Significance
Prior work has suggested that the ability of miR-33, a microRNA involved in regulation of lipid metabolism, to regulate liver function underlies its effects on obesity and/or atherosclerosis. In this work, we selectively remove miR-33 from the liver and demonstrate that, unlike mice globally deficient for miR-33, mice lacking miR-33 in the liver are not predisposed to diet-induced obesity and are actually protected from hepatic insulin resistance and fibrosis. While loss of liver miR-33 increases circulating high-density lipoprotein and increases reverse cholesterol transport in mice on a chow diet, it is not sufficient to decrease atherosclerotic plaque burden under hyperlipidemic conditions where hepatic miR-33 is already repressed. This information will help improve efforts to develop novel therapies against cardiometabolic diseases.
Keywords: miRNA, metabolism, atherosclerosis, obesity, fibrosis
Abstract
miR-33 is an intronic microRNA within the gene encoding the SREBP2 transcription factor. Like its host gene, miR-33 has been shown to be an important regulator of lipid metabolism. Inhibition of miR-33 has been shown to promote cholesterol efflux in macrophages by targeting the cholesterol transporter ABCA1, thus reducing atherosclerotic plaque burden. Inhibition of miR-33 has also been shown to improve high-density lipoprotein (HDL) biogenesis in the liver and increase circulating HDL-C levels in both rodents and nonhuman primates. However, evaluating the extent to which these changes in HDL metabolism contribute to atherogenesis has been hindered by the obesity and metabolic dysfunction observed in whole-body miR-33–knockout mice. To determine the impact of hepatic miR-33 deficiency on obesity, metabolic function, and atherosclerosis, we have generated a conditional knockout mouse model that lacks miR-33 only in the liver. Characterization of this model demonstrates that loss of miR-33 in the liver does not lead to increased body weight or adiposity. Hepatic miR-33 deficiency actually improves regulation of glucose homeostasis and impedes the development of fibrosis and inflammation. We further demonstrate that hepatic miR-33 deficiency increases circulating HDL-C levels and reverse cholesterol transport capacity in mice fed a chow diet, but these changes are not sufficient to reduce atherosclerotic plaque size under hyperlipidemic conditions. By elucidating the role of miR-33 in the liver and the impact of hepatic miR-33 deficiency on obesity and atherosclerosis, this work will help inform ongoing efforts to develop novel targeted therapies against cardiometabolic diseases.
Increased circulating levels of low-density lipoprotein cholesterol (LDL-C) is the primary risk factor for the development of atherosclerotic plaques. While the use of statins to lower LDL-C levels has proven an effective treatment for patients at risk for developing cardiovascular disease (CVD), heart disease remains the leading cause of death in developed countries. More recently, researchers have sought to develop treatments able to regulate other factors related to atherosclerosis, including reverse cholesterol transport (RCT), inflammation, and plaque stability. Among the novel approaches to treat CVD, microRNAs (miRNAs) have shown a great deal of potential due to their ability to target many different mRNAs involved in processes related to plaque development. For example, inhibition of miR-148 has been shown to both decrease circulating LDL-C levels and increase circulating high-density lipoprotein cholesterol (HDL-C) levels through targeting of multiple different mRNAs (1). However, the promiscuous nature of miRNAs also increases the potential for unintended effects, necessitating an in-depth exploration of the impact of altering miRNA expression/activity in different organs and tissues.
miR-33 is an intronic miRNA encoded within the Sterol regulatory element-binding protein 2 (Srebf2) gene (2–4). The protein encoded by this gene, SREBP2, is the primary transcription factor responsible for regulating cellular cholesterol levels by promoting cholesterol uptake and synthesis in response to low intracellular sterol levels. miR-33 is transcribed along with its host gene, and has also been found to be an important regulator of cholesterol metabolism, by targeting ATP Binding Cassette Subfamily A Member 1 (Abca1), a cholesterol transporter critical for HDL-C biogenesis. miR-33 has also been shown to target numerous other mRNAs, including key factors in other functions related to atherogenesis. In macrophages, which make up the bulk of atherosclerotic plaques, miR-33 has been shown to regulate a number of important functions related to plaque development, including fatty acid oxidation (FAO), mitochondrial function, polarization, efferocytosis, and cholesterol efflux (2–9). Cholesterol efflux is the first step in the RCT pathway, the process by which macrophages are able to remove cholesterol from the plaque for transport to the liver via circulating HDL-C. In addition to this, within the liver, miR-33 has been shown to further regulate RCT by targeting factors involved in HDL-C biogenesis (Abca1) and the final step of the RCT process, bile acid secretion and synthesis, which facilitates removal of cholesterol from the body (10, 11).
These findings demonstrated that miR-33 is in a unique position to regulate many different factors related to atherosclerotic plaque formation and could provide an important therapeutic target for the treatment of CVD. Consistent with this, initial work with both rodents and nonhuman primates was able to show that inhibition of miR-33 could increase circulating HDL levels (2–4, 12–14). Moreover, miR-33 inhibition or genetic ablation was shown to reduce atherosclerotic plaque size in mouse models of atherosclerosis (15–17). However, long-term treatment with miR-33 inhibitors was found to increase circulating triglycerides (TAGs) and promote hepatic steatosis (18, 19). Moreover, genetic ablation of miR-33 resulted in increased susceptibility to obesity and metabolic dysfunction (20, 21). We have demonstrated that transplantation of bone marrow from miR-33–deficient animals into the Ldl receptor-knockout (Ldlr−/−) mouse model of atherosclerosis was sufficient to promote macrophage RCT and reduce plaque burden. Moreover, specific disruption of the interaction between miR-33 and Abca1 largely mimicked the effects of miR-33 deficiency on atherosclerosis without impacting body weight or metabolic function.
While these findings demonstrate the importance of macrophage miR-33 for promoting plaque formation, prior work has been unable to address how the regulation of HDL biogenesis and bile acid metabolism by miR-33 in the liver impacts atherosclerosis due to the metabolic alterations observed in whole-body knockout mice. Additionally, it is not clear whether loss of miR-33 in the liver may contribute to these metabolic phenotypes. In this work, we have developed a conditional miR-33 knockout model and used this to selectively remove miR-33 from the liver (LKO). Through characterization of this model, we demonstrate that liver-specific loss of miR-33 increases circulating HDL-C and improves in vivo RCT. Moreover, we demonstrate that hepatic miR-33 deficiency does not result in the predisposition to diet-induced obesity and metabolic dysfunction observed in whole-body knockout animals. Conversely, we find that, after long-term high-fat diet (HFD) feeding, LKO mice have an improved ability to regulate metabolic homeostasis and reduced expression of factors related to hepatic fibrosis and inflammation. Our data further indicate that LKO mice are protected from CCl4-induced liver fibrosis, which may be in part mediated by up-regulation of Ski, a negative regulator of TGFβ signaling (22). However, despite the beneficial alterations observed in LKO mice, we did not observe any differences in atherosclerotic plaque size. These findings support the conclusion that the proatherogenic effects of miR-33 are primarily due to direct effects on macrophages within the atherosclerotic plaque. This work also indicates that adverse metabolic effects observed in global miR-33–knockout mice are unlikely to be due to loss of miR-33 in the liver, which aids in the development of new approaches for treating atherosclerosis and obesity.
Results
Loss of Hepatic miR-33 Increases Circulating HDL-C and RCT Capacity.
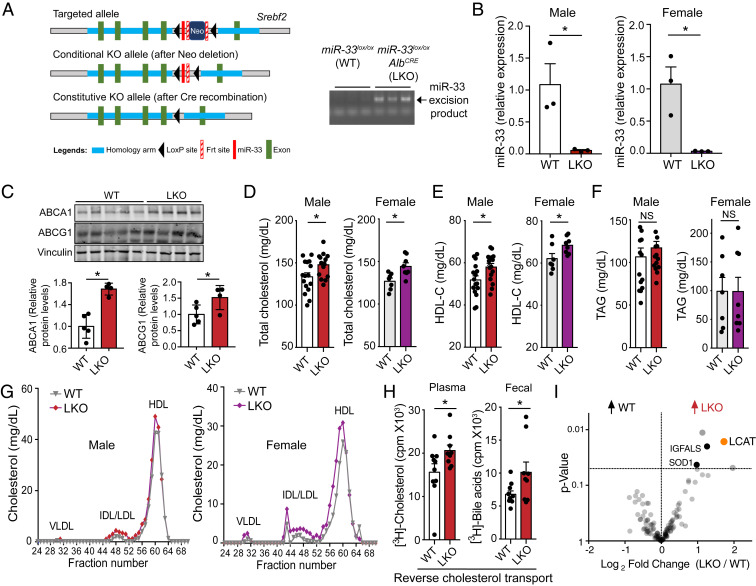
To determine the specific impact of hepatic miR-33 on obesity, metabolic function, and atherogenesis, we generated a conditional miR-33 knockout model (miR-33loxP/loxP). In these mice, the intronic region of the Srebf2 gene encoding miR-33 is flanked by loxP sites, so Cre recombinase activity leads to the excision of this region, causing miR-33 deficiency (Fig. 1 A, Left). By crossing these mice to well-established Albumin-Cre strain, we have generated liver-specific miR-33–knockout mice (LKO). Excision of the floxed region could be visualized by PCR amplification followed by agarose gel electrophoresis (Fig. 1 A, Right), and loss of miR-33 in the liver was confirmed by qPCR analysis (Fig. 1B). While the amount of miR-33 detected in LKO mice was very low, residual expression that was observed is likely due to expression of miR-33 in other less abundant cell types found within the liver. Kupffer cells were found to express an appreciable amount of miR-33, but this was lower than the expression in primary hepatocytes (SI Appendix, Fig. S1A). Deletion of miR-33 in hepatocytes did not alter hepatic Srebf2 mRNA levels or SREBP2-regulated gene expression (SI Appendix, Fig. S1B). Consistent with the known role of miR-33 in suppressing the posttranscriptional expression of ABCA1 and ABCG1, LKO mice expressed higher levels of both transporters in the liver compared to WT mice (Fig. 1C). As expected by the derepression of ABCA1, we also observed an increase in circulating levels of total cholesterol and HDL-C in both male and female LKO mice (Fig. 1 D and E), while circulating TAG levels were not altered (Fig. 1F). FPLC fractionation further demonstrates an increase in the HDL peak of LKO mice (Fig. 1G).
Fig. 1.
Loss of miR-33 in the liver increases circulating HDL levels and improves RCT capacity. (A) Schematic diagram depicting the generation of conditional miR-33–knockout mice. The construct is composed of a short flippase recombination enzyme (Flp)-recognition target (FRT), reporter, and a Cre recombinase recognition target (loxP). The first loxP site is followed by the miR-33 coding region, then the first FRT site, the neomycin selection cassette driving the neomycin resistance gene, a second FRT site, and the second loxP site (Top). Mice with the floxed allele but lacking the neo cassette were generated by crossing with flp recombinase-deleter mice (Middle). Subsequently, these mice were bred with mice expressing Cre recombinase to produce tissue-specific miR-33–knockout mice (Bottom). PCR amplification of the excised genomic region containing miR-33 in the liver of LKO mice (Right). (B) qPCR analysis of miR-33 expression in the liver of miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) animals (n = 3). (C) Representative Western blots and densitometric analysis of ABCA1, ABCG1, and housekeeping standard Vinculin in liver lysates from male WT and LKO mice. (D–F) Levels of total cholesterol (D), HDL-C (E), and TAGs (F) in plasma of WT and LKO mice (male, n = 15 to 16; female, n = 7 to 8). (G) Cholesterol content of FPLC-fractionated lipoproteins from pooled plasma of n = 5 to 8 WT and LKO mice. (H) The [3H]-cholesterol in plasma (Left) and in fecal bile acids (Right) from male WT and LKO mice injected IP with [3H]-cholesterol–labeled BMDMs (n = 9 to 10). (I) Plasma proteome analysis from male WT and LKO mice. The graph indicates the significantly increased proteins found in WT and LKO plasma (n = 5). Data represent the mean ± SEM (*P ≤ 0.05 compared with WT animals).
We next sought to directly assess whether these changes were sufficient to improve RCT capacity in vivo. To accomplish this, we isolated peritoneal macrophages from wild-type (WT) mice and preloaded them with radioactively labeled cholesterol. These cells were then injected intraperitoneally (IP) into LKO and control mice. By measuring the amount of radioactively labeled cholesterol present in the plasma and feces of these mice, we were able to show that LKO mice have an increased ability to take up excess cholesterol into the plasma from peripheral cells and remove it from the body through the fecal bile acids (Fig. 1H). In an attempt to determine protein factors that could be related to the increase in circulating HDL-C and RCT observed above in LKO animals, a quantitative mass spectrometry (MS)-based proteomic approach was taken. Plasma from WT and LKO mice was analyzed to quantify global plasma proteome alterations. Of interest, lecithin:cholesterol acyltransferase (LCAT), an enzyme responsible for the esterification and incorporation of cholesterol within HDL (23), was significantly enriched within the plasma of LKO mice (Fig. 1I). The higher levels of circulating LCAT observed in LKO mice was likely due to increased plasma HDL-C observed in these animals, since the 3′UTR of Lcat does not have a predicted binding site for miR-33 (SI Appendix, Fig. S2A) and the hepatic LCAT mRNA and protein expression was similar in both groups of mice (SI Appendix, Fig. S1 B and C). Together, these findings demonstrate that LKO mice have an improved circulating lipid profile, which may contribute to the reduced atherosclerotic plaque size observed in studies using miR-33 inhibitors.
Hepatic miR-33 Deficiency Does Not Promote Obesity, and Actually Improves Metabolic Function after HFD Feeding.
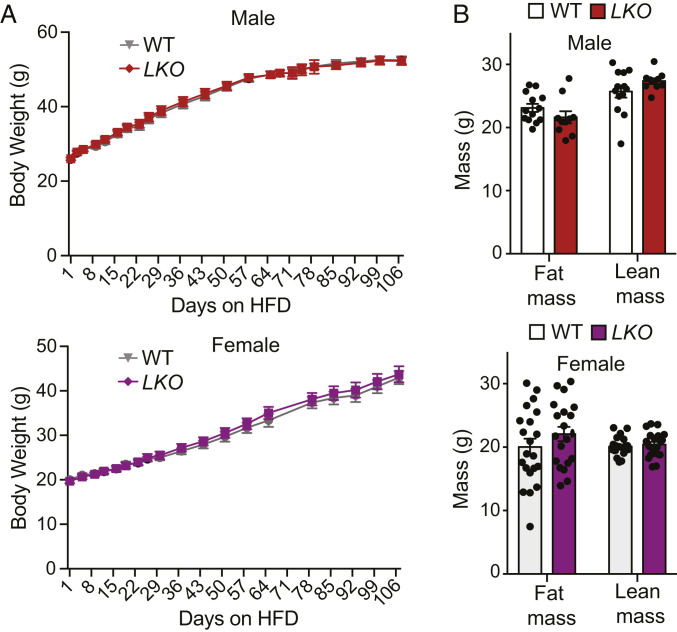
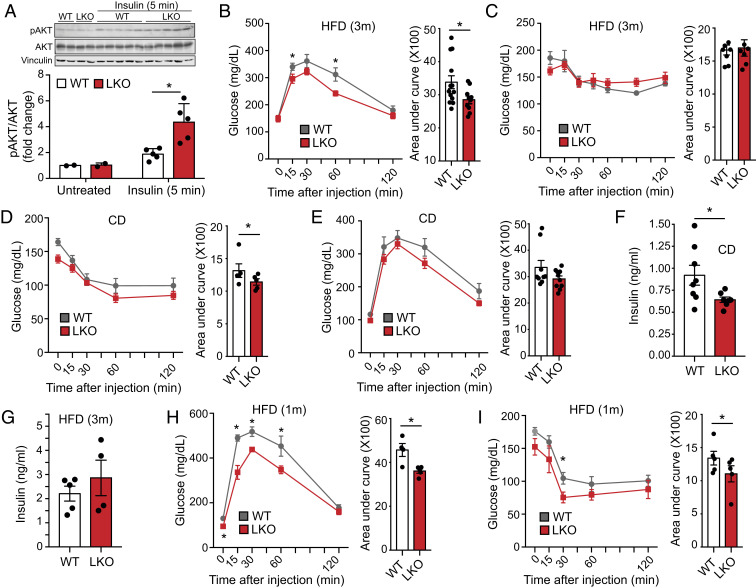
As global miR-33–knockout mice are predisposed to the development of obesity and metabolic dysfunction, we sought to determine the extent to which hepatic miR-33 contributes to this phenotype. However, we did not observe any differences in body weight (Fig. 2A) or body composition (Fig. 2B) in male or female LKO mice after HFD feeding. Moreover, we observed that insulin sensitivity is actually improved in LKO mice, as the phosphorylation of AKT (pAKTs473) and the ratio of pAKTs473/total were increased in the livers of these mice following injection of insulin (Fig. 3A). Interestingly, we also observed an increase in pAKTs473 and pAKTs473/total in the skeletal muscle of LKO mice (SI Appendix, Fig. S3A), suggesting that the improved metabolic regulation by the liver in these mice could lead to a secondary improvement in insulin sensitivity in other tissues. However, this response is not global, as visceral white adipose tissue did not show a similar response (SI Appendix, Fig. S3B). Consistent with this, we find that male LKO mice have an improved ability to regulate circulating blood glucose levels (Fig. 3B), and female LKO mice show a very similar trend that does not quite reach statistical significance (SI Appendix, Fig. S4A). This is in stark contrast to global miR-33–deficient animals that show a profound impairment in regulation of glucose homeostasis after HFD feeding (20, 21).
Fig. 2.
Hepatic miR-33 deficiency does not predispose mice to diet-induced obesity. (A) Body weight trajectories in miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) mice fed an HFD (male, n = 14 to 19; female, n = 21 to 24). (B) Body composition analysis of fat mass and lean mass in HFD-fed WT and LKO mice (male, n = 10 to 13; female, n = 20 to 21). Data represent the mean ± SEM.
Fig. 3.
Loss of miR-33 in the liver improves metabolic function. Characterization of metabolic function in male miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) mice fed chow diet or subjected to short-term (1 mo) or long-term (3 mo) HFD feeding. (A) Representative Western blots of total AKT (tAKT), phosphorylated AKT (pAKTs473), and housekeeping standard Vinculin in liver lysates from WT and LKO mice injected with insulin 5 min prior to euthanasia (Top). Densitometric analysis of the ratio of pAKTs473/total (Bottom; basal, n = 2; insulin, n = 5). Data represent the mean ± SEM. GTTs (B) and ITTs (C) in WT and LKO mice fed an HFD for 3 mo with areas under the curve (Right; n = 9 to 13). ITT (D) and GTT (E) in chow diet-fed WT and LKO mice with areas under the curve (Right; GTT, n = 9 to 10; ITT, n = 5). (F) Plasma insulin levels in chow diet-fed WT and LKO mice (n = 7 to 8). (G) Plasma insulin levels in WT and LKO mice fed an HFD for 3 mo (n = 4 to 5). GTT (H) and ITT (I) in WT and LKO mice fed an HFD for 1 mo with areas under the curve (Right; n = 5). Data represent the mean ± SEM (*P ≤ 0.05 compared with WT animals).
To better understand the metabolic changes that occur in LKO mice, a pyruvate tolerance test (PTT) was performed to assess hepatic gluconeogenesis, but this did not reveal any differences between LKO and control animals (SI Appendix, Fig. S4B). Additionally, despite the improved induction of pAKT that we observe, no differences were found in the ability of LKO mice to modulate blood glucose levels in response to a bolus of insulin [i.e., insulin tolerance test (ITT); Fig. 3C]. To determine whether the metabolic differences between LKO and control mice were diminished due to the severe insulin resistance that develops after prolonged HFD feeding (3 mo), we performed additional characterization of metabolic function in mice fed a chow diet and after a shorter period (1 mo) of HFD feeding. Chow-fed mice were more responsive to insulin during an ITT (Fig. 3D). Glucose tolerance tests (GTTs) in chow-fed animals also revealed a trend toward improved regulation of glucose homeostasis, but this was not found to be statistically significant (Fig. 3E). Importantly, circulating insulin levels were found to be reduced in chow diet-fed LKO mice, which likely contributed to the less substantial response to glucose administration (Fig. 3F). Insulin levels were increased after 3 mo of HFD feeding in male mice, and the difference between WT and LKO mice was no longer observed (Fig. 3G). In female mice, the increase in insulin levels in response to HFD was less pronounced and tended to be even lower in LKO mice compared to controls. This may in part account for the more moderate effects observed in regulation of glucose homeostasis (SI Appendix, Fig. S4C).
Metabolic characterization of mice fed an HFD for only 1 mo also shows that LKO animals have improved regulation of glucose homeostasis during a GTT (Fig. 3H). Additionally, although the differences are less pronounced than those observed in chow diet-fed mice, regulation of glucose levels in response to insulin was also significantly improved (Fig. 3I). While the metabolic phenotype in these animals is not dramatic, our original results and the additional data we have provided support the conclusion that LKO mice have improved insulin sensitivity. In relatively healthy control mice fed a chow diet, this difference is largely offset by increasing circulating insulin levels, but HFD feeding leads to an impaired ability to regulate glucose homeostasis. As such, the improved insulin sensitivity of LKO mice results in a significant improvement in regulation of glucose homeostasis after HFD feeding.
To examine other potential explanations for the obesity phenotype associated with global loss of miR-33, we also assessed the impact of adipocyte- and macrophage-specific miR-33 deficiency. Our characterization of miR-33loxP/loxP mice crossed tob Adiponectin-Cre animals (ATKO) did not reveal any differences in body weight or body composition in male or female mice fed chow or HFD (SI Appendix, Fig. S5 A–C). ATKO mice also did not show the differences in fat tolerance tests or GTTs that were observed in whole-body miR-33–knockout mice (SI Appendix, Fig. S5 D–F), and no differences were observed in circulating HDL-C levels under either chow- or HFD-fed conditions (SI Appendix, Fig. S5G). Additionally, miR-33loxP/loxP mice were crossed with LysM-Cre animals to generate mice deficient for miR-33 specifically in macrophages and neutrophils (MKO). As our prior work has demonstrated that bone marrow transplant from miR-33–deficient animals was sufficient to reduce atherosclerotic plaque formation, these animals were characterized primarily in the context of hyperlipidemia. Similar to what was observed after HFD feeding, whole-body miR-33–knockout mice showed a pronounced body-weight and insulin-resistance phenotype under these conditions (24). MKO mice were not found to have any differences in body weight with either chow or Western diet (WD; SI Appendix, Fig. S6A). Circulating levels of HDL-C were also not different in these mice (SI Appendix, Fig. S6B). Together, these findings demonstrate that neither the macrophages nor adipose tissue contribute substantially to the obesity and metabolic dysfunction observed in mice lacking miR-33 globally.
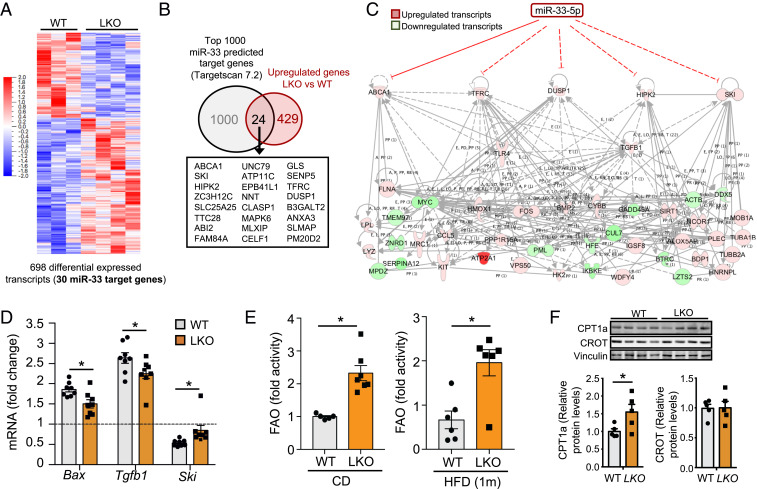
To assess what factors contribute to the improved metabolic function we observe in LKO mice, we performed RNA sequencing (RNA-seq) analysis in hepatic tissue from LKO and control animals on chow or HFD. Principal component analysis of this dataset reveals clear separation based on genotype (Fig. 4A). In HFD-fed animals, there were 735 genes whose expression was significantly altered in LKO mice, among which 278 genes were up-regulated and 457 down-regulated (Fig. 4B). We then used Ingenuity Pathway Analysis to assess the relative functions of these dysregulated genes and were able to identify a number of canonical pathways that were overrepresented in this dataset (Fig. 4C). The top pathway in this analysis was the hepatic fibrosis and stellate cell activation pathway, which included numerous genes involved in promoting hepatic fibrosis that showed reduced expression in LKO mice compared to controls (Fig. 4D). Further analysis revealed inflammatory response as one of the top diseases and functions associated with loss of miR-33 in the liver of HFD-fed mice, which was predicted to be decreased (SI Appendix, Table S1). Consistent with this, leukocyte extravasation (Fig. 4E) and other pathways related to inflammation (Fig. 4C) were also down-regulated in the liver of these animals. Upstream regulator analysis identified decreased activity of a number of cytokines (TNF, IL1β), as well as the profibrotic factor TGFβ1. These key factors are responsible for regulation of numerous other upstream regulators, and therefore likely to be responsible for many of the transcriptional changes observed (SI Appendix, Fig. S7). As expression of miR-33 in stellate cells was not found to be substantially impacted by Albumin-Cre–specific excision (SI Appendix, Fig. S1 A and B), it is likely that the changes in genes related to stellate cell activation and inflammation were secondary to alterations in hepatocyte function, which have been shown to have an important impact on stellate cell activation and fibrosis (25). As hepatic fibrosis and inflammation can promote chronic liver disease and impair liver function, these changes may directly contribute to the improved metabolic function we observe in these animals.
Fig. 4.
Liver-specific miR-33 deficiency reduces the expression of genes related to hepatic fibrosis and inflammation. RNA-seq from livers of male miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) mice fed an HFD. (A) Principal component analysis of RNA-seq data from WT and LKO mice. (B) Heat map depicting differentially expressed genes between WT and LKO mice. (C) Top 15 canonical pathways overrepresented among differentially expressed genes in RNA-seq analysis of WT and LKO mice. Orange bars indicate pathways in which genes are consistently up-regulated, blue indicates pathways in which genes are consistently down-regulated, and gray indicates pathways in which some genes are significantly altered in both directions. (D and E) Heat maps depicting differentially expressed genes involved in hepatic fibrosis and hepatic stellate cell activation (D) and leukocyte extravasation (E) pathways.
Hepatic miR-33 Deficiency Protects against Acute and HFD-Induced Liver Fibrosis.
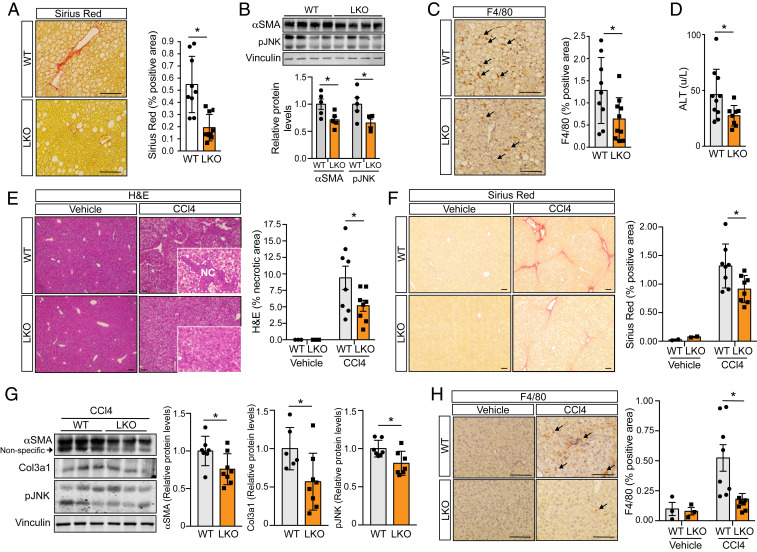
To directly evaluate whether miR-33 deficiency can protect against HFD-induced liver fibrosis, we performed picrosirius red staining in histologic liver sections. This analysis demonstrated that collagen accumulation was significantly reduced in the livers of LKO mice compared to controls after HFD feeding (Fig. 5A). Consistent with this, Western blot analysis revealed a significant reduction in the protein levels of markers of fibrosis (αSMA) and inflammation (pJNK; Fig. 5B). F4/80 staining also revealed a decrease in macrophage accumulation within the livers of LKO mice (Fig. 5C), and circulating levels of alanine aminotransferase (ALT), a common biomarker of liver damage, were reduced (Fig. 5D). The reduction of hepatic macrophage content in LKO mice was independent of circulating leukocyte distribution, which was similar in WT and LKO mice fed an HFD for 1 mo (SI Appendix, Fig. S9). To further assess the extent to which miR-33 deficiency protects against hepatic fibrosis, we treated LKO and control animals with carbon tetrachloride (CCl4), a commonly used model of acute liver fibrosis. While the extent of fibrosis induced using this model was much greater than that observed in our HFD-fed animals, the protection elicited from hepatic miR-33 deficiency was similarly pronounced. Histologic assessment of hepatic sections demonstrated that LKO mice had a significant reduction in the accumulation of both necrotic regions (Fig. 5E) and collagen fibers after CCl4 treatment (Fig. 5F). Western blot analysis also revealed a reduction in αSMA and pJNK, as well as Col3a1, in LKO mice treated with CCl4 compared to controls (Fig. 5G). Finally, in keeping with our findings in HFD-fed mice, the accumulation of F4/80-positive macrophages in response to CCl4 treatment was reduced in LKO mice (Fig. 5H). Together, these findings demonstrate that loss of miR-33 protects against the development of hepatic fibrosis, which may account in part for the improved metabolic function of LKO mice on HFD.
Fig. 5.
Loss of miR-33 in liver protects against HFD- and CCl4-induced hepatic fibrosis. Characterization of factors related to fibrosis, inflammation, and liver function in male miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) mice. (A) Representative images and quantification of Sirius red staining in liver sections from WT and LKO mice fed an HFD (n = 9 to 10). (B) Representative Western blots and densitometric analysis of αSMA, pJNK, and housekeeping standard Vinculin in WT and LKO mice fed an HFD (n = 5). (C) Representative images and quantification of F4/80 staining in liver sections from WT and LKO mice fed an HFD (n = 9 to 10). Arrows indicate F4/80-positive cells. (D) Serum ALT levels in WT and LKO mice fed an HFD (n = 8 to 10). (E) Representative images of H&E staining in liver sections from WT and LKO mice treated with vehicle or CCl4 for 6 wk. (Insets) Increased-magnification views of the presence or lack of necrotic areas, which are also quantified (Right; vehicle, n = 3; CCl4, n = 8). (F) Representative images and quantification of Sirius red staining in liver sections from WT and LKO mice treated with vehicle or CCl4 for 6 wk (vehicle, n = 2; CCl4, n = 8). (G) Representative Western blots and densitometric analysis of αSMA, Col3a1, pJNK, and housekeeping standard Vinculin in WT and LKO mice treated with CCl4 for 6 wk (n = 7 to 8). (H) Representative images and quantification of F4/80 staining in liver sections from WT and LKO mice treated with vehicle or CCl4 for 6 wk (vehicle, n = 3; CCl4, n = 8). Arrows indicate F4/80-positive cells. Data represent the mean ± SEM (*P ≤ 0.05 compared with WT animals).
Identification of Differentially Expressed miR-33 Target Genes.
We next used RNA-seq data to assess what known and predicted targets of miR-33 may be involved in mediating these effects. As previous reports have demonstrated that miR-33 is repressed in the liver along with SREBP2 under conditions where hepatic lipid levels are elevated (4, 26), we assessed changes in predicted miR-33 targets in liver samples from mice under chow diet conditions to identify target genes that may be altered in these conditions and contribute to the protection from HFD-induced fibrosis and metabolic dysfunction that we observe. Principal component analysis demonstrated a clear separation between control and LKO, and genes from a number of pathways were found to be significantly enriched in this dataset (SI Appendix, Fig. S10). In chow diet-fed animals, loss of miR-33 led to significant alterations in the expression of 698 genes, 30 of which were identified as strong potential targets of miR-33 based on the TargetScan prediction algorithm (Fig. 6A). Among these predicted targets, a higher proportion of genes (24 of 30) were up-regulated than would be predicted based on the rest of the dataset (Fig. 6B). Two of these predicted targets, Dusp1 and Tfrc, were identified as likely upstream regulatory factors in this dataset. Two of the top transcriptional regulators in this analysis were Tlr4 and Tgfb1, and these targets, as well as other known (Abca1) and predicted (Ski and Hipk2) miR-33 targets, have been linked to TLR4 and TGFβ signaling. Together, these five potential miR-33 targets are predicted to directly regulate a number of other genes identified as being dysregulated in LKO mice under chow diet-fed conditions, and many of these were also identified as important upstream regulators, demonstrating the ability of miR-33 to directly mediate many of the transcriptional changes observed under chow diet conditions (Fig. 6C). For comparison, under HFD-fed conditions, 28 predicted miR-33 targets were found to be significantly altered, but only 11 of these were up-regulated, and, among these, only ABCA1 was identified as a potential upstream regulator (SI Appendix, Fig. S11). Of particular relevance, SKI has been demonstrated to be an important negative regulator of TGFβ signaling. Since TGFβ signaling is considered the primary pathway responsible for induction of fibrotic response, impairment in this signaling pathway due to derepression of Ski could play a direct role in mediating the antifibrotic response observed in LKO mice. Consistent with this hypothesis, expression of Ski was also found to be elevated in LKO mice compared to controls following treatment with CCl4, while expression of the proapoptotic factor Bcl2-associated protein X (Bax), which is induced by fibrosis and TGFβ, was reduced (Fig. 6D).
Fig. 6.
RNA-seq in chow diet-fed mice identifies potential targets of miR-33 that are up-regulated in LKO mice and could mediate effects on gene expression and functional changes. RNA-seq from livers of male miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) mice fed a chow diet. (A) Heat map depicting differentially expressed genes between WT and LKO mice. (B) Venn diagrams depicting the overlap between miR-33 target genes and total up-regulated transcripts. (C) Network depicting known and predicted miR-33 targets that are up-regulated in LKO mice and predicted to contribute to changes in other dysregulated genes. (D) qPCR analysis of mRNA expression of Bax, Tgfb1, and Ski in WT and LKO mice treated with CCl4 for 6 wk (n = 8). (E) Analysis of FAO in the livers of male WT and LKO mice on a chow diet (Left) or HFD (Right; n = 5 to 7). (F) Representative Western blots and densitometric analysis of CPT1a, CROT, and housekeeping standard Vinculin in male WT and LKO mice fed an HFD. Data represent the mean ± SEM (*P ≤ 0.05 compared with WT animals).
Consistent with the repression of miR-33 and SREBP2 under hyperlipidemic conditions (26), most of the miR-33 targets that were identified as being up-regulated in the liver of LKO mice under chow diet conditions were not found to be altered after prolonged HFD feeding, although ABCA1 was still elevated both at the mRNA and protein level (SI Appendix, Fig. S12). As such, many of the changes that we observe after HFD feeding are likely secondary to differences in metabolic regulation and liver function. Enhanced FAO has previously been demonstrated to protect against fibrosis (27), and we observed a dramatic increase in the capacity for FAO in LKO mice on both chow and HFD (Fig. 6E). miR-33 has been shown to regulate a number of factors involved in FAO in the liver as well as other tissues. Consistent with this, we observe increased protein expression of CPT1a in the livers of our LKO mice (Fig. 6F). Despite this, we do not observe any differences in hepatic TAGs between LKO and control animals on HFD, and only a trend toward reduced TAGs in chow diet-fed mice (SI Appendix, Fig. S13). Overall, our findings demonstrate that hepatic miR-33 targets a number of factors related to the regulation of lipid metabolism, fibrosis, and inflammatory response that together lead to improved metabolic regulation by the liver.
Loss of miR-33 in the Liver Is Not Sufficient to Reduce Atherosclerotic Plaque Size.
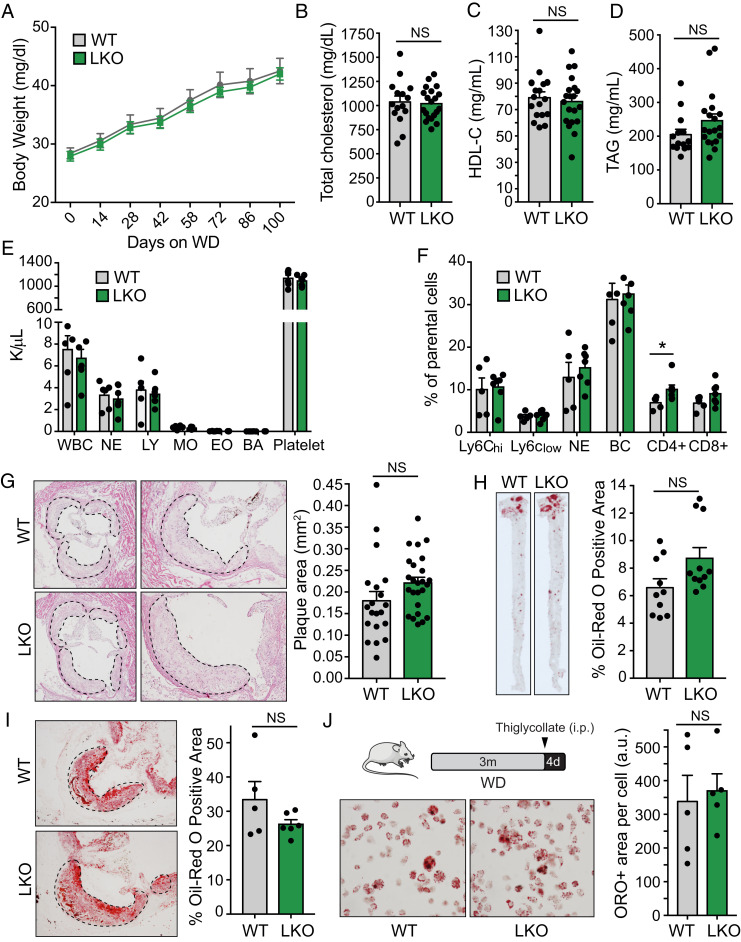
In our previous work, the obesity and metabolic dysfunction observed in whole-body miR-33–knockout mice prevented any meaningful assessment of how changes in HDL biogenesis and RCT in the liver impact atherosclerosis. As these phenotypes are not observed in our LKO mice, we next sought to determine whether these animals were protected from the development of atherosclerotic plaques. To do this, we injected LKO and control mice with an adenovirus encoding a gain-of-function mutant of the proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme (AAV8-PCSK9). This approach has previously been demonstrated to cause degradation of the LDL receptor (LDLR), thereby promoting hypercholesterolemia and atherosclerotic plaque formation in mice fed a WD (40% fat Kcal, 1.25% cholesterol). Similar to what we observed in mice without hypercholesterolemia, LKO mice in this study did not show any differences in body weight even after WD feeding (Fig. 7A). However, following AAV8-PCSK9 injection and WD feeding, we no longer observed any differences in circulating levels of total cholesterol or HDL-C between control and LKO mice (Fig. 7 B and C). TAGs were once again unaltered (Fig. 7D). These effects are consistent with what we previously observed in miR-33–knockout mice in an Ldlr-knockout background and are likely due to the fact that transcription of Srepf2, and therefore miR-33, is repressed under hyperlipidemic conditions (4, 26). Similarly, we did not observe any differences in total cholesterol, HDL-C, or TAGs in LKO mice after HFD feeding (SI Appendix, Fig. S14). Assessment of circulating leukocytes by Hemavet (Fig. 7E) and fluorescence-activated cell sorting (FACS; Fig. 7F) did not reveal differences in most immune cells between LKO mice and control animals, but CD4+ cells were found to be elevated in LKO mice. As LKO mice do not show the pronounced obesity and hyperlipidemia phenotype observed in mice deficient for miR-33 globally, this model provides an excellent opportunity to assess the impact of hepatic miR-33 on atherogenesis.
Fig. 7.
Hepatic miR-33 deficiency does not impact atherosclerotic plaque development. Characterization of male miR-33loxP/loxP (WT) and miR-33loxP/loxP/AlbuminCre (LKO) mice treated with AAV8-PCSK9 and fed a WD for 16 wk. (A) Body weight trajectories of WT and LKO mice fed a WD (n = 12 to 13). (B–D) Levels of total cholesterol (B), HDL-C (C), and TAGs (D) in plasma of WT and LKO mice following AAV8-PCSK9 treatment and WD feeding (n = 15 to 20). (E) Peripheral blood counts from WT and LKO mice following AAV8-PCSK9 treatment and WD feeding using a Hemavet hematology analyzer (n = 6 to 7). (F) Flow cytometry analysis of circulating leukocytes from WT and LKO mice following AAV8-PCSK9 treatment and WD feeding. Data are expressed as percentages of live cells (n = 5). (G) Representative images (enlarged, Right) and quantification of H&E-stained atherosclerotic plaques within the aortic root of WT and LKO mice following AAV8-PCSK9 treatment and WD feeding (n = 20 to 25). (H) Representative images and quantification of Oil Red O-stained en face preps of WT and LKO mice following AAV8-PCSK9 treatment and WD feeding (n = 10 to 11). (I) Representative images and quantification of Oil Red O-stained atherosclerotic plaques within the aortic root of WT and LKO mice following AAV8-PCSK9 treatment and WD feeding (n = 5 to 6). (J) Representative images and quantification of Oil Red O staining in peritoneal macrophages from WT and LKO mice treated with AAV8-PCSK9 and fed a WD for 12 wk (n = 5). Data represent the mean ± SEM (*P ≤ 0.05 compared with WT animals).
Analysis of the atherosclerotic plaque formation in LKO and control animals also did not demonstrate any significant differences in the size of plaques within the aortic root (Fig. 7G). Lipid accumulation, as assessed by Oil Red O staining of the aorta en face (Fig. 7H) and in histological sections of the aortic root (Fig. 7I), was also unaltered. In vivo foam cell formation was also unchanged in LKO mice, consistent with the similar levels of circulating lipids (Fig. 7J). Overall, this work demonstrates that loss of miR-33 in the liver does not lead to increased body weight and actually improves measures of liver function and whole-body metabolic regulation. Despite this, and the increased circulating HDL-C and RCT observed in chow-diet conditions, LKO mice did not show any protection from the formation of atherosclerotic plaques.
Discussion
While miRNAs represent an entirely new level of regulation that could provide novel therapeutic approaches for the treatment of human diseases, the promiscuous nature of these miRNAs and our limited understanding of the mechanisms by which they function has raised concerns over the potential utility of miRNA-based therapies. miR-33 is an excellent example of both the potential utility and pitfalls of this type of approach. Work by numerous groups has demonstrated that loss or inhibition of miR-33 can promote macrophage cholesterol efflux, increase circulating HDL-C, and limit the development of atherosclerotic plaques in mouse models. Recent work has also demonstrated that loss or inhibition of miR-33 could also impede the development of fibrosis in both the heart and kidney (28, 29). However, global loss of miR-33 leads to the development of obesity, dyslipidemia, and insulin resistance (20, 21). In Ldlr−/− mice, these detrimental whole-body effects were found to offset the beneficial changes observed in plaque macrophages (24). We have shown that pH low insertion peptides can be used to deliver miR-33 inhibitors specifically to the kidney and other low-pH microenvironments (28), and other recent work by our group has demonstrated that this approach may also be effective for delivering miR-33 inhibitors to macrophages within the tumor microenvironment (30). While this type of approach may prove very important for limiting unintended consequences of miRNA-based therapies, it will still be necessary to explore in detail the specific role of miRNAs in different tissues to properly assess the potential benefits and hazards of manipulating these miRNAs in human patients.
To this end, we generated a conditional miR-33 knockout mouse model (miR-33loxP/loxP) and used this to selectively eliminate miR-33 from key metabolic tissues. Our initial efforts have focused on the liver, as alterations in hepatic lipid accumulation and biosynthesis have been suggested to be responsible for some of the negative effects associated with inhibition or loss of miR-33 (19, 20). Our recent work demonstrated that the ability of the liver to regulate lipid synthesis in response to changes in nutrient status was not impacted by miR-33, and that changes in food consumption were primarily responsible for driving the obesity and metabolic dysfunction observed in these animals (21). However, as the liver is one of the primary tissues responsible for sending signals to the brain to coordinate feeding behavior and other metabolic functions, we crossed our miR-33loxP/loxP mice to the well-established Albumin-Cre mouse line to generate animals that lack miR-33 selectively in the liver. Additionally, we used Adiponectin-Cre and LysM-Cre to selectively remove miR-33 from the adipose tissue and macrophages, respectively.
Initial characterization of these animals clearly demonstrated that loss of miR-33 in the liver, adipose tissue, and macrophages was not responsible for promoting the obesity and metabolic dysfunction observed in mice with global miR-33 deficiency. Indeed, male LKO mice actually had significantly enhanced insulin sensitivity and an improved ability to regulate glucose homeostasis after HFD feeding. Consistent with this, we observe an increase in FAO in the liver of LKO mice. While the results of our PTT indicate that the capacity for hepatic gluconeogenesis is similar between LKO and control animals, the response to insulin, as measured by AKT phosphorylation, is improved in LKO animals. This indicates that the ability to suppress gluconeogenesis in response to insulin is likely enhanced, which could mediate the improved metabolic function that is observed. Additionally, we also observe enhanced AKT phosphorylation in the skeletal muscle of LKO animals, demonstrating that the improved hepatic function observed in LKO mice can also improve insulin sensitivity in other important metabolic organs, consistent with the liver’s role as a master regulator of lipid and glucose metabolism. These peripheral effects may also contribute to some extent to the improved metabolic function that is observed.
A similar trend toward improved glucose homeostasis was observed in female LKO mice, although this did not reach statistical significance. It is possible that hormonal factors or other differences between the sexes may have contributed to the somewhat greater variability in females, but we have no direct evidence to suggest that this is the case. Alternatively, it is possible that the window for observing metabolic alterations in the male mice was larger. Our data show that the ability to restore blood glucose levels was impaired to a greater degree by HFD in male mice than in their female counterparts, consistent with previous comparisons between sexes, suggesting this may have contributed to the more significant increase observed in male mice. Similarly, the levels of circulating insulin after HFD were higher in male mice. Female animals show a trend toward reduced insulin levels in the LKO mice, similar to what is observed in chow diet-fed animals, which may also contribute to the slightly less substantial changes in regulation of glucose homeostasis. As our metabolic phenotype was more consistent in male animals and our breeding strategy provided more male mice for analysis, some of the follow-up analyses and experiments on fibrosis and atherosclerosis were performed only in male mice.
RNA-seq revealed a reduction in the expression of genes and pathways related to inflammation and fibrosis after HFD feeding. Further assessment demonstrated that LKO mice were protected against both HFD- and CCl4-induced hepatic fibrosis. Mechanistically, we have identified known targets of miR-33 (ABCA1, CPT1) related to lipid metabolism, as well as miR-33 targets more directly involved in fibrotic response (SKI, HIPK2), that may be involved in mediating these effects. These findings indicate that, rather than being detrimental to hepatic function, inhibition of miR-33 in the liver may actually have beneficial effects. This suggests that targeted miR-33 inhibition in the liver could provide a viable therapeutic approach both for metabolic disorders associated with impaired liver function and more acute cases of liver damage. This is important, as miR-33 inhibitors have been shown to effectively target miR-33 in the liver. Moreover, approaches such as triantennary N-acetylgalactosamine–conjugated antisense oligonucleotides have been demonstrated to be effective at targeted delivery specifically to the liver (31).
We have previously demonstrated that loss of miR-33 in macrophages and other hematopoietic cells is sufficient to reduce atherosclerotic plaque burden. These effects appear to be primarily due to targeting of ABCA1, as selective disruption of the interaction between ABCA1 and miR-33 was found to largely mimic these effects. However, the increased feeding and corresponding predisposition to obesity and metabolic dysfunction of miR-33–deficient mice has prevented any meaningful assessment of whether the enhanced biogenesis of HDL in the liver of miR-33–deficient animals also contributes to the beneficial effects on delaying atherogenesis. As LKO mice were not found to have altered body weight or metabolic function, we sought to address this question in these animals. Consistent with previous work, we found that loss of miR-33 in the liver resulted in increased circulating HDL-C levels and enhanced RCT in young mice on a chow diet. We further reveal, through a proteomic approach, a potential secondary mechanism whereby miR-33 can directly regulate HDL function through the regulation of LCAT, which may also contribute to the increased RCT capacity of LKO mice. As LCAT levels were not found to be altered in the liver, and LCAT in circulation is primarily bound to circulating HDL-C, it is likely that the increased LCAT we observe in the plasma of LKO animals is a reflection of the increased HDL-C levels observed in these animals. However, differences in HDL-C were not observed under hyperlipidemic conditions. It is therefore not surprising that these mice did not show any protection from atherosclerotic plaque development. While it is certainly possible that enhanced HDL biogenesis and other effects of miR-33 in the liver could contribute to the reduced plaque burden observed with inhibition of miR-33, our findings clearly demonstrate that these changes are not sufficient to drive this effect.
These findings indicate that the ability of miR-33 to alter cholesterol efflux and other functions within the plaque macrophages is the primary mechanism by which miR-33 impacts atherosclerotic plaque formation. It is important to note that mice have only one isoform of miR-33, while humans have two isoforms, miR-33a and miR-33b. Like the mouse isoform, miR-33a is encoded within the Srebf2 gene and would be expected to be repressed under hyperlipidemic conditions. However, miR-33b is encoded within the Srebf1 gene, which is regulated in an independent manner (26). This suggests that, in humans, hepatic miR-33b may play a more important role in regulation of atherosclerosis than we observe in mice. In the future, it will be important to determine how miR-33 impacts the function of other key metabolic tissues and how this contributes to the obesity and metabolic dysfunction observed in whole-body miR-33–deficient mice. In future work, it will also be important to determine the specific tissues and cell types that are responsible for mediating the obesity and feeding phenotypes observed in whole-body knockout animals as well as the mechanisms through which miR-33 promotes these effects.
Materials and Methods
The plasma proteomics, RNA-seq analysis, and the isolation, cellular purification, and statistical methods are described in SI Appendix, Materials and Methods.
Animals.
Generation of conditional miR-33–knockout mice (miR-33loxP/loxP) was accomplished with the assistance of Cyagen Biosciences. The success of this approach has been verified by Southern blotting and confirmed by PCR-based genotyping using specific primers. To generate liver-specific miR-33–knockout mice, miR-33loxP/loxP mice were crossed with transgenic mice expressing Cre recombinase under the control of tissue-specific promoters: Albumin promoter (JAX stock 003574), AdipoQ promoter (JAX stock 010803), and Lyz2 promoter (JAX stock 004781). For diet-induced obesity experiments, mice were fed a standard chow diet for 8 to 10 wk, after which they were either switched to an HFD containing 60% calories from fat (D12492; Research Diets) for 8 to 20 wk or maintained on chow diet. For atherosclerosis experiments, proprotein convertase subtilisin/kexin type 9 (PCSK9) adenoassociated virus (AAV8-PCSK9) was injected IP (1.0 × 1011 VC) to promote the degradation of LDLR and increase circulating cholesterol levels. Accelerated atherosclerosis was induced by feeding mice for 12 to 16 wk with a WD containing 1.25% cholesterol (D12108; Research Diets). Body weights were measured throughout HFD or WD feeding studies, and analysis of body composition was performed by Echo MRI (Echo Medical System). Mice used in all experiments were sex- and age-matched and kept in individually ventilated cages in a pathogen-free facility. All of the experiments were approved by the institutional animal care use committee of Yale University School of Medicine.
RNA Isolation and Real-Time qPCR.
Total RNA and miRNAs from liver tissue were isolated using the miRNeasy miRNA Isolation Kit according to the manufacturer’s instructions. For mRNA expression analysis, cDNA was synthesized using iScript RT Supermix (Bio-Rad) following the manufacturer’s protocol. Real-time qPCR analysis was performed in duplicate using SsoFast EvaGreen Supermix (BioRad) on an iCycler Real-Time Detection System (Eppendorf). The mRNA levels were normalized to 18S. To quantify miR-33 levels, RNAs were reverse-transcribed using the TaqMan MicroRNA Reverse Transcription Kit and quantified with the miRNA-specific TaqMan miRNA assay (Life Technologies/Invitrogen). The amount of the indicated miRNA was normalized to the amount of U6 RNA.
Lipoprotein Profile and Lipid Measurements.
Mice were fasted for 12 to 16 h overnight before blood samples were collected by retroorbital venous plexus puncture, and plasma was separated by centrifugation. HDL-C was isolated by precipitation of non–HDL-C, and both HDL-C fractions and total plasma were stored at −80 °C. Total plasma cholesterol and TAGs were enzymatically measured (Wako Pure Chemicals) according to the manufacturer’s instructions. The lipid distribution in plasma lipoprotein fractions was assessed by fast-performed liquid chromatography (LC) gel filtration with two Superose 6 HR 10/30 columns (Pharmacia Biotech).
In Vivo Macrophage-Specific RCT.
Bone marrow-derived macrophages (BMDMs) from WT mice were differentiated in vitro. After 7 d of differentiation, cells were cultured in 75-cm tissue culture flasks at 5 × 106 cells per flask and grown to 90% confluence in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum. Mouse macrophages were incubated for 48 h in the presence of 5 μCi/mL of [1α,2α(n)-3H]-cholesterol (GE Healthcare), 100 μg/mL of acetylated LDL, and 10% lipoprotein-depleted serum. These cells were washed, equilibrated, detached by gently pipetting, and resuspended in 0.9% (wt/vol) saline and pooled before being injected IP into control or LKO mice. Equal plasma counts per minute were injected in both groups of mice. Mice were then individually housed, and stools were collected over the next 2 d. Plasma counts per minute were determined at 48 h by liquid scintillation counting. At that time, mice were euthanized. Fecal lipids were extracted with isopropyl alcohol/hexane (2:3; vol/vol). The lipid layer was collected and evaporated and [3H]-cholesterol radioactivity measured by liquid scintillation counting. The [3H]-tracer detected in fecal bile acids was determined in the remaining aqueous portion of fecal material extracts.
Metabolic Characterization.
GTTs were performed following overnight fasting (16 h) by IP injection of glucose at a dose of 2 g/kg for chow diet and 1-mo HFD-fed mice or 1 g/kg for 3-mo HFD-fed mice. Blood glucose was measured at 0, 15, 30, 60, and 120 min post injection. ITTs were performed following a 6-h fast by IP injection of 0.75 U/kg insulin. Blood glucose measurements were taken 0, 15, 30, 60, and 120 min after injection of insulin. PTTs were performed following overnight fasting (16 h) by IP injection of 1.5 g/kg pyruvate. Blood glucose measurements were taken 0, 15, 30, 60, and 120 min after injection of pyruvate. Plasma insulin levels were measured with Mercodia Mouse Insulin ELISA (no. 10-1247-01) according to manufacturer instructions. Fat tolerance test was performed as previously described (32). Briefly, mice were fasted for 4 h beginning at 7:00 AM, followed by oral gavage of 10 μL olive oil per gram body weight. Blood samples were collected from the tail vein 0, 1, 2, 4, and 6 h after administration.
Western Blot Analysis.
Tissues were homogenized by manual disruption and the Bullet Blender Homogenizer in ice-cold buffer containing 50 mM Tris⋅HCl, pH 7.5, 0.1% sodium dodecyl sulfate (SDS), 0.1% deoxycholic acid, 0.1 mM EDTA, 0.1 mM EGTA, 1% Nonidet P-40, 5.3 mM NaF, 1.5 mM NaP, 1 mM orthovanadate, 1 mg/mL protease inhibitor mixture (Roche), and 0.25 mg/mL 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Roche). Lysates were sonicated and rotated at 4 °C for 1 h before the insoluble material was removed by centrifugation at 12,000 × g for 10 min. After normalizing for equal protein concentration, cell lysates were resuspended in SDS sample buffer before separation by SDS polyacrylamide gel electrophoresis. Following transfer of the proteins onto nitrocellulose membranes, the membranes were probed with the following antibodies: ABCA1 (Abcam no. 18180; 1:1,000), ABCG1 (Novus no. 400–132; 1:1,000), AKT (Cell Signaling no. 4691; 1:500), phosphorylated-AKT(S473) (Cell Signaling no. 9271; 1:500), αSMA (Sigma A5228; 1:2,000), COLIII (Sigma ab7778; 1:1,000), CROT (Novus no. 3144; 1:1,000); CPT1A (Abnova H00001374-P01; 1:1,000), Vinculin (Sigma V9131; 1:2,000), and LCAT (Abcam no. 109417; 1:1,000). Protein bands were visualized using the Odyssey Infrared Imaging System (LI-COR Biotechnology), and densitometry was performed using ImageJ software.
Liver Histology.
Mouse livers were perfused with phosphate-buffered saline (PBS) solution and fixed in 4% paraformaldehyde (PFA) overnight. After PFA, livers were washed three times in PBS and transferred to 70% EtOH. Then, livers were submitted for standard paraffin processing though the Yale Pathology Tissue Services core. Sections were stained with H&E, Sirius red, and F4/80 to evaluate liver morphology and global injury associated with liver inflammation and fibrosis. For each quantification, pictures from eight randomly selected fields were taken with an EVOS microscope. Quantification of stained areas was performed with ImageJ software.
CCl4-Induced Liver Fibrosis.
CCl4 (Sigma-Aldrich no. 289116; NH2COCH2) was administered by IP injection at a dose of 0.6 mL/kg once per week for a total of 6 wk. CCl4 was diluted in corn oil at a final volume of 50 µL. Untreated mice received weekly doses of 50 µL of corn oil. After 6 wk, mice were euthanized, and liver and blood were collected for analysis. For CCl4 administration, age-matched WT (n = 8) and LKO (n = 8) mice were used. For the control untreated groups, WT (n = 3) and LKO (n = 3) were included in the study.
ALT Measurements.
ALT activity was determined in serum with the ALT Activity Assay Kit (Sigma-Aldrich MAK052) following the manufacturer’s recommendations.
FAO.
FAO was assayed as previously described (33). In brief, livers were removed from control and LKO mice and homogenized in five volumes of chilled STE buffer (pH 7.4, 0.25 M sucrose, 10 mM Tris⋅HCl, and 1 mM EDTA). The homogenate was immediately centrifuged, and the pellet was resuspended and incubated with a reaction mixture containing 0.5 mmol/L palmitate (conjugated to 7% BSA/[14C]-palmitate at 0.4 μCi/mL) for 30 min. After this incubation period, the resuspended pellet-containing reaction mixture was transferred to an Eppendorf tube, the cap of which housed a Whatman filter paper disk that had been presoaked with 1 mol/L sodium hydroxide. The 14CO2 trapped in the reaction mixture media was then released by acidification of media using 1 mol/L perchloric acid and gentle agitation of the tubes at 37 °C for 1 h. Radioactivity that had become adsorbed onto the filter disk was then quantified by liquid scintillation counting in a β-counter.
Circulating Leukocyte Analysis.
Blood was collected by retroorbital puncture in heparinized microhematocrit capillary tubes. Measurement of total circulating numbers of blood leukocytes was performed using a Hemavet system. For further FACS analysis, erythrocytes were lysed with ACK lysis buffer (155 mM ammonium chloride, 10 mM potassium bicarbonate, and 0.01 mM EDTA, pH 7.4). White blood cells were resuspended in 3% fetal bovine serum (FBS) in PBS, blocked with 2 μg mL−1 of FcgRII/III, then stained with a mixture of antibodies. Monocytes were identified as CD115hi and subsets as Ly6-Chi and Ly6-Clo; neutrophils were identified as CD11bhiLy6Ghi; B cells were identified as CD19hiB220hi; T cells were identified as CD4hi or CD8hi. The following antibodies were used (all from BioLegend): FITC-Ly6-C (HK1.4), PE-CD115 (AFS98), APC- Ly6-G (1A8), PB-CD11b (M1/70), APC-CD19 (6D5), PE/Cy7-B220 (RA3-6B2), APC/Cy7-CD4 (RM4-5), and BV421-CD8a (53-6.7). All antibodies were used at 1:300 dilutions.
Histology, Immunohistochemistry, and Morphometric Analyses of Atherosclerotic Plaques.
Mouse hearts were perfused with PBS and put in 10 mL 4% paraformaldehyde for 4 h. After incubation in paraformaldehyde, hearts were washed with PBS, left with PBS for 1 h, and put in 30% sucrose overnight. Finally, hearts were embedded in OCT compound and frozen. Serial sections were cut at 6-µm thickness using a cryostat. Every fourth slide from the serial sections was stained with hematoxylin and eosin, and each consecutive slide was stained with Oil Red O for quantification of the lesion area and lipid accumulation, respectively. Aortic lesion size of each animal was obtained by averaging the lesion areas in at least nine sections from the same mouse.
En Face Oil Red O Staining.
Oil Red O stock solution (35 mL; 0.2% wt/vol in methanol) was mixed with 10 mL of 1 M NaOH and filtered. Aortas opened up longitudinally were briefly rinsed with 78% methanol, stained with 0.16% Oil Red O solution for 50 min, and then destained in 78% methanol for 5 min. The lesion area was quantified as percent of Oil Red O staining area in total aorta area.
Cell Culture.
For in vivo foam cell formation experiments, mice were fed a WD for 12 wk, and cells were harvested by peritoneal lavage 4 d after IP injection of thioglycollate (3% wt/vol). Cells were plated in RPMI medium 1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin. After 2 h, nonadherent cells were removed and remaining cells were stained for 30 min with 0.3% Oil Red O solution in 60% isopropanol. The mean area of Oil Red O-stained region per cell was quantified from six or more fields of containing at least 200 total cells using the ImageJ software from the NIH.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R35HL135820 to C.F.-H.; R01HL105945, R01HL135012 to Y.S.; and 1K01DK120794 to N.L.P.), the American Heart Association (16EIA27550005 to C.F.-H.), Yale Liver Center (P30 DK34989), and the Programa Postdoctoral de Perfecionamiento de Personal del Govierno Vasco (Spain) (to P.F.-T.). M.M. is a British Heart Foundation (BHF) Chair Holder (CH/16/3/32406) with BHF programme grant support (RG/16/14/32397).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2006478118/-/DCSupplemental.
Data Availability.
The genomic data were deposited in the Gene Expression Omnibus (accession no. GSE164517) (34). All study data are included in the article and SI Appendix.
References
- 1.Goedeke L., et al. , MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat. Med. 21, 1280–1289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marquart T. J., Allen R. M., Ory D. S., Baldán A., miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. U.S.A. 107, 12228–12232 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Najafi-Shoushtari S. H., et al. , MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328, 1566–1569 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayner K. J., et al. , MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570–1573 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karunakaran D., et al. , Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ. Res. 117, 266–278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouimet M., et al. , microRNA-33 regulates macrophage autophagy in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, 1058–1067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouimet M., et al. , MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Invest. 125, 4334–4348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dávalos A., et al. , miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. U.S.A. 108, 9232–9237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerin I., et al. , Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J. Biol. Chem. 285, 33652–33661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T., Francl J. M., Boehme S., Chiang J. Y., Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology 58, 1111–1121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen R. M., et al. , miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol. Med. 4, 882–895 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horie T., et al. , MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 17321–17326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayner K. J., et al. , Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 478, 404–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottiers V., et al. , Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci. Transl. Med. 5, 212ra162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayner K. J., et al. , Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Invest. 121, 2921–2931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotllan N., Ramírez C. M., Aryal B., Esau C. C., Fernández-Hernando C., Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr-/- mice–Brief report. Arterioscler. Thromb. Vasc. Biol. 33, 1973–1977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horie T., et al. , MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE-/- mice. J. Am. Heart Assoc. 1, e003376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen R. M., Marquart T. J., Jesse J. J., Baldán A., Control of very low-density lipoprotein secretion by N-ethylmaleimide-sensitive factor and miR-33. Circ. Res. 115, 10–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goedeke L., et al. , Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol. Med. 6, 1133–1141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horie T., et al. , MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat. Commun. 4, 2883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price N. L., et al. , Genetic ablation of miR-33 increases food intake, enhances adipose tissue expansion, and promotes obesity and insulin resistance. Cell Rep. 22, 2133–2145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tecalco-Cruz A. C., Ríos-López D. G., Vázquez-Victorio G., Rosales-Alvarez R. E., Macías-Silva M., Transcriptional cofactors Ski and SnoN are major regulators of the TGF-β/Smad signaling pathway in health and disease. Signal Transduct. Target. Ther. 3, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousset X., Vaisman B., Amar M., Sethi A. A., Remaley A. T., Lecithin: Cholesterol acyltransferase–From biochemistry to role in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 16, 163–171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price N. L., et al. , Genetic dissection of the impact of miR-33a and miR-33b during the progression of atherosclerosis. Cell Rep. 21, 1317–1330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan S. S., et al. , Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 43, 435–443 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Nishino T., et al. , SREBF1/MicroRNA-33b Axis exhibits potent effect on unstable atherosclerotic plaque formation in vivo. Arterioscler. Thromb. Vasc. Biol. 38, 2460–2473 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Simon N., Hertig A., Alteration of fatty acid oxidation in tubular epithelial cells: From acute kidney injury to renal fibrogenesis. Front. Med. (Lausanne) 2, 52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price N. L., et al. , Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight 4, e131102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiga M., et al. , MicroRNA-33 controls adaptive fibrotic response in the remodeling heart by preserving lipid raft cholesterol. Circ. Res. 120, 835–847 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Sahraei M., et al. , Suppressing miR-21 activity in tumor-associated macrophages promotes an antitumor immune response. J. Clin. Invest. 129, 5518–5536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash T. P., et al. , Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 42, 8796–8807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordts P. L., et al. , ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Invest. 126, 2855–2866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynh F. K., Green M. F., Koves T. R., Hirschey M. D., Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 542, 391–405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price N. L., Fernandez-Hernando C., Loss of hepatic miR-33 improves metabolic homeostasis and liver function without altering body weight or atherosclerosis. Gene Expression Omnibus. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164517. Deposited 10 January 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomic data were deposited in the Gene Expression Omnibus (accession no. GSE164517) (34). All study data are included in the article and SI Appendix.