Abstract
Schizophrenia is a severe neuropsychiatric disorder associated with a wide array of transcriptomic and neurobiochemical changes. Genome-wide transcriptomic profiling conducted in postmortem brain have provided novel insights into the pathophysiology of this disorder, and identified biological processes including immune/inflammatory-related responses, metabolic, endocrine, and synaptic function. However, few studies have investigated whether similar changes are present in peripheral tissue. Here, we used RNA-sequencing to characterize transcriptomic profiles of lymphocytes in 18 nonpsychotic controls and 19 individuals with schizophrenia. We identified 2819 differentially expressed transcripts (Pnominal < .05) in the schizophrenia group when compared to controls. Bioinformatic analyses conducted on a subset of 293 genes (Pnominal < .01 and |log2 FC| > 0.5) highlighted immune/inflammatory responses as key biological processes in our dataset. Differentially expressed genes in lymphocytes were highly enriched in gene expression profiles associated with cortex layer 5a and immune cells. Thus, we investigated whether the changes in transcripts levels observed in lymphocytes could also be detected in the prefrontal cortex (PFC, BA10) in a second replication cohort of schizophrenia subjects. Remarkably, mRNA levels detected in the PFC and lymphocytes were in strong agreement, and measurements obtained using RNA-sequencing positively correlated with data obtained by reverse transcriptase-quantitative polymerase chain reaction analysis. Collectively, our work supports a role for immune dysfunction in the pathogenesis of schizophrenia and suggests that peripheral markers can be used as accessible surrogates to investigate putative central nervous system disruptions.
Keywords: RNA-seq, inflammation, immune system, schizophrenia, lymphocytes, postmortem brain
Introduction
Schizophrenia is a severe neuropsychiatric disorder characterized by a cluster of symptoms, including delusions, hallucinations, and cognitive impairment; it is one of the most devastating mental illnesses among young adults.1 The global incidence of this disorder is approximately 1% of the world’s population.2 The pathophysiology of schizophrenia is thought to be the result of a complex interplay between genetic and environmental stimuli.3,4 Both central and peripheral epigenetic dysregulations of the genome may play key roles in mediating the clinical manifestations and progression of the disorder.5–11 Current treatments do not address the full spectrum of symptoms and the response rate to antipsychotics remain partial in many patients.12,13 Thus, a better understanding of the molecular mechanisms underlying the development and progression of this disorder is likely to allow for novel diagnostic tools and the potential for personalized treatments in schizophrenia.
Several studies have investigated the transcriptional changes associated with schizophrenia using microarray or RNA-sequencing (RNA-seq) in peripheral tissue and postmortem human brain. While dysregulation of multiple biological processes (eg, GABAergic neurotransmission, synaptic and mitochondrial function) have been associated with schizophrenia,14 many transcriptomic studies in postmortem brain of individuals with schizophrenia highlighted changes in genes related to the immune system and inflammatory responses in corticolimbic regions including the prefrontal cortex.15–19 Collectively, these studies suggest that alterations of the immune system might contribute to the pathogenesis of the disorder. While the precise role of the immune system in neuropsychiatric disorders remains somewhat controversial, evidence from genomic, blood, postmortem and in vivo imaging studies are leaning toward a consensus that immune activation has involved the pathophysiology of schizophrenia.20–22 Disturbances in the blood-brain barrier may also explain the synergistic increases in inflammatory markers observed in brain and blood of individuals suffering from schizophrenia.23,24 Interestingly, genome-wide association studies have identified risk polymorphisms in the major histocompatibility complex region25–27 and the gene for complement component 4 (C4)28 in individuals with schizophrenia. A positive genetic association with the genetic region encoding the cytokines interleukin (IL)-1α, and IL-1β and IL1RA has also been reported,29 strongly implicating the immune system in schizophrenia. A growing body of evidence indicates that alterations of the inflammatory system could be one of the important upstream factors producing biological abnormalities in schizophrenia. Early-life adverse events, including maternal infection and perinatal stress have been shown to increase the risk of schizophrenia and increase cytokine levels,30–32 providing further evidence that infection-related physiological mechanisms might alter neurodevelopmental processes and consequently lead to increased susceptibility to schizophrenia later in life.33
The development of schizophrenia follows a complex natural course over time, starting with a prodrome, the first episode in adolescence or early adulthood, and progressive deterioration over adult years. Given the natural history of this neuropsychiatric disorder, postmortem brain studies may not adequately unveil the molecular underpinnings of the disease. Thus, to investigate whether the transcriptomic changes observed in postmortem brains of schizophrenia subjects are mirrored in patient lymphocytes, we conducted RNA-seq in lymphocytes of individuals with schizophrenia and unaffected controls. Bioinformatic analyses were performed to identify enriched gene sets. Importantly, the most significant differentially expressed genes identified in lymphocytes were investigated in prefrontal cortex of an independent case–control cohort to establish the concordance and reproducibility of the transcriptomic changes observed in peripheral tissues.
Methods and Materials
Subjects
Lymphocytes were obtained from the Nathan S. Kline Institute (NKI) for Psychiatric Research as part of a cohort of 18 controls and 19 individuals with schizophrenia participating in a larger study. Both control and schizophrenia subjects met strict inclusion criteria and attempts were made to match control cases for sex and age. Schizophrenia patients had a long history of illness with several hospitalizations and/or years of outpatient clinic treatment (although we did not have precise data on specific years of illness for many subjects), and were diagnosed by review of hospital records, using checklists for DSM-IV34 and later DSM-V35, and supplemented by SCID diagnostic interviews when these were available from conjoint or prior studies. Schizophrenia subjects were associated with a State mental institution (Rockland Psychiatric Center) or its affiliated outpatient clinic and residences. All schizophrenia subjects are treated with first generation and/or second generation antipsychotics and some were also treated with additional mood medications. Control individuals were recruited from subjects screened at the NKI for Psychiatric Research outpatient clinic rolls or were recruited from the local community by advertisements. Control subjects who never met criteria for schizophrenia, bipolar disorder, major depressive disorder, schizophreniform disorder, or drug-induced psychosis. Control subjects had no history of antipsychotic or antidepressant medication. Subjects who had a history of opiates, cocaine, ecstasy or methamphetamine abuse/use in the past 2 months or had a positive urine toxicology test for these substances, heavy marijuana use or consistent daily smoking as well as heavy cigarette smoking were excluded.5,36 In addition, control subjects with positive urine toxicology for antidepressants or antipsychotics were also excluded from the study. Subjects with a history of brain trauma injury, diagnosis of Down syndrome, Alzheimer’s disease or related specific dementias were excluded as well.
Frozen human postmortem brain samples from prefrontal cortex (BA10) were obtained from the University of Maryland Brain and Tissue Bank, the Human Brain and Spinal Fluid Resource Center (VA Greater Los Angeles Healthcare System), and the University of Pittsburgh Brain Tissue Donation Program, which are brain and tissue repositories of the NIH-funded NeuroBioBank (request #1418) and included 10 control and 10 schizophrenia patients. Demographic details for both cohorts are provided in Table 1.
Table 1.
Demographics of Lymphocyte and BA10 Samples From Patients With Schizophrenia and Control
| Lymphocytes Samples | |||
|---|---|---|---|
| Characteristic | Schizophrenia | Nonpsychotic Control | Test |
| N | 19 | 18 | NA |
| Age | 44.4 ± 9.7 | 34.6 ± 11.6 | t 1,35 = 2.87, P = .0009 |
| Sex (M/F) | 15/4 | 11/7 | FET = 0.295 |
| Race (W/B/H/A) | 4/12/2/1 | 5/11/1/1 | LR = 0.468, df = 3, P = .926 |
| Current cigarette smoker (Y/N) | 12/7 | 4/14 | FET = 0.020 |
| Cigarettes smoked per week | 29.8 ± 40.9 | 12.4 ± 26.3 | t 1,35 = 1.53, P = 0.135 |
| Marijuana – urine toxicology positive | 0/14a | 3/18 | FET = 0.238 |
| PANSS total | 73.6 ± 17.5 | NA | NA |
| PANSS positive | 18.1 ± 6.3 | NA | NA |
| PANSS negative | 21.3 ± 7.7 | NA | NA |
| MATRICS overall composite score | 24.0 ± 14.9b | 40.2 ± 8.6 | T w = 3.92, df = 25.3, P =. 001 |
| Antipsychotic treatment type (first generation/second generation/combined first and second generation) (n) | 3/10/6 | NA | NA |
| On clozapine (Y/N) | 6/13 | 0/18 | FET = 0.02 |
| On valproate (Y/N) | 4/15 | 0/18 | FET = 0.105 |
| On lithium (Y/N) | 1/18 | 0/18 | FET = 1.00 |
| On antidepressants (Y/N) | 2/17 | 0/18 | FET = 0.486 |
| On benzodiazepines (Y/N) | 5/14 | 0/18 | FET = 0.046 |
| Postmortem BA10 samples | |||
| Count | 10 | 10 | NA |
| Age | 49.8 ± 2.6 | 53.6 ± 2.1 | t 1,19 = 1.13, P = 0.272 |
| Sex | M | M | NA |
| Race (W/B) | 9/1 | 8/2 | NA |
| PMI (h) | 24.8 ± 9.1 | 16.1 ± 1.7 | t 1,19 = 0.94, P = .359 |
| RIN | 7.11 ± 0.55 | 7.4 ± 0.52 | t 1,19= 0.33, P = .745 |
| Cause of death | |||
| Suicide | 4 | 0 | NA |
| Natural | 4 | 9 | NA |
| Accidental | 1 | 0 | NA |
| Undetermined | 1 | 1 | NA |
Note: Lymphocytes samples were provided by Nathan Klein Institute for psychiatric research. Postmortem brain samples were provided by NIH NeuroBioBank. M = male; F = female; N = number; race: W = white, Caucasian, B = black or African American, H = nonblack Hispanic surname, A = Asian; statistics: T = two-sample t-test, Tw = t-test for unequal variances, FET = Fisher’s exact test; LR = likelihood ratio; NA = not applicable.
aOnly 14 of 19 schizophrenics received urine toxicology; five inpatients were assumed not to be ingesting marijuana.
b n = 17. Indicated values are mean ± SD or number of subjects.
*P < 0.05, Student’s t-test.
Clinical Assessments
Individuals with schizophrenia who donated lymphocytes were evaluated within one week from donation for the current level of psychopathology by the Positive and Negative Syndrome Scale (PANSS) by trained research psychiatrists for assessment of levels of current psychopathology.37 The National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) was also assessed to test cognitive performances in schizophrenia and control subjects.38
Lymphocyte Sample Collection and Processing
Subject’s blood was collected in 4–8 lavender top collection tubes (EDTA) (~8 ml blood per tube), and processed using Balance Salt Solution (BSS, see Ficoll-Paque Plus instructions for reagent preparation). Blood samples were then layered over cold Ficoll-Paque Plus, and centrifuged using a swinging bucket rotor at 400g for 45 min at 4°C. The lymphocyte layer was removed into sterile collection tubes, centrifuged at 100g for 10 min at 4°C and loose lymphocyte pellet washed with BSS.
mRNA Sequencing
Total RNA was isolated from lymphocytes and postmortem brain samples using the Qiagen miRNeasy Mini Kit (Qiagen, CA). RNA Integrity Number (RIN) was determined with the Agilent 2100 Bioanalyzer (Agilent Technologies, CA) and varied from 7.9 to 9.6. RNA-seq libraries were constructed using the Illumina TruSeq Stranded RNA sample Prep kit (Illumina, CA) following the manufacturer’s instructions. A single library was prepared from each sample. All libraries were then quantitated by qPCR, combined in equimolar concentration into three pools of 11–16 individual (half C and half S), and each pool was sequenced on two lanes of a HiSeq2500. Fastq files were processed and demultiplexed with bcltofastq 1.8.4 for pools 1 and 2 and bcltofastq 2.17.1.14 for pool 3. Raw reads were then checked for quality using FASTQC (v 0.11.2), trimmed and filtered using Trimmomatic (v 0.33) to remove residual adapter content and low-quality bases (Phred quality score < 28). Trimmed/filtered reads were aligned to UCSC-hg38 genome and gene models using STAR (v 3.5.0a). Postalignment gene counts were then determined using featureCounts (subread v 1.4.6-p4) with multimapping reads excluded. The gene-level read counts were then imported into R (v 3.4.2) for statistical analyses. After processing the third pool, USCS gene models were updated to NCBI Annotation release 108 using RefSeq transcript IDs or gene symbols/alias in Bioconductor’s org.Hs.eg.db package39 (v 3.5.0). Trimmed mean of M values (TMM) normalization40 in the edgeR package41 (v 3.20.6) was used to normalize the counts to log2-transformed counts per million (log CPM), using the cpm function with prior count = 3. 12 290 genes without log CPM > log2 (1) in at least three samples were filtered out, leaving 14 154 genes to be analyzed for differential expression. TMM-values were recalculated and expression values were normalized using the limma-voom + TMM method,42 then analyzed using a statistical model that accounted for processing batch, sex, and age. Multiple hypothesis testing adjustments for schizophrenia vs control comparison were done using the false discovery rate method.43 Multidimensional scaling was performed using the limma R package44 (v 3.42.2) using the 5000 most variable genes between each pair of samples after correcting for batch, sex, and age. The method of Buja and Eyuboglu45 implemented in the sva R package (v 3.34.0) indicated that six latent variables could be used to model unexplained variation in gene expression. These six variables were then estimated using the removal of unwanted variation (RUV) procedure implemented in the RUVNormalize R package46,47 (v 1.20.1) using 87 genes with P > .5 for a disease state, batch, sex, and age as negative controls.
The pipeline of our analysis is illustrated in figure 1A. Data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus48 and are accessible through GEO Series accession number GSE165604 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165604).
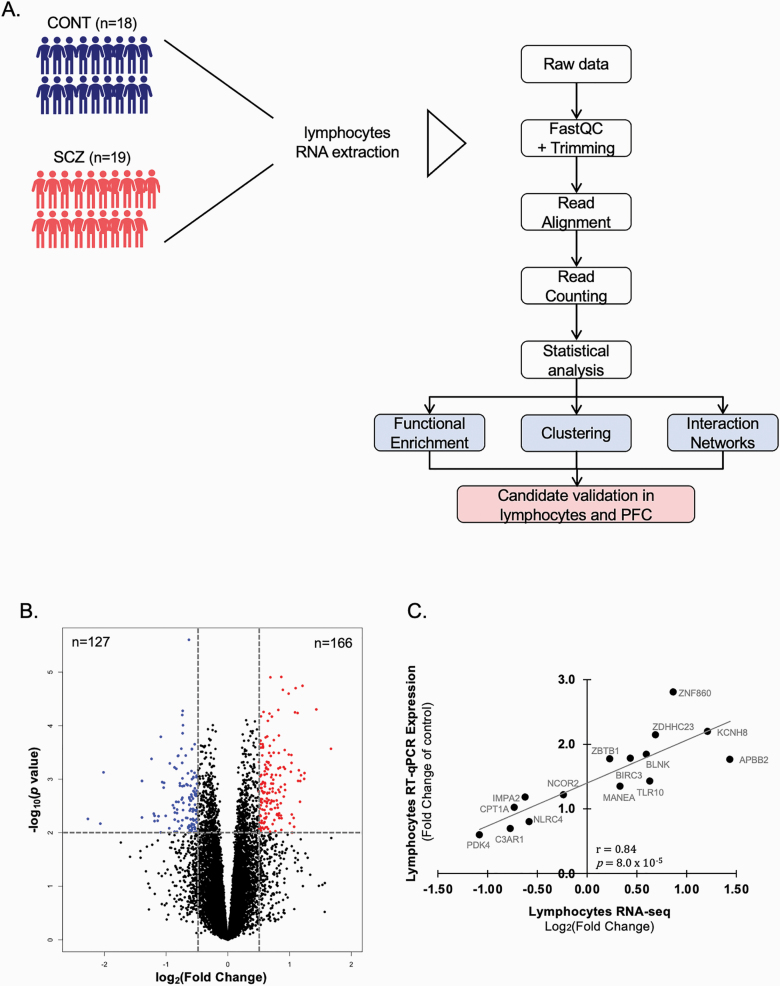
Fig. 1.
Differentially expressed transcripts in lymphocytes of schizophrenia subjects. (A) Experimental and analytical procedures flowchart. RNAs were extracted from the lymphocytes of 18 control and 19 individuals with schizophrenia. Raw data for the RNA-seq were then processed and differentially expressed genes statistically analyzed. Bioinformatic analyses were then performed to assess a functional enrichment and gene clustering of the differentially expressed genes. Several of the most significant genes were validated in both lymphocytes and prefrontal cortex (PFC) of control and schizophrenia subjects. (B) Volcano plot of the effect size [log2(fold change)] vs log10(P-value) of 14 154 detected transcripts. Enrichment and functional analyses were conducted on 293 transcripts identified with |log2FC| > 0.5 and P-value < .01. Blue dots represent hypo-expressed transcripts, red dots correspond to hyperexpressed transcripts in the schizophrenia group. (C) Pearson’s correlation analysis of the lymphocytes transcripts validated in the same subjects by RT-q-PCR.
Gene Ontology and Network Analysis
Functional analysis of differentially expressed genes between control and schizophrenia was assessed for 293 transcripts identified with |log2 FC| > 0.5 and P-value < .01, which was equivalent to FDR < 0.139 (figure 1B). Biological processes significantly overrepresented in our dataset were identified using the Database for Annotation, Visualization and Integrated Discovery (DAVID).49 Enriched regulatory pathways were assessed using Kyoto Encyclopedia of Gene and Genomes (KEGG). Biological processes identified with FDR < 0.05 were considered significant. Top upstream regulators, canonical pathways and gene networks were analyzed by Qiagen’s Ingenuity Pathway Analysis (IPA, Qiagen, CA).50 Network analysis was used to evaluate highly connected molecules from our list in the Qiagen knowledge base, with a size constraint of 35 focus molecules per network. Both direct and indirect relationships were considered. ConsensusPathDB was used to investigate interacting network modules enriched in our dataset.51 Cell Specific Expression Analysis Tool (CSEA) was used to identify genes potentially enriched in the central nervous system (CNS) cellular subtypes.52
Reverse Transcriptase-Quantitative Polymerase Chain Reaction
mRNA expression in lymphocytes (n = 37) and BA10 (n = 20) were assessed as previously described using reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR).53 Briefly, RNA was first converted to cDNA then amplified using specific primers listed in supplementary table S1. Changes in expression were determined using the ∆∆Ct method. Three reference genes (β-actin, β-2-microglobulin [B2M], and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were used for normalization of mRNA levels. The geometric mean of the Cq value obtained for each reference gene was used for normalization. Data are presented as average fold change of controls.
Statistical Analysis
For all experiments other than those involving sequencing data, statistical differences were assessed with two-tailed Student t tests and comparisons were considered statistically significant at P < .05. When variables did not follow a normal distribution, they were transformed using log, Ln, or square root. If transformed (log, Ln, square root) variable still did not follow normal distribution, nonparametric (Mann–Whitney U, chi-square) analyses were performed where appropriate. ANCOVA was performed for adjusting covariants on the results. Correlation analyses were performed using two-tailed Pearson’s correlation analysis. All statistical tests were run using PASW v.18 software (SPSS) or GraphPad Prism version 8 (GraphPad Software, CA). A hypergeometric distribution was calculated in R and used to assess the significance of coexpression across different RNA-seq datasets. Additionally, multidimensional scaling analysis was used to address the impact of drug treatment on gene expression in the schizophrenia group. Drugs, or groups of drugs, taken by at least three subjects were considered in the analysis. Removal of unwanted variation was also considered to identify latent variables to include in the analysis.47 Using the Buja and Eyuboglu method45 implemented in the num.sv function of the sva package46 six latent variables were identified and included in the model.
Results
Differentially Expressed Genes in Lymphocytes of Individuals With Schizophrenia
We used RNA sequencing to profile transcriptomic changes in lymphocytes of 18 control and 19 schizophrenia subjects which revealed 2819 differentially expressed transcripts with 1378 upregulated and 1441 downregulated transcripts (Pnominal < .05). 1018 differentially expressed transcripts were observed when considering Pnominal < .01. The distribution of effect sizes (log2 FC vs log10(P-value)) is shown in the volcano plot (figure 1B). For downstream bioinformatic analyses, we selected a subset of 293 genes (Pnominal < .01 and |log2 FC| > .5) with 166 upregulated (highlighted in red) and 127 downregulated (highlighted in blue, figure 1B).
Validation of RNA-seq Findings
To confirm the findings of our RNA-seq dataset, we selected 15 of the most significantly dysregulated genes for independent validation by RT-qPCR analysis. These included nine upregulated transcripts in schizophrenia compared to controls (ie, in order of descending significance in the RNA-seq dataset, ZNF860, ZDHHC23, KCNH8, APBB2, BIRC3, MANEA, ZBTB1, BLNK, TLR10), and six downregulated transcripts (ie, IMPA2, NCOR2, PDK4, CPT1A, NLRC4, C3AR1). The expression levels detected by RT-qPCR and RNA-seq were in agreement, and measurements obtained with these two methods showed a strong correlation (r = .80, P = 8.0 × 10–5; figure 1C). The mRNA levels for all validated genes are presented in supplementary table S2.
Functional Enrichment Analysis of Differentially Expressed Genes
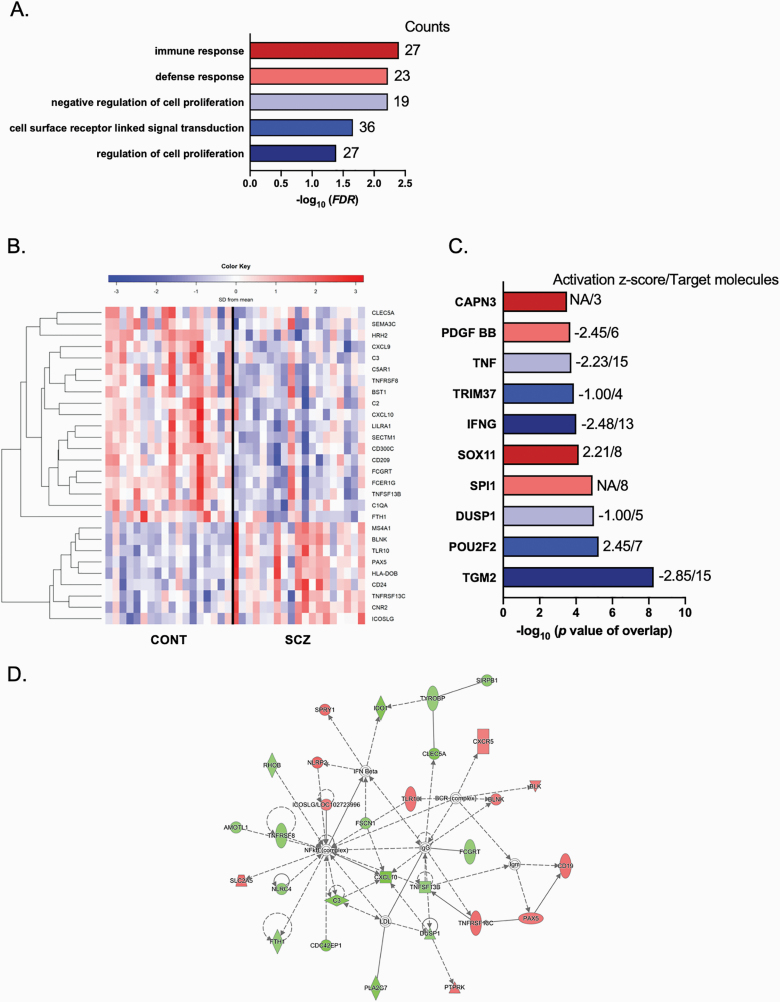
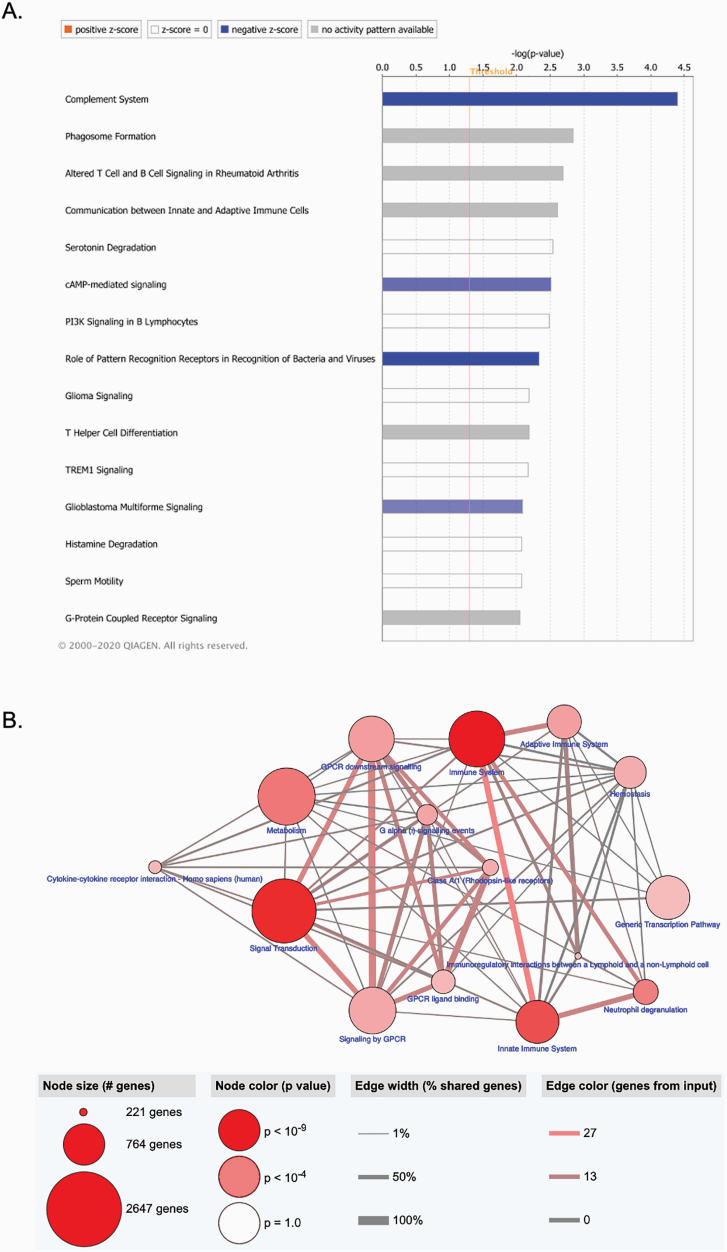
To evaluate the function of differentially expressed genes we used both gene ontology and pathway analysis tools. Gene ontology enrichment analysis using DAVID highlighted significantly enriched biological processes related to the immune system and regulation of cellular processes, including “immune response” (GO: 0006955, FDR = 0.004) and “defense response” (GO: 0006952, FDR = 0.006; figure 2A and supplementary table S3). The hierarchical clustering of “immune response” term sorted by the group showed that the expression levels for ~70% (18/27) of the genes were reduced in schizophrenia when compared to controls (figure 2B). The pathway analysis using KEGG showed enrichment in immune-related function, including “Staphylococcus aureus infection” and “Complement and coagulation cascades” (supplementary table S4). To explore upstream transcriptional regulators of these gene expression changes, we used IPA. This analysis identified transcriptional regulators, including transglutaminase 2 (TGM2), POU class 2 homeobox 2 (POU2F2), dual specificity phosphatase 1 (DUSP1), Spi-1 proto-oncogene (SPI1), SRY-box transcription factor 11 (SOX11), interferon gamma (IFNG), tripartite motif containing 37 (TRIM37), tumor necrosis factor (TNF), platelet-derived growth factor subunit B (PDGF BB), and calpain 3 (CAPN3, figure 2C, supplementary table S5). Of note, several of these transcriptional regulators play important roles in immune/inflammatory responses. We also used IPA to highlight relevant gene networks. This analysis revealed 22 gene networks (the top ten networks are shown in supplementary table S6), and the most significant of these networks were regulators/mediators of the immune response (figure 2D). Biological function related to the immune response and inflammatory processes was a recurrent finding in the IPA canonical pathway analysis (figure 3A). Several pathways involved in immune function contained genes enriched in the lymphocytes of schizophrenia subjects, eg, complement system, altered T cell and B cell signaling, communication between innate and adaptative immune cells (supplementary table S7). Furthermore, pathway analysis using ConsensusPathDB highlighted enrichment in several immune-related processes, with the “immune system” being one of the most significant processes (figure 3B).
Fig. 2.
Functional enrichment analysis of differentially expressed genes in lymphocytes of schizophrenia subjects. (A) Top biological processes of DAVID gene ontology classification for biological processes (FDR < 0.05) are indicated with the number of gene counts per category. (B) Hierarchical clustering of 27 genes across 18 control and 19 schizophrenia subjects using a colored heatmap based on expression levels of immune response transcripts observed in DAVID (blue to red: low-to-high expression). (C) Ingenuity Pathway Analysis (IPA, Qiagen, CA) top 10 upstream regulators with activation score and number of target molecules. (D) IPA interaction network for “Inflammatory response.” Lines between genes represent known interactions (solid—direct; dashed—indirect). Genes are referred to as nodes and the intensity of the node color indicates the degree of hyper- (red) or hypo- (green) expression of a given gene.
Fig. 3.
Enriched pathways of differentially expressed genes in lymphocytes of schizophrenia subjects. (A) Significant Canonical Ingenuity Pathway Analysis (IPA, Qiagen, CA) pathways enriched from our dataset are displayed along the x-axis. The y-axis displays the −log of P-value which is calculated by Fisher’s exact test right-tailed, with taller bars corresponding to increased significance. The bars show predicted pathway inhibition (blue) based on the z-score. White bars have a z-score at or very close to 0. Gray bars indicate pathways where no prediction can currently be made. (B) ConsensusDB pathway-based analysis. Nodes represent functional groups of gene sets. Their size is proportional to the number of genes enriched in our dataset. Node color is indicative of the P-value. Edge thickness is proportional to the overlap between gene sets in the enrichment map.
Interestingly, the multidimensional scale analysis of the RNA-seq dataset revealed a trend towards individuals treated with haloperidol clustering more with the controls (supplementary figure S1A), while patients on mood medications (valproic acid, lithium, trazadone, or citalopram) did not (supplementary figure S1B). However, these trends did not hold up to statistical testing, likely due to the sample size in our analysis. Similarly, subjects with a higher MATRICS overall composite score (supplementary figure S1C) or a lower PANSS (supplementary figure S1D) tended to cluster more with the control group. In addition, the first latent variable identified while removing unwanted variation was associated with valproic acid use (P = .01; supplementary figure S1E).
Differentially Expressed Genes in the Brain of Individuals With Schizophrenia
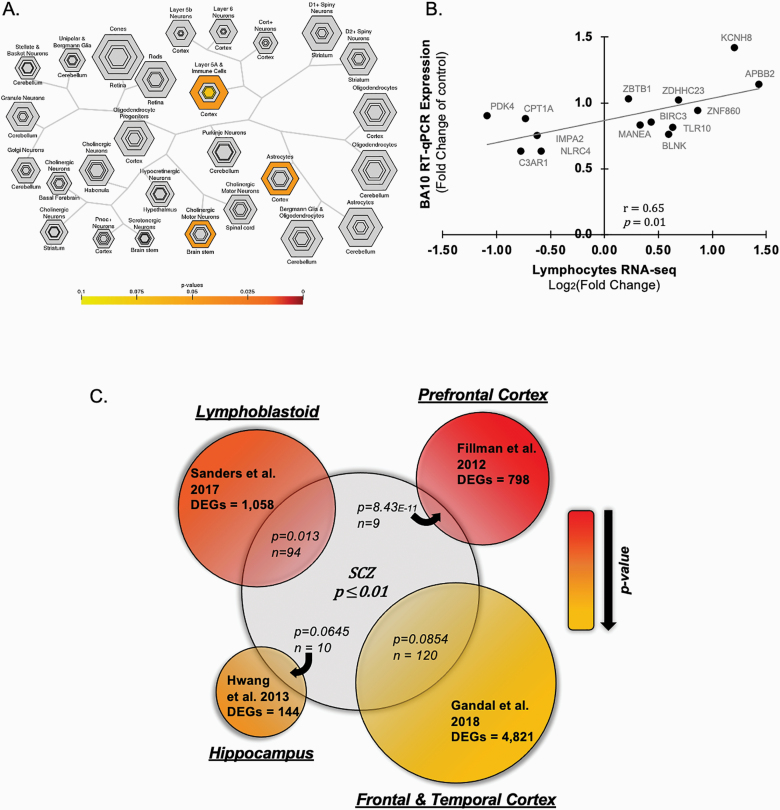
To investigate how the observed changes in lymphocytes from individuals with schizophrenia could relate to alterations observed in brain cellular subtypes, we used the CSEA tool. We detected a highly significant enrichment of the differentially expressed lymphocytes genes in the transcriptomic profile from the cortex layer 5a a immune cells, cortical astrocytes, and cholinergic motor neurons of the brain stem (figure 4A). Based on these findings, we investigated whether the changes in transcripts levels we observed in lymphocytes could also be detected in the prefrontal cortex of schizophrenia subjects. Thus, the transcripts measured for the RNA-seq validation in lymphocytes (ie, IMPA2, ZNF860, ZDHHC23, KCNH8, APBB2, BIRC3, CPT1A, MANEA, ZBTB1, BLNK, TLR10, PDK4, NLRC4, C3AR1) were also assessed in the prefrontal cortex (BA10) of a different cohort of individuals with schizophrenia (supplementary table S8). Transcript levels detected in BA10 were positively correlated with the lymphocytes mRNA levels measured by both RNA-seq (figure 4B, r = .65, P = .01) and RT-qPCR (supplementary figure S2, r = .58, P = .03).
Fig. 4.
Differentially expressed genes in the brain of individuals with schizophrenia. (A) Cell Specific Expression Analysis (CSEA) hierarchical clustering of cell types by transcript levels of our lymphocytes dataset. The size of the bullseye is scaled to the number of specific and enriched transcripts. Bullseyes are color-coded based on Fisher’s exact test P-values. (B) Pearson’s correlation analysis of the lymphocytes transcripts obtained in the RNA-seq and transcripts measure in postmortem prefrontal cortex (BA10) of schizophrenia subjects by RT-q-PCR. (C) Venn diagram showing the overlap of our dataset (lymphocytes RNAseq 1018 transcripts; Pnominal < .01) with previously published RNA-seq studies conducted in the prefrontal cortex of schizophrenia subjects,17 lymphoblastoid cell lines of European ancestry schizophrenia cohort,54 the hippocampus of schizophrenia subjects18 and in the transcriptome-wide analysis from postmortem brain samples from individuals with schizophrenia.55 The size of each circle is proportional to the number of differentially expressed genes (DEGs) detected in each study, while overlapping regions between datasets are proportional to the size of the overlap. The P-value of the enrichment analysis obtained by hypergeometric-based test and the number of common genes is indicated for each overlap.
The correlation between gene expression changes in lymphocytes and prefrontal cortex in the schizophrenia of the genes measured, suggested that there may be a more general concordance of transcriptional signatures in these tissues. We therefore used gene set analysis to assess the overlap between the RNA-seq data obtained in lymphocytes and transcriptomic datasets in the schizophrenia brain. We observed a significant overlap between the 1018 differentially expressed genes in our dataset (Pnominal < .05) and the RNA-seq analysis performed by Fillman et al17 in the prefrontal cortex of schizophrenia subjects (figure 4C). Similarly, a significant overlap was detected with differentially expressed genes detected in lymphoblastoid cell lines in the schizophrenia cohort of European ancestry.54 We also found common differentially expressed genes in the hippocampus of schizophrenia subjects18 and transcriptome-wide analysis of postmortem brain samples from individuals with schizophrenia. However, these comparisons did not pass our significance threshold (P = .0645 and P = .0854, respectively, figure 4C).55
Discussion
During the last 50 years, clinical neuroimaging and molecular studies suggest that immune dysfunction is one of the factors contributing to the pathogenesis of schizophrenia.56,57 Mounting evidence shows that inflammation has a potential role in the development and maintenance of the disease58–61 and immune and inflammatory mediators could serve as potential biomarkers for schizophrenia.1,62 One hypothesis is that immune alterations observed in the CNS could originate from changes in the peripheral immune system.63,64 Using next-generation sequencing (ie, RNA-seq) as an unbiased approach, we show that alterations in the expression patterns of immune-related genes can be observed in the lymphocytes of individuals suffering from schizophrenia, as previously reported.17,54 Here, we further show that immune/inflammatory mediators altered in peripheral blood lymphocytes are also changed in the prefrontal cortex of individuals with schizophrenia.
While an array of biological mechanisms, including neurodevelopment and epigenetic processes, have been linked with the pathophysiology of schizophrenia, the molecular underpinnings of these alterations remain to be elucidated. Increasing number of studies have attempted to unravel the association between immune dysregulations and the development of schizophrenia,65 a link that genome-wide association studies (GWAS) have also highlighted by pointing to a strong relationship between genes regulating the immune/inflammatory response and this psychiatric disorder.66 The role of infection and inflammation in schizophrenia psychopathology has been strengthened by evidence showing that prenatal infections during gestation as well as high levels of proinflammatory cytokines increase the risk of schizophrenia in the offspring.32,67 Subclinical chronic inflammation has also been observed in adults with schizophrenia.32 In addition, several transcriptomic studies highlighted the dysregulation of immune-related genes in blood samples of individuals with schizophrenia.68–70 Chronic inflammatory comorbidity across the lifespan of schizophrenia patients may predispose to a wide variety of physical diseases associated with the higher mortality rate in these patients.71,72
Altogether, these studies support the view that the immune system is an interesting axis to add to existing therapeutic strategies as well as providing an indicator of a disease state. In line with this view, Miller et al73 showed that cytokines, including IL-1β, IL-6, and TGF-β can serve as state-related markers for schizophrenia. These cytokines are increased in the blood of schizophrenia subjects during the acute phase of psychosis and are normalized with treatment. However, the levels of IL-12, IFN-γ, TNF-α, and sIL-2R are increased in schizophrenia subjects and were not corrected with treatment, suggesting that changes in these factors may serve as trait markers.73 Additionally, IFN-γ, TNF-α, IL-2, IL-10,74 and IL-675 have been identified as genetic biomarkers for schizophrenia. Of note, several of these immune-related markers, eg, cytokines such as IFN-γ, TNF-α were identified as upstream regulators in the IPA analysis of our RNA-seq data, suggesting that changes in these immune-related factors could explain the observed gene expression changes detected in our dataset.
Similar to what we observed in our lymphocytes dataset, studies conducted in postmortem brains of long-term treated schizophrenia patients have repeatedly highlighted alterations in immune-related markers15,17–19 showing that current treatment approaches are not sufficient to correct immune manifestations of the disease. Immune dysregulation has also been observed in proteomic studies of cerebrospinal fluid, where increase release of S100B indicates activation of astrocytes in schizophrenia patients.76,77 Interestingly, using CSEA, we were able to show significant enrichment of the differentially expressed genes observed in lymphocytes in the expression profile of cortical astrocytes. Moreover, we also showed a highly significant correlation for the mRNA expression of differentially expressed genes in prefrontal cortex and lymphocytes. Despite our promising observation, one limitation of our study is due to the small number of subjects by current standards. Additional studies are needed to establish a strong correlation in a larger cohort. However, these findings are supported by previous studies that have reported similarities between blood and CNS samples,78 suggesting that peripheral marks can be used as accessible surrogates to investigate putative CNS disruption. Important overlap has also been observed between our data set and transcriptome-wide analyses performed on lymphoblastoid cell lines54 and postmortem brain areas from individuals with schizophrenia, including the prefrontal cortex17,55 and the hippocampus.18
It is commonly thought that antipsychotic treatments reduce the levels of inflammatory cytokines.20,22 However, the effects of antipsychotic medication on inflammatory cytokines are not sufficient to correct the multifaceted symptoms of schizophrenia. The cohort used in our study did not allow us to assay any significant effects of differential antipsychotic drug treatments, because of the low number of subjects in each drug treatment category. Since all subjects with schizophrenia were treated with antipsychotic medication, no data on pre vs. post phases of antipsychotic treatment were available to enable the effects of drug-treatment in our RNA-seq analysis. Nonetheless, treatment with haloperidol and valproic acid seemed to induce gene expression profiles that were more similar to the control group (supplementary figure S1), but the underlying explanation for this trend is unclear. It is important to note here that two of the five subjects treated with haloperidol also received olanzapine or clozapine. Further investigation is needed to determine whether the inflammation/immune alterations observed in schizophrenia are a pathophysiological feature of the disorder itself, which would likely be present also in prodromal stage and/or early first-episode patients, or a secondary effect which becomes prominent only during the chronic developmental course of the illness. The multidimensional scaling analysis suggesting that patients with lesser severity of cognitive and symptomatic abnormalities may be more similar to controls also suggests that the current severity of illness may also be a factor. Additional studies of RNA-seq in lymphocytes of subjects in the prodromal or first-episode stage of the illness would help answer this question. Interestingly, several anti-inflammatory agents have been tested for their efficacy in alleviating schizophrenia symptoms.79,80 A recent meta-analytic investigation by Cho and colleagues79 shows that some drugs with anti-inflammatory properties can reduce psychiatric symptoms measured with PANSS when they are used as an adjunctive treatment. Similar results were found in a meta-analysis by Çakici et al81 indicating beneficial effects of anti-inflammatory agents on symptom severity in schizophrenia subjects.
Overall, our study highlights significant associations between transcriptomic changes in lymphocytes for genes involved in the immune/inflammatory system and schizophrenia. Several of the alterations we observed in the periphery were confirmed in postmortem prefrontal cortex samples from the second cohort of schizophrenia subjects, suggesting that those genes may contribute to the pathophysiology of the disease. This work provides further evidence for the contribution of the immune system in schizophrenia and supports the concept that alterations in inflammatory gene expression observed in the periphery may serve as putative biomarkers for schizophrenia.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the patients from the NKI for agreeing to participate in our study. Moreover, we are grateful the NIH NeuroBioBank for kindly providing the postmortem brain samples. RNA-sequencing was performed by Dr Gloria Rendon at the Roy J. Carver Biotechnology Center at the University of Illinois, Urbana-Champaign. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the National Institute of Mental Health (NIMH) grant R01MH101043 (to A.G.) and by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant P50AA022538 (Center for Alcohol Research in Epigenetics component 4) (to A.G.).
References
- 1. Rodrigues-Amorim D, Rivera-Baltanás T, López M, Spuch C, Olivares JM, Agís-Balboa RC. Schizophrenia: a review of potential biomarkers. J Psychiatr Res. 2017;93:37–49. [DOI] [PubMed] [Google Scholar]
- 2. Jablensky A Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Psychiatry Clin Neurosci. 2000;250(6):274–285. [DOI] [PubMed] [Google Scholar]
- 3. Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18(12):727–740. [DOI] [PubMed] [Google Scholar]
- 4. European Network of National Networks studying Gene-Environment Interactions in Schizophrenia (EU-GEI), van Os J, Rutten BP, et al. Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40(4):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auta J, Smith RC, Dong E, et al. DNA-methylation gene network dysregulation in peripheral blood lymphocytes of schizophrenia patients. Schizophr Res. 2013;150(1):312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38(1):138–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guidotti A, Auta J, Davis JM, et al. Toward the identification of peripheral epigenetic biomarkers of schizophrenia. J Neurogenet. 2014;28(1-2):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javidfar B, Park R, Kassim BS, Bicks LK, Akbarian S. The epigenomics of schizophrenia, in the mouse. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirnics K, Middleton FA, Lewis DA, Levitt P. Delineating novel signature patterns of altered gene expression in schizophrenia using gene microarrays. ScientificWorldJournal. 2001;1:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oh G, Petronis A. Environmental studies of schizophrenia through the prism of epigenetics. Schizophr Bull. 2008;34(6):1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akbarian S Special volume: the genomics and epigenomics of schizophrenia. Schizophr Res. 2020;217:1–3. [DOI] [PubMed] [Google Scholar]
- 12. Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8(11):303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485–515. [DOI] [PubMed] [Google Scholar]
- 14. Iwamoto K, Kato T. Gene expression profiling in schizophrenia and related mental disorders. Neuroscientist. 2006;12(4):349–361. [DOI] [PubMed] [Google Scholar]
- 15. Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62(7):711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang X, Liu Y, Hahn CG, Gur RE, Sleiman PMA, Hakonarson H. RNA-seq analysis of amygdala tissue reveals characteristic expression profiles in schizophrenia. Transl Psychiatry. 2017;7(8):e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. [DOI] [PubMed] [Google Scholar]
- 18. Hwang Y, Kim J, Shin JY, et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry. 2013;3:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erbağci AB, Herken H, Köylüoglu O, Yilmaz N, Tarakçioglu M. Serum IL-1beta, sIL-2R, IL-6, IL-8 and TNF-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators Inflamm. 2001;10(3):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganguli R, Yang Z, Shurin G, et al. Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Res. 1994;51(1):1–10. [DOI] [PubMed] [Google Scholar]
- 22. Theodoropoulou S, Spanakos G, Baxevanis CN, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47(1):13–25. [DOI] [PubMed] [Google Scholar]
- 23. Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry—novel perspectives on brain disorders. Nat Rev Neurol. 2019;15(6):317–328. [DOI] [PubMed] [Google Scholar]
- 24. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stefansson H, Ophoff RA, Steinberg S, et al. ; Genetic Risk and Outcome in Psychosis (GROUP) Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu M, He L. Convergent evidence shows a positive association of interleukin-1 gene complex locus with susceptibility to schizophrenia in the Caucasian population. Schizophr Res. 2010;120(1-3):131–142. [DOI] [PubMed] [Google Scholar]
- 28. Coelewij L, Curtis D. Mini-review: update on the genetics of schizophrenia. Ann Hum Genet. 2018;82(5):239–243. [DOI] [PubMed] [Google Scholar]
- 29. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Labouesse MA, Dong E, Grayson DR, Guidotti A, Meyer U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics. 2015;10(12):1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cattane N, Richetto J, Cattaneo A. Prenatal exposure to environmental insults and enhanced risk of developing Schizophrenia and Autism Spectrum Disorder: focus on biological pathways and epigenetic mechanisms. Neurosci Biobehav Rev. 2018;117:253–278. doi: 10.1016/j.neubiorev.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 32. Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert Rev Neurother. 2007;7(7):789–796. [DOI] [PubMed] [Google Scholar]
- 33. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub; 2013. [Google Scholar]
- 36. Satta R, Maloku E, Zhubi A, et al. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci USA. 2008;105(42):16356–16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Opler MGA, Yavorsky C, Daniel DG. Positive and Negative Syndrome Scale (PANSS) Training: challenges, solutions, and future directions. Innov Clin Neurosci. 2017;14(11-12):77–81. [PMC free article] [PubMed] [Google Scholar]
- 38. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 39. Huber W, Carey VJ, Gentleman R, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12(2):115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 44. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buja A, Eyuboglu N. Remarks on parallel analysis. Multivariate Behav Res. 1992;27(4):509–540. [DOI] [PubMed] [Google Scholar]
- 46. Leek JT, Storey JD. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet. 2007;3(9):1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacob L, Gagnon-Bartsch JA, Speed TP. Correcting gene expression data when neither the unwanted variation nor the factor of interest are observed. Biostatistics. 2016;17(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. [DOI] [PubMed] [Google Scholar]
- 50. Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30(4):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herwig R, Hardt C, Lienhard M, Kamburov A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat Protoc. 2016;11(10):1889–1907. [DOI] [PubMed] [Google Scholar]
- 52. Xu X, Wells AB, O’Brien DR, Nehorai A, Dougherty JD. Cell type-specific expression analysis to identify putative cellular mechanisms for neurogenetic disorders. J Neurosci. 2014;34(4):1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gatta E, Grayson DR, Auta J, et al. Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1). Mol Psychiatry. Published online June 25, 2019. doi: 10.1038/s41380-019-0449-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanders AR, Drigalenko EI, Duan J, et al. Transcriptome sequencing study implicates immune-related genes differentially expressed in schizophrenia: new data and a meta-analysis. Transl Psychiatry. 2017;7(4):e1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gandal MJ, Zhang P, Hadjimichael E, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420):eaat8127. doi: 10.1126/science.aat8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heath RG, Krupp IM, Byers LW, Lijekvist JI. Schizophrenia as an immunologic disorder. 3. Effects of antimonkey and antihuman brain antibody on brain function. Arch Gen Psychiatry. 1967;16(1):24–33. [DOI] [PubMed] [Google Scholar]
- 57. Benros ME, Mortensen PB. Role of infection, autoimmunity, atopic disorders, and the immune system in schizophrenia: evidence from epidemiological and genetic studies. Curr Top Behav Neurosci. 2020;44:141–159. [DOI] [PubMed] [Google Scholar]
- 58. Fond G, Lançon C, Korchia T, Auquier P, Boyer L. The role of inflammation in the treatment of schizophrenia. Front Psychiatry. 2020;11:160. doi: 10.3389/fpsyt.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Müller N Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44(5):973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zakharyan R, Boyajyan A. Inflammatory cytokine network in schizophrenia. World J Biol Psychiatry. 2014;15(3):174–187. [DOI] [PubMed] [Google Scholar]
- 62. Noto CS, Gadelha A, Belangero SI, et al. Association of biomarkers and depressive symptoms in schizophrenia. Neurosci Lett. 2011;505(3):282–285. [DOI] [PubMed] [Google Scholar]
- 63. Kirch DG, Alexander RC, Suddath RL, et al. Blood-CSF barrier permeability and central nervous system immunoglobulin G in schizophrenia. J Neural Transm Gen Sect. 1992;89(3):219–232. [DOI] [PubMed] [Google Scholar]
- 64. Kirch DG, Kaufmann CA, Papadopoulos NM, Martin B, Weinberger DR. Abnormal cerebrospinal fluid protein indices in schizophrenia. Biol Psychiatry. 1985;20(10):1039–1046. [DOI] [PubMed] [Google Scholar]
- 65. Tomasik J, Rahmoune H, Guest PC, Bahn S. Neuroimmune biomarkers in schizophrenia. Schizophr Res. 2016;176(1):3–13. [DOI] [PubMed] [Google Scholar]
- 66. Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry. 2014;75(4):276–283. [DOI] [PubMed] [Google Scholar]
- 67. Brown AS, Hooton J, Schaefer CA, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161(5):889–895. [DOI] [PubMed] [Google Scholar]
- 68. Gardiner EJ, Cairns MJ, Liu B, et al. Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J Psychiatr Res. 2013;47(4):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sainz J, Mata I, Barrera J, et al. Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry. 2013;18(10):1056–1057. [DOI] [PubMed] [Google Scholar]
- 70. Xu J, Sun J, Chen J, et al. RNA-Seq analysis implicates dysregulation of the immune system in schizophrenia. BMC Genomics. 2012;13 Suppl 8:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brink M, Green A, Bojesen AB, Lamberti JS, Conwell Y, Andersen K. Excess medical comorbidity and mortality across the lifespan in schizophrenia.: a nationwide Danish register study. Schizophr Res. 2019;206:347–354. [DOI] [PubMed] [Google Scholar]
- 72. Laursen TM Causes of premature mortality in schizophrenia: a review of literature published in 2018. Curr Opin Psychiatry. 2019;32(5):388–393. [DOI] [PubMed] [Google Scholar]
- 73. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Freudenreich O, Brockman MA, Henderson DC, et al. Analysis of peripheral immune activation in schizophrenia using quantitative reverse-transcription polymerase chain reaction (RT-PCR). Psychiatry Res. 2010;176(2–3):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maekawa M, Yamada K, Toyoshima M, et al. Utility of scalp hair follicles as a novel source of biomarker genes for psychiatric illnesses. Biol Psychiatry. 2015;78(2):116–125. [DOI] [PubMed] [Google Scholar]
- 76. Rothermundt M, Ponath G, Glaser T, Hetzel G, Arolt V. S100B serum levels and long-term improvement of negative symptoms in patients with schizophrenia. Neuropsychopharmacology. 2004;29(5):1004–1011. [DOI] [PubMed] [Google Scholar]
- 77. Steiner J, Bielau H, Bernstein HG, Bogerts B, Wunderlich MT. Increased cerebrospinal fluid and serum levels of S100B in first-onset schizophrenia are not related to a degenerative release of glial fibrillar acidic protein, myelin basic protein and neurone-specific enolase from glia or neurones. J Neurol Neurosurg Psychiatry. 2006;77(11):1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2006;141B(3):261–268. [DOI] [PubMed] [Google Scholar]
- 79. Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry. 2019;53(8):742–759. [DOI] [PubMed] [Google Scholar]
- 80. Kelly WRK, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Deanna L. A review of anti-inflammatory agents for symptoms of schizophrenia—William R Keller, Lionel M Kum, Heidi J Wehring, Maju Mathew Koola, Robert W Buchanan, Deanna L Kelly, 2013. J Psychopharmacol (Oxf). Published online November 13, 2012. Accessed June 17, 2020 http://journals.sagepub.com/doi/10.1177/0269881112467089?icid=int.sj-full-text.similar-articles.2 [DOI] [PMC free article] [PubMed]
- 81. Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019;49(14):2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.