Significance
Reports suggest that at least 25% of women suffering from reproductive disorders are due to hypothalamic dysfunction. Pulsatile gonadotropin-releasing hormone (GnRH) regimens are used for infertile women. Thus, elucidation of the GnRH/gonadotropin pulse generator is warranted to improve therapeutic approaches for these disorders. Normal pulsatile gonadotropin release and folliculogenesis were rescued by transfecting the kisspeptin gene (Kiss1) into the hypothalamic neurokinin B (NKB) neurons in global Kiss1 knockout infertile female rats but not by transfecting outside the NKB neurons. Our results demonstrate that kisspeptin/NKB/dynorphin A (KNDy) neurons serve as the GnRH pulse generator, and >20% KNDy neurons are enough to maintain GnRH/gonadotropin pulses and folliculogenesis in female rats. The findings provide a potential therapeutic aspect for hypothalamic reproductive disorders.
Keywords: conditional Kiss1 KO, KNDy rescue, kisspeptin, LH pulses, neurokinin B
Abstract
The gonadotropin-releasing hormone (GnRH) pulse is fundamental for mammalian reproduction: GnRH pulse regimens are needed as therapies for infertile women as continuous GnRH treatment paradoxically inhibits gonadotropin release. Circumstantial evidence suggests that the hypothalamic arcuate KNDy neurons expressing kisspeptin (encoded by Kiss1), neurokinin B (encoded by Tac3), and dynorphin A serve as a GnRH pulse generator; however, no direct evidence is currently available. Here, we show that rescuing >20% KNDy neurons by transfecting Kiss1 inside arcuate Tac3 neurons, but not outside of these neurons, recovered folliculogenesis and luteinizing hormone (LH) pulses, an indicator of GnRH pulses, in female global Kiss1 knockout (KO) rats and that >90% conditional arcuate Kiss1 KO in newly generated Kiss1-floxed rats completely suppressed LH pulses. These results first provide direct evidence that KNDy neurons are the GnRH pulse generator, and at least 20% of KNDy neurons are sufficient to maintain folliculogenesis via generating GnRH/gonadotropin pulses.
Reproductive function is precisely orchestrated by hypothalamic neuropeptides—kisspeptin (encoded by the Kiss1 gene) and gonadotropin-releasing hormone (GnRH)—, pituitary gonadotropins, and peripheral sex hormones. These work together in order to ensure follicular development and ovulation in females and spermatogenesis in males, thus leading to offspring in mammals, including humans and domestic animals (1–6). Crucially, the brain mechanism responsible for the generation of GnRH pulses, which governs gonadotropin pulses and consequent follicular development/spermatogenesis (7, 8), is fundamentally important. This is because GnRH is able to stimulate gonadotropin release only when GnRH is secreted in a pulsatile manner at a physiological frequency. Indeed, chronic administration of GnRH paradoxically suppresses gonadotropin and consequently sex hormone release (9, 10), thus, chronic GnRH treatment is therapeutically used to inhibit sex steroid secretion in patients suffering from endometriosis as well as prostate cancer (10). Furthermore, at least 25% of women suffering from reproductive disorders are due to anovulation, which is likely due to a central disfunction regarding pulsatile GnRH release (11–13). Thus, patients could potentially take pulsatile GnRH administration by an attached pump as a medical treatment to enhance follicular development and recover ovulation (14). Furthermore, social, physical, or nutritional stress often suppresses gonadal function via suppression of GnRH/luteinizing hormone (LH) pulses in primates, ruminants, and rodents (15–19). This wealth of evidence indicates the importance of the brain mechanism in generating GnRH pulses at a physiological frequency. However, the mechanism generating GnRH/LH pulses has not been elucidated in detail, and clarifying the central mechanism could lead to new therapeutic aspects for reproductive disorders in humans as well as domestic animals.
Circumstantial evidence accumulated in the last 15 y suggests that the caudal hypothalamic population of kisspeptin neurons, located in the arcuate nucleus (ARC), play a key role in controlling pulsatile GnRH release in female mammals, including primates (20), ruminants (21–23), and rodents (24–26). Specifically, ARC kisspeptin neurons are also referred to as KNDy neurons because the ARC kisspeptin neurons express neurokinin B (NKB, encoded by the Tac3 gene) and dynorphin A (Dyn, encoded by the Pdyn gene) (22, 27, 28). Importantly, almost complete colocalization of kisspeptin- and NKB-immunoreactive cell bodies (97%) was reported in female rats (29). In addition, rhythmic increases in the multiple unit activity (MUA volley), which are detected from the electrodes placed in close proximity to ARC KNDy neurons, synchronized with LH pulses in goats (21, 30). The frequency of this MUA volley was increased and decreased by a central administration of NKB and Dyn, respectively (22). Taken together, these results suggest that KNDy neurons could be an intrinsic GnRH pulse generator, and this pulse generation is controlled by NKB and Dyn in an autocrine/paracrine manner. However, no direct evidence proving the role of KNDy neurons as the GnRH pulse generator has been provided yet because previous studies showed that gene-modified mice bearing low levels of Kiss1 expression in the ARC (and also in the anteroventral periventricular nucleus [AVPV]) still exhibited puberty, estrous cyclicity, and fertility (31, 32). Therefore, from a clinical point of view, it is noteworthy to clarify the threshold of the number of KNDy neurons to maintain reproductive function via generating GnRH pulses in mammals. Although LH pulses completely disappeared in global Kiss1 knockout (KO) rats (24), the role of the ARC KNDy neurons in GnRH pulse generation could not be exactly distinguished from that of the other populations of kisspeptin neurons located in the AVPV/preoptic area (POA) or medial amygdala in the brain (33–38). Thus, it needs to be elucidated if the rescue of KNDy neurons by transfecting the Kiss1 gene into the ARC NKB neurons, or solely Kiss1 rescue regardless of ARC NKB neurons, could recover LH pulses and folliculogenesis to clarify the role of KNDy neurons (but not solely Kiss1-expressing cells) as the GnRH pulse generator.
The present study aims to investigate whether the ARC KNDy neurons serve as a GnRH pulse generator and, if so, what percentage/threshold of KNDy neurons are required to maintain folliculogenesis and GnRH/LH pulses. To this end, we rescued KNDy neurons by transfecting CAG-promoter–driven Kiss1 complementary DNA (cDNA) targeted into the ARC Tac3-expressing neurons in global Kiss1 KO female rats, whose blood gonadotropin levels are undetectable (24), to prove that KNDy neurons are served as the GnRH pulse generator and to determine the threshold of the rate of KNDy neurons to maintain GnRH/LH pulse generation and follicular development. Further, we engineered a conditional ARC Kiss1 KO in newly generated Kiss1-floxed female rats to confirm the notion obtained by KNDy neuron-rescue experiment. Both the KNDy neuron-rescued global Kiss1 KO rats and the conditional ARC Kiss1 KO rats were subjected for analyses of ARC Kiss1 expression, pulsatile LH release, pituitary gonadotropin content, and gene expression of Lhb, Fshb, and Gnrhr, which encode LH and follicle-stimulating hormone (FSH) β-subunits and the GnRH receptor, respectively, in the pituitary. Vaginal opening and vaginal smears were monitored, and ovaries were subjected to analysis for gene expression of the steroidogenesis marker and gonadotropin receptors and to histological analysis to determine if the rescue of KNDy neurons could recover ovarian function and follicular development in global Kiss1 KO rats. In addition, the estrogen-induced LH surge was analyzed to ascertain whether the GnRH surge generating mechanism remains in the conditional ARC Kiss1 KO rats.
Results
Rescue of >20% KNDy Neurons Recovered Pulsatile LH Release in Female Global Kiss1 KO Rats.
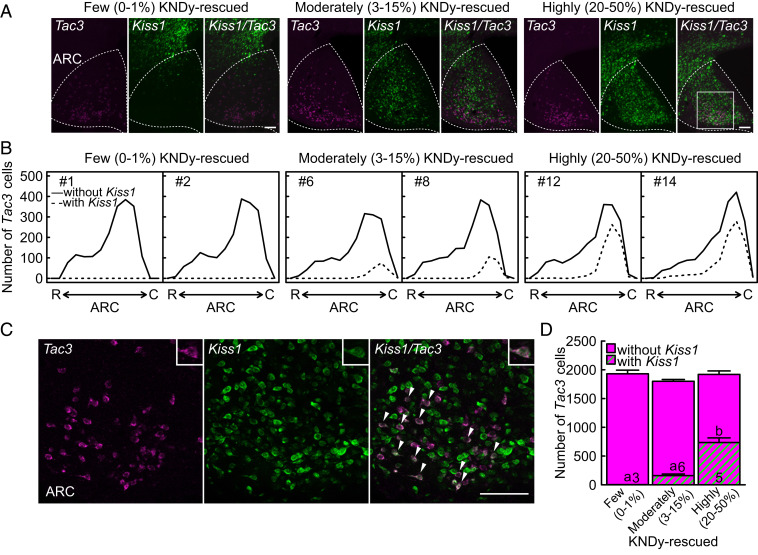
Global Kiss1 KO female rats (n = 14) were transfected with Kiss1 cDNA utilizing an adeno-associated virus (AAV) vector carrying CAG-promoter–driven Kiss1 (AAV-Kiss1) into the ARC to rescue KNDy neurons. A large number of Kiss1-expressing cells were found both inside and outside of the ARC in the mediobasal hypothalamus of ovariectomized (OVX) global Kiss1 KO rats infected with AAV-Kiss1 (Fig. 1A). Tac3-positive cells coexpressing Kiss1 were mainly found in the caudal part of ARC of global Kiss1 KO rats infected with AAV-Kiss1 (Fig. 1 B and C). According to the rescue rates of KNDy neurons, that is the Kiss1- and Tac3-coexpressing rate out of the total ARC Tac3-expressing cells, the AAV-Kiss1–treated rats were divided into three groups: 20 to 50% KNDy neuron-rescued (highly KNDy-rescued, n = 5), 3 to 15% KNDy neuron-rescued (moderately KNDy-rescued, n = 6), and 0 to 1% KNDy neuron-rescued (few KNDy-rescued, n = 3) global Kiss1 KO rats (see SI Appendix, Fig. S1 for the rescue rates of KNDy neurons in each individual). The number of ARC Tac3-positive cells was comparable between groups, whereas the number of Kiss1- and Tac3-coexpressing cells was significantly higher in the highly KNDy-rescued rats than those in the moderately and few KNDy-rescued rats (Fig. 1D).
Fig. 1.
Rescue of KNDy neurons in female global Kiss1 KO rats. (A) Kiss1 (green)- and Tac3 (magenta)-expressing cells in the mediobasal hypothalamus of three representative OVX global Kiss1 KO rats infected with AAV-Kiss1 targeted into the ARC (indicated by the dotted line). Note that a large number of Kiss1-expressing cells were found inside of the ARC of highly and moderately KNDy-rescued global Kiss1 KO rats, whereas Kiss1-expressing cells were mainly found outside of the ARC of few KNDy-rescued rats. (B) Distribution of ARC Tac3-expressing cells with (dashed lines) or without (solid lines) Kiss1 expression throughout the ARC of two representative few, moderately, and highly KNDy-rescued rats. R, the rostral part of ARC and C, the caudal part of ARC. (C) Higher magnification of the squared area in panel A showing Kiss1- and Tac3-coexpressing cells (arrowheads). Insets show a representative Kiss1- and Tac3-coexpressing cell. (D) The number of Tac3-expressing cells with or without Kiss1 expression in the ARC of few, moderately, and highly KNDy-rescued rats. Values expressed in the bar graph are mean ± SEM. Numbers in each column indicate the number of animals used. The values with different letters were significantly different from each other (P < 0.05) based on one-way ANOVA followed by Tukey's HSD test. (Scale bars, 100 μm.)
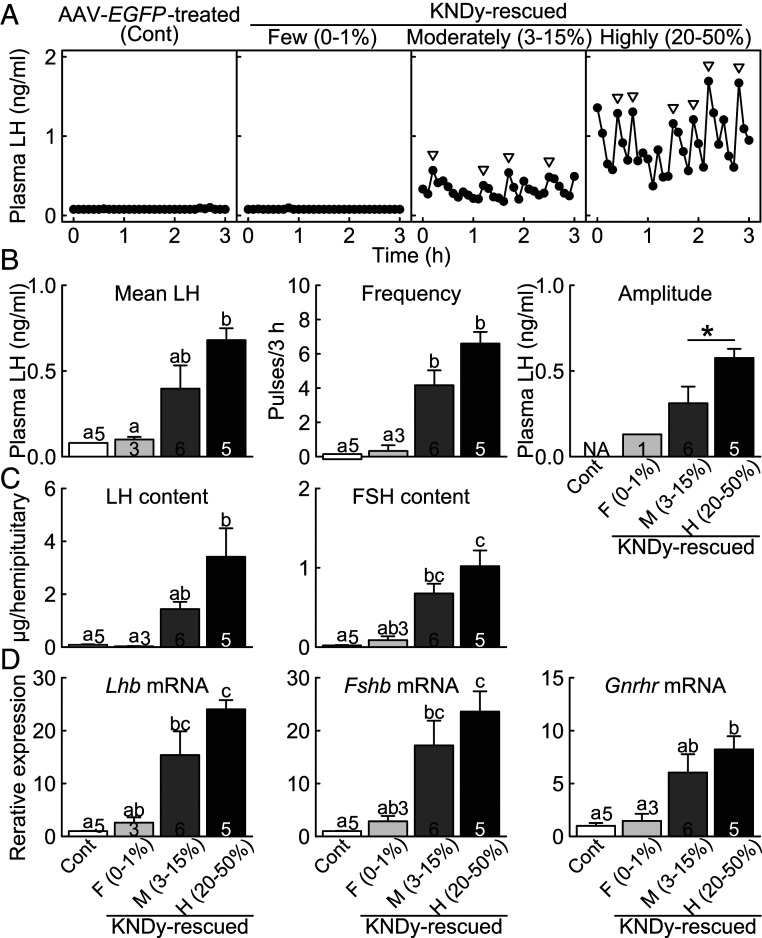
Pulsatile LH release, an indicator of GnRH pulses, was apparently recovered in the highly KNDy-rescued rats and was modestly recovered in the moderately KNDy-rescued rats in the OVX condition (Fig. 2A). On the other hand, the few KNDy-rescued rats and AAV-enhanced green fluorescent protein (EGFP)-treated global Kiss1 KO control rats (n = 5) showed a complete lack of LH pulses in the OVX condition. Importantly, even the few KNDy-rescued rats showed a number of solely Kiss1-expressing cells outside of the ARC Tac3-expressing cells but not inside of the ARC Tac3-expressing cells (Fig. 1 A and B), indicating that the rescue of KNDy neurons, but not Kiss1 transfection solely, could recover the pulsatile LH release in global Kiss1 KO rats. The mean LH concentration was significantly higher in the highly KNDy-rescued rats than those in the AAV-EGFP–treated global Kiss1 KO control and few KNDy-rescued rats (Fig. 2B). The frequency of LH pulses was significantly higher in both the highly and moderately KNDy-rescued rats than those in the AAV-EGFP–treated control and few KNDy-rescued rats. The amplitude of LH pulses was significantly higher in the highly KNDy-rescued rats than that in the moderately KNDy-rescued rats. The profile of LH pulses in the highly KNDy-rescued rats was similar to that in wild-type rats and AAV-EGFP–treated Kiss1-floxed (KNDy intact) rats as shown in SI Appendix, Fig. S2.
Fig. 2.
Recovery of gonadotropin synthesis/release and Gnrhr mRNA expression in the KNDy neuron-rescued female rats. (A) Plasma LH profiles in representative OVX few, moderately, and highly KNDy-rescued global Kiss1 KO rats and OVX AAV-EGFP–treated global Kiss1 KO control rats (Cont). Arrowheads indicate LH pulses identified with the PULSAR computer program. Mean LH concentration and the frequency and amplitude of LH pulses (B), the pituitary LH and FSH content (C), and the pituitary Lhb, Fshb, and Gnrhr mRNA expression (D) in the few, moderately, and highly KNDy-rescued rats and control rats. Values expressed in the bar graphs are mean ± SEM. Numbers in (or on) each column indicate the number of animals used. The values with different letters were significantly different from each other (P < 0.05) based on one-way ANOVA followed by Tukey's HSD test. Asterisk indicates statistically significant differences (P < 0.05) between the highly and moderately KNDy-rescued rats based on Welch's t test. Note that LH pulses were detected in zero out of five AAV-EGFP–treated Kiss1 KO control rats and in one out of three few KNDy-rescued rats. NA, not applicable.
Rescue of >20% of KNDy Neurons Recovered Pituitary Gonadotropin Synthesis and Pituitary Gnrhr Expression in Female Global Kiss1 KO Rats.
The rescue of more than 20% of ARC KNDy neurons recovered LH and FSH content in the pituitary of the global Kiss1 KO rats (Fig. 2C). Specifically, the pituitary LH and FSH content were significantly higher in the highly KNDy-rescued rats than those in the AAV-EGFP–treated global Kiss1 KO control and few KNDy-rescued rats. Additionally, the pituitary FSH content in the moderately KNDy-rescued rats was significantly higher than that in the AAV-EGFP–treated control rats.
Rescue of ARC KNDy neurons also recovered Lhb, Fshb, and Gnrhr messenger RNA (mRNA) expression in the pituitary of global Kiss1 KO rats (Fig. 2D). Specifically, the pituitary Lhb and Fshb mRNA expression were significantly higher in the highly KNDy-rescued rats than those in the AAV-EGFP–treated control and few KNDy-rescued rats. In addition, the pituitary Lhb and Fshb mRNA expression in the moderately KNDy-rescued rats were significantly higher than those in the AAV-EGFP–treated control rats. Pituitary Gnrhr mRNA expression was significantly higher in the highly KNDy-rescued rats than those in the AAV-EGFP–treated control and few KNDy-rescued rats.
Rescue of >20% of KNDy Neurons Recovered Follicular Development and Ovarian Estrogen Synthase (Aromatase) mRNA Expression in Female Global Kiss1 KO Rats.
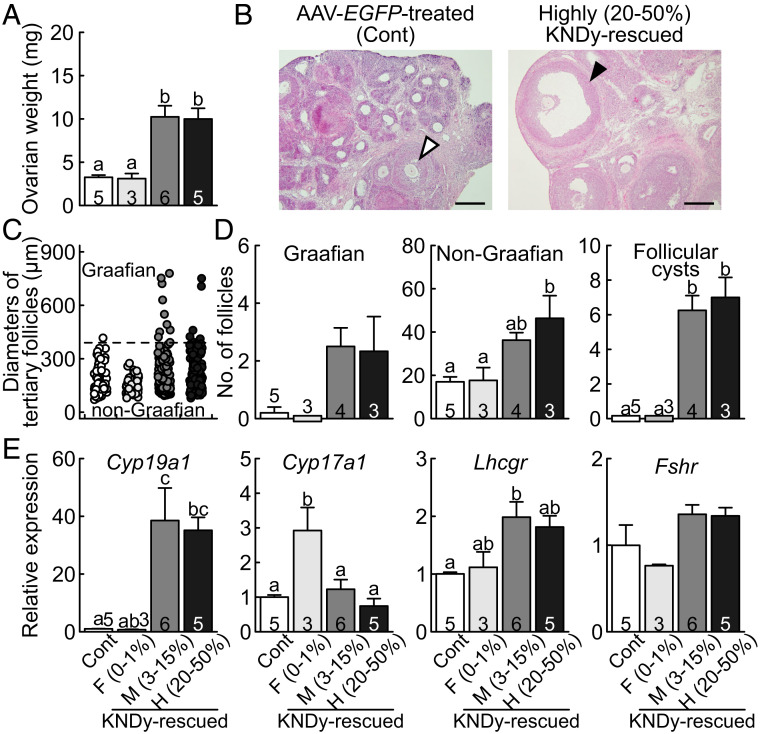
Ovarian weight was increased and follicular development up to the Graafian follicles was induced by the rescue of the KNDy neurons in the global Kiss1 KO rats (Fig. 3 A and B). Specifically, the ovarian weight was significantly higher in both the highly and moderately KNDy-rescued rats compared to those in the AAV-EGFP–treated global Kiss1 KO control and few KNDy-rescued rats. Follicular development as shown by the existence of the Graafian follicles was evident in the highly KNDy-rescued rats, but only non-Graafian tertiary follicles at most were found in the AAV-EGFP–treated control rats (Fig. 3B). Both the highly and moderately KNDy-rescued rats showed both Graafian (a diameter greater than 390 µm) and a number of non-Graafian tertiary follicles, while the AAV-EGFP–treated control and few KNDy-rescued rats showed a moderate number of non-Graafian tertiary follicles (Fig. 3C). The number of Graafian follicles tended to be higher in both the highly and moderately KNDy-rescued rats than those in the AAV-EGFP–treated control and few KNDy-rescued rats (Fig. 3D). The number of non-Graafian tertiary follicles was significantly higher in the highly KNDy-rescued rats than those in the AAV-EGFP–treated control and few KNDy-rescued rats (Fig. 3D). In addition, estrogen synthase (aromatase, coded by Cyp19a1) mRNA expression in the ovaries was significantly higher in the highly and moderately KNDy-rescued rats than the AAV-EGFP–treated control rats (Fig. 3E). The ovarian LH receptor (coded by Lhcgr) mRNA expression was significantly higher in the moderately KNDy-rescued rats and tended to be higher in the highly KNDy-rescued rats than that of AAV-EGFP–treated control rats. On the other hand, androgen synthase (encoded by Cyp17a1) and FSH receptor (coded by Fshr) mRNA expression were comparable between highly, moderately KNDy-rescued rats and AAV-EGFP–treated control rats. Cyp17a1 mRNA expression was significantly higher in few KNDy-rescued rats than other groups.
Fig. 3.
Recovery of follicular development and ovarian Cyp19a1 and Lhcgr mRNA expression in the KNDy neuron-rescued female rats. (A) Ovarian weight of the few, moderately, and highly KNDy-rescued global Kiss1 KO rats and AAV-EGFP–treated global Kiss1 KO control rats (Cont). (B) A Graafian follicle (solid arrowhead) in the ovary of a representative highly KNDy-rescued rat and a non-Graafian tertiary follicle (open arrowhead) in the ovary of a representative control rat. (Scale bars, 200 μm.) (C) The diameter in each Graafian and non-Graafian tertiary follicle in the few, moderately, and highly KNDy-rescued rats and control rats. The tertiary follicle with a diameter greater than 390 µm (horizontal dashed line) was termed as Graafian. (D) The mean numbers of Graafian and non-Graafian tertiary follicles and follicular cysts in each group. (E) The ovarian Cyp19a1, Cyp17a1, Lhcgr, and Fshr gene expression in each group. Values expressed in the bar graphs are mean ± SEM. Numbers in (or on) each column indicate the number of animals used. The values with different letters were significantly different from each other (P < 0.05) based on one-way ANOVA followed by Tukey’s HSD test.
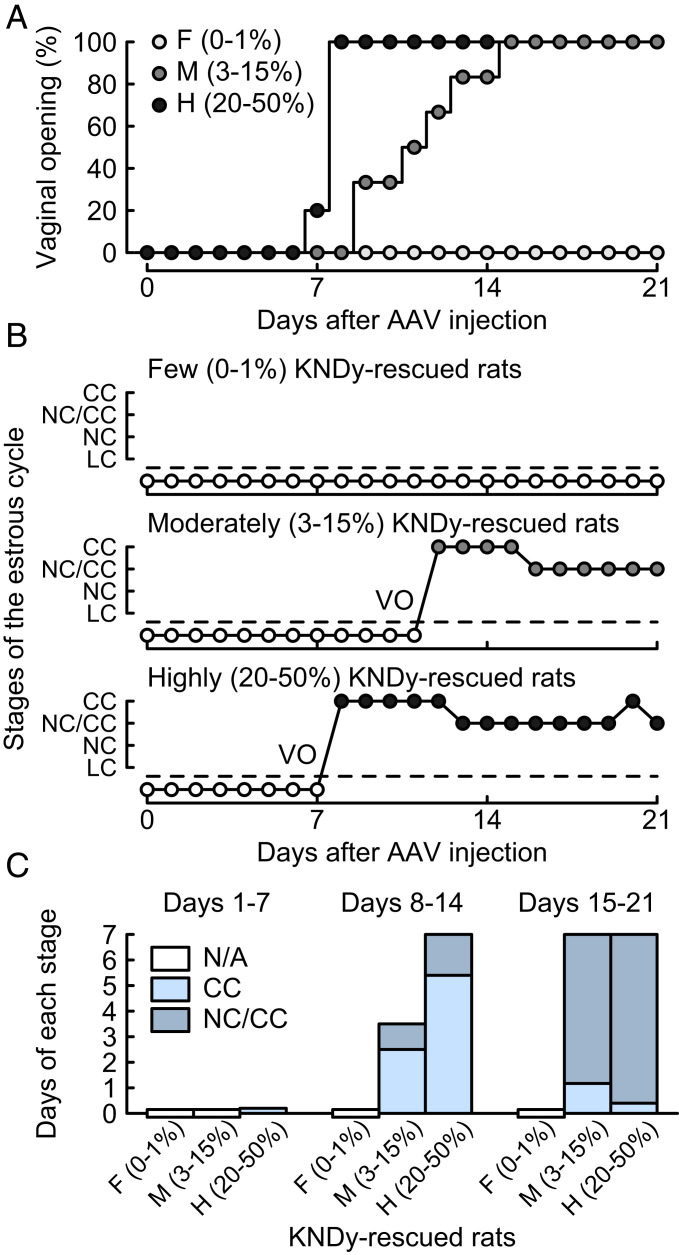
Importantly, the highly and moderately KNDy-rescued rats showed follicular cysts (follicles greater than 1.5 mm in diameter) and no corpora lutea, indicating that they failed to show an LH surge and ovulation. The number of follicular cysts was significantly higher in both the highly and moderately KNDy-rescued rats than those in the AAV-EGFP–treated control and few KNDy-rescued rats (Fig. 3D). Further, the highly and moderately KNDy-rescued rats showed vaginal opening 7 to 8 and 9 to 15 d after the AAV-Kiss1 injection, respectively (Fig. 4A), and they showed persistent estrus thereafter (Fig. 4 B and C). This suggests that their ability to synthesize ovarian estrogen was, at least partly, restored; however, they failed to show an LH surge and ovulation. There was no significant difference in the numbers of the primordial, primary, secondary, and atretic follicles between groups (SI Appendix, Fig. S3).
Fig. 4.
Recovery of vaginal opening (VO) and occurrence of persistent estrus in the KNDy neuron-rescued female rats. (A) Days at the VO (expressed as a percentage of the total number of animals per group). There were significant differences in the occurrence probability of the VO between the groups (P < 0.05, Kaplan–Meier analysis and log-rank test). (B) Vaginal smear pattern in a representative animal in each group. CC, cornified cells; NC, nucleated cells; and LC, leukocytes. (C) Days that animals showed CC and a mixture of NC and CC (NC/CC) in the first, second, and third weeks after the AAV-Kiss1 injection. Note that no animal showed a LC smear pattern. N/A, not applicable because animals failed to show VO.
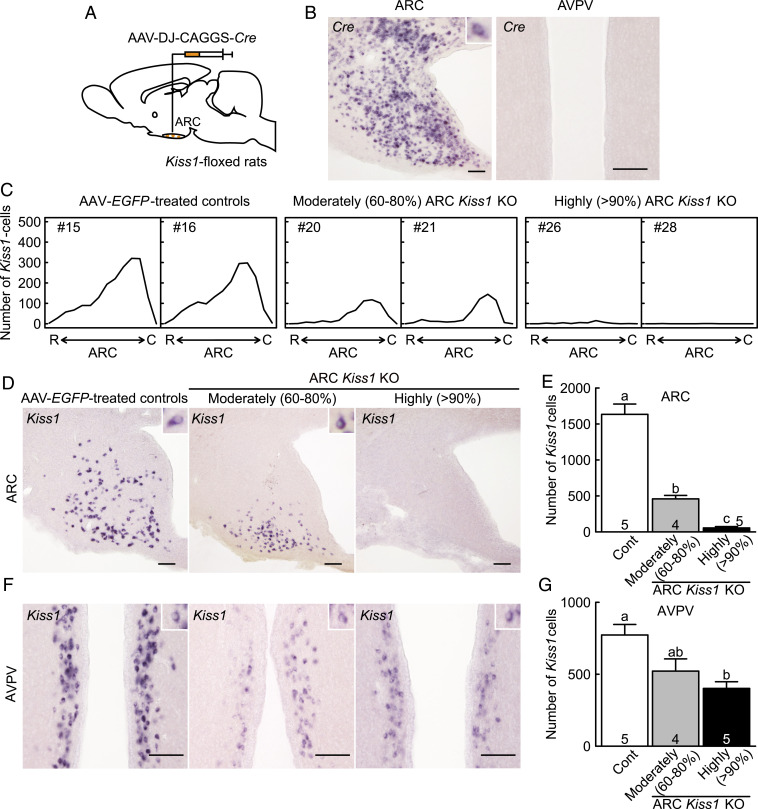
Evaluation of Conditional ARC Kiss1 KO Female Rats with the Cre-loxP System.
An AAV vector carrying CAG-promoter–driven Cre (AAV-Cre) was transfected into the ARC of Kiss1-floxed rats (n = 9) to delete Kiss1 gene in the ARC (Fig. 5A). Cre mRNA was successfully expressed in the ARC, but not in the AVPV, of the AAV-Cre–treated Kiss1-floxed female rats (Fig. 5B). Few or a moderate number of Kiss1-expressing cells were found throughout the ARC of AAV-Cre–treated Kiss1-floxed rats in the OVX + low estradiol-17β (E2) condition, whereas a large number of Kiss1-expressing cells were found throughout the ARC of AAV-EGFP–treated Kiss1-floxed control rats (n = 5, Fig. 5 C and D). According to the severity of ARC conditional Kiss1 KO, AAV-Cre–treated Kiss1-floxed rats were divided into the two groups: >90% (highly) ARC Kiss1 KO rats (n = 5), in which less than 10% Kiss1-expressing cells remained in the ARC compared to the AAV-EGFP–treated Kiss1-floxed control rats, and 60 to 80% (moderately) ARC Kiss1 KO rats (n = 4), in which 20 to 40% Kiss1-expressing cells remained in the ARC (see SI Appendix, Fig. S4 for the severity of ARC Kiss1 KO in each individual). The number of Kiss1-expressing cells was significantly lower in the ARC of both highly and moderately ARC Kiss1 KO rats than that of AAV-EGFP–treated Kiss1-floxed control rats (Fig. 5E). Besides, the number of ARC Kiss1-expressing cells was significantly lower in the highly ARC Kiss1 KO rats than that in the moderately ARC Kiss1 KO rats (Fig. 5E). Note that Kiss1-expressing cells mainly remained in the caudal part of the ARC of moderately ARC Kiss1 KO rats (Fig. 5C).
Fig. 5.
Evaluation of conditional ARC Kiss1 KO female rats. (A) Schematic illustration of the injection of AAV-Cre into the ARC. (B) Cre-expressing cells in the ARC and AVPV of a representative OVX + low E2 AAV-Cre–treated Kiss1-floxed rats. (C) Distribution of ARC Kiss1-expressing cells throughout the ARC of two representative OVX + low E2 moderately and highly ARC Kiss1 KO rats and OVX + low E2 AAV-EGFP–treated Kiss1-floxed control rats (Cont). R, the rostral part of ARC and C, the caudal part of ARC. (D) Kiss1-expressing cells in the ARC of representative moderately and highly ARC Kiss1 KO rats and control rat. Insets show representative Kiss1-expressing cells. (E) The number of Kiss1-expressing cells in the ARC. (F) Kiss1-expressing cells in the AVPV of representative animals. (G) The number of Kiss1-expressing cells in the AVPV. Values expressed in the bar graphs are mean ± SEM. Numbers in (or on) each column indicate the number of animals used. The values with different letters were significantly different from each other (P < 0.05) based on one-way ANOVA followed by Tukey's HSD test. (Scale bars, 100 μm.)
Interestingly, a moderate number of Kiss1-expressing cells with relatively weak Kiss1 signals were found in the AVPV of highly and moderately ARC Kiss1 KO rats (51.9 ± 6.0% and 67.5 ± 11.0% compared to the AAV-EGFP–treated Kiss1-floxed control rats, Fig. 5F), although no Cre mRNA expression was found in the AVPV (Fig. 5B). A large number of Kiss1-expressing cells were shown in the AVPV of AAV-EGFP–treated control rats in the same OVX + low E2 condition (Fig. 5F). The number of AVPV Kiss1-expressing cells was significantly lower in the highly ARC Kiss1 KO rats than that in AAV-EGFP–treated control rats (Fig. 5G).
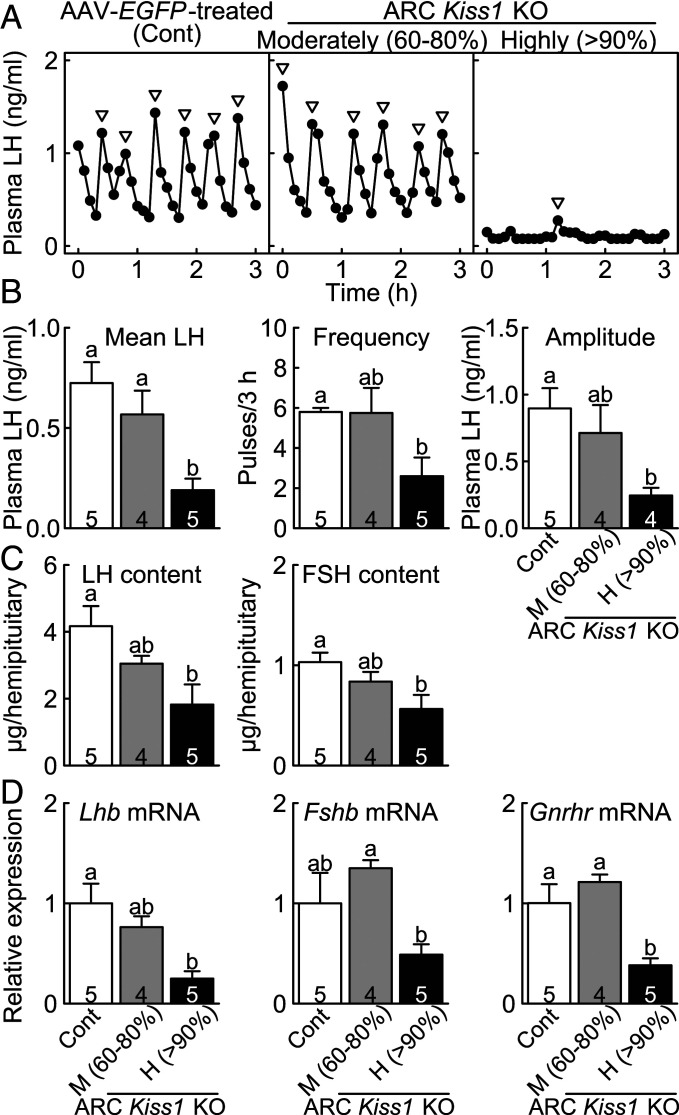
Suppression of Pulsatile LH Release in the Conditional ARC Kiss1 KO Female Rats.
The highly ARC Kiss1 KO rats showed either a lack or severe suppression of pulsatile LH release (Fig. 6A), whereas the AAV-EGFP–treated Kiss1-floxed control and moderately ARC Kiss1 KO rats showed frequent LH pulses in the OVX + low E2 condition. The mean LH concentration was significantly lower in the highly ARC Kiss1 KO rats than those in the AAV-EGFP–treated control and moderately ARC Kiss1 KO rats (Fig. 6B). The frequency and amplitude of LH pulses in the highly ARC Kiss1 KO rats were significantly lower than those in AAV-EGFP–treated control rats.
Fig. 6.
Suppression of gonadotropin synthesis/release and Gnrhr mRNA expression in the conditional ARC Kiss1 KO female rats. (A) Plasma LH profiles in representative OVX + low E2 moderately and highly ARC Kiss1 KO rats and AAV-EGFP–treated Kiss1-floxed control rat (Cont). Arrowheads indicate LH pulses identified with PULSAR computer program. Mean LH concentration and the frequency and amplitude of LH pulses (B), the pituitary LH and FSH content (C), and the pituitary Lhb, Fshb, and Gnrhr mRNA expression (D) in the moderately and highly Kiss1 KO rats and control rats. Values expressed in the bar graphs are mean ± SEM. Numbers in each column indicate the number of animals used. The values with different letters were significantly different from each other (P < 0.05) based on one-way ANOVA followed by Tukey’s HSD test.
Reduction of Pituitary Gonadotropin Synthesis, Gnrhr Expression, and Ovarian Weight in the Conditional ARC Kiss1 KO Female Rats.
The pituitary LH and FSH content were significantly lower in the highly ARC Kiss1 KO rats than those in the AAV-EGFP–treated Kiss1-floxed control rats in the OVX + low E2 condition (Fig. 6C). The pituitary Lhb mRNA expression was significantly lower in the highly ARC Kiss1 KO rats than the AAV-EGFP–treated control rats (Fig. 6D). The pituitary Fshb mRNA expression in the highly ARC Kiss1 KO rats tended to be lower than the AAV-EGFP–treated control rats and were significantly lower than the moderately ARC Kiss1 KO rats. The pituitary Gnrhr mRNA level was significantly lower in the highly ARC Kiss1 KO rats than those in the AAV-EGFP–treated control and moderately ARC Kiss1 KO rats. The ovarian weight in the highly ARC Kiss1 KO rats (62.8 ± 5.4 mg) was significantly lower than that in the AAV-EGFP–treated control rats (95.9 ± 7.5 mg).
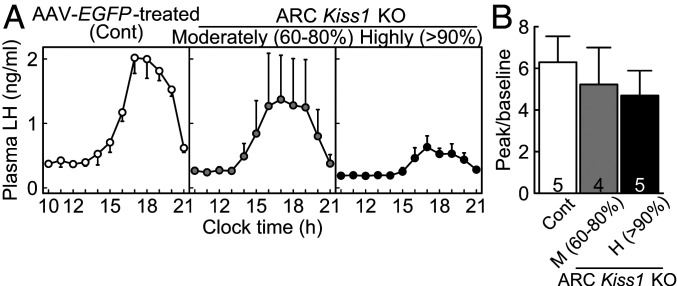
Estrogen-Induced Surge-Like Increase in Plasma LH Concentrations in the Conditional ARC Kiss1 KO Female Rats.
A surge-like increase in plasma LH levels was found in the afternoon 2 d after the E2 treatment at a positive feedback level in both highly and moderately ARC Kiss1 KO OVX rats as well as in the AAV-EGFP–treated Kiss1-floxed control OVX rats (Fig. 7A), indicating that the GnRH/LH surge generator had functioned even in the highly ARC Kiss1 KO rats. It seemed, the fewer the number of remaining ARC Kiss1-expressing cells, the lower the baseline and peak levels of LH, indicating that KNDy neurons are necessary to induce an LH surge with high amplitude. Importantly, the ratios of peak and baseline of plasma LH concentration (Fig. 7B) were comparable between groups.
Fig. 7.
A positive feedback level of E2-induced surge-like increase in plasma LH levels in the conditional ARC Kiss1 KO female rats. (A) Mean plasma LH profiles in the moderately and highly ARC Kiss1 KO rats and AAV-EGFP–treated Kiss1-floxed control rats (Cont). (B) The ratios of peak to baseline of plasma LH levels. Values expressed are mean ± SEM. Numbers in each column indicate the number of animals used. No significant differences were detected in the ratio by one-way ANOVA.
Discussion
The present study provides direct evidence to indicate that the ARC KNDy neurons are the GnRH pulse generator and that the presence of more than 20% of ARC KNDy neurons was able to rescue reproductive function because frequent LH pulses and follicular development were evident in the global Kiss1 KO rats only when the Kiss1 expression was rescued in more than 20% of ARC Tac3-expressing cells. It should be noted that Kiss1 transfection outside of the ARC Tac3-expressing cells failed to recover LH pulses and follicular development, suggesting that KNDy neurons are the intrinsic source of GnRH pulse generation, and the rescue of solely Kiss1 expression in the mediobasal hypothalamus could not function as the pulse generator. This notion was further confirmed by the current conditional ARC Kiss1 KO rats, in which LH pulses disappeared only when more than 90% of the ARC Kiss1-expressing cells were knocked out. The current study provides direct evidence that the ARC KNDy neurons serve as the GnRH pulse generator, a key central mechanism for mammalian reproduction, and to show that relatively small portion (>20%) of KNDy neurons are enough to maintain ovarian function via the maintaining of pulsatile GnRH/gonadotropin release. The current results provide a further understanding of the brain mechanism regulating follicular development and steroidogenesis to contribute to further therapeutic approaches in both women and female domestic animals suffering from reproductive disorders.
The present results were largely consistent with previous studies suggesting that the ARC KNDy neurons would be the GnRH pulse generator: the MUA volley synchronized with LH pulses were detected from the electrodes placed in close proximity to the ARC KNDy neurons in goats (21, 30); the mouse ARC KNDy neurons exhibited rhythmic increases in intracellular Ca2+ that correspond to LH pulses by using the fiber photometry and that optogenetic stimulation or inhibition of mouse ARC KNDy neurons induced or suppressed LH pulses, respectively (25, 26). Based on this knowledge, the advantage of the current study was that we were able to show that the recovery of Kiss1 expression in the ARC Tac3-expressing neurons was capable of rescuing GnRH/LH pulses and follicular development. Thus, by the use of two approaches with gene-modified rats, we have provided direct evidence to confirm the threshold of the portion of KNDy neurons.
The present study demonstrated that the profile of pulsatile LH release is largely dependent on the threshold of the number of KNDy neurons. The LH pulses in the highly KNDy-rescued global Kiss1 KO rats was quite similar to that in wild-type female and the AAV-EGFP–treated Kiss1-floxed control rats: the LH pulse parameters, that is, mean LH concentration and the frequency and amplitude of LH pulses, seemed comparable between these groups (SI Appendix, Fig. S2). Frequent LH pulses were also found if 20 to 40% of KNDy neurons remained in the Kiss1-floxed rats. Importantly, LH pulses were severely inhibited when more than 90% of Kiss1 were knocked out in the ARC of Kiss1-floxed rats, and the rescue of 3 to 15% KNDy neurons showed weak LH pulses. These results suggest that 20% of KNDy neurons are enough to drive GnRH pulse generation in female rats and that the 20% of KNDy neurons synchronize each other via autocrine/paracrine NKB stimulation and/or gap junction–mediated cell–cell communication, as described previously (39). The notion is consistent with previous and recent studies showing that gene-modified mice with a low amount of Kiss1 expression in the ARC (and also AVPV) still exhibited puberty, estrous cyclicity, fertility, and LH pulses (31, 32, 40). Further, it seemed that the caudal population of KNDy neurons play a key role in generating GnRH pulses because rescuing KNDy neurons in the caudal ARC successfully recovered pulsatile LH release. Furthermore, remaining KNDy neurons in the caudal ARC showed regular LH pulses in the current conditional Kiss1 KO rats. The current results also suggest the functional redundancy of the KNDy neuronal population. Interestingly, Herbison et al. (41) showed that 34% GnRH neurons are enough to maintain puberty, ovulation, and fertility in mutant mice, suggesting the functional redundancy of the GnRH neuronal population. It is tempting to speculate that the redundancy of ARC KNDy neurons as well as GnRH neurons may ensure the acute switching on of the activity of GnRH neurons and reproductive cascade to avoid missing the chance of reproduction. Indeed, our previous study showed that the positive energetic cues triggered the pubertal onset of kisspeptin-GnRH-gonadotropin cascade within 1 d in growth-retarded female rats (42).
The present study also demonstrated that rescue of 20% of KNDy neurons successfully ameliorated follicular development and estrogen synthesis ability in the ovary of global Kiss1 KO rats. The highly and moderately KNDy-rescued rats showed a greater number of Graafian and non-Graafian tertiary follicles and higher aromatase mRNA expression than the AAV-EGFP–treated global Kiss1 KO rats. The ARC KNDy-dependent follicular development from the early tertiary stage to the Graafian stage was also found in growth-retarded rats, in which ad libitum feeding acutely induced ARC Kiss1 expression and follicular development (42). Taken together, these results suggest that KNDy neurons play a crucial role in the control of follicular development from the secondary/early tertiary stage to the Graafian follicles via induction of pulsatile GnRH/gonadotropin release.
Interestingly, the current study also demonstrates that KNDy neurons are necessary to induce the LH surge with a high enough amplitude in female rats. The smaller amplitude of LH surge in the current highly ARC Kiss1 KO rats than others would be due to an inhibition of GnRH pulse generation, resulting in a significant decrease in LH and FSH content and mRNA levels of LH β-subunit and GnRH receptor in the pituitary. In other words, the ARC KNDy neurons play a role in maintaining the pituitary gonadotropin synthesis and responsiveness to GnRH via pulsatile GnRH stimulation so that they contribute to the amplification of LH surge. Importantly, it is unlikely that ARC KNDy neurons mediate E2-induced LH surge generation because even the highly ARC Kiss1 KO rats showed a surge-like increase in LH release in the afternoon when the animals were treated with a positive feedback level of E2. Further, the rescue of KNDy neurons in the global Kiss1 KO rats showed a persistent estrous smear pattern, follicular cysts, and no corpora lutea, indicating the lack of LH surge and ovulation. This suggests that ARC KNDy neurons are largely responsible for GnRH/LH pulse generation but not surge generation. This implication is consistent with previous studies, showing that GnRH/LH surge generation is dependent on the AVPV/POA kisspeptin neurons in several mammalian species (33, 34, 36, 37) and that the follicular cysts are the result of continuous stimulation of follicles by tonic gonadotropins without an LH surge in cows (43).
The present study suggests a possibility that ARC KNDy neurons may regulate Kiss1 expression in AVPV kisspeptin neurons because AVPV Kiss1 expression was attenuated in the current highly ARC Kiss1 KO rats even though no Cre-expressing cells were detected in the AVPV of Kiss1-floxed rats infected with AAV-Cre into the ARC. It is unlikely that kisspeptin secreted from ARC KNDy neurons directly up-regulates Kiss1 expression in the AVPV kisspeptin neurons because the kisspeptin receptor (also known as GPR54) was not detected in the AVPV region by Gpr54 mapping study in female rats (44). Thus, it is speculated that the AVPV Kiss1 mRNA expression would be somehow up-regulated by non-kisspeptin signal(s) from the ARC KNDy neurons and/or the interneuron(s) under the control of the ARC KNDy neurons. This notion is consistent with our recent study, showing that AVPV Kiss1 expression was also deprived in conditional ARC Kiss1 KO female mice (45). Indeed, a previous anterograde tracing study indicated the projection of ARC KNDy neurons toward AVPV kisspeptin neurons (46). Further studies are needed to address the role of ARC KNDy neurons in the regulation of the AVPV kisspeptin neurons.
In conclusion, the present study provides direct evidence showing that KNDy neurons serve as the intrinsic source of the GnRH pulse generator and that at least 20% of endogenous ARC KNDy neurons are enough to maintain reproductive function in female rats via retaining biosynthesis and pulsatile release of gonadotropins from the pituitary via generation of GnRH pulses in order to orchestrate reproduction in mammals. These findings have shown insights into the brain mechanism underlying mammalian reproduction and also provided a potential therapeutic aspect to shed light on the reproductive disorders in women and female domestic animals.
Materials and Methods
Animals.
Wild-type, global Kiss1 KO, and Kiss1-floxed rats were maintained in a room with a 14:10 h light/dark cycle (lights on 05:00 h) at 22 ± 3 °C and had free access to food (CE-2; CLEA Japan) and water. Female rats having two consecutive regular 4- or 5-d estrous cycles, determined by vaginal smears, were mated overnight with male rats on the day of proestrus. Pregnant females, determined by the presence of vaginal plugs, were housed individually. The day that newborn litter was found at noon was designated postnatal day 0. Genotypes were analyzed by PCR with DNA obtained from newborn pups. The primers are listed in SI Appendix, Table S1. The litter size was adjusted to eight by postnatal day 5 to minimize the growth variation within and between litters. The female pups were weaned on postnatal day 20. Care of the animals and all the experimental procedures performed in the present study were reviewed and approved by the Animal Experiment Committees of Nagoya University and the National Institute of Physiological Sciences.
Gene Targeting and Generation of Kiss1-Floxed Rats.
To generate Kiss1-floxed rats, a targeting vector was designed to insert two loxP sites encompassing exons 2 and 3 of the Kiss1 gene coding for 52-amino acid rat kisspeptin and a neomycin-resistance gene into the Kiss1 locus in rat embryonic stem (ES) cells via homologous recombination (SI Appendix, Fig. S5A). Rat ES cells, WDB/Nips-ES1 (Rat Genome Database identification: 10054010), were cultured, and gene targeting was performed by electroporation, as described previously (47). ES clones were selected in a neomycin-supplemented medium. Targeted ES clones were confirmed by PCR and Southern blot analysis (SI Appendix, Fig. S5 B and C). The primers for the ES selection are listed in SI Appendix, Table S1. A correctly targeted ES clone was used to generate germline chimera rats. Targeted ES cells were microinjected into the blastocoelic cavity of blastocysts collected from Crlj:WI rats (Charles River Laboratories). Those blastocysts were then transferred to pseudopregnant female rats under isoflurane anesthesia (2 to 3% in air). Four foster mother rats that received the embryo transfer were transported from the National Institute of Physiological Sciences to Nagoya University. The resultant chimeric males were coupled with Iar:Wistar-Imamichi females (Institute for Animal Reproduction). Four out of the eight chimera rats produced germline pups with the ES cell-derived genome. The Kiss1fl/+ males and females were mated in order to generate Kiss1-floxed homozygous rats (Kiss1fl/fl rats). The Kiss1fl/fl female rats showed normal 4- or 5-d estrous cycles.
Preparation of AAV Vectors.
AAV [type DJ, type 2/type 8/type 9 chimera (48)] vectors carrying either CAG-promoter–driven Kiss1 (AAV-Kiss1), Cre (AAV-Cre), or EGFP (AAV-EGFP) were prepared and purified as described previously (49). AAV vectors were stored at −80 °C.
Rescue of KNDy Neurons by a Recovery of Kiss1 Expression in ARC Tac3-Expressing Neurons of Global Kiss1 KO Rats.
AAV-Kiss1 (5.5 × 109 viral genome [vg]/µL) were bilaterally injected into the posterior ARC of 25- or 26-day-old global Kiss1 KO female rats generated previously (24) under anesthesia with a mixture of ketamine (26.7 mg/kg)-xylazine (5.3 mg/kg) and isoflurane inhalation (1 to 3% in air). The stereotaxic coordinate was 3.3 mm posterior and 9.4 mm ventral to the bregma and 0.5 mm lateral to the midline according to the rat brain atlas (50). AAV-EGFP (5.5 × 109 vg/µL) was injected as a control in the same manner. After the AAV-Kiss1 injection, the vaginal opening and then vaginal smear pattern were examined in order to monitor stages of the estrous cycle for 3 wk.
Blood Sampling for Analyses of Pulsatile LH Release in the KNDy Neuron-Rescued Rats.
Three weeks after the AAV injection, the female rats were OVX, and the ovaries were subjected to analysis for gene expression and to histological analysis as described later. Two weeks after the OVX, the animals were subjected to blood sampling to determine LH pulses. Note that the OVX rats had an advantage to avoid the individual differences in endogenous ovarian steroids. A silicon cannula (inner diameter 0.5 mm and outer diameter 1.0 mm; Shin-Etsu Polymer) was inserted into the right atrium through the jugular vein 1 d before the onset of blood sampling. Blood samples (100 μL) were collected from freely moving conscious OVX rats every 6 min for 3 h (13:00 to 16:00 h), and plasma samples were collected after the centrifugation at 4 °C. An equivalent volume of rat red blood cells, which were taken from donor rats and diluted with heparinized saline, was replaced through the atrial catheter after each blood collection to keep the hematocrit constant.
Brain and Pituitary Sampling from the KNDy Neuron-Rescued Rats.
One day after blood sampling, the animals were deeply anesthetized with sodium pentobarbital (40 mg/kg, Kyoritsu Seiyaku) and then intracardially perfused with 4% paraformaldehyde (Sigma-Aldrich Japan). The brains were immediately removed and postfixed in the same fixative overnight at 4 °C. The brains were then immersed in 30% sucrose in 0.05 M phosphate buffer until the brains sank at 4 °C. Frozen frontal sections containing the ARC (50-μm thickness) were prepared using a cryostat (CM1800, Leica Biosystems). Every fourth ARC section was used for double in situ hybridization (ISH) to visualize Kiss1 and Tac3. The pituitary was also collected and the hemipituitaries were stored at −80 °C until analyses for LH and FSH content and Lhb, Fshb, and Gnrhr mRNA expression, respectively.
Double ISH for Kiss1 and Tac3 Expression in the KNDy Neuron-Rescued Rats.
The double ISH was performed as described previously (51, 52). Briefly, the sections were hybridized overnight at 60 °C with a fluorescein isothiocyanate (FITC)-labeled anti-sense complementary RNA (cRNA) probe for Kiss1 (position 33–349, AY196983) and a digoxigenin (DIG)-labeled anti-sense cRNA probe for Tac3 (position 180–304, NM_019162). The hybridized FITC-labeled probe was detected with a peroxidase (POD)-conjugated anti-FITC antibody (Roche Diagnostics) and TSA Plus Fluorescein System (1:100; PerkinElmer), and the hybridized DIG-labeled probe was detected by a POD-conjugated anti-DIG antibody (Roche Diagnostics), TSA Plus Biotin Kit (1:100; PerkinElmer), and DyLight 594-conjugated streptavidin (Thermo Fisher Scientific). The fluorescent images were examined under a fluorescence microscope with ApoTome optical sectioning (ZEISS). The number of the Tac3-expressing cells and Kiss1- and Tac3-coexpressing cells throughout the ARC (from 1.72 to 4.36 mm posterior to the bregma) were bilaterally counted. The specificity of anti-sense cRNA probes for Kiss1 and Tac3 were verified by control experiments using sense cRNA probes. No signals were found in the sections incubated with sense cRNA probes (33, 42).
Quantitative Analysis of Follicular Development in the KNDy Neuron-Rescued Rats.
The ovaries were weighed, fixed with 10% neutral buffered formalin for 24 h, and embedded with paraffin as described previously (42). Serial 8-µm sections of ovaries were made by using a microtome (RM2235, Leica Biosystems) and stained with hematoxylin and eosin. The number of follicles were counted every fifth section under a light microscope (BX53, Olympus) according to previous studies (42, 53). The stages of follicles were classified according to previous studies (54–56).
Evaluation of Pulsatile and Surge-Mode of LH Release in the AAV-Cre-Induced ARC Kiss1 KO Rats.
AAV-Cre (5.0 × 109 vg/µL) were bilaterally injected into the anterior and posterior ARC of 10- to 12-week-old Kiss1fl/fl female rats under anesthesia, as described above. The stereotaxic coordinates were 2.8 mm (anterior ARC) and 3.8 mm (posterior ARC) posterior to the bregma, 9.6 mm ventral to the bregma, and 0.6 mm lateral to the midline according to the rat brain atlas (50). AAV-EGFP was injected as a control in the same manner. Two weeks after the AAV injection, the animals were OVX and immediately received Silastic tubing (inner diameter 1.57 mm, outer diameter 3.18 mm, and length 26 mm; Dow Corning) filled with E2 dissolved in peanut oil at 20 μg/mL to produce a negative feedback level of plasma E2 (57) for 1 wk. The OVX + low E2 condition was chosen so as to avoid the individual differences in endogenous estrogens and to detect both AVPV and ARC Kiss1 mRNA expression. The animals were subjected to frequent blood sampling to determine LH pulses in the same manner of the KNDy neuron-rescued rat experiment. One day after the frequent blood sampling, the animals were anesthetized with isoflurane inhalation, and the estrogen tubing was replaced with another one containing E2 dissolved in peanut oil at 1000 μg/mL to produce a positive feedback level of plasma E2 (58). Blood samples (100 μL) were collected every 1 h from 10:00 to 21:00 h at 2 d after the E2 replacement to determine the afternoon LH surge.
Brain and Pituitary Sampling from the AAV-Cre-Induced ARC Kiss1 KO Rats.
One day after the second blood sampling, the estrogen tubing was replaced with another one containing E2 dissolved in peanut oil at 20 μg/mL. One week later, the animals were deeply anesthetized with sodium pentobarbital and then intracardially perfused with 4% paraformaldehyde. The brains were collected, and the frontal sections containing the AVPV and ARC (50-μm thickness) were prepared using a cryostat. Every second AVPV section and every fourth ARC section were used for ISH to visualize Kiss1 and Cre. The pituitary was collected and processed as described above.
ISH for Kiss1 and Cre Expression in the AAV-Cre-Induced ARC Kiss1 KO Rats.
The ISH was performed as described previously (33, 45, 59, 60). Briefly, the sections were hybridized overnight at 60 °C with DIG-labeled anti-sense cRNA probe for either Kiss1 (position 33–349, AY196983) or Cre (position 485–1516, X03453). The hybridized probe was detected with an alkaline phosphatase-conjugated anti-DIG antibody (1:1,000; Roche Diagnostics) and a chromogen solution (337 μg/mL 4-nitro blue tetrazolium chloride and 175 μg/mL 5-bromo-4-chloro-3-indolyl-phosphate, Roche Diagnostics). The Kiss1-expressing cells throughout the AVPV (from 0.12 mm anterior to 0.60 mm posterior to the bregma) and ARC (as described above) were bilaterally counted under a light microscope, BX53. The specificity of anti-sense cRNA probes for Kiss1 and Cre were verified by control experiments using sense cRNA probes. No signals were found in the sections incubated with sense Kiss1 (33) and Cre (SI Appendix, Fig. S6) cRNA probes.
Radioimmunoassay and LH Pulse Parameter Analysis.
Plasma LH concentrations were determined by a double-antibody radioimmunoassay (RIA) with a rat LH-RIA kit provided by the National Hormone and Peptide Program (NHPP). The concentrations were expressed in terms of rat LH-RP3. The least detectable level of LH assay was 3.9 pg/tube, and the intra- and interassay coefficients of variation were 4.3 and 9.1% at 69 pg/tube, respectively. LH pulses were identified by the PULSAR computer program (61). Mean LH concentration and the frequency and amplitude of LH pulses were calculated during the 3-h sampling period for each individual and then for the group. As for the E2-induced LH surge, a ratio of peak to baseline of LH surge was calculated in each individual and then in the group.
The pituitary LH and FSH content were also determined by RIA with rat LH-RIA and rat FSH-RIA kits. The fixed hemipituitaries were homogenized and sonicated in RIA buffer (0.05 M phosphate-buffered saline) and LH and FSH concentrations in the supernatant were determined. The FSH concentrations were expressed in terms of the rat FSH-RP2. The least detectable level of FSH assay was 15.6 pg/tube, and the intra- and interassay coefficients of variation were 5.8 and 7.2% at 564 pg/tube, respectively.
qRT-PCR for Analyses of Pituitary and Ovarian Gene Expression.
Total RNA was extracted from the fixed hemipituitary tissues by using the RNeasy FFPE kit (QIAGEN) in accordance with the manufacturer’s instruction. Total RNA was extracted from the ovaries by using ISOGEN (Nippon Gene). The cDNA from each sample was synthesized with oligo (deoxythymidine) primer at 37 °C by using the high-capacity cDNA reverse transcription kit (Applied Biosystems). Gene expression levels were determined using the 7500 real-time PCR system (Applied Biosystems) with Thunderbird SYBR Green qPCR Mix (TOYOBO) as described previously (45, 58). Forward and reverse primers for Lhb, Fshb, Gnrhr, Cyp19a1, Cyp17a1, Lhcgr, Fshr, and Actb (encoding β-actin) are listed in SI Appendix, Table S1. The specificity of the amplification products was confirmed by the dissociation curve analysis and electrophoresis on 1.5% agarose gels. The relative gene expression levels were normalized to Actb, and the fold changes between the treatment and control groups were calculated using the 2-ΔΔCT method.
Statistical Analysis.
Statistical differences in the number of ARC Tac3-expressing cells and Kiss1- and Tac3-coexpressing cells, the number of ARC and AVPV Kiss1-expressing cells, the LH pulse parameters, the pituitary LH and FSH content, the pituitary Lhb, Fshb, and Gnrhr gene expression, the ovarian weight, the number of follicles, ovarian Cyp19a1, Cyp17a1, Lhcgr, and Fshr gene expression, and the ratio of peak to baseline of LH surge between the groups were determined by one-way ANOVA followed by Tukey's honestly significant difference (HSD) test by using R version 3.6.2 (https://www.R-project.org/). Statistical differences in the amplitude of LH pulses between the highly and moderately KNDy-rescued rats were determined by Welch's t test (see details in the legend of Fig. 2). Statistical differences in timing of vaginal opening between the groups were determined by Kaplan–Meier analysis and log-rank test. Differences were considered statistically significant when P < 0.05.
Supplementary Material
Acknowledgments
We respectfully acknowledge the contributions of the late Professor Kei-ichiro Maeda, PhD, DVM, of The University of Tokyo, who suddenly passed away on February 3, 2018. His leadership, supervision, and original ideas contributed greatly to this work. The authors are grateful to the NHPP, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. A.F. Parlow for providing the LH and FSH assay kits. The radioimmunoassays were performed at the Nagoya University Radioisotope Center. We thank Dr. Nicola Skoulding for editorial assistance. This work was supported in part by Japan Society for the Promotion of Science KAKENHI grant numbers 18H03973, 18K19267 (to H. Tsukamura), 19H03103 (to N.I.), and 20H03127 (to Y.U.) and the Cooperative Study Program of National Institute for Physiological Sciences. This study was also supported in part by the Graduate Program of Transformative Chem-Bio Research at Nagoya University, supported by MEXT (WISE Program). This study was also supported in part by the following research grants to the late Professor Kei-ichiro Maeda: the Program for Promotion of Basic Research Activities for Innovative Biosciences and the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009156118/-/DCSupplemental.
Data Availability.
All study data are included in the article and/or SI Appendix.
References
- 1.Seminara S. B., et al. , The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Topaloglu A. K., et al. , Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 366, 629–635 (2012). [DOI] [PubMed] [Google Scholar]
- 3.de Roux N., et al. , Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U.S.A. 100, 10972–10976 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skorupskaite K., George J. T., Anderson R. A., The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 20, 485–500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franssen D., Tena-Sempere M., The kisspeptin receptor: A key G-protein-coupled receptor in the control of the reproductive axis. Best Pract. Res. Clin. Endocrinol. Metab. 32, 107–123 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Ieda N., et al. , Kisspeptin: A central regulator of reproduction in mammals. SVU-int. J. Vet. Sci. 3, 10–26 (2019). [Google Scholar]
- 7.Dyer R. G., Robinson J. E., The LHRH pulse generator. J. Endocrinol. 123, 1–2 (1989). [DOI] [PubMed] [Google Scholar]
- 8.Maeda K., et al. , The LHRH pulse generator: A mediobasal hypothalamic location. Neurosci. Biobehav. Rev. 19, 427–437 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Belchetz P. E., Plant T. M., Nakai Y., Keogh E. J., Knobil E., Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202, 631–633 (1978). [DOI] [PubMed] [Google Scholar]
- 10.Plosker G. L., Brogden R. N., Leuprorelin. A review of its pharmacology and therapeutic use in prostatic cancer, endometriosis and other sex hormone-related disorders. Drugs 48, 930–967 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Smith S., Pfeifer S. M., Collins J. A., Diagnosis and management of female infertility. JAMA 290, 1767–1770 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Barbieri R. L., “Female infertility” in Yen and Jaffe’s Reproductive Endocrinology, Strauss J. F., Barbieri R. L., Gargiulo A. G., Eds. (Elsevier, Amsterdam, ed. 8, 2019), pp. 556–581. [Google Scholar]
- 13.Barbieri R. L., Clinical applications of GnRH and its analogues. Trends Endocrinol. Metab. 3, 30–34 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Filicori M., Cognigni G. E., Ovulation induction with pulsatile gonadotropin releasing hormone: Missing in action. Fertil. Steril. 109, 621–622 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Matsuwaki T., Kayasuga Y., Yamanouchi K., Nishihara M., Maintenance of gonadotropin secretion by glucocorticoids under stress conditions through the inhibition of prostaglandin synthesis in the brain. Endocrinology 147, 1087–1093 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Maeda K., Tsukamura H., Neuroendocrine mechanism mediating fasting-induced suppression of luteinizing hormone secretion in female rats. Acta Neurobiol. Exp. (Warsz.) 56, 787–796 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Tilbrook A. J., Turner A. I., Clarke I. J., Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 5, 105–113 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Tilbrook A. J., Turner A. I., Clarke I. J., Stress and reproduction: Central mechanisms and sex differences in non-rodent species. Stress 5, 83–100 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Chatterton R. T., The role of stress in female reproduction: Animal and human considerations. Int. J. Fertil. 35, 8–13 (1990). [PubMed] [Google Scholar]
- 20.Terasawa E., Guerriero K. A., Plant T. M., Kisspeptin and puberty in mammals. Adv. Exp. Med. Biol. 784, 253–273 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkura S., et al. , Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J. Neuroendocrinol. 21, 813–821 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi Y., et al. , Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 30, 3124–3132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman R. L., et al. , Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 154, 4259–4269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uenoyama Y., et al. , Lack of pulse and surge modes and glutamatergic stimulation of LH release in Kiss1 knockout rats. J. Neuroendocrinol. 27, 187–197 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Han S. Y., McLennan T., Czieselsky K., Herbison A. E., Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc. Natl. Acad. Sci. U.S.A. 112, 13109–13114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarkson J., et al. , Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. U.S.A. 114, E10216–E10223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman R. L., et al. , Kisspeptin neurons in the arcuate nucleus of the Ewe express both dynorphin A and neurokinin B. Endocrinology 148, 5752–5760 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Lehman M. N., Coolen L. M., Goodman R. L., Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151, 3479–3489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.True C., Kirigiti M., Ciofi P., Grove K. L., Smith M. S., Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J. Neuroendocrinol. 23, 52–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamura H., et al. , Kisspeptin and GnRH pulse generation. Adv. Exp. Med. Biol. 784, 297–323 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Popa S. M., et al. , Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology 154, 2784–2794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer C., Boehm U., Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat. Neurosci. 14, 704–710 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Adachi S., et al. , Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 53, 367–378 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Homma T., et al. , Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol. Reprod. 81, 1216–1225 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Tsukamura H., Homma T., Tomikawa J., Uenoyama Y., Maeda K., Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann. N. Y. Acad. Sci. 1200, 95–103 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y., et al. , Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J. Neuroendocrinol. 26, 909–917 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Matsuda F., et al. , The LH surge-generating system is functional in male goats as in females: Involvement of kisspeptin neurones in the medial preoptic area. J. Neuroendocrinol. 27, 57–65 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Comninos A. N., et al. , Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct. Funct. 221, 2035–2047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikegami K., et al. , Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J. Neuroendocrinol. 29, 1–14 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Minabe S., et al. , Inducible Kiss1 knockdown in the hypothalamic arcuate nucleus suppressed pulsatile secretion of luteinizing hormone in male mice. J. Reprod. Dev. 66, 369–375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbison A. E., Porteous R., Pape J. R., Mora J. M., Hurst P. R., Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149, 597–604 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majarune S., et al. , Ad libitum feeding triggers puberty onset associated with increases in arcuate Kiss1 and Pdyn expression in growth-retarded rats. J. Reprod. Dev. 65, 397–406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneko H., et al. , Perturbation of estradiol-feedback control of luteinizing hormone secretion by immunoneutralization induces development of follicular cysts in cattle. Biol. Reprod. 67, 1840–1845 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Higo S., Honda S., Iijima N., Ozawa H., Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus. J. Neuroendocrinol., 28 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Ikegami K., et al. , Conditional kisspeptin neuron-specific Kiss1 knockout with newly generated Kiss1-floxed and Kiss1-Cre mice replicates a hypogonadal phenotype of global Kiss1 knockout mice. J. Reprod. Dev. 66, 359–367 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeo S. H., Herbison A. E., Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152, 2387–2399 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Hirabayashi M., et al. , Ability of tetraploid rat blastocysts to support fetal development after complementation with embryonic stem cells. Mol. Reprod. Dev. 79, 402–412 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Grimm D., et al. , In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 82, 5887–5911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi K., et al. , Survival of corticostriatal neurons by Rho/Rho-kinase signaling pathway. Neurosci. Lett. 630, 45–52 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G., Watson C., The Rat Brain in Stereotaxic Coordinates (Academic Press, San Diego, ed. 6, 2008). [Google Scholar]
- 51.Assadullah, et al. , Co-expression of the calcitonin receptor gene in the hypothalamic kisspeptin neurons in female rats. Reprod. Med. Biol. 17, 164–172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ieda N., et al. , GnRH(1-5), a metabolite of gonadotropin-releasing hormone, enhances luteinizing hormone release via activation of kisspeptin neurons in female rats. Endocr. J. 67, 409–418 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Yamada T., et al. , Exposure to 1-bromopropane causes ovarian dysfunction in rats. Toxicol. Sci. 71, 96–103 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Pedersen T., Peters H., Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 17, 555–557 (1968). [DOI] [PubMed] [Google Scholar]
- 55.Hirshfield A. N., A. R. Midgley, Jr, Morphometric analysis of follicular development in the rat. Biol. Reprod. 19, 597–605 (1978). [DOI] [PubMed] [Google Scholar]
- 56.Bogovich K., Follicle-stimulating hormone plays a role in the induction of ovarian follicular cysts in hypophysectomized rats. Biol. Reprod. 47, 149–161 (1992). [DOI] [PubMed] [Google Scholar]
- 57.Cagampang F. R., Maeda K., Tsukamura H., Ohkura S., Ota K., Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J. Endocrinol. 129, 321–328 (1991). [DOI] [PubMed] [Google Scholar]
- 58.Horihata K., Inoue N., Uenoyama Y., Maeda K., Tsukamura H., Retinoblastoma binding protein 7 is involved in Kiss1 mRNA upregulation in rodents. J. Reprod. Dev. 66, 125–133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada S., et al. , Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 148, 2226–2232 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Minabe S., et al. , Long-term neonatal estrogen exposure causes irreversible inhibition of LH pulses by suppressing arcuate kisspeptin expression via estrogen receptors α and β in female rodents. Endocrinology 158, 2918–2929 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Merriam G. R., Wachter K. W., Algorithms for the study of episodic hormone secretion. Am. J. Physiol. 243, E310–E318 (1982). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.