Abstract
Perivascular space (PVS) is associated with neurodegenerative diseases, while its effect on Parkinson’s disease (PD) remains unclear. We aimed to investigate the clinical and neuroimaging significance of PVS in basal ganglia (BG) and midbrain in early-stage PD. We recruited 40 early-stage PD patients and 41 healthy controls (HCs). Both PVS number and volume were calculated to evaluate PVS burden on 7 T magnetic resonance imaging images. We compared PVS burden between PD and HC, and conducted partial correlation analysis between PVS burden and clinical and imaging features. PD patients had a significantly more serious PVS burden in BG and midbrain, and the PVS number in BG was significantly correlated to the PD disease severity and L-dopa equivalent dosage. The fractional anisotropy and mean diffusivity values of certain subcortical nuclei and white matter fibers within or nearby the BG and midbrain were significantly correlated with the ipsilateral PVS burden indexes. Regarding to the midbrain, the difference between bilateral PVS burden was, respectively, correlated to the difference between fiber counts of white fiber tract passing through bilateral substantia nigra in PD. Our study suggests that PVS burden indexes in BG are candidate biomarkers to evaluate PD motor symptom severity and aid in predicting medication dosage. And our findings also highlight the potential correlations between PVS burden and both grey and white matter microstructures.
Subject terms: Parkinson's disease, Parkinson's disease
Introduction
Perivascular spaces (PVSs) are fluid-filled spaces surrounding blood vessels that course from the brain surface through brain parenchyma1. It’s a normal anatomical structure in the central nervous system, commonly observed in basal ganglia (BG), white matter centrum semiovale, midbrain and hippocampus2. Although the results of PVS studies remained controversial, the PVS burden had been shown to correlate with aging, cognitive capacity3,4, sleep quality5, and depressive states6. It was described as a characteristic of neurological diseases including, small vessel disease7, Alzheimer’s disease (AD)8, multiple sclerosis9, as well as Parkinson’s disease (PD)10,11.
PD is a chronic neurodegenerative disorder, characterized by motor symptoms (resting tremor, bradykinesia, rigidity, and gait abnormalities) and non-motor symptoms (depression, anxiety, cognition decline, etc.)12. The pathological hallmarks of PD are dopaminergic neuron degeneration and abnormal α‐synuclein deposition in the substantia nigra (SN)13. Recent studies indicated that the PVS system played a disease-modifying role in PD10,11,14,15. Since the PVS system is involved in the glymphatic drainage system to eliminate metabolic waste16,17, and there was evidence showing that PVS system helped drive out soluble proteins involved in neurodegenerative diseases from the brain interstitial fluid18, we also assume that PVS participates in the clearance of abnormal α‐synuclein19 and its dysfunction may aggravate the pathology of PD.
Enlarged PVS became visible and measurable through magnetic resonance imaging (MRI) techniques. PVS burden was defined as indexes of PVS quantification to represent severity of PVS enlargement, including PVS number and volume20. Previous studies reported that PVS might contribute to the characteristic asymmetry of motor symptoms10, and could be a useful neuroimaging biomarker for evaluating cognitive decline in PD10,15. The physiological and pathophysiological mechanisms of PVS may be reflected in its neighboring white matter, as PVS burden had shown correlation with white matter hyperintensity21.
These studies partly explained the significance of PVS, however, it was previously subject to the limitation of MRI resolution. Only PVSs with larger diameters were visible, and most studies focused primarily on the potential pathogenicity of enlarged PVSs. Due to the higher resolution and signal-to-noise ratio (SNR) of 7 Tesla (7 T) MRI, more small size PVSs can be detected and the PVS numbers were markedly higher than conventional 1.5 or 3 T MRI studies1. Therefore, the small PVS should also be taken into account in studies of neurodegenerative diseases, which could alter the correlations between PVS burden and clinical features compared to previous studies1.
This study aimed to compare the PVS burden in early-stage PD to those in healthy controls (HCs), with the influence of smaller PVSs included. We performed a quantitative evaluation of PVS burden on 7 T MRI images to explore the prevalence of PVS and characterize the correlation patterns of PVS burden with clinical and imaging features to further understand its pathophysiological mechanism in PD.
Results
Participant characteristics
The demographic and clinical characteristics of the PD and HC participants are shown in Table 1. There were no significant differences between the PD and HC groups in age, sex, education, Hamilton Rating Scale for Anxiety (HAMA) score, Mini-Mental State Examination (MMSE) score, Montreal Cognitive Assessment (MoCA) score, Parkinson Neuropsychometric Dementia Assessment (PANDA) score, or prevalence of vascular risk factors such as hypertension, hyperlipidemia, diabetes mellitus, and smoking. The PD patients had significantly higher Hamilton Rating Scale for Depression (HAMD) score than the HC participants (p = 0.009), indicating the depressive state was more severe in the PD patients.
Table 1.
Demographic and clinical characteristics of participants.
| PD (n = 40) | HC (n = 41) | p | |
|---|---|---|---|
| Age (years) | 52.5 ± 1.2 | 49.8 ± 2.0 | 0.242 |
| Sex, male (%) | 47.5 | 51.2 | 0.738 |
| Education (years) | 8.8 ± 0.7 | 10.8 ± 0.7 | 0.059 |
| Hypertension (%) | 25.0 | 19.5 | 0.553 |
| Diabetes mellitus (%) | 5.0 | 4.9 | 0.626 |
| Hyperlipidemia (%) | 0.0 | 4.9 | 0.485 |
| Smoking (%) | 17.5 | 19.5 | 0.816 |
| Disease duration (years) | 5.4 ± 1.0 | – | – |
| MDS-UPDRS I | 5.5 ± 0.5 | – | – |
| MDS-UPDRS II | 8.4 ± 0.6 | – | – |
| MDS-UPDRS III | 23.4 ± 2.0 | – | – |
| MDS-UPDRS IV | 1.9 ± 0.5 | – | – |
| MDS-UPDRS total | 39.1 ± 3.0 | – | – |
| H-Y stage | 1.8 ± 0.1 | – | – |
| LEDD (mg) | 535.0 ± 39.5 | – | – |
| HAMA | 3.7 ± 0.5 | 2.7 ± 0.5 | 0.083 |
| HAMD | 5.8 ± 0.6 | 3.8 ± 0.6 | 0.009** |
| MMSE | 26.5 ± 0.5 | 27.3 ± 0.4 | 0.272 |
| MoCA | 22.6 ± 0.8 | 23.8 ± 0.7 | 0.285 |
| PANDA | 20.7 ± 0.9 | 21.3 ± 1.1 | 0.672 |
Results are expressed as means ± standard error of the mean for the continuous variables and as frequencies for the categorical variables.
PD Parkinson’s disease, HC healthy control, MDS-UPDRS Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale, H-Y stage Hoehn & Yahr stage, LEDD L-dopa equivalent daily dose, HAMA Hamilton Rating Scale for Anxiety, HAMD Hamilton Rating Scale for Depression, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, PANDA Parkinson neuropsychometric dementia assessment.
**Indicates p < 0.01.
Distribution of PVSs
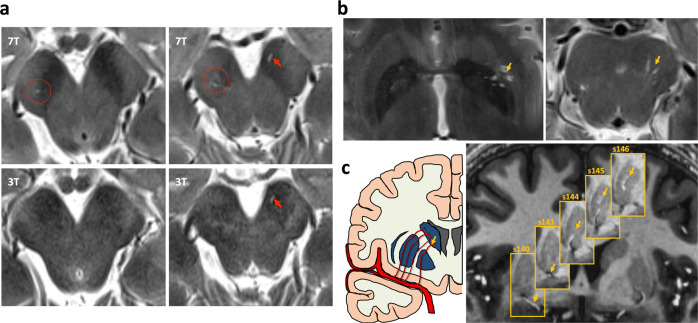
Although our 3 T MRI sequence already achieved a relatively high resolution, 7 T MRI images could expose PVSs more clearly, particularly for small size PVSs due to its much higher SNR compared to 3 T MRI images (Fig. 1a). The PVS quantification was performed by a single reader (T.S.) blind to the participants’ clinical status. Before the formal study, reader (T.S.) was trained by a senior researcher (Y.Y.) on a training set of 20 cases21. Intra-class correlation coefficients between two readers reached 0.81 for PVSs in BG and 0.89 for PVSs in midbrain.
Fig. 1. Visualization of the perivascular space.
a Comparison of detectable PVS in one subject on 7 T images and 3 T images. b PVSs in the BG and midbrain on T2 images. c Visualization of the PVS system and its spatially corresponding lenticulostriate artery in the BG on MP2RAGE imaging.
In this study, the PVS burden indexes, PVS number and PVS volume, in the right-hemispheric BG (BGR), left-hemispheric BG (BGL), right-hemispheric midbrain (MidR), and left-hemispheric midbrain (MidL) were shown in Table 2. Both PVS number and volume in BGR were significantly larger in the PD group than those in the HC group (p = 0.035 for number, p = 0.015 for volume). And the PVS number in bilateral midbrain (p = 0.042 for MidR, p = 0.018 for MidL) and the PVS volume in MidR (p = 0.041) were significantly larger in the PD group compared to the HC group, as shown in Fig. 2.
Table 2.
PVS distribution in PD and HC.
| PD (n = 40) | HC (n = 41) | p | |
|---|---|---|---|
| BGR | |||
| Number | 12.4 ± 0.8 | 9.81 ± 0.7 | 0.035* |
| Volume | 41.5 ± 3.9 | 29.1 ± 2.7 | 0.015* |
| BGL | |||
| Number | 11.9 ± 0.8 | 11.1 ± 0.8 | 0.555 |
| Volume | 36.9 ± 3.3 | 35.6 ± 3.4 | 0.921 |
| MidR | |||
| Number | 8.2 ± 0.6 | 6.2 ± 0.6 | 0.042* |
| Volume | 13.5 ± 1.2 | 9.6 ± 1.4 | 0.041* |
| MidL | |||
| Number | 6.6 ± 0.5 | 4.6 ± 0.5 | 0.018* |
| Volume | 9.1 ± 1.0 | 6.8 ± 1.1 | 0.145 |
Results are expressed as means ± standard error of the mean.
PVS perivascular space, PD Parkinson’s disease, HC healthy control, BG basal ganglia, Mid midbrain, R right-hemisphere, L left-hemisphere.
*Indicates p < 0.05.
Fig. 2. Comparison of the perivascular space burden between PD and HC groups.
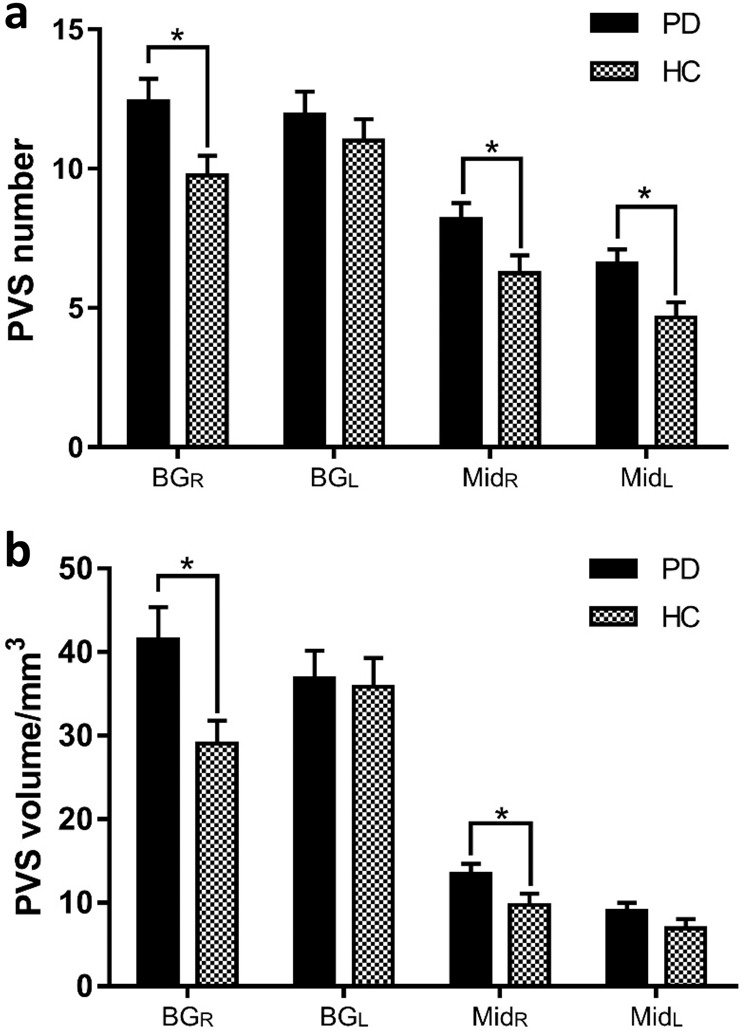
a PVS number and b PVS volume in BG and midbrain (Mid). *Indicates p < 0.05. The results are presented as means ± standard error of mean.
Correlation analysis with clinical features
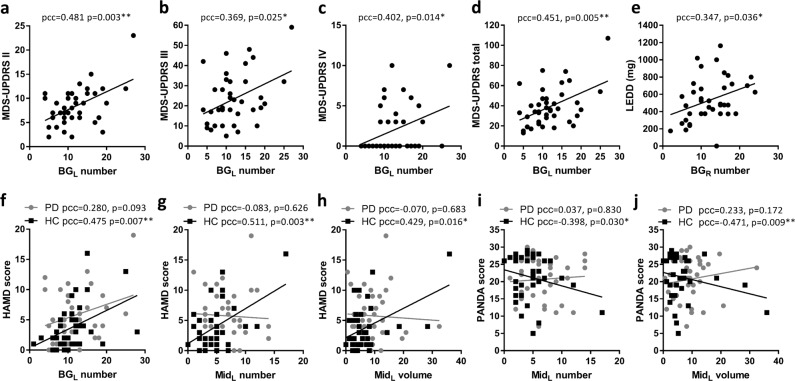
After regressing out the effect of age, sex, and total brain volume, the PVS number of BGL was positively correlated with Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) II subscore (partial correlation coefficients (pcc) = 0.481, p = 0.003), III subscore (pcc = 0.369, p = 0.025), IV subscore (pcc = 0.402, p = 0.014), and total score (pcc = 0.451, p = 0.005) in the PD group (Fig. 3a–d), while the PVS volume of BGL was not correlated to MDS-UPDRS scores. The PVS number of BGR was positively correlated to L-dopa equivalent daily dose (LEDD) (pcc = 0.347, p = 0.036, Fig. 3e). However, the PVS burden indexes were not correlated to HAMA, HAMD, MMSE, MoCA, or PANDA scores.
Fig. 3. Correlations of perivascular space burden indexes in BG and midbrain with clinical features.
Correlations with a−d MDS-UPDRS scores and e LEDD in PD. Correlations with f−h HAMD score in PD and HC. Correlation with i−j PANDA score in PD and HC. *Indicates the partial correlation coefficient (pcc) < 0.05 and ** indicates pcc < 0.01.
In the HC group, the PVS number of BGL (pcc = 0.475, p = 0.007), the PVS number of MidL (pcc = 0.511, p = 0.003) and the PVS volume of MidL (pcc = 0.429, p = 0.016) were positively correlated with the HAMD score after regressing out the effect of age, sex, and total brain volume (Fig. 3f–h). Furthermore, the PVS burden indexes in MidL were negatively correlated to the PANDA score (pcc = −0.398, p = 0.030 for PVS number; pcc = −0.471, p = 0.009 for PVS volume), as shown in Fig. 3i, j.
Correlation analysis with FA and MD values
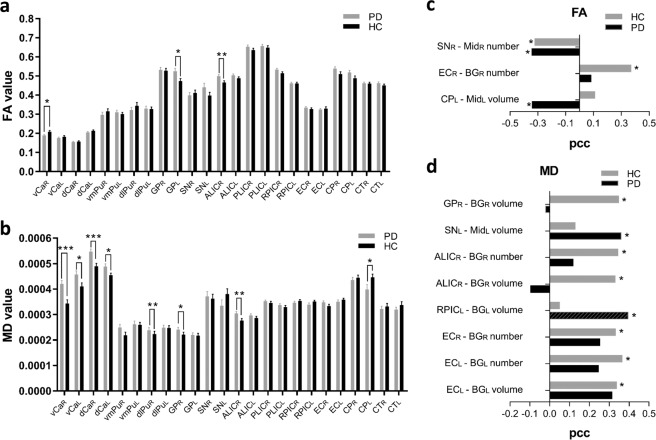
Results of the regions of interest (ROI)-based analysis showed that the PD group had significantly lower fractional anisotropy (FA) value in right-hemispheric ventral caudate (vCa) (p = 0.031) and higher FA value in left-hemispheric globus pallidus (GP) (p = 0.031), as well as higher mean diffusivity (MD) values in bilateral vCa (p = 0.0003 for right-hemisphere, p = 0.030 for left-hemisphere), bilateral dorsal caudate (dCa) (p = 0.0006 for right-hemisphere, p = 0.021 for left-hemisphere), right-hemispheric ventromedial putamen (vmPu) (p = 0.027), and right-hemispheric GP (p = 0.049). In the fiber of interest (FOI)-based analysis, we also found significantly higher FA value in right-hemispheric anterior limb of internal capsule (ALIC) (p = 0.005), as well as higher MD value in right-hemispheric ALIC (p = 0.005) and lower MD values in left-hemispheric cerebral peduncle (CP) (p = 0.029) in PD group compared to HC group (Fig. 4a, b). The results of right vCa and right dCa were survived after false discovery rate (FDR) correction.
Fig. 4. Fractional anisotropy (FA) values and mean diffusivity (MD) values analysis.
a and b Comparison of FA and MD values between PD and HC groups. c and d Correlations between PVS burden indexes and FA, MD values in PD and HC. * Indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. The results are presented as means ± standard error of mean. Abbreviations: vCa ventral caudate, dCa dorsal caudate, vmPu ventromedial putamen, dlPu dorsolateral putamen, GP globus pallidus, SN substantia nigra, ALIC anterior limb of internal capsule, PLIC posterior limb of internal capsule, RPIC retrolenticular part of internal capsule, EC external capsule, CP cerebral peduncle, CT corticospinal tract, pcc partial correlation coefficient.
Correlations between the FA values and the PVS burden indexes were presented in Fig. 4c. The FA value of subcortical nuclei, such as the right-hemispheric SN within midbrain was negatively correlated with the PVS number for the MidR (pcc = −0.342, p = 0.044) in PD group, whereas a similar presentation was also found in HC group (pcc = −0.322, p = 0.049). Regarding to the white matter fibers, the FA value of the left-hemispheric CP was negatively correlated with the PVS volume of MidL (pcc = −0.341, p = 0.045) in the PD group. And in the HC group, the PVS number of BGR was positively correlated with FA values of right-hemispheric external capsule (EC) (pcc = 0.369, p = 0.023).
Correlations between MD values and PVS burden indexes were presented in Fig. 4d. The MD value of left-hemispheric SN was positively correlated with the PVS volume of MidL (pcc = 0.359, p = 0.034) in the PD group. In the HC group, the MD value of right-hemispheric GP was positively correlated with the PVS volume of BGR (pcc = 0.346, p = 0.033). In regard to the white matter fibers, the PVS volume of BGL was positively correlated with the MD value of left-hemispheric retrolenticular part of internal capsule (RPIC) (pcc = 0.393, p = 0.019) in the PD group. In the HC group, the MD value of right-hemispheric ALIC was positively correlated with the PVS number (pcc = 0.342, p = 0.035) and volume (pcc = 0.330, p = 0.043) of BGR. The MD value of right-hemispheric EC was positively correlated to the PVS number of BGR (pcc = 0.330, p = 0.043), and the MD value of left-hemispheric EC was positively correlated to the PVS number (pcc = 0.363, p = 0.025) and volume (pcc = 0.337, p = 0.039) of BGL.
Correlation analysis with white matter fibers
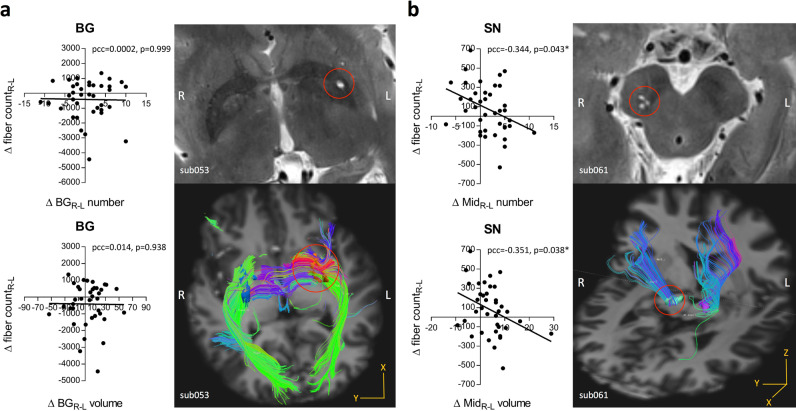
There was no significant difference of the fiber counts of white fiber tract passing through the nuclei within BG and midbrain between PD and HC groups, and we did not find significant correlations between PVS burden and fiber counts. Regarding to the BG region, the difference between bilateral PVS burden indexes (both PVS number and volume) were not significantly correlated to the difference between fiber counts of white matter fiber tract passing through bilateral subcortical nuclei, including the vCa, dCa, vmPu, dorsolateral putamen (dlPu), GP, and whole BG in both PD and HC groups (only results of whole BG in PD was shown in Fig. 5a). And with respect to the midbrain region, the difference between bilateral PVS burden indexes were, respectively, correlated to the difference between fiber counts of white fiber tract passing through bilateral SN (pcc = −0.344, p = 0.043 for PVS number; pcc = −0.351, p = 0.038 for PVS volume) after controlling for total brain volume, age, and sex in the PD group (Fig. 5b). Although there was no statistical significance, the HC group still showed a negative correlation trend.
Fig. 5. White matter fibers tacking analysis.
a Correlations of the difference between bilateral PVS burden indexes in BG (ΔBGR-L number and volume) and the difference between fiber counts of white fiber tract passing through the bilateral BG in PD. An example of fiber tacking result of one PD patient (sub053) with severer PVS burden in left-hemispheric putamen. b Correlations of the difference between bilateral PVS burden indexes in midbrain (ΔMidR-L number and volume) and the difference between fiber counts of white fiber tract passing through the bilateral midbrain in PD. An example of fiber tacking result of one PD patient (sub061) showing severe PVS burden and sparser fiber in right midbrain. * Indicates the partial correlation coefficient (pcc) with p < 0.05. Δfiber countR-L difference between fiber counts of white fiber tract passing through the bilateral BG or midbrain.
Discussion
In this study, we explored the PVS burden considering both number and volume in BG and midbrain in the early-stage PD patients. We detected significantly heavier PVS burden in PD group than the HC group on 7 T MRI images, as well as provided fresh data to support the relationship between PVS burden and motor symptom severity in PD. Along with previous studies, we also found that PVS burden was significantly correlated with cognitive function and emotional status. Furthermore, changes of diffusion tensor imaging (DTI)-derived features of certain subcortical nuclei and white matter fibers were also associated with PVS burden.
Considering the potential functions of the PVS system, studies had proved that the PVS waste drainage system contributed to a larger portion of interstitial solutes clearance, such as amyloid beta (Aβ), preventing Aβ deposition that was thought to be pathogenic in AD18,22. Similar to AD, abnormal aggregated protein α-synuclein that forms Lewy bodies or Lewy neuritis is the main neuropathological characteristics of PD13. PVS may also contribute to clearance of abnormal α-synuclein in the brain19. On the one hand, the failure of perivascular clearance might cause increased α-synuclein deposition, formation of Lewy pathology and damage of dopaminergic neurons, which might lead to modification of PD pathology and clinical features. On the other hand, dopaminergic neuronal degeneration might produce excessive metabolic wastes and accumulated in PVS, lead to perivascular blockage and heavier PVS burden, which might contribute to the pathological process in PD23. Unfortunately, previous studies linking PVS and dopamine transporter (DAT) uptake showed inconsistent results. Several case reports and case-control studies had shown that the existence of PVS might relate to lower DAT uptake24–26. And it was also proposed that enlarged PVSs would cause downstream dysfunction from the presynaptic nigrostriatal dopaminergic system and led to incomplete response to levodopa25,26. Other study showed no significant correlation between PVS burden and DAT positron emission tomography (PET) abnormality11. To explain this phenomena, studies had identified two types of PVS according to the histological appearance of the surrounding tissue: type 1 with normal surrounding nervous tissue, and type 2 with surrounding rarefication and abnormal gliosis11,27, suggesting that these inconsistent results might be due to different types of detected PVS in these studies. The most commonly detected PVSs on MRI images have diameters <3 mm, are used to be considered as type 1 with normal physiological functions. Accordingly, the present study mainly focused on the potential role of these PVSs.
Previous studies mainly focused on reporting PD cases with enlarged PVS14,25,28,29 and studying the populations of PD patients without comparing to the normal controls15,30,31. We compared the PVS burden of PD group to those of HCs, and found a more severe PVS burden in PD. Another difference with previous studies was that those studies mainly focused on middle-stage and late-stage PD patients with average of ages over 60 years old10,11, whereas we intended to investigate the PVS burden in early-stage PD patients with average of age about 50 years old. We observed more severe PVS burden in the PD group, therefore we revealed that the exacerbation of PVS burden was already existed in the early phase of PD disease process. Statistically significant differences were mainly found in PVS burden in the right-hemisphere, the left hemisphere only showed an increasing trend in PD. To a certain extent, our results might provide new evidence for hemispheric lateralization among PD. The progress of PVS burden in PD might start from right-hemisphere, and irrelevant to symptom onset-side. Previous studies have pointed out the lateralization of certain brain function, the right-hemisphere is dominant for controlling limb position and posture, whereas the left-hemisphere is the dominant for appendicular movements32,33. The neurodegenerative process of PD also initials asymmetrically, might exhibit left-hemispheric predominance of subclinical nigrostriatal deficit34,35. Moreover, PD patients with freezing of gait were presented with more affected right-hemisphere circuitry, especially in the pedunculopontine nucleus within midbrain36,37. However, the mechanism of lateralization for motor features and neuro-pathophysiology of PD still requires more evidence. In the present study, significant correlations between the PVS burden in left BG and MDS-UPDRS scores were also found in the PD patients. We suggested that the PVS number in left BG could reflect severity and progression of PD motor symptoms. In addition, our results showed that PD patients with more severe PVS burden in right hemisphere needed higher dosage of anti-PD drugs, which is in line with the hypothesis that possible dysfunction of the PVS pathway existed in PD, as well as the incomplete response to levodopa caused by PVS-related dysfunction of presynaptic nigrostriatal dopaminergic system25,26. The right striatum might have lower quantities of dopamine than those of left, which lead to vulnerability to nigrostriatal denervation in right-hemisphere38. Interestingly, PD patients with relatively heavier right-hemisphere dysfunction displayed greater medication doses39, which was consistent with our results. Besides, since only the PVS number of BG was correlated to these clinical features, the PVS number rather than PVS volume in BG might be a more important measure to represent severity of motor symptoms, which was consistent with one previous study declaring that PVS number was more meaningful than PVS volume21.
A previous study reviewed that the prefrontal-limbic network in depression is modulated by hypothalamus, BG, and midbrain40. Increased PVS burden over time significantly associated with higher incidence of depressive symptoms6 and might be able to predict resistance to antidepressant monotherapy in elderly patients with depression41. An animal study supported this assumption with revealing that dilated PVS might exacerbate depression-like behaviors by increasing the levels of inflammatory factors42. We found significant association between HAMD score and PVS burden of both BG and midbrain in the HC group, indicating that poor emotional states like depression might aggravate the PVS burden. But the emotional status of most HCs was at a normal level, the correlation might be in different extent while focusing on patients with depression. As PVS dysfunction might relate to neuro-neurophysiology and clinical motor features of PD, it also related to cognitive decline in PD3,4,15. The PVS burden, particularly correlated with executive functioning4 and visuospatial ability43, which were frequently involved cognitive domains in PD. Possible mechanism of PVS might be decreased clearance of metabolic waste, lead to accumulation of Aβ, α-synuclein relating to cognitive decline. In patients with cognition decline, brain atrophy was often accompanied by cerebrospinal fluid (CSF) space enlargement and cerebral ventricular dilatation, might following by a secondary ex-vacuo effect as compensation3,44. Therefore, the PVS might be an earlier imaging feature than brain atrophy during the aging and cognition decline process3. Studies of PD provided evidence that PVS burden in BG could be regarded as biomarker to evaluate cognitive decline in PD15,30. We also found that healthy elderly with more severe PVS burden in midbrain, had more decreased cognitive function. However, the PVS burden did not show a significant correlation with the emotional state and cognitive function in PD patients, which might be caused by the possible presence of other influential factors that covered these correlations.
PVS is a microscopic tubular structure that occupies extra-vascular space, in which water molecules move freely45. Regarding to white matter fiber tracts, they are spatially parallelizing with PVSs46. Except for vessels, water molecules inside the PVS are also hindered by surrounding brain tissue. DTI-derived features reflect the diffusion properties of both the surrounding brain tissue and fluid from PVS, thus the enlargement of PVS might have substantial influence on DTI features45. At the same time, the activity of the glymphatic system could be evaluated by calculating diffusivity along the PVS, providing more evidence that the PVS burden related to the impairment of white matter fiber47. In addition to the relationship between PVS and dopaminergic terminals that we have mentioned above11, gray and white matter microstructures might also be an important part of the pathophysiological mechanism of PVS.
We used the FA value to quantify the degree of directional dependence of diffusion that depends on the number and density of axons48. The MD value was also used to describe the overall amount of isotropic diffusion within brain tissue49, and its increasing might be caused by enlargement of extracellular space, suggesting degeneration of brain tissue50. However, towards the changes of white matter microstructure in PD, the alterations of FA and MD due to PD remain controversial. Most studies focusing on advanced-stage PD patients reported decreased FA and/or increased MD, indicating demyelination and axonal degeneration of white matter microstructure51. Other results like increased FA and decreased MD were defined as microstructural compensation in prodromal-stage and early-stage of PD, which was distinguished from pathological findings52. Our results showed significantly decreased FA in caudate accompanied by increased MD in caudate and putamen, demonstrating degeneration in these respective regions. And significantly increased FA and MD values in GP and ALIC were detected, indicating possible selective neurodegeneration or a potential compensatory reorganization after treatment50.
Back to the correlation between PVS burden and surrounding brain tissues, the PVS number in BG was correlated with the load of deep white matter hyperintensity, which was corresponding to the demyelination and sparseness of axons21. And some PD cases reported that the DTI features of fibers adjacent to enlarged PVS were changed compared to the normal contralateral fibers28. Similarly, our results indicated that PVS burden severity was related to the integrity of adjacent white matter fibers. The participants with higher PVS volume in midbrain had a more decreased FA and more increased MD in SN, revealed that the PVS might increase the diffusivity of water molecules and even exacerbate the degeneration of SN. The MD values of GP, ALIC, RPIC, and EC were also increasing with the PVS burden indexes of BG, indicating PVS burden in BG also influenced the adjacent white matter microstructure. We also investigated the correlation between the fiber counts of white matter fiber track passing through bilateral subcortical nuclei and bilateral PVS burden. For the midbrain in PD patients, we found that the hemisphere with more severe PVS burden in midbrain showed a sparser fiber track passing through SN than the contralateral hemisphere. Since the HCs had less severe PVS burden than PD patients, they just showed a negative correlation trend, with no statistical significance was attained. PVS burden in unilateral cerebral hemisphere was significantly correlated to ipsilateral DTI-derived features, while the same index in contralateral hemisphere only showed a correlation trend. Possibly, we quantitated the PVS burden in the whole BG and midbrain, within which the distribution of PVS might vary in different subcortical nuclei and white matter fibers. Although without statistical significance, these results still indicating slightly positive or negative correlations between PVS burden and both gray and white matter microstructure. Based on these results, smaller PVS burden that was previously considered as type 1 “normal” structure might still exhibit physiological dysfunctions, which was mainly reflected by causing an indispensable important effect on the brain parenchyma, particularly the DTI-derived features. However, this phenomenon might not be unique to PD. As a possible biomarker for cerebral small-vessel disease affecting nearby brain parenchyma21, PVS burden indexes might be able to play a disease-modifying role in a certain disease by involving related brain regions.
Growing evidence had shown that insufficient clearance of pathologic protein deposits through brain drainage system including the PVS pathway might serve as new targets for promising modifier treatment and even prevention for neurodegenerative disorders53. The “perivascular pump” driven by arterial pulsation might be a powerful convection enhanced delivery method, by which viral particles for gene-therapy or therapeutic agents could be administered directly into central nervous system54. Furthermore, focused ultrasound combined with microbubbles could enhance the brain blood barrier opening along with expansion and contraction of PVS, which might induce bulk flow and lead to increased penetration of drugs into the brain tissue55. Another important therapeutic target is enhancing the brain clearance mechanisms by reducing in reactive astro- and microgliosis, normalizing the perivascular aquaporin-4 polarization, inducing fibrinolysis of fibrin clots in PVS, which are still beyond clinical application so far56. Since the clearance of interstitial solutes increases by 60% during the sleep state and exercise could also increase glymphatic influx and clearance, sleep improvement, and voluntary exercise provide easily implemented strategies to improve glymphatic function56. Moreover, increased PVS burden is associated with hypertension56, thus, improved hypertension control might also help in slowing down the aggravation of PVS burden and its potential role in neurodegenerative disorders.
In conclusion, the present study demonstrated that the PVS burden in the BG and the midbrain were already aggravated in early-stage PD patients. The PVS burden indexes, including PVS number and volume, could be considered as a biomarker to evaluate severity and progression of PD motor symptom, and aid in predicting medication dosage. Furthermore, the PVS burden might also reflect microstructural alteration of surrounding brain tissues. Despite the limited sample size, with higher spatial resolution and SNR of MRI data, our study provides preliminary evidence to support the potential role of PVS in the pathophysiology of PD. Future work will focus on reviewing the correlations between PVS burden and the progression of clinical feature, as well as the continuing impact of PVS burden on brain parenchyma through longitudinal research during the course of PD.
Methods
Participants and clinical data collection
This study was approved by the ethics committee of Second Affiliated Hospital of Zhejiang University School of Medicine. Written informed consent was obtained from all the participants. We recruited 40 PD patients from the outpatient clinic of the Department of Neurology of Second Affiliated Hospital of Zhejiang University School of Medicine, and 41 age-matched and sex-matched healthy controls (HCs) from neighboring communities.
PD patients met the Movement Disorder Society (MDS) Clinical Diagnostic Criteria for PD57. HCs had no history of neurological or psychiatric disease and no family history of PD or related neurodegenerative disorders. Participants with small vessel disease such as white matter hyperintensity, lacunar infarctions, and cerebral microbleeds were excluded. All the participants underwent a clinical evaluation, including clinical histories (self-report or medical records), neurological, and neuropsychological examinations. PD disease severity was assessed using MDS-UPDRS and Hoehn & Yahr stage (H-Y stage) during off-stage, early-stage PD was defined as H-Y stage between 1 and 2.558. We separately analyzed the MDS-UPDRS subscores for more detailed information, including the subscore I for non-motor experiences of daily living, subscore II for motor experiences of daily living, subscore III for motor examination, and subscore IV for motor complications59. We applied the HAMA and HAMD to assess emotional status, the MMSE, MoCA, and PANDA to evaluate cognitive function.
MRI images acquisition
MRI images were acquired with a 7 T Magnatom research system (Siemens Healthcare, Erlangen, Germany) with prototype sequences, including a magnetization prepared with two rapid gradient echoes (MP2RAGE) sequence (voxel size: 0.7 × 0.7 × 0.7 mm3, TR = 5000 ms, TI1/TI2 = 900/2750 ms, TE = 2.3 ms, α1/α2 = 5°/3°, and two times generalized autocalibrating partial parallel acquisition (GRAPPA) acceleration), a T2-weighted turbo spin echo (TSE) sequence (voxel size: 0.5 × 0.5 × 2.4 mm3, TR = 7000 ms, and TE = 66 ms) and a multiband echo-planar DTI sequence (voxel size: 1.5 × 1.5 × 1.5 mm, TR = 6000 ms, TE = 87.4 ms). A patient underwent an extra 3 T MRI scan to obtain another T2 image on a 3 T Prisma research system (Siemens Healthcare, Erlangen, Germany) using a T2-weighted TSE sequence (voxel size: 0.5 × 0.5 × 2.8 mm3, TR = 9360 ms, and TE = 100 ms) for comparsion.
MRI images processing
In this study, we focused on the effects of small size PVSs. Therefore, a PVS was defined as a small (<3 mm), smooth, linear-shaped, ovoid-shaped, or round-shaped space with isointense CSF that ran along with perforating arteries60–62. We acquired a high-resolution MP2RAGE image to visualize the PVS system and its spatially corresponding lenticulostriate artery (LAS) simultaneously, as shown in Fig. 1c, with PVSs showing low intensity similar to CSF and the blood vessels showing high intensity. The PVS burden was defined to represent the severity of PVSs, which was assessed on axial T2-weighted images in the BG and midbrain (Fig. 1b). According to previous studies15,63, we performed PVS quantification in the slice containing the maximum amount of PVSs in the BG region60, of which the anterior and posterior border zones were the anterior end of the insula and the posterior end of the thalamus1. For the midbrain region, the PVSs were counted in the whole volume64. The PVS counting, segmentation, and volume calculation were performed using the ITK-SNAP software version 3.8 (http://www.itksnap.org/). We separately counted PVS number and calculated the PVS volume in four defined regions: BGR, BGL, MidR, and MidL. We manually delineated along the boundary of all identified PVSs. The ITK-SNAP software automatically gave the voxel number of identified PVSs in each region, and the PVS volume was calculated as the sum of the individual volume of identified PVS in each region per mm3. Then we obtained the PVS number and the PVS volume of each region, regarded as the PVS burden indexes.
Total brain volume that was expressed as the sum of total gray and white matter volume was estimated on MP2RAGE images using VBM8 embedded in SPM8 software (Statistical Parametric Mapping; https://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The DTI data was processed using FSL version 6.0.1 (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl) to generate fractional anisotropy (FA) and mean diffusivity (MD) maps. The Brainnetome Atlas65 adding additional nuclei within midbrain, and the ICBM-DTI-81 white matter atlas66 were separately transformed into each participant’s DTI space67. Then we used “fslmeants” command of FSL to obtain 12 subcortical nuclei ROIs, including the bilateral vCa, dCa, vmPu, dlPu, GP and SN, as well as 12 FOIs, including the bilateral ALIC, posterior limb of internal capsule (PLIC), RPIC, EC, CP, and corticospinal tract (CT) from these two atlases. Then the averages of FA and MD values were extracted for subsequent analysis.
Deterministic tractography was conducted using Diffusion Toolkit version 0.6.4 and TrackVis version 0.6.1 (http://trackvis.org) in each participant’s DTI space. We applied a DWI mask threshold and an angular threshold of 35° to limit deviations from main path. We loaded ROIs from transformed atlas including bilateral vCa, dCa, vmPu, dlPu, GP, and SN to track white matter fibers passing through these subcortical nuclei, and the fiber counts of each track were given for subsequent analysis.
Statistical analysis
The results were expressed as mean ± standard error of mean (SEM) for continuous variables and as percentages for the categorical variables. The Student’s two-sample t-test was used for the comparison of continuous variables including clinical and DTI features between groups. The chi-squared test was used for the comparison of categorical variables. The difference between right- and left-hemispheric PVS burden indexes, and the differences between fiber counts of white matter fibers passing through the right- and left-hemispheric nuclei within BG and midbrain were also calculated. Partial correlation analysis was performed to calculate pcc and evaluate the correlations between PVS number, PVS volume and MDS-UPDRS, H-Y stage, LEDD, MMSE, MoCA, PANDA, HAMA, HAMD scores, and DTI parameters. Age, sex, education, and total brain volume were entered as covariates due to their potential influence. These analyses were considered significant at p < 0.05, uncorrected. At the same time, we further used a FDR corrected p < 0.05 for multiple testing.
Statistical analyses were conducted with SPSS Statistics software version 22 (IBM Corporation, Armonk, NY, USA). Statistical plots were generated by GraphPad Prism version 7 (GraphPad Inc., San Diego, CA, USA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFA0701400), the National Natural Science Foundation of China (61673346, 81520108010), the National Major Scientific Instruments Development Project of China (81527901), the Science and Technology Project of Zhejiang Province (2016C34006), the Fundamental Research Funds for the Central Universities (2019XZZX001-01-21) and Zhejiang Lab (No. 2018EB0ZX01). The authors gratefully thank all study participants for their participation in this study. And we would like to acknowledge Zhejiang University 7 T Brain Imaging Research Center for all the help and support.
Author contributions
T.S., H.-Y.L., and B.R.Z. designed this study. Y.X.C., J.T., and W.L. were responsible for diagnosis and clinical evaluation. Y.-C.H. and T.K. designed MRI sequences. C.-Y.L. contributed to the DTI sequence modification. Y.M.Y., J.J.X., and S.Z. collected MRI data. T.S. and Y.M.Y. analyzed MRI data. T.S. wrote the manuscript. H.-Y.L. and B.R.Z. contributed to the revision of the manuscript.
Data availability
Clinical and neuroimaging data can be shared on reasonable request from qualified investigators by contacting the corresponding authors. Sharing and reuse of our data require the expressed written permission of the authors.
Code availability
As previously mentioned in the “MRI images processing” subsection, the PVS quantification was performed using the ITK-SNAP version 3.8 (http://www.itksnap.org/). Total brain volume was estimated using VBM8 embedded in SPM8 software (Statistical Parametric Mapping; https://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The DTI data was processed using FSL 6.0.1 (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl). Deterministic tractography was conducted using Diffusion Toolkit 0.6.4 and TrackVis 0.6.1 (http://trackvis.org). Statistical analyses were conducted with SPSS Statistics software version 22 (IBM Corporation, Armonk, NY, USA). Statistical plots were generated by GraphPad Prism version 7 (GraphPad Inc., San Diego, CA, USA).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Baorong Zhang, Email: brzhang@zju.edu.cn.
Hsin-Yi Lai, Email: laihy@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-021-00155-0.
References
- 1.Bouvy WH, et al. Perivascular spaces on 7 Tesla brain MRI are related to markers of small vessel disease but not to age or cardiovascular risk factors. J. Cereb. Blood Flow Metab. 2016;36:1708–1717. doi: 10.1177/0271678X16648970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams HH, et al. A priori collaboration in population imaging: The Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement consortium. Alzheimer’s Dement. 2015;1:513–520. doi: 10.1016/j.dadm.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niazi M, et al. Quantitative MRI of perivascular spaces at 3T for early diagnosis of mild cognitive impairment. Am. J. Neuroradiol. 2018;39:1622–1628. doi: 10.3174/ajnr.A5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passiak BS, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology. 2019;92:e1309–e1321. doi: 10.1212/WNL.0000000000007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berezuk C, et al. Virchow–Robin spaces: correlations with polysomnography-derived sleep parameters. Sleep. 2015;38:853–858. doi: 10.5665/sleep.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Sloten TT, et al. Cerebral small vessel disease and association with higher incidence of depressive symptoms in a general elderly population: The AGES-Reykjavik Study. Am. J. Psychiatry. 2015;172:570–578. doi: 10.1176/appi.ajp.2014.14050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potter GM, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int. J. Stroke. 2015;10:376–381. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee G, et al. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain. 2017;140:1107–1116. doi: 10.1093/brain/awx003. [DOI] [PubMed] [Google Scholar]
- 9.Favaretto, A. et al. Enlarged Virchow Robin spaces associate with cognitive decline in multiple sclerosis. PLoS ONE12, ARTN https://doi.org/e018562610.1371/journal.pone.0185626 (2017). [DOI] [PMC free article] [PubMed]
- 10.Laitinen LV, et al. Dilated perivascular spaces in the putamen and pallidum in patients with Parkinson’s disease scheduled for pallidotomy: a comparison between MRI findings and clinical symptoms and signs. Mov. Disord. 2000;15:1139–1144. doi: 10.1002/1531-8257(200011)15:6<1139::AID-MDS1012>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Lee D, Hong IK, Ahn TB. Dilated Virchow–Robin space and dopamine transporter imaging in the striatum of patients with Parkinsonism. Can. J. Neurol. Sci. Le. J. Can. Sci. Neurol. 2015;42:248–254. doi: 10.1017/cjn.2015.43. [DOI] [PubMed] [Google Scholar]
- 12.Shen T, et al. Early-onset Parkinson’s disease caused by PLA2G6 compound heterozygous mutation, a case report and literature review. Front. Neurol. 2019;10:915. doi: 10.3389/fneur.2019.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 14.Fenelon G, et al. Parkinsonism and dilatation of the perivascular spaces (etat crible) of the striatum: a clinical, magnetic resonance imaging, and pathological study. Mov. Disord. 1995;10:754–760. doi: 10.1002/mds.870100609. [DOI] [PubMed] [Google Scholar]
- 15.Park, Y. W. et al. Magnetic resonance imaging-visible perivascular spaces in basal ganglia predict cognitive decline in Parkinson’s disease. Mov. Disord.10.1002/mds.27798 (2019). [DOI] [PubMed]
- 16.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 17.Wuerfel J, et al. Perivascular spaces–MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332–2340. doi: 10.1093/brain/awn171. [DOI] [PubMed] [Google Scholar]
- 18.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundaram S, et al. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neurosci. Biobehav. Rev. 2019;103:305–315. doi: 10.1016/j.neubiorev.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Castro V, et al. Reliability of an automatic classifier for brain enlarged perivascular spaces burden and comparison with human performance. Clin. Sci. 2017;131:1465–1481. doi: 10.1042/CS20170051. [DOI] [PubMed] [Google Scholar]
- 21.Laveskog A, et al. Perivascular spaces in old age: assessment, distribution, and correlation with white matter hyperintensities. Am. J. Neuroradiol. 2018;39:70–76. doi: 10.3174/ajnr.A5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherian I, et al. Exploring the Virchow–Robin spaces function: a unified theory of brain diseases. Surg. Neurol. Int. 2016;7:S711–S714. doi: 10.4103/2152-7806.192486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez J, et al. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell. Mol. Neurobiol. 2016;36:289–299. doi: 10.1007/s10571-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, et al. Dilated perivascular space in the midbrain may reflect dopamine neuronal degeneration in Parkinson’s disease. Front. Aging Neurosci. 2020;12:161. doi: 10.3389/fnagi.2020.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta SH, et al. Dilated Virchow–Robin spaces and Parkinsonism. Mov. Disord. 2013;28:589–590. doi: 10.1002/mds.25474. [DOI] [PubMed] [Google Scholar]
- 26.Mestre TA, et al. Can isolated enlarged Virchow–Robin spaces influence the clinical manifestations of Parkinson’s disease? Mov. Disord. Clin. Pract. 2014;1:67–69. doi: 10.1002/mdc3.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancardi GL, et al. Lacunae and cribriform cavities of the brain. Correlations with pseudobulbar palsyand Parkinsonism. Eur. Neurol. 1988;28:11–17. doi: 10.1159/000116220. [DOI] [PubMed] [Google Scholar]
- 28.Conforti R, et al. Dilated Virchow–Robin space and Parkinson’s disease: a case report of combined MRI and diffusion tensor imaging. Radiol. Case Rep. 2018;13:871–877. doi: 10.1016/j.radcr.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DG, Oh SH, Kim OJ. A case of disseminated polycystic dilated perivascular spaces presenting with dementia and parkinsonism. J. Clin. Neurol. 2007;3:96–100. doi: 10.3988/jcn.2007.3.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata K, Sugiura M, Nishimura Y, Sakura H. The effect of small vessel disease on motor and cognitive function in Parkinson’s disease. Clin. Neurol. Neurosurg. 2019;182:58–62. doi: 10.1016/j.clineuro.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Wan, Y. et al. Exploring the association between Cerebral small-vessel diseases and motor symptoms in Parkinson’s disease. Brain Behav. 910.1002/brb3.1219 (2019). [DOI] [PMC free article] [PubMed]
- 32.Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 2006;7:160–166. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- 33.Lizarraga KJ, et al. Asymmetric neuromodulation of motor circuits in Parkinson’s disease: the role of subthalamic deep brain stimulation. Surg. Neurol. Int. 2017;8:261. doi: 10.4103/sni.sni_292_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherfler C, et al. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain. 2012;135:3348–3354. doi: 10.1093/brain/aws253. [DOI] [PubMed] [Google Scholar]
- 35.Iranzo A, et al. Left-hemispheric predominance of nigrostriatal deficit in isolated REM sleep behavior disorder. Neurology. 2020;94:e1605–e1613. doi: 10.1212/WNL.0000000000009246. [DOI] [PubMed] [Google Scholar]
- 36.Fling BW, et al. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136:2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam S, et al. Does dominant pedunculopontine nucleus exist? Brain. 2015;138:e323. doi: 10.1093/brain/awu225. [DOI] [PubMed] [Google Scholar]
- 38.Haaxma CA, et al. Side of symptom onset affects motor dysfunction in Parkinson’s disease. Neuroscience. 2010;170:1282–1285. doi: 10.1016/j.neuroscience.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Hanna-Pladdy B, Pahwa R, Lyons KE. Paradoxical effect of dopamine medication on cognition in Parkinson’s disease: relationship to side of motor onset. J. Int. Neuropsychol. Soc. 2015;21:259–270. doi: 10.1017/S1355617715000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett MR. The prefrontal-limbic network in depression: modulation by hypothalamus, basal ganglia and midbrain. Prog. Neurobiol. 2011;93:468–487. doi: 10.1016/j.pneurobio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Patankar TF, et al. Virchow–Robin space dilatation may predict resistance to antidepressant monotherapy in elderly patients with depression. J. Affect. Disord. 2007;97:265–270. doi: 10.1016/j.jad.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, et al. Dilated Virchow–Robin spaces in the hippocampus impact behaviors and effects of anti-depressant treatment in model of depressed rats. J. Affect. Disord. 2017;219:17–24. doi: 10.1016/j.jad.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 43.Maclullich AM, et al. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J. Neurol. Neurosurg. Psychiatry. 2004;75:1519–1523. doi: 10.1136/jnnp.2003.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudie JD, Rauschecker AM, Nabavizadeh SA, Mohan S. Neuroimaging of dilated perivascular spaces: from benign and pathologic causes to mimics. J. Neuroimaging. 2018;28:139–149. doi: 10.1111/jon.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sepehrband F, et al. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. Neuroimage. 2019;197:243–254. doi: 10.1016/j.neuroimage.2019.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai K, et al. The feasibility of quantitative MRI of perivascular spaces at 7 T. J. Neurosci. Methods. 2015;256:151–156. doi: 10.1016/j.jneumeth.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taoka T, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol. 2017;35:172–178. doi: 10.1007/s11604-017-0617-z. [DOI] [PubMed] [Google Scholar]
- 48.Zasler, N. D. & Kaplan, P. E. Fractional Anisotropy (Springer: New York, 2016).
- 49.Vos SB, et al. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. NeuroImage. 2012;59:2208–2216. doi: 10.1016/j.neuroimage.2011.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkinson-Clement C, Pinto S, Eusebio A, Coulon O. Diffusion tensor imaging in Parkinson’s disease: review and meta-analysis. NeuroImage. 2017;16:98–110. doi: 10.1016/j.nicl.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andica C, et al. Neurocognitive and psychiatric disorders-related axonal degeneration in Parkinson’s disease. J. Neurosci. Res. 2020;98:936–949. doi: 10.1002/jnr.24584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanjari Moghaddam, H., Dolatshahi, M., Mohebi, F. & Aarabi, M. H. Structural white matter alterations as compensatory mechanisms in Parkinson’s disease: a systematic review of diffusion tensor imaging studies. J. Neurosci. Res.10.1002/jnr.24617 (2020). [DOI] [PubMed]
- 53.Pahnke J, Langer O, Krohn M. Alzheimer’s and ABC transporters–new opportunities for diagnostics and treatment. Neurobiol. Dis. 2014;72 Part A:54–60. doi: 10.1016/j.nbd.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadaczek P, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol. Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen, H. et al. Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor. Sci. Rep.610.1038/Srep28599 (2016). [DOI] [PMC free article] [PubMed]
- 56.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017;131:2257–2274. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Postuma RB, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1599. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 58.Kwon DY, Kwon Y, Kim JW. Quantitative analysis of finger and forearm movements in patients with off state early stage Parkinson’s disease and scans without evidence of dopaminergic deficit (SWEDD) Parkinsonism Relat. Disord. 2018;57:33–38. doi: 10.1016/j.parkreldis.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 60.Liang Y, et al. Enlarged perivascular spaces in the centrum semiovale are associated with poststroke depression: a 3-month prospective study. J. Affect. Disord. 2018;228:166–172. doi: 10.1016/j.jad.2017.11.080. [DOI] [PubMed] [Google Scholar]
- 61.Wardlaw JM, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jimenez-Balado J, et al. Prevalence of hippocampal enlarged perivascular spaces in a sample of patients with hypertension and their relation with vascular risk factors and cognitive function. J. Neurol. Neurosurg. Psychiatry. 2018;89:651–656. doi: 10.1136/jnnp-2017-316724. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, C. Q. et al. Risk factors of dilated Virchow–Robin spaces are different in various brain regions. PLoS ONE910.1371/journal.pone.0105505 (2014). [DOI] [PMC free article] [PubMed]
- 64.Dubost F, et al. Enlarged perivascular spaces in brain MRI: automated quantification in four regions. Neuroimage. 2019;185:534–544. doi: 10.1016/j.neuroimage.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 65.Fan L, et al. The human Brainnetome Atlas: a new Brain Atlas based on connectional architecture. Cereb. Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mori S, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui Z, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013;7:42. doi: 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical and neuroimaging data can be shared on reasonable request from qualified investigators by contacting the corresponding authors. Sharing and reuse of our data require the expressed written permission of the authors.
As previously mentioned in the “MRI images processing” subsection, the PVS quantification was performed using the ITK-SNAP version 3.8 (http://www.itksnap.org/). Total brain volume was estimated using VBM8 embedded in SPM8 software (Statistical Parametric Mapping; https://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The DTI data was processed using FSL 6.0.1 (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl). Deterministic tractography was conducted using Diffusion Toolkit 0.6.4 and TrackVis 0.6.1 (http://trackvis.org). Statistical analyses were conducted with SPSS Statistics software version 22 (IBM Corporation, Armonk, NY, USA). Statistical plots were generated by GraphPad Prism version 7 (GraphPad Inc., San Diego, CA, USA).