Abstract
Background
IL-1 plays a pivotal role in the inflammatory response during cytokine storm syndromes.
Objective
Our aim was to analyze the efficacy and safety of early anti-inflammatory treatment (AIT) with intravenous anakinra with or without glucocorticoids in coronavirus disease 2019 (COVID-19) pneumonia.
Methods
We performed a retrospective single-center cohort study of patients admitted for COVID-19 pneumonia from February 26 to April 29, 2020, to assess the efficacy of early AIT with intravenous anakinra (100 mg every 8 hours for 3 days, with tapering) alone or in combination with a glucocorticoid (intravenous methylprednisolone, 1-2 mg/kg daily, with tapering). The standard of care (SOC) treatment was hydroxychloroquine and/or azithromycin with or without antivirals and anticoagulants. Late rescue AIT with anakinra or tocilizumab was also evaluated. Treatment effect on overall survival was assessed by a propensity score–adjusted Cox model.
Results
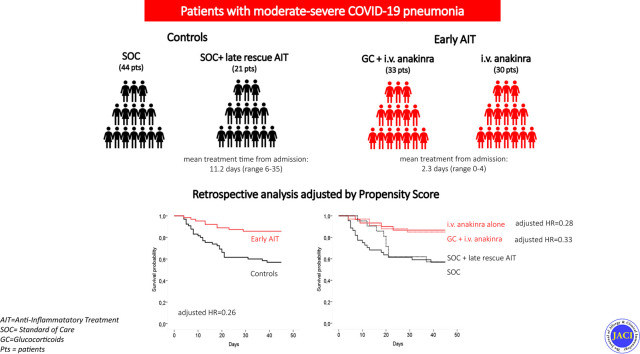
A total of 128 patients were analyzed; 63 patients received early AIT (30 received anakinra alone and 33 received anakinra plus a glucocorticoid) at admission, and 65 patients did not receive early AIT and were used as controls; of the latter 65 patients, 44 received the SOC treatment alone and 21 received the SOC treatment plus late rescue AIT. After adjustment for all the unbalanced baseline covariates, early AIT reduced the hazard of mortality by 74% (adjusted hazard ratio [HR] = 0.26; P < .001). The effect was similar in patients receiving anakinra alone (adjusted HR = 0.28; P = .04) and anakinra plus a glucocorticoid (adjusted HR = 0.33; P = .07). Late rescue treatment did not show a significant advantage over SOC treatment alone (adjusted HR = 0.82; P = .70).
Conclusions
This study suggests, on a larger series of patients with COVID-19 pneumonia, the potential efficacy and safety of the early use of high doses of intravenous anakinra with or without glucocorticoids.
Key words: IL-1, anakinra, glucocorticoid, early treatment, COVID-19 pneumonia
Abbreviations used: AIT, Anti-inflammatory treatment; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; Fio2, Fraction of inspired oxygen; HR, Hazard ratio; ICU, Intensive care unit; IPW, Inverse probability of treatment weight; LDH, Lactate dehydrogenase; MAS, Macrophage activation syndrome; OS, Overall survival; PS, Propensity score; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SOC, Standard of care
Graphical abstract
Coronavirus disease 2019 (COVID-19) might be associated with severe atypical pneumonia. Factors associated with intensive care unit (ICU) admission and death include lymphopenia, as well as elevated levels of transaminases, lactate dehydrogenase (LDH), d-dimer, ferritin, and soluble IL-2 receptor.1 These features are reminiscent of cytokine storm syndrome, in which hyperinflammation and multiorgan disease arise through excessive cytokine release from uncontrolled immune activation, similar to a condition named macrophage activation syndrome (MAS).2, 3, 4 Identification of key mediators driving MAS, including IL-1β, IL-6, IL-18, and IFN-γ, has inaugurated a new era of cytokine neutralization enabling reduction in mortality.3 , 4 The use of anticytokine drugs in the context of COVID-19 pneumonia has been suggested as an efficacious strategy allowing prevention of respiratory failure, mechanical ventilation, and death.2 , 3 Tocilizumab (anti–IL-6 receptor) is effective in cytokine release syndrome associated with chimeric antigen receptor T-cell therapy,5 a syndrome notably reminiscent of COVID-19 in that many patients develop acute respiratory distress syndrome and has been extensively used during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic with variable results.6, 7, 8, 9
Recent studies have demonstrated the role of NLRP3 receptors in response to coronaviruses. The virus regulates NLRP3 inflammasome, inducing maturation and secretion of the inflammatory cytokines IL-1β and IL-18.10 Moreover, studies in bats, which are considered the natural reservoir of the whole Betacoronavirus genus and survive the infection without evident disease, demonstrated a lack of IL-1β secretion following the stimulation with canonic NLRP3 pathway activators,11 suggesting that a dampened inflammatory response during SARS-CoV-2 infection might represent an evolutionary advantage for survival.12
Recombinant IL-1 receptor antagonist (anakinra) was used during the first wave of pandemic in small series, in different regimens and different disease phases.13, 14, 15, 16, 17, 18, 19, 20, 21 In a recent pilot study, we provided the first evidence of the safety and efficacy of the use of high-doses of intravenous anakinra in severe COVID-19 pneumonia in the first days after hospital admission.14 In the present study, we have reported the results of the early use of anti-inflammatory treatment with intravenous anakinra in a consecutive series of 63 patients with COVID-19, providing the first evidence of its efficacy as monotherapy when compared with the combination of its and glucocorticoids.
Methods
Patients
Beginning on March 18, 2020, patients were included in an off-label treatment protocol approved by the institutional review board of Ente Ospedaliero Galliera, Genoa, Italy. At admission, patients were eligible to receive early AIT with high doses of intravenous anakinra with or without a glucocorticoid according to the following inclusion criteria: (1) severe COVID-19–related pneumonia at lung computed tomography scan with positive or pending evidence of SARS-CoV-2 by RT-PCR assay; (2) presence of 1 of 3 parameters, namely, the need for respiratory assistance with a Pao 2-to–fraction of inspired oxygen (Fio 2) ratio (Pao 2/Fio 2) of 300 mm Hg or lower and elevation of C-reactive protein (CRP) or ferritin level to 3 times the normal limits; and (3) lymphocyte count lower than1000/mm3 and elevation of d-dimer or LDH level to 3 times the normal range. Early AIT was defined as treatment assigned at admission after the screening for inclusion criteria. All patients were provided with information about anakinra plus a glucocorticoid and were the able to either accept or refuse this treatment. Their choice was noted in the medical record.
The starting dose of anakinra (Swedish Orphan Biovitrum, Stockholm, Sweden) was 100 mg every 8 hours for 3 days, followed by tapering (100 mg every 12 hours for 1-3 days, followed by 100 mg every 24 hours for 1-3 days) according to the clinical response, for a maximum of 9 days.14 , 22 , 23 Glucocorticoid treatment (intravenous methylprednisolone, 1 to 2 mg/kg once or twice daily, with tapering) was used as the first drug in patients admitted to the emergency room because of the impossibility of starting anakinra treatment immediately. The control group was retrospectively identified among patients admitted from February 26 to March 25, 2020, with consideration of all patients receiving the standard of care (SOC) treatment who, for various reasons, were not treated with the early AIT despite satisfying the inclusion criteria. The SOC treatment consisted of hydroxychloroquine (400 mg twice daily on the first day followed by 200-400 mg twice daily for 7 days) and/or azithromycin (500 mg daily for 7 days). Antivirals (lopinavir/ritonavir [400/100 mg twice daily] or darunavir [800 mg]/ritonavir [100 mg daily] for 7 days, and enoxaparin [4000 IU per day] adapted according to body weight, kidney function, and d-dimer levels) were also used in some patients. Anakinra, intravenous tocilizumab (8 mg/kg to a maximum dose of 800 mg), and glucocorticoids were also used as a late rescue AIT in patients who did not receive early AIT. The exclusion criteria for study analysis were as follows: (1) incomplete clinical information; (2) death within 4 days; (3) previous admission for other conditions (eg, major surgery), with subsequent in-hospital COVID-19 infection; and (4) ultrafragile patient older than 90 years. The retrospective analysis of data was approved by the Ethical Review Board of Regione Liguria (IMMUNOCOVID-19, 221/2020-DB id 10564).
Outcomes and statistical analysis
A post hoc power calculation was run, showing that a sample size of 130 patients would provide a power of 80% (at a 95% CI) to detect a 55% mortality reduction (assuming a mortality of 40% in the control group). All patients were followed until recovery or death. The primary end point was overall survival (OS), calculated from the day of hospitalization. Patients were included in the early AIT group if the treatment was started within 4 days from hospital admission; patients who died before day 4 were excluded. The exclusion of early deaths with a landmark of 4 days was motivated by the need to control the immortal time bias: patients must survive long enough to be treated, and those dying soon after admission are more likely to be included in the untreated group (for details, see Supplementary Appendix available in the Online Repository at www.jacionline.org). Some patients (who entered the ICU at hospitalization) received anakinra treatment after ICU admission. ICU admission was therefore considered to be a time-dependent covariate in the analysis.
To minimize the impact of the treatment indication bias inherent to nonrandomized studies, OS was compared between treatment groups by a propensity score (PS)-adjusted Cox model. The PS was derived by a logistic regression model that included the following baseline variables: age; sex; comorbidities (cardiovascular, diabetes, hypertension, chronic obstructive pulmonary disease, and malignancies); smoking status; Pao 2/Fio 2 ratio; time from the onset of symptoms to hospital admission; serum LDH, ferritin, CRP, and d-dimer levels; lymphocyte counts; treatment with antivirals; and treatment with anticoagulants. The assumption of positivity of the PS was checked after the calculation. For each patient, the inverse probability of treatment weight (IPW) based on PS was calculated. To assess the improvement in balance of covariate distribution between the 2 groups after determination of the IPW, the Cohen standardized mean differences (SMDs) were compared between the 2 groups in the original samples and after weighting (the lower the SMD, the highest the balance), with an SMD between 0.10 and 0.25 indicating an acceptable balance.24
To make efficient use of the available data, we applied an advanced multiple imputation of missing values strategy (10 imputations) for missing baseline data25 before running the PS-adjusted and multivariate analyses. CRP, d-dimer, LDH, and ferritin levels were log-transformed before the analysis to normalize their skewed distributions. Respiratory supportive care was classified according to a 5-point score, with a score of 0 indicating ambient air, a score of 1 indicating an Fio 2 value of 24% to 32% (nasal cannula), a score of 2 indicating an Fio 2 value of 35% to 60% (Venturi mask), a score of 3 indicating continuous positive airway pressure, and a score of 4 indicting mechanical invasive ventilation. The unadjusted cumulative probability of death was displayed by means of Kaplan-Meier survival curves. A multivariate Cox model was run as a sensitivity analyses on OS and to assess treatment in the 4 groups (see the Supplementary Appendix for details). A linear mixed model with random intercept and random slope was used to assess longitudinal change in the clinical parameters (CRP level, LDH level, Pao 2/Fio 2 ratio, and respiratory score) after anakinra treatment in the experimental group. The interaction between time and treatment group was tested in the model to assess whether the parameters' behavior over time was different between the 2 arms of treated patients (treated with anakinra alone or anakinra plus a glucocorticoid).
Results
Patients
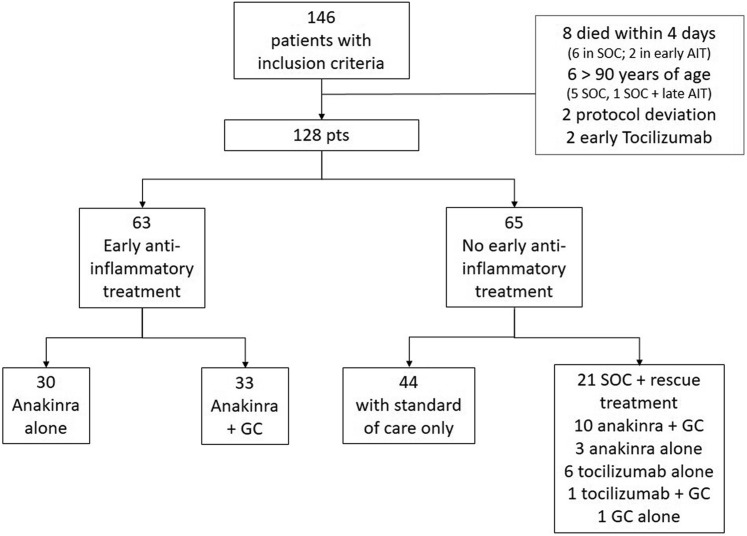
From February 26 to April 29, 2020, 146 consecutive patients with complete clinical information at baseline and fulfilling the inclusion criteria were evaluated. In all, 18 patients were excluded for the following reasons: 8 died within 4 days after admission; 6 were older than 90 years; 2 deviated from the protocol (administration of low doses of anakinra at day 1 and 2 only); and 2 received early tocilizumab treatment (Fig 1 ).
Fig 1.
Flowchart of the patients analyzed in the study. GC, Glucocorticoid; pts, patients.
A final cohort of 128 patients was therefore included in the analysis (65 controls and 63 patients who received early AIT). In the early AIT group, 30 patients received anakinra alone, whereas 33 received anakinra plus a glucocorticoid. The glucocorticoid was started 1 or 2 days before the anakinra; in sporadic cases, they were administered in combination. Of the 65 controls, 44 received the SOC treatment alone and 21 received the SOC treatment followed by late rescue AIT.
The demographic and clinical features of the patients are reported in Table I . Additional details about the characteristics of the 4 subgroups (SOC treatment only, SOC treatment plus late rescue AIT, early AIT with anakinra alone, and early AIT with anakinra plus a glucocorticoid) are reported in Table E1 (available in this article's Online Repository at www.jacionline.org). The mean time from admission to therapy start of patients receiving early AIT was 2.3 days (range 0-4) as compared with 11.2 days (range 6-35) in the case of those receiving late rescue AIT. All patients received hydroxychloroquine and/or azithromycin as the SOC treatment. Most of the baseline variables were unbalanced between groups (SMD > 0.10) (Table I). The patients who received early AIT were younger than the controls but generally had a more severe inflammatory status (higher ferritin, CRP, and d-dimer levels) and a lower Pao 2/Fio 2 ratio. There was also an imbalance in comorbidities, with a higher proportion of patients with comorbidities in the control group, which was in line with their older age. Also, the proportion of patients receiving antiviral therapy was higher among the controls than in the early AIT group, whereas the opposite was true for enoxaparin.
Table I.
Baseline demographic and clinical characteristics
| Characteristic | Control group (n = 65) | Early AIT group (n = 63) | SMD | P value |
|---|---|---|---|---|
| Age (y), mean (SD) Median, range |
67.3 (16.0) 68 (27-89) |
60.7 (15.7) 59 (23-88) |
0.42 | .020 |
| Sex (M/F), no. (%) | 45/20 (69.2/30.8) | 42/21 (66.7-33.3) | 0.055 | .85 |
| Comorbidities (no.), mean (SD) Median (range) |
1.5 (1.5) 1 (0-5) |
1.0 (1.1) 1 (0-4) |
0.34 | .13 |
| Time of first symptom/admission (d), median (IQR) | 5 (2-7) | 7 (4-9) | 0.30 | .004 |
| Ferritin level (ng/mL), mean (SD) Median (IQR) |
645 (707) 470 (202-810) |
1174 (810) 951 (595-1543) |
0.70 | <.001 |
| CRP level (mg/dL), mean (SD) Median (IQR) |
7.5 (7.5) 4.1 (1.9-11.0) |
9.8 (6.3) 8.7 (4.4-13.5) |
0.33 | .007 |
|
d-Dimer level (ng/mL), mean (SD) Median (IQR) |
1870 (2215) 1142 (785-2267) |
2226 (5499) 813 (535-1570) |
0.085 | .15 |
| LDH level (U/L), mean (SD) Median (IQR) |
306 (146) 278 (198-398) |
378 (137) 349 (272-453) |
0.51 | .002 |
| Lymphocyte count (per mm3), mean (SD) Median (IQR) |
1.33 (1.34) 1.05 (0.8-1.35) |
0.98 (0.50) 0.85 (0.67-1.28) |
0.34 | .055 |
| Pao2/Fio2 ratio (mm Hg), median (IQR) | 301 (217-374) | 223 (147-300) | 0.69 | <.001 |
| Pao2/Fio2 ratio < 300 mm Hg, no. (%) | 33 (50) | 48 (76.2) | 0.56 | .006 |
| Diabetes, no. (%) | 17 (26.2) | 11 (17.5) | 0.21 | .29 |
| Hypertension, no. (%) | 27 (41.5) | 22 (34.9) | 0.14 | .47 |
| Cardiovascular disease, no. (%) | 19 (29.2) | 8 (12.7) | 0.41 | .03 |
| COPD, no. (%) | 10 (15.4) | 5 (7.9) | 0.23 | .27 |
| Malignancy, no. (%) | 9 (13.9) | 13 (20.6) | 0.18 | .35 |
| Smoker, no. (%) | 8 (12.3) | 5 (7.9) | 0.14 | .56 |
| Anticoagulant therapy, no. (%) | 50 (76.9) | 60 (95.2) | 0.54 | .004 |
| Antiviral therapy, no. (%) | 42 (64.6) | 24 (38.1) | 0.55 | .004 |
| Treated with CPAP at admission, no. (%) | 9 (13.9) | 22 (34.9) | 0.50 | .007 |
| Mechanical ventilation at admission, no. (%) | 5 (7.7) | 7 (11.1) | 0.12 | .56 |
COPD, Chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; IQR, interquartile range.
Mortality and progression to invasive mechanical ventilation
In all, 37 patients (29%) died. The number of deaths in the AIT group was 9 of 63 (14%), including 4 of 30 (13%) in the anakinra-alone group and 5 of 33 (15%) in the anakinra plus a glucocorticoid group. The number of deaths in the control group was 28 of 65 (43%), including 19 of 44 (43%) who received the SOC treatment only and 9 of 21 (43%) who received the SOC treatment plus late rescue AIT.
A total of 28 patients (13 in the early AIT group and 15 in the control group) were admitted to the ICU. Seven patients who were intubated at admission received early AIT (6 received anakinra alone and 1 received anakinra plus a glucocorticoid) while in the ICU (see Fig E1 in this article's Online Repository at www.jacionline.org).
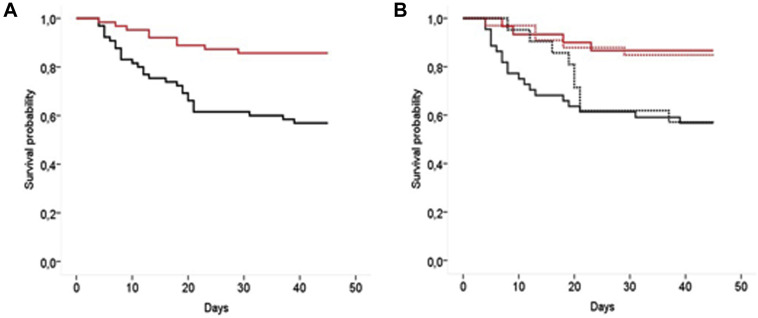
Unadjusted survival in the early AIT and control groups is reported in Fig 2 , A; in Fig 2, B the treatment group is split into the subgroup receiving anakinra alone (solid line) and the subgroup treated with anakinra in combination with a glucocorticoid (dashed line), whereas the control group is split into the subgroup receiving the SOC treatment only (solid line) and the subgroup receiving the SOC treatment and late rescue AIT (dashed line).
Fig 2.
A, Unadjusted Kaplan-Meyer survival curves in the early AIT (red) and in the control group (black). B, The treatment group is split in the group receiving anakinra alone (solid line) or in combination with a glucocorticoid (GC) (dashed line), whereas the control group is split in the group receiving the SOC treatment only (solid line) and in the group receiving the SOC treatment and late rescue AIT (dashed line).
The baseline demographic and clinical characteristics and unbalanced reduction after IPW are reported in Table E2 (available in this article's Online Repository at www.jacionline.org).
In the IPW-Cox model including admission to ICU as a time-dependent covariate, early AIT reduced the hazard of mortality by 74% (adjusted HR = 0.26 [95% CI = 0.10-0.66]; P < .001). In a multivariate Cox model adjusting for all the selected baseline characteristics as detailed in Table E3 (available in this article's Online Repository at www.jacionline.org), the effect was similar in the group receiving anakinra alone (adjusted HR = 0.28; P = .04) and in the 1 patient receiving anakinra plus a glucocorticoid (adjusted HR = 0.33; P = .07) (see the Supplementary Appendix and Table E4 available in this article's Online Repository at www.jacionline.org). Receiving late rescue AIT did not show a significant advantage in OS over receiving the SOC treatment alone (adjusted HR = 0.82; P = .70).
Behavior of the inflammatory and respiratory parameters and comparison between anakinra alone and anakinra plus a glucocorticoid in responding patients
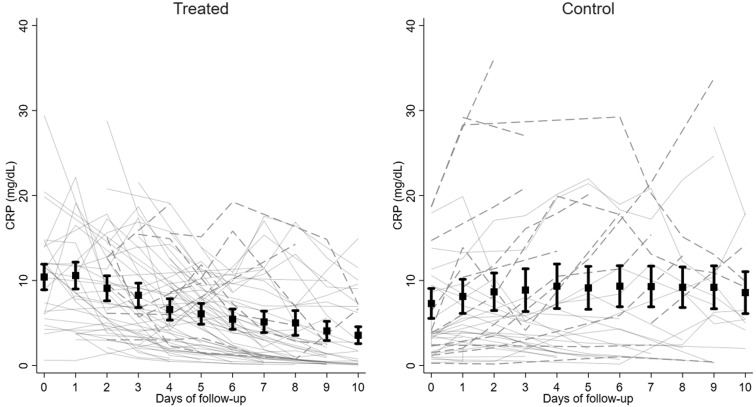
Fig 3 , A shows the behavior of CRP level in patients receiving early AIT compared with that in the controls (Fig 3, B). Fig E2, A (available in the Online Repository at www.jacionline.org) shows the amelioration of Pao 2/Fio 2 ratio, and Fig E2, B shows the average score for respiratory supportive care over time in all surviving patients receiving early AIT. The graphs were stopped at day 10 because the high number of missing data points after day 10 would have made the graphs misleading. The whole follow-up was considered for the mixed model analysis, fitting a linear trend from day 0 to the end of follow-up and comparing the slopes of improvement between the anakinra-alone and anakinra plus glucocorticoid arms.
Fig 3.
CRP level over time in the early AIT (treated) group and in the control group. Dotted lines represent patients who died during follow-up; solid lines represent patients who recovered. Squares are the mean values at each time point with its 95% CI.
The rapid improvement in all the inflammatory and respiratory parameters is clear after the start of early AIT, with no significant difference between the anakinra-alone and the anakinra plus glucocorticoid groups for Pao 2/Fio 2 ratio (P = .11), LDH level (P = .85), and respiratory supportive care score (P = .94).
The improvement in CRP level was more rapid in the anakinra plus glucocorticoid group (mean time for CRP level normalization (CRP level < 0.5 mg/dL) (mean time = 9.8 days ± 8.1 days) than in the anakinra-alone group (mean time = 16.8 days ± 10.8 days [P = .011]) (see Fig E3 available in the Online Repository at www.jacionline.org).
Safety
None of the patients in early and late AIT groups discontinued the treatment because of an elevation of liver enzyme levels. Sudden death occurred in 1 patient in the early AIT group and in 1 patient during the SOC treatment. In both patients, acute pulmonary thromboembolism was suspected despite ongoing enoxaparin prophylaxis. A total of 6 patients (2 in the early anakinra group, 1 in early anakinra plus a glucocorticoid group, 2 in the SOC treatment–only group, and 1 in the SOC treatment plus late AIT group) displayed bacteremia during their hospitalization. Four patients receiving AIT (3 treated with anakinra alone and 1 treated with anakinra and plus a glucocorticoid) displayed candidemia. None of those patients died of sepsis.
Discussion
The present study has reported on the use of early AIT with anakinra in patients with severe COVID-19 pneumonia treated during the first wave of the pandemic, and it provides the first data on the efficacy of anakinra as monotherapy in comparison with treatment consisting of a combination of anakinra plus a glucocorticoid.
After the promising results of a first pilot study,14 the treatment protocol was adopted as the main therapeutic approach in our hospital. The aim was to aggressively tackle the inflammatory response in patients with severe pulmonary involvement and clear signs of inflammation since the first days after their admission. The early AIT was associated with a reduction in mortality with respect to patients who received the SOC treatment exclusively or to whom anti-inflammatory drugs (anakinra or tocilizumab) were administered as a rescue treatment later during the disease course. The use of a glucocorticoid in combination with anakinra did not change the probability of survival, but it did prompt a faster decrease in the acute-phase reactants in the responding patients.
A growing body of evidence is suggesting a pathogenic role for monocyte cells and related inflammatory secreted molecules in COVID-19.26, 27, 28 IL-1β is a proinflammatory cytokine secreted by monocytes in response to different stimuli (including Toll-like receptor agonists, activated complement components, and IL-1β by itself in an autocrine way) that induce fever and neutrophilia and increase adhesion molecules' gene expression on endothelial cells.29 In vitro stimulation of the PBMCs of patients with COVID-19 or isolated monocytes has shown an increased activation of the IL-1β pathway30 in a subset of patients, and serum analysis has allowed a correlation between longitudinal IL-1β levels and disease severity and risk of death.31 Whole blood32 and single-cell gene expression analyses have confirmed increased transcription of the IL1B gene at least in a subset of circulating monocytes26 and the activation of IL-1 response genes in neutrophils of patients with severe COVID-19.27
In addition to the control of IL-1β oversecretion by circulating and resident monocytes and macrophages, the use of recombinant IL-1 receptor antagonist implies the possibility of also blocking IL-1α. Endothelial cells and the type 2 epithelium of the lung display high levels of constitutive IL-1α precursor. The IL-1 α precursor is active and does not need processing by NLRP3; therefore, the lytic effect of the viral infection might result in the IL-1α precursor release contributing to initiation and perpetuation of the inflammatory response at the tissue level.33 A number of previous studies using a retrospective design have pointed out the positive effect of anakinra in severe pulmonary COVID-19 infection13, 14, 15, 16, 17, 18, 19, 20, 21 (Table II ). Most of them have described the response to treatment in a selected group of patients without comparison with a control group (Table II). Other studies have used a control group of patients who had similar conditions but were not receiving the anti-inflammatory drug for different reasons.13 , 17 , 18 , 21 In our study, we used the same approach, trying to mitigate the intrinsic selection bias of nonrandomized studies by applying a PS-adjusted analysis, as was recently recommended for similar studies.34 An additional limitation of retrospective studies comparing treated patients with untreated patients is a potential immortal time bias: patients must survive long enough to be treated, and those dying soon after admission are more likely to be included in the untreated group. The exclusion of early deaths with a landmark of 4 days was motivated by the need to control the immortal time bias.
Table II.
Studies on the use of anakinra in COVID-19 pneumonia
| Study | No. of patients | Anakinra treatment | Control group | Statistical analysis | Outcome | Adverse events |
|---|---|---|---|---|---|---|
| Cavalli et al13 | 36 in total 29 7 |
iv 5 mg/kg 2×/d → tapering sc 100 mg 2×/d |
16 (SOC) | Unadjusted Kaplan-Meyer curve | Unadjusted HR = 0.20 for mortality; 0.50 for ICU admission (for iv treatment only) | 1 instance of bacteremia, 3 instances of increased liver enzyme levels (with discontinuation), and 3 instances of APC thromboembolism |
| Pontali et al14 | 5 | iv 300 mg/d for 3 d → tapering | No | Descriptive | All alive | No instances of bacteremia or increased liver enzyme levels |
| Aouba et al15 | 9 | sc 100 mg 2×/d for 3 d → 100 mg/d for 10 d |
No | Descriptive | All alive | 3 instances of increased liver enzyme levels and 1 instance of acute respiratory failure (discontinuation) |
| Dimopoluos et al16 | 8 | iv 200 mg 3×/d for 7 d | No | Descriptive | 3 deaths, 5 alive | No bacteremia |
| Huet et al17 | 52 | sc 100 mg 2×/d for 3 d → 100 mg/d for 7 | 44 (SOC) | Adjusted Cox model | Adjusted HR = 0.22 for mortality or ICU admission | 7 instances of increased liver enzyme levels (4 in the controls) 10 thromboembolic events (5 in the controls) No bacteremia |
| Cauchois et al18 | 12 | iv 300 mg/d for 5 d → 200 mg/d for 2 d → 100 mg/d for 1 d | 10 (SOC) | Descriptive | Anakinra: all alive Controls: 1 death |
NR |
| Navarro-Millan et al19 | 11 | sc 100 mg 3×/d → tapering | 3 (SOC) | Descriptive | Anakinra: 1 death Controls: all alive |
1 injection site reaction 4 bacterial infections 3 instances of increased liver enzyme levels |
| Langer-Gould et al20 | 41 | sc variable schedule (mean duration 9 d; mean cumulative dose 1500 mg) + glucocorticoid | 51 (tociluzumab) | Adjusted Cox mode | Risk of death: 22% with anakinra vs 46% with tocilizumab Adjusted HR = 0.46 (NS) |
NR |
| Bozzi et al21 | 65 | iv 600 mg/d for 3 d → 300 mg/d for 11 d + a glucocorticoid |
55 | Adjusted Cox model | Adjusted HR = 0.18 for mortality | Increased liver enzyme levels (6.2%) Neutropenia (1.5%) 9 instances of bacteremia (anakinra + glucocorticoid) vs 4 instances in the control group) |
| Present study | Total 63 30 33 |
Early treatment iv 300 mg/d for 3 d →tapering iv 300 mg/d for 3 d → tapering + a glucocorticoid 1-2 mg/kg per day → tapering |
Total 65 44 (SOC) 21 (SOC + late rescue AIT) |
IPW-Cox model | Total, adjusted HR = 0.26 for mortality Adjusted HR = 0.28 for mortality Adjusted HR = 0.33 for mortality |
1 instance of sudden death in anakinra (1 in the controls) 3 instances of bacteremia (2 who received the SOC, 1 who received late AIT) 4 instances of candidemia (3 in the anakinra-only group; 1 in the anakinra + glucocorticoid group) |
APC, Arterial pulmonary circulation; iv, intravenous; NR, not reported; NS, not significant; sc, subcutaneous.
Despite these statistical methods to adjust the baseline unbalances, we cannot exclude that part of the survival benefit observed in patients treated with early AIT could be due to a progressive optimization of the general management of critically ill patients during the weeks during which the study was performed.
Overall, the present data suggest that a high-dose AIT used in the first phase of the disease—as soon as a severe inflammatory pulmonary response necessitating oxygen or hospitalization becomes evident—is able to provide a prompt amelioration of the inflammatory and respiratory parameters in the majority of patients with severe COVID-19 pneumonia.
The use of glucocorticoids was controversial because it was done during the first weeks of the COVID-19 pandemic.35 , 36 Some retrospective studies37 and data from the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial38 pointed out the possible protective role of glucocorticoids, especially in patients with a severe acute respiratory distress syndrome. The hypothesis of a possible positive effect of early administration of a glucocorticoid in combination with anakinra comes from the daily practice in patients with severe cytokine storms, such as MAS.3 In a recent study, the combination of anakinra plus a glucocorticoid in 41 patients was associated with a risk of death of 22%; with respect to the 46% of patients treated with tocilizumab, however, this combination was mainly used in intubated patients.20 Similar results were recently reported by Bozzi et al.21
In our study no randomization was used to assign patients to glucocorticoid treatment. The choice was essentially based on the logistic organization in the hospital, prompting the immediate start of administration of a glucocorticoid in all patients who fulfilled the inclusion criteria and were temporarily admitted in the emergency room, where anakinra was not available.
The group receiving an additional glucocorticoid did not present substantial baseline demographic, inflammatory, or clinical differences from those of the group receiving anakinra alone; the longitudinal analysis of the inflammatory parameters after the start of treatment suggests that an adjuvant glucocorticoid treatment could reduce the time required for control of the inflammatory response, with an undefined impact in terms of safety, especially in regard to opportunistic infections. In this respect, the good safety profile of the use of anakinra in COVID-19 that was observed in the present study is in line with the findings in previous reports (Table E3). Along with the present study, more than 300 patients with COVID-19 treated with anakinra have been reported (Table E3). Despite all of the methodologic limitations of retrospective studies, the cumulative evidence coming from all of the currently available reports indicates anakinra as a possible safe strategy to control the inflammatory response in severe COVID-19 pneumonia. It is conceivable that the availability of different studies of the use of anakinra will allow a meta-analysis approach aimed at the identification of those subsets of patients with the higher probability to benefit from the treatment and at the optimization for a proper design in future randomized clinical trials. In particular, the design of new clinical trials using a combination of high doses of anakinra plus a glucocorticoid should be focused on patients in the early phases of their severe inflammatory pulmonary disease—as soon as they require oxygen assistance and hospitalization. Conversely, the inclusion in such studies of patients with milder disease and a higher probability of recovery even without the need for a more aggressive AIT might dilute the actual effect of this approach in COVID-19 pneumonia.
In conclusion, our study confirms the potential efficacy and safety of the early use of high doses of intravenous anakinra with or without a glucocorticoid on the largest series of patients with severe COVID-19 pneumonia reported so far; their concomitant use may represent a valid approach to be tested in future randomized trials in severely affected patients.
Clinical implications.
High doses of anakinra plus a glucocorticoid should be used in the early phases of severe COVID-19 pneumonia, as soon as the patients require oxygen assistance and hospitalization.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. S0140-673630566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metha P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunesuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravelli A., Davì S., Minoia F., Martini A., Cron R.Q. Macrophage activation syndrome. Hematol Oncol Clin North Am. 2015;29:927–941. doi: 10.1016/j.hoc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toniati P., Piva S., Cattalini M. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capra R., De Rossi N., Mattioli F. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colaneri M., Bogliolo L., Valsecchi P. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siu K.L., Yuen K.S., Castaño-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn M., Anderson D.E., Zhang Q., Tan C.W., Lim B.L., Luko K. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat Microbiol. 2019;4:789–799. doi: 10.1038/s41564-019-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Veerdonk F.L., Netea M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit Care. 2020;24:44. doi: 10.1186/s13054-020-03166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;14:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M. Favorable anakinra responses in severe Covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123.e1. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cauchois R., Koubi M., Delarbre D., Manet C., Carvelli J., Blasco V.B. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117:18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro-Millán I., Sattui S.E., Lakhanpal A., Zisa D., Siegel C.H., Crow M.K. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. 2020;172:1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer-Gould A., Smith J.B., Gonzales E.G., Castillo R.D., Garza Figueroa J., Ramanathan A. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020;99:291–297. doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzi G, Mangioni D, Minoia F, Aliberti S, Grasselli G, Barbetta L, Castelli V et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: an observational cohort study. J Allergy Clin Immunol 147:561-566. [DOI] [PMC free article] [PubMed]
- 22.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–278. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eloseily E.M., Weiser P., Crayne C.B., Haines H., Mannion M.L., Stoll M.L. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 24.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart E., Lee B.K., Leacy F.P. Prognostic score–based balance measures for propensity score methods in comparative effectiveness research. J Clin Epidemiology. 2013;66(suppl 8):S84–S90. doi: 10.1016/j.jclinepi.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulte-Schrepping J., Reusch N., Paclik D., Bassler K., Schlickeiser S., Zhang B.W. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418.e8. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann E., Menon M., Blandin Knight S., Konkel E.J., Jagger C., Shaw N.T. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garlanda C., Dinarello C.A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong E.Z.Y., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Mohideen S.M.H. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27:879–882.e2. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello C.A., Ikejima T., Warner S.J., Orencole S.F., Lonnemann G., Cannon J.C. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 34.Kooistra E.J., Waalders N.J.B., Kox M., Pickker P. Effect of anakinra in COVID-19. Lancet Rheumatol. 2020;2:e23–e24. doi: 10.1016/S2665-9913(20)30235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 38.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [e-pub ahead of print]. N Engl J Med https://doi.org/10.1056/NEJMoa2021436. Accessed July 17, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.