Abstract
Objective
We assessed the performance of the ratio of peripheral arterial oxygen saturation to the inspired fraction of oxygen (SpO2/FiO2) to predict the ratio of partial pressure arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) among patients admitted to our emergency department (ED) during the SARS-CoV-2 outbreak.
Methods
We retrospectively studied patients admitted to an academic-level ED in France who were undergoing a joint measurement of SpO2 and arterial blood gas. We compared SpO2 with SaO2 and evaluated performance of the SpO2/FiO2 ratio for the prediction of 300 and 400 mmHg PaO2/FiO2 cut-off values in COVID-19 positive and negative subgroups using receiver-operating characteristic (ROC) curves.
Results
During the study period from February to April 2020, a total of 430 arterial samples were analyzed and collected from 395 patients. The area under the ROC curves of the SpO2/FiO2 ratio was 0.918 (CI 95% 0.885–0.950) and 0.901 (CI 95% 0.872–0.930) for PaO2/FiO2 thresholds of 300 and 400 mmHg, respectively. The positive predictive value (PPV) of an SpO2/FiO2 threshold of 350 for PaO2/FiO2 inferior to 300 mmHg was 0.88 (CI95% 0.84–0.91), whereas the negative predictive value (NPV) of the SpO2/FiO2 threshold of 470 for PaO2/FiO2 inferior to 400 mmHg was 0.89 (CI95% 0.75–0.96). No significant differences were found between the subgroups.
Conclusions
The SpO2/FiO2 ratio may be a reliable tool for hypoxemia screening among patients admitted to the ED, particularly during the SARS-CoV-2 outbreak.
Keywords: Respiratory insufficiency, Oximetry, COVID-19, Triage, ROC curve
1. Introduction
1.1. Background
Acute respiratory distress is a common reason for emergency department (ED) admission. Since the end of 2019, healthcare workers have been facing the coronavirus disease (COVID-19) pandemic due to SARS-CoV-2, with a major risk of overcrowding in the ED. Severe COVID-19 infections are associated with hypoxemia, the severity of which can quickly become life-threatening.
The development of tools for the initial assessment of the degree of hypoxemia among patients with respiratory symptoms is essential. Such a tool should reduce both under-triage, allowing critical patients to quickly receive the appropriate level of care, and over-triage, maintaining critical care capacity despite an influx of patients. An appropriate triage tool should also be applicable for all causes of respiratory distress, whether related to COVID-19 or not. Limited reliable data are available for the triage of hypoxemic patients in the ED, yet it could be particularly useful during the COVID-19 pandemic.
1.2. Importance
Early diagnosis of critical hypoxemia is important for both COVID-19 infection and Acute Respiratory Distress Syndrome (ARDS) based on the ratio of partial pressure arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) [1,2]. This indicator has been validated in many pathological situations [3,4] but requires an arterial blood sample and therefore is often not immediately accessible upon the patient's admission. Arterial puncture is also a source of pain and complications [[5], [6], [7], [8]].
Validation of a noninvasive tool could allow us to improve triage time and patient orientation upon admission. In particular, the ratio of peripheral arterial oxygen saturation to the inspired fraction of oxygen (SpO2/FiO2) has been proposed by several authors [[9], [10], [11], [12]]. However, diagnostic performance of this index has not yet been evaluated.
1.3. Goals of this investigation
This study evaluated the performance of the SpO2/FiO2 ratio for predicting mild (PaO2/FiO2 superior to 400 mmHg) or moderate (PaO2/FiO2 inferior to 300 mmHg) hypoxemia, and evaluated the performance of this index among patients admitted during the COVID-19 outbreak, with subgroup analyses distinguishing SARS-CoV-2 positive and negative patients.
2. Methods
2.1. Study design and setting
We performed a retrospective observational cohort study to assess the predictive value of SpO2/FiO2 for the PaO2/FiO2 ratio among adult patients admitted to our ED from February to April 2020. The study center is an academic-level hospital in France and was the regional referral center for COVID-19 patient management during the pandemic.
We followed the Standards for Reporting of Diagnostic Accuracy criteria for diagnostic performance studies [13]. Our study is in accordance with the current regulations in France relative to protection of personal health and privacy data.
2.2. Data collection
We extracted the following from our computer data system (DxCare® software, France): data on demographics, medical history, vital signs at admission and time of arterial blood sampling, presence of oxygen therapy, oxygen flow rate (QO2) at arterial puncture, SARS-CoV-2 virological status, final diagnosis, and blood gas results for each arterial sample during ED admission including PaO2, arterial hemoglobin saturation (SaO2), and arterial carbon dioxide partial pressure (PaCO2). Data collection was planned subsequent to admission of the study population for the study period. All patients admitted during the study period and from whom an arterial blood sampling was performed were reviewed. The diagnosis of COVID-19 infection was retained in our analyses in cases of a positive PCR and/or typical lesions in chest CT scans (when performed).
FiO2 was estimated from the interface used for oxygen delivery (nasal cannulas, single mask, high-concentration mask, non-invasive ventilation) and oxygen flow rate. For nasal-cannula or single-mask oxygen therapy, FiO2 was estimated from the data presented by Wettstein et al [14] using the formula FiO2 = 4.QO2 + 21%. For the high-concentration mask, FiO2 was estimated at 80%. For non-invasive ventilation, the FiO2 value selected was the one set on the ventilator.
Pulsed oxygen saturation was measured using pulse oximeters (Space Labs Healthcare Qube™, USA). The quality of the measurement was controlled by a nurse with simultaneous visualization of the plethysmography curve. Arterial blood gas analyses were performed immediately after sampling with a Werfen Instrumentation Laboratory GEM Premier 5000™ (Spain).
2.3. Choice of PaO2/FiO2 thresholds
The PaO2/FiO2 thresholds chosen to plot ROC curves were 300 and 400 mmHg, respectively. The 300 mmHg threshold corresponds to the definition of both severe COVID-19 [15], and ARDS [16]. The 400 mmHg threshold corresponds to changes in hematosis relative to observed normal [17] and is used for early detection of hypoxemia, such as in the SOFA score [18].
2.4. Inclusion and exclusion criteria
This study included patients over 18 years of age who were admitted to our ED and underwent vital sign recording and arterial blood gas sampling less than 1 h apart. Exclusion criteria were the absence of recording of one of the parameters studied, a suspicion of blood gas analyses using venous samples (defined as SaO2 inferior to 75% with SpO2 superior to 95%), and the presence of hyperoxygenation (defined as the presence of oxygen therapy despite SaO2 superior to 99%). We did not exclude samples collected for extra-respiratory indications.
2.5. Statistical analyses
Calculation of the SpO2/FiO2 values and determination of the mean areas under the receiver-operating characteristic (ROC) curves of SpO2/FiO2 for PaO2/FiO2 threshold values of 300 and 400 mmHg, as well as their confidence intervals, were performed using R software version 3.6.3 (pROC package) [19]. Agreement between SpO2/FiO2 and PaO2/FiO2 ratios was assessed using Spearman's rank correlation coefficient. Subgroup analysis of COVID-19 positive and negative patients was conducted using Delong's test of equivalence on ROC curves of both subgroups for PaO2/FiO2 threshold values of 300 and 400 mmHg. SpO2/FiO2 thresholds were determined using the weighted closest topleft method (pROC package).
3. Results
3.1. Characteristics of the study subjects
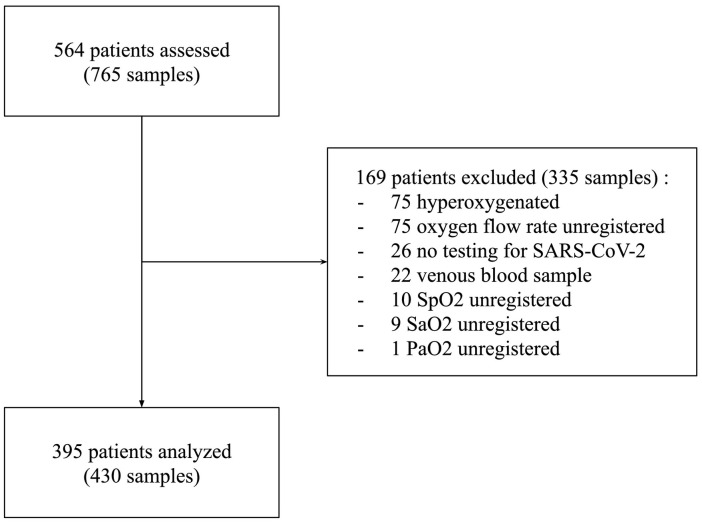
During the study period, 564 patients underwent one or more blood gas analyses for a total of 765 blood samples. After application of the exclusion criteria, 430 blood samples from 395 patients were included in statistical analyses. Fig. 1 shows the flow chart of the study. The characteristics of the patients and blood samples are presented in Table 1 .
Fig. 1.
Flowchart of the study.
Table 1.
Characteristics of study subjects
| Patients characteristics | N = 395 |
| Age, median (IQR) | 60 (44–78) |
| Gender, male, N (%) | 193 (48.9%) |
| Comorbidities: | |
| cardiac, N (%) | 91 (23.0%) |
| heart failure, N (%) | 71 (18.0%) |
| atrial fibrillation, N (%) | 40 (10.1%) |
| coronary artery disease, N (%) | 30 (7.6%) |
| other cardiac disease, N (%) | 33 (8.4%) |
| pulmonary, N (%) | 118 (29.9%) |
| asthma, N (%) | 48 (12.2%) |
| Chronic obstructive pulmonary disease(COPD), N (%) | 51 (12.9%) |
| lung carcinosis, N (%) | 10 (2.53%) |
| restrictive lung disease, N (%) | 16 (4.05%) |
| other, N (%) | 10 (2.53%) |
| Acute kidney failure, N (%) | 33 (8.4%) |
| Chronic kidney disease, N (%) | 29 (7.34%) |
| COVID-19 status | |
| positive, N (%) | 90 (22.8%) |
| negative, N (%) | 305 (77.2%) |
| Diagnosis at discharge | |
| pulmonary disease, N (%) | 291 (73.7%) |
| SARS-CoV-2, N (%) | 90 (22.8%) |
| COPD exacerbation, N (%) | 21 (5.32%) |
| Acute asthma, N (%) | 18 (4.56%) |
| Pneumonia, N (%) | 49 (12.4%) |
| Cardiogenic pulmonary oedema, N (%) | 21 (5.32%) |
| respiratory acute viral syndrome (undetermined), N (%) | 72 (18.3%) |
| others, N (%) | 20 (5.06%) |
| Extra-respiratory disease, N (%) | 104 (26.3%) |
| Vital signs: | |
| Heart rate, median (IQR) | 88 (77–101) |
| Systolic blood pressure, median (IQR) | 129 (115–144) |
| Diastolic blood pressure, median (IQR) | 77 (68–87) |
| SpO2, median (IQR) | 97 (95–99) |
| Ventilatory rate, median (IQR) | 22 (18–28) |
| Temperature (°C), median (IQR) | 37 (36.6–37.5) |
| Blood gas analysis | N = 430 |
| Sample from COVID-19 patient, N (%) | 94 (21.9%) |
| FiO2 (%), median (IQR) | 21 (21–29) |
| PaO2 (mmHg), median (IQR) | 83.3 (71.3–97.5) |
| SaO2 (%), median (IQR) | 97.9 (96.4–98.9) |
| PaCO2 (mmHg), median (IQR) | 36.0 (32.3–40.5) |
| pH, median (IQR) | 7.44 (7.41–7.47) |
| PaO2/FiO2 (mmHg), median (IQR) | 364.3 (269.5–450) |
| PaO2/FiO2 > 400, N (%) | 164 (38.1%) |
| PaO2/FiO2 300–400, N (%) | 132 (30.7%) |
| PaO2/FiO2 < 300, N (%) | 134 (31.2%) |
| SaO2/FiO2, median (IQR) | 461.2 (339.0–470.5) |
| SpO2/FiO2, median (IQR) | 452.4 (337.9–466.7) |
3.2. Main results
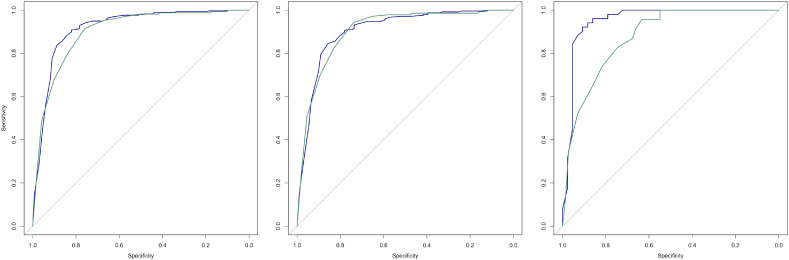
Spearman's rank correlation between SpO2/FiO2 and PaO2/FiO2 ratios was 0.799 (CI 95% 0.747–0.842). The ROC curves of the SpO2/FiO2 ratio among overall (N = 430), COVID-19 positive (N = 94) and negative (N = 336) subgroups for PaO2/FiO2 threshold values of 300 and 400 mmHg are shown in Fig. 2 . Delong's test for comparison of ROC curves showed no significant difference between the subgroups for PaO2/FiO2 threshold values of 300 mmHg (D = 1.46, p = 0.15) and 400 mmHg (D = 0.68, p = 0.50).
Fig. 2.
ROC curves of the SpO2/FiO2 ratio for PaO2/FiO2 threshold values of 300 mmHg (blue) and 400 mmHg (green), for overall (left), COVID-19 negative (center) and positive (right) patients. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The AUCs of ROC curves of overall patients were 0.918 (CI 95% 0.885–0.950) for a PaO2/FiO2 threshold of 300 mmHg and 0.901 (CI 95% 0.872–0.930) for a PaO2/FiO2 threshold of 400 mmHg. Analysis of SpO2/FiO2 performance shows a specificity of SpO2/FiO2 superior to 470 of 0.98 (CI95% 0.96–0.99) with a positive predictive value (PPV) of 0.89 (CI95% 0.75–0.96) for PaO2/FiO2 superior to 400 mmHg. Specificity of SpO2/FiO2 inferior to 350 was 0.95 (CI95% 0.91–0.97) with PPV of 0.88 (CI95% 0.84–0.91) for PaO2/FiO2 inferior to 300 mmHg.
4. Discussion
4.1. Synthesis of relevant results
The SpO2/FiO2 ratio in our cohort showed a good association with the PaO2/FiO2 ratio. In this series, the use of thresholds of 370 and 450 showed satisfactory diagnostic performances for the positive diagnosis of PaO2/FiO2 < 300 mmHg and exclusion of a PaO2/FiO2 < 400 mmHg. Performance was not significantly different between COVID-19 positive and negative patients.
Our results suggest that SpO2/FiO2 can be used for the estimation of the degree of hypoxemia on admission to the emergency room, allowing the patient's severity to be assessed prior to confirmation of viral status. Without other signs of respiratory distress (including increased work of breathing and/or altered mental status), SpO2/FiO2 superior to 450 could be used as a decision threshold for outpatient management, whereas SpO2/FiO2 inferior to 370 could require referral to intensive care units. An SpO2/FiO2 between 370 and 450 would require further clinical evaluation and possibly arterial blood gas.
4.2. Relationship to previous results
Our results support data published by Lu et al. [20], who found that a significant association between SpO2/FiO2 decreases and increases mortality risk. Similarly, results recently published by Hamlovich et al. [21] showed the prognostic value of SpO2 and FiO2 in the initial assessment of the severity of COVID-19 patients. In this context, our results support the possibility of using SpO2/FiO2 by admission nurses as a tool for triage and early referral of patients admitted for respiratory symptoms.
4.3. Limitations
This study had several limitations. First, its retrospective design is subject to several biases. In particular, the SARS-CoV-2 virological status is based on the presence of a positive PCR and/or CT scan with typical lesions. The number of false positives is likely to be small; thus, we can assume the existence of many false negatives, particularly in patients who have not had a CT scan or if the CT scan was performed too early, which may be negative during the first few days of symptom progression [22].
The population sample collected over this period cannot be considered representative of a standard sample of patients consulted in an ED. Significantly more patients were admitted for respiratory symptoms during the period. In addition, relatively fewer patients were admitted for other indications as they may have avoided consultations to reduce their exposure to viral contamination.
Blood gas measurements performed for acute respiratory distress were included, as well as for non-respiratory reasons, considering that validation of SpO2/FiO2 in non-hypoxemic patients was necessary to exclude significant hypoxemia at triage. Hyperoxygenated patients were excluded from the analyses, in whom the SpO2/FiO2 ratio is artificially lowered by a nonlinear evolution of SpO2 (limited between 99% and 100%) compared to FiO2. This choice limits the use of these results for triage, where pre-hospital treatment may have imposed significant oxygen therapy during transport to the hospital center, leading to hyperoxygenation at admission to the ED. The use of SpO2/FiO2 then requires a prior decrease in FiO2 to obtain an SpO2 of less than 99%.
Finally, this study evaluated the performance of SpO2/FiO2 based on its association with a biological criterion. An evaluation based on a clinical criterion would be more relevant and would allow for a better assessment of the interest of this index in practice. In particular, previous studies [23,24] have shown that the relationship between PaO2 and FiO2 is not linear. Thus, the PaO2/FiO2 ratio depends on the FiO2, and its evolution in cases of FiO2 variation depends on the fraction of shunt and the ventilation/perfusion mismatch. As such, a perfect correlation between SaO2/FiO2 and PaO2/FiO2 would mean that SaO2/FiO2 has the same drawbacks.
5. Conclusion
In summary, our results suggest that SpO2/FiO2 could be a useful index for triage upon admission of patients consulting for acute respiratory symptoms, particularly with suspicion of COVID-19, and would identify patients who could be managed on an outpatient basis and patients requiring admission to the intensive care unit. The use of this index nevertheless requires oxygen titration to ensure the absence of hyperoxygenation, and does not take into account the other dimensions of the respiratory assessment (including work of breathing and mental status).
Future studies are required for a prospective evaluation of the diagnostic performance of SpO2/FiO2 among COVID-19 patients, as well as in the acute respiratory tract. In particular, the use of a clinical criterion such as mortality, length of hospitalization, the need for admission to intensive or conventional care could allow us to more accurately assess the contribution of SpO2/FiO2 in emergency medicine triage, providing options in addition to PaO2/FiO2.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
References
- 1.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;385(25):1451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 2.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Offner P.J., Moore E.E. Lung injury severity scoring in the era of lung protective mechanical ventilation: the PaO2/FIO2 ratio. J Trauma. 2003;55(2):285–289. doi: 10.1097/01.TA.0000078695.35172.79. [DOI] [PubMed] [Google Scholar]
- 4.Esteve F., Lopez-Delgado J.C., Javierre C., Skaltsa K., Carrio M.L., Rodríguez-Castro D., et al. Evaluation of the PaO2/FiO2 ratio after cardiac surgery as a predictor of outcome during hospital stay. BMC Anesthesiol. 2014;14:83. doi: 10.1186/1471-2253-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford A. An audit of the patient’s experience of arterial blood gas testing. Br J Nurs. 2004;13(9):529–532. doi: 10.12968/bjon.2004.13.9.12963. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen J.D. Clinical sequelae from arterial needle puncture, cannulation, and incision. Circulation. 1967;35(6):1118–1123. doi: 10.1161/01.cir.35.6.1118. [DOI] [PubMed] [Google Scholar]
- 7.Giner J., Casan P., Belda J., González M., Miralda R.M., Sanchis J. Pain during arterial puncture. Chest. 1996;110(6):1443–1445. doi: 10.1378/chest.110.6.1443. [DOI] [PubMed] [Google Scholar]
- 8.Halpern A.A., Mochizuki R., Long C.E., 3rd. Compartment syndrome of the forearm following radial-artery puncture in a patient treated with anticoagulants. JBJS. 1978;60(8):1136–1137. [PubMed] [Google Scholar]
- 9.Sanz F., Dean N., Dickerson J., Jones B., Knox D., Fernández-Fabrellas E., et al. Accuracy of PaO2 /FiO2 calculated from SpO2 for severity assessment in ED patients with pneumonia. Respirol Carlton Vic. 2015;20(5):813–818. doi: 10.1111/resp.12560. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande P.P., Shintani A.K., Hagerman H.E., St Jacques P.J., Rice T.W., Sanders N.W., et al. Derivation and validation of SpO2/FiO2 ratio to impute for PaO2/FiO2 ratio in the respiratory component of the sequential organ failure assessment (SOFA) score. Crit Care Med. 2009;37(4):1317–1321. doi: 10.1097/CCM.0b013e31819cefa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsuya H., Sakanashi Y. Simple and noninvasive indicator of pulmonary gas exchange impairment using pulse oximetry. J Clin Monit. 1989;5(2):82–86. doi: 10.1007/BF01617878. [DOI] [PubMed] [Google Scholar]
- 12.Brown S.M., Grissom C.K., Moss M., Rice T.W., Schoenfeld D., Hou P.C., et al. Nonlinear imputation of Pao2/Fio2 from Spo2/Fio2 among patients with acute respiratory distress syndrome. Chest. 2016;150(2):307–313. doi: 10.1016/j.chest.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bossuyt P.M., Reitsma J.B., Bruns D.E., Gatsonis C.A., Glasziou P.P., Irwig L., et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351 doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wettstein R.B., Shelledy D.C., Peters J.I. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50(5):604–609. [PubMed] [Google Scholar]
- 15.China National Health Commission . 7th ed. 2020. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment. [Google Scholar]
- 16.Definition Task Force A.R.D.S., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012 Jun 20;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Trulock E.P. In: Clinical methods: The history, physical, and laboratory examinations. 3rd ed. Walker H.K., Hall W.D., Hurst J.W., editors. Butterworths; Boston: 1990. Arterial blood gases. [PubMed] [Google Scholar]
- 18.Vincent J.-L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J., et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(77) doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Jiang L., Chen T., Wang Y., Zhang B., Hong Y., et al. Continuously available ratio of SpO2/FiO2 serves as a noninvasive prognostic marker for intensive care patients with COVID-19. Respir Res. 2020;21(1):194. doi: 10.1186/s12931-020-01455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haimovich A., Ravindra N.G., Stoytchev S., Young H.P., PerryWilson F., van Dijk D., et al. Development and validation of the quick COVID-19 severity index (qCSI): a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76(4):442–453. doi: 10.1016/j.annemergmed.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y.-C., Luo H., Liu S., Huang S., Zhou Z., Yu Q., et al. Dynamic evolution of COVID-19 on chest computed tomography: experience from Jiangsu Province of China. Eur Radiol. 2020;30(11):6194–6203. doi: 10.1007/s00330-020-06976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aboab J., Louis B., Jonson B., Brochard L. Relation between PaO2/FIO2 ratio and FIO2: a mathematical description. Intensive Care Med. 2006;32(10):1494–1497. doi: 10.1007/s00134-006-0337-9. [DOI] [PubMed] [Google Scholar]
- 24.Karbing D.S., Kjærgaard S., Smith B.W., Espersen K., Allerød C., Andreassen S., et al. Variation in the PaO2/FiO2 ratio with FiO2: mathematical and experimental description, and clinical relevance. Crit Care. 2007;11(6):R118. doi: 10.1186/cc6174. [DOI] [PMC free article] [PubMed] [Google Scholar]