and HABbaits1 showed similar variability with depth amongst each other (Figs. 5, 6; Supplementary Material Fig. 1, 2), suggesting that the hybridisation capture technique reliably preserves the DNA damage patterns of the original sample (represented by Shotgun) and is well-suited for the capture of past marine eukaryote sedaDNA. Further, HOPS provided a valid approach to authenticate sedaDNA from marine eukaryotes. We were unable to generate clear sedaDNA damage profiles from the upper ~ 25 to 35 cmbsf in both MCS3 (spanning the last ~ 145 years; Fig. 5; Supplementary Material Fig. 1) and GC2 (~ 1500 years, Fig. 6; Supplementary Material Fig. 2), indicating that sedaDNA damage is not as pronounced in the upper (younger) sediment layers at our study location and detectable only below that depth. Below ~ 35 cmbsf in GC2 the E. huxleyi DNA damage profiles assumed a typical U-shape as the number of mismatches at the end of DNA fragments increases (Fig. 6; Supplementary Material Fig. 2). Our E. huxleyi sedaDNA damage profiles are the first generated for a marine eukaryote—and extend over an ~ 8950-year timescale.
Discussion
We applied two new RNA bait sets and hybridisation capture to inshore and offshore marine sediments to investigate marine eukaryotes more broadly (Planktonbaits1) and a more tailored approach focused on selected common and harmful taxa in our study region (HABbaits1). Our results show that hybridisation capture improves the yield of target eukaryote sedaDNA, and preserves DNA damage patterns, allowing us to assess sedaDNA authenticity, and generate the first ancient DNA damage profiles of a keystone marine phytoplankton organism, the ubiquitous coccolithophore Emiliania huxleyi.
Both Planktonbaits1 and HABbaits1 successfully captured sedaDNA of eukaryote organisms in two sediment cores collected off the Tasmanian east coast. Eukaryote sedaDNA has been repeatedly shown to be present in low amounts in seafloor sediments, limiting the metagenomic analysis and detailed reconstruction of past marine ecosystems. While both Planktonbaits1 and HABbaits1 achieved a considerable enrichment in Eukaryota for our inshore site MCS3 (4- to 9-fold, respectively), this increase was about half at the offshore site GC2. This difference may be due to the initial difference in proportions of Eukaryota DNA at the two sites. Shotgun showed Eukaryota contributed ~ 5% to the total pool of sedaDNA at MCS3, while contributing ~ 28% at GC2. The latter high proportion is primarily a result of a sharp increase in the relative abundance of Eukaryota in GC2 below 35 cmbsf. This initially relatively high proportion of Eukaryota sequences in GC2 sedaDNA extracts may have saturated the baits in our hybridisation reaction and would explain the less pronounced increase in GC2 Eukaryota proportions using either bait set. To further increase the Eukaryota signal in future studies, it may be beneficial to add a larger volume of baits (> 3 µL) to sedaDNA extracts expected (e.g., from shallow shotgun sequencing prior to enriching) to have a relatively high Eukaryota sedaDNA content.
The HOPS bioinformatic tool23 proved highly valuable in identifying and analysing ancient eukaryote sequences in our sedaDNA. The HOPS generated output of our ‘Eukaryota’ (taxa list a) run enabled the determination of ‘% eukaryote sedaDNA damage’, a parameter that can be used as a proxy of sedaDNA authenticity in the future. Here, the % eukaryote sedaDNA damage was in the lower quarter (1–24%), with a relatively high proportion of reads passing the default filtering criteria. The latter criteria used a minimum percent identity (mpi) level of 95%, a relatively stringent cut-off while still retaining the majority of reads. Lowering the mpi cut-off may have resulted in higher % eukaryote sedaDNA damage due to more reads passing the filtering criteria, however, this would also result in increased inaccuracies in taxa-assignments. It might be that ~ 24% comprises the maximum possible degree of eukaryote sedaDNA damage, for example, due to chromatin structures that may protect the majority of DNA regions while exposing others to degradation32–34. The latter might also explain the plateauing of the % eukaryote sedaDNA damage below ~ 30 cmbsf in GC2, however, this speculative and requires further investigation.
At both our inshore and offshore site, we observed a significant increase in % eukaryote sedaDNA damage with subseafloor depth, demonstrating that eukaryote sedaDNA shows increased DNA damage with increasing age of sediments. However, at both sites the sedaDNA damage was consistently lower in the upper 25–35 cmbsf (< 7% and < 15% at MCS3 and GC2, respectively), then increased sharply down-core from this depth (to ~ 6 to 11% and ~ 22% in MCS3 and GC2, respectively), and remained at this level towards the bottom of in GC2. At sites MCS3 and GC2, subseafloor depths of ~ 30 cmbsf correspond to sediment ages of ~ 124 and ~ 1271 years, respectively, which explains the much higher % eukaryote sedaDNA damage in GC2 (~ 22%) relative to MCS3 (< 10%) at this depth. It is possible that physical and/or chemical factors contribute to the increased preservation of sedaDNA damage patterns at depth, such as increased sediment compaction associated with less pore water movement and less oxygen exposure, as well as microbial activity35–37, and that the latter factors might play a greater role below 30 cmbsf at our sites. However, the above points on potential physico-chemical contributing factors to the variability in % eukaryote sedaDNA damage with subseafloor depth are speculative. Further research is required to determine whether reaching a ‘critical depth’ at which the % eukaryote sedaDNA damage first accelerates and the plateaus is a pattern characteristic of our study location, or of wider importance. If the latter holds true, then this ‘critical depth’ could be used as a guide to define ‘recent’ versus ‘ancient’ sediments, a distinction that has remained unclear in the sedaDNA research field to date. Future sedaDNA studies should also investigate how the % eukaryote sedaDNA damage varies in much older sediment records (older than Holocene) and depending on sediment properties (e.g., clay-rich sediments that appear to benefit DNA preservation38).
The strong positive correlation between % eukaryote sedaDNA damage amongst Shotgun, Planktonbaits1 and HABbaits1, demonstrates that DNA damage signals present in sedaDNA are preserved throughout the hybridisation capture approach. This is important as it allows the authentication of sedaDNA using bioinformatic tools, which any ancient DNA study should incorporate23. Through hybridisation capture more target sequences are available as input for DNA damage analysis software such as HOPS, which increases the robustness of such analyses23, thus is strongly recommended for sedaDNA analyses. While future refinement of the ‘% eukaryote sedaDNA damage’ measure may be necessary, our analyses show that it can be used as a proxy for sedaDNA authenticity in sediment records. Generally, for marine sedaDNA investigations of eukaryote taxa, the capacity to assess DNA damage provides a crucial advantage over metabarcoding where deamination patterns are removed throughout the amplification process and thus prevent damage-based authenticity assessments.
Running our data through the HOPS pipeline (taxa list b) and MEx-IPA allowed us to generate DNA damage plots for a key marine phytoplankton species, Emiliania huxleyi. This ubiquitous calcareous nanoplankton has thrived in the oceans since the Cretaceous, is one of the most abundant phytoplankton species in the global ocean and is ubiquitous from tropical to temperate to Antarctic Australian waters39,40. Consistent with its biogeographic distribution in the modern ocean, we expected to detect traces of this species in our sedaDNA, and in higher relative abundances offshore39,40.
We retained the maximum number of reads throughout our analyses (by examining proportions rather than rarefying our data), which enabled us to generate E. huxleyi DNA damage profiles from all three datasets, Shotgun, Planktonbaits1 and HABbaits1. The damage profiles generated by Shotgun, Planktonbaits1 and HABbaits1 for the same sample were very similar, indicating preservation of DNA damage patterns in our original sample (Shotgun) and in our enriched samples after hybridisation capture. Consistent with our finding of low eukaryote-derived sedaDNA damage in the upper 25–30 cmbsf, no clear E. huxleyi damage patterns could be determined from these depths. sedaDNA damage patterns with a typical U-shape were found only below ~ 25 cmbsf in GC2, again suggesting the existence of a critical depth below which DNA degradation becomes more pronounced, reinforcing the importance of investigating whether this phenomenon is of wider importance, and possibly influenced by physical or chemical properties, sedimentation rates, and/or microbial activity.
The study of marine sedaDNA offers huge potential for the comprehensive reconstruction of past marine ecosystems (including viruses, archaea, prokaryotes and eukaryotes). Eukaryotes (phytoplankton and higher organisms) are particularly popular study organisms due to their importance as primary producers and use as environmental indicators. However, sedaDNA studies focussing on eukaryotes have been severely limited by the very low amounts of DNA preserved in the subseafloor, and the lack of bioinformatic tools to authenticate these miniscule amounts of eukaryote sedaDNA in metagenomic data. To date, no marine sedaDNA studies have included authentication of sedaDNA (i.e., that the DNA recovered is ancient and not substantially impacted by contamination with modern DNA) a routine procedure for ancient DNA studies focussing on humans and megafauna. Our study provides a key advance in that we (1) used a hybridisation capture technique to enrich target marine eukaryote sedaDNA independent of DNA fragment size, and (2) applied the recently developed bioinformatic tool HOPS for sedaDNA damage analysis and to bioinformatically authenticate our marine sedaDNA. These advances provide a critical benefit to studies of paleo-community composition from sedaDNA, and the detailed investigation of both model and non-model organisms within these communities.
Conclusions
In this study we show the reliability of the hybridisation capture as a novel tool for investigating changing patterns of abundance of marine eukaryotes from their sedaDNA in seafloor sediments. We furthermore applied a recently developed bioinformatic tool for metagenomic DNA damage analysis (HOPS) to our sedaDNA, which allowed us to develop a new measure for sedaDNA authenticity (‘% eukaryote sedaDNA damage’) that changes with subseafloor depth. Through our sedaDNA damage analysis were also able to generate sedaDNA damage profiles of the ubiquitous coccolithophore E. huxleyi, the first ever such profiles generated for a marine eukaryote—extending over an 8000-year timescale. Our study provides a major step forward for the future investigation of eukaryotes from marine sedaDNA, enabling detailed insights into past marine ecosystem composition over geological timescales.
Supplementary Information
Acknowledgements
We are grateful to Brian Brunelle from Arbor Biosciences, USA, for his expert assistance with designing Planktonbaits1 and HABbaits1. We thank Oscar Estrada-Santamaria, Steve Richards, Holly Heiniger, Nicole Moore and Steve Johnson for their help and advice during extractions, library preparations, and hybridisation capture. We are grateful to Raphael Eisenhofer, Vilma Pérez, Yassine Souilmi, Yichen Liu, and Ron Hübler for their help with on bioinformatic analyses. We thank the Marine National Facility, the crew of RV Investigator voyage IN2018_T02 and the scientific team for their support during field work (2018 MNF Grant H0025318), and Prof. Andrew McMinn for assistance with collection of the cores. This study was funded through the 2017 Australian Research Council (ARC) Discovery Project DP170102261. AC was funded by ARC Laureate Fellowship FL140100260.
Author contributions
L.A. designed this research, carried out laboratory work, bioinformatic and statistical analyses and wrote the first draft of the manuscript. G.H., C.B., and C.W. collected and provided the core samples. C.W. provided sediment core dating. A.C. provided guidance on the hybridisation capture technique and ancient DNA analyses. G.H., C.B. and A.C. assisted with data interpretation. A.C., G.H. and C.B. developed the conceptual approach and secured the funding for the project. All co-authors provided comments and feedback on manuscript drafts, and edited the final manuscript submitted for publication.
Data availability
The demultiplexed raw sequencing data analysed during this study are not yet publicly available due to ongoing manuscript preparations but are available from the corresponding author upon reasonable request. Once publications are finalised, the data will be made openly available via the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82578-6.
References
- 1.Szczuciński W, et al. Ancient sedimentary DNA reveals past tsunami deposits. Mar. Geol. 2016;381:29–33. doi: 10.1016/j.margeo.2016.08.006. [DOI] [Google Scholar]
- 2.More KD, et al. A 43 kyr record of protist communities and their response to oxygen minimum zone variability in the Northeastern Arabian Sea. Earth Planet. Sci. Lett. 2018;496:248–256. doi: 10.1016/j.epsl.2018.05.045. [DOI] [Google Scholar]
- 3.De Schepper S, et al. The potential of sedimentary ancient DNA for reconstructing past sea ice evolution. ISME J. 2019;13:2566–2577. doi: 10.1038/s41396-019-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbrecht LH, et al. Ancient DNA from marine sediments: Precautions and considerations for seafloor coring, sample handling and data generation. Earth-Sci. Rev. 2019;196:102887. doi: 10.1016/j.earscirev.2019.102887. [DOI] [Google Scholar]
- 5.Verity PG, Smetacek V. Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar. Ecol. Prog. Ser. 1996;130:277–293. doi: 10.3354/meps130277. [DOI] [Google Scholar]
- 6.Hays GC, Richardson AJ, Robinson C. Climate change and marine plankton. Trends Ecol. Evol. 2005;20:337–344. doi: 10.1016/j.tree.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Armbrecht L, et al. An optimized method for the extraction of ancient eukaryote DNA from marine sediments. Mol. Ecol. Resour. 2020;20:906–919. doi: 10.1111/1755-0998.13162. [DOI] [PubMed] [Google Scholar]
- 8.Armbrecht L. The potential of sedimentary ancient DNA to reconstruct past ocean ecosystems. Oceanography. 2020 doi: 10.5670/oceanog.2020.211. [DOI] [Google Scholar]
- 9.Taberlet P, Coissac E, Pompanon F, Brochmann C, Willerslev E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 2012;21:2045–2050. doi: 10.1111/j.1365-294X.2012.05470.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen MW, et al. Ancient and modern environmental DNA. Philos. Trans. R Soc. B. Biol. Sci. 2015;370:20130383. doi: 10.1098/rstb.2013.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyrich LS, et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544:357–361. doi: 10.1038/nature21674. [DOI] [PubMed] [Google Scholar]
- 12.Horn S. Target enrichment via DNA hybridisation capture. In: Shapiro B, Hofreiter M, editors. Ancient DNA, Methods and Protocols. Berlin: Springer; 2012. pp. 177–188. [Google Scholar]
- 13.Foster NR, Gillanders BM, Jones AR, Young JM, Waycott M. A muddy time capsule: using sediment environmental DNA for the long-term monitoring of coastal vegetated ecosystems. Mar. Freshw. Res. 2020;71:869–876. doi: 10.1071/MF19175. [DOI] [Google Scholar]
- 14.Paijmans JLA, Gilbert MTP, Hofreiter M. Mitogenomic analyses from ancient DNA. Mol. Phylogenet. Evol. 2013;69:404–416. doi: 10.1016/j.ympev.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Li C, et al. DNA capture reveals transoceanic gene flow in endangered river sharks. Proc. Natl. Acad. Sci. USA. 2015;112:13302–13307. doi: 10.1073/pnas.1508735112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murchie T, et al. PalaeoChip Arctic1. 0: An optimised eDNA targeted enrichment approach to reconstructing past environments. bioRxiv. 2019 doi: 10.1101/730440. [DOI] [Google Scholar]
- 17.MyBaits Manual v.4.01—Hybridization Capture for Targeted NGS, 2018. https://arborbiosci.com/wp-content/uploads/2019/08/myBaits-Manual-v4.pdf.
- 18.Ginolhac A, Rasmussen M, Gilbert MTP, Willerslev E, Orlando L. mapDamage: testing for damage patterns in ancient DNA sequences. Bioinformatics. 2011;27:2153–2155. doi: 10.1093/bioinformatics/btr347. [DOI] [PubMed] [Google Scholar]
- 19.Jónsson H, Ginolhac A, Schubert M, Johnson PLF, Orlando L. MapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics. 2013;29:1682–1684. doi: 10.1093/bioinformatics/btt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llamas B, et al. Late Pleistocene Australian marsupial DNA clarifies the affinities of extinct megafaunal kangaroos and wallabies. Mol. Biol. Evol. 2015;32:574–584. doi: 10.1093/molbev/msu338. [DOI] [PubMed] [Google Scholar]
- 21.Tobler R, et al. Aboriginal mitogenomes reveal 50,000 years of regionalism in Australia. Nature. 2017;544:180–184. doi: 10.1038/nature21416. [DOI] [PubMed] [Google Scholar]
- 22.Collin TC, et al. An open-sourced bioinformatic pipeline for the processing of Next-Generation Sequencing derived nucleotide reads: Identification and authentication of ancient metagenomic DNA. bioRxiv. 2020 doi: 10.1101/2020.04.20.050369. [DOI] [Google Scholar]
- 23.Hübler R, et al. HOPS: automated detection and authentication of pathogen DNA in archaeological remains. Genome Biol. 2019;20:1–13. doi: 10.1186/s13059-019-1903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willerslev E, Cooper A. Ancient DNA. Proc. R. Soc. B Biol. Sci. 2005;272:3–16. doi: 10.1098/rspb.2004.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vargas, C. et al. Eukaryotic plankton diversity in the sunlit ocean. Science348, 1261605–1/11 (2015). [DOI] [PubMed]
- 26.Quast C, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillou L, et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013;41:597–604. doi: 10.1093/nar/gks1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grzebyk, D., Audic, S., Decelle, J. & de Vargas, C. PHYTOPK28-D1D2: A curated database of 28S rRNA gene D1–D2 domains from eukaryotic organisms dedicated to metabarcoding analyses of marine phytoplankton samples. Mendeley Data1. 10.17632/mndb4h87yg.1 (2017).
- 29.Ratnasingham S, Hebert PDN. The Barcode of life data system. Mol. Ecol. Notes. 2007;7:355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer M, Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. 2010;6:pdb.prot5448. doi: 10.1101/pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- 31.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 32.Takata H, et al. Chromatin compaction protects genomic DNA from radiation damage. PLoS ONE. 2013;8:1–11. doi: 10.1371/journal.pone.0075622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kistler L, Ware R, Smith O, Collins M, Allaby RG. A new model for ancient DNA decay based on paleogenomic meta-analysis. Nucleic Acids Res. 2017;45:6310–6320. doi: 10.1093/nar/gkx361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gornik SG, Hu I, Lassadi I, Waller RF. The biochemistry and evolution of the dinoflagellate nucleus. Microorganisms. 2019;7:245. doi: 10.3390/microorganisms7080245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eglinton G, Logan GA. Molecular preservation. Philos. Trans. R. Soc. London B. 1991;333:315–328. doi: 10.1098/rstb.1991.0081. [DOI] [PubMed] [Google Scholar]
- 36.Palchevskiy V, Finkel SE. Escherichia coli competence gene homologs are essential for competitive fitness and the use of DNA as a nutrient. J. Bacteriol. 2006;188:3902–3910. doi: 10.1128/JB.01974-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corinaldesi C, Beolchini F, Dell’Anno A. Damage and degradation rates of extracellular DNA in marine sediments: Implications for the preservation of gene sequences. Mol. Ecol. 2008;17:3939–3951. doi: 10.1111/j.1365-294X.2008.03880.x. [DOI] [PubMed] [Google Scholar]
- 38.Vuillemin A, et al. Archaea dominate oxic subseafloor communities over multimillion-year time scales. Sci. Adv. 2019;5:1–12. doi: 10.1126/sciadv.aaw4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallegraeff GM. Coccolithophorids (calcareous nanoplankton) from Australian waters. Bot. Mar. 1984;27:229–247. [Google Scholar]
- 40.Cubillos JC, et al. Calcification morphotypes of the coccolithophorid Emiliania huxleyi in the Southern Ocean: Changes in 2001 to 2006 compared to historical data. Mar. Ecol. Prog. Ser. 2007;348:47–54. doi: 10.3354/meps07058. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The demultiplexed raw sequencing data analysed during this study are not yet publicly available due to ongoing manuscript preparations but are available from the corresponding author upon reasonable request. Once publications are finalised, the data will be made openly available via the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra).