Abstract
The vast majority of patients with advanced breast cancer present skeletal complications that severely compromise their quality of life. Breast cancer cells are characterized by a strong tropism to the bone niche. After engraftment and colonization of bone, breast cancer cells interact with native bone cells to hinder the normal bone remodeling process and establish an osteolytic “metastatic vicious cycle”. The sympathetic nervous system has emerged in recent years as an important modulator of breast cancer progression and metastasis, potentiating and accelerating the onset of the vicious cycle and leading to extensive bone degradation. Furthermore, sympathetic neurotransmitters and their cognate receptors have been shown to promote several hallmarks of breast cancer, such as proliferation, angiogenesis, immune escape, and invasion of the extracellular matrix. In this review, we assembled the current knowledge concerning the complex interactions that take place in the tumor microenvironment, with a special emphasis on sympathetic modulation of breast cancer cells and stromal cells. Notably, the differential action of epinephrine and norepinephrine, through either α- or β-adrenergic receptors, on breast cancer progression prompts careful consideration when designing new therapeutic options. In addition, the contribution of sympathetic innervation to the formation of bone metastatic foci is highlighted. In particular, we address the remarkable ability of adrenergic signaling to condition the native bone remodeling process and modulate the bone vasculature, driving breast cancer cell engraftment in the bone niche. Finally, clinical perspectives and developments on the use of β-adrenergic receptor inhibitors for breast cancer management and treatment are discussed.
Subject terms: Bone cancer, Neurophysiology, Bone cancer
Introduction
Under physiological conditions, the sympathetic nervous system (SNS) is involved in the so-called “fight-or-flight” response to acute stress. Upon perceiving threats to internal homeostasis, the SNS acts on multiple molecular and cellular processes throughout the body that ensure a coordinated adaptive response to different stressors. Physical mobility is boosted through an increase in heart and respiratory rates, as well as through energy mobilization from adipose tissue and the liver1,2. On the other hand, anabolic processes such as digestion, gastrointestinal motility and reproduction are hampered2–4. Sympathetic signaling is mainly achieved through peripheral release of norepinephrine (NE) by sympathetic nerve terminals or systemic release of epinephrine (Epi) into the circulation by the adrenal glands. These catecholamines are the endogenous ligands of α/β adrenoreceptors (α-AR, β-AR), which exhibit widespread expression in a multitude of cell types and tissues5–8. This family of receptors is composed of a total of nine G protein-coupled receptors (GPCRs): Gq-coupled α1A, α1B, and α1D ARs; Gi-coupled α2A, α2B, and α2C ARs; and finally, Gs-coupled β1, β2, and β3 ARs.
Breast cancer is still a major socioeconomic issue and was the leading cause of cancer-specific death in women in 2018 (https://gco.iarc.fr/today/home). It is a highly heterogeneous disease that is usually characterized by estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor 2 (HER2) status of the primary tumor. Advances in diagnostic and adjuvant therapies have increased the life expectancy of patients with breast cancer, but this condition remains incurable in later stages of disease progression9. Surgery and radiation therapy are the gold standards for the treatment of early-stage breast cancer, as are hormone therapy and the HER2-targeting antibody trastuzumab for HER2-positive cancers. Systemic administration of hormone therapy, targeted therapy, chemotherapy or a combination of these is usually the preferred treatment approach for late-stage metastatic breast cancer. However, the 5-year survival rate of women diagnosed with distant metastasis is 27% (https://www.cancer.org/cancer/breast-cancer). These treatments are still ineffective and commonly associated with toxic side effects; therefore, there is still a need for improved therapeutic options. A better understanding of the pathological processes through which breast cancer thrives in the host is of paramount importance to discovering new therapeutic targets.
In the past decade, the physiological mechanisms that govern the response to stress have emerged as potential therapeutic targets in breast cancer due to findings from several epidemiologic and preclinical studies10–12. In particular, the action of NE and Epi on their cognate receptors has raised important considerations regarding their role in breast cancer progression, analogous to observations in other bone-tropic cancers such as prostate cancer13–17. However, the adrenergic regulation of the multiple cellular processes that drive breast cancer remains a matter of intense debate.
In this review, we discuss the current knowledge found in the literature concerning preclinical and clinical data on SNS modulation of breast cancer. Most patients with metastatic breast cancer present severe skeletal complications such as hypercalcemia, pain, and an increased incidence of fractures18. Therefore, insight into the sympathetic regulation of bone metastatic disease is also discussed in the following sections.
Breast cancer and the SNS: a complex picture
Adrenoreceptors (ARs) have been reported to be expressed in a wide range of breast cancer cell lines (Table 1) as well as in tumor samples from patients with breast cancer19–21. AR overexpression, particularly β2-AR overexpression, was found to be correlated with poor prognosis of ER− breast cancer patients in a recent study by Kurozumi et al.21, where immune biomarkers, such as the grades of tumor-infiltrating lymphocytes and programmed death ligand 1 expression, were shown to be significantly reduced in these patients. Another report by Liu et al.19 demonstrated that the β2-AR level was correlated with lower disease-free survival and higher lymph node metastasis rates in a small cohort of HER2+ breast cancer patients. Both of these studies point to a putative role of β2-AR in breast cancer pathology, but scrutinizing the mechanisms by which it promotes disease progression is still a complex exercise. In this section, we assemble the available data regarding the effect of multiple ARs on breast cancer, from primary tumor proliferation and survival to extracellular matrix (ECM) invasion and entry into the systemic circulation.
Table 1.
AR expression in human breast cancer cell lines
| Cell line | Molecular subtype | AR(s) expressed | Reference |
|---|---|---|---|
| T47D | Luminal A (ER+, PR+, HER2−) | α2A-AR, α2B-AR, α2C-AR | 130 |
| MCF7 | Luminal A (ER+, PR+, HER2−) | α1-AR, α2B-AR, α2C-AR, β1-AR, β2-AR | 19,50,131,132 |
| ZR-75 | Luminal A (ER+, PR+, HER2−) | β1-AR, β2-AR | 131 |
| BT474 | Luminal B (ER+, PR+, HER2+) | β2-AR | 19 |
| SKBR3 | HER2 (ER−, PR−, HER2+) | β2-AR | 19 |
| MDA-MB-453 | HER2 (ER−, PR−, HER2+) | β2-AR | 131 |
| MDA-MB-231 | Basal (ER−, PR−, HER2−) | β2-AR | 11,47,132,133 |
| MDA-MB-468 | Basal (ER−, PR−, HER2−) | β1-AR, β2-AR | 131,133 |
| HS578T | Basal (ER−, PR−, HER2−) | α2A-AR | 132 |
Proliferation and survival
Cancer cell proliferation and apoptosis inhibition are crucial hallmarks of cancer22. Adrenergic signaling has been implicated in several apoptosis pathways, and it has been previously suggested that endogenous catecholamines directly exert prosurvival effects on breast cancer cells23–25 (Figs. 1, 2). Epi was described as an antiapoptotic stimulus in human breast cancer cells in vitro, inactivating the proapoptotic protein BAD through phosphorylation in a PKA-dependent manner24. Furthermore, another in vitro experiment by Reeder et al. showed that NE and Epi decrease the efficacy of commonly used drugs targeting proliferating cells, such as paclitaxel, since these catecholamines arrest MDA-MB-231 breast cancer cells in G1 phase, decelerating the cell cycle25. These results are consistent with evidence from other in vitro studies showing that β2-AR agonists inhibit triple-negative breast cancer cell proliferation and DNA synthesis23,26,27. Strikingly, low concentrations of Epi increased MCF7 and MDA-MB-231 cell proliferation, while the β2-AR agonist isoproterenol decreased the proliferation of both cell lines27. These findings could be explained by the observation that Epi was shown to differentially bind to distinct ARs depending on its concentration, with greater affinity for α2-AR at nanomolar concentrations and shifting to β2-AR binding at micromolar concentrations23. Moreover, the increase in proliferation evoked by low concentrations of Epi was abrogated by the addition of the α2-AR antagonist rauwolscine23. Exciting questions remain, such as the following: what is the impact of fluctuations in Epi or NE levels in the tumor microenvironment on breast cancer progression, and how can this knowledge be translated to a clinical setting? There is already recent in vivo evidence that sheds some light on the impact of circulating Epi on tumor growth; Walker and colleagues have shown that adrenal denervation and inhibition of Epi release do not impact disease progression28.
Fig. 1.
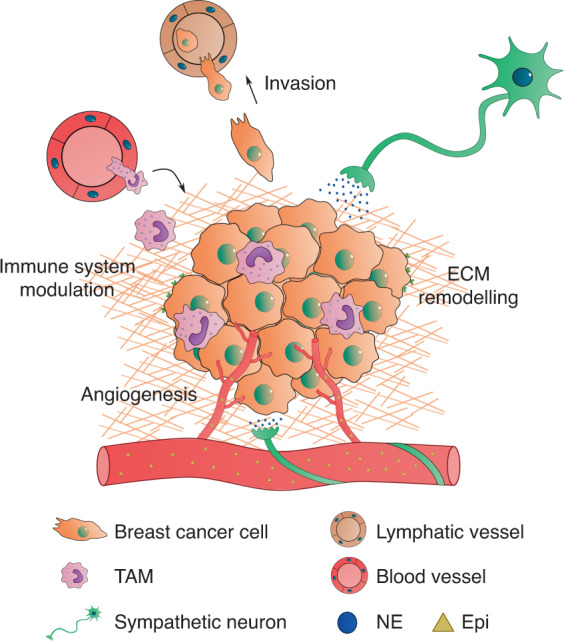
Sympathetic control of breast cancer progression. NE released from sympathetic neurons closely associated with blood vessels, as well as Epi that diffuses from the circulation, modulate several important hallmarks of breast cancer such as survival, angiogenesis, immune surveillance escape, ECM remodeling and invasion. NE, norepinephrine; Epi, epinephrine; TAM, tumor-associated macrophage; ECM, extracellular matrix
Fig. 2.
Adrenergic receptor downstream signaling. β2-AR activation triggers several downstream signaling pathways, mediated by an increase in intracellular cAMP, leading to Ca2+ release, apoptosis inhibition through phosphorylation of BAD, and cytoskeletal rearrangement. β2-ARs are quickly desensitized by β-arrestins after ligand binding and signal transduction. Alternatively, α1-AR stimulation has also been described to promote invasive phenotypes through PKC-mediated signaling pathways. NE, norepinephrine; TAM, tumor-associated macrophage; ECM, extracellular matrix
Some observations from in vivo studies point to a negligible effect of β-ARs on primary tumor growth, since compared to vehicle control treatment, isoproterenol stimulation of orthotopic breast cancer tumors did not change primary tumor proliferation11,23,29,30. It is unclear whether these results arose from the direct action of β2-AR on tumor cell proliferation, inhibition of tumor growth by other cell types in the stroma or even a combination of direct and indirect effects. Another study using human xenografts in immunocompromised mice reported increased ER+/PR+ breast cancer tumor growth after inoculation with the α2-AR agonist clonidine31. The increase in tumor growth was accompanied by a similar increase in the proliferation of tumor-associated fibroblasts, and thus, an indirect effect of α2-AR agonism through the tumor microenvironment cannot be ruled out31. It is also intriguing that Thaker et al. reported an increase in MDA-MB-231 tumor growth after chronic stress induction in an orthotopic breast cancer model32, contrasting with the studies previously discussed. Notably, pharmacological β-AR activation seems to inhibit primary tumor growth11,23, while endogenous chronic stress either causes negligible effects or increases tumor growth29,30,32–34. This observation raises important questions, such as whether compensatory mechanisms are exerted by other ARs in endogenous stress models, since Epi and NE can stimulate both α-ARs and β-ARs. In fact, α2-AR antagonists were shown to counteract the increase in tumor growth evoked by restraint stress33. Lamkin and colleagues also showed that in the absence of chronic stress, α2-AR blockade recapitulated the tumor growth observed when the SNS was endogenously activated33, adding another layer of complexity to the impact of SNS signaling on breast cancer. This effect probably arises because presynaptic α2-ARs in peripheral SNS neurons establish a negative feedback loop to control NE release from neuronal terminals35. Thus, blockade of α2-ARs in the absence of chronic stress increases the release of NE in the tumor microenvironment, mirroring endogenous activation of the SNS.
Angiogenesis
As breast tumors proliferate and grow, the need for nutrients and oxygen rises concordantly. These needs are met by the sprouting of new blood vessels that give rise to a network of often aberrant vasculature in the tumor microenvironment36. The SNS has emerged as an important player in neoangiogenesis, since it has already been shown that sympathetic outflow can induce the secretion of proangiogenic factors, namely, vascular endothelial growth factor (VEGF), by breast cancer cells29,37–39. In addition, direct cell–cell contact between breast cancer cells and endothelial cells leads to increased formation of capillary structures in vitro, a result markedly potentiated by the addition of NE38. This effect was suggested to be mediated by the β2-AR/PKA/mTOR pathway and by upregulation of the Notch ligand Jagged-1, directly augmenting Notch signaling in endothelial cells38. Interestingly, there seems to be a cell-specific response to β2-AR agonists in terms of VEGF expression that is not entirely due to differential β2-AR expression37. β2-AR agonists were found to increase VEGF production in a brain-tropic variant of the MDA-MB-231 cell line in vitro but not in the parental cell line or in cells with low β2-AR expression, such as MCF7 cells37. Distinct targets of downstream effectors of the β2-AR/PKA pathway in the different cell lines might explain the disparity in terms of angiogenic responses.
Other players have recently been suggested to be involved in the sympathetic regulation of tumor angiogenesis. Activation of peroxisome proliferator-activated receptor γ (PPARγ) was shown to markedly decrease VEGF expression in 4T1 murine breast cancer cells in vitro, and NE was shown to inhibit PPARγ expression in these cells39. This inhibition was abrogated by the addition of ICI118551, pointing towards a β2-AR-mediated effect39.
In addition to the in vitro data previously discussed, accumulating evidence from several in vivo studies indicates that chronic stress modulates neoangiogenesis and the lymphatic vasculature in breast cancer. Chronic restraint stress, as a model of endogenous SNS activation, was found to increase VEGFC secretion from MDA-MB-231 orthotopic tumors in immunocompromised mice, as well as from 66cl4 tumors in immunocompetent mice, leading to increased tumor lymphatic vessel density29. This effect was recapitulated or abrogated by isoproterenol or propranolol treatment, respectively, suggesting the existence of a β-AR-specific signaling pathway29. Stress-induced production of VEGF in 66cl4 primary breast tumors in mice and a consequent increase in vascularization were also described30. The increased tumor vasculature was also suggested to be an additional route of cancer cell escape29,30 (Fig. 1), facilitating metastasis, as discussed in the following sections.
Immune system modulation
The crosstalk between the SNS and the immune system in the regulation of inflammation is already recognized. Dendritic cells and monocytes express both the α-AR and β-AR subtypes, and adrenergic activation in these cells leads to downregulation of tumor necrosis factor α (TNF-α), IL-1, and IL-6, resulting in the promotion of an immunosuppressive phenotype40. The effect of the SNS on the different immune cell populations in the context of inflammation and hematopoiesis has already been previously reviewed41.
Among the many cellular components of the tumor microenvironment that are affected by SNS catecholamines, tumor-associated macrophages (TAMs) are crucial for cancer progression. SNS signaling prompts breast cancer cells to secrete proinflammatory cytokines, such as IL-637 and M-CSF30, which can enhance the recruitment and infiltration of macrophages into the primary tumor (Fig. 1). On the other hand, β2-AR activation in macrophages increases the expression of cancer progression-promoting factors, such as transforming growth factor β (TGF-β), matrix metalloproteinase (MMP) 9, VEGF and cyclooxygenase-2 (COX2), in vivo30. Macrophage expression of COX2 and consequent secretion of prostaglandin E2 (PGE2) further drives the production of VEGFC by cancer cells to induce lymphangiogenesis29. In addition, in an orthotopic breast cancer model, peripheral sympathetic nerve ablation using 6-hydroxydopamine led to inhibition of TAM recruitment and to a decrease in tumor IL-6 levels42.
Upon chronic stress induction in syngeneic breast cancer mouse models, TAMs are mostly primed towards an immunosuppressive M2 phenotype: genes such as Arginase-1 and IL-10 are overexpressed, while M1 phenotype-characteristic genes are conversely downregulated30,43. In addition, Bucsek et al. reported a significant decrease in tumor-infiltrating effector cytotoxic CD8+ T cells upon β-AR activation and concomitant 4T1 breast cancer tumor growth44. Immunosuppressive CD4+ Treg cells and splenic myeloid-derived suppressor cells were also elevated in stressed mice44.
Furthermore, and in agreement with the reports discussed above, Kamiya et al. elegantly illustrated the influence of tumor sympathetic innervation on immune checkpoint expression and cancer progression34. With a viral vector-based tool, the authors were able to specifically denervate the tumor stroma without affecting surrounding tissues34. The subsequent decrease in tumor NE content abrogated tumor growth and metastatic spread. Moreover, sympathetic denervation downregulated immune checkpoint molecules, such as programmed death 1 (PD-1), in β2-AR-expressing CD4+ and CD8+ tumor-infiltrating lymphocytes. The authors observed the same outcomes in chemically induced and spontaneous breast cancer models and reported correlations between the density of sympathetic fibers, PD-1 expression and tumor recurrence in a small cohort of human breast cancer patients34.
These observations reinforce the hypothesis that SNS-driven immunosuppression and subsequent evasion of immune surveillance play an important role in breast cancer progression.
Extracellular matrix invasion
As the disease progresses, a cascade of cellular events triggers the ability of breast cancer cells to remodel and invade adjacent tissues, eventually escaping into the circulation through intravasation into blood or lymphatic vessels45. Crosstalk between the tumor microenvironment and breast cancer cells is crucial for the acquisition of invasive features, and the SNS has been directly linked to the process of epithelial-to-mesenchymal transition (EMT)46.
Adrenergic signaling, namely, through β2-AR, has been shown to directly modulate several cellular processes in breast cancer cell lines. Isoproterenol stimulation led to increased invasive capacity of highly metastatic MDA-MB-231 cells in vitro, and this effect was β2-AR specific46. Interestingly, overexpression of β2-AR in MCF7 cells resulted in increases in the number of invadopodia and the invasive capacity after incubation with isoproterenol46.
The molecular mechanisms that govern this adrenergic response have begun to be elucidated in recent years. Stimulation of β2-AR in vitro causes the accumulation of intracellular cAMP through the Gαs/adenylyl cyclase pathway and consequent dephosphorylation of ERK1/247. This increase in cAMP activates PKA and exchange protein directly activated by cAMP, leading to increased mobilization of Ca2+ in a feedforward loop that ultimately drives cell invasion mechanisms47 (Fig. 2). In other in vitro studies, β2-AR activation led to increased motility and invasiveness of MDA-MB-231 cells, partially through changes in actin remodeling and contractility and an increase in plasma membrane protrusions48,49. Interestingly, the β-AR agonist isoproterenol reduced the number of focal adhesions while increasing the number of invadopodia, favoring motility in three-dimensional spaces but not on two-dimensional surfaces48.
Although most of the available data in the literature are from experiments with β2-AR and MDA-MB-231 cells, other cell lines and ARs should not be overlooked. Dezong et al. reported that invasion and migration mediated by the proto-oncogenic tyrosine protein kinase Src were modulated by different ARs in the MDA-MB-231 and MCF7 cell lines in vitro, namely, β2-ARs and α1-ARs, respectively50. Src was found to be targeted for phosphorylation via different signaling pathways, i.e., PKA in MDA-MB-231 cells and PKC in MCF7 cells50. These data might explain the seemingly contradictory results observed in previous studies, where the migration capacity of MCF7 cells was described to be decreased upon stimulation with the β-AR agonist isoproterenol27. The same study reported a decrease in MDA-MB-231 cell migration after isoproterenol stimulation27, possibly because a parental MDA-MB-231 cell line was used instead of a highly metastatic variant of the MDA-MB-231 cell line47–49.
In addition to the direct effects of NE on breast cancer cells, stimulation of tumor stromal α2-AR was reported to promote breast cancer progression and invasion. Pharmacological activation of α2-AR but not α1-AR or β-AR increased the rate of metastasis in a syngeneic orthotopic breast cancer model51. These changes were correlated with altered collagen structure and were cancer cell independent, since the cell line used did not respond to NE in vitro51. However, no insight was provided on the stromal players targeted by α2-AR agonizts that are involved in collagen remodeling.
As can be appreciated by the collective results of previous studies, the interplay between breast cancer and the SNS is extremely complex. Clearly, knowledge concerning the combination of α-AR and β-AR signaling on cancer progression, as well as on the distinct cellular players in the tumor microenvironment, is still scarce. Therefore, careful consideration should be exercised when designing experiments and therapeutic interventions.
Breast cancer metastasis and the bone niche
After escaping into the vasculature, breast cancer cells disseminate and travel towards distant organs in a complex multistep process that has not yet been fully elucidated. Breast cancer exhibits specific tropism for organs such as the lung, brain, liver and bone, and there are indications that this tropism is associated with breast cancer receptor status52. Luminal A/B tumors are the most prevalent subtype in patients with breast cancer, and they mostly metastasize to bone53,54. Luminal A/B bone metastases are typically indolent in the first years of follow-up, and patients presenting only bone metastases have higher overall survival rates than patients presenting metastasis to other distant sites53,54. However, ~70% of all late-stage breast cancer patients exhibit bone metastatic foci leading to severe complications such as hypercalcemia, pain and bone fractures52,55. Metastatic foci are found mostly in long bones, ribs, the pelvis, and vertebrae, which contain abundant marrow and provide an immune context favorable for cancer cell survival; the bone marrow microenvironment is crucial for the maintenance of the hematopoietic stem cell niche56. In addition, bone stromal cells secrete a combination of cytokines and growth factors that favor breast cancer cell homing, survival, and proliferation57. Breast cancer cells establish close interactions with bone cells, namely, osteoclasts and osteoblast-lineage cells, and the SNS can potentiate this crosstalk.
The metastatic vicious cycle
The skeletal system plays a critical role in all stages of human development. The skeleton is responsible for locomotion; it is the preferential site for hematopoiesis, regulates mineral homeostasis and protects vital organs, such as the brain, heart and lungs. It is therefore crucial to maintain skeletal structural integrity and function throughout life. This maintenance is achieved mainly through a highly dynamic bone remodeling process, where the bone matrix is degraded and subsequently replaced by new mineralized bone in a coordinated fashion. Osteoclasts are specialized multinucleated cells of the hematopoietic lineage that are able to demineralize and resorb the bone matrix using a combination of secreted enzymes, such as cathepsin K (CatK)58 and tartrate resistant acid phosphatase59. During resorption, factors secreted from osteoclasts and byproducts of bone matrix degradation recruit precursors of bone-forming cells, coupling bone resorption and bone formation. These precursors of a mesenchymal lineage differentiate into mature osteoblasts, which are then responsible for the deposition of high amounts of ECM proteins and for their mineralization60. Osteoblasts can then entomb themselves in the matrix that they produce and transform into osteocytes. These cells account for more than 90% of the cells present in cortical bone and have long extensions, creating an interconnecting network between osteocytes themselves and cells in the bone marrow61. Osteocytes are thought to have an endocrine62 and mechanosensitive role63,64 in bone, participating in complex adaptations to internal and external stimuli.
Breast cancer often leads to highly osteolytic bone metastases, where cancer cells exploit the normal bone remodeling process and shift the balance towards increased bone resorption. Parathyroid hormone-related protein (PTHrP), MMPs and PGE2 are some of the factors released by tumor cells that modulate the expression of receptor activator of NF-κB ligand (RANKL) by osteoblasts, which is a master regulator of osteoclast differentiation65,66. Increased RANKL production by osteoblasts and osteocytes in turn enhances osteoclast differentiation and activity, leading to extensive bone degradation. On the other hand, bone matrix-embedded factors released during resorption, such as TGF-β, insulin growth factor, and platelet-derived growth factor, further stimulate tumor growth and perpetuate a “vicious cycle” of bone destruction67. Biphosphonates and denosumab (an anti-RANKL human monoclonal antibody) are commonly used as adjuvant therapies for the treatment of metastatic bone disease to normalize the level of osteoclastic activity68. However, although these treatments alleviate skeleton-related symptoms, new and more effective therapeutic targets are needed to suppress the establishment of the vicious cycle.
The SNS and bone metastatic disease
Bones are highly innervated organs, with a high density of sensory and sympathetic nerve fibers in the periosteum and along blood vessels in the bone marrow69. A physical and functional association of nerve fibers and bone cells is to be expected70, since the nerve fiber density is usually increased near surfaces with enhanced bone turnover71.
Although cells of osteoblast and osteoclast lineages have been reported to express α-AR mRNA, its relative expression compared to that of the β2-AR subtype is greatly reduced72–74. β2-AR but not β1-AR or β3-AR is widely expressed in primary osteoclasts and osteoclastic cell lines5,75, as well as in osteoblast lineage cells76–78. β2-AR is fully functional in bone cells, since β2-AR agonism triggers an increase in intracellular cAMP in vitro77. Interestingly, cells of the osteoblast lineage also express monoamine oxidase (MAO)α and MAOβ79, as well as the NE transporter80, and are thus able to take up and catabolize NE from the external milieu.
β-AR activation in bone triggers osteoclastic differentiation, diminished bone formation and consequent bone loss (reviewed in81), mostly due to an increase in RANKL production by osteoblast lineage cells in vivo82,83 (Fig. 3). Similarly, β2-AR agonism was reported to increase RANKL production by the MLO-Y4 osteocytic cell line in vitro and consequently to induce the differentiation of the RAW264.7 osteoclastic cell line in coculture experiments84. Although osteocytes have received increasing attention in recent years regarding their role in the modulation of breast cancer progression85–88, data on the action of adrenergic signaling pathways on osteocytes in this context are still scarce. Osteocytes express β2-AR, and as they are the most common cell type in bone, the importance of their putative crosstalk with the SNS in breast cancer should not be overlooked. Regardless, SNS activation of osteoblast-lineage cells seems to further potentiate the establishment of a metastatic vicious cycle upon bone metastatic colonization of breast cancer.
Fig. 3.
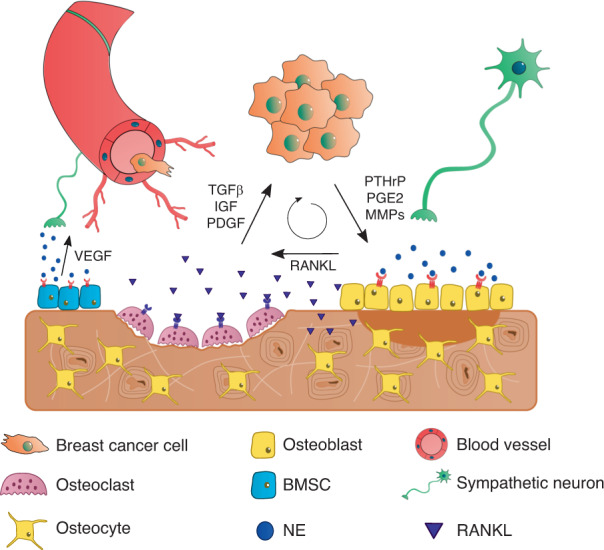
The bone metastatic niche and the metastatic vicious cycle. Once engrafted in the bone, breast cancer cells secrete pro-osteoclastic factors such as PTHrP, PGE2, and MMPs, which induce the expression of RANKL by osteoblasts and osteocytes, promoting osteoclast differentiation and activity. In turn, factors released from the bone matrix enhance the growth of cancer cells, establishing a metastatic vicious cycle that leads to extensive bone degradation. NE, norepinephrine; BMSC, bone marrow stromal cell
Campbell and colleagues have made important contributions to this field of research. In a mouse model of bone metastasis established by intracardiac injection of bone-tropic MDA-MB-231 cells, the authors showed that adrenergic stimulation of the bone stroma potentiated the establishment of the metastatic vicious cycle11. Chronic immobilization stress, as a model of endogenous sympathetic activity, was used to demonstrate that augmented catecholamine levels led to the formation of larger osteolytic lesions, an effect mediated by β2-AR11. Moreover, isoproterenol administration before injection of breast cancer cells increased the numbers of tumor foci and lesions in bone, suggesting that sympathetic triggering in the bone microenvironment facilitated breast cancer cell engraftment. The authors suggested that this effect was partially due to RANKL signaling and its chemotactic action on MDA-MB-231 cells11.
In addition to augmented RANKL signaling, adrenergic stimuli promoted breast cancer extravasation and retention in the bone through modulation of the bone vasculature. Nude mice subjected to either chronic immobilization stress or isoproterenol administration showed increased VEGF-A expression by bone marrow stromal cells (BMSCs) and consequent angiogenesis, which resulted in the promotion of breast cancer cell colonization89. Furthermore, incubation of BMSCs with isoproterenol led to the release of IL-1β, which in turn activated E/P-selectin expression in endothelial cells and enabled the adhesion and retention of breast cancer cells in vitro90 (Fig. 4).
Fig. 4.

Breast cancer cell extravasation into the bone niche. NE stimulation of stromal β2-AR is associated with an increased release of VEGF and IL-1β, which leads to augmented angiogenesis and expression of P- and E-selectins in endothelial cells. The latter event promotes breast cancer cell extravasation from the circulation into the bone marrow
Interestingly, the interplay between the SNS and breast cancer in the bone metastatic niche is not unidirectional. Not only is the SNS capable of inducing breast cancer cell engraftment and proliferation through RANKL and VEGF-A signaling, but conversely, breast cancer may also be able to regulate AR dynamics in the bone niche. Breast cancer cell-secreted PTHrP is a well-known modulator of bone turnover in the metastatic niche (reviewed in91). PTHrP binds to PTH receptor 1 (PTHR1) expressed in osteoblasts and upregulates RANKL expression to promote osteoclastogenesis, driving the vicious metastatic cycle92. Interestingly, PTHR1, β2-AR, and their corresponding downstream pathways in osteoblastic cells seem to be intimately connected. Using germline β2-AR knockout mice, Hanyu et al. demonstrated that β2-AR expression is required for the osteoanabolic effect of PTH and that β2-AR modulates the expression of PTHR1 target genes, such as RANKL, alkaline phosphatase, bone sialoprotein, and osteoprotegerin (a RANKL decoy receptor), in osteoblasts93. On the other hand, PTH was shown to directly downregulate β2-AR expression in osteoblast-like MC3T3-E1 cells in vitro94. This interdependency might be explained by common intracellular downstream effectors that are triggered by binding of their corresponding ligands. Both PTHR1 and β2-AR are GPCRs that signal through the adenylyl cyclase/PKA axis and promote the phosphorylation of cAMP-response element binding protein to induce transcription of target genes94. Furthermore, after ligand binding, both receptors are rapidly desensitized through pathways dependent on β-arrestin and β-adrenergic kinase 195–97, which can also act as protein scaffolds that subsequently lead to the activation of the mitogen-activated protein kinases ERK1/2 and several other effector molecules98. However, while these interactions have been described to occur between PTHR1 and β2-AR in the context of intermittent PTH treatment, it is still unknown whether breast cancer-secreted PTHrP can elicit the same response in the context of bone metastatic disease. Although PTHrP and PTH share the same receptor, there are several described noncanonical pathways for the action of PTHrP whose importance is still poorly understood91. Therefore, more data on the interplay between PTHrP and β2-AR in breast cancer bone metastasis are urgently required, since this knowledge could change our understanding of the dynamics of β2-AR expression in bone throughout the progression of this disease and facilitate the design of new, more effective therapeutic options.
Breast cancer and beta-blockers: a clinical perspective
Although preclinical data are extremely valuable for understanding the many processes that control breast cancer progression and metastatic spread, it is crucial to translate the results into a clinical setting. In the past decade, increasing attention has been devoted to the effect of sympathetic activity on breast cancer patient survival and breast cancer recurrence10,99. In this section, we will review the published epidemiologic and clinical data on the effect of several β-AR antagonists (henceforth called beta-blockers) on breast cancer and discuss the limitations associated with the interpretation of the reported results.
Epidemiologic studies have previously suggested that patients with cancer subjected to high levels of psychosocial stress usually have a poorer prognosis and survival than those not subjected to these conditions100. SNS-targeting beta-blockers are thus potential therapeutic options for cancer and are already widely used in other pathological settings, such as the treatment of asthma and hypertension101,102. The safety profile of these drugs is well described, and they are not associated with an increased incidence of breast cancer, as evidenced by previous epidemiologic studies103,104.
A proof-of-principle study performed by Powe et al. analyzed the effect of beta-blocker prescription prior to breast cancer diagnosis on patient survival10. Reduced tumor recurrence and metastasis incidence and increased patient survival rates were reported in the beta-blocker-treated group, with no significant differences in tumor stage, tumor size, tumor grade or vascular invasion between the treated and placebo groups.
However, the population size in that study was relatively small, and no distinction between the type of beta-blockers used was included in the analysis10. Atenolol and bisoprolol are β1-AR specific, while propranolol and timolol are nonspecific β1/2-AR antagonists; therefore, the contributions of the different ARs to the reported results cannot be isolated. In fact, another population-based study by Barron et al. showed a beneficial effect of propranolol but not atenolol on breast cancer metastasis and patient survival105. Interestingly, Melhem-Bertrand et al. reported a beneficial effect of the β1-AR-targeting drugs metoprolol and atenolol on the recurrence of triple-negative breast cancer (TNBC) but not on ER-positive breast cancer, highlighting the importance of breast cancer receptor status on the response to beta-blockers106. Thus, it is still unclear which receptors are the main contributors to the reported beneficial effects of beta-blockers on breast cancer recurrence, and this topic is a matter of intense debate. However, we hypothesize that a broader acting beta-blocker, such as propranolol, could be even more beneficial than specific beta-blockers in managing breast cancer recurrence and metastasis.
Several studies have suggested that beta-blocker usage could be explored as an adjuvant therapy in breast cancer treatment10,29,105–109. However, these studies have some limitations, such as a retrospective design, small population size, difficulties in the assessment of beta-blocker treatment duration and compliance, or a lack of access to data on comorbidities and other medications. Other retrospective studies reported no correlation between beta-blocker usage and reduced breast cancer-specific mortality or recurrence99,110–112, and thus, the benefits of these drugs remain controversial (for more details, refer to Table 2). Randomized clinical trials are warranted to assess the clinical relevance of beta-blockers for breast cancer treatment.
Table 2.
Summary of epidemiologic studies regarding the influence of β-blockers on breast cancer outcomes
| Treated group size/total size | β-Blocker used (population size) |
Improved patient survival (HR; CI) | Reduced tumor recurrence (HR; CI) | Reduced incidence of metastasis (HR; CI) | Reference |
|---|---|---|---|---|---|
| 43/466 | Atenolol (25) | Yes (0.291; 0.119–0.715) | Yes (−) | Yes (0.430; 0.200–0.926) | 10 |
| Propranolol (7) | |||||
| Bisoprolol (7) | |||||
| Timolol (4) | |||||
| 595/4 738 | Atenolol (525) | Yes (0.19; 0.06–0.60) | N.D. | Yes (−) | 105 |
| Propranolol (70) | |||||
| 204/1 779 | Atenolol (−) | No (0.76; 0.44–1.33) | No (0.86; 0.57–1.32) | N.D. | 134 |
| Metoprolol (−) | |||||
| Propranolol (−) | |||||
| Others (−) | |||||
| 102/1 413 | Metoprolol (43) | No (0.64; 0.38–1.07) | Yes (0.52; 0.31–0.88) | N.D. | 106 |
| Atenolol (38) | |||||
| Others (21) | |||||
| 74/800 | Carvedilol (11) | Yes (0.42; 0.18–0.97) | Yes (0.52; 0.28–0.97) | Yes (0.32; 0.12–0.90) | 108 |
| Sotalol (3) | |||||
| Atenolol (27) | |||||
| Betaxolol (1) | |||||
| Bisoprolol (11) | |||||
| Metoprolol (8) | |||||
| Nebivolol (13) | |||||
| 3 660/18 733 |
Metoprolol (1 793) Atenolol (622) |
N.D. | No (1.3; 1.1–1.5) | N.D. | 111 |
| Propranolol (586) | |||||
| Others (659) | |||||
| 1 770/55 252 | Propranolol (1 770) | No (0.94; 0.77–1.16) | N.D. | N.D. | 99 |
| 1 443/5 754 | Carvedilol (22) | No (1.11; 0.94–1.32) | N.D. | N.D. | 112 |
| Sotalol (84) | |||||
| Atenolol (854) | |||||
| Bisoprolol (189) | |||||
| Metoprolol (45) | |||||
| Propranolol (249) | |||||
| 153/1 144 | Bisoprolol (59) | No (1.05; 0.85–1.29) | Yes (0.81; 0.66–0.99) | N.D. | 109 |
| Metoprolol (48) | |||||
| Atenolol (28) | |||||
| Propranolol (13) | |||||
| Others (5) | |||||
| 93/956 | N.D. | Yes (0.48; 0.23–0.99) | No (0.93; 0.39–2.25) | Yes (0.40; 0.17–0.93) | 29 |
HR hazard ratio, CI 95% confidence interval (lower limit–higher limit), N.D. no data
To our knowledge, the only results from phase II placebo-controlled clinical trials published to date address the effect of perioperative propranolol administration on several metastatic biomarkers in patients with early breast cancer. Zhou et al. reported decreased immunosuppression after the administration of propranolol compared to placebo controls during the perioperative period of breast cancer surgery113. Propranolol was also shown to block the proliferation of patient-derived regulatory T cells113. Shaashua114 and Haldar115 reported a reduction in the expression of EMT-related genes in resected primary tumors from patients simultaneously treated with propranolol and the COX-2 inhibitor etodolac. The resected tumors also showed reduced expression of prometastatic, antiapoptotic and proliferation markers; increased infiltration of B-cells; and a decreased population of TAMs. Propranolol- and etodolac-treated patients also presented reduced levels of the circulating inflammatory cytokines IFNγ and IL-6 and increased levels of NK cell activation during treatment114. Another randomized clinical trial by Hiller et al. showed similar results with the administration of propranolol for one week before surgical resection of the primary breast tumor116. In this study, compared to placebo-treated controls, patients treated with propranolol before surgery showed reduced EMT gene expression and increased dendritic cell infiltration and M1 macrophage polarization in the resected tumors. Interestingly, compared to clinically responsive patients, patients clinically nonresponsive to propranolol (i.e., without significant reductions in blood pressure and heart rate after beta blockade) showed decreased tumor EMT gene expression, although immune cell infiltration in the primary tumor was changed116. These clinical trials pointed to a possible beneficial effect of propranolol on reducing the metastatic potential of primary breast tumors. However, adequately powered clinical trials with a focus on overall survival and cancer recurrence are still needed before propranolol can be used for breast cancer treatment.
Conclusion and future perspectives
Despite the advances made in recent years, knowledge on the impact of endogenous stress on the complex interactions governing breast cancer disease progression is still incomplete. This review summarizes and combines the available data regarding SNS signaling in the orchestration of breast cancer.
To date, adrenergic signaling has been implicated in several steps of disease progression, promoting tumor growth, angiogenesis, immunosuppression and invasion (Fig. 1). While several in vitro studies and animal models have illustrated the intricate control exerted by the SNS over cancer cellular processes, the contributions of the different ARs expressed in the multiple cellular components of the tumor microenvironment remain puzzling. Furthermore, the inherent heterogeneity of breast cancer presents an additional challenge in modeling this disease. The distinctive AR expression patterns in breast cancer cell lines widely used in the various experimental models are certainly relevant, and more information on the adrenergic control of disease progression in different cell lines is urgently needed.
Modeling the various cellular and structural components of the cancer niche is still technically challenging. The use of immunodeficient mice is required for xenograft models, but the contribution of the immune system is not considered in these models. Thus, current in vitro and in vivo models do not completely recapitulate the complexity of the disease, but as new, more complicated models are developed, discerning the specific contributions of each cell type becomes increasingly difficult. Specific deletion of β2-AR in not only breast cancer cells46 but also osteoblasts89 and macrophages117 could be used as an important tool to elucidate the role of this receptor in various models of the disease, although no models of conditional β2-AR knockout specifically in osteoclasts or osteocytes have been described to date. Furthermore, microfluidic systems have several advantages when compared to traditional in vitro models since they allow the compartmentalization of different cell types and the introduction of fluid flow, which can be physiologically relevant. Microfluidic platforms have already been developed for the study of breast cancer metastasis to bone118–121, but modeling the SNS in these platforms is still challenging.
Metastatic tropism for bone is an evident feature of breast cancer, and bone is the most common site of metastasis in luminal breast cancer patients52. Although adrenergic stimulation of the bone microenvironment is thought to increase osteolysis and potentiate the metastatic vicious cycle, the SNS-controlled interactions between breast cancer and bone cells remain mostly unexplored, apart from the contributions of Elefteriou and his group11,89,90. Although the use of luminal A breast cancer cell lines in bone metastasis models presents technical challenges due to the less invasive phenotype of these cell lines, it is crucial to understand the molecular changes that might be elicited by the SNS in these cells. Furthermore, since luminal A tumors are the most common subtype of breast tumors in patients, the use of luminal subtype breast cancer cells in in vitro and in vivo models of this disease is certainly more clinically relevant than the currently widespread use of aggressive TNBC cells.
Future developments in novel targeted therapeutic strategies, such as tumor-specific denervation via viral vectors34, are exciting fields of research that will require input from various areas of expertise before becoming applicable in a clinical setting. It is still unclear whether this technique can be applied to locally and specifically denervate bone in preclinical studies. In addition, other denervation techniques, such as chemical sympathectomy by local delivery of guanethidine into the femoral bone marrow via an osmotic minipump, have been established122, which could help to clarify the role of sympathetic nerves in bone metastasis.
Finally, clinical observations on the usage of beta-blockers for the treatment of breast cancer suggest that interfering with SNS signaling could have beneficial effects on patients, particularly in the control of metastatic spread. However, systemic administration of beta-blockers can also have unforeseen consequences on the progression of breast cancer, and adequately powered clinical trials are needed before their therapeutic implementation. Targeted drug delivery systems could address the currently unmet clinical challenge of circumventing the disadvantages of systemic beta-blocker administration. The unique biochemical and biophysical characteristics of the bone microenvironment provide the means for targeted drug delivery to bone metastatic tumors. Bisphosphonates123, acidic amino acid peptidic sequences124, liposomes125, organic126, and inorganic127 nanoparticles, chimeric peptides targeting CatK128 and HER2-targeting nanoparticles129 have been previously used to achieve bone metastasis-specific drug and gene delivery in vivo. Whether these strategies can be used to deliver SNS-targeting drugs specifically to the bone microenvironment and whether they can be translated into a clinical benefit remain to be elucidated.
Taken together, the data summarized in this review highlight the importance of SNS activation in breast cancer. In the next few years, exciting new developments are expected that would allow us to complement our understanding of the molecular cues that drive breast cancer progression.
Acknowledgements
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and Portuguese funds through FCT/MCTES in the framework of the project “SproutOC” (POCI-01-0145-FEDER-030158, PTDC/MED-PAT/30158/2017). F.C. is a recipient of the Ph.D. fellowship SFRH/BD/128771/2017. D.M.S. is a recipient of the postdoctoral fellowship SFRH/BPD/115341/2016.
Competing interests
The authors declare no competing interests.
References
- 1.Lyons CE, et al. Optogenetic-induced sympathetic neuromodulation of brown adipose tissue thermogenesis. FASEB J. 2020;34:2765–2773. doi: 10.1096/fj.201901361RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem. Cell Egr. Bone Marrow Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Mani BK, Osborne-Lawrence S, Vijayaraghavan P, Hepler C, Zigman JM. β1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J. Clin. Investig. 2016;126:3467–3478. doi: 10.1172/JCI86270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai M, Nagasawa T, Koshihara Y, Yamamoto S, Togari A. Effects of β-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2003;1640:137–142. doi: 10.1016/S0167-4889(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang HH, Brennan TC, Muir MM, Mason RS. Functional α1- and β2-adrenergic receptors in human osteoblasts. J. Cell Physiol. 2009;220:267–275. doi: 10.1002/jcp.21761. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, et al. Bidirectional role of β2-adrenergic receptor in autoimmune diseases. Front. Pharmacol. 2018;9:1313. doi: 10.3389/fphar.2018.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali DC, et al. β-Adrenergic receptor, an essential target in cardiovascular diseases. Heart Fail. Rev. 2020;25:343–354. doi: 10.1007/s10741-019-09825-x. [DOI] [PubMed] [Google Scholar]
- 9.Galvano A, et al. Denosumab for bone health in prostate and breast cancer patients receiving endocrine therapy? A systematic review and a meta-analysis of randomized trials. J. Bone Oncol. 2019;18:100252. doi: 10.1016/j.jbo.2019.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powe DG, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JP, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLOS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obradović MMS, et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567:540–544. doi: 10.1038/s41586-019-1019-4. [DOI] [PubMed] [Google Scholar]
- 13.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control CCC. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 14.Hassan S, et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Investig. 2013;123:874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnon C, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 16.Decker AM, et al. Sympathetic signaling reactivates quiescent disseminated prostate cancer cells in the bone marrow. Mol. Cancer Res. 2017;15:1644–1655. doi: 10.1158/1541-7786.MCR-17-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coarfa C, et al. Influence of the neural microenvironment on prostate cancer. Prostate. 2018;78:128–139. doi: 10.1002/pros.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez-Andrade JM, et al. Bone cancer pain. Ann. N. Y. Acad. Sci. 2010;1198:173–181. doi: 10.1111/j.1749-6632.2009.05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, et al. A Her2-let-7-β2-AR circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer. 2015;15:832. doi: 10.1186/s12885-015-1869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivero EM, et al. Prognostic significance of α- and β2-adrenoceptor gene expression in breast cancer patients. Br. J. Clin. Pharmacol. 2019;85:2143–2154. doi: 10.1111/bcp.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurozumi S, et al. β2-Adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2019;177:603–610. doi: 10.1007/s10549-019-05341-6. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RobertA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Pérez Piñero C, Bruzzone A, Sarappa M, Castillo L, Lüthy I. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012;166:721–736. doi: 10.1111/j.1476-5381.2011.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sastry KSR, et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- 25.Reeder A, et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br. J. Cancer. 2015;112:1461–1470. doi: 10.1038/bjc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slotkin TA, et al. β-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2000;60:153–166. doi: 10.1023/A:1006338232150. [DOI] [PubMed] [Google Scholar]
- 27.Gargiulo L, et al. Differential á2-adrenergic receptor expression defines the phenotype of non-tumorigenic and malignant human breast cell lines. Oncotarget. 2014;5:10058–10069. doi: 10.18632/oncotarget.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker AK, et al. Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology. 2019;99:191–195. doi: 10.1016/j.psyneuen.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Le CP, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan EK, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruzzone A, et al. α2-Adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr. Cancer Drug Targets. 2011;11:763–774. doi: 10.2174/156800911796191051. [DOI] [PubMed] [Google Scholar]
- 32.Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 33.Lamkin DM, et al. 2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology. 2015;51:262–270. doi: 10.1016/j.psyneuen.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamiya A, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019;22:1289–1305. doi: 10.1038/s41593-019-0430-3. [DOI] [PubMed] [Google Scholar]
- 35.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 36.Nagy JA, Chang S-H, Shih S-C, Dvorak AM, Dvorak HF. Heterogeneity of the Tumor Vasculature. Semin Thromb. Hemost. 2010;36:321–331. doi: 10.1055/s-0030-1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden KS, Szpunar MJ, Brown EB. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 2011;130:747–758. doi: 10.1007/s10549-011-1348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr Relat Cancer. 2014;21:783–795. doi: 10.1530/ERC-14-0236. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, et al. Activation of β2-adrenergic receptor promotes growth and angiogenesis in breast cancer by down-regulating PPARγ. Cancer Res. Treat. 2020;52:830–847. doi: 10.4143/crt.2019.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360–373. doi: 10.1016/j.neuron.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szpunar MJ, Belcher EK, Dawes RP, Madden KS. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav. Immun. 2016;53:223–233. doi: 10.1016/j.bbi.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamkin DM, et al. β-Adrenergic-stimulated macrophages: comprehensive localization in the M1-M2 spectrum. Brain, Behav., Immun. 2016;57:338–346. doi: 10.1016/j.bbi.2016.07.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bucsek MJ, et al. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639–5651. doi: 10.1158/0008-5472.CAN-17-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bill R, Christofori G. The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 2015;589:1577–1587. doi: 10.1016/j.febslet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Chang A, et al. β2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav. Immun. 2016;57:106–115. doi: 10.1016/j.bbi.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pon CK, Lane JR, Sloan EK, Halls ML. The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. 2016;30:1144–1154. doi: 10.1096/fj.15-277798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creed SJ, et al. β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015;17:145. doi: 10.1186/s13058-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim T-H, et al. Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J. Cell Sci. 2016;129:4563–4575. doi: 10.1242/jcs.194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dezong G, Zhongbing M, Qinye F, Zhigang Y. Carvedilol suppresses migration and invasion of malignant breast cells by inactivating Src involving cAMP/PKA and PKCδ signaling pathway. J. Cancer Res. Ther. 2014;10:991–997. doi: 10.4103/0973-1482.137664. [DOI] [PubMed] [Google Scholar]
- 51.Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev. Res. 2013;6:1262–1272. doi: 10.1158/1940-6207.CAPR-13-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soni A, et al. Breast cancer subtypes predispose the site of distant metastases. Am. J. Clin. Pathol. 2015;143:471–478. doi: 10.1309/AJCPYO5FSV3UPEXS. [DOI] [PubMed] [Google Scholar]
- 53.Ignatov A, Eggemann H, Burger E, Ignatov T. Patterns of breast cancer relapse in accordance to biological subtype. J. Cancer Res. Clin. Oncol. 2018;144:1347–1355. doi: 10.1007/s00432-018-2644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H, et al. Impact of molecular subtypes on metastatic behavior and overall survival in patients with metastatic breast cancer: a single-center study combined with a large cohort study based on the surveillance, epidemiology and end results database. Oncol. Lett. 2020;20:87. doi: 10.3892/ol.2020.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J. Bone Miner. Res. 2018;33:2099–2113. doi: 10.1002/jbmr.3618. [DOI] [PubMed] [Google Scholar]
- 57.Kang Y. Dissecting tumor-stromal interactions in breast cancer bone metastasis. Endocrinol. Metab. 2016;31:206–212. doi: 10.3803/EnM.2016.31.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drake FH, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996;271:12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 59.Halleen JM, et al. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J. Bone Miner. Res. 2000;15:1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 60.Ecarot-Charrier B, Glorieux FH, van der Rest M, Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J. Cell Biol. 1983;96:639–643. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–149. doi: 10.1016/S8756-3282(00)00421-X. [DOI] [PubMed] [Google Scholar]
- 62.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robling AG, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of sost/sclerostin. J. Biol. Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 64.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone. 2010;47:872–881. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin JJ, et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Pape F, Vargas G, Clézardin P. The role of osteoclasts in breast cancer bone metastasis. J. Bone Oncol. 2016;5:93–95. doi: 10.1016/j.jbo.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 68.Fizazi K, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 69.Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178–190. doi: 10.1016/j.neuroscience.2018.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leitão L, et al. Osteoblasts are inherently programmed to repel sensory innervation. Bone Res. 2020;8:20. doi: 10.1038/s41413-020-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayilekshmy M, et al. Innervation is higher above bone remodeling surfaces and in cortical pores in human bone: lessons from patients with primary hyperparathyroidism. Sci. Rep. 2019;9:5361. doi: 10.1038/s41598-019-41779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonseca TL, et al. Double disruption of α2A- and α2C -adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J. Bone Miner. Res. 2011;26:591–603. doi: 10.1002/jbmr.243. [DOI] [PubMed] [Google Scholar]
- 73.Nishiura T, Abe K. α1-Adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor κB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch. Oral. Biol. 2007;52:778–785. doi: 10.1016/j.archoralbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Hirai T, Tanaka K, Togari A. α1-adrenergic receptor signaling in osteoblasts regulates clock genes and bone morphogenetic protein 4 expression through up-regulation of the transcriptional factor nuclear factor IL-3 (Nfil3)/E4 promoter-binding protein 4 (E4BP4) J. Biol. Chem. 2014;289:17174–17183. doi: 10.1074/jbc.M113.546135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kondo H, Takeuchi S, Togari A. β-Adrenergic signaling stimulates osteoclastogenesis via reactive oxygen species. Am. J. Physiol.-Endocrinol. Metab. 2013;304:E507–E515. doi: 10.1152/ajpendo.00191.2012. [DOI] [PubMed] [Google Scholar]
- 76.Moore RE, Smith CK, Bailey CS, Voelkel EF, Tashjian AH. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23:301–315. doi: 10.1016/S0169-6009(08)80105-5. [DOI] [PubMed] [Google Scholar]
- 77.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/S0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 78.Togari A, et al. Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 1997;233:125–128. doi: 10.1016/S0304-3940(97)00649-6. [DOI] [PubMed] [Google Scholar]
- 79.Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI. β2-Adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophysics. 2009;482:96–103. doi: 10.1016/j.abb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Ma Y, et al. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J. Biol. Chem. 2013;288:30105–30113. doi: 10.1074/jbc.M113.481309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018;98:1083–1112. doi: 10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 83.Liang H, et al. Selective β2-adrenoreceptor signaling regulates osteoclastogenesis via modulating RANKL production and neuropeptides expression in osteocytic MLO-Y4 cells. J. Cell. Biochem. 2019;120:7238–7247. doi: 10.1002/jcb.27998. [DOI] [PubMed] [Google Scholar]
- 84.Yao Q, et al. Beta-adrenergic signaling affect osteoclastogenesis via osteocytic MLO-Y4 cells’ RANKL production. Biochem. Biophysi. Res. Commun. 2017;488:634–640. doi: 10.1016/j.bbrc.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 85.Chen A, et al. Attraction and compaction of migratory breast cancer cells by bone matrix proteins through tumor-osteocyte interactions. Sci. Rep. 2018;8:5420. doi: 10.1038/s41598-018-23833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S, et al. Osteocyte-driven downregulation of snail restrains effects of Drd2 inhibitors on mammary tumor cells. Cancer Res. 2018;78:3865–3876. doi: 10.1158/0008-5472.CAN-18-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan Y, et al. Skeletal loading regulates breast cancer-associated osteolysis in a loading intensity-dependent fashion. Bone Res. 2020;8:9. doi: 10.1038/s41413-020-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maroni P, Bendinelli P. Bone, a secondary growth site of breast and prostate carcinomas: role of osteocytes. Cancers. 2020;12:1812. doi: 10.3390/cancers12071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulcrone PL, et al. Skeletal colonization by breast cancer cells is stimulated by an osteoblast and β2AR-dependent neo-angiogenic switch. J. Bone Miner. Res. 2017;32:1442–1454. doi: 10.1002/jbmr.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clément-Demange L, Mulcrone PL, Tabarestani TQ, Sterling JA, Elefteriou F. β2ARs stimulation in osteoblasts promotes breast cancer cell adhesion to bone marrow endothelial cells in an IL-1β and selectin-dependent manner. J. Bone Oncol. 2018;13:1–10. doi: 10.1016/j.jbo.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin, T. J. & Johnson, R. W. Multiple actions of parathyroid hormone-related protein in breast cancer bone metastasis. Br. J. Pharmacol. 1–13 (2019). 10.1111/bph.14709. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 92.Thomas RJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation1. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 93.Hanyu R, et al. Anabolic action of parathyroid hormone regulated by the β2-adrenergic receptor. Proc. Natl Acad. Sci. 2012;109:7433–7438. doi: 10.1073/pnas.1109036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moriya S, et al. PTH regulates β2-adrenergic receptor expression in osteoblast-Like MC3T3-E1 cells. J. Cell. Biochem. 2015;116:142–148. doi: 10.1002/jcb.24953. [DOI] [PubMed] [Google Scholar]
- 95.Bianchi EN, Ferrari SL. β-arrestin2 regulates parathyroid hormone effects on a p38 MAPK and NFκB gene expression network in osteoblasts. Bone. 2009;45:716–725. doi: 10.1016/j.bone.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spurney RF. Regulated expression of G protein-coupled receptor kinases (GRK’s) and β-arrestins in osteoblasts. Calcif. Tissue Int. 2003;73:153–160. doi: 10.1007/s00223-002-1018-5. [DOI] [PubMed] [Google Scholar]
- 97.Fukayama S, Kong G, Benovic JL, Meurer E. & Jr, A. H. T. β-adrenergic receptor kinase-1 acutely regulates PTH/PTHrP receptor signalling in human osteoblastlike cells. Cell. Signal. 1997;9:469–474. doi: 10.1016/S0898-6568(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 98.Gesty-Palmer D, et al. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 Activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 99.Cardwell CR, et al. Propranolol and survival from breast cancer: a pooled analysis of European breast cancer cohorts. Breast Cancer Res. 2016;18:119. doi: 10.1186/s13058-016-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 101.Lawrence DS, Sahay JN, Chatterjee SS, Cruickshank JM. Asthma and beta-blockers. Eur. J. Clin. Pharmacol. 1982;22:501–509. doi: 10.1007/BF00609622. [DOI] [PubMed] [Google Scholar]
- 102.Paterson JW, Dollery CT. Effect of propranolol in MILD hypertension. Lancet. 1966;288:1148–1150. doi: 10.1016/S0140-6736(66)90471-5. [DOI] [PubMed] [Google Scholar]
- 103.Fryzek JP, et al. A cohort study of antihypertensive medication use and breast cancer among Danish women. Breast Cancer Res. Treat. 2006;97:231–236. doi: 10.1007/s10549-005-9091-x. [DOI] [PubMed] [Google Scholar]
- 104.Li CI, et al. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer. 2003;98:1504–1513. doi: 10.1002/cncr.11663. [DOI] [PubMed] [Google Scholar]
- 105.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 106.Melhem-Bertrandt A, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montoya A, et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2016;8:6446–6460. doi: 10.18632/oncotarget.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Botteri E, et al. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 2013;140:567–575. doi: 10.1007/s10549-013-2654-3. [DOI] [PubMed] [Google Scholar]
- 109.Spera G, et al. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann. Oncol. 2017;28:1836–1841. doi: 10.1093/annonc/mdx264. [DOI] [PubMed] [Google Scholar]
- 110.Shah SM, et al. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br. J. Clin. Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sørensen GV, et al. Use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J. Clin. Oncol. 2013;31:2265–2272. doi: 10.1200/JCO.2012.43.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cardwell CR, Coleman HG, Murray LJ, Entschladen F, Powe DG. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK Clinical Practice Research Datalink cohort. Int. J. Epidemiol. 2014;42:1852–1861. doi: 10.1093/ije/dyt196. [DOI] [PubMed] [Google Scholar]
- 113.Zhou L, et al. Propranolol attenuates surgical stress–induced elevation of the regulatory Tt cell response in patients undergoing radical mastectomy. J. Immunol. 2016;196:3460–3469. doi: 10.4049/jimmunol.1501677. [DOI] [PubMed] [Google Scholar]
- 114.Shaashua L, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 2017;23:4651–4661. doi: 10.1158/1078-0432.CCR-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haldar R, et al. Perioperative inhibition of β-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav. Immun. 2018;73:294–309. doi: 10.1016/j.bbi.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 116.Hiller JG, et al. Pre-operative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a Phase II randomized trial. Clin. Cancer Res. 2020;26:1803–1811. doi: 10.1158/1078-0432.CCR-19-2641. [DOI] [PubMed] [Google Scholar]
- 117.Lechtenberg KJ, Meyer ST, Doyle JB, Peterson TC, Buckwalter MS. Augmented β2-adrenergic signaling dampens the neuroinflammatory response following ischemic stroke and increases stroke size. J. Neuroinflammation. 2019;16:112. doi: 10.1186/s12974-019-1506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mei X, et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr. Biol. 2019;11:119–129. doi: 10.1093/intbio/zyz008. [DOI] [PubMed] [Google Scholar]
- 119.Hao S, et al. A Spontaneous 3D bone-on-a-chip for bone metastasis study of breast cancer cells. Small. 2018;14:1702787. doi: 10.1002/smll.201702787. [DOI] [PubMed] [Google Scholar]
- 120.Bersini S, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jeon JS, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl Acad. Sci. USA. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubový P, et al. Local chemical sympathectomy of rat bone marrow and its effect on marrow cell composition. Autonom. Neurosci.: basic Clin. 2017;206:19–27. doi: 10.1016/j.autneu.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Wu H, Luo Y, Xu D, Ke X, Ci T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromolecules. 2020;164:2583–2597. doi: 10.1016/j.ijbiomac.2020.08.068. [DOI] [PubMed] [Google Scholar]
- 124.Jiang B, et al. Dual-targeting delivery system for bone cancer: synthesis and preliminary biological evaluation. Drug Deliv. 2012;19:317–326. doi: 10.3109/10717544.2012.714809. [DOI] [PubMed] [Google Scholar]
- 125.Zhang G, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012;18:307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 126.Salerno M, et al. Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr. Cancer Drug Targets. 2010;10:649–659. doi: 10.2174/156800910793605767. [DOI] [PubMed] [Google Scholar]
- 127.Wang C, et al. Trifolium-like platinum nanoparticle-mediated photothermal therapy inhibits tumor growth and osteolysis in a bone metastasis model. Small. 2015;11:2080–2086. doi: 10.1002/smll.201403315. [DOI] [PubMed] [Google Scholar]
- 128.Wang X, et al. Peptide decoration of nanovehicles to achieve active targeting and pathology-responsive cellular uptake for bone metastasis chemotherapy. Biomater. Sci. 2014;2:961–971. doi: 10.1039/c4bm00020j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kievit FM, et al. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano. 2012;6:2591–2601. doi: 10.1021/nn205070h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Castillo LF, Rivero EM, Goffin V, Lüthy IA. Alpha2-adrenoceptor agonists trigger prolactin signaling in breast cancer cells. Cell. Signal. 2017;34:76–85. doi: 10.1016/j.cellsig.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Cakir Y, Plummer HK, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int. J. Oncol. 2002;21:153–157. [PubMed] [Google Scholar]
- 132.Vázquez SM, et al. Human breast cell lines exhibit functional α2-adrenoceptors. Cancer Chemother. Pharmacol. 2005;58:50–61. doi: 10.1007/s00280-005-0130-4. [DOI] [PubMed] [Google Scholar]
- 133.Strell C, et al. Norepinephrine promotes the β1-integrin–mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROα release. Mol. Cancer Res. 2012;10:197–207. doi: 10.1158/1541-7786.MCR-11-0130. [DOI] [PubMed] [Google Scholar]
- 134.Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res. Treat. 2011;129:549–556. doi: 10.1007/s10549-011-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]



