Abstract
Patients with brain tumors have an increased risk for depression, whose underlying pathomechanism may involve dysregulated tryptophan/kynurenine metabolism. In this study, we analyzed the relation of depressive symptoms to clinical and tumor characteristics as well as cerebral and systemic tryptophan metabolism in patients with primary brain tumors. Sixty patients with newly-diagnosed or recurrent primary brain tumor underwent testing with the Beck Depression Inventory-II (BDI-II), and 34 patients also had positron emission tomography (PET) imaging with alpha-[11C]methyl-L-tryptophan (AMT). BDI-II scores were correlated with clinical and tumor-related variables, cerebral regional AMT metabolism measured in the non-tumoral hemisphere, and plasma tryptophan metabolite levels. Sixteen patients (27%) had BDI-II scores indicating depression, including 6 with moderate/severe depression. High BDI-II scores were independent of clinical and tumor-related variables except lower Karnofsky Performance Status scores. In patients with recurrent malignant gliomas, depression was associated with shorter survival (hazard ratio: 3.7; p=0.048). High BDI-II total and somatic subscale scores were associated with higher frontal cortical and thalamic AMT metabolic values measured on PET. In contrast, plasma tryptophan and kynurenine metabolite levels did not correlate with the BDI-II scores. In conclusion, our results confirm previous data that depression affects more than ¼ of patients with primary brain tumors, it is largely independent of tumor characteristics and is associated with shorter survival in patients with recurrent malignant gliomas. On PET imaging, higher tryptophan metabolism in the frontal cortex and thalamus was found in those with brain tumor-associated depression and supports the role of dysregulated tryptophan/kynurenine metabolism in this condition.
Keywords: brain tumor, depression, PET, tryptophan, serotonin, kynurenine pathway
INTRODUCTION
Patients with brain tumors have an increased risk for depression or depressive symptoms. A recent review found a pooled depression prevalence of 21.7% in brain tumor patients (Huang et al., 2017). Still, the relation between depression and cancer, including brain tumors, remains poorly understood. While depression can develop as a reaction to the disease, depressive symptoms may also precede the clinical diagnosis of cancer (Bortolato et al., 2017; Cosci et al., 2015; Huang et al., 2017). Moreover, depression in patients with brain tumor is often not diagnosed and, therefore, not treated in clinical practice (Litofsky et al., 2004). While most previous studies found no clear association between brain tumor-related depression and clinical variables, including gender, functional status, tumor size, lobe, laterality, or treatment approaches (Rooney et al., 2011), depression was associated with reduced physical function, cognitive impairment, and impaired quality of life (Pelletier, et al., 2002; Rooney et al., 2011). Moreover, in patients with high-grade glioma, depression was associated with shorter survival (Shi etal., 2018).
Tumor-associated depressive symptoms may be related to overlapping molecular mechanisms involved in tumor pathology and depression (Mugge et al., 2018). Among potential mechanisms, the involvement of tryptophan metabolism is commonly implicated. A systematic shunt between serotonin and kynurenine metabolism of tryptophan (“kynurenine shunt”) can result in depleted serotonin levels and accumulation of toxic kynurenine metabolites (Mangoni, 1974). Immune-mediated activation of indoleamine 2,3-dioxygenase 1 (IDO1), a key enzyme of the immunosuppressive kynurenine pathway, and increased metabolism via the 3-hydroxykynurenine branch of the pathway can lead to elevated levels of the neurotoxic quinolinic acid. This has been associated with major depressive disorder (MDD) and suicidality (Platten et al., 2019) and implicated in intervening pathomechanisms of systemic inflammation, cancer, and depressive symptoms (Cervenka et al., 2017; Sforzini et al., 2019).
Tryptophan metabolism in the brain can be noninvasively evaluated in vivo by positron emission tomography (PET) using alpha-[11C]methyl-L-tryptophan (AMT), which accumulates in serotonergic neurons and is also metabolized by IDO1 of the kynurenine pathway (Chugani & Muzik, 2000; Diksic et al., 1990; Muzik et al., 1997). AMT-PET studies in patients with MDD or a history of suicide attempts found focal abnormalities in the fronto-limbic structures, mostly in cingulate and frontal/prefrontal cortex (Berney et al., 2008; Frey et al., 2010; Leyton et al, 2005; Rosa-Neto et al., 2004), indicating abnormal serotonergic activity in these regions. AMT-PET can also provide localizing and prognostic information in pre- and post-treatment brain tumors (Alkonyi et al., 2012; Juhasz et al., 2006; Kamson et al., 2013). Our previous studies also showed abnormal AMT uptake and kinetic variables not only in brain tumors, where its values correlated with key enzymes of the kynurenine pathway in resected tumor samples (Batista et al., 2009; Bosnyak et al., 2015; Guastella et al., 2018; Guastella et al., 2016), but also in the contralateral non-tumoral hemisphere (Juhasz et al., 2012; Kamson et al., 2014). Moreover, high uptake in the thalamus was associated with shorter survival in post-treatment gliomas (Kamson et al., 2014). In a preliminary study, tryptophan transport and metabolic rates in the non-tumoral thalamus, striatum, and frontal cortex, measured by AMT-PET, were associated with depression in patients with brain tumors (Bosnyak et al., 2015).
Previous studies suggested systemic dysregulation of tryptophan/kynurenine metabolism, reflected by altered blood tryptophan metabolite levels and increased kynurenine/tryptophan ratios (an often-used surrogate measure of systemic IDO activity, although activity of tryptophan 2,3-dioxygenase (TDO), present in the liver, may also affect its value) in patients with glioblastoma and other cancers (Adams et al., 2014; Lyon et al., 2011; Suzuki et al., 2010). However, only a few studies investigated blood tryptophan and kynurenine metabolite levels in the context of cancer-associated depression (Botwinick et al., 2014; Herrstedt et al., 2019). While serum kynurenine, tryptophan, or their ratio did not correlate with depression scores in non-cancer patients (Hestad et al., 2017), the kynurenic acid/tryptophan ratio correlated negatively with such scores in patients with pancreatic cancer (Botwinick et al., 2014).
The aim of the present study was to evaluate the relation of clinical and tumor characteristics to depression scores in patients with newly-diagnosed and recurrent primary brain tumors. In addition, we used AMT-PET to evaluate regional cortical and subcortical tryptophan metabolism as a potential imaging marker of brain tumor-associated depression and also assessed if the imaging variables are specific for any of the three major aspects of depression (somatic, affective, cognitive), as defined by the Beck Depression Inventory, Second Edition (BDI-II) (Beck et al., 1996). Finally, we also tested if BDI-II scores are associated with variations in plasma tryptophan metabolites in this patient group.
MATERIALS AND METHODS
Subjects
A total of 60 patients with a primary brain tumor (37 males, mean age: 51±15 years) were prospectively recruited to complete a BDI-II questionnaire during pre-treatment evaluation as part of a research protocol between April 2012 and February 2019. Patients with bilateral tumors, language difficulties, global cognitive impairment, history of MDD, or Karnofsky Performance Status (KPS) score <60 were excluded from the study. Of the 60 patients, 34 had a newly-diagnosed brain tumor and the remaining 26 were diagnosed with post-treatment tumor progression or recurrent tumor (Table 1) based on serial follow-up MRI scans (Wen et al., 2010). Tumor types included gliomas (n=49), meningiomas (n=10), and dysembryoplastic neuroepithelial tumor (DNET; n=1) (Table 1). Thirty patients (50%) were on antiepileptic medication, 19 patients (32%) were on steroid treatment, and 10 patients (17%) were on selective serotonin reuptake inhibitor (SSRI) therapy at the time of their BDI-II testing. Out of the 60 patients, 34 underwent dynamic AMT-PET scanning with blood radioactivity measurements (used for tracer kinetic analysis) on the day of the BDI-II testing. Tumor types in this group included gliomas (n=25), meningiomas (n=8), and DNET (n=1) (Table 1). All patients had a tumor in one cerebral hemisphere, while the contralateral side showed no abnormality. The study was approved by the Institutional Review Board of Wayne State University, and written informed consent was obtained from all participants.
Table 1.
Tumor type and grade distribution in the whole group (n=60), in the subgroup with AMT-PET (n=34), and those with plasma tryptophan and metabolite levels (n=28).
| Whole group | Patients with AMT-PET | Patients with plasma tryptophan metabolites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Newly-diagnosed | Recurrent | Total | Newly-diagnosed | Recurrent | Total | Newly-diagnosed | Recurrent | Total | |
| Glioma | |||||||||
| Astrocytoma (Gr. II/III) | 2 (−/2) | 1 (1/−) | 3 | - | 1 (1/−) | 1 | - | 1 (1/−) | 1 |
| Oligodendroglioma (Gr. II/III) | 6 (3/3) | 3 (1/2) | 9 | 3 (2/1) | 1 (−/1) | 4 | 3 (2/1) | - | 3 |
| Mixed oligo-astrocytoma (Gr. II/III) | 1 (1/−) | 2 (−/2) | 3 | - | - | - | - | - | - |
| Glioblastoma (Gr. IV) | 13 | 18 | 31 | 6 | 12 | 18 | 5 | 9 | 14 |
| Other or unknown glioma type | 2 | 1 | 3 | 2 | - | 2 | 2 | - | 2 |
| Meningioma (Gr. I/II) | 9 (6/1)a | 1 (−/1) | 10 | 7 (4/1)a | 1 (−/1) | 8 | 6 (4/1)a | 1 (−/1) | 7 |
| DNET | 1 | - | 1 | 1 | - | 1 | 1 | - | 1 |
| Total | 34 | 26 | 60 | 19 | 15 | 34 | 17 | 11 | 28 |
: not all patients had surgery to establish tumor grade
Abbreviations: AMT-PET: alpha-[11C]methyl-L-tryptophan positron emission tomography; Gr.:WHO Grade; DNET: dysembryoplastic neuroepithelial tumor.
AMT-PET scanning
All AMT-PET studies were performed using a GE Discovery STE PET/CT scanner (GE Medical Systems, Milwaukee, WI) located at the PET Center of the Children’s Hospital of Michigan in Detroit. The scanner has a 15 cm field of view and generates 47 image planes with a slice thickness of 3 mm. The reconstructed image in-plane resolution obtained is 7.5±0.4 mm (isotropic) at full-width half-maximum, and images were reconstructed with an iterative reconstruction (2 iterations, 16 subsets, 8 mm axial smoothing). The AMT tracer was synthesized using a high-yield procedure as previously described (Chakraborty et al., 1996). The procedure for AMT-PET scanning has been described in previous studies (Bosnyak et al., 2015; Juhasz et al., 2006). In short, following 6 hours of fasting, a slow bolus of AMT (3.7 MBq/kg) was injected intravenously over 2 minutes. In the initial 20 minutes of the scan following tracer injection, a dynamic PET scan of the heart was performed to obtain the blood input function from the left cardiac ventricle noninvasively. The blood input function was continued beyond these 20 minutes by using venous blood samples (0.5 mL/sample, collected at 20, 30, 40, 50, and 60 minutes after AMT injection). At 25 minutes after tracer injection, a dynamic emission scan of the brain (7Õ5 min) was obtained. Measured attenuation correction, scatter, and decay correction were applied to all PET images. For visualization of AMT uptake, averaged activity images 30–55 minutes post-injection were created and converted to an AMT standardized uptake value (SUV) image. These images were then co-registered to anatomic MR images acquired the closest to the PET scans (within 2 weeks) for further analysis.
AMT-PET kinetic analysis
For quantification of AMT accumulation in the brain, a Patlak graphical analysis was performed (Patlak et al., 1983) on each PET image, as described previously (Alkonyi et al., 2012; Bosnyak et al., 2015; Juhasz et al., 2006; Kamson et al., 2013). This procedure yields two major kinetic variables, including AMT K and volume of distribution (VD). The AMT K value (expressed as ml/g/min) reflects the unidirectional uptake of tracer into tissue (Chugani et al., 1998; Muzik et al., 1997), which is proportional to the metabolism of tryptophan via the serotonin and/or the kynurenine pathways (Chugani & Muzik, 2000). VD characterizes the net tracer transport from blood to tissue. Our initial screening found no relation between regional VD or SUV and BDI-II scores (p>0.4 in all correlations), therefore, these variables was not included in any further analyses.
For analysis of regional cortical and subcortical AMT K values, the 3D Slicer 3.6.3 software suite was used (Brigham and Women’s Hospital, Boston, MA) as described previously (Bosnyak et al., 2015; Kamson et al., 2014; Kamson et al., 2014). In brief, T2-weighted and/or fluid-attenuated inversion recovery (FLAIR) as well as volumetric post-contrast T1-weighted images were co-registered to and fused with the PET image volumes. Cortical and subcortical regions of interest (ROIs), contralateral to the tumor, were delineated and analyzed as described in our previous study (Bosnyak et al., 2015). The ROIs included MRI-defined gray matter voxels: a single plane for the thalamus and putamen with the largest axial diameter, and multiple planes for the cortical ROIs including the frontal (three planes), temporal (two planes), and parietal (two planes) lobes. None of these ROIs showed abnormal contrast enhancement or abnormal T2/FLAIR signal on the co-registered MR images. The occipital cortex, which often shows high physiologic AMT uptake, was not included in the analyses. Tumoral values were also not included in the analyses, as they showed no associations with BDI-II scores in initial screening. All cortical and subcortical ROIs were applied on the co-registered dynamic PET images, and AMT K values were obtained for each region as the average of the values measured in each plane belonging to a region.
Assessment of depression
Symptoms of depression were assessed using the BDI-II, which is a self-reported measure and a widely accepted screening instrument for symptoms corresponding to the diagnostic criteria for depressive disorders outlined in the Diagnostic and Statistical Manual for Mental Disorders, 4th edition. BDI-II has 21 items, each rated on a 4-point Likert scale (0–3). The measure yields a total score (0–63), and the following cutoff scores were used to estimate depression severity: <13 = no/minimal mood disorder, 13–19 = mild depression, 20–28 = moderate depression, and >28 = severe depression. The psychometric properties of the scale have been well established, and the measure is widely used with both clinical and research samples of cancer (including brain tumor) patient groups (Bosnyak et al., 2015; Mainio et al., 2005; Noll et al., 2019; Pelletier et al., 2002; Rooney et al., 2011; Storch et al., 2004). In addition to the total scores, we also calculated cognitive, affective, and somatic subscale scores. Since the factor structure of the 21 questions is variable across models (McElroy et al., 2018), we used a model similar to Steer et al. (Steer et al., 1999) splitting the non-cognitive section into affective and somatic subscales based on the nature of the questions thus including 8 cognitive (questions 2,3,5–9,14), 6 affective (questions 1,4,10-13), and 7 somatic items (questions 15-21). The somatic items include questions related to loss of energy, changes in sleeping, changes in appetite, irritability, difficulty in concentration, tiredness or fatigue, and loss of interest in sex. All BDI-II scores were made available for the referring physicians, who made decisions regarding further clinical testing and changes in therapy; however, such changes did not affect the results of the present study.
Plasma tryptophan and metabolite measurements
In 28 of the 34 patients who had a dynamic AMT-PET scan (see tumor type distribution of this subgroup in Table 1), fasting blood samples collected in conjunction with the PET scans (right before tracer injection) were available and used for metabolite analysis using a validated liquid chromatography with tandem mass spectrometry method for the assay of plasma levels of tryptophan, kynurenine, kynurenic acid, 3-hydroxykynurenine and 5-hydroxyindoleacetic acid (a serotonin metabolite). Details on blood sample preparation, chromatographic and mass spectrometry conditions are described in the Supplementary material.
Statistical analysis
First, in the whole group (n=60), BDI-II depression scores were compared between binary clinical variables, including gender, tumor type (gliomas vs. meningiomas), newly-diagnosed vs. recurrent tumors, presence vs. absence of antiepileptic, steroid, or SSRI treatment, using the Mann-Whitney U test. The same BDI-II scores were also compared among tumor grades, lateralization (left, right, bilateral/midline), and lobar localization using the Kruskal-Wallis test. Furthermore, BDI-II depression scores were correlated with age and KPS scores in the whole group as well as in the newly-diagnosed and recurrent tumor subgroups separately using Spearman’s rank correlations. Cox regression analyses were performed in the malignant glioma (WHO grade III-IV) subgroups to obtain a hazard ratio (HR) for overall survival of depressed (BDI-II scores ≥13) vs. non-depressed patients (BDI-II <13). Since post-treatment KPS was reported to predict survival in glioblastoma patients (Chambless et al., 2015), we repeated the Cox regression analysis in those with KPS scores of >70 vs. ≤70.
In the second set of analyses, cerebral cortical and subcortical regional AMT K values from the PET analysis were compared between gliomas vs. non-gliomas (meningiomas and DNET), newly-diagnosed vs. recurrent tumors, and depressed vs. non-depressed patients using the Mann-Whitney U test. Correlations between AMT K and BDI-II scores were performed using Spearman’s rank correlations. The same tests were used for the plasma tryptophan and metabolite levels for the 28 patients, where these values were measured. Statistical analyses were performed using IBM SPSS Statistics, version 24. A p value less than 0.05 (with Bonferroni correction, where applicable), was considered to be significant.
RESULTS
Relation between BDI-II scores, clinical variables, and tumor characteristics
In the whole set of patients (n=60), mean total BDI-II score was 10±8 (range: 0–37). Sixteen patients (27%) had BDI-II scores ≥13, indicating depressive disorder, including 10 with mild depression (scores 13-19) and 6 with scores consistent with moderate to severe depression (scores >19). Only 5 of these patients were on SSRI treatment at the time of the testing.
Total BDI-II scores were not different between tumor types (meningioma vs. glioma), between newly-diagnosed vs. recurrent tumors, or across tumor grades and did not differ according to tumor lateralization or lobar localization (Table 2). Likewise, antiepileptic and steroid treatment was not associated with the BDI-II scores. However, higher BDI-II scores were noted in patients with ongoing SSRI therapy (15±10 vs. 8±7, p=0.017, Table 2).
Table 2.
Comparison of the total BDI-II scores in various subgroups in the total study population (N=60). P values refer to results of Mann-Whitney U tests, unless otherwise indicated.
| Total BDI-II score | |||
|---|---|---|---|
| Number of subjects | Mean ± SD | p value | |
| Gender | |||
| male | 37 | 10 ± 8 | 0.83 |
| female | 23 | 10 ± 9 | |
| Tumor group | |||
| newly-diagnosed | 34 | 9 ± 9 | 0.31 |
| recurrent | 26 | 10 ± 7 | |
| Tumor typea | |||
| glioma | 49 | 9 ± 8 | 0.88 |
| meningioma | 10 | 11 ± 10 | |
| DNET | 1 | (10) | |
| Tumor gradeb,c | |||
| Grade I | 7 | 10 ± 9 | 0.58 |
| Grade II | 9 | 12 ± 8 | |
| Grade III | 10 | 7 ± 4 | |
| Grade IV | 31 | 10 ± 8 | |
| Tumor lateralizationc | |||
| left | 23 | 11 ± 9 | 0.64 |
| right | 34 | 9 ± 8 | |
| bilateral/midline | 3 | 9 ± 8 | |
| Tumor localizationc | |||
| frontal | 29 | 11 ± 8 | 0.26 |
| temporal | 13 | 8 ± 10 | |
| parietal | 9 | 9 ± 8 | |
| occipital | 5 | 5 ± 4 | |
| otherd | 4 | 11 ± 12 | |
| Antiepileptic treatment | |||
| yes | 30 | 10 ± 9 | 0.82 |
| no | 30 | 9 ± 8 | |
| SSRI treatment | |||
| yes | 10 | 15 ± 10 | 0.017 |
| no | 50 | 8 ± 7 | |
| Steroid usee | |||
| yes | 19 | 11 ± 8 | 0.43 |
| no | 40 | 9 ± 8 | |
: DNET has not included;
: three patients with no surgery have not been included;
: Kruskal-Wallis test;
: located outside the cerebral lobes (all meningiomas);
: one patient had no data available on steroid use. SD: standard deviation.
Total BDI-II scores did not correlate with age (p=0.25) but showed a moderate inverse correlation with the KPS scores (Spearman’s rho [r]=−0.32, p=0.014). KPS score also showed a similar inverse correlation with somatic subscale scores (r=−0.31, p=0.017), while no correlation was observed with cognitive (p=0.11) or affective subscale scores (p=0.08). Since the KPS scores were significantly lower in the recurrent compared to the newly-diagnosed tumor group (84±12 vs. 91±11, respectively, p=0.014), the correlations were repeated in these two subgroups separately. A strong negative correlation with the total BDI-II scores was found in the recurrent tumor group only (r=−0.60, p=0.002), which was also present for both the somatic and cognitive subscale scores (r=−0.60, p=0.002 and r=−0.43, p=0.036, respectively). No similar correlation was found in the newly-diagnosed group (r=−0.06, p=0.73 for the total BDI-II scores).
In recurrent grade III-IV gliomas (n=18), the mean overall survival after the BDI-II testing was 9.5±6.7 months, with 6 patients (33%) being alive at last follow-up (mean follow-up: 12 months [4–21 months]). Total BDI-II scores ≥13 were associated with shorter survival in Cox regression analysis (HR, 3.7; 95% Cl: 1.0–13.6; p=0.048; Fig. 1). Estimated overall survival was 6.6 (SEM: 0.9) months in patients with high versus 17.0 (SEM: 3.9) months in those with low BDI-II scores. In the same subgroup, overall survival was not significantly different between patients with >70 vs. ≤70 KPS scores (HR, 0.23; 95% Cl: 0.05-1.17; p=0.08). Survival analysis was not performed in the newly-diagnosed high-grade glioma group, where only two patients had BDI-II scores ≥13.
Fig. 1.

Kaplan-Meier survival curves for total BDI-II scores in patients with recurrent high-grade gliomas. Patients with BDI-II scores ≥13 (solid line) had a shorter overall survival than those with BDI-II scores <13 (dashed line) (hazard ratio: 3.7; 95% CI: 1.0–13.6; p=0.048)
BDI-II scores and regional AMT metabolic rates measured by PET
In the 34 patients with AMT-PET data, the K values were not different in any regions between glioma and non-glioma patients or between newly-diagnosed and recurrent glioma groups (Table 3). Total BDI-II scores in the whole group showed a strong positive correlation with frontal cortical and thalamic AMT K values (Table 4). When patients with SSRI treatment (n=5) were excluded, the correlations were even stronger for the whole group, and significant correlations (after Bonferroni correction) included the temporal cortex (Table 4, Fig. 2a). The glioma subgroup showed a somewhat weaker correlation in the same regions (Table 4).
Table 3.
Comparison of regional AMT K-values in the contralateral (non-tumoral) hemisphere between patients with gliomas vs. non-glioma tumors and between newly-diagnosed vs. recurrent gliomas. None of the differences were significant.
| Brain region | Mean K values (SD) |
p value | Glioma mean K values (SD) |
p value | ||
|---|---|---|---|---|---|---|
| Gliomas | Non-gliomas | Newly-diagnosed | Recurrent | |||
| frontal cortex | 0.0069 (0.0028) | 0.0059 (0.0014) | 0.54 | 0.0066 (0.0028) | 0.0071 (0.0029) | 0.63 |
| temporal cortex | 0.0070 (0.0028) | 0.0056 (0.0020) | 0.62 | 0.0065 (0.0026) | 0.0074 (0.0029) | 0.18 |
| parietal cortex | 0.0067 (0.0027) | 0.0055 (0.0016) | 0.71 | 0.0063 (0.0025) | 0.0071 (0.0028) | 0.30 |
| putamen | 0.0085 (0.0035) | 0.0070 (0.0017) | 0.49 | 0.0080 (0.0030) | 0.0089 (0.0039) | 0.47 |
| thalamus | 0.0078 (0.0032) | 0.0063 (0.0018) | 0.31 | 0.0069 (0.0026) | 0.0084 (0.0036) | 0.13 |
Table 4.
Correlations between total BDI-II scores and regional AMT K values in the whole group and subgroup (all tumors and gliomas separately) with selective serotonin reuptake inhibitor (SSRI) treatment.
| Brain region | Whole group (n=34) | Group with no SSRI treatment |
||||
|---|---|---|---|---|---|---|
| all tumors (n=29) | gliomas (n=21) | |||||
| r value | p value | r value | p value | r value | p value | |
| frontal cortex | 0.49 | 0.004* | 0.55 | 0.002* | 0.51 | 0.02 |
| temporal cortex | 0.36 | 0.038 | 0.52 | 0.004* | 0.44 | 0.04 |
| parietal cortex | 0.35 | 0.046 | 0.47 | 0.011 | 0.38 | 0.1 |
| putamen | 0.34 | 0.053 | 0.42 | 0.023 | 0.39 | 0.08 |
| thalamus | 0.53 | 0.001* | 0.61 | <0.001* | 0.55 | 0.01 |
Significant after Bonferroni correction.
Fig. 2.
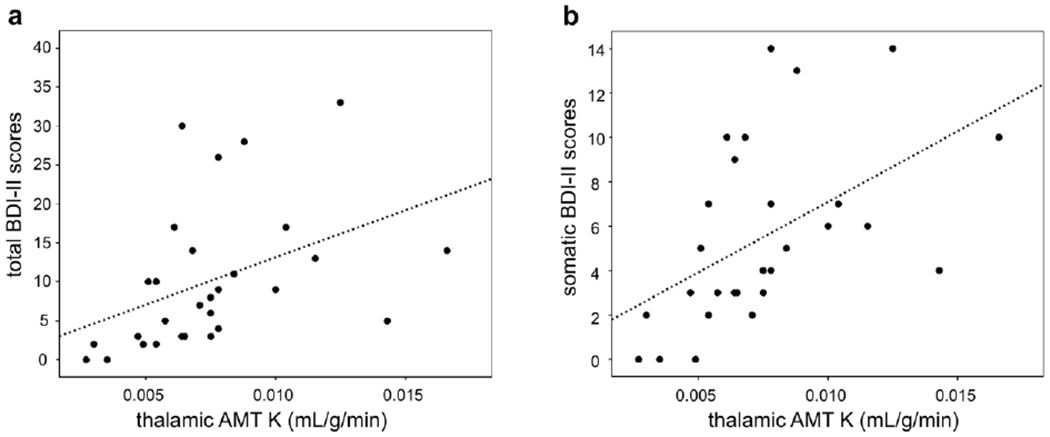
Correlations between thalamic AMT K values and total (a) and somatic subscale (b) scores from BDI-II in patients with no SSRI treatment (n=29). Both correlations were highly significant (Spearman’s rho [r]=0.61, p<0.001, and r=0.62, p<0.001, respectively)
BDI-II subscale analysis of patients with no SSRI treatment showed that somatic subscale scores had the strongest association with the AMT K values, while weaker correlations were found with affective and no correlation with cognitive sub scale scores (Table 5). The strongest correlations of these scores was observed with the frontal cortical and thalamic K values (Table 5, Fig. 2b)
Table 5.
Correlations between BDI-II subscale scores and regional AMT K values in patients with no SSRI treatment (n=29).
| cognitive | affective | somatic | ||||
|---|---|---|---|---|---|---|
| Brain region | r value | p value | r value | p value | r value | p value |
| frontal cortex | 0.27 | 0.17 | 0.43 | 0.023 | 0.59 | 0.001* |
| temporal cortex | 0.30 | 0.11 | 0.40 | 0.034 | 0.53 | 0.003* |
| parietal cortex | 0.23 | 0.24 | 0.38 | 0.044 | 0.47 | 0.011 |
| putamen | 0.18 | 0.35 | 0.30 | 0.12 | 0.47 | 0.011 |
| thalamus | 0.35 | 0.07 | 0.50 | 0.006* | 0.62 | <0.001* |
Significant after Bonferroni correction.
BDI-II scores and plasma tryptophan metabolites
In the 28 patients where plasma tryptophan metabolites were available, tryptophan or its metabolite levels were not different between depressed and non-depressed or SSRI-treated and non-treated patient subgroups (p≥0.18 in all comparisons). Levels of tryptophan, its metabolites, or the kynurenine/tryptophan ratio, also did not correlate with the total BDI-II (Table 6) or with the KPS scores (p>0.16) for the whole group; the same was found when only patients with glioma were included in the analysis (data not shown).
Table 6.
Average plasma tryptophan and metabolite levels (± standard deviation [SD]), and their correlations with total BDI-II scores (n=28).
| Mean ± SD (μmol/L) | r value | p value | |
|---|---|---|---|
| tryptophan | 30.12 ± 5.33 | −0.14 | 0.47 |
| kynurenine | 1.34 ± 0.44 | −0.31 | 0.11 |
| kynurenic acid | 0.12 ± 0.06 | −0.06 | 0.77 |
| 3-hydroxy kynurenine | 0.029 ± 0.008 | −0.24 | 0.22 |
| 5-hydroxyindoleacetic acid | 0.045 ± 0.014 | −0.21 | 0.29 |
| kynurenine/tryptophan ratio | 0.045 ± 0.013 | −0.26 | 0.19 |
DISCUSSION
Our study demonstrates the high prevalence of depression (27%) in patients with primary brain tumors, although only a portion of them (17%) was on antidepressant treatment, typically started by a neuro-oncologist without a formal psychiatric evaluation and diagnosis based on DSM-IV criteria. Mild depressive symptoms were common in this cohort and they can be missed without formal depression screening. Patients with moderately lower KPS score are at higher risk for depression. The positive association of BDI-II scores with frontal cortical and thalamic PET-derived AMT K values may indicate abnormal tryptophan metabolism in these remote non-tumoral regions, but the underlying mechanism is yet to be determined. The lack of a correlation between depression scores and plasma tryptophan and kynurenine metabolites suggests that the observed depressive symptoms (or some of its components such as somatic symptoms) are more specifically related to alterations of tryptophan metabolism in specific brain regions rather than to systemic changes that can be affected by multiple enzymes in the brain and extracerebral sites, including activity of IDO 1 and TD02 (abundant in the liver).
In our first set of analysis in 60 patients with primary brain tumors, there were no significant associations between BDI-II scores and clinical or tumor-related variables except KPS scores. This is in line with the conclusion of a recent review noting the lack of clear associations between brain tumor-related depression and various clinical factors (Rooney et al., 2011). Previous studies also found no association of depression with gender (Brown et al., 2006; Litofsky et al., 2004; Rooney et al., 2009), antiepileptic or steroid treatment (Brown et al., 2006; Rahman et al., 2015), or tumor-related variables in various tumor cohorts (Brown et al., 2006; Hahn et al., 2003; Litofsky et al., 2004; Pelletier et al., 2002). The robust association of lower KPS scores with higher BDI-II scores, especially in the recurrent tumor group, may be explained by the effect of somatic symptoms and impaired quality of life on the patients’ mood. Some studies raised concerns about the use of BDI-II and other depression screening tools in patients with advanced cancer, and demonstrated that BDI-II total and somatic sub scale scores may be better attributed to the somatic symptoms rather than true depression (Warmenhoven et al., 2013; Wedding et al., 2007). However, another study showed that not only the somatic but also the non-somatic component of BDI-II was correlated with quality of life scores in patients with head and neck cancer (D’Antonio et al., 1998). Importantly, multiple studies utilized BDI-II in brain tumor populations and reported depression scores to be very similar across studies, including the present study, with mean scores to be 10-11 (Bunevicius, 2017; Noll et al., 2019; Pelletier et al., 2002). In a recent cohort of 102 brain tumor patients (Noll et al., 2019), the BDI-II-detected prevalence of at least mild depression was 27%, i.e., identical to our findings. These data suggest that screening with BDI-II provides a remarkably consistent rate of depression across various brain tumor populations. Our findings are also in line with previous reports [summarized in a recent meta-analysis (Shi et al., 2018)] demonstrating that depression, even with relatively mild symptoms that can be easily missed by routine clinical assessment, is associated with shorter overall survival. These data, including the ones from the present study, suggest that screening, early recognition, and treatment of depressive symptoms may improve glioma-associated survival, although this needs to be tested in prospective studies.
Our second main finding is that higher BDI-II scores were associated with higher frontal cortical and thalamic (and, to a lesser degree, temporal cortical) AMT K values measured by PET imaging in the non-tumor-affected hemisphere. These findings refine the results of our previous smaller PET study which found correlations between BDI-II scores and AMT K and/or VD (transport-related) values in more widespread regions, including frontal, temporal, and parietal cortex, as well as striatal and thalamic regions (Bosnyak et al., 2015). The present results suggest that the clinical/imaging associations are specific for tryptophan metabolic rates and confined to frontal (temporal) cortex and thalamus. The difference between the two studies may be explained by the bigger and more homogeneous patient population in the present study. The additional subscale analysis also suggests that the somatic aspect of depression may be the main driver of these correlations.
Previous studies in MDD patients using AMT-PET reported decreased K values in the anterior cingulate and mesial temporal cortex (Rosa-Neto et al., 2004), while decreased orbital and ventromedial prefrontal cortex values were found in patients with a history of suicide attempts (Leyton et al., 2005). Decreased regional K values were attributed to decreased serotonin synthesis, which was associated with higher depression scores and higher number of suicide attempts (Frey et al., 2010; Leyton et al., 2005). One AMT-PET study also showed an interval increase of tryptophan metabolism in the medial prefrontal and anterior cingulate cortex 24 days after combined antidepressant treatment (SSRI + pindolol) suggesting normalization of serotonin synthesis rates (Berney et al., 2008). Overall, these studies identified the frontal lobe as a key brain region affected by decreased tryptophan metabolism in depressive states. While previous studies have implicated the frontal cortex to be involved in mood regulation and depression (Millan et al., 2016), there are also data to support the role of the thalamus, which showed the strongest association between BDI-II scores and AMT K values in the present study. A functional MRI study showed increased functional connectivity in the thalamus, orbitofrontal cortex, and subgenual cingulate in depressed patients (Greicius et al., 2007), while a postmortem study of patients with major depressive disorder reported elevated neuron number in the limbic thalamus (Young et al., 2004). Whether similar thalamic functional and microstructural abnormalities exist in patients with brain tumor-associated depression, requires future studies.
Unlike the previous PET studies in MDD populations, our findings show a positive correlation between regional AMT K values and BDI-II scores in patients with primary brain tumors. The exact mechanism of this association remains to be determined. One plausible explanation may involve the activity of IDO1 that can be overexpressed constitutively in malignant tumors (including gliomas) (Uyttenhove et al., 2003) and can be also upregulated by pro-inflammatory cytokines as a result of chronic inflammatory responses in cancers and also depression (Cervenka et al., 2017; Sforzini et al., 2019). The elevated cytokine release in the brain may be originated from activated microglia, which is known to be associated with depression (Reus et al., 2015; Schiepers et al., 2005). Indeed, a postmortem study in suicide patients with MDD demonstrated increased microglia density in the mediodorsal thalamus, dorsolateral prefrontal cortex, and anterior cingulate cortex (Steiner et al., 2008), i.e., brain regions overlapping with regions showing a relation between depressive symptoms and tryptophan metabolism in the present study.
Although tumor treatments, especially radiation therapy, may also induce brain inflammatory responses (Schaue et al., 2015), there was no difference in the AMT K values between newly-diagnosed and recurrent (with prior treatment, including radiotherapy in most cases) tumor patient groups, suggesting no major treatment effects on the findings. Microscopic glioma cell infiltration, which can occur even in remote brain regions, may be also a source of increased tryptophan metabolic values; however, these values were not different between patients with glioma and those with non-infiltrating meningiomas, thus making this latter explanation unlikely.
Increased activity of key enzymes of the kynurenine pathway could be a treatment target in patients with brain tumor. A number of potent IDO1 enzyme inhibitors have been developed and are being tested in various malignant cancers (including gliomas) with the intent of curbing tumoral immunosuppression (Komiya & Huang, 2018). IDO1 inhibition may potentiate the antitumor effects of other checkpoint inhibitors in glioblastoma treatment (Sordillo et al., 2017; Wainwright et al., 2014). Due to the increasing body of data suggesting the role of the activated kynurenine pathway in both cancer progression and co-morbid depression, recent studies discussed the notion of the potential antidepressant efficacy of IDO inhibition (Sforzini et al., 2019). This hypothesis can be tested prospectively as such inhibitors enter in human clinical trials. As shown in a recent proof-of-concept study with the IDO inhibitor indoximod (Lukas et al., 2019), AMT-PET may be well suited to monitor the effects of such inhibitors on cerebral tryptophan metabolism. Inhibition of TDO2 (Kozlova & Frederick, 2019) may also be relevant in this respect, as it is also commonly upregulated in human gliomas (Opitz et al., 2011). However, TDO2 is a much more substrate-selective enzyme than IDO1 and is not known to show substantial activity on AMT (Basran et al. 2008); to overcome this, there have been recent efforts to develop novel, F-18-labeled tryptophan analog PET tracers labeled on the benzene ring to evaluate TDO2 activity in vivo (reviewed recently in John et al., 2019).
We found no association between depression and plasma tryptophan and kynurenine metabolite levels. Only a few previous studies investigated the alterations of plasma tryptophan metabolites in patients with cancer-related depression (Botwinick et al., 2014; Herrstedt et al., 2019), and neither of them focused on brain tumors. One study reported a negative correlation between plasma kynurenic acid/tryptophan ratio and mood score in 17 patients with pancreatic cancer (Botwinick et al., 2014), while another found an exercise-dependent attenuation of plasma kynurenine levels and associated reduced depression in 50 patients with gastro-esophageal junction cancers (Herrstedt et al., 2019). Our data did not reveal a similar association in brain tumor patients, although this negative finding may be affected the small sample size, heterogeneous tumor group, and the potential effects of both cerebral and extracerebral enzyme activities on the blood metabolite levels.
Our study has some limitations. We investigated a mixed primary brain tumor patient population, including newly-diagnosed and recurrent tumors. The sample size was relatively small for the whole group and even smaller for those who had PET scan or blood metabolite measures available. Our analysis did not include some potentially relevant brain regions (e.g., cingulate cortex or amygdala) due to limited spatial resolution of AMT-PET, which makes assessment of detailed tracer kinetics in small regions difficult and unreliable. The regions in our study were defined on native PET images rather than in a template space, because the use of standardized normal brain templates may introduce inaccuracies when applied to brains with space-occupying lesions such as brain tumors and resection cavities. Also, AMT is not a specific tracer to IDO1 activity, as it can also be metabolized via the serotonin pathway (Diksic et al.,1990; Muzik et al., 1997). However, increased serotonin synthesis would not be expected to lead to depressive symptoms, as both preclinical and clinical data demonstrated an opposite association (Lopez-Munoz & Alamo, 2009). PET radioligands more specific for the kynurenine pathway are being developed and could clarify this issue once they enter in human studies. Future studies with other, non-tryptophan PET tracers (e.g., F-18-labeled fluoroethyl-L-tyrosine, used increasingly in pre- and post-treatment brain tumor imaging) (Langen et al., 2017) could determine if the observed effects are tryptophan-specific or could be replicated with other amino acid derivative PET radiotracers. Finally, the presence and severity of depression was evaluated by BDI-II to minimize the burden to the patients. Although BDI-II is a self-reported measure and is generally used as a screening tool for depression, the overall BDI-II score is validated and has been widely utilized in previous brain tumor populations (Mainio et al., 2005; Noll et al., 2019; Pelletier et al., 2002; Rooney et al., 2011); also, the use of this tool is practical, as patients with brain tumor often refuse to undergo a formal psychiatric evaluation (Litofsky & Resnick, 2009). It is also notable that the majority of depressed subjects had mild/moderate depression, thus limiting the overall range of BDI-II scores, which could potentially affect the observed correlations. Finally, ongoing SSRI treatment may affect cerebral serotonin synthesis rates and the measured AMT K values. To eliminate this confounding effect, we repeated our analysis with SSRI-nai’ve patients and found the correlations between BDI-II scores and PET variables to be stronger, thus supporting that the observed associations were not due to SSRI effects.
CONCLUSIONS
These findings confirm that depressive symptoms in patients with primary brain tumor are common although often mild, and they are largely independent of age, gender, antiepileptic and steroid treatment, as well as major tumor-related characteristics. Consistent with previous data, depression was underdiagnosed and undertreated in our patients, and this could be particularly relevant in the recurrent glioma group where high BDI-II scores were associated with shorter survival. While most of our analyses relied on correlations that do not provide a definitive causal relation, the observed molecular imaging abnormalities likely indicate an imbalance between the serotonin and kynurenine pathways in the brain, and higher tryptophan metabolism in the frontal cortex and thalamus may serve as an imaging marker of depression in this patient population. The results of this study can facilitate future efforts to target dysregulated tryptophan metabolism in patients with primary brain tumors.
Supplementary Material
Acknowledgement
We thank Edit Bosnyák, MD, PhD, who assisted in data collection. We are grateful to the entire staff at the PET Center, Children’s Hospital of Michigan, Detroit Medical Center, who provided invaluable technical help in patient scheduling and performing the PET scans. We also thank Thomas Mangner, PhD, who performed the AMT radiosynthesis, as well as Xun Bao and Jing Li, PhD, at the Karmanos Cancer Institute Pharmacology Core for performing the blood tryptophan metabolite measurements.
Funding: This study was supported by grants from the National Cancer Institute (R01 CA123451 and P30 CA022453).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interests: The authors declare that they have no conflict of interest.
Ethical approval: The study was approved by the Wayne State University Institutional Review Board. All procedures performed in this study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Written informed consent was obtained from all individual participants included in the study.
REFERENCES
- Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, et al. (2014). Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One, 9(11), e112945. doi: 10.1371/journal.pone.0112945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkonyi B, Barger GR, Mittal S, Muzik O, Chugani DC, Bahl G, et al. (2012). Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of alpha-11C-methyl-L-tryptophan PET. J Nucl Med, 53(7), 1058–1064. doi: 10.2967/jnumed.111.097881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkonyi B, Mittal S, Zitron I, Chugani DC, Kupsky WJ, Muzik O, et al. (2012). Increased tryptophan transport in epileptogenic dysembryoplastic neuroepithelial tumors. J Neurooncol, 107(2), 365–372. doi: 10.1007/s11060-011-0750-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basran J, Rafice SA, Chauhan N, Efimov I, Cheesman MR, Ghamsari L, et al. (2008). A kinetic, spectroscopic, and redox study of human tryptophan 2,3-dioxygenase. Biochemistry, 47(16), 4752–4760. doi: 10.1021/bi702393b [DOI] [PubMed] [Google Scholar]
- Batista CE, Juhasz C, Muzik O, Kupsky WJ, Barger G, Chugani HT, et al. (2009). Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol, 11(6), 460–466. doi: 10.1007/s11307-009-0225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for The Beck Depression Inventory Second Edition (BDI-II). San Antonio: Psychological Corporation. [Google Scholar]
- Berney A, Nishikawa M, Benkelfat C, Debonnel G, Gobbi G, & Diksic M (2008). An index of 5-HT synthesis changes during early antidepressant treatment: alpha-[11C]methyl-L-tryptophan PET study. Neurochem Int, 52(4-5), 701–708. doi: 10.1016/j.neuint.2007.08.021 [DOI] [PubMed] [Google Scholar]
- Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, et al. (2017). Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev, 52, 58–70. doi: 10.1016/j.ctrv.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Bosnyak E, Kamson DO, Behen ME, Barger GR, Mittal S, & Juhasz C (2015). Imaging cerebral tryptophan metabolism in brain tumor-associated depression. EJNMMI Res, 5(1), 56. doi: 10.1186/s13550-015-0136-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnyak E, Kamson DO, Guastella AR, Varadarajan K, Robinette NL, Kupsky WJ, et al. (2015). Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro Oncol, 17(9), 1284–1292. doi: 10.1093/neuonc/nov098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ, & Chabot JA (2014). A biological basis for depression in pancreatic cancer. HPB (Oxford), 16(8), 740–743. doi: 10.1111/hpb.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, et al. (2006). Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol, 76(3), 283–291. doi: 10.1007/s11060-005-7020-9 [DOI] [PubMed] [Google Scholar]
- Bunevicius A (2017). Reliability and validity of the SF-36 Health Survey Questionnaire in patients with brain tumors: a cross-sectional study. Health Qual Life Outcomes, 15(1), 92. doi: 10.1186/s12955-017-0665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka I, Agudelo LZ, & Ruas JL (2017). Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science, 557(6349). doi: 10.1126/science.aaf9794 [DOI] [PubMed] [Google Scholar]
- Chakraborty PK, Mangner TJ, Chugani DC, Muzik O, & Chugani HT (1996). A high-yield and simplified procedure for the synthesis of alpha-[11C]methyl-L-tryptophan. Nucl Med Biol, 25(8), 1005–1008. [DOI] [PubMed] [Google Scholar]
- Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, & Thompson RC (2015). The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol, 121(2), 359–364. doi: 10.1007/s11060-014-1640-x [DOI] [PubMed] [Google Scholar]
- Chugani DC, & Muzik O (2000). Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab, 20(1), 2–9. doi: 10.1097/00004647-200001000-00002 [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Chakraborty P, Mangner T, & Chugani HT (1998). Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-L-tryptophan. Synapse, 28(1), 33–43. doi: [DOI] [PubMed] [Google Scholar]
- Cosci F, Fava GA, & Sonino N (2015). Mood and anxiety disorders as early manifestations of medical illness: a systematic review. Psychother Psychosom, 84(1), 22–29. doi: 10.1159/000367913 [DOI] [PubMed] [Google Scholar]
- D’Antonio LL, Long SA, Zimmerman GJ, Peterman AH, Petti GH, & Chonkich GD (1998). Relationship between quality of life and depression in patients with head and neck cancer. Laryngoscope, 108(6), 806–811. doi: 10.1097/00005537-199806000-00006 [DOI] [PubMed] [Google Scholar]
- Diksic M, Nagahiro S, Sourkes TL, & Yamamoto YL (1990). A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab, 10(1), 1–12. doi: 10.1038/jcbfm.1990.2 [DOI] [PubMed] [Google Scholar]
- Frey BN, Skelin I, Sakai Y, Nishikawa M, & Diksic M (2010). Gender differences in alpha-[(11)C]MTrp brain trapping, an index of serotonin synthesis, in medication-free individuals with major depressive disorder: a positron emission tomography study. Psychiatry Res, 183(2), 157–166. doi: 10.1016/j.pscychresns.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry, 62(5), 429–437. doi: 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AR, Michelhaugh SK, Klinger NV, Fadel HA, Kiousis S, Ali-Fehmi R, et al. (2018). Investigation of the aryl hydrocarbon receptor and the intrinsic tumoral component of the kynurenine pathway of tryptophan metabolism in primary brain tumors. J Neurooncol, 139(2), 239–249. doi: 10.1007/s11060-018-2869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AR, Michelhaugh SK, Klinger NV, Kupsky WJ, Polin LA, Muzik O, et al. (2016). Tryptophan PET Imaging of the Kynurenine Pathway in Patient-Derived Xenograft Models of Glioblastoma. Mol Imaging, May 5;15. doi: 10.1177/1536012116644881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, & Halperin EC (2003). Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys, 55(4), 992–999. doi: 10.1016/s0360-3016(02)04205-0 [DOI] [PubMed] [Google Scholar]
- Herrstedt A, Bay ML, Simonsen C, Sundberg A, Egeland C, Thorsen-Streit S, et al. (2019). Exercise-mediated improvement of depression in patients with gastro-esophageal junction cancer is linked to kynurenine metabolism. Acta Oncol, 1–9. doi: 10.1080/0284186X.2018.1558371 [DOI] [PubMed] [Google Scholar]
- Hestad KA, Engedal K, Whist JE, & Farup PG (2017). The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front Psychol, 8, 1561. doi: 10.3389/fpsyg.2017.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zeng C, Xiao JX, Zhao DW, Tang H, Wu HS, et al. (2017). Association between depression and brain tumor: a systematic review and meta-analysis. Oncotarget, 8(55), 94932–94943. doi: 10.18632/oncotarget.19843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John F, Muzik O, Mittal S, & Juhasz C (2019). Fluorine-18-Labeled PET Radiotracers for Imaging Tryptophan Uptake and Metabolism: a Systematic Review. Mol Imaging Biol. doi: 10.1007/s11307-019-01430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Barger GR, Kupsky WJ, Chakraborty PK, Muzik O, et al. (2012). Quantitative PET imaging of tryptophan accumulation in gliomas and remote cortex: correlation with tumor proliferative activity. Clin Nucl Med, 37(9), 838–842. doi: 10.1097/RLU.0b013e318251e458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Muzik O, Wu D, Sloan AE, Barger G, et al. (2006). In vivo uptake and metabolism of alpha-[11C]methyl-L-tryptophan in human brain tumors. J Cereb Blood Flow Metab, 26(3), 345–357. doi: 10.1038/sj.jcbfm.9600199 [DOI] [PubMed] [Google Scholar]
- Kamson DO, Lee TJ, Varadarajan K, Robinette NL, Muzik O, Chakraborty PK, et al. (2014). Clinical significance of tryptophan metabolism in the nontumoral hemisphere in patients with malignant glioma. J Nucl Med, 55(10), 1605–1610. doi: 10.2967/jnumed.114.141002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamson DO, Mittal S, Buth A, Muzik O, Kupsky WJ, Robinette NL, et al. (2013). Differentiation of glioblastomas from metastatic brain tumors by tryptophan uptake and kinetic analysis: a positron emission tomographic study with magnetic resonance imaging comparison. Mol Imaging, 12(5), 327–337. [PMC free article] [PubMed] [Google Scholar]
- Kamson DO, Mittal S, Robinette NL, Muzik O, Kupsky WJ, Barger GR, et al. (2014). Increased tryptophan uptake on PET has strong independent prognostic value in patients with a previously treated high-grade glioma. Neuro Oncol, 16(10), 1373–1383. doi: 10.1093/neuonc/nou042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya T, & Huang CH (2018). Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front Oncol, 8, 423. doi: 10.3389/fonc.2018.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova A, & Frederick R (2019). Current state on tryptophan 2,3-dioxygenase inhibitors: a patent review. Expert Opin Ther Pat, 29(1), 11–23. doi: 10.1080/13543776.2019.1556638 [DOI] [PubMed] [Google Scholar]
- Langen KJ, Stoffels G, Filss C, Heinzel A, Stegmayr C, Lohmann P, et al. (2017). Imaging of amino acid transport in brain tumours: Positron emission tomography with O-(2-[(18)F]fluoroethyl)-L-tyrosine (FET). Methods, 130, 124–134. doi: 10.1016/j.ymeth.2017.05.019 [DOI] [PubMed] [Google Scholar]
- Leyton M, Diksic M, & Benkelfat C (2005). Brain regional alpha-[11C]methyl-L-tryptophan trapping correlates with post-mortem tissue serotonin content and [11C]5-hydroxytryptophan accumulation. Int J Neuropsychopharmacol, 8(4), 633–634. doi: 10.1017/S1461145705005420 [DOI] [PubMed] [Google Scholar]
- Litofsky NS, Farace E, Anderson F Jr., Meyers CA, Huang W, Laws ER Jr., et al. (2004). Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery, 54(2), 358–366. doi: 10.1227/01.neu.0000103450.94724.a2 [DOI] [PubMed] [Google Scholar]
- Litofsky NS, & Resnick AG (2009). The relationships between depression and brain tumors. J Neurooncol, 94(2), 153–161. doi: 10.1007/s11060-009-9825-4 [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, & Alamo C (2009). Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des, 15(14), 1563–1586. doi: 10.2174/138161209788168001 [DOI] [PubMed] [Google Scholar]
- Lukas RV, Juhasz C, Wainwright DA, James CD, Kennedy E, Stupp R, et al. (2019). Imaging tryptophan uptake with positron emission tomography in glioblastoma patients treated with indoximod. J Neurooncol, 141(1), 111–120. doi: 10.1007/s11060-018-03013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DE, Walter JM, Starkweather AR, Schubert CM, & McCain NL (2011). Tryptophan degradation in women with breast cancer: a pilot study. BMC Res Notes, 4, 156. doi: 10.1186/1756-0500-4-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainio A, Hakko H, Timonen M, Niemela A, Koivukangas J, & Rasanen P (2005). Depression in relation to survival among neurosurgical patients with a primary brain tumor: a 5-year follow-up study. Neurosurgery, 56(6), 1234–1241. doi: 10.1227/01.neu.0000159648.44507.7f [DOI] [PubMed] [Google Scholar]
- Mangoni A (1974). The “kynurenine shunt” and depression. Adv Biochem Psychopharmacol, 11(0), 293–298. [PubMed] [Google Scholar]
- McElroy E, Casey P, Adamson G, Filippopoulos P, & Shevlin M (2018). A comprehensive analysis of the factor structure of the Beck Depression Inventory-II in a sample of outpatients with adjustment disorder and depressive episode. Ir J Psychol Med, 35(1), 5361. doi: 10.1017/ipm.2017.52 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Rivet JM, & Gobert A (2016). The frontal cortex as a network hub controlling mood and cognition: Probing its neurochemical substrates for improved therapy of psychiatric and neurological disorders. J Psychopharmacol, 30(11), 1099–1128. doi: 10.1177/0269881116672342 [DOI] [PubMed] [Google Scholar]
- Mugge L, Mansour TR, Crippen M, Alam Y, & Schroeder J (2018). Depression and glioblastoma, complicated concomitant diseases: a systemic review of published literature. Neurosurg Rev. doi: 10.1007/s10143-018-1017-2 [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Chakraborty P, Mangner T, & Chugani HT (1997). Analysis of [C-11]alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab, 17(6), 659–669. doi: 10.1097/00004647-199706000-00007 [DOI] [PubMed] [Google Scholar]
- Noll KR, Sullaway CM, & Wefel JS (2019). Depressive symptoms and executive function in relation to survival in patients with glioblastoma. J Neurooncol, 142(1), 183191. doi: 10.1007/s11060-018-03081-z [DOI] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478(7368), 197–203. doi: 10.1038/nature10491 [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, & Fenstermacher JD (1983). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab, 3(1), 1–7. doi: 10.1038/jcbfm.1983.1 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Verhoef MJ, Khatri N, & Hagen N (2002). Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol, 57(1), 41–49. doi: 10.1023/a:1015728825642 [DOI] [PubMed] [Google Scholar]
- Platten M, Nollen EAA, Rohrig UF, Fallarino F, & Opitz CA (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. doi: 10.1038/s41573-019-0016-5 [DOI] [PubMed] [Google Scholar]
- Rahman Z, Wong CH, Dexter M, Olsson G, Wong M, Gebsky V, et al. (2015). Epilepsy in patients with primary brain tumors: The impact on mood, cognition, and HRQOL. Epilepsy Behav, 48, 88–95. doi: 10.1016/j.yebeh.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience, 300, 141–154. doi: 10.1016/j.neuroscience.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Rooney AG, Carson A, & Grant R (2011). Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst, 103(1), 61–76. doi: 10.1093/jnci/djq458 [DOI] [PubMed] [Google Scholar]
- Rooney AG, van Nieuwenhuizen D, Reijneveld JC, & Grant R (2009). Female gender is not a proven risk factor for depression in glioma. J Neurooncol, 95(3), 449. doi: 10.1007/s11060-009-9947-8 [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Diksic M, Okazawa H, Leyton M, Ghadirian N, Mzengeza S, et al. (2004). Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Arch Gen Psychiatry, 61(6), 556–563. doi: 10.1001/archpsyc.61.6.556 [DOI] [PubMed] [Google Scholar]
- Schaue D, Micewicz ED, Ratikan JA, Xie MW, Cheng G, & McBride WH (2015). Radiation and inflammation. Semin Radiat Oncol, 25(1), 4–10. doi : 10.1016/j.semradonc.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, & Maes M (2005). Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry, 29(2), 201–217. doi: 10.1016/j.pnpbp.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Sforzini L, Nettis MA, Mondelli V, & Pariante CM (2019). Inflammation in cancer and depression: a starring role for the kynurenine pathway. Psychopharmacology (Berl). doi: 10.1007/s00213-019-05200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Lamba N, Zheng LJ, Cote D, Regestein QR, Liu CM, et al. (2018). Depression and survival of glioma patients: A systematic review and meta-analysis. Clin Neurol Neurosurg, 172, 8–19. doi: 10.1016/j.clineuro.2018.06.016 [DOI] [PubMed] [Google Scholar]
- Sordillo PP, Sordillo LA, & Helson L (2017). The Kynurenine Pathway: A Primary Resistance Mechanism in Patients with Glioblastoma. Anticancer Res, 37(5), 2159–2171. doi: 10.21873/anticanres.11551 [DOI] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, & Beck AT (1999). Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol, 55(1), 117–128. doi: [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. (2008). Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res, 42(2), 151–157. doi: 10.1016/j.jpsychires.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, & Roth DA (2004). Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depress Anxiety, 19(3), 187–189. doi: 10.1002/da.20002 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, et al. (2010). Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer, 67(3), 361–365. doi: 10.1016/j.lungcan.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med, 9(10), 1269–1274. doi: 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, et al. (2014). Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res, 20(20), 5290–5301. doi: 10.1158/1078-0432.CCR-14-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmenhoven F, van Weel C, Vissers K, & Prins J (2013). Screening instruments for depression in advanced cancer patients: what do we actually measure? Pain Pract, 13(6), 467–475. doi: 10.1111/papr.12012 [DOI] [PubMed] [Google Scholar]
- Wedding U, Koch A, Rohrig B, Pientka L, Sauer H, Hoffken K, et al. (2007). Requestioning depression in patients with cancer: contribution of somatic and affective symptoms to Beck’s Depression Inventory. Ann Oncol, 18(11), 1875–1881. doi : 10.1093/annonc/mdm353 [DOI] [PubMed] [Google Scholar]
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. (2010). Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol, 28(11), 1963–1972. doi: 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- Young KA, Holcomb LA, Yazdani U, Hicks PB, & German DC (2004). Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry, 161(7), 1270–1277. doi: 10.1176/appi.ajp.161.7.1270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


