In an elegant analysis of resource utilization data from 500 eukaryote species, Kiørboe and Thomas (1) describe a positive relationship between the rate at which organisms acquire and ingest food. From this, they infer that “perhaps the most commonly assumed trade-off in ecology—between relative performance at low and high resource (food) levels—does not exist.” Notwithstanding the independent value of investigating trade-offs in foraging activities, we argue that this interpretation is inconsistent with the original and prevailing contemporary definition of the gleaner-opportunist trade-off (2–5).
Whereas Kiørboe and Thomas (1) define the trade-off as “between the capacities for searching for food and for acquiring and processing food,” the original definition was explicitly based on population growth rate, and, more specifically, the trade-off between maximum growth rate and minimum resource requirement () (2, 3). To quote from Grover (2), “I call the one with the lower a gleaner, the one with the higher maximal growth rate an opportunist, and I call this relation between two competitors the gleaner-opportunist trade-off.” As such, the extent to which Kiørboe and Thomas’s (1) data can be used to draw inference on the gleaner-opportunist trade-off is contingent on a close relationship between ingestion rate and population growth rate.* However, many factors may contribute to the emergent population growth rate, and even the simplest consumer-resource models typically incorporate at least two other critical parameters: mortality rate and conversion efficiency (7, 8).
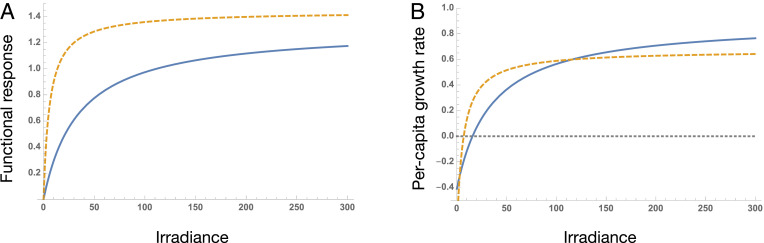
Even when functional responses for ingestion rates do not cross, allowing for additive species-specific mortality rates can result in intersecting per capita growth functions (4) (Fig. 1). Indeed, this is the case that would arise under Kiørboe and Thomas’s (1) alternative hypothesis of a trade-off between foraging and predation risk. Here, “fast” strategists have a lower , while “slow” strategists have a higher maximum growth rate. This is, by definition, a gleaner-opportunist trade-off. Note that it is also possible for the reverse to arise, where slow gleaners trade off against fast opportunists (8).
Fig. 1.
The difference between functional responses and per capita growth rates in an empirically parameterized model [adapted from Litchman and Klausmeier (4)]. (A) The functional responses of two phytoplankton species (Nitzschia sp. and Sphaerocystis schroeteri) for light show the slow-fast continuum as in ref. 1. (B) The realized per capita growth rates after accounting for species specific mortality show the gleaner-opportunist trade-off.
Unlike mortality, the contribution of conversion efficiency (conversion of energy into offspring) to population growth rate is typically treated as multiplicative with ingestion rate. As such, differences in conversion efficiency can significantly change the shape of the population growth response, including the emergence of the gleaner-opportunist trade-off when fast species have lower conversion efficiencies. Although Kiørboe and Thomas (1) do show a positive relationship between ingestion rate and somatic growth rate on a subset of their data, we caution against inferring population growth rate directly from change in individual biomass.
Finally, it is valuable to recognize that, even if we interpret a foraging-predation risk trade-off as distinct from the gleaner-opportunist trade-off, both can yield fluctuation-dependent coexistence via the same underlying mechanism, relative nonlinearity (9, 10). As such, irrespective of terminology, it would be premature to rule out the importance of resource fluctuations for diversity maintenance.
Footnotes
The authors declare no competing interest.
*Note that the synonymous gleaner-exploiter trade-off was also first discussed in reference to population growth rate (6).
References
- 1.Kiørboe T., Thomas M. K., Heterotrophic eukaryotes show a slow-fast continuum, not a gleaner–exploiter trade-off. Proc. Natl. Acad. Sci. U.S.A. 117, 24893–24899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grover J. P., Resource competition in a variable environment: Phytoplankton growing according to Monod’s model. Am. Nat. 136, 771–789 (1990). [Google Scholar]
- 3.Grover J. P., Resource Competition (Springer Science & Business Media; ) (1997) vol. 19. [Google Scholar]
- 4.Litchman E., Klausmeier C. A., Competition of phytoplankton under fluctuating light. Am. Nat. 157, 170–187 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Mittelbach G. G., McGill B. J., Community Ecology (Oxford University Press, 2019). [Google Scholar]
- 6.Fredrickson A. G., Stephanopoulos G., Microbial competition. Science 213, 972–979 (1981). [DOI] [PubMed] [Google Scholar]
- 7.Tilman D., Resource Competition and Community Structure (Princeton University Press, 1982). [PubMed] [Google Scholar]
- 8.Abrams P. A., Brassil C. E., Holt R. D., Dynamics and responses to mortality rates of competing predators undergoing predator–prey cycles, Theor. Popul. Biol. 64, 163–176 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Armstrong R. A., McGehee R., Competitive exclusion, Am. Nat. 115, 151–170 (1980). [Google Scholar]
- 10.Yuan C., Chesson P., The relative importance of relative nonlinearity and the storage effect in the lottery model, Theor. Popul. Biol. 105, 39–52 (2015). [DOI] [PubMed] [Google Scholar]