Abstract
Fragrance is an integral part of cosmetic products and is often regarded as an overriding factor in the selection of cosmetics among consumers. Fragrances also play a considerable role in masking undesirable smells arising from fatty acids, oils and surfactants that are commonly used in cosmetic formulations. Essential oils are vital assets in the cosmetic industry, as along with imparting pleasant aromas in different products, they are able to act as preservatives and active agents and, simultaneously, offer various benefits to the skin. Moreover, the stimulating demand for natural ingredients has contributed massively to a renewed interest in cosmetic and wellness industries in plant derivatives, especially essential oils. This has led popular cosmetic companies to endorse natural fragrances and opt for minimally processed natural ingredients, given the potentially adverse health risks associated with artificial fragrance chemicals, which are major elements of cosmetics. Among the high-valued essential oils used as fragrances are citrus, lavender, eucalyptus, tea tree and other floral oils, among others, while linalool, geraniol, limonene, citronellol, and citral are much-appreciated fragrance components used in different cosmetics. Thus, this review aimed to highlight the enormous versatility of essential oils as significant sources of natural fragrances in cosmetics and cosmeceuticals. Moreover, a special focus will be laid on the different aspects related to essential oils such as their sources, market demand, chemistry, fragrance classification, aroma profile, authenticity and safety.
Keywords: essential oils, natural fragrances, cosmetics
1. Introduction
The use of fragrance is ubiquitous and is a global human phenomenon. Over the course of time, countless numbers of flavors and fragrances have found their way into everyday life, notably into foods, beverages and confectionery items; into personal care products (soaps, toothpastes, mouthwashes, deodorants, bath lotions and shampoos), perfumes, and other cosmetics as well as pharmaceutical formulations. Indeed, flavors and aromas are added to make such products more attractive or to mask the taste or smell of less pleasant ones [1]. In recent times, green consumerism and the resurgence of the use of “naturals” have given a fresh impetus to the development of plant-based products, especially in beauty and wellness industries [2].
Fragrances play a particularly important role in increasing the attractiveness of cosmetics. Pleasant smells influence the comfortability and the effect of the products and also impact significantly on the overall evaluation of cosmetics. Therefore, along with the shape and design of the container, the smell is also one of the characteristics of cosmetics that the consumer experiences [3] and look forward to in the selection of those items. Among natural fragrances, essential oils, which are complex mixtures of terpenes and other aromatic or aliphatic compounds, produced as secondary metabolites in specialized secretory tissues of aromatic plants [4], are the most popular. In nature, essential oils play very important roles in plant defense and signaling processes. For instance, they are involved in plant defense against insects, herbivores, and microorganisms, including attraction of pollinating insects and fruit-dispersing animals, water regulation and allelopathic interactions [5,6,7]. Once the plants have been harvested, the essential oil is extracted by means of steam distillation, expression (physical crushing of essential oil glands situated in fruit rinds or outermost waxy layer of fruit’s peel) [8], microwave-assisted extraction, solvent extraction, or enfleurage (transfer of the essential oil from flower petals to fat). Of these methods, steam distillation is the most common overall, while expression is most commonly used to obtain essential oils from the peels of citrus fruits such as orange, lemon, bergamot, etc.) [9].
Indeed, the volatile nature of essential oils makes them likely to be useful as fragrances but does not preclude other functions for them in cosmetics [10]. In fact, their intended use has been for a long time principally industrial, whereby standardization of fragrance requiring the blending of essential oils from different plants with the aim of obtaining a specific scent has been a common practice [11]. It has been reported that 300 essential oils out of about 3000 plant species are commercially available in the flavors and fragrances market [12]. The essential oils produced predominantly for industrial purposes are those from orange, corn mint, citronella, eucalyptus, peppermint, and lemon [13].
Essential oils are widely incorporated into modern skincare products because of their complexity of active compounds, strongly fragrant properties and natural marketing image. Moreover, over the years, they have shown several scientifically proven cosmetic properties. For instance, their importance in cosmetic preparations as natural preservative agents, owing to their antimicrobial properties, alone or in combination with other preservatives is established [14], whereby they assure protection against bacteria and fungi. Hence conveniently, added essential oils are able to enhance the dermato-cosmetic properties of the final product, not only by protecting against microbial infections but also contributing to the preservation of the cosmetic formulation [15,16]. Interestingly, they are also used to induce additional benefits to the skin such as anti-acne, anti-aging, skin lightening, and sun protection, among others, in cosmeceutical products, thus rendering them highly valuable to cosmetic industries [17,18,19,20,21,22].
Many industries use synthetic fragrances that are developed in a laboratory to mimic the aromatic and chemical constituents of natural, plant-based oils that are more expensive to manufacture. Nevertheless, synthetic fragrances may not contain the beneficial aspects of natural plant-based essential oils and could even be unsafe for human applications [23]. For instance, chemicals found in man-made fragrances include phthalates, which are endocrine disruptors [24] and known carcinogens such as benzene derivatives [25]. On the other hand, the global natural fragrances market is witnessing a high growth due to increasing usage of natural fragrances such as essential oils over synthetic fragrances as a result of their associated numerous health benefits such as in aromatherapy which is expected to drive the growth of the market in the coming years. For instance, the global essential oils market size was found to exceed USD 7.51 billion in 2018 and is expected to grow at greater than 9% compound annual growth rate (CAGR) between 2019 and 2026 [26]. Moreover, famous cosmetic brands use various essential oils in cosmetics, and the most expensive perfumes contain pure essential oils, which give them special and unique scents and make them unique as exclusive fragrances [27]. However, although the effectiveness of essential oils in health and beauty is widely acknowledged, they may not be completely free from contraindications and allergic effects [28].
Indeed, large-scale harvesting of aromatic plants for commercial purposes can cause species loss, exposing them to the danger of extinction. It is, therefore, vital that systematic cultivation of these plants be introduced in order to conserve biodiversity and protect endangered species [29]. Sustainable extraction of natural products, making use of innovative technologies, process intensification and agro-based solvents constitute the way out in the development of eco-conceived fragrant ingredients covering every olfactory family without putting biodiversity at risk any further [30].
Hence, this review has aimed to provide a spotlight on the versatility of these botanical essences as an explicit source of natural fragrances commonly used in cosmetic and cosmeceutical products. Additionally, other pertinent aspects related to essential oils, such as their sources, market demand, chemistry, fragrance classifications, aroma profiles, as well as their authenticity and safety, have been briefly discussed.
2. Sources of Essential Oils
There are 400,000 known plant species of both aromatic and medicinal plants, of which about 2000 species come from nearly 60 botanical families of essential oils bearing plants [31]. The plant families composed of species yielding the majority of the most economically important essential oils are not limited to one specialized taxonomic group but can be found distributed among all plant classes [32] (Table 1).
Table 1.
Main sources of essential oils in plant families [32].
| Type of Vascular Plants | Plant Families | Examples of Essential Oil-Bearing Plants |
|---|---|---|
| 1. Gymnosperms | Cupressaceae | Cedar leaf, cedarwood, juniper |
| Pinaceae | Fir and pine | |
| 2. Angiosperms | ||
| Monocots | Acoraceae | Calamus |
| Poaceae | Vetiver and aromatic grass | |
| Zingiberaceae | Ginger and cardamom | |
| Dicots | Apiaceae | Coriander and fennel |
| Asteraceae | Tarragon, chamomile and wormwood | |
| Geraniaceae | Geranium | |
| Illiciaceae | Star anise | |
| Lamiaceae | Lavender, patchouli, mint, oregano | |
| Lauraceae | Litsea, cinnamon, camphor, sassafras | |
| Myristicaceae | Mace and nutmeg | |
| Myrtaceae | Allspice, myrtle and clove | |
| Oleaceae | Jasmine | |
| Rosaceae | Rose | |
| Santalaceae | Sandalwood |
Several plants contain essential oils; however, parts of plants which serve as the major source of essential oil, can be different. These include flowers and inflorescences (e.g., chamomile, lavender, rose, ylang-ylang), leaves (e.g., basil, laurel, lemongrass, peppermint, rosemary), fruits (black pepper, nutmeg), peel (orange, bergamot, lemon, tangerine), seeds (anise, cumin, cardamom, fennel), berries (allspice, juniper), bark (cinnamon, cassia, sassafras), wood (cedarwood, camphor, sandalwood), root and rhizomes (ginger, vetiver, turmeric), and resin (myrrh, frankincense) [33].
3. Demand for Essential Oils in Cosmetic Industries
Essential oils have been widely used all over the world, and their use is growing due to the strong demand for pure natural ingredients in various fields. Thus, large quantities of essential oils are produced globally to fuel the industries of fragrances and flavors, cosmetics, as well as phytomedicine and aromatherapy [34,35].
Demand comes mostly from the following markets: food and beverage (35%), fragrances, cosmetics and aromatherapy (29%), household (16%), and pharmaceutical (15%) [13]. As consumers have become increasingly conscious of the health benefits of essential oils, inclinations for foods and beverages containing these volatile oils as additives have developed. The global essential oils market has also been led by the growth in demand for organic and natural hygienic products owing to increasing awareness of health problems among consumers. Moreover, natural flavors and fragrances demand in perfumes, cosmetics, thermal and relaxation applications are likely to stimulate the demand for essential oils. Essential oils and oleoresins are not merely used in the food processing and industrial seasoning sectors but are also vital in the perfume and flavoring industries. Globally, operating flavors and fragrance manufacturers are among the main buyers of essential oils. Their sales present an indication of developments in their market and subsequent demand for essential oils [13].
Particularly in cosmeceutical industries, essential oils such as that from orange has garnered maximum revenue as it offers multiple health remedies such as skin elasticity, firmness and treats scars, acne, and stretch marks. Lemon and orange essential oils also have antiseptic properties, which make them ideal ingredients in skin and hair care. Furthermore, the antimicrobial properties of these oils have opened new avenues for further business growth [26].
Some essential oils are produced on a very large-scale (e.g., in 2008, production of orange oils was ~51,000 tons, corn mint oils ~32,000 tons, and lemon oils ~9200 tons). Essential oils obtained from a variety of fruits from the genus Citrus are the most popular natural essential oils and account for the largest part of commercial natural flavors and fragrances [36]. The production of some others at a much smaller scale due to their rarity is, however, traded at very elevated prices [e.g., agarwood oil (6000–11,000 €/kg), iris (6200–100,000 €/kg depending on the concentration of irones), or rose oil (6000–10,000 €/kg)]. These prices vary and may be related to the scarcity of the raw materials, harvesting issues, climate dependence, or yield of extraction. Essential oil industries cumulative sales represented several billion US$ in 2008 [34,37].
Europe accounts for the greatest share of the global essential oils market, with the Asia Pacific region and North America tying for second place. Essential oils play a key role in the manufacturing of scented candles, oils, and other products. In 2017, the essential oils industry in the United States witnessed a boost in revenue of nearly 10% and was expected to reach around 7.3 billion dollars in market value by 2024. The essential oils that make up the largest share of the US market are orange, corn mint, and eucalyptus essential oils. Moreover, as a country with a rich history of perfume manufacturing, France is also the top exporter of essential oils worldwide. The export value of essential oils and resinoids from the US, in recent years, has grown by nearly two billion US dollars. Moreover, some countries that cannot produce certain varieties of essential oils in huge quantities to satisfy the local demands must import them from other countries. For instance, Lebanon imported the most essential oils of any country globally, at around 13.75 billion US dollars in 2017, while Northern European countries such as Germany, the Netherlands, and the United Kingdom, accounted for almost 80% of the orange oil imported to Europe [38].
4. Chemistry of Essential Oils
Although the term “aromatic” in modern usage describes the quality of giving off an aroma that is either pleasant or odious to the nose, an aromatic compound or moiety, in the language of chemistry, has a chemical arrangement that results in delocalization of electrons, producing greater molecular stability. Thus, essential oils may be a mixture of aromatic and aliphatic (non-aromatic) compounds, all of which contribute to the perceived aroma [39]. Essential oils are soluble in alcohol, ether, and fixed oils but insoluble in water. These volatile oils are generally liquid and colorless at room temperature. They have a characteristic odor and have a density less than unity, with the exception of a few cases (cinnamon, sassafras, clove, and vetiver). Moreover, they have a refractive index and a very high optical activity [40].
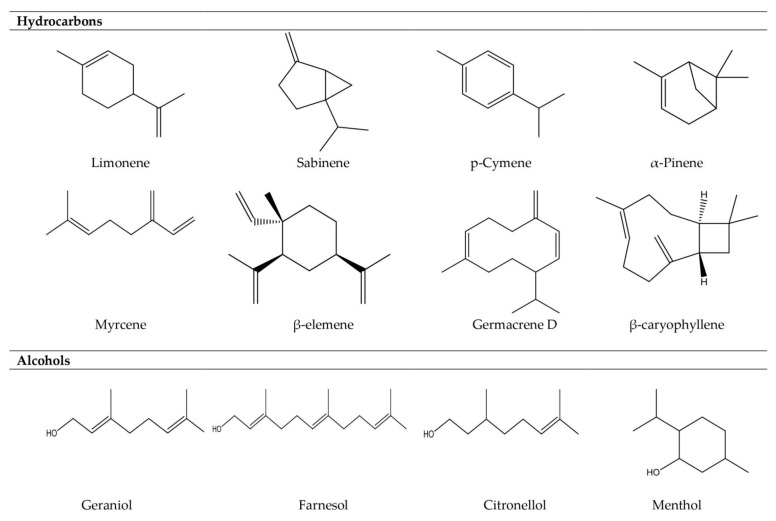
Essential oils can be composed of only a few to up to more than 100 single substances [41]. Being natural mixtures of a very complex nature, essential oils may consist of about 20–60 components at quite different concentrations, with two or three major components being present at fairly high concentrations (20–70%), compared with other components present in trace amounts [42]. The flavor contribution of single compounds, though, does not strictly depend on their respective concentration but relies on the specific odor threshold that is determined by structure and volatility. Consequently, even minor components deriving from oxidation or degradation reactions may have a strong impact on the flavor if their aroma value is high enough [43]. For the most part, essential oil components can be assigned as lipophilic terpenoids, phenylpropanoids, or short-chain aliphatic hydrocarbon derivatives of low molecular weight, with the first being the most frequent and characteristic constituents. Among these, allylic, mono-, bi-, or tricyclic mono- and sesquiterpenoids of different chemical classes make up a major part of essential oils, such as hydrocarbons, ketones, alcohols, oxides, aldehydes, phenols, or esters [41] (Figure 1). The absence of even one component may change the aroma. In general, monoterpene hydrocarbons are less valuable regarding their contribution to the fragrance of the essential oil than oxygenated compounds, which are highly odoriferous [44]. In addition to their chemical properties, essential oils can be seen to vary greatly in their physical properties in terms of their density, refractive index, and rotatory power. For instance, the essential oils of the different organs (limbo, foliar sheath, and rhizomes) of Siphonochilus ethiopicus were reported to significantly differ in those physical properties. These properties contribute to a better characterization of plant essential oils [45].
Figure 1.
Examples from major classes of compounds in essential oils.
The terpenes are the largest group of natural fragrances, and their classification is mainly based on the number of isoprene units present in their structure. Depending on the number of C5 units, the terpenes are classified into hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15) and diterpenes (C20) [4,46,47]. Monoterpenes are the most abundant in essential oils (about 90%) with a great variety of structures. Geraniol/nerol, linalool, citronellol, citronellal and citral are the most important terpenes and are widely used in the perfume industries [48,49]. Biogenetically, terpenoids and phenylpropanoids have different primary metabolic precursors and are generated through different biosynthetic routes. The pathways involved in terpenoids are the mevalonate and mevalonate-independent (deoxyxylulose phosphate) pathways, whereas phenylpropanoids originate through the shikimate pathway [40].
Many components of essential oils contain one or more asymmetric carbon atoms exhibiting optical activity. These chiral compounds of natural origin (mono- and sesquiterpenes) are usually found in characteristic enantiomeric distributions as they have evolved via enzymatically controlled biosynthetic synthesis. They also have different uses in the food, pharmaceutical as well as fragrance industries. Moreover, the chirality of the odorants exerts an influence on their mode of action, suggesting that, in analogy with other pharmaceuticals, the antipodes may act differently. In addition to variations in their biological, therapeutic, and pharmacological potentials, odor (olfactory) between chiral compounds may differ [50]. For example, the R-(−)- and S-(+)-enantiomers of the monoterpene ketone carvone differ in odor and taste; S-(+)-carvone has an aromatic flavor, while R-(−)-carvone has a characteristic minty flavor. Furthermore, in the case of limonene, the odor of (+)- limonene was rated to be significantly more pleasant and more activating than that of (−)-limonene [50]. As for -(+)- and -(−)-menthol, while both enantiomers have a peppermint odor quality, (−)- menthol has a much stronger cooling effect than (+)-menthol [51].
The chemical composition of essential oils can vary greatly because of physiological (plant organ, ontogenesis), environmental (soil composition, weather conditions), and genetic factors [52]. Other factors that may influence the oils’ chemical composition are geographical variation [53], the plant parameters (e.g., species, cultivated or wild plants), harvest and postharvest/predistillation parameters (e.g., season of harvest, pretreatment of biomass, and storage conditions of source plant materials), and production parameters (e.g., mode of production, distillation parameters, commercial oil, or laboratory-produced), and other parameters (e.g., storage condition and storage time of essential oils, age of essential oil, aging by exposure to oxygen and ultraviolet light, and analytical parameters) [54].
5. Classification of Essential Oils Fragrances
Interest in the research of essential oils is mainly championed by the perfume industry. The interest is reflected economically by the readily available unmet demand for fragrances and flavors. This market demand has given rise to the production of synthetic fragrances and flavors. However, the need for natural products has not been overtaken by the supply of synthetic products. In fact, this also has gradually extended to the replacement of purely synthetic products [55].
Essential oils can be classified as top, middle and base “notes” according to their odorous characteristics, diffusion rate in air and volatility [56]. For instance, the top notes are those that are the most volatile and the first perceptible odors [56] that are detected and fade first while providing freshness to the blend. They are responsible for customer first impressions, and therefore, are the selling note of a perfume. They are light scents, lasting 5–10 min or remain for a maximum of 30 min [57]. These include bergamot, juniper, cinnamon, and gardenia.
Middle notes are those essential oils tending to be spicy or floral and give body to blends; their duration time is also brief and can remain up to 1 h. These include ylang-ylang, geranium, lavender, jasmine, and clove.
In contrast, the base notes give a perfume the depth and last the longest. These are the least volatile essential oils that remain longer up to several hours [56,57]. Some essential oils that are used as base notes are myrrh, vanilla, sandalwood, and frankincense [56].
The incorporation of new aromas into perfumes and fragrances and the development of new products are critical to the success of the perfumer and flavor chemist [58]. Indeed, essential oils are important components of perfumes, offering a wide variety of choices for perfume formulations. Perfumes are formulated mostly using alcohol. Eau de types of perfumes are mostly formulated using the essential oils and are usually amber color because of their natural oils color, but they are normally clear [56]. Perfume types can be defined by the amount of essential oil included, e.g., Eau de parfum, Eau de cologne, Eau de toilette, etc. (Table 2).
Table 2.
Varying percentage of essential oils and alcohol in different perfume types [56].
| Type of Perfume | Fragrance/Essential Oil | Alcohol |
|---|---|---|
| Eau de parfum | 8–15% | 80–90% |
| Splash colognes | 1–3% | 80% |
| Eau de cologne | 3–5% | 70% |
| Eau de toilette | 4–8% | 80–90% |
6. Aroma Profiles of Essential Oils and Their Individual Compounds
Essential oils, as well as their isolated compounds, are widely used in cosmetic products as they offer a variety of benefits, including their pleasant aromas. However, the main reason for their usage in cosmetics is their pleasant aromas. Fatty acids, fatty oils and surfactants used in the production-process of cosmetic products often exhibit an unpleasant scent [28]. Essential oils and their terpenoids are one of the most important natural products used by the cosmetic and perfumery industries. In fact, it has been revealed that there is a predominance of essential oils, especially terpenes and terpenoids applied as active ingredients for the products and processes patented in the areas of cosmetics and perfumery [59].
Indeed, essential oils derived from a huge array of aromatic plants have been documented to be used as significant sources of fragrance in perfumes and cosmetics, offering a wide selection of natural scents with unique notes. Hence, in this section, the aroma profiles of essential oils commonly used in perfume and cosmetic industries are presented, along with their principal components, color range, the scientific/common names of plants from which they are derived, and the parts that have been used (Table 3). Besides, the odor description of some individual essential oil fragrances is reported in Table 4.
Table 3.
Aroma profile of essential oils used as fragrances in cosmetics and/or perfumes.
| Plant Species from Which Essential Oils are Derived | Common Name | Part(s) Used | Principal Componentsa | Color Range of Oils | Aroma Description | References |
|---|---|---|---|---|---|---|
| 1. Amyris balsamifera L. | Balsam torchwood | Wood | Elemol, 10,γ-epi-eudesmol, γ-eudesmol, valerianol, α-eudesmol, 7-α-epi-eudesmol, β-eudesmol, drimenol | Pale yellow to amber yellow | Characteristic, woody | [60] |
| 2. Angelica archangelica | Angelica | Roots and rhizomes | Phellandrene, pinene, limonene, linalool and borneol; rich in coumarins including osthol, Angelicin, bergapten and imperatorin | Colorless or pale yellow | Rich herbaceous-earthy body note | [61] |
| Seed | Colorless | Fresher, spicy top note | ||||
| 3. Aniba rosaeodora Ducke | Rosewood | Wood/leaves | α-Pinene, β-pinene, limonene, 1,8-cineole, trans-linalool oxide (furanoid), cis-linalool oxide (furanoid), linalool, trans-linalool oxide (pyranoid), α-terpineol, α-copaene, β-selinene, α-selinene, spathulenol, caryophyllene oxide, benzyl benzoate | Pale-yellow | Floral, sweet, woody, and citric | [62,63] |
| 4. Boswellia sacra Flueck. | Frankincense | Gum | α-Pinene (2.0–64.7%), α-thujene (0.3–52.4%), β-pinene (0.3–13.1%), myrcene (1.1–22.4%), sabinene (0.5–7.0%), limonene (1.3–20.4%), p-cymene (2.7–16.9%) and β-caryophyllene (0.1–10.5%) | Clear or yellow | Balsamic, camphor-like, spicy, woody, slightly lemony | [64,65] |
| 5. Cananga odorata Hook. F. and Thoms) | Ylang ylang | Flowers | Prenyl acetate, p-cresyl methyl ether, methyl benzoate, linalool, benzyl acetate, geraniol, geranyl acetate, (E)-cinnamyl acetate, β-caryophyllene, germacrene-D, (E,E)-α-farnesene, (E,E)-farnesol, benzyl benzoate, (E,E)-farnesyl acetate and benzyl salicylate | Pale yellow to dark yellow | Characteristic, floral, recalling jasmine | ISO3063:2004(E) |
| 6. Carum carvi L. | Caraway | Ripe fruit | Myrcene, limonene, cis-dihyrocarvone, trans-carveol, cis-carveol, carvone | Colorless to pale yellow | Fresh, herbaceous, spicy | [66] |
| 7. Cedrus atlantica (Endl.) Manetti ex Carrière | Cedarwood | Wood/sawdust | Himachalene, α-himachalene, β-himachalene, cis-bisabolene, himachalol, allo-himachalol, α-atlantone, ƴ-atlantone, himachalene oxide | Yellowish to orange-yellow or deep amber-colored | Camphoraceous–cresylic top note with a sweet, tenacious woody undertone, reminiscent of cassie and mimosa | [67] |
| 8. Cinnamomum verum J. Presl | Cinnamon | Bark | (E)-Cinnamaldehyde, eugenol, β-caryophyllene, β-phellandrene, linalool | Reddish-brown | Characteristically spicy burning | [54,64] |
| 9. Citrus aurantiifolia (Christm.) Swingle | Lime | Peel | Limonene, β-pinene, γ-terpinene, citral | Colorless to greenish-yellow | Mild citrus, floral | [10,68] |
| 10. Citrus aurantium var. amara L. | Neroli/bitter orange | Flowers | Linalool, β-pinene, α-terpineol, limonene, sabinene, nerol, nerolidol, linalyl acetate and α-pinene | Pale yellow to coffee brown | Sweet, fresh and floral odor | [28,69] |
| 11. Citrus bergamia Risso | Bergamot | Rind of fruit | Linalyl acetate, limonene, α-terpinene, β-pinene, γ-terpinene, geraniol, linalool, lactones, bergaptene, β-bisabolene | Light green-yellow | Light, delicate citrus-like scent with slightly floral/sweet/balsamic character (top note) | [11] |
| 12. Citrus limon (L.) Osbeck | Lemon | Peel | Limonene, β-pinene, γ-terpinene, sabinene, α-pinene, geranial, neral, α-thujene, β-bisabolene, terpinolene, trans-α-bergamotene, α-terpineol, α-terpinene, neryl acetate, linalool, p-cymene, citronellal, trans-caryophyllene, terpineol-4, nerol, camphene, nonanal, geraniol, octyl aldehyde, α-phellandrene, and cis-sabinene-hydrate | Colorless to pale yellow | Fresh lemon peel | [10,70] |
| 13. Citrus reticulata Blanco | Tangerine | Peel | Limonene, γ-terpinene, terpinolene, myrcene, α-pinene, p-cymene, α-thujene | Dark orange to reddish-orange or brownish-orange | Orange-like | [10,54] |
| 14. Citrus sinensis (L.) Osbeck | Sweet orange | Peel | Limonene, α-pinene, β-pinene, sabinene, myrcene, geranial, n-octanal, n-decanal, n-nonanal, neral, β-sinensal, valencene, linalool | Yellow to reddish-yellow | Characteristic, orange peel odor | [71] |
| 15. Citrus paradisi Macfad. | Grapefruit | Peel of fruit | Limonene, citronellal, citral, sinensal, geranyl acetate, paradisol, geraniol, ketones, lactones, coumarins such as auraptene, limettin and sesquiterpenes such as cadinene | Yellow or greenish | Fresh, sharp, citrus aroma (top note) | [11] |
| 16. Commiphora myrrha (Nees) Engl. | Myrrh | Gum | Furanoeudesma-1,3-diene, curzerene, β-elemene, lindestrene, furanodiene | Yellow to reddish-brown | Pleasant balsamic, camphor-like, musty, incense-like | [54,64] |
| 17. Coriandrum sativum L. | Coriander | Fruit | Borneol, 2E-decenal, camphor, dodecanal, n-decanal, neryl acetate, geraniol, linalool | Colorless to pale yellow | Floral, balsamic undertone and peppery-woody suave top-note | [72,73] |
| 18. Cupressus sempervivens L. | Cypress | Leaves | α-Pinene, Δ-carene, limonene, sesquiterpene, α-terpinene, sabinene, carvone | Yellowish | Pleasingly smoky, woody smell, and amber-like | [74,75] |
| 19. Cymbopogon citratus (DC.) Stapf | Lemongrass | Grass leaf | Citral, myrcene, dipentene, linalool, geraniol, nerol, citronellol, and farnesol, sesquiterpenes, methyl heptenone, esters, acids and others | Yellow or pale sherry-colored | Intense sweet lemony, reminiscent of lemon drops (top note) | [11] |
| 20. Cymbopogon martini (Roxb.) W. Watson | Palmarosa | Leaves | Myrcene, linalool, β-caryophyllene, α-terpineol, geranyl acetate, geranyl isobutyrate, geraniol, (E,Z) farnesol, geranyl hexanoate | Yellow | Fresh rose-like | [64,76] |
| 21. Daucus carota L. | Carrot seed | Seeds | β-Bisabolene, elemicin, geranyl acetate, and sabinene | Yellowish-brown color | Soft, sweet earthy grounding aroma | [77] |
| 22. Elettaria cardamomum (L.) Maton | Cardamom | Fruits | α-Pinene, sabinene, myrcene, limonene, 1,8-cineole, linalool, linalyl acetate, terpinen-4-ol, α-terpineol, terpinyl acetate, trans-nerolidol | Almost colorless to pale yellow | Characteristic, spicy, cineolic | [78] |
| 23. Eucalyptus globulus Labill. | Eucalyptus | Leaves | 1,8-Cineole, α-pinene, limonene, aromadendrene, p-cymene, globulol | Yellow to red | Fresh balsamic camphor-like | [54,64] |
| 24. Feniculum vulgare Mill. | Fennel | Seeds | Phenols, trans-anethole, methyl chavicol, α-pinene, α-thujene, γ-terpinene, limonene, myrcene, phellandrene, fenchone, 1,8-cineole, fenchol, acids, lactones and coumarins | Yellowish | Strong, anisey, camphoric | [11,64] |
| 25. Helichrysum italicum (Roth) G. Don | Immortelle | Aerial parts | Neryl acetate, g-curcumene, neryl propionate and ar-curcumene | Pale yellow to red | Strong, honey-like aroma | [28,79] |
| 26. Hyssopus officinalis L. | Hyssop | Aerial parts | Isopinocamphone, pinocamphone, β-pinene | Light-yellow | Herbaceous, camphor-like odors with warm and spicy undertones | [80] |
| 27. Illicium verum Hook.f. | Star anise | Fruit | Trans-anethole (80–90%) | Pale yellow | Warm, spicy, extremely sweet, licorice-like scent | [61] |
| 28. Jasminum officinale L. | Jasmine | Flowers | Benzyl acetate, linalyl acetate, benzyl benzoate, methyl jasmonate, methyl anthranilate, linalool, nerol, geraniol, benzyl alcohol, farnesol, terpineol, phytols, eugenol, cis-jasmone; acids, aldehydes and others | Dark, orange-brown | Highly intense, rich, sweet, floral odor (base note) | [11] |
| 29. Juniper communis L. | Juniper | Ripe berries | α-Pinene (20–50%), sabinene (<20%), β-pinene (1–12%), β-myrcene 1–35%, α-phellandrene (<1%), limonene 2–12%, terpinen-4-ol (0.5–10%), bornyl acetate (<2%), and β-caryophyllene (< 7%) | Colorless or pale yellow-green | Fresh terebinth or turpentine-like/conifer-like aroma | [74,81] |
| 30. Laurus nobilis L. | Laurel | Leaves | 1,8-Cineole, sabinene, α-terpinyl acetate, linalool, eugenol, methyl eugenol, α-pinene | Yellow | Aromatic, spicy | [82] |
| 31. Lavandula angustifolia Mill. | Lavender | Flowering tops | Linalyl acetate (25–47%), linalool (max. 45%), terpinen-4-ol (max. 8%), camphor (max. 1.5%), limonene (max. 1%) and 1,8-cineole (max. 3%) | Colorless to pale yellow | Sweet floral aroma | [28] |
| 32. Litsea cubeba (Lour.) Pers. | Litsea | Fruits | Citral (neral and geranial) (78.7–87.4%), d-limonene (0.7–5.3%) | Pale yellow | An intense lemon-like, spicy aroma | [83] |
| 33. Matricaria chamomilla L. | German chamomile | Flowers and flower heads | (E)-β-Farnesene (4.9–8.1%), terpene alcohol (farnesol), chamazulene (2.3–10.9%), α-bisabolol (4.8–11.3%), and α-bisabolol oxides A (25.5–28.7%) and α-bisabolol oxides B (12.2–30.9%) | Blue | Warm, fruity scent Sweet herbaceous odor (middle note) | [84] |
| 34. Melaleuca alternifolia (Maiden and Betche) Cheel | Tea tree | Leaves | Terpinen-4-ol (30–48%), γ-terpinene (10–28%), 1,8-cineole (traces-15%), α-terpinene (5–13%), α-terpineol (1.5–8%), p-cymene (0.5–8%), α-pinene (1–6%), terpinolene (1.5–5%), sabinene (traces-3.5%), aromadendrene (traces-3%), δ-cadinene (traces-3%), viridiflorene (traces-3%), limonene (0.5–1.5%), globulol (traces-1%), viridiflorol (traces-1%) [ISO 4730 (2004)] | Colorless to pale yellow | Intensive aromatic fresh camphoraceous odor | [28] |
| 35. Melaleuca leucadendra (L.) L. | Cajeput | Fresh leaves and twigs | 1,8-Cineole (14–65%), terpenes (45%), alcohols (5%), esters | Pale yellow-green | Camphorous, highly penetrating odor with a slightly fruity note (top note) | [11] |
| 36. Mentha x piperita L. | Peppermint | Aerial parts | Menthol, menthone, isomenthone, menthofuran, neomenthol, pulegone, menthyl acetate, 3-octanal, 1,8-cineole, limonene, trans-sabinene hydrate, β-caryophyllene | Almost colorless to pale greenish yellow | Characteristic of mint, sweet, menthol-like | [85] |
| 37. Myristica fragrans Houtt. | Nutmeg | Seeds | Myristicin, α-pinene, sabinene, limonene, elemicin, eugenol, safrol and β-pinene | Pale yellow to nearly colorless | Spicy, sweet, woody | [86] |
| 38. Ocimum basilicum L. | Basil | Flowering herb | Linalool, citronellol, geraniol, terpinen-4-ol, α-terpineol, methyl chavicol, eugenol, ethyl eugenol, limonene, camphene, α-pinene, β-pinene, γ-terpinene, p-cymene, cis-ocimene, 1,8-cineole, linalyl acetate, fenchyl acetate, methyl cinnamate, β-caryophyllene | Colorless or pale yellow | Light sweet, spicy odor reminiscent of anise or clove (top note) | [11] |
| 39. Pelargonium graveolens L’Hér. | Geranium | Aerial parts | Citronellol, geraniol, linalool, citronellyl formate, geranyl formate, citral, guaiazulene, β-caryophyllene, cis-rose oxide, phellandrene, limonene, α-pinene, menthone | Pale or olive green | Sweet rose-like odor with a hint of mint or “greenness” (middle note) | [11] |
| 40. Pimenta dioica (L.) Merr. | Allspice | Leaf | Mainly eugenol, less in the fruit (60–80%) than in the leaves (up to 96%), also methyl eugenol, cineole, phellandrene and caryophyllene, among others | Yellowish-red or brownish liquid | Powerful sweet, spicy scent, similar to cloves | [61] |
| Berry | Pale yellow liquid | Sweet, warm balsamic, spicy body note (middle note) and fresh, clean top note | ||||
| 41. Pimpinella anisum L. | Aniseed | Seeds | Trans-anethole (75–90%) | Colorless to pale yellow | Warm, spicy-sweet characteristic scent | [61] |
| 42. Pinus massoniana Lamb. | Turpentine | Gum resin | α-Pinene, camphene, β-pinene, δ-3-carene, myrcene, limonene, p-cymene, longifolene, β-caryophyllene, caryophyllene oxide | Colorless | Characteristic of gum turpentine | [87] |
| 43. Piper nigrum L. | Black pepper | Unripe berries (peppercorns) | Sesquiterpenes (20–30%) and monoterpenes (70–80%) such as bisabolene, β-caryophyllene, thujene, pinene, camphene, sabinene, terpinene, myrcene, limonene, phellandrene, small amounts of ketone, phenols, alcohols and others | Light to pale olive | Dry, woody, warm, spicy, oriental (base note) | [11] |
| 44. Pogostemon cablin (Blanco) Benth. | Patchouli | Leaves | α-Pinene (0.01–0.3%), β-pinene (0.02–1%), Limonene (0.01–0.3%), δ-elemene (0.01–1.9%), β-patchoulene (0.03–12%), β-elemene (0.18–1.9%), cycloseychellene (0.02–0.8%), (E)-β-caryophyllene (0.75–6.8%), α-guaiene (2.9–23%), seychellene (2.3–13%), α-humulene (0.05–2%), α-patchoulene (1.2-13%), pogostone (0.1–27.7%), patchoulol (11–72%), pogostol (0.2–6.2%), α-bulnesene (2.9–23%), aciphyllene (0.7–4.2%), norpatchoulenol (0.11–4.0%), caryophyllene oxide (0.0–4.6%), germacrene D (0.0–0.2%) | Yellow to reddish-brown | Earthy, woody and camphoraceous | [88] |
| 45. Rosa damascena Mill. | Rose | Flowers | Citronellol, geraniol, nerol, nonadecane, heneicosane | Yellow to yellow-green | Sweet, floral, rosaceous | [89,90,91] |
| 46. Rosmarinus officinalis L. | Rosemary | Leaves | Eucalyptol and α-pinene, camphor, bornyl acetate, camphene, β-pinene, β-myrcene, limonene and borneol | Colorless to pale yellow | Strong, warm, woody, balsamic aroma | [28,92] |
| 47. Salvia sclarea L. | Clary sage | Inflorescences | Linalool, linalyl acetate | Yellow | Characteristic herbaceous odor | [93,94] |
| 48. Santalum album L. | Sandalwood | Heartwood | 90% Santalol content (cis-α-santalol and cis-β-santalol) (ISO 3518:2002) | Yellow to light brown | Sweet woody | [95] |
| 49. Syzygium aromaticum (L.) Merrill et. Perry | Clove | Bud | Eugenol (70–95%), eugenol acetate (up to 20%) and β-caryophyllene (12–17%) | Colorless or pale yellow | Clove aroma | [96] |
| 50. Vetiveria zizanioides (Linn.) Nash | Vetiver | Roots | α-Vetivone (8.4–13.3%), khusimol (0.6–8.9%), β-vetivone (2.2–3.7%), khusian-2-ol (1.8–2.3%) and khusimone (1.2–2.3%) | Pale-yellow to dark brown, olive or amber | Deep, smoky, earthy, and woody with a sweet persistent undertone | [30,97,98] |
a Essential oils’ chemical composition is subject to variation according to changes in extrinsic and extrinsic factors [40].
Table 4.
Odor characteristics of some individual compounds of essential oils.
| Essential Oil Compounds | Odor Description | Reference |
|---|---|---|
| Anethole | Pleasant odor of anise oil | [28] |
| Bisabolol | Sweet floral odor | [28] |
| Bornyl acetate | Woody, camphor, mentholic, spicy | [99] |
| Carvone | Minty herbaceous | [100] |
| Citral | Lemony | [28] |
| Citronellol | Strong floral, rose, sweet like | [101] |
| Cuminal | Spicy harsh | [101] |
| Decanal | Sweet, aldehydic, orange, waxy, citrus rind | [99] |
| Farnesol | Flowery, weak-citrus odor | [28] |
| Geraniol | Fresh, sweet, rose-like | [101] |
| Geranyl acetate | Pleasant, floral rose, herbal | [101] |
| Germacrene D | Woody, spice | [99] |
| Limonene | Strong odor of orange | [28] |
| Linalool | Floral, grassy, pleasant, citrus | [101] |
| Linalyl acetate | Floral, sweet citrus | [100] |
| Menthol | Sweet minty, cooling and fresh scent | [28] |
| Myrcene | Pleasant floral | [101] |
| Myristicin | Spice, warm, balsam, woody | [99] |
| Neral | Citric, green | [100] |
| p-Cymene | Fresh, citrus, terpene, woody, spice | [99] |
| Sabinene | Woody, terpene, citrus, pine, spice | [99] |
| Terpineol | Sweet, lilac odor | [101] |
| Terpinolene | Fresh, woody, sweet, pine, citrus | [99] |
| α-Pinene | Fresh, camphor, sweet, pine, earthy, woody | [99] |
| β-Phellandrene | Mint, turpentine | [99] |
| β-Pinene | Woody, turpentine | [101] |
| γ-Terpinene | Woody, terpene, lemon, lime, tropical, herbal | [99] |
7. Safety of Essential Oils
Mainly due to their perceived safety, the use of essential oils and other botanically derived products has become more popular. However, there have been recent concerns about the standardization and safety of these natural products frequently found in cosmetics and sought as a substitute for synthetic chemicals. For instance, essential oils are challenging to standardize because of the variable growing conditions, genetics, and harvesting of botanicals [102]. In fact, in recent years, several studies have been published with regard to the safety profiles on essential oils [103,104].
Although the vast majority of the most common essential oils have been well tried and tested and safety levels have been determined, there are clearly some essential oils that are more likely to cause adverse reactions than others, and the presence and concentration of a relatively potent allergen is a major factor in allergic contact dermatitis. The presence of adulterants or contaminants may also be a factor. Besides, the oxidation of essential oil constituents can increase risk of causing skin reactions because the oxides and peroxides formed are more reactive. This can be seen with (+)-limonene, δ-3-carene and α-pinene and arise due to the formation of oxidation products, some of which are more sensitizing than the parent compound [103]. For this reason, proper storage of essential oils is required to preserve their effectiveness and decrease the risk of adverse reactions. Accordingly, essential oils should be properly stored in a dark, cool place or refrigerator, in firmly sealed brown bottles [104].
Nevertheless, the fact that an essential oil constituent is capable of being oxidized does not automatically imply that the use of essential oil containing it presents a significant allergenic risk [103]. On the other hand, many essential oils that are considered to be non-toxic can have a toxic effect on some people; this can be influenced by preceding sensitization to a particular essential oil, a group of essential oils having similar components or some adulterants in an essential oil [105].
Photosensitization may also occur when a phototoxin in the essential oil is applied to the skin in the presence of sunlight or ultraviolet A (UVA) light. For instance, furanocoumarins are phototoxins that can be found mainly in expressed citrus peel oils, although they may be present in angelica root (Angelica archangelica L.), rue (Ruta graveolens L.), parsley leaf (Petroselinum crispum (Mill.) Fuss), and marigold (Tagetes minuta L.) essential oils. The most common furanocoumarins are psoralen and bergapten. Inflammatory skin reactions occurring when an essential oil containing these phototoxic compounds applied to the skin is exposed to UVA light can vary from pigmentation, blistering to severe full-thickness burns [106]. While some essential oils are known as dermal irritants, their severity may depend on their concentration. These should not be used on any inflammatory or allergic skin condition and should always be appropriately diluted in vegetable oils. Moreover, since some essential oils can make the skin more sensitive when exposed to the sun and UV rays like those encountered in a tanning bed, exposure to the sun or tanning salon should be avoided for at least 24 h after application of photosensitizing essential oils [107].
Twenty-six possible allergenic fragrances have been defined [108] (Table 5), of which 18 can be found as ingredients of essential oils, and for this reason, they must be declared on the packaging or in the information leaflet if the concentration of these allergenic fragrances is higher than the permissible concentration of 0.01% in shower gels and baths (rinse-off products) and higher than 0.001% in body oils, massage oils and creams (leave-on products) [28]. For example, methyl eugenol should not be intentionally added as a cosmetic ingredient. However, when fragrance compounds containing methyl eugenol present naturally in essential oils are used as components in cosmetic products, the highest concentration of methyl eugenol in the finished products must not go beyond 0.01% in fine fragrance, 0.004% in eau de toilette, 0.002% in a fragrance cream, 0.0002% in other leave-on products and in oral hygiene products, and 0.001% in rinse-off products [109].
Table 5.
List of 26 possible allergenic fragrances [28].
| Amylcinnamal | Geraniol |
| Amylcinnamyl alcohol | Farnesol |
| Anisyl alcohol | Hexyl cinnamaldehyde |
| Benzyl alcohol | Hydroxy-citronellal |
| Benzyl benzoate | Hydroxy-methylpentylcyclohexenecarboxaldehyde |
| Benzyl cinnamate | Isoeugenol |
| Benzyl salicylate | D-Limonene |
| Cinnamyl alcohol | Linalool |
| Cinnamal | Methyl heptin carbonate |
| Citral | 3-Methyl-4-(2,6,6-tri-methyl-2-cyclohexen-1-yl)-3-buten-2-one |
| Citronellol | Oakmoss and treemoss extract |
| Coumarin | treemoss extract |
| Eugenol | 2-(4-tert-Butylbenzyl) propionaldehyde |
Regulatory authorities such as the Food and Drug Administration (FDA) do not require approval for a fragrance that may contain essential oil since fragrances are regarded as cosmetic ingredients. Compared to food products and drugs, the regulation process of the FDA for essential oils that are used as fragrance ingredients is not that rigorous. Consequently, cosmetic manufacturers have an important responsibility to provide high-quality products. The FDA always requires a list of ingredients to ensure product quality; though, it will not oblige the manufacturer to divulge cosmetic secrets because the FDA does not have the authority to require allergen labeling for cosmetics. This situation may be disadvantageous to consumers, particularly to those with less familiarity with essential oils [110].
Furthermore, the International Organization for Standardization (ISO) codifies the composition of each essential oil with an individual identification (e.g., ISO1342:1988 for Rosmarinus officinalis L. and ISO11043:1998 for Ocimum basilicum L.). In ISO/Technical committee 54, ISO has also standardized many analytical methods for controlling the quality of these specifications along with requirements for labeling, transport, and marking. ISO/TC 54 emphasizes global trade in essential oils, thus focusing on improving the quality of the essential oil market and protecting the health of consumers who buy products containing essential oils; to increase the safety level of these oils [110].
Additionally, the International Fragrance Association (IFRA) defines which essential oils and which components represent a potential allergy risk and determines their maximum concentration to produce safe cosmetic products [28]. IFRA also issues recommendations for the safe use of fragrance ingredients, which are published in the IFRA Code of Practice and its guidelines [111].
Essential oil safety is monitored in a variety of different ways, all of which have been geared to the perfumery, cosmetics and food industries. The synthesis of new aroma chemicals and their widespread usage in “natural essential oils” together with many diluents, has brought about many problems, the worst being dermatologic sensitivity [112].
Because of the complexity of essential oils, however, it is important to test specific oils, given that fragrance mixes alone are not sufficient for the elimination of essential oil contact allergy [54,113]. It is often difficult to accurately determine the responsible allergen due to the complexities of the chemical compositions and the co-reactivity with other fragrances. While the screening series includes several popular essential oils, it is important to test patients with their own products, as their composition may have been altered by the aging process. Testing can be achieved safely for most essential oils by the formulation of 2% to 5% oil concentrations in petrolatum [54]. Patch testing can do much to detect and avoid skin reactions. The amount of essential oil used is usually measured in drops or percentage. On average, there are 20 drops of essential oil in 1 mL. Patch tests can be used to test for irritation and are suggested for all potential-risk patients. For this purpose, the essential oil is diluted at double the concentration desired to be used and applied on an adhesive bandage and placed on the forearm. If irritation is bound to happen, it will do so quickly [106].
8. Authenticity of Essential Oils
One of the chief problems with large-scale essential oils’ production is the relatively small yield per unit mass of raw materials that seemingly result in the generally high prices that the oils fetch on the international market [55]. This, coupled with the increasing demand for essential oils, are one of the obvious reasons for essential oil adulteration. Adulteration could feasibly increase toxicity, mainly in the area of skin reactions. The co-presence of both adulterants and contaminants is also a matter of concern [103]. Authentication is therefore of crucial significance for both consumers and chemical companies [114]. Hence, this part of the review will cover some of the known cases of adulterations in essential oils and some existing analytical techniques adopted for their detection.
In the course of time, a great number of techniques have been developed and applied for essential oils’ analyses. While part of them has been replaced by either easier or easier-to-handle techniques, other methods have maintained their importance and have been continuously improved [115].
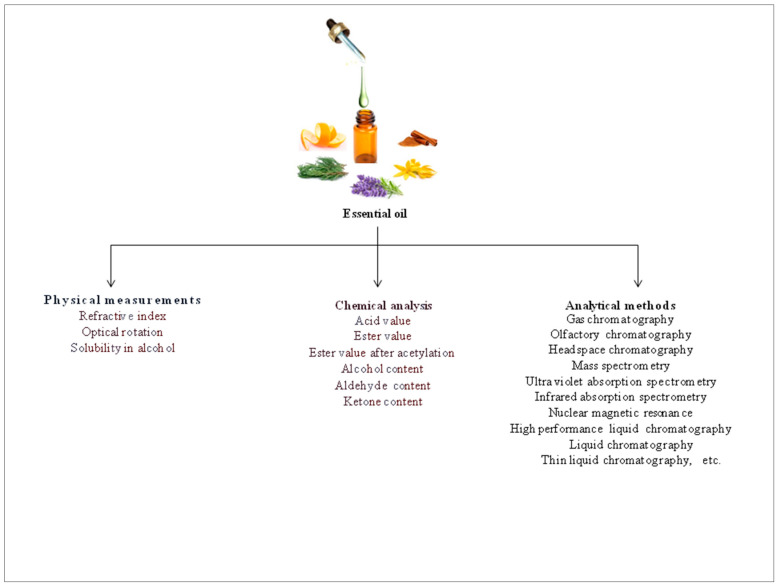
Essential oils have long been analyzed by measuring their physicochemical constants. These values indicate the quality of the oils and are critical for the detection of adulteration. The physical constants include the specific gravity, refractive index and optical rotation. Solubility is expressed in terms of solubility in ethyl alcohol and has a practical significance. On the other hand, the chemical constants used to assess essential oils are acid value, ester value after acetylation, as well as alcohol, aldehyde, and ketone contents, etc. These parameters demonstrate the characteristics of essential oils and are greatly helpful in evaluating their quality (Figure 2) [3].
Figure 2.
Analysis of essential oils.
Gas chromatography (GC) and mass spectrometry (MS) are the most common and important tools used to analyze the constituents of essential oils and can add further improvements to essential oil analysis where broad categories of monoterpenes and varying isomers strongly resembling each other in terms of their chemical structures can be individually detected [116]. In fact, structural isomers, as well as cis–trans isomers, are generally separated in GC with standard columns and hence, is the method of choice for the analysis of essential oils [117]. In addition, optically active compounds are detectable by chiral chromatography [118]. GC is ideally suited to volatile compounds and has revolutionized the detection of minor chemical constituents, especially when used in conjunction with MS and nuclear magnetic resonance (NMR) spectroscopy. MS looks at the fragmentation patterns of compounds under ionizing conditions, and this information is used to deduce their structures, while NMR elucidates the structures of molecules by examining the environment of specific atoms such as hydrogen, by looking at their characteristic nuclear spins. The sensitivity of analytical techniques for organic compounds has considerably increased over recent years to the point whereby even trace constituents, including pollutants such as pesticides, can be detected as well [103].
A variety of chromatographic columns and detectors are used in GC that has indeed helped to expand its applicability to various analytical problems, including essential oil analysis. Different types of detectors (selective or not) can be used to generate signals. The flame ionization detector (FID) is the most popular detector for GC for its reliability and its sensitivity in the detection of organic vapors and is non-selective compared to many specific detectors. FID detectors are mass rather than concentration sensitive and can detect most carbon-containing substances, which makes them very convenient for the analysis of the majority of essential oils [119].
There is no simple generalization about the key odorants in essential oils; the odor of some essential oils is due to a large amount of a single compound, although trace amounts could determine the odor of others, and in general, the true odor is the manifestation of a complex mixture of compounds [120]. Gas-chromatography-olfactometry (GC-O) is a well-known standard technique that enables the assessment of odor-active components in complex mixtures, based on the correlation between the chromatographic peaks of the eluted substances perceived simultaneously by two detectors, one of them being the human olfactory system. Over the years, the GC-O has been frequently employed in essential oil analysis [100,121,122]. Results of GC-O analysis can give information about the presence or absence of odor in a compound, measure the duration of odor activity, describe the quality of the odor perceived, and quantify the intensity of specific odorant which may have an ultimate purpose in the application of such compounds in the flavor and fragrance industries. GC-O is also used to locate the odor-active regions in the chromatogram and to generate an odor profile for the whole essential oil sample [123].
High-Performance liquid chromatography-Ultraviolet (HPLC-UV) may represent a supplementary or even alternative method for analysis of volatile oils because of their sensitivity, versatility, and selectivity [124]. Usually, HPLC is the method of choice in the analysis of less volatile constituents of essential oils [125]. The use of HPLC-MS provides valuable information about the content and nature of constituents of natural complex matrices, such as essential oils [126].
Indeed, several reported cases of essential oil adulteration have been documented, such as the addition of a non-volatile ingredient, cheap synthetic compounds, volatile or essential oil from other natural sources, as well as vegetable oils so as to increase their weight. Other than that, the total or partial substitution of part of the original plant from which the essential oil is obtained by other plants has also been reported [52,127]. All these adulteration methods can degrade the quality of the essential oils and, by adding one or more synthetic compounds, adulteration can lead to safety issues or non-compliance with the natural labels. Consequently, authentication is an important topic for consumer protection and the quality of essential oil production [128]. Adulteration of essential oils can also have an effect on the regulatory aspect, as an essential oil may no longer conform to specifications of standardization. Most of the time, adulterants are added at a low level (5–8%) to avoid detection by common analytical methods [129].
9. Conclusions
Essential oils have seen a revival in popularity in the last few years. They are widely used in the cosmetic industries as a fragrance and active components. Moreover, their ability to impart a wide range of unique and pleasant aromas in cosmetic products and at the same time acting as bioactive agents (anti-aging, antimicrobial, sun protection, and whitening) make them prized and highly valued ingredients in cosmetics and cosmeceutical products.
Moreover, the ‘’back to nature” trend has enormously amplified the use of botanical extracts and oils at the cost of artificial and synthetic derivatives, thought to be hazardous to human health. Furthermore, unlike artificial fragrances, which can mimic some herbal fragrances, essential oils are nonetheless more in demand given the increasing awareness of their health benefits supported by scientific findings, thus making them more tempting and attractive to consumers. On that note, the future of the essential oils industry seems promising, with lucrative avenues in the cosmetic and perfume industries.
However, although essential oils are generally regarded as safe, as complex mixtures of compounds, some of which known to be allergens and skin sensitizing agents, need to be indicated on cosmetics labels, especially for consumers having sensitive, allergic-prone skin or existing skin disorders and could opt for patch testing before using products containing them.
Author Contributions
Conceptualization, J.B.S.; F.M.M.; methodology, G.Z.; F.M.; software, G.Z.; validation, J.B.S.; F.M.M.; formal analysis, F.M.M.; investigation, J.B.S.; F.M.M.; resources; G.Z.; data curation, F.M.M.; writing—original draft preparation, J.B.S.; F.M.M.; writing—review and editing, J.B.S.; F.M.M.; visualization, G.Z.; supervision, F.M.M.; project administration, G.Z.; FM.; funding acquisition, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of essential oils are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coppen J.J. Flavours and Fragrances of Plant Origin. FAO; Rome, Italy: 1995. [Google Scholar]
- 2.Amberg N., Fogarassy C. Green consumer behavior in the cosmetics market. Resources. 2019;8:137. doi: 10.3390/resources8030137. [DOI] [Google Scholar]
- 3.Mitsui T. New Cosmetic Science. Elsevier; Amsterdam, The Netherlands: 1997. [Google Scholar]
- 4.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 5.Benelli G., Pavela R., Petrelli R., Cappellacci L., Canale A., Senthil-Nathan S., Maggi F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod. 2018;124:236–243. doi: 10.1016/j.indcrop.2018.07.048. [DOI] [Google Scholar]
- 6.Pouresmaeil M., Nojadeh M.S., Movafeghi A., Maggi F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 2020;155:112785. doi: 10.1016/j.indcrop.2020.112785. [DOI] [Google Scholar]
- 7.Zuzarte M., Salgueiro L. Bioactive Essential Oils and Cancer. Springer; Berlin, Germany: 2015. Essential oils chemistry; pp. 19–61. [Google Scholar]
- 8.Burger P., Plainfossé H., Brochet X., Chemat F., Fernandez X. Extraction of natural fragrance ingredients: History overview and future trends. Chem. Biodivers. 2019;16:e1900424. doi: 10.1002/cbdv.201900424. [DOI] [PubMed] [Google Scholar]
- 9.Loo M. Integrative Medicine for Children. Elsevier Health Sciences; Amsterdam, The Netherlands: 2008. [Google Scholar]
- 10.Burnett C.L., Fiume M.M., Bergfeld W.F., Belsito D.V., Hill R.A., Klaassen C.D., Liebler D.C., Marks J.G., Jr., Shank R.C., Slaga T.J. Safety Assessment of Citrus-Derived Peel Oils as Used in Cosmetics. Int. J. Toxicol. 2019;38:33S–59S. doi: 10.1177/1091581819862504. [DOI] [PubMed] [Google Scholar]
- 11.Pitman V. Aromatherapy: A Practical Approach. Nelson Thornes; Cheltenham, UK: 2004. [Google Scholar]
- 12.Hussain H., Al-Harrasi A., Green I.R. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; Amsterdam, The Netherlands: 2016. Frankincense (Boswellia) Oils; pp. 431–440. [Google Scholar]
- 13.Barbieri C., Borsotto P. In: Essential Oils: Market and Legislation. El-Shemy H., editor. Potential of Essential Oils, IntechOpen; London, UK: 2018. pp. 107–127. [Google Scholar]
- 14.Dreger M., Wielgus K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013;59:142–156. doi: 10.2478/hepo-2013-0030. [DOI] [Google Scholar]
- 15.Herman A., Herman A.P., Domagalska B.W., Młynarczyk A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013;53:232–237. doi: 10.1007/s12088-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manou I., Bouillard L., Devleeschouwer M., Barel A. Evaluation of the preservative properties of Thymus vulgaris essential oil in topically applied formulations under a challenge test. J. Appl. Microbiol. 1998;84:368–376. doi: 10.1046/j.1365-2672.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- 17.Aumeeruddy-Elalfi Z., Lall N., Fibrich B., Van Staden A.B., Hosenally M., Mahomoodally M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018;26:232–243. doi: 10.1016/j.jfda.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra A., Mishra A., Chattopadhyay P. Assessment of in vitro sun protection factor of Calendula officinalis L.(asteraceae) essential oil formulation. J. Young Pharm. 2012;4:17–21. doi: 10.4103/0975-1483.93575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wongsukkasem N., Soynark O., Suthakitmanus M., Chongdiloet E., Chairattanapituk C., Vattanikitsiri P., Hongratanaworakit T., Tadtong S. Antiacne-causing Bacteria, Antioxidant, Anti-Tyrosinase, Anti-Elastase and Anti-Collagenase Activities of Blend Essential Oil comprising Rose, Bergamot and Patchouli Oils. Nat. Prod. Commun. 2018;13:639–642. doi: 10.1177/1934578X1801300529. [DOI] [Google Scholar]
- 20.Winkelman W.J. Aromatherapy, botanicals, and essential oils in acne. Clin. Dermatol. 2018;36:299–305. doi: 10.1016/j.clindermatol.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Mazzarello V., Gavini E., Rassu G., Donadu M.G., Usai D., Piu G., Pomponi V., Sucato F., Zanetti S., Montesu M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020;13:233. doi: 10.2147/CCID.S236956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao H., Yang G., Wang Y., Liu J.P., Smith C.A., Luo H., Liu Y. Complementary therapies for acne vulgaris. Cochrane Database Syst. Rev. 2015;1:CD009436. doi: 10.1002/14651858.CD009436.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavanaugh J.L. Ph.D. Thesis. University of Colorado; Denver, CO, USA: 2013. Examining the Differential Effects of Natural and Synthetic Aromas of Lavender and Peppermint on Cognition, Mood, and Subjective Workload. [Google Scholar]
- 24.Olujimi O., Fatoki O.S., Odendaal J.P., Okonkwo J. Endocrine disrupting chemicals (phenol and phthalates) in the South African environment: A need for more monitoring. Water SA. 2010;36:5. doi: 10.4314/wsa.v36i5.62001. [DOI] [Google Scholar]
- 25.Carson P.A. Hazardous Chemicals Handbook. Elsevier; Amsterdam, The Netherlands: 2002. [Google Scholar]
- 26.Ahuja K., Singh S. Essential Oils Market Size by Application (Orange oil, Lemon oil, Eucalyptus oil, Clove oil, Peppermint oil, Jasmine oil, Rosemary oil, Cornmint oil, Citronella oil, Geranium, Spearmint oil, Lavender oil, Tea tree oil and others) by Application (Food & beverage, Aromatherapy, Cosmetics & Toiletries, Pharmaceuticals, Cleaning & Home care, Animal Feed, Fragrances and Others) Industry Analysis Report, Regional Outlook, Growth Potential, Competitive Market Share & Forecast, 2019–2026. Global Market Insights, Inc. [(accessed on 11 December 2020)];2019 Available online: https://www.gminsights.com/industry-analysis/essential-oil-market.
- 27.Butnariu M., Sarac I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018;1:35. doi: 10.14302/issn.2576-6694.jbbs-18-2489. [DOI] [Google Scholar]
- 28.Sarkic A., Stappen I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics. 2018;5:11. [Google Scholar]
- 29.De Silva T. Development of essential oil industries in developing countries In A Manual on the Essential Oil Industry. United Nations Ndustrlal Development Organization; Vienna, Austria: 1995. Chapter 1. [Google Scholar]
- 30.Burger P., Landreau A., Watson M., Janci L., Cassisa V., Kempf M., Azoulay S., Fernandez X. Vetiver Essential Oil in Cosmetics: What Is New? Medicines. 2017;4:41. doi: 10.3390/medicines4020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babita S., Sellam P., Jayoti M., Puja R. Floral essential oils: Importance and uses for mankind. HortFlora Res. Spectr. 2014;3:7–13. [Google Scholar]
- 32.Franz C., Novak J. Sources of essential oils. In: Hüsnü Can Baser K., Buchbauer G., editors. Handbook of Essential Oils and Science, Technology and Applications. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2010. pp. 39–81. [DOI] [Google Scholar]
- 33.Tongnuanchan P., Benjakul S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014;79:R1231–R1249. doi: 10.1111/1750-3841.12492. [DOI] [PubMed] [Google Scholar]
- 34.Baser K.H.C., Buchbauer G. Handbook of Essential Oils: Science, Technology, and Applications. CRC Press; Boca Raton, FL, USA: 2015. [Google Scholar]
- 35.Başer K.H.C., Demirci F. Chemistry of essential oils. In: Berger R.G., editor. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Springer; New York, NY, USA: 2007. pp. 43–86. [Google Scholar]
- 36.Sawamura M. Citrus Essential Oils: Flavor and Fragrance. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 37.Adlard E. Handbook of Essential Oils. Science, Technology and Applications. Springer; Berlin, Germany: 2010. [Google Scholar]
- 38.Ridder M. Essential Oils Market Worldwide-Statistics & Facts. [(accessed on 23 December 2020)];2020 Available online: https://www.statista.com/topics/5174/essential-oils/
- 39.Sadgrove N., Jones G. A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian agriculture. Agriculture. 2015;5:48–102. doi: 10.3390/agriculture5010048. [DOI] [Google Scholar]
- 40.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turek C., Stintzing F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013;12:40–53. doi: 10.1111/1541-4337.12006. [DOI] [Google Scholar]
- 42.Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grosch W. Flavours and Fragrances. Springer; Berlin, Germany: 2007. Gas Chromatography—Olfactometry of Aroma Compounds; pp. 363–378. [Google Scholar]
- 44.Kusuma H.S., Mahfud M. Chemical Composition of Essential Oil of Indonesia Sandalwood Extracted by Microwave-Assisted Hydrodistillation. AIP Conf. Proc. 2016;1755:050001-1–050001-6. [Google Scholar]
- 45.Noudogbessi J., Yedomonhan H., Alitonou G., Chalard P., Figueredo G., Adjalian E., Avlessi F., Chalchat J., Sohounhloué D. Physical characteristics and chemical compositions of the essential oils extracted from different parts of Siphonochilusaethiopicus (Schweinf.) BL Burtt (Zingiberaceae) harvested in Benin. J. Chem. Pharm. Res. 2012;4:4845–4851. [Google Scholar]
- 46.Ashour M., Wink M., Gershenzon J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. Annu. Plant Rev. 2018;40:258–303. [Google Scholar]
- 47.Llana-Ruiz-Cabello M., Pichardo S., Maisanaba S., Puerto M., Prieto A.I., Gutierrez-Praena D., Jos A., Camean A.M. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem. Toxicol. 2015;81:9–27. doi: 10.1016/j.fct.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 48.Pavela R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015;76:174–187. [Google Scholar]
- 49.Sell C.S. The Chemistry of Fragrances: From Perfumer to Consumer. Royal Society of Chemistry; London, UK: 2006. [Google Scholar]
- 50.Lahlou M. Essential oils and fragrance compounds: Bioactivity and mechanisms of action. Flavour Fragr. J. 2004;19:159–165. doi: 10.1002/ffj.1288. [DOI] [Google Scholar]
- 51.Laska M., Teubner P. Olfactory discrimination ability of human subjects for ten pairs of enantiomers. Chem. Senses. 1999;24:161–170. doi: 10.1093/chemse/24.2.161. [DOI] [PubMed] [Google Scholar]
- 52.Salgueiro L., Martins A., Correia H. Raw materials: The importance of quality and safety. A review. Flavour Fragr. J. 2010;25:253–271. doi: 10.1002/ffj.1973. [DOI] [Google Scholar]
- 53.Şanli A., Karadoğan T. Geographical impact on essential oil composition of endemic Kundmannia anatolica Hub.-Mor. (Apiaceae) Afr. J. Tradit. Complementary Altern. Med. 2017;14:131–137. doi: 10.21010/ajtcam.v14i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Groot A.C., Schmidt E. Essential oils, part IV: Contact allergy. Dermatitis. 2016;27:170–175. doi: 10.1097/DER.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 55.Masango P. Ph.D. Thesis. University of Surrey; Guildford, UK: 2001. Towards Understanding Steam Distillation of Essential Oils by Differential Quantification of Principal Components Using Capillary Gas Chromatography. [Google Scholar]
- 56.Irshad M., Subhani M.A., Ali S., Hussain A. Biological Importance of Essential Oils, Essential Oils—Oils of Nature, Hany A. El-Shemy. [(accessed on 15 December 2020)];2020 Available online: https://www.intechopen.com/books/essential-oils-oils-of-nature/biological-importance-of-essential-oils.
- 57.Vankar P.S. Essential oils and fragrances from natural sources. Resonance. 2004;9:30–41. doi: 10.1007/BF02834854. [DOI] [Google Scholar]
- 58.Simon J. Essential oils and culinary herbs, Advances in new crops; Proceedings of the First National Symposium ’New Crops: Research, Development, Economics’; Indianapolis, IN, USA. 3–26 October 1988; Portland, OR, USA: Timber Press; 1990. pp. 472–483. [Google Scholar]
- 59.Da Silva-Santos A., Antunes A., D’avila L., Bizzo H., Souza-Santos L. The use of essential oils and terpenics/terpenoids in cosmetics and perfumery: A study identifies the major uses of essential oils and terpenics/terpenoids through granted patents. Perfum. Flavor. 2005;30:50–55. [Google Scholar]
- 60.ISO 3525: 2008(E) Oil of Amyris Balsamifera L. [(accessed on 15 December 2020)];2008 Available online: https://www.sis.se/api/document/preview/910039/
- 61.Lawless J. The Encyclopedia of Essential Oils: The Complete Guide to the Use of Aromatic oils in Aromatherapy, Herbalism, Health, and Well Being. Conari Press; San Francisco, CA, USA: 2013. [Google Scholar]
- 62.Maia J.G.S., Andrade E.H.A., Couto H.A.R., Da Silva A.C.M., Marx F., Henke C. Plant sources of Amazon rosewood oil. Quim. Nova. 2007;30:1906–1910. doi: 10.1590/S0100-40422007000800021. [DOI] [Google Scholar]
- 63.Maia J.G.S., Mourão R.H.V. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; Amsterdam, The Netherlands: 2016. Amazon Rosewood (Aniba rosaeodora Ducke) Oils; pp. 193–201. [Google Scholar]
- 64.Lavabre M. Aromatherapy Workbook. Inner Traditions/Bear & Co; Windsor County, VT, USA: 1996. [Google Scholar]
- 65.Van Vuuren S.F., Kamatou G.P., Viljoen A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010;76:686–691. doi: 10.1016/j.sajb.2010.06.001. [DOI] [Google Scholar]
- 66.ISO 8896: 2016(E) Essential Oil of Caraway (Carum carvi L.) 2016. [(accessed on 15 December 2020)]; Available online: https://www.sis.se/api/document/preview/920364/
- 67.Battaglia S. Essential oil Monograph: Atlas Cedarwood. [(accessed on 15 December 2020)];2019 Available online: http://www.salvatorebattaglia.com.au/images/pdf/A4_Monograph_Atlas_Cedarwood.pdf.
- 68.Jain S., Arora P., Popli H. A comprehensive review on Citrus aurantifolia essential oil: Its phytochemistry and pharmacological aspects. Braz. J. Nat. Sci. 2020;3:354. doi: 10.31415/bjns.v3i2.101. [DOI] [Google Scholar]
- 69.Wang K., Zhu R.-Z., Qu R.-F., Li Z.-Y. Comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry for the analysis of volatile components in Neroli essential oil. Mendeleev Commun. 2012;1:45–46. doi: 10.1016/j.mencom.2012.01.018. [DOI] [Google Scholar]
- 70.Aguilar-Hernández M.G., Sánchez-Bravo P., Hernández F., Carbonell-Barrachina Á.A., Pastor-Pérez J.J., Legua P. Determination of the Volatile Profile of Lemon Peel Oils as Affected by Rootstock. Foods. 2020;9:241. doi: 10.3390/foods9020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ISO 3140:2019 (E) Essential Oil of Sweet Orange Expressed (Citrus sinensis L.) [(accessed on 15 December 2020)];2019 Available online: https://www.sis.se/api/document/preview/80011724/
- 72.Arctander S. Perfume and flavor materials of natural origin. Perfum. Flavor Mater. Nat. Origin. 1960:370 [Google Scholar]
- 73.Soares B.V., Morais S.M., dos Santos Fontenelle R.O., Queiroz V.A., Vila-Nova N.S., Pereira C.M., Brito E.S., Neto M.A., Brito E.H., Cavalcante C.S., et al. Antifungal activity, toxicity and chemical composition of the essential oil of Coriandrum sativum L. fruits. Molecules. 2012;17:8439–8448. doi: 10.3390/molecules17078439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groom N.F. In: The Perfume Handbook. Groom N., editor. Springer Netherlands; Dordrecht, The Netherlands: 1992. pp. 81–89. [Google Scholar]
- 75.Mahmood Z., Ahmed I., Saeed M.U.Q., Sheikh M.A. Investigation of physico-chemical composition and antimicrobial activity of essential oil extracted from lignin-containing Cupressus sempervirens. BioResources. 2013;8:1625–1633. doi: 10.15376/biores.8.2.1625-1633. [DOI] [Google Scholar]
- 76.Rao B.R.R., Rajput D.K., Patel R.P., Purnanand S. Essential oil yield and chemical composition changes during leaf ontogeny of palmarosa (Cymbopogon martinii var. motia) Nat. Prod. Commun. 2010;5:1947–1950. doi: 10.1177/1934578X1000501224. [DOI] [PubMed] [Google Scholar]
- 77.Rokbeni N., M’rabet Y., Dziri S., Chaabane H., Jemli M., Fernandez X., Boulila A. Variation of the chemical composition and antimicrobial activity of the essential oils of natural populations of Tunisian Daucus carota L.(Apiaceae) Chem. Biodivers. 2013;10:2278–2290. doi: 10.1002/cbdv.201300137. [DOI] [PubMed] [Google Scholar]
- 78.ISO 4733: 2004(E) Essential Oil of Cardamom. [(accessed on 15 December 2020)];2004 Available online: https://www.sis.se/api/document/preview/904961/
- 79.Kladar N.V., Anačkov G.T., Rat M.M., Srđenović B.U., Grujić N.N., Šefer E.I., Božin B.N. Biochemical characterization of Helichrysum italicum (Roth) G. Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy, and antioxidant properties. Chem. Biodivers. 2015;12:419–431. doi: 10.1002/cbdv.201400174. [DOI] [PubMed] [Google Scholar]
- 80.Judžentienė A. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; Amsterdam, The Netherlands: 2016. Hyssop (Hyssopus officinalis L.) Oils; pp. 471–479. [Google Scholar]
- 81.Majewska E., Kozłowska M., Kowalska D., Gruczyńska E. Characterization of the essential oil from cone-berries of Juniperus communis L. (Cupressaceae) Herba Pol. 2017;63:48–55. doi: 10.1515/hepo-2017-0018. [DOI] [Google Scholar]
- 82.Alejo-Armijo A., Altarejos J., Salido S. Phytochemicals and biological activities of Laurel tree (Laurus nobilis) Nat. Prod. Commun. 2017;12:743–757. doi: 10.1177/1934578X1701200519. [DOI] [PubMed] [Google Scholar]
- 83.Si L., Chen Y., Han X., Zhan Z., Tian S., Cui Q., Wang Y. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules. 2012;17:7057–7066. doi: 10.3390/molecules17067057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh O., Khanam Z., Misra N., Srivastava M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ISO 856:2006, Oil of Peppermint (Mentha x piperita L.) [(accessed on 13 December 2020)];2006 Available online: https://www.sis.se/api/document/preview/907351/
- 86.Periasamy G., Karim A., Gibrelibanos M., Gebremedhin G. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; Amsterdam, The Netherlands: 2016. Nutmeg (Myristica fragrans Houtt.) oils; pp. 607–616. [Google Scholar]
- 87.ISO 21389:2004(E), Oil of Gum Turpentine (Pinus Massoniana Lamb.) [(accessed on 18 December 2020)];2004 Available online: https://cdn.standards.iteh.ai/samples/35861/a65f5ee52cc5413197cbc3e49c675fda/ISO-21389-2004.pdf.
- 88.Van Beek T.A., Joulain D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018;33:6–51. doi: 10.1002/ffj.3418. [DOI] [Google Scholar]
- 89.Atanasova T., Kakalova M., Stefanof L., Petkova M., Stoyanova A., Damyanova S., Desyk M. Chemical composition of essential oil from Rosa Damascena mill., growing in new region of Bulgaria. Ukr. Food J. 2016;5:492–498. [Google Scholar]
- 90.Jalali-Heravi M., Parastar H., Sereshti H. Development of a method for analysis of Iranian damask rose oil: Combination of gas chromatography–mass spectrometry with Chemometric techniques. Anal. Chim. Acta. 2008;623:11–21. doi: 10.1016/j.aca.2008.05.078. [DOI] [PubMed] [Google Scholar]
- 91.Verma R.S., Padalia R.C., Chauhan A., Singh A., Yadav A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011;25:1577–1584. doi: 10.1080/14786419.2010.520162. [DOI] [PubMed] [Google Scholar]
- 92.Tomi K., Kitao M., Konishi N., Murakami H., Matsumura Y., Hayashi T. Enantioselective GC–MS analysis of volatile components from rosemary (Rosmarinus officinalis L.) essential oils and hydrosols. Biosci. Biotechnol. Biochem. 2016;80:840–847. doi: 10.1080/09168451.2016.1146066. [DOI] [PubMed] [Google Scholar]
- 93.Pešić P.Ž., Banković V.M. Investigation on the essential oil of cultivated Salvia sclarea L. Flavour Fragr. J. 2003;18:228–230. [Google Scholar]
- 94.Joy P.P., Thomas J., Mathew S., Skaria B.P. Tropical aromatic and medicinal plants. Aromat. Med. Plants Res. Stn. Odakkali Asamannoor PO Kerala India. 1998:83–85. [Google Scholar]
- 95.Howes M.-J.R., Simmonds M.S., Kite G.C. Evaluation of the quality of sandalwood essential oils by gas chromatography–mass spectrometry. J. Chromatogr. 2004;1028:307–312. doi: 10.1016/j.chroma.2003.11.093. [DOI] [PubMed] [Google Scholar]
- 96.Nurdjannah N., Bermawie N.C. Cloves. Indonesian Agency for Agriculture Research and Development (IAARD) Indonesia. 2012:197–215. [Google Scholar]
- 97.Chomchalow N. The Utilization of Vetiver as Medicinal and Aromatic Plants with Special Reference to Thailand. PRVN/ORDPB; Bangkok, Thailand.: 2001. (Tech. Bull. No. 2001/1). [Google Scholar]
- 98.David A., Wang F., Sun X., Li H., Lin J., Li P., Deng G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Vetiveria zizanioides (L.) Nash Essential Oil Extracted by Carbon Dioxide Expanded Ethanol. Molecules. 2019;24:1897. doi: 10.3390/molecules24101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Zaeddi H., Martínez-Tomé J., Calín-Sánchez Á., Burló F., Carbonell-Barrachina Á.A. Volatile composition of essential oils from different aromatic herbs grown in mediterranean regions of Spain. Foods. 2016;5:41. doi: 10.3390/foods5020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.D’Acampora Zellner B., Lo Presti M., Barata L.E.S., Dugo P., Dugo G., Mondello L. Evaluation of leaf-derived extracts as an environmentally sustainable source of essential oils by using gas chromatography− mass spectrometry and enantioselective gas chromatography− olfactometry. Anal. Chem. 2006;78:883–890. doi: 10.1021/ac051337s. [DOI] [PubMed] [Google Scholar]
- 101.Ravi R., Prakash M., Bhat K.K. Aroma characterization of coriander (Coriandrum sativum L.) oil samples. Eur. Food Res. Technol. 2007;225:367–374. doi: 10.1007/s00217-006-0425-7. [DOI] [Google Scholar]
- 102.Mortimer S., Reeder M. Botanicals in dermatology: Essential oils, botanical allergens, and current regulatory practices. Dermatitis. 2016;27:317–324. doi: 10.1097/DER.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 103.Tisserand R., Young R. Essential Oil Safety A Guide for Health Care Professionals. 2nd ed. Elsevier; London, UK: 2014. Essential oil profiles; pp. 187–482. [Google Scholar]
- 104.Vostinaru O., Heghes S.C., Filip L. Essential Oils-Bioactive Compounds, New Perspectives and Applications. IntechOpen; London, UK: 2020. Safety Profile of Essential Oils. [Google Scholar]
- 105.Bouchiha H., Rouabhi R., Bouchama K., Djebar-Berrebbah H., Djebar M. Potential toxicity of essential oil extracted from medicinal plant (Mentha piperita) on an alternative cellular model paramecium sp. Rev. Kasmera. 2015;43:114–130. [Google Scholar]
- 106.Buckle J. Clinical Aromatherapy (Third Edition) Essential Oils in Healthcare. Churchill Livingstone; London, UK: 2016. Essential Oil Toxicity and Contraindications; pp. 73–94. [Google Scholar]
- 107.Balick R. A peek into safe use of essential oils. Pharm. Today. 2019;25:22–23. doi: 10.1016/j.ptdy.2019.07.013. [DOI] [Google Scholar]
- 108.Van Oosten E.J., Schuttelaar M.L.A., Coenraads P.J. Clinical relevance of positive patch test reactions to the 26 EU-labelled fragrances. Contact Dermat. 2009;61:217–223. doi: 10.1111/j.1600-0536.2009.01605.x. [DOI] [PubMed] [Google Scholar]
- 109.SCCS, Reference: Scientific Committee on Consumer Safety (SCCS) Opinion on Fragrance Allergens in Cosmetic Products. [(accessed on 26 December 2020)];2012 Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_102.pdf.
- 110.Es I., Khaneghah A.M., Akbariirad H. Essential Oils in Food Processing: Chemistry, Safety and Applications. Wiley-Blackwell; Hoboken, NJ, USA: 2017. Global regulation of essential oils; pp. 327–338. [Google Scholar]
- 111.SCCNFP The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers, Fragrance Allergy in Consumers. A Review of the Problem. [(accessed on 22 December 2020)];Analysis of the Need for Appropriate Consumer Information and Identification of Consumer Allergens. 1999 Available online: https://ec.europa.eu/health/ph_risk/committees/sccp/documents/out98_en.pdf.
- 112.Lis-Balchin M. Aromatherapy Science: A Guide for Healthcare Professionals. Pharmaceutical Press; London, UK: 2006. [Google Scholar]
- 113.Warshaw E.M., Zug K.A., Belsito D.V., Fowler J.F., Jr., DeKoven J.G., Sasseville D., Maibach H.I., Mathias C.T., DeLeo V.A., Taylor J.S. Positive patch-test reactions to essential oils in consecutive patients from North America and Central Europe. Dermatitis. 2017;28:246–252. doi: 10.1097/DER.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 114.Do T.K.T., Hadji-Minaglou F., Antoniotti S., Fernandez X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015;66:146–157. [Google Scholar]
- 115.Kubeczka K.-H. Handbook of Essential Oils. CRC Press; Boca Raton, FL, USA: 2020. History and Sources of Essential Oil Research; pp. 3–39. [Google Scholar]
- 116.Chin S.T., Nolvachai Y., Marriott P.J. Enantiomeric Separation in Comprehensive Two-Dimensional Gas Chromatography with Accurate Mass Analysis. Chirality. 2014;26:747–753. doi: 10.1002/chir.22280. [DOI] [PubMed] [Google Scholar]
- 117.Chizzola R. Regular monoterpenes and sesquiterpenes (essential oils) Nat. Prod. 2013;10:2973–3008. [Google Scholar]
- 118.Fiorini D., Scortichini S., Bonacucina G., Greco N.G., Mazzara E., Petrelli R., Torresi J., Maggi F., Cespi M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crops Prod. 2020;154:112688. [Google Scholar]
- 119.Mani B.K., Murthy V., Boland M., Yee K. Analysis of constituents in different Fractions collected during distillation of Cardamom oil for flavour and fragrance applications. J. Appl. Pharm. Sci. 2017;7:177–183. doi: 10.7324/JAPS.2017.70125. [DOI] [Google Scholar]
- 120.Clery R. High-impact odorants in essential oils. Flavour Fragr. J. 2010;25:117–120. doi: 10.1002/ffj.1980. [DOI] [Google Scholar]
- 121.Benzo M., Gilardoni G., Gandini C., Caccialanza G., Finzi P.V., Vidari G., Abdo S., Layedra P. Determination of the threshold odor concentration of main odorants in essential oils using gas chromatography–olfactometry incremental dilution technique. J. Chromatogr. 2007;1150:131–135. doi: 10.1016/j.chroma.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 122.Breme K., Tournayre P., Fernandez X., Meierhenrich U.J., Brevard H., Joulain D., Berdague J.L. Identification of odor impact compounds of Tagetes minuta L. essential oil: Comparison of two GC-olfactometry methods. J. Agric. Food Chem. 2009;57:8572–8580. doi: 10.1021/jf9016509. [DOI] [PubMed] [Google Scholar]
- 123.Eyres G., Marriott P.J., Dufour J.-P. The combination of gas chromatography–olfactometry and multidimensional gas chromatography for the characterisation of essential oils. J. Chromatogr. 2007;1150:70–77. doi: 10.1016/j.chroma.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 124.Da S. Rauber, C.; Guterres, S.S.; Schapoval, E.E. LC determination of citral in Cymbopogon citratus volatile oil. J. Pharm. Biomed. Anal. 2005;37:597–601. doi: 10.1016/j.jpba.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 125.Lockwood G. Techniques for gas chromatography of volatile terpenoids from a range of matrices. J. Chromatogr. 2001;936:23–31. doi: 10.1016/S0021-9673(01)01151-7. [DOI] [PubMed] [Google Scholar]
- 126.Dugo P., Mondello L., Dugo L., Stancanelli R., Dugo G. LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J. Pharm. Biomed. Anal. 2000;24:147–154. doi: 10.1016/S0731-7085(00)00400-3. [DOI] [PubMed] [Google Scholar]
- 127.König W.A., Hochmuth D.H. Enantioselective gas chromatography in flavor and fragrance analysis: Strategies for the identification of known and unknown plant volatiles. J. Chromatogr. Sci. 2004;42:423–439. doi: 10.1093/chromsci/42.8.423. [DOI] [PubMed] [Google Scholar]
- 128.Mosandl A. Authenticity assessment: A permanent challenge in food flavor and essential oil analysis. J. Chromatogr. Sci. 2004;42:440–449. doi: 10.1093/chromsci/42.8.440. [DOI] [PubMed] [Google Scholar]
- 129.Pellati F., Orlandini G., van Leeuwen K.A., Anesin G., Bertelli D., Paolini M., Benvenuti S., Camin F. Gas chromatography combined with mass spectrometry, flame ionization detection and elemental analyzer/isotope ratio mass spectrometry for characterizing and detecting the authenticity of commercial essential oils of Rosa damascena Mill. Rapid Commun. Mass Spectrom. 2013;27:591–602. doi: 10.1002/rcm.6489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.