Abstract
Chronic methamphetamine use is linked to abnormalities in brain structure, which may reflect neurotoxicity related to metabolism of the drug. As the cytochrome P450 2D6 (CYP2D6) enzyme is central to the metabolism of methamphetamine, genotypic variation in its activity may moderate effects of methamphetamine on brain structure and function. This study explored the relationship between CYP2D6 genotype and measures of brain structure and cognition in methamphetamine users. Based on the function of genetic variants, a CYP2D6 activity score was determined in 82 methamphetamine-dependent (DSM-IV criteria) and 79 healthy-control participants who completed tests of cognitive function (i.e., attention, memory and executive function); most were also evaluated with structural MRI (66 methamphetamine-dependent and 52 control). The relationship between CYP2D6 activity score and whole brain cortical thickness differed by group (interaction p=0.024), as increasing CYP2D6 activity was associated with thinner cortical thickness in the methamphetamine users (β=−0.254; p=0.035), but not in control subjects (β=0.095; p=0.52). Interactions between CYP2D6 activity and group were non-significant for hippocampal volume (ps>0.05), but both hippocampi showed trends similar to those observed for cortical thickness [negative relationships in methamphetamine users (ps<0.05) and no relationships in controls (ps>0.50)]. Methamphetamine users had lower cognitive scores than control subjects (p=0.007), but there was no interaction between CYP2D6 activity score and group on cognition (p>0.05). Results suggest that CYP2D6 genotypes linked to higher enzymatic activity may confer risk for methamphetamine-induced deficits in brain structure. The behavioral consequences of these effects are unclear and warrant additional investigation.
Keywords: CYP2D6, metabolism, methamphetamine, cognition, brain structure
1. INTRODUCTION
Preclinical evidence indicates that methamphetamine consumption can be neurotoxic, particularly when administered in large doses1. However, methamphetamine-induced changes in the brain can be moderated by a variety of biological and drug-specific factors2–5. The efficiency through which methamphetamine is metabolized, in particular, affects concentrations of the drug and its metabolic products in the brain, leading to individual differences in the degree to which users are subjected to potentially harmful compounds6,7.
In humans, approximately 37%–54% of methamphetamine is excreted unchanged in urine8, depending upon urinary pH9. Phase I metabolism (oxidation) in the liver is primarily regulated by the enzyme cytochrome P450 2D6 (CYP2D6), which catalyzes the conversion of methamphetamine to the p-hydroxylated metabolite, para-hydroxymethamphetamine (p-OHMA), and the N-demethylated product, amphetamine7,10. Both metabolites occur in the plasma of human methamphetamine users10. Whereas amphetamine can have its own psychoactive effects, p-OHMA is not psychoactive but has hypertensive and other adrenergic effects11.
The CYP2D6 enzyme is genetically polymorphic, such that individuals differ in degree of CYP2D6 activity, ranging from absent or poor enzymatic activity (two non-functional alleles) to ultrarapid (two functional alleles with gene copy number variation). Building on existing functional data12, consensus guidelines have been developed to assign a phenotypic enzyme activity score based on CYP2D6 genotype and to translate this activity score to categorical metabolizer status13. The guidelines dictate that poor metabolizers carry two non-functional CYP2D6 alleles (activity score 0); intermediate metabolizers carry one null and one partial function allele (activity score 0.5) or two partial function alleles (activity score 1); extensive metabolizers carry one partial function allele and one wildtype allele (activity score 1.5) or two wildtype alleles (activity score 2); and ultrarapid metabolizers are identical to extensive metabolizers but carry one or more additional functional or partially functional copies (activity score > 2).
Research has confirmed that urinary concentrations of p-OHMA are associated with CYP2D6 genotype14,15. An autopsy study demonstrated that methamphetamine users with the CYP2D6 intermediate metabolizer phenotype have higher levels of methamphetamine in urine and bone marrow than methamphetamine users with the extensive metabolizer phenotype16.
Yet little research has evaluated whether variation in CYP2D6 genotype affects the clinical or neurobiological consequences of chronic methamphetamine use. Cherner and colleagues17 hypothesized that low CYP2D6 activity would confer greater risk for developing methamphetamine-induced cognitive deficits due to greater exposure to the drug relative to those with greater metabolic activity. However, contrary to expectations, they found that methamphetamine-dependent participants with the extensive metabolizer phenotype had greater cognitive impairment than those with the poor or intermediate phenotype, particularly in the domains of memory and executive function17. Because extensive metabolizers are proportionally exposed to higher levels of p-OHMA and amphetamine than poor metabolizers, this suggested the possibility that these metabolic byproducts may be more harmful to neurocognition than the parent compound.
To our knowledge, no other studies of the effect of CYP2D6 on behavior or brain function of methamphetamine users are available. However, a previous study found that methamphetamine users with higher CYP2D6 activity scores (based on genotype) tended to be more likely to have heart failure than those with lower activity scores18, again suggesting that greater CYP2D6 activity may confer increased risk of untoward outcome.
Given evidence that greater CYP2D6 activity is associated with cognitive deficits in methamphetamine-dependent participants17, we sought to evaluate whether genotypes that confer greater CYP2D6 activity would be associated with alterations in brain structure in methamphetamine-dependent subjects. We also aimed to determine whether the reported relationship between CYP2D6 and cognitive performance in methamphetamine users is replicable. CYP2D6 genotype was assayed in 82 methamphetamine-dependent participants and 79 healthy control participants who completed cognitive tests in the domains of attention, memory and executive function. Most of the participants also received structural MRI (methamphetamine N = 66; healthy control N = 52). Healthy control subjects were included to test whether potential relationships between CYP2D6 and cognition or brain structure were specific to methamphetamine users.
We hypothesized that, in methamphetamine users, genotypes with greater CYP2D6 activity would be associated with worse performance on a battery of cognitive tests, particularly tests of memory and executive function; in controls, we expected no such relationships. We also hypothesized that methamphetamine users but not control participants would show a negative relationship between CYP2D6 activity scores and whole brain cortical thickness. Memory deficits have been associated with thinner whole-brain cortical thickness in methamphetamine users19. Similarly, given the association between memory and hippocampal structure20,21, we hypothesized that greater CYP2D6 activity would be associated with smaller hippocampal volume in methamphetamine users but unrelated to hippocampal volume in control participants.
2. METHODS AND MATERIALS
2.1. Participants
The participants were 82 currently methamphetamine-dependent subjects who were not seeking treatment, and 79 healthy control subjects. Participants were recruited using Internet and local newspaper advertisements, and they received monetary compensation. After receiving a detailed description of the protocol, they provided written informed consent, following the guidelines of the UCLA Office for Protection of Research Subjects. All control participants completed the study on an outpatient basis. Fifty-four methamphetamine users completed the study as inpatients in the UCLA General Clinical Research Center (GCRC), where they maintained supervised abstinence from drugs of abuse; 28 completed the study as outpatients after closure of the GCRC (in which case abstinence was reinforced through compensation). All methamphetamine participants had urine toxicology tests positive for methamphetamine at study entry, but negative for methamphetamine and other illicit substances (amphetamine, opiates, cocaine, benzodiazepines) during cognitive and MRI assessments, which occurred following approximately one week of abstinence (see Table 1). Control participants tested negative for all drugs except marijuana. Given the long duration in which marijuana can be detected through urinalysis, brief abstinence from marijuana for outpatients was verified through saliva testing [Oratect; Grapevine, Texas], with all participants endorsing at least 4 days of abstinence at testing. Participants were originally recruited to complete other studies of cognition and brain structure/function in methamphetamine dependence e.g.,19,22,23–25, but none had CYP2D6 analyzed until the present study. All participants were fluent in English and were administered the Structured Clinical Interview for the DSM-IV (SCID) for Axis I diagnosis26. The exclusion criteria, based on interview and laboratory tests, were: neurological disease (e.g., stroke, head trauma with loss of consciousness > 30 min); frank structural brain abnormalities on MRI; systemic disease; cardiovascular disease; pulmonary disease; HIV infection (HIV1/HIV2 antibody screen); abnormal laboratory tests (hematocrit, plasma electrolytes, markers for hepatic and renal function); use of psychotropic medications; diagnosis of current abuse or dependence for any substance other than methamphetamine, marijuana or nicotine; and any current non-substance-induced Axis I psychiatric conditions (with the exception of one methamphetamine user with social phobia and one control subject with specific phobia). Six control participants and eight methamphetamine users met criteria for current marijuana abuse or dependence. Demographic characteristics of the participants are shown in Table 1.
Table 1.
Characteristics of Research Participants
| Healthy Control | Methamphetamine-Dependent | |
|---|---|---|
| Sample size | 79 | 82 |
| Age | 32.3 ± 8.0 (19 to 54) | 33.2 ± 9.1 (18 to 52) |
| Education (yrs.) | 13.5 ± 1.9 (9 to 18) | 12.5 ± 1.6** (8 to 18) |
| Premorbid IQ (vocabulary score) | 31.0 ± 4.0 (19 to 38) | 27.7 ± 5.8** (12 to 38) |
| Ethnicity | ||
| Caucasian | 31 | 40 |
| African Am. | 9 | 4 |
| Hispanic | 21 | 26 |
| Asian/Pacific Islander | 11 | 5 |
| Other | 7 | 7 |
| Gender | ||
| Male/female | 42/37 | 42/40 |
| Cigarette Smokers (yes/no) | 38/41 | 74/8** |
| Cigarette Pack-Years (smokers only) | 9.4 ± 8.0 (0.35 to 35.0) | 10.5 ± 12.7 (0.02 to 59.5) |
| Days Alcohol/Past 30 | 4.8 ± 7.5 (0 to 30) | 3.7 ± 5.8 (0 to 30) |
| Days Marijuana/Past 30 | 1.4 ± 4.8 (0 to 28) | 3.7 ± 7.9* (0 to 30) |
| Days Methamphetamine/Past 30 | -- | 22.3 ± 8.0 (5 to 30) |
| Duration of Heavy Methamphetamine Use (yr.) | -- | 7.7 ± 6.7 (0.2 to 24) |
| Grams Methamphetamine/week | -- | 3.0 ± 2.6 (0.13 to 14.5) |
| Days Abstinent at Cognitive Testing | -- | 7.4 ± 2.8 (3 to 17) |
| Days Abstinent at MRI Scanning | -- | 6.9 ± 3.2 (3 to 19) |
| Preferred Route of Methamphetamine | ||
| Administration | ||
| Smoke | -- | 58 |
| Inject | -- | 12 |
| Intranasal | -- | 8 |
| Other/No Preference | -- | 4 |
Note. Values are means ± SDs, where appropriate, with the range in parentheses. The symbols * and ** indicate significant difference from the control group at p < 0.05 and p < 0.01, respectively. Premorbid IQ was estimated with the Shipley Vocabulary Test55. Heavy methamphetamine use defined as using at least three times a week, or twice weekly binges. When cognitive testing was completed on two days, duration of abstinence was taken as the average of the two days.
2.3. Genotyping
Genomic DNA was extracted from whole blood using QiaAmp DNA Blood Mini Kits (Qiagen, Valencia, CA). Genotyping was performed using the TaqMan genotyping platform (Life Technologies, Grand Island, NY) with Qiagen Type-it Fast SNP Probe PCR Kit according to manufacturer’s protocols. All markers were in Hardy-Weinberg equilibrium, 10% of the dataset was genotyped in duplicate with perfect concordance, and allele frequencies were consistent with those reported by the HapMap Consortium (https://www.genome.gov/10001688/international-hapmap-project). The four most common functional SNPs in CYP2D6 that are associated with >90% of intermediate metabolizer and poor metabolizer phenotypes in Caucasian and African American individuals were genotyped (rs1065852 [missense], rs3892097 [splice donor], rs16947 [missense], rs28371706 [missense]), and gene copy number variation (CNV) was determined using Taqman real-time PCR. Therefore, we were able to detect gene deletions and duplications and distinguish *4, *5, *10, *17, and *29 from wildtype alleles. We did not test for rare polymorphisms/alleles (<1% minor allele frequency), since these, if present at all, would have represented a negligible portion of our sample. While over 90 known alleles have been described in CYP2D6, the two most common variants in Caucasian and Hispanic populations account for over 94% of the genetic variation27. In order to further validate the TaqMan genotyping method, a subset of 24 genotypes from our sample (4 samples from each of 3 genotype groups for each variant) were verified by dideoxy sequencing on a 3730 DNA Analyzer (Life Technologies, Grand Island, NY). TaqMan-generated genotypes matched sequence-derived genotypes in all cases.
Activity score was assigned by summing the number of null (0), partial function (0.5), and wild-type (1) alleles plus or minus CNV duplication or deletion according to the combined Clinical Pharmacogenetics Consortium (CPIC) and Dutch Pharmacogenetics Working Group (DPWG) consensus guideline13. For metabolizer status assignment, extensive metabolizers had a score of 1.5–2, intermediate metabolizers 0.5–1, poor metabolizers 0 and ultrarapid >212,13. For statistical analysis, we used the activity score as the variable of analysis so that the data could be kept in its most granular form; however, we also provide figures with the data shown categorically so that trends can be observed in the different metabolizer types.
2.3. Cognitive Tests
The cognitive battery was an abbreviated version of a battery that we have used before19,28. It included measures of processing speed/attention: Trailmaking Part A29, Stroop Color and Word identification30; learning/memory: Selective Reminding Test – Total Recall31; and executive function: Stroop Color-Word Interference30; Trailmaking Part B29, Controlled Oral Word Fluency FAS,32, Stop Signal Task – Stop Signal Reaction Time33 and the Wisconsin Card Sorting Task – Perseverative Errors34. IQ was estimated with the vocabulary score from the Shipley Hartford Test35 (this score was not included in the overall cognitive battery score, see below). Based on prior recommendation36, scores on the Stop-Signal Task from participants with stop signal reaction times greater than 3 standard deviations above the overall mean were excluded (3 controls and 1 methamphetamine user). One control participant’s score on Trailmaking Part B was also excluded because it was 7 standard deviations slower than the overall mean.
Because data were not available from all participants for all cognitive tests, an overall cognitive battery score was calculated for only those participants who completed at least three separate tests (N = 141; Control N = 76; Methamphetamine N = 65). As described previously28,37, the overall cognitive battery score was created by centering and scaling each of the test scores based on the mean and standard deviation of the control group, and then averaging the resulting standardized scores (tests on which lower scores indicated better performance were multiplied by −1 to maintain consistent directionality of scores). Standardizing to the control sample is simply a scale change applied equally to all subjects, designed to put equal weights on the measures in the composite score. It does not represent self-referential use of the data or influence the detection of group differences. Standardizing to the control group, rather than to published norms, has the advantage of not introducing variation in scores based on differences in normative datasets used for different tests; see38; it also allows for standardization of tests for which there are no published normative data (e.g., Stop Signal Task).
2.4. Structural MRI
Useable MRI data were available form 118 participants. High-resolution magnetization-prepared rapid gradient imaging (MPRAGE) was performed at 1.5 Tesla on a Siemens Sonata scanner (TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, voxel size = 1 mm3). Cortical thickness and hippocampal volumes were measured using FreeSurfer (version 5.3). Briefly, images were normalized to remove bias field, non-brain tissue was removed from the image39, and subcortical white matter and deep gray matter volumetric structures were segmented40,41. To generate cortical surfaces, a tessellation was formed along the white-matter surface and was grown outward towards the intensity gradient separating the gray matter from the cerebrospinal fluid. White-matter and pial surfaces were visually inspected for accuracy, and manually corrected in cases of where the white matter was not accurately classified and in cases where the pial surface included dura, sinus, or skull. Whole- brain cortical thickness was taken as the weighted average of right and left hemisphere cortical thickness, weighted by cortical volume.
2.4. Data Analysis
Group differences in demographic characteristics were evaluated using t-tests or Chi-square tests, as appropriate. Multiple regression was used to evaluate the relationship between group, CYP2D6 activity score and their interaction on whole brain cortical thickness. In this analysis, the main effects of group and activity score were entered in step one, while the interaction between group and activity score was added to the main effects in step two. Analysis of the other dependent variables (i.e., hippocampal volume, cognitive battery scores) were carried out in a similar fashion to the analysis of cortical thickness. Age, years of education, estimated IQ and recent marijuana use (days of marijuana use in the last 30 days) were included as covariates in all models. Total intracranial volume (calculated by FreeSurfer) was also included as a covariate in the analyses of brain structure.
Following significant effects in whole brain cortical thickness, exploratory correlations were conducted on specific regions of interest. Based on prior research in methamphetamine users, these included regions of the orbitofrontal cortex22,42, inferior frontal gyrus21,43, middle frontal gyrus44,45, superior frontal gyrus22,42, cingulate cortex21,22, precentral cortex22, and precuneus22,46.
RESULTS
3.1. Group comparisons of demographic characteristics
The methamphetamine user and control groups did not significantly differ in age, gender, ethnicity, days of alcohol use in the last 30 days, intracranial volume, CYP2D6 activity score or CYP2D6 metabolizer type (ps > 0.05). In the methamphetamine group, CYP2D6 activity score was not significantly correlated with age of first methamphetamine use, years of heavy methamphetamine use, grams of methamphetamine used per week, days of methamphetamine used in the last month, days of marijuana used in the last month, or pack years of cigarette smoking (ps > 0.05; in methamphetamine users, these variables also did not differ significantly among the different CYP2D6 metabolizer types, ps > 0.05). The CYP2D6 activity score did not differ by preferred route of methamphetamine administration (p > 0.05), nor was categorical CYP2D6 metabolizer type associated with route of administration (p > 0.05).
The methamphetamine group had a higher proportion of individuals who smoked cigarettes than the control group (p < 0.01); only eight methamphetamine users did not smoke cigarettes (because group and smoking status were confounded, smoking status was not included as a covariate in group analyses). Compared to control subjects, the methamphetamine users also had fewer years of education, lower estimated IQ, and more frequent recent marijuana use (days used in last 30; ps < 0.05), so these variables were included as covariates in subsequent analyses. The demographic composition of the groups is shown in Table 1.
3.2. CYP2D6 activity score and brain structure
There were no significant main effects of group and CYP2D6 activity score on whole brain cortical thickness (ps > 0.05), but these findings were limited by a significant interaction between group and CYP2D6 activity score (F change (1, 109) = 5.25; p = 0.024). Follow-up regressions revealed that CYP2D6 activity score was negatively related to whole brain cortical thickness in methamphetamine users (β = −0.254; p = 0.035), but was not significantly related to cortical thickness in control subjects (β = 0.095; p = 0.52); see Figure 1. Cortical thickness by categorical metabolizer type is also shown in Figure 2.
Figure 1.
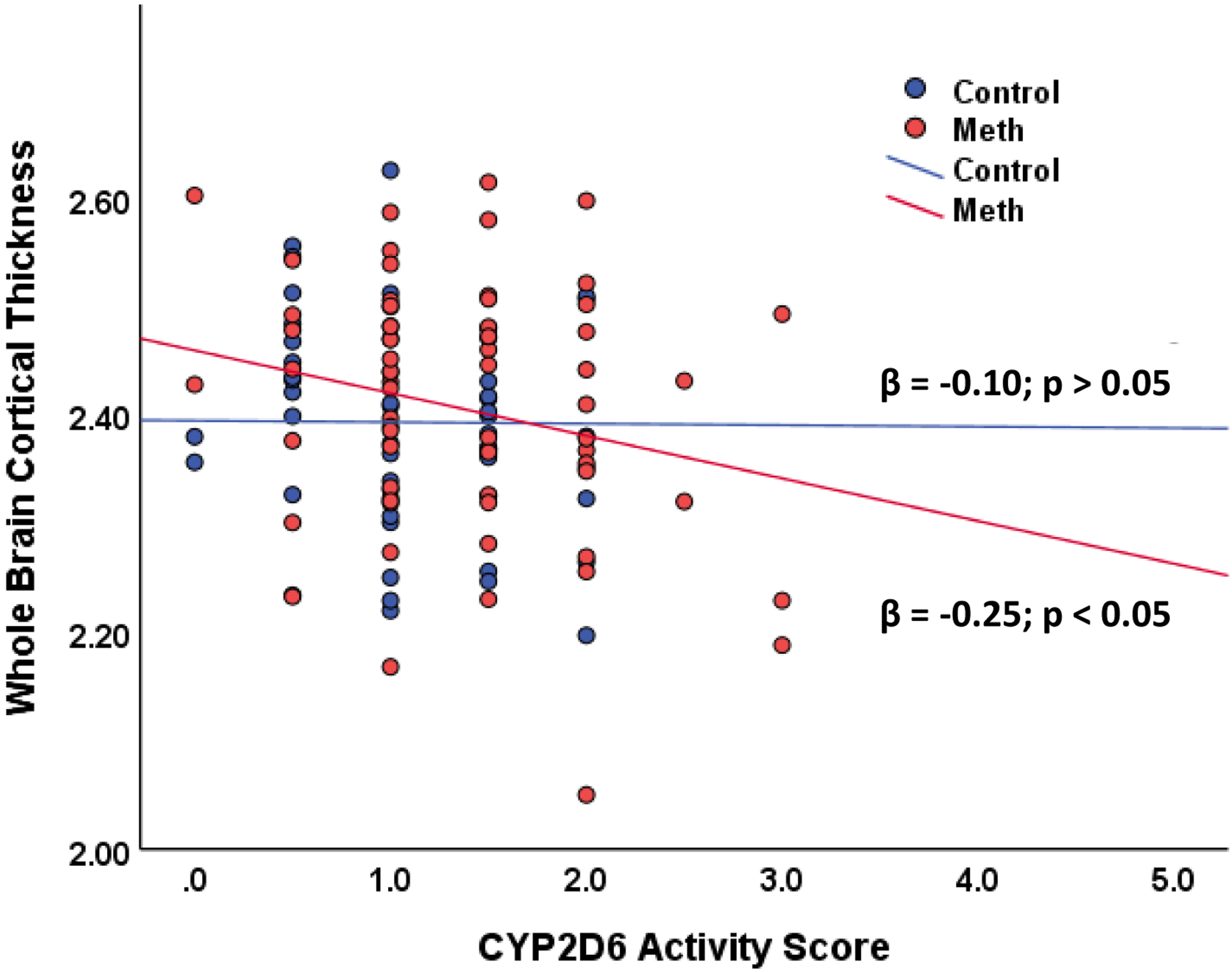
The relationship between CYP2D6 activity score and whole brain cortical thickness.
Note: Methamphetamine N = 66; healthy control N = 52. Cortical thickness measured in millimeters.
Figure 2.
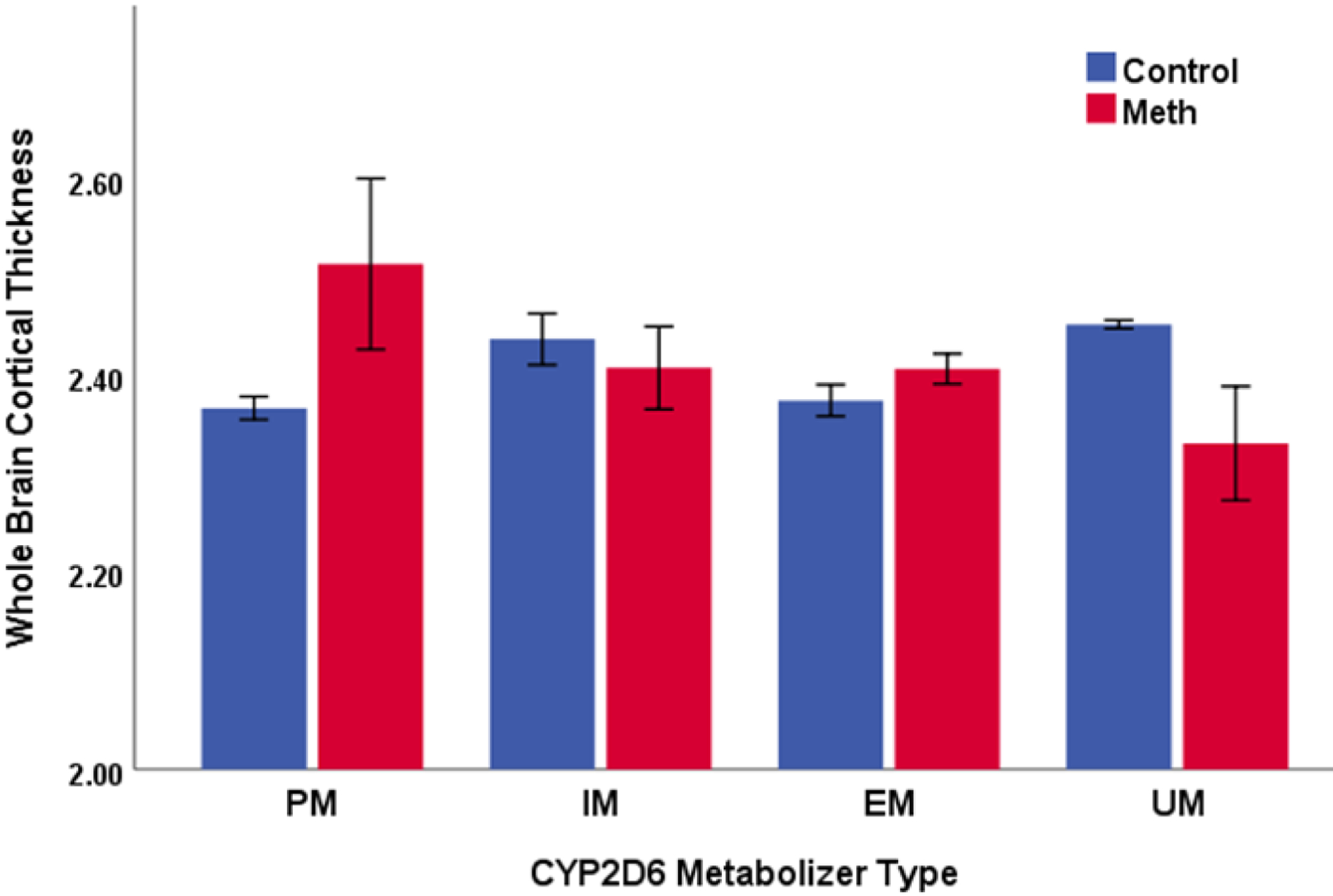
The relationship between CYP2D6 metabolizer type and whole brain cortical thickness.
Note: Methamphetamine N = 66; healthy control N = 52. PM = poor metabolizer (Meth N = 2; control N = 2). IM = intermediate metabolizer (Meth N = 7; control N = 12). EM = extensive metabolizer (Meth N = 52; control N = 36). UM = ultrarapid metabolizer (Meth N = 5; control N = 2). Cortical thickness measured in millimeters. Error bars reflect +/− 1 SEM.
With respect to left hippocampal volume, there was a main effect of CYP2D6 activity score (β = −0.208; p = 0.008), but no main effect of group (β = −0.043; p = 0.60). The interaction between group and CYP2D6 activity score was a non-significant trend (F change (1, 108) = 2.99; p = 0.087). Follow-up regressions revealed that CYP2D6 activity score was negatively related to left hippocampal volume in methamphetamine users (β = −0.306; p = 0.004), but was not significantly related to left hippocampal volume in control subjects (β = −0.076; p = 0.53); see Figures 3 and 4 (left panel).
Figure 3.
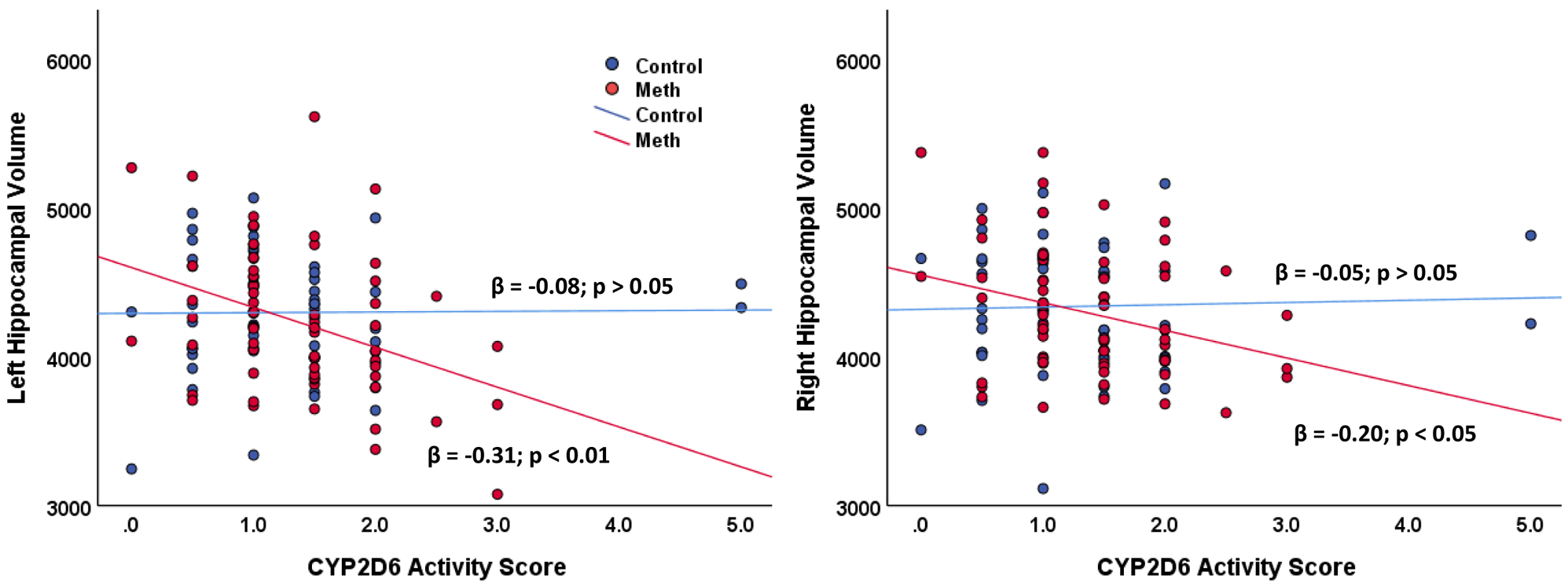
The relationship between CYP2D6 activity score and hippocampal volume.
Note: Methamphetamine N = 66; healthy control N = 52. Volume measured in cubic millimeters.
Figure 4.
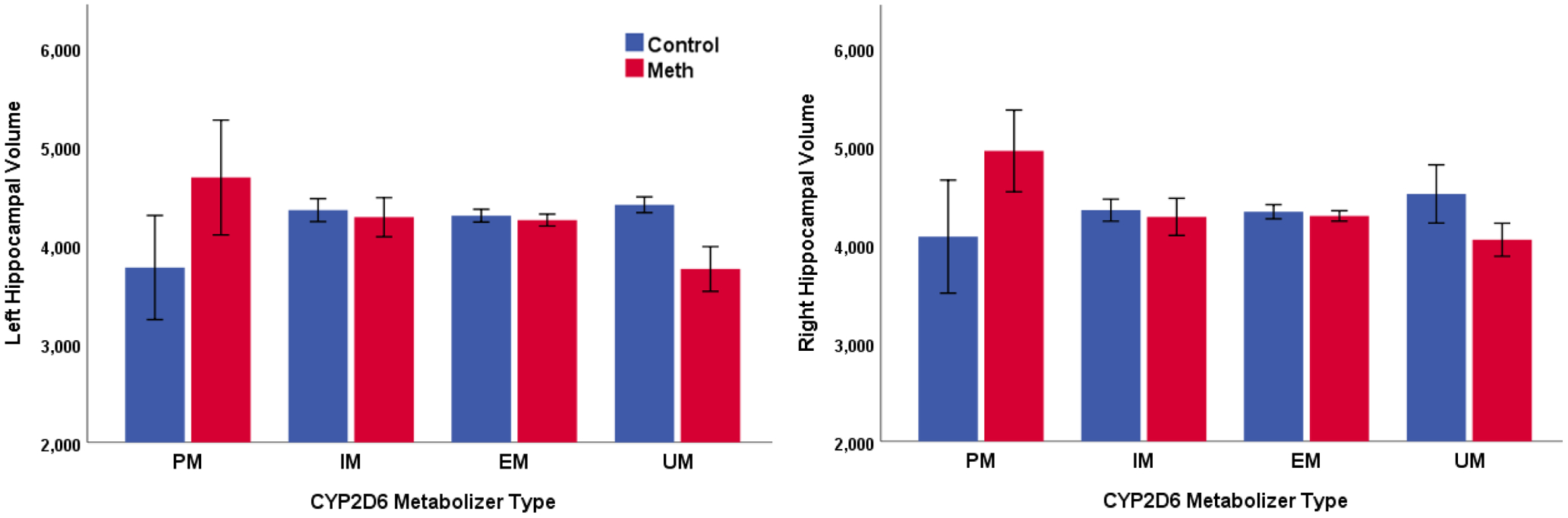
The relationship between CYP2D6 metabolizer type and hippocampal volume.
Note: Methamphetamine N = 66; healthy control N = 52. PM = poor metabolizer (Meth N = 2; control N = 2). IM = intermediate metabolizer (Meth N = 7; control N = 12). EM = extensive metabolizer (Meth N = 52; control N = 36). UM = ultrarapid metabolizer (Meth N = 5; control N = 2).Volume measured in cubic millimeters. Error bars reflect +/− 1 SEM.
Regarding right hippocampal volume, there were no main effects of group or CYP2D6 activity score (ps > 0.05), nor was there a significant interaction between group and CYP2D6 activity score (F change (1, 108) = 0.70; p = 0.41). However, as seen in the left hemisphere, methamphetamine users showed a negative relationship between CYP2D6 activity score and right hippocampal volume (β = −0.195; p = 0.042), whereas control subjects showed no relationship (β = −0.045; p = 0.71); see Figures 3 and 4 (right panel).
Given relationships observed between whole brain cortical thickness and CYP2D6 activity score in methamphetamine users, exploratory partial correlations (controlling for age, education, recent marijuana use, intracranial volume and estimated IQ) were conducted between the CYP2D6 activity score and cortical regions of interest in methamphetamine users. The results are shown in supplemental materials (Supplementary Table 1).
Lastly, we examined whether depressive symptoms, pack-years of cigarette smoking or years of heavy methamphetamine use were independently related to measures of brain structure (whole-brain cortical thickness and hippocampal volume) in methamphetamine users (while controlling for age and estimated intracranial volume); if any of these variables were significant (p < 0.05), they were included as covariates into the aforementioned regressions relating CYP2D6 activity score to brain structure.
In methamphetamine users, scores on the Beck Depression Inventory (BDI) administered at study admission were not significantly related to measures of brain structure (whole-brain cortical thickness and volume of the hippocampi; ps > 0.05). Pack-years of cigarette smoking was negatively related to the volume of the right (β = −0.318; p = 0.006) and left hippocampi (β = −0.271; p = 0.037) in the methamphetamine group, but was not significantly related to whole-brain cortical thickness (p > 0.05). However, when pack-years of smoking was included as a covariate in the regressions relating CYP2D6 activity score to hippocampal volume in methamphetamine users, CYP2D6 remained a significant predictor (ps < 0.05, bilaterally). Years of heavy methamphetamine use showed a trend-level negative relationship with whole-brain cortical thickness (β = −0.253, p = 0.068), but was not significantly related to volume of the hippocampi (ps > 0.05). CYP2D6 activity score remained a significant predictor of whole-brain cortical thickness (p = 0.033) when years of heavy methamphetamine use was added to the regression.
3.3. CYP2D6 activity score and cognition
There was a significant main effect of group on overall cognitive battery scores (β = −0.231; p = 0.007), but no main effect of CYP2D6 activity score (β = −0.096; p = 0.22) and no group by CYP2D6 interaction (F change (1, 133) = 0.91; p = 0.34). Methamphetamine users (mean = −0.41) had significantly lower overall cognitive battery scores than control subjects (mean = −0.01).
For exploratory purposes, main effects and the interactions between group and CYP2D6 activity score were examined for each cognitive test. In these analyses, no main effects of CYP2D6 activity score were observed. No interactions were found between group and activity score for any cognitive test, with the exception of a trend-level finding on verbal fluency (p = 0.05). This relationship indicated that verbal fluency was negatively related to the activity score in control subjects (β = −0.271, p < 0.01), but not in methamphetamine users (β = 0.069, p > 0.05). Main effects of group were found for measures of attentional control, with methamphetamine users having lower performance than control subjects in each instance (i.e., Stroop Word, Stroop Color, Stroop Color-Word Interference ps ≤ 0.01). Trend-level main effects were also found for stop signal reaction time (p = 0.07) and Selective Reminding (p = 0.08). None of the exploratory findings were significant when correcting for multiple comparisons. Cognitive scores for the participants are shown in Table 2.
Table 2.
Cognitive Performance of Research Participants
| Healthy Control | Methamphetamine-Dependent | |
|---|---|---|
| Cognitive Battery Overall Mean** | −0.01 ± 0.59 (−1.81 to 1.24) 5% “low” | −0.41 ± 0.56 (−2.2 to 0.7) 14% “low” |
| Processing Speed/Attention | ||
| Trailmaking Part Aa | 24.7 ± 7.6 (13 to 44) 15% “low” | 25.8 ± 7.3 (13 to 47) 13% “low” |
| Stroop Word Naming* | 104.7 ± 15.9 (68 to 148) 15% “low” | 95.2 ± 14.4 (60 to 124) 21% “low” |
| Stroop Color Naming* | 77.0 ± 11.7 (54 to 103) 14% “low” | 70.9 ± 10.2 (48 to 92) 20% “low” |
| Learning/Memory | ||
| Selective Reminding Task – Total Score† | 119.8 ± 14.2 (68 to 139) 13% “low” | 112.3 ± 15.3 (70 to 140) 20% “low” |
| Executive Function | ||
| Stroop Word-Color Interference* | 49.3 ± 10.3 (33 to 72) 22% “low” | 43.7 ± 9.2 (22 to 68) 22% “low” |
| Trailmaking Part Ba | 60.8 ± 26.2 (26 to 155) 10% “low” | 67.3 ± 21.5 (36 to 119) 16% “low” |
| Verbal Fluency (F, A, S) | 44.8 ± 12.9 (17 to 74) 14% “low” | 38.9 ± 11.1 (15 to 68) 23% “low” |
| Wisconsin Card Sorting (Perseverative Errors)a | 10.5 ± 8.3 (3 to 38) 14% “low” | 13.3 ± 10.5 (4 to 52) 17% “low” |
| Stop Signal Reaction Timea† | 222.6 ± 73.5.2 (79.7 to 505.1) 11% “low” | 253.5 ± 80.9 (89.1 to 534.7) 21% “low” |
Note. Values are means ± SDs, with the range in parentheses.
Higher scores indicate worse performance. The “low” percentage is the percent of the scores that placed ≥ 1 SD below the control sample mean. The symbols *, ** and † indicate difference between the groups at p < 0.05, p < 0.01 and p < 0.10 (trend), uncorrected for multiple comparisons.
3. DISCUSSION
Based on previous research, we hypothesized that genotypes conferring increased CYP2D6 activity would be associated with abnormalities in cognition and brain structure in methamphetamine users. Our hypotheses were partially supported. Activity scores based on CYP2D6 genotype were negatively related to whole brain cortical thickness in methamphetamine users, whereas no such relationship was present in healthy control subjects. Interactions between group and the CYP2D6 activity score were non-significant in the analyses of hippocampal volume; however, the pattern of relationships was similar—CYP2D6 activity scores were negatively related to bilateral hippocampal volume in methamphetamine users (ps < 0.05), while relationships in control subjects were non-significant (ps > 0.50). We did not find significant relationships between cognitive performance and CYP2D6 activity in methamphetamine users, although methamphetamine users did perform significantly worse than control subjects overall.
The data presented here suggest that the cortical effect of CYP2D6 in methamphetamine users is generalized, as it is similar in the entire cortical ribbon and both hippocampi. Further, the CYP2D6 activity score showed mild to minimal negative relationships across different cortical regions, without any areas of particularly strong effect (rather, the relationship between the activity score and whole brain was stronger than in any of the regions evaluated; see Supplementary Table 1). With respect to whole-brain cortical thickness and volume of the hippocampi, the effect size of the relationships with CYP2D6 in methamphetamine users was small to moderate (see47). Thus, to the extent that CYP2D6 activity promotes methamphetamine-induced cortical changes, this suggests that it does so subtly and globally.
Structural findings support the hypothesis that increased CYP2D6 enzymatic activity increases the risk of subtle brain changes in methamphetamine users, although it is important to underscore that neither enzymatic activity per se nor changes in brain over time were evaluated in the current study. If CYP2D6 activity increases the risk of methamphetamine-induced brain changes, several potentially overlapping processes may play a role. First, extensive metabolizers of methamphetamine are exposed to relatively higher levels of the metabolic byproducts p-OHMA and amphetamine. Peripherally, p-OHMA leads to increased blood pressure, heart rate and body temperature, and can be toxic in very large doses11. Hypertension can cause vascular remodeling and impaired cerebral autoregulation that can ultimately lead to cerebral atrophy48; chronic exposure to p-OHMA may hasten these effects. Indeed, methamphetamine users with higher CYP2D6 activity scores are at increased risk of heart failure18.
It also is notable that p-OHMA is an indirectly acting sympathomimetic49, and is likely to stimulate release of dopamine from neural terminals and the periphery, similar to what has been shown for para-hydroxyamphetamine (p-OHA)50. Therefore, relative to poor metabolizers, extensive metabolizers may be exposed to an exaggerated phasic dopaminergic response to methamphetamine. Interestingly, striatal D1-type dopamine receptor availability is negatively correlated with whole brain cortical thickness in methamphetamine users, consistent with the view that phasic dopamine release produces cortical neurotoxicity51. Thus, dopaminergic action may contribute to the moderating effect of CYP2D6 on cortical thickness.
The CYP2D6 enzyme is present in the brain as well as the liver, with evidence of expression in cortical neurons, cerebellum, midbrain, striatum, hippocampus and thalamus, particularly at the level of the synaptic terminal52. CYP2D6 in brain is involved in the conversion of tyramine to dopamine53,54 and in the regeneration of serotonin from 5-methoxytryptamine55,56. Its action on endogenous compounds raises the possibility that CYP2D6 may not only affect the metabolism of methamphetamine but also potentiate the effects of methamphetamine on monoaminergic neurotransmitter systems.
Methamphetamine users in this study had deficits in cognitive battery scores relative to the control subjects, consistent with prior research4,19,57. However, we did not find significant relationships between CYP2D6 activity score and cognitive performance in methamphetamine users, failing to replicate a prior finding17 (In fact, on a test of verbal fluency we found a trend-level interaction suggesting that performance was negatively related to CYP2D6 in control subjects but not in methamphetamine users. This result did not survive correction for multiple comparisons, however). The reason for the discrepancy between the two studies is unclear. Because both studies used grossly similar test batteries (some of the tests were identical between studies), it is unlikely that differences can be accounted for by different cognitive tests. However, participants in the Cherner et al. study had been abstinent from methamphetamine for an average of more than 100 days, whereas methamphetamine users in the current study had been abstinent for approximately one week prior to testing. Withdrawal symptoms significantly decrease between baseline and one week of abstinence, although elevated levels can persist for up to a month or longer58. Research also indicates that methamphetamine withdrawal explains some variance in cognitive test scores in the early stage of abstinence19. Withdrawal may have thus had some impact on test scores in the current study, but consistent measures of withdrawal were not available to investigate this issue further. Our null findings regarding cognition and CYP2D6 may pertain only to the stage of early abstinence, not protracted abstinence. Additional research is needed to investigate whether or not CYP2D6 genotype is reliably associated with cognitive function in methamphetamine users, although findings regarding brain structure support this possibility. Additional research should also examine whether CYP2D6 moderates other aspects of behavioral function.
This study is not without limitations. Because it combined data from prior studies, complete sets of cognitive data and/or MRI scans were not obtained from all participants, although sample sizes were generally acceptable given the limited state of the literature. The data acquired here cannot establish a causal role between CYP2D6 activity and brain structure in methamphetamine users. Negative findings in control subjects, however, support the consideration that the effects are linked to methamphetamine use. Because cigarette smoking status was confounded with group (methamphetamine and control), we could not control for smoking status in group-level analyses. However, in the methamphetamine group alone, controlling for pack-years of cigarette smoking did not significantly attenuate the relationships we found between CYP2D6 and brain structure. Finally, the behavioral consequences of the relationships between CYP2D6 genotype and brain structure are unclear.
Nonetheless, the current findings are consistent with prior literature showing that greater CYP2D6 activity confers risk for deleterious outcome in methamphetamine users. If this is the case, harm reduction strategies can be explored to mitigate these consequences, such as treatment with CYP2D6 inhibitors.
Supplementary Material
Acknowledgements:
The authors of this manuscript declare no conflicts of interest. Research was supported by NIH grants R21 DA034928 (Dean), K23 DA 927734 (Dean), UL1TR000124 (UCLA CTSI), P20 DA 022539 (London), R01 DA 020726 (London), and R01 DA 15179 (London), and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies and the Marjorie M. Greene Trust (London). Data available on request from the authors.
REFERENCES
- 1.Yang X, Wang Y, Li Q, et al. The Main Molecular Mechanisms Underlying Methamphetamine- Induced Neurotoxicity and Implications for Pharmacological Treatment. Frontiers in molecular neuroscience. 2018;11:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowyer JF, Gough B, Slikker W Jr., Lipe GW, Newport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacology Biochemistry and Behavior. 1993;44(1):87–98. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol. 2010;21(7):602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(2):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teuchert-Noodt G, Dawirs RR. Age-related toxicity in prefrontal cortex and caudate-putamen complex of gerbils (Meriones unguiculatus) after a single dose of methamphetamine. Neuropharmacology. 1991;30(7):733–743. [DOI] [PubMed] [Google Scholar]
- 6.Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21(1):21–34. [DOI] [PubMed] [Google Scholar]
- 7.Lin LY, Di Stefano EW, Schmitz DA, et al. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos. 1997;25(9):1059–1064. [PubMed] [Google Scholar]
- 8.Kim I, Oyler JM, Moolchan ET, Cone EJ, Huestis MA. Urinary pharmacokinetics of methamphetamine and its metabolite, amphetamine following controlled oral administration to humans. Therapeutic drug monitoring. 2004;26(6):664–672. [DOI] [PubMed] [Google Scholar]
- 9.Cook CE, Jeffcoat AR, Sadler BM, et al. Pharmacokinetics of oral methamphetamine and effects of repeated daily dosing in humans. Drug Metab Dispos. 1992;20(6):856–862. [PubMed] [Google Scholar]
- 10.Shima Katagi M, Kamata H, et al. Conjugates of p-hydroxymethamphetamine and 4-hydroxy-3- methoxymethamphetamine in blood obtained from methamphetamine and 3,4-methylenedioxymethamphetamine users: Analysis by LC-MS-MS. Forensic Toxicology. 2008;26:58–65. [Google Scholar]
- 11.Romhild W, Krause D, Bartels H, Ghanem A, Schoning R, Wittig H. LC-MS/MS analysis of pholedrine in a fatal intoxication case. Forensic Sci Int. 2003;133(1–2):101–106. [DOI] [PubMed] [Google Scholar]
- 12.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83(2):234–242. [DOI] [PubMed] [Google Scholar]
- 13.Caudle KE, Sangkuhl K, Whirl-Carrillo M, et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clinical and translational science. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda GE, Sordo M, Salazar AM, et al. Determination of amphetamine, methamphetamine, and hydroxyamphetamine derivatives in urine by gas chromatography-mass spectrometry and its relation to CYP2D6 phenotype of drug users. J Anal Toxicol. 2007;31(1):31–36. [DOI] [PubMed] [Google Scholar]
- 15.Smith RL. Human genetic variations in oxidative drug metabolism. Xenobiotica. 1986;16:361–365. [PubMed] [Google Scholar]
- 16.Matsusue A, Ikeda T, Tani N, et al. Association between cytochrome P450 2D6 polymorphisms and body fluid methamphetamine concentrations in Japanese forensic autopsy cases. Forensic Sci Int. 2018;289:33–39. [DOI] [PubMed] [Google Scholar]
- 17.Cherner M, Bousman C, Everall I, et al. Cytochrome P450–2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. J Int Neuropsychol Soc. 2010;16(5):890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutter ME, Gaedigk A, Albertson TE, et al. Polymorphisms in CYP2D6 may predict methamphetamine related heart failure. Clin Toxicol (Phila). 2013;51(7):540–544. [DOI] [PubMed] [Google Scholar]
- 19.Dean AC, Morales AM, Hellemann G, London ED. Cognitive deficit in methamphetamine users relative to childhood academic performance: link to cortical thickness. Neuropsychopharmacology. 2018;43(8):1745–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichenbaum H A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. [DOI] [PubMed] [Google Scholar]
- 21.Thompson PM, Hayashi K, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125(3):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71(3):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36(5):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B, London ED, Poldrack RA, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29(47):14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IP). Washington, D.C.: American Psychiatric Press; 1995. [Google Scholar]
- 27.Klein TE, Chang JT, Cho MK, et al. Integrating genotype and phenotype information: an overview of the PharmGKB project. Pharmacogenetics Research Network and Knowledge Base. Pharmacogenomics J. 2001;1(3):167–170. [DOI] [PubMed] [Google Scholar]
- 28.Dean AC, Hellemann G, Sugar CA, London ED. Educational attainment is not a good proxy for cognitive function in methamphetamine dependence. Drug Alcohol Depend. 2012;123:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- 30.Golden CJ. Stroop color and word test: A manual for clinical and experimental uses. Wood Dale, IL: Stoelting Company; 1978. [Google Scholar]
- 31.Buschke H Retention in immediate memory estimated without retrieval. Science. 1963;140:56–57. [DOI] [PubMed] [Google Scholar]
- 32.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 33.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. JExpPsycholHumPerceptPerform. 1984;10(2):276–291. [DOI] [PubMed] [Google Scholar]
- 34.Heaton RK. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- 35.Shipley WC, Burlingame CC. A convenient self-administering scale for measuring intellectual impairment in psychotics. American Journal of Psychiatry. 1941;97(6):1313–1325. [Google Scholar]
- 36.Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Frontiers in psychology. 2012;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED. Denial in methamphetamine users: Associations with cognition and functional connectivity in brain. Drug Alcohol Depend. 2015;151:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitrushina M, Boone KB, Razani J, D’Elia LF, eds. Handbook of normative data for neuropsychological assessment. second ed. Oxford: Oxford University Press; 2005. [Google Scholar]
- 39.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- 40.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 41.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral cortex (New York, NY : 1991). 2004;14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 42.Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106(8):1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabibnia G, Monterosso JR, Baicy K, et al. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31(13):4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SJ, Lyoo IK, Hwang J, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. IntJNeuropsychopharmacol. 2006;9(2):221–228. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz DL, Mitchell AD, Lahna DL, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50(4):1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162(8):1461–1472. [DOI] [PubMed] [Google Scholar]
- 47.Acock AC. A Gentle Introduction to Stata. Fourth edition ed. College Station, Texas2014. [Google Scholar]
- 48.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Current hypertension reports. 2003;5(3):255–261. [DOI] [PubMed] [Google Scholar]
- 49.Bates AT, Chamberlain S, Champion M, et al. Pholedrine: a substitute for hydroxyamphetamine as a diagnostic eyedrop test in Horner’s syndrome. Journal of neurology, neurosurgery, and psychiatry. 1995;58(2):215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208(2):203–209. [PubMed] [Google Scholar]
- 51.Okita K, Morales AM, Dean AC, et al. Striatal dopamine D1-type receptor availability: no difference from control but association with cortical thickness in methamphetamine users. Mol Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chinta SJ, Pai HV, Upadhya SC, Boyd MR, Ravindranath V. Constitutive expression and localization of the major drug metabolizing enzyme, cytochrome P4502D in human brain. Brain research Molecular brain research. 2002;103(1–2):49–61. [DOI] [PubMed] [Google Scholar]
- 53.Bromek E, Haduch A, Daniel WA. The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: An in vitro study. Eur J Pharmacol. 2010;626(2–3):171–178. [DOI] [PubMed] [Google Scholar]
- 54.Hiroi T, Imaoka S, Funae Y. Dopamine formation from tyramine by CYP2D6. Biochem Biophys Res Commun. 1998;249(3):838–843. [DOI] [PubMed] [Google Scholar]
- 55.Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A, Gonzalez FJ. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics. 2003;13(3):173–181. [DOI] [PubMed] [Google Scholar]
- 56.Yu AM, Idle JR, Gonzalez FJ. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab Rev. 2004;36(2):243–277. [DOI] [PubMed] [Google Scholar]
- 57.Zhong N, Jiang H, Du J, et al. The cognitive impairments and psychological wellbeing of methamphetamine dependent patients compared with healthy controls. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2016;69:31–37. [DOI] [PubMed] [Google Scholar]
- 58.Zorick T, Nestor L, Miotto K, et al. Withdrawal symptoms in abstinent methamphetamine‐dependent subjects. Addiction. 2010;105(10):1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


