Significance
Understanding the initiating mechanisms of cardiac injury associated with doxorubicin is fundamental to the development of novel preventative strategies. Evidence suggests that expression of membrane transporters contributes to the initial accumulation of doxorubicin in cardiomyocytes and, subsequently, cellular injury. Using patient-derived cardiomyocytes, we identified a candidate membrane transporter contributing to this debilitating phenotype and performed validation studies using engineered cell-based and animal models to demonstrate that targeting this transport mechanism can afford cardio-protection without compromising the anticancer properties of doxorubicin. Our findings provide insights that addresses an unmet therapeutic need in cancer patients receiving doxorubicin-based treatment.
Keywords: solute carriers, slc22a3, doxorubicin, cardiotoxicity, s100 proteins
Abstract
Doxorubicin is a commonly used anticancer agent that can cause debilitating and irreversible cardiac injury. The initiating mechanisms contributing to this side effect remain unknown, and current preventative strategies offer only modest protection. Using stem-cell–derived cardiomyocytes from patients receiving doxorubicin, we probed the transcriptomic landscape of solute carriers and identified organic cation transporter 3 (OCT3) (SLC22A3) as a critical transporter regulating the cardiac accumulation of doxorubicin. Functional validation studies in heterologous overexpression models confirmed that doxorubicin is transported into cardiomyocytes by OCT3 and that deficiency of OCT3 protected mice from acute and chronic doxorubicin-related changes in cardiovascular function and genetic pathways associated with cardiac damage. To provide proof-of-principle and demonstrate translational relevance of this transport mechanism, we identified several pharmacological inhibitors of OCT3, including nilotinib, and found that pharmacological targeting of OCT3 can also preserve cardiovascular function following treatment with doxorubicin without affecting its plasma levels or antitumor effects in multiple models of leukemia and breast cancer. Finally, we identified a previously unrecognized, OCT3-dependent pathway of doxorubicin-induced cardiotoxicity that results in a downstream signaling cascade involving the calcium-binding proteins S100A8 and S100A9. These collective findings not only shed light on the etiology of doxorubicin-induced cardiotoxicity, but also are of potential translational relevance and provide a rationale for the implementation of a targeted intervention strategy to prevent this debilitating side effect.
The anthracycline doxorubicin is commonly used in many regimens for the treatment of solid tumors and hematological malignancies (1, 2). The clinical utility of doxorubicin is limited by the onset of dose-dependent and irreversible cardiac injury that predisposes patients to an increased risk for congestive heart failure (1–3). The apparent lack of inherent regenerative capacity in the heart (4) increases cardiovascular-related mortality and is particularly evident in adolescent and young adult cancer survivors receiving doxorubicin. Moreover, symptomatic cardiac failure can occur even up to 20 y after the cessation of therapy (5, 6). The clinical manifestations of the cardiovascular side effects range from asymptomatic decline in ventricular ejection fraction to symptomatic cardiomyopathy and congestive heart failure (2). Due to pleiotropic mechanisms such as oxidative damage, inhibition of topoisomerase II-β, and intracellular calcium dysregulation, which all contribute to cardiomyocyte cell death (7), current preventative and treatment strategies, such as dexrazoxane, offer only modest protection in the management of doxorubicin-induced cardiac injury and only stabilize patients at a lower level of cardiac function (7, 8). Furthermore, the decision to act on a cardiac injury marker is hampered by the lack of alternative treatment options to replace doxorubicin and the recognition that individualized reductions in the dose of doxorubicin administered to patients will negatively affect disease management. Therefore, the development of novel effective strategies to prevent the occurrence of doxorubicin-induced cardiac injury is urgently needed.
We hypothesized that identification of a transporter-mediated mechanism of cellular uptake of doxorubicin in cardiomyocytes may shed light on the etiology of cardiac injury and may lead to the development of novel therapeutic interventions aimed at targeting this initiating mechanism to limit exposure of the heart to doxorubicin. Drug transporters are the second largest family of membrane proteins that regulate a cell’s internal physiology in the extracellular environment (9). In prior studies evaluating the transmembrane transport of doxorubicin, it was concluded that the predominant mechanism of accumulation is associated with passive diffusion of the unionized drug (10, 11). However, the saturable uptake process of doxorubicin in cell-based models and the cationic properties of doxorubicin at physiological pH support instead the existence of one or more, currently unknown, carrier-mediated mechanisms (12, 13). Various metabolizing enzymes and ATP-dependent efflux transporters have been identified that contribute to doxorubicin-induced cardiac toxicity (14), and certain cationic-type uptake transporters are now recognized as essential carriers in the heart that regulate intracellular levels of cardiac hormones (e.g., catecholamines) and pharmacological response to cardiovascular drugs (15–21). However, direct evidence supporting a role of transporters in the uptake of doxorubicin and subsequent cellular injury in cardiomyocytes remains unknown (22).
We set out to remedy this knowledge deficit by probing the transcriptomic landscape of solute carriers (SLCs) using cardiomyocytes derived from human induced pluripotent stem-cell–derived cardiomyocytes (hiPSC-CMs) of pediatric cancer patients receiving doxorubicin that either did or did not experience cardiotoxicity, based on the hypothesis that differential gene expression between these two groups will allow the identification of SLCs capable of transporting doxorubicin in a manner that would influence susceptibility to injury (23). This analysis resulted in the identification of the organic cation transporter 3 (OCT3) (SLC22A3) as a previously unrecognized uptake transporter of doxorubicin in cardiomyocytes that can be targeted genetically and pharmacologically in order to afford cardio protection without negatively influencing the antitumor properties of doxorubicin.
Results
SLC Transportome in Cardiomyocytes of Pediatric Cancer Patients.
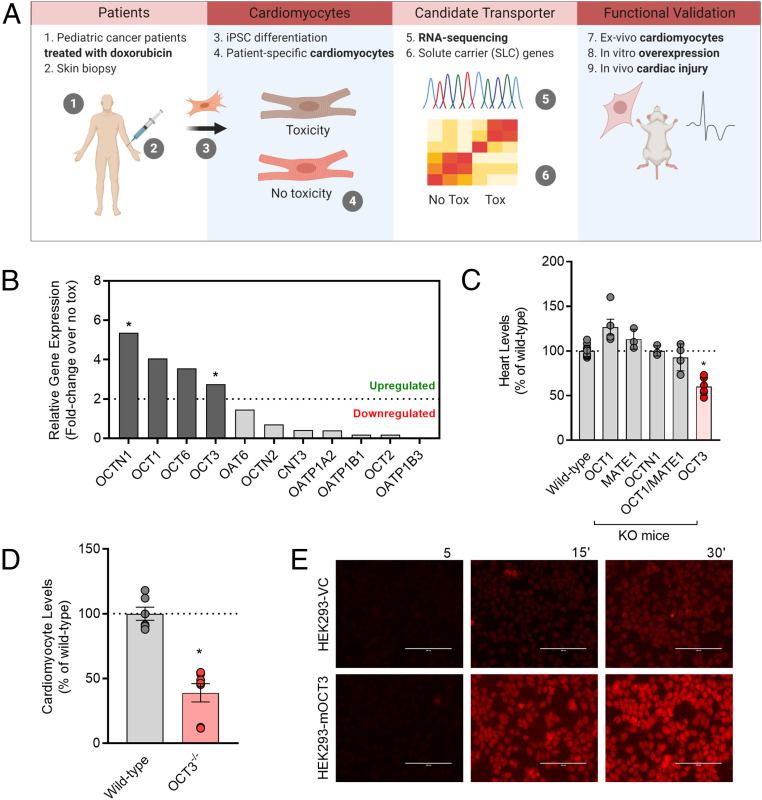
We hypothesized that doxorubicin-induced cardiac injury and its associated pathophysiology is dependent on a carrier-mediated mechanism of doxorubicin entry into cardiomyocytes. In order to comprehensively evaluate the expression of SLC genes in cardiomyocytes, we utilized hiPSC-CMs due to their unique ability to recapitulate patient-specific expression signatures in vivo (23). We first performed transcriptomic sequencing to evaluate the landscape of SLCs in hiPSC-CMs from pediatric cancer patients receiving doxorubicin who did or did not experience clinically annotated cardiotoxicity (Fig. 1A and SI Appendix, Tables S1 and S2 and Fig. S1A). Further investigation indicated that several SLCs are differentially expressed (more than a two-fold change) in hiPSC-CMs of patients who experienced cardiotoxicity. In particular, we found that expression of the cationic transporters OCT1 (SLC22A1), OCT3 (SLC22A3), OCTN1 (SLC22A4), and OCT6 (SLC22A16) was substantially up-regulated in patients experiencing cardiotoxicity (Fig. 1B). The transcriptomic landscape from control hiPSC-CMs derived from healthy volunteers recapitulated the expression levels across structurally similar and diverse subfamilies of SLCs, compared to adult heart tissue samples (SI Appendix, Fig. S1B), and we found that the observed gene expression changes are not causally dependent on exposure to doxorubicin (SI Appendix, Fig. S1C). These findings are in line with the notion that the baseline expression of certain SLCs could influence susceptibility to doxorubicin-induced cardiac injury and are consistent with published reports confirming the high expression of several of the identified cationic-type transporters in the heart (3, 16, 18, 21, 24, 25).
Fig. 1.
Identification of a doxorubicin uptake transporter. (A) Initial workflow in identifying a doxorubicin uptake transporter from the SLC transportome of pediatric cancer patients receiving doxorubicin-based therapy. (B) Differential gene expression of various SLCs after doxorubicin treatment in hiPSCs-CMs derived from patients who experienced cardiotoxicity and patients who did not experience cardiotoxicity (n = 3 per group) and expressed as a fold change. Statistical analysis was performed using the gene-level differential expression comparison: *P < 0.05; 0.0003, 0.07, 0.43, and 0.04 for OCTN1, OCT1, OCT6, and OCT3, respectively. (C) Relative levels of doxorubicin in heart tissues isolated from wild-type or transporter-deficient mice following a single intravenous injection of doxorubicin at a dose of 5 mg/kg (n = 4 to 6 per group). (D) Ex vivo levels of doxorubicin in cardiomyocytes isolated from wild-type or OCT3-deficient mice (n = 4 to 6 per group). Data presented represent the mean ± SEM and are expressed as a percentage over wild-type (males and females combined). Statistical analysis was performed using an unpaired two-sided Student’s t test with Welch’s correction: *P < 0.05, compared to wild-type values. (E) Fluorescent images illustrating time-dependent accumulation of doxorubicin in HEK293 cells engineered to overexpress a vector control (VC) or mouse OCT3 (mOCT3). (Scale bar: 100 µm.)
Identification of OCT3 as a Cardiomyocyte Transporter of Doxorubicin.
To investigate whether any of the identified transporters contribute to the accumulation of doxorubicin, we utilized mice with a genetic deficiency of OCT1/OCT2 (SLC22A1/SLC22A2), OCT3 (SLC22A3), OCTN1 (SLC22A40), or the functionally related transporter MATE1 (SLC47A1). This approach was based on the expectation that the lack of one or more of these transporters would lead to the diminished levels of doxorubicin in the heart. Preliminary studies using RNA sequencing revealed that the relative expression of these transporters in heart tissues of wild-type mice resembled levels observed in human adult heart tissues and hiPSC-CMs, with the exception of OCT2 and OCT6 being undetectable in mice (SI Appendix, Table S3). An initial analysis demonstrated that the in vivo uptake of doxorubicin was significantly diminished only in heart tissues of OCT3-deficient mice (Fig. 1C and SI Appendix, Fig. S2A). Furthermore, the deficiency of OCT3 was associated with reduced doxorubicin levels in primary murine cardiomyocytes ex vivo (Fig. 1D). This observation is consistent with recent reports showing reduced cardiac levels of other cationic substrates, such as tetraethylammonium, dehydrocorydaline, and metformin, using the same OCT3 knockout mouse model (18, 26–28). To provide further support for OCT3 being a carrier of doxorubicin in the heart, we verified the high expression of this SLC in hiPSC-CMs (SI Appendix, Fig. S1 B and C) and demonstrated that doxorubicin preferentially accumulates in and is cytotoxic against cells engineered to overexpress either the mouse or human homolog of OCT3 (Fig. 1E and SI Appendix, Fig. S2 B–E).
Targeting OCT3 Attenuates Doxorubicin-Induced Cardiac Injury in Mice.
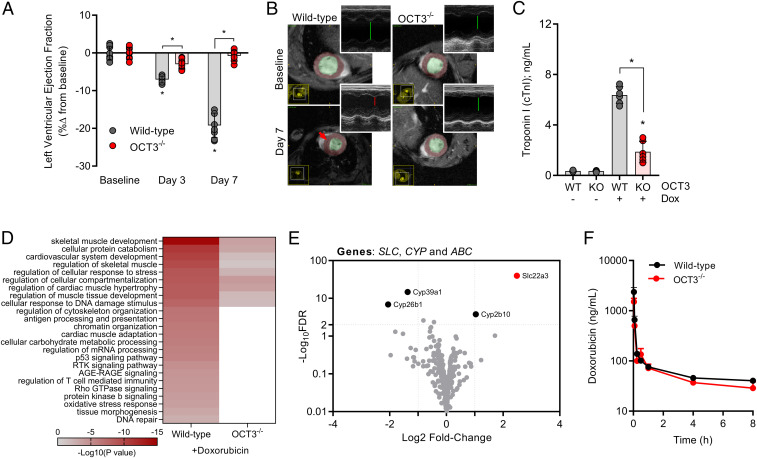
Since genetic deficiency of OCT3 was associated with diminished accumulation in vivo in cardiac tissue and ex vivo in cardiomyocytes, we hypothesized that the pathophysiology of doxorubicin-induced cardiac injury would also be dependent on OCT3-genotype status. To test this hypothesis, we evaluated cardiac function in wild-type or OCT3-deficient mice using two independent modalities based on cardiac ultrasound or magnetic resonance imaging by measuring LVEF as a marker of cardiovascular performance after an acute challenge with doxorubicin. Consistent with our hypothesis, OCT3-deficient mice were significantly protected from a reduction in LVEF (Fig. 2A and SI Appendix, Fig. S3A). Representative m-mode and epicardial surface images and measurements of ventricular wall dimensions are consistent and supportive of the development of cardiomyopathy that is dependent on OCT3-genotype status (Fig. 2B and SI Appendix, Fig. S3 B–F). Furthermore, elevated levels of troponin I, a clinically used serum marker for cardiomyocyte apoptosis, and up-regulation of signaling transduction pathways associated with doxorubicin-induced cardiac injury and cardiovascular remodeling, were markedly attenuated in OCT3-deficient mice (Fig. 2 C and D). Moreover, protection against cardiac injury was also observed in OCT3-deficient mice following a chronic challenge with doxorubicin (SI Appendix, Fig. S4 A–C). We further confirmed that the observed protection is independent of concurrent compensatory alterations in the expression of transporters phylogenetically linked to OCT3, other known doxorubicin-related target genes (e.g., TOP2β, CBR1/3, or ATP-binding cassette transporters), or potentially altered plasma levels of doxorubicin that result from the deletion of OCT3 (Fig. 2 E and F and SI Appendix, Fig. S5 A–D and Table S4). Since several known endogenous substrates of OCT3 are hormones essential in cardiovascular signaling (e.g., catecholamines), we also confirmed that OCT3-deficient mice did not have intrinsically abnormal pathological or biochemical changes in the heart or serum at baseline (SI Appendix, Fig. S6 and Table S5).
Fig. 2.
OCT3 deficiency attenuates doxorubicin-induced cardiac injury. (A) Ultrasound evaluation of acute cardiac dysfunction (left ventricular ejection fraction) at baseline, day 3, or day 7 (n = 6 per group). (B) Representative images from cardiac magnetic resonance imaging or ultrasound (Insets) illustrating the ventricular dimensions of the heart. (C) Serum concentrations of cardiac troponin I at day 7 (n = 6 per group). Male wild-type or OCT3-deficient mice received multiple intraperitoneal injections of doxorubicin at a dose of 3 mg/kg consecutively for 7 d (21 mg/kg cumulatively). Data presented represent the mean ± SEM and are expressed as a percentage change compared to baseline values. (D) Categorizing differentially expressed genes using the criteria ≥2 Log10FDR and Log2 fold change of greater and less than positive and negative 2, respectively. Signal transduction pathways were identified using Gene Ontology and KEGG: Kyoto Encyclopedia of Genes and Genomes annotation and analysis with Metascape. (E) Volcano plot of differentially expressed SLC and ABC transporter genes, and CYP metabolizing genes in untreated wild-type or OCT3-deficient mice. Positive fold change indicates higher expression in wild-type mice. Dotted lines indicate a Log10FDR threshold of >2 or Log2FC (fold change) of ±2. RNA-sequencing analysis was performed on cardiomyocytes of naive or doxorubicin-treated wild-type or OCT3-deficient mice (n = 3 per group). (F) Plasma concentration-time profile of doxorubicin in male wild-type or OCT3-defcient mice following a single intraperitoneal dose of 5 mg/kg (n = 6 per group). Statistical analysis was performed using an unpaired two-sided Student’s t test with Welch’s correction: *P < 0.05, compared to baseline or wild-type values or between treatment groups.
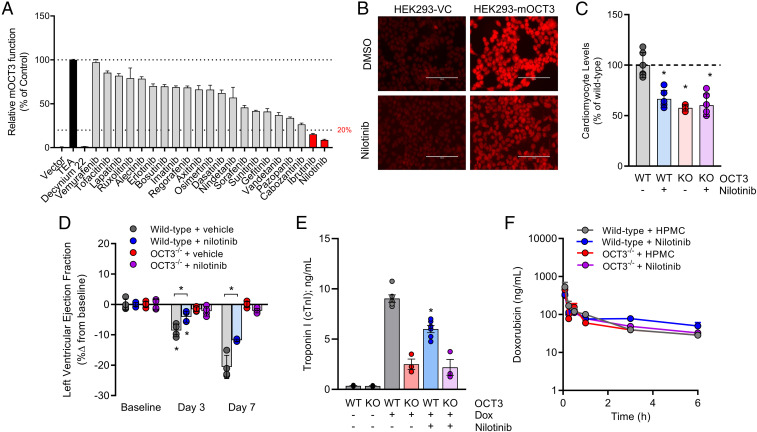
Next, we evaluated whether pharmacological targeting of OCT3 would preserve cardiac function after doxorubicin administration in a manner similar to that observed in mice with a genetic deletion of OCT3. In our previously reported high-throughput screen of inhibitors against OCTs, tyrosine kinase inhibitors (TKIs) were identified as a class of agents with potent transporter-modulatory properties (29). Due to their unique inhibitory activity against OCTs, we performed a screen of Food and Drug Administration (FDA)-approved TKIs and sought to determine whether these agents are modifiers of transport function in cells overexpressing OCT3. This screen revealed that TKIs also have a differential capacity to inhibit the activity of murine OCT3, and nilotinib was found to be the most potent inhibitor (Fig. 3A). Pretreatment of cells with nilotinib, a Bcr-Abl inhibitor used in the treatment of certain leukemias, was found to modulate OCT3 activity with submicromolar inhibitory activity (IC50 = 0.87 µM) (SI Appendix, Fig. S7 A–D). Interestingly, even short-term exposure to nilotinib followed by its removal in a washout experiment resulted in sustained inhibition of OCT3 for more than 24 h (SI Appendix, Fig. S7E). Furthermore, nilotinib exhibited similar inhibitory properties against the murine and human homolog of OCT3, using the well-characterized xenobiotic substrate of OCT3, metformin (SI Appendix, Fig. S8 A–D). In line with the supposition that pharmacological targeting of this transporter would preserve cardiac function, we found that nilotinib significantly diminished the accumulation of doxorubicin in cells overexpressing mouse OCT3 (Fig. 3B) and in primary murine cardiomyocytes to levels similar to those observed in cells that are OCT3-deficient (Fig. 3C).
Fig. 3.
Inhibition of OCT3 with nilotinib preserves cardiovascular function. (A) In vitro identification of nilotinib as a potent inhibitor of mouse OCT3. Tetraethylammonium (TEA) and decynium-22 were used as positive control substrate or inhibitor, respectively (n = 12 per group). (B) Representative fluorescent images of nilotinib-mediated inhibition of doxorubicin uptake in HEK293 cells overexpressing vector control (VC) or mouse OCT3 (mOCT3). (C) Ex vivo levels of doxorubicin in cardiomyocytes isolated from male and female wild-type or OCT3-deficient mice (n = 6 per group) in the presence or absence of nilotinib pretreatment. (D) Ultrasound evaluation of cardiac dysfunction (left ventricular ejection fraction) at baseline, day 3, or day 7 (n = 6 per group). (E) Serum concentrations of cardiac troponin I at day 7 (n = 3 to 6 per group). Male wild-type or OCT3-deficient mice received multiple intraperitoneal injections of doxorubicin at a dose of 3 mg/kg consecutively for 7 d (21 mg/kg cumulatively). Mice were pretreated with vehicle (hydroxypropyl methylcellulose) or nilotinib (15 mg/kg) 30 min before every doxorubicin injection. (F) Plasma concentration-time profile of doxorubicin (5 mg/kg) in male wild-type or OCT3-deficient mice pretreated for 30 min with vehicle or nilotinib (15 mg/kg) (n = 6 per group). Data presented represent the mean ± SEM and are expressed as a percentage change to control, wild-type, or baseline values. Statistical analysis was performed using an unpaired two-sided Student’s t test with Welch’s correction: *P < 0.05, compared to baseline or wild-type values or between treatment groups.
We next utilized an acute model of doxorubicin-induced cardiac injury in wild-type or OCT3-deficient mice receiving a single oral dose of nilotinib 30 min before administration of the anthracycline. This study showed that pharmacological targeting of OCT3 with nilotinib significantly preserved cardiovascular performance and reduced levels of circulating troponin I without causing concurrent alterations in the plasma levels of doxorubicin (Fig. 3 D–F and SI Appendix, Table S4). These results are in alignment with a prior study using a rat model of doxorubicin-induced cardiac injury in which pretreatment with nilotinib was also observed to afford cardio protection by an unverified mechanism (30).
OCT3-Dependent Gene Changes in Hearts of Doxorubicin-Treated Mice.
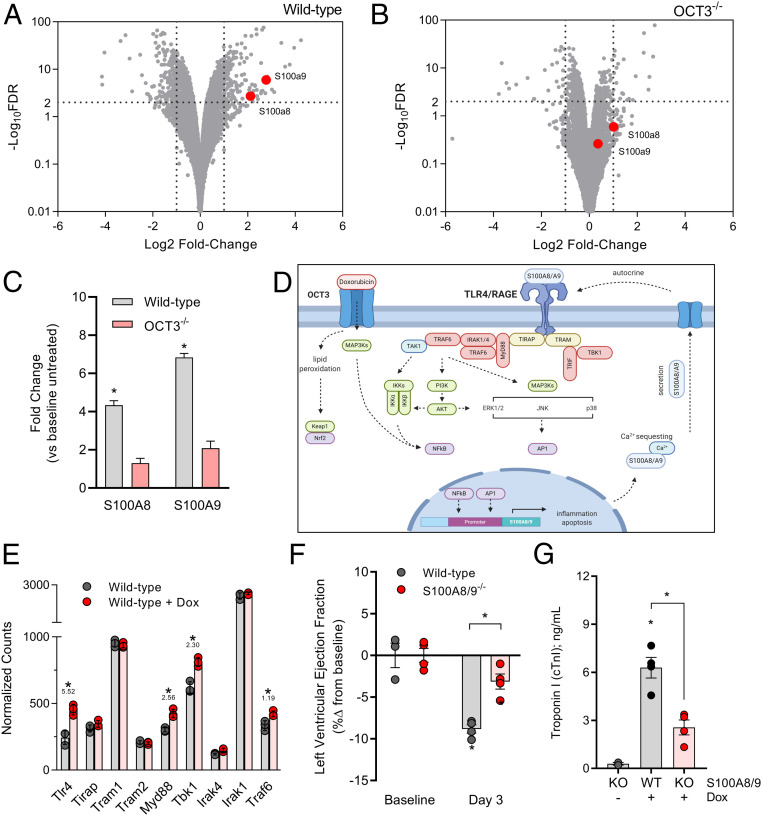
Since the targeting of the initial OCT3-dependent entry of doxorubicin in cardiomyocytes is essential for the following pathological changes associated with cardiac injury, we sought to identify affected transcriptomic signatures that are dependent on OCT3-genotype status. RNA sequencing of heart tissue from wild-type mice treated with doxorubicin revealed that the genes encoding the calcium binding proteins S100A8 and S100A9 genes were especially highly up-regulated (Fig. 4A), and this up-regulation is dramatically reduced in OCT3-deficient mice (Fig. 4 B and C). Similarly, genes along the signal transduction axis of RAGE/TLR4, which are activated by S100A8 and S100A9, were concurrently dysregulated (Fig. 4 D and E). Since calcium dysregulation is a hallmark of doxorubicin-related cardiac injury, we validated these observations by the demonstration that S100A8 or S100A9 protein levels were also up-regulated in an OCT3-dependent manner (SI Appendix, Fig. S9 A–D) and that genetic deficiency of S100A8 and S100A9 in mice was associated with preserved cardiovascular function and reduced levels of troponin I following an acute challenge with doxorubicin (Fig. 4 F and G).
Fig. 4.
RNA sequencing reveals OCT3-dependent transcriptional changes. RNA sequencing of heart apexes isolated from male (A) wild-type or (B) OCT3-deficient mice treated with a single intraperitoneal dose (15 mg/kg) of doxorubicin (n = 3 per group). Positive fold change indicates higher expression post doxorubicin treatment in genotype status. Dotted lines indicate a Log10FDR threshold of >2 or Log2FC (fold change) of ±2. (C) Interpolated fold changes in doxorubicin-treated wild-type or OCT3-deficient mice compared to treatment naive mice. (D) Schematic illustrating the proposed mechanism of an OCT3-dependent S100A8/A9 signaling cascade in doxorubicin-induced cardiac injury. (E) Relative expression of genes involved in the TLR4/RAGE-signaling pathway using normalized counts from sequencing data. Values underneath asterisk indicate Log10FDR. (F) Left ejection fraction and (G) serum cardiac troponin I as markers of doxorubicin-induced cardiac toxicity on day 3 following three consecutive doxorubicin injections at a dose of 3 mg/kg (cumulative 9 mg/kg) in dual S100A8/A9 knockout mice (n = 4 per group). All data presented represent the mean ± SEM. Statistical analysis was performed using an unpaired two-sided Student’s t test with Welch’s correction: *P < 0.05, compared to baseline or wild-type values.
OCT3 Inhibition as an Adjunct to Doxorubicin Therapy.
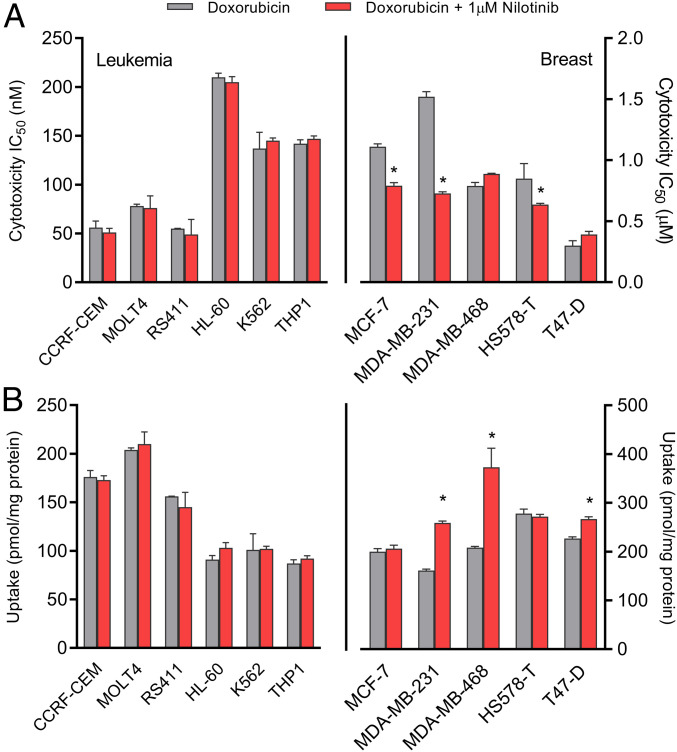
Although combining doxorubicin with an OCT3 inhibitor could potentially reduce the incidence and severity of cardiac injury, it is important to verify that such strategy does not negatively influence the antitumor properties of doxorubicin. The success of a preventative intervention with an OCT3 inhibitor such as nilotinib would be dependent on the dosing/scheduling strategy, as well as the expression status of OCT3 and related cationic transporters in cancer cells. In order to gain preliminary insights, we evaluated the transcriptional expression profile of OCT3 and other drug transporters in human tumor specimens and cancer cell lines, using publicly available sequencing data from The Cancer Genome Atlas (TCGA) and the National Cancer Institute’s Developmental Therapeutics Program. This revealed that OCT3 is detected at low levels in leukemias and breast cancer, the primary indications for doxorubicin (SI Appendix, Fig. S10 A and B). This suggests that pharmacological targeting of OCT3 can potentially reduce the incidence of doxorubicin-induced cardiac injury without negatively influencing antitumor efficacy. In support of this notion, we found that concentrations of nilotinib similar to the observed IC50 for OCT3 function (∼1 µM) did not influence the cytotoxicity of doxorubicin in a panel of leukemia and breast cancer cell lines (Fig. 5A and SI Appendix, Fig. S11 A–M) and that the cellular entry of doxorubicin into these cells occurs in an OCT3-independent manner that is insensitive to nilotinib (Fig. 5B).
Fig. 5.
Nilotinib does not influence doxorubicin activity in leukemia or breast cancer. (A) Cytotoxicity of doxorubicin in leukemia (Left) or breast cancer cells (Right) in the presence of absence of nilotinib (1 µM). Cytotoxicity was measured by an MTT assay following continuous 72-h exposure of doxorubicin (n = 12 per group). A nonlinear regression model was used to generate a sigmoidal curve and IC50 values. (B) Uptake of doxorubicin in leukemia (Left) or breast cancer cells (Right) in the presence or absence of nilotinib (1 µM) (n = 6). Uptake data are normalized to protein concentrations. All data presented represent the mean ± SEM. Statistical analysis was performed using a sample Student’s t test: *P < 0.05, compared to doxorubicin alone.
Discussion
In the present study, we identified the organic cation transporter OCT3 as an important, previously unrecognized contributor to doxorubicin uptake in cardiomyocytes. In particular, we found that mice deficient for OCT3 are protected from both acute and chronic forms of doxorubicin-induced cardiac injury. In addition, we found that nilotinib, an FDA-approved TKI used in the treatment of certain leukemias, is a potent modulator of OCT3 activity, and that pretreatment of mice with nilotinib can preserve cardiac function after the administration of doxorubicin.
In the precloning era, the cellular accumulation of doxorubicin and other cationic anthracyclines such as daunorubicin was widely believed to be dependent exclusively on a passive diffusion-mediated mechanism. In the late 1990s, the molecular entities responsible for the uptake of cationic xenobiotics were finally identified as the polyspecific, bidirectional, and facilitative diffusional transporters OCT1, OCT2, and OCT3. These transporters have a relatively broad substrate profile that is at least partially overlapping, but their tissue expression profile is diverse such that OCT3 is generally regarded as a less important contributor to uptake of xenobiotic substrates than OCT1 in the liver or OCT2 in the kidney. Due to the dominance of the other OCTs in these particular organs of elimination, OCT3 has traditionally received relatively scant attention (31). More recently, however, reduced oral bioavailability and attenuated pharmacological response to certain substrate drugs such as metformin have been detected in OCT3 knockout mice, and a 3′-untranslated region variant of OCT3 was found to be associated with reduced metformin response in humans, suggesting that OCT3 may significantly contribute to the distribution of certain substrate drugs to OCT3-expressing tissues (32). Studies involving OCT3 knockout mice have also highlighted that OCT3 is an important organic cationic transporter in the heart (26, 28, 33, 34), where it may regulate the cardiac uptake of xenobiotic substrates. This prior knowledge is consistent with our current observation that OCT3 is the sole cationic transporter driving the cardiac uptake of doxorubicin and its subsequent injury and provides a mechanistic explanation for the observation that the expression of the OCT3 gene is particularly highly up-regulated in hiPSC-CMs from pediatric cancer patients who experienced a clinically annotated cardiotoxic event after treatment with doxorubicin. In this context, it is noteworthy that RNA-sequencing analysis revealed an absence of OCT3 alterations at the transcript level both in hiPSC-CMs from patients and in the hearts of wild-type mice treated with doxorubicin. This suggests that doxorubicin-induced cardiac injury in cancer patients may be a direct consequence of inherent expression differences in OCT3 at baseline and that genotyping of OCT3 status may predict patient susceptibility to cardiac injury. Recently, polymorphic variants in OCT3 have been demonstrated to contribute to the incidence and severity of coronary artery disease (35–37), while the functional consequence of these variants has resulted in reduced transport of known OCT3 substrates (38, 39). Therefore, the mechanistic basis in gain- or loss-of-function activity due to these variants may provide a future rationale for clinical genotyping of SLC22A3 status to predict interindividual variability in cardiac response to doxorubicin. It should be pointed out that the initial identification of OCT3 as a putative target of doxorubicin was based on specimens obtained from a relatively small number of pediatric patients who received combination chemotherapy and not single-agent doxorubicin. Additional studies involving larger cohort sizes in various populations, including adult patients, are currently ongoing to validate our observations.
An additional limitation of our study includes the fact that not all genes related to the transport of doxorubicin were investigated. However, a direct clinically relevant contribution of other cationic-type carriers identified from our hiPSC-CMs to doxorubicin-induced cardiac injury is not supported by our current findings. For example, a role of OCT1 in doxorubicin-induced cardiac injury is unlikely based on the observation that its expression in heart tissue is 10-fold lower than that of OCT3 and also because OCT3-deficient mice, which express functional OCT1, remain completely protected from the expected cardiac injury. Likewise, our RNA-sequencing data demonstrate that OCT6 transcripts are undetectable in the hearts of mice; however, it is present at levels that are 100-fold lower in adult human heart samples in comparison with OCT3. While previous studies have linked polymorphic variants in the OCT6 gene to interindividual variability in measures of systemic exposure to doxorubicin (40, 41), and in vitro overexpression of OCT6 in leukemic and ovarian cancer cells increased doxorubicin-induced cytotoxicity (42, 43), our current findings using an OCT6 overexpression model reveal that doxorubicin is not a transported substrate. Collectively, these observations suggest that OCT6 may contribute to doxorubicin phenotypes other than those related to cardiac injury. In our studies, we also found that OCTN1 deficiency was not associated with a protection against doxorubicin-induced cardiac injury, although this transporter is known to be highly expressed in murine and human heart tissue (16, 17) and is highly up-regulated in pediatric cancer patients who experience cardiotoxicity. While immunoreactivity of OCTN1 has been detected on mitochondria and plasma membrane of cardiac myocytes, presumably due to a role in the transport of its known substrates L-carnitine and ergothioneine (15, 44), the apparent transport of doxorubicin by OCTN1 (45) may merely reflect a mechanism of dysregulation involving mitochondrial activity and/or regulation of cardiac energy metabolism.
The demonstration that OCT3 plays a pivotal role in a clinically relevant, potentially life-threatening cardiotoxicity warrants the further exploratory use of specific transport inhibitors in doxorubicin-containing regimes. Specifically, our findings suggest that translational interventions targeting this transport mechanism could be strategically exploited as a preventative strategy and may offer benefits over alternative, previously proposed approaches that target one of many pleiotropic signaling pathways that may also influence apoptotic pathways in tumor cells. The success of our proposed strategy depends at least in part on optimizing local exposure to cardiomyocytes by applying the right dose and schedule of OCT3 inhibitors. Such agents would ideally have high potency, high specificity, low drug–drug interaction potential, and favorable pharmaceutical properties. We hypothesized that the class of TKIs is of particular interest in this context, as these agents have many of the above features, and several members of the class are known to potently inhibit drug transporters related to OCT3, such as OCT2 (29, 46). The ultimate identification of nilotinib as an exquisitely potent inhibitor of OCT3 is consistent with recent findings indicating that several TKIs can exert selective and potent inhibitory effects on OCT3. Comparison of the IC50 values for OCT3 inhibition with the unbound plasma levels of these drugs that can be achieved in patients suggests that potential clinically significant transport inhibition might take place by these TKIs that impact the tissue distribution of drugs that are substrates of OCT3 (47). The mechanism by which nilotinib affects the function of OCT3 requires further investigation, although the noncompetitive nature of this effect supports the possibility that it involves the inhibition of a presently unknown regulatory kinase that phosphorylates the transporter, as reported previously for effects of TKIs on OCT2 (29).
The translational potential of using an intervention concept based on the use of OCT3 inhibitors to prevent cardiac toxicity is further supported by our observations that the cytotoxicity of doxorubicin against leukemia or breast cancer cells is not negated by nilotinib. In addition, the plasma pharmacokinetic profile of doxorubicin is unaffected by genetic or pharmacological inhibition of OCT3, consistent with the limited contribution of this transporter to the uptake of substrates in organs of elimination that would drive alterations in systemic exposure. In this context, it should be pointed out that, while most side effects associated with Bcr‐Abl inhibitors are mild, reversible, and easily managed, the nilotinib prescribing information carries a black box warning for QT prolongation, which could hinder the immediate clinical implementation of the proposed intervention. It is worth noting, however, that the median time from the start of nilotinib therapy using a conventional chronic regimen (e.g., once or twice daily dosing without interruption) to the onset of an adverse cardiac event is >14 mo (range, 2 to 68 mo) (48). Since we aim to interrogate the response of healthy tissues and cancer cells to the nilotinib–doxorubicin combination following acute or intermittent exposure to the TKI to restrict access of doxorubicin to cardiomyocytes, we anticipate that nilotinib will not be intrinsically toxic in the context of such a strategy. This would be consistent with recent findings from a phase I clinical trial in which an intermittent regimen of nilotinib was administered as an adjuvant treatment with doxorubicin in patients with sarcomas and in which no cardiac toxicity was observed (49). Collectively, this prior clinical experience with nilotinib demonstrates that targeting OCT3 can be exploited to achieve cardio protection in cancer patients receiving doxorubicin and provides a rationale for the future discovery and development of alternative OCT3 inhibitors with increased specificity and selectivity and an improved safety profile.
As a next step toward understanding the molecular mechanisms contributing to the lack of severe doxorubicin‐induced cardiac injury in OCT3-deficient mice, we performed RNA-sequencing analyses on heart biopsies following doxorubicin administration in vivo. Transcriptional profiling of key pathway genes implicated as potential biomarkers of doxorubicin‐induced cardiac injury could clearly distinguish mouse genotype groups and identify complex gene expression changes and a drug-response signature in wild-type mice that is largely absent in OCT3-deficient mice. In particular, we found strong dysregulation of the calcium-binding proteins S100A8 and S100A9 that have been implicated in a wide range of biological responses involved in inflammation, immune diseases, and chemotherapy resistance to anticancer drugs (50–52). Although the connection between these proteins and cardiac injury induced by doxorubicin remains unclear, previous studies have suggested that the plasma concentrations of S100A8 and S100A9 are also increased in hearts of doxorubicin-treated diabetic mice (53) and that elevated S100A8 and S100A9 levels have been implicated as a predictive biomarker for individuals with chronic heart failure (54). The biological importance and contribution of these proteins to doxorubicin-induced cardiac injury is further substantiated by a prior study where activation of S100A8 and S100A9 was pivotal for the development of post ischemic heart failure mediated by RAGE and TLR4 signaling. Furthermore, cardiac dysfunction is preserved in doxorubicin-treated TLR4-deficient mice (55), and our present study demonstrates that the deletion of both of S100A8 and S100A9 also significantly attenuated cardiac dysfunction. This suggests that involvement of these genes in cardiomyocytes include a p38 MAPK and NFκB-signaling axis that leads to the release of proinflammatory cytokines (e.g., IL-6), resulting in a local inflammatory response. Interestingly, S100A8 and S100A9 are also secreted proteins that behave in an autocrine manner and lead to the propagation of this response that is dependent on OCT3.
Collectively, we identified a previously unrecognized pathway of doxorubicin-induced cardiac toxicity that is initiated by OCT3-mediated transport and results in a downstream signaling cascade involving the calcium-binding proteins S100A8 and S100A9. The function of OCT3 can be inhibited by nilotinib through a noncompetitive mechanism without antagonizing the anticancer properties of doxorubicin in multiple translationally relevant models of leukemia and breast cancer. These findings not only shed light on the etiology of doxorubicin-induced cardiac toxicity, but also provide a rationale for the identification of targeted intervention strategies to prevent this debilitating side effect.
Materials and Methods
See also SI Appendix, Detailed Materials and Methods.
Patient-Derived iPSC Cardiomyocytes.
All pluripotent and reprogramming cell cultures were maintained accordingly to previously published methodology (23). The experimental protocol was approved by Northwestern University Human Institutional Review Board under the protocol STU00204341, and all subjects provided informed consent/assent. RNA-sequencing analysis was performed on hiPSC-CMs from six pediatric patients diagnosed with Wilm’s tumor, acute lymphoblastic leukemia, Ewing’s sarcoma, or hepato-carcinoma (SI Appendix, Table S1 and S2). Patients did not receive other chemotherapeutic agents with a black box warning for potential to cause cardiotoxicity and the cumulative anthracycline was calculated using the doxorubicin isotoxic equivalent.
Animal Models.
All animals were housed in a temperature-controlled environment with a 12-h light/12-h dark cycle, given standard chow diet and water ad libitum, and handled according to the Animal Care and Use Committee of The Ohio State University under an approved protocol (2015A00000101-R1). For all experiments, age- and sex-matched wild-type or knockout mice (8 to 12 wk) were used.
Statistical Analyses.
Data presented represent the mean ± SEM before and/or after normalization to baseline values and are expressed as a percentage. All experiments were performed using multiple replicates and were performed independently on at least two independent occasions. An unpaired two-sided Student’s t test with Welch’s correction was used for comparisons between two groups (control/baseline vs. treatment/genotype), and a one-way ANOVA with Dunnett’s post hoc test was used for comparing more than two groups. A P < 0.05 was used as the statistical cutoff across all analyses.
Supplementary Material
Acknowledgments
We thank the Small Animal Imaging Core (SAIC) at The Ohio State University for providing access to the instrumentation for imaging studies; the Genetically Engineered Mouse Modeling Core (GEMMC) for the re-derivation of S100A8/A9 knockout mice; the Comparative Pathology and Mouse Phenotyping Shared Resource (CPMPSR); and the Genomics Shared Resource (GSR) for RNA library generation and sequencing. This project was supported in part by Robert-Bosch Stiftung and the Duetsche Forschungsgemeinschaft under Germany’s Excellence Strategy Grants EXC2180–290900677 (to A.T.N.) and Grants P30CA016058 (SAIC, CPMPSR, GEMMC, and GSR), R01CA215802 (to A.S.), R01CA187176 (to A.S.), R01CA238946 (to M.B.L. and S.H.), R01GM066233 (to J.W.), R50CA211524 (to P.S.Y.); and the Comprehensive Cancer Center at The Ohio State University using Pelotonia funds (K.M.H. and M.Z.T.). The content is solely the responsibility of the authors and does not represent the official views of the funding agencies.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020168118/-/DCSupplemental.
Data Availability.
RNA sequencing data have been deposited in the Gene Expression Omnibus database (GSE157904). The RNA data on human tumor samples are based upon publicly available data generated by the TCGA Research Network (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga) (56).
References
- 1.Swain S. M., Whaley F. S., Ewer M. S., Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 97, 2869–2879 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Carvalho C., et al. , Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 16, 3267–3285 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff D. D., et al. , Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91, 710–717 (1979). [DOI] [PubMed] [Google Scholar]
- 4.Lionetti V., Ventura C., Regenerative medicine approach to repair the failing heart. Vascul. Pharmacol. 58, 159–163 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Kremer L. C., van Dalen E. C., Offringa M., Ottenkamp J., Voûte P. A., Anthracycline-induced clinical heart failure in a cohort of 607 children: Long-term follow-up study. J. Clin. Oncol. 19, 191–196 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Lipshultz S. E.et al.; American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Basic Cardiovascular Sciences, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Radiology , Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American heart association. Circulation 128, 1927–1995 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Renu, K., Abilash V. G., Tirupathi Pichiah P. B., Arunachalam S., Molecular mechanism of doxorubicin-induced cardiomyopathy: An update. Eur. J. Pharmacol. 818, 241–253 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Octavia Y., et al. , Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 52, 1213–1225 (2012). [DOI] [PubMed] [Google Scholar]
- 9.César-Razquin A., et al. , A call for systematic research on solute carriers. Cell 162, 478–487 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Bachur N. R., Steele M., Meriwether W. D., Hildebrand R. C., Cellular pharmocodynamics of several anthrocycline antibiotics. J. Med. Chem. 19, 651–654 (1976). [DOI] [PubMed] [Google Scholar]
- 11.Dalmark M., Storm H. H., A Fickian diffusion transport process with features of transport catalysis. Doxorubicin transport in human red blood cells. J. Gen. Physiol. 78, 349–364 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usansky J. I., Liebert M., Wedemeyer G., Grossman H. B., Wagner J. G., The uptake and efflux of doxorubicin by a sensitive human bladder cancer cell line and its doxorubicin-resistant subline. Sel. Cancer Ther. 7, 139–150 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Sasaya M., et al. , Uptake of doxorubicin by cultured kidney epithelial cells LLC-PK1. Biol. Pharm. Bull. 21, 527–529 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Lal S., Mahajan A., Chen W. N., Chowbay B., Pharmacogenetics of target genes across doxorubicin disposition pathway: A review. Curr. Drug Metab. 11, 115–128 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Iwata D., et al. , Involvement of carnitine/organic cation transporter OCTN2 (SLC22A5) in distribution of its substrate carnitine to the heart. Drug Metab. Pharmacokinet. 23, 207–215 (2008). [DOI] [PubMed] [Google Scholar]
- 16.McBride B. F., et al. , The organic cation transporter, OCTN1, expressed in the human heart, potentiates antagonism of the HERG potassium channel. J. Cardiovasc. Pharmacol. 54, 63–71 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamhonwah A. M., Wong J., Tam C., Mai L., Tein I., Organic cation/carnitine transporter family expression patterns in adult murine heart. Pathol. Res. Pract. 205, 395–402 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Solbach T. F., Grube M., Fromm M. F., Zolk O., Organic cation transporter 3: Expression in failing and nonfailing human heart and functional characterization. J. Cardiovasc. Pharmacol. 58, 409–417 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Miyake T., et al. , Involvement of organic cation transporters in the kinetics of trimethylamine N-oxide. J. Pharm. Sci. 106, 2542–2550 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Grube M., et al. , Uptake of cardiovascular drugs into the human heart: Expression, regulation, and function of the carnitine transporter OCTN2 (SLC22A5). Circulation 113, 1114–1122 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Grube M., et al. , Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am. J. Pathol. 178, 2547–2559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang K. M., Hu S., Sparreboom A., Drug transporters and anthracycline-induced cardiotoxicity. Pharmacogenomics 19, 883–888 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Burridge P. W., et al. , Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koepsell H., Lips K., Volk C., Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 24, 1227–1251 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Nishimura M., Naito S., Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 23, 22–44 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Zwart R., Verhaagh S., Buitelaar M., Popp-Snijders C., Barlow D. P., Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol. Cell. Biol. 21, 4188–4196 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee N., et al. , Taste of a pill: Organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. J. Biol. Chem. 289, 27055–27064 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., et al. , Organic cation transporter 1 and 3 contribute to the high accumulation of dehydrocorydaline in the heart. Drug Metab. Dispos. 48, 1074–1083 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Sprowl J. A., et al. , A phosphotyrosine switch regulates organic cation transporters. Nat. Commun. 7, 10880 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z. Y., et al. , Evaluation of the pharmacokinetics and cardiotoxicity of doxorubicin in rat receiving nilotinib. Toxicol. Appl. Pharmacol. 272, 238–244 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Koepsell H., Organic cation transporters in health and disease. Pharmacol. Rev. 72, 253–319 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Chen E. C., et al. , Targeted disruption of organic cation transporter 3 attenuates the pharmacologic response to metformin. Mol. Pharmacol. 88, 75–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng N., et al. , Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 1063, 69–76 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., et al. , Intracellular beta1-adrenergic receptors and organic cation transporter 3 mediate phospholamban phosphorylation to enhance cardiac contractility. Circ Res., 10.1161/CIRCRESAHA.120.317452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., et al. , Functional variant in the SLC22A3-LPAL2-LPA gene cluster contributes to the severity of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 36, 1989–1996 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Zhao Q., et al. , PHACTR1 and SLC22A3 gene polymorphisms are associated with reduced coronary artery disease risk in the male Chinese Han population. Oncotarget 8, 658–663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paquette M., Bernard S., Baass A., SLC22A3 is associated with lipoprotein (a) concentration and cardiovascular disease in familial hypercholesterolemia. Clin. Biochem. 66, 44–48 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Al-Eitan L. N., Almomani B. A., Nassar A. M., Elsaqa B. Z., Saadeh N. A., Metformin pharmacogenetics: Effects of SLC22A1, SLC22A2, and SLC22A3 polymorphisms on glycemic control and HbA1c levels. J. Pers. Med. 9, E17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakata T., et al. , Functional analysis of human organic cation transporter OCT3 (SLC22A3) polymorphisms. J. Pharmacol. Sci. 113, 263–266 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Lal S., et al. , Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics 8, 567–575 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Faraji A., Dehghan Manshadi H. R., Mobaraki M., Zare M., Houshmand M., Association of ABCB1 and SLC22A16 gene polymorphisms with incidence of doxorubicin-induced febrile neutropenia: A survey of Iranian breast cancer patients. PLoS One 11, e0168519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okabe M., et al. , Characterization of the organic cation transporter SLC22A16: A doxorubicin importer. Biochem. Biophys. Res. Commun. 333, 754–762 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Ota K., et al. , Expression of organic cation transporter SLC22A16 in human epithelial ovarian cancer: A possible role of the adriamycin importer. Int. J. Gynecol. Pathol. 26, 334–340 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Smith E., et al. , Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 106, 691–697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okabe M., et al. , Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol. Cancer Ther. 7, 3081–3091 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang K. M., et al. , Neuronal uptake transporters contribute to oxaliplatin neurotoxicity in mice. J. Clin. Invest. 130, 4601–4606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minematsu T., Giacomini K. M., Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol. Cancer Ther. 10, 531–539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim T. D., et al. , Clinical cardiac safety profile of nilotinib. Haematologica 97, 883–889 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alemany R., et al. , Nilotinib as coadjuvant treatment with doxorubicin in patients with sarcomas: A phase I trial of the Spanish group for research on sarcoma. Clin. Cancer Res. 24, 5239–5249 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Sedaghat F., Notopoulos A., S100 protein family and its application in clinical practice. Hippokratia 12, 198–204 (2008). [PMC free article] [PubMed] [Google Scholar]
- 51.Donato R., et al. , Functions of S100 proteins. Curr. Mol. Med. 13, 24–57 (2013). [PMC free article] [PubMed] [Google Scholar]
- 52.Bresnick A. R., Weber D. J., Zimmer D. B., S100 proteins in cancer. Nat. Rev. Cancer 15, 96–109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pei X. M., et al. , S100A8 and S100A9 are associated with doxorubicin-induced cardiotoxicity in the heart of diabetic mice. Front. Physiol. 7, 334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma L. P., et al. , S100A8/A9 complex as a new biomarker in prediction of mortality in elderly patients with severe heart failure. Int. J. Cardiol. 155, 26–32 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Riad A., et al. , Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur. J. Heart Fail. 10, 233–243 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Weinstein J. N.et al., The cancer genome atlas pan-cancer analysis project. Nature Genetics 45, 1113–11120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data have been deposited in the Gene Expression Omnibus database (GSE157904). The RNA data on human tumor samples are based upon publicly available data generated by the TCGA Research Network (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga) (56).